Abstract
Background
Time trends in overweight and obesity in the general population have been well documented; however, temporal patterns in type 1 diabetes (T1D) have not been thoroughly investigated. We therefore assessed temporal patterns in overweight and obesity and predictors of weight change in 589 individuals from the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study, a cohort of childhood onset T1D.
Methods
Participants were first seen in 1986–1988, when mean age and diabetes duration were 29 and 20 years, respectively, and biennially thereafter for 18 years. Overweight was defined as 25≤BMI<30 kg/m2. Obese was defined as a BMI≥30 kg/m2.
Results
At baseline, the prevalence of overweight and obesity were 28.6% and 3.4%, respectively. After 18 years of follow-up, the prevalence of overweight increased by 47% while the prevalence of obesity increased 7-fold. Seven percent were on intensive insulin therapy (≥3 insulin injections per day or on insulin pump) at baseline; by 2004–2007, this was 82%. Predictors of weight change were a higher baseline HbA1c, symptomatic autonomic neuropathy (inversely), overt nephropathy (inversely), and going onto intensive insulin therapy during follow-up.
Conclusion
These data demonstrate dramatic weight gain in T1D and underscore the complexity of weight change in this disease.
Time trends in overweight and obesity in the general population have been well documented [1–3]; however, such temporal patterns in populations with pre-existing disease, such as type 1 diabetes (T1D), have not been as thoroughly investigated. Traditionally, the phenotype of T1D was normal or underweight; however, there is evidence that this may be changing. A lower prevalence of overweight and obesity in the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study, relative to the general population has been reported, although the incidence (12%) in both populations was similar over a mean of 7 years of follow-up [4].
The effect of weight gain on cardiovascular risk factors in T1D diabetes has been noted to interact with intensive insulin therapy and improved glycemic control. Reports from the Diabetes Complications and Control Trial/ Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) [5] and the EDC [4] study have demonstrated an adverse lipid and hemodynamic profile with increased adiposity as well as cross-sectional associations with long-term complications [6]. However, the EDC study also showed that excess weight in association with improved glycemic control was less harmful in terms of cardiovascular disease risk profile [4]. As T1D is a disease characterized by insulin deficiency leading to an abnormal fuel utilization and acute complications such as ketoacidosis, weight loss or a lower weight may partially reflect inadequate insulin therapy. Furthermore, some of the long term complications like neuropathy and nephropathy may also be associated with anorexia, wasting, and weight loss. Thus the causes, and consequences, of weight change in T1D may be different than in the general population, and add complexity to the concern about a rise in overweight and obesity in T1D.
The purpose of this report was therefore to extend our previous EDC follow-up and determine the prevalence and incidence of overweight and obesity over eighteen years of follow-up, as well as to identify potential risk factors for weight gain in T1D.
Methods
The EDC study is a prospective study of a well-defined cohort (n=658) with childhood-onset (<17 years) type 1 diabetes, first diagnosed between January 1, 1950 and May 30, 1980 at Children’s Hospital of Pittsburgh, the United States. Participants were first seen for baseline examination between November 1, 1986 and October 31, 1988 and biennially thereafter for ten years, after which they were followed by survey with exams limited to certain subgroups. Between 2004 and 2007, an eighteen year follow-up was conducted for all participants. The design and methods of the study have been previously described [7]. Participants were thus followed for eighteen years. For this report, because of the variability introduced by the growth spurt, and differing body compositions in children and adolescents, and to be consistent with our recent report showing a protective benefit of weight change in adults aged 18 years and older [8], we focus on the population 18 years and older (n=589 at baseline) for the weight change analyses. For the descriptive analyses of temporal trends, for comparability with National Center for Health Statistics data, we use those aged 20 years and older at each time period.
Before each cycle of examinations, information was collected by questionnaire concerning demographic characteristics, medical history, and health care behaviors as previously reported [7]. Participants were weighed in light clothing and without shoes on a balance beam scale. Height was measured using a wall-mounted stadiometer. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters. For the first ten years, all height and weight were measured. Beginning in 1998, where height and weight data were less available from follow-up clinical exams, self-reported data from the medical history questionnaire were used, representing approximately 20% of the data for this time period. Overweight was defined as a BMI ≥25 kg/m2. Obesity was defined as a BMI ≥30 kg/m2. Only measured height and weight were used to determine weight change.
Fasting blood samples were assayed for lipids, lipoproteins, and glycosylated hemoglobin. Stable glycosylated hemoglobin A1 (HbA1) was originally measured in saline-incubated samples by microcolumn cation exchange chromatography (Isolab, Akron, Ohio, USA). On October 26, 1987, the method was changed to high-performance liquid chromatography (HPLC) (Diamat, Bio-Rad Laboratories, Hercules, CA, USA). The two methods were highly correlated (r = 0.95; Diamat HbA1 = 0.18±1.00 Isolab HbA1). Beginning in 1998, HbA1c was measured using the DCA2000 analyzer. Original HbA1 (1986 to 1998) and A1c (1998 to 2004) were converted to DCCT aligned HbA1c values using regression formulas derived from duplicate analyses (DCCT HbA1c = [0.83 * EDC HbA1] + 0.14; DCCT HbA1c = [EDC HbA1c − 1.13]/0.81). Insulin dose was defined as the total daily units of insulin divided by the body weight in kilograms. Intensive insulin therapy was defined as having three or more insulin injections per day or using an insulin pump. A family history of type 2 diabetes was defined as diabetes diagnosed after the age of 30 in a first degree relative without immediate insulin use. Physical activity was determined by number of flights climbed per day, city blocks or equivalent walked per day, and mets calculated from sports/exercise*minutes of participation*number of times per week. Physical activity is expressed in kilocalories. Late complications of diabetes (coronary artery disease, overt nephropathy, proliferative retinopathy, symptomatic autonomic neuropathy, and lower extremity arterial disease) were assessed as previously described [7].
Statistical analyses
The validity of the reported weight was assessed by conducting a Pearson’s intraclass correlation test between the measured and reported weight of all participants providing both. Self-reported and measured BMI were highly correlated in both 2000–2004 (intraclass correlation=0.92, 0.10–0.94), median difference=0.4 kg/m2, n=199) and 2004–2007 (intraclass correlation=0.82, 0.05–0.85), median difference 0.7 kg/m^2, n=299).
Weight change was represented as the residuals of baseline BMI regressed on BMI at last follow-up. General linear models were used to determine the predictors of weight change. All analyses were adjusted for follow-up time. Cox proportional hazards models were used to determine predictors of time to the first gain of 5 kg/m2 or loss of 2 kg/m2, while controlling for baseline BMI. Results are expressed as per standard deviation change in continuous variables. General linear models were used to determine differences in continuous variables by BMI category and to test for a linear trend across BMI category. Linear regression analysis with fully stepwise selection, i.e. a modification of the forward stepwise procedure [9], was used to determine the independent predictors of weight change. Criteria for selection of variables available for multivariable analysis were a p-value less than 0.05 or a strong biological plausibility in a type 1 diabetes population (i.e. intensive insulin therapy). Criteria for model inclusion was a p-value of <0.05. All analyses were conducted using SAS version 9.1 (Cary, North Carolina). All procedures were approved by the Institutional Review Board of the University of Pittsburgh, and all participants provided informed consent.
Results
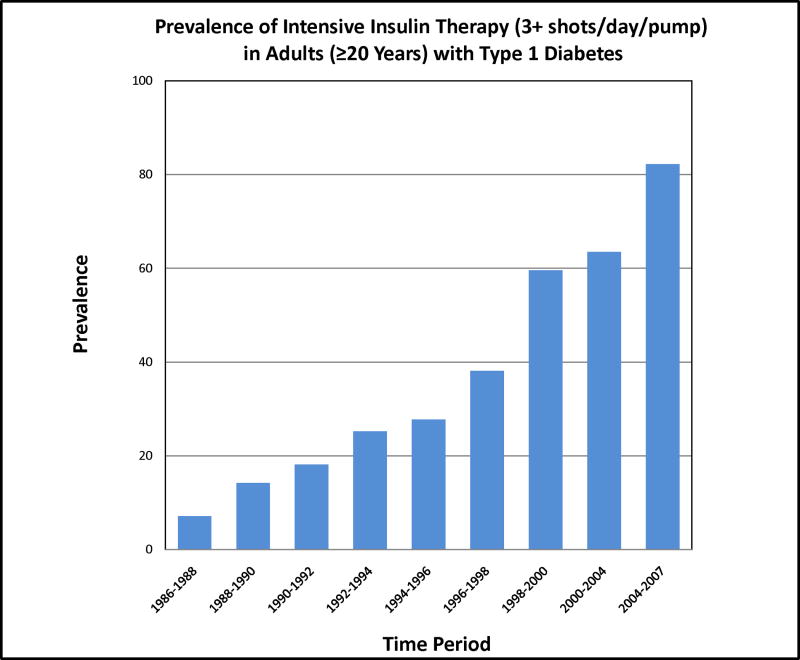
Participants were followed for a median of 18 years (range: 1.5–20.6 years), with follow-up data for 95% (n=625) of the original cohort. Mean age at baseline was 29.1 years (range: 18.0–48.0 years). During the course of follow-up, 142 (24%) participants died (mean age at death was 44.7 years). Mean and median increase in BMI over the course of follow-up was 2.6 and 2.2 kg/m2, respectively, ranging from a change of −6.7 to a change of 29.3 kg/m2. This did not vary by sex (p=0.96). One hundred and eighty-three participants gained five or more kg/m2 over the course of follow-up. Twenty-six participants had a gain of ten or more kg/m2. Seven percent of the population was on intensive insulin therapy (IIT) at baseline; by 2004–2007, this had increased to 82% and median insulin injection frequency among non pump users increased from 2 to 4 per day. Nevertheless, although injection frequency increased, total daily insulin dose decreased from 0.76 to 0.62 Units/kg/day.
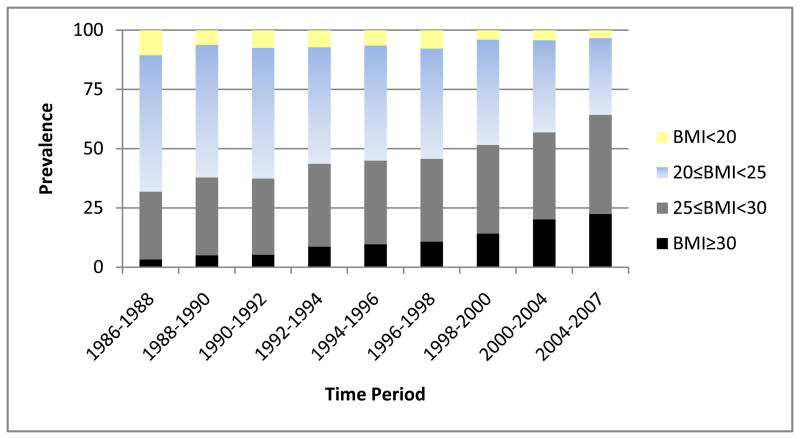
Overall 3.4 % of the participants 18 years and older were obese at baseline. By 2004–2007 this had risen seven-fold to 22.7%. The prevalence of being overweight, but not obese, rose from 28.6% in 1986–1988 to 42.0% in 2004–2007, a 47% increase. Figures 1 and 2 show the temporal patterns in the prevalences of being overweight and obese and the use of IIT. As these data may be influenced by the aging of the cohort and survivorship, age-group specific prevalences of underweight, normal weight, overweight, and obese were also examined by time-period. As an example, Table 1 presents these data for the 40–49 year-old age group, 25% of whom were overweight or obese in 1986–1988. By 2004–2007, 68.2 % in that age group, i.e. 40–49 years old, were overweight or obese. The age-period table of Appendix Table 1 further demonstrates that this rise in the prevalence of overweight and obesity was not due to the aging of the cohort. Appendix Table 2 shows both full age and gender prevalences by time period. Prevalences over time were similar in men and women. The incidence of overweight and obesity were also examined. In those with a BMI <25 in 1986–1988, the incidence of being overweight or obese was 49.1%, while the incidence of obesity, for those with a BMI <30 at baseline, was 20.9%, during the 18 years of follow-up. Twenty percent of the population gained at least 5 kg/m2 over the course of follow-up; seven percent lost at least 2 kg/m2.
Figure 1.
Temporal patterns in overweight and obesity in type 1 diabetes.
Figure 2.
Temporal patterns in intensive insulin therapy in adults with type 1 diabetes.
Table 1.
Age-specific Prevalence of Underweight, Normal weight, Overweight, and Obese for those Aged 40–49 in Each Time Period, n (%)
| BMI<20 (Underweight) | 20≤BMI<25 (Normal weight) | 25≤BMI<30 (Overweight) | BMI≥30 (Obese) | |
|---|---|---|---|---|
| 1986–1988 | 4 (9.1) | 29 (65.9) | 10 (22.7) | 1 (2.3) |
| 1988–1990 | 3 (5.8) | 29 (55.8) | 17 (32.7) | 3 (5.8) |
| 1990–1992 | 6 (8.5) | 43 (60.6) | 18 (25.4) | 4 (5.6) |
| 1992–1994 | 10 (12.2) | 39 (47.6) | 27 (32.9) | 6 (7.3) |
| 1994–1996 | 14 (11.9) | 58 (49.2) | 35 (29.7) | 11 (9.3) |
| 2004–2007 | 5 (2.9) | 50 (28.9) | 79 (45.7) | 39 (22.5) |
The baseline predictors (and subsequent use of intensive insulin therapy) of change in BMI from baseline to last follow-up are shown in Table 2. A higher HbA1c at baseline and the subsequent use of intensive insulin therapy during follow-up were predictive of weight gain, while overt nephropathy and symptomatic autonomic neuropathy predicted weight loss. In multivariable linear regression analyses with stepwise selection, intensive insulin therapy was reduced to marginal significance (p=0.07) and overt nephropathy was not selected as an independent predictor (Table 3).
Table 2.
Predictors of Weight Change Adjusted for Follow-up Time in Adults with Type 1 Diabetes
| Weight change | ||
|---|---|---|
| β (SE) | p-value | |
| Age (years) | −0.19 (0.15) | 0.21 |
| Diabetes duration (years) | −0.12 (0.15) | 0.43 |
| HbA1c (%) | 0.35 (0.15) | 0.03 |
| Insulin dose (U/kg/dy) | 0.10 (0.16) | 0.54 |
| Insulin injections/day* | −0.02 (0.15) | 0.88 |
| Physical activity (kcal/week)* | 0.15 (0.16) | 0.35 |
| Sex (female) | −0.16 (0.30) | 0.58 |
| Coronary artery disease | −0.21 (0.58) | 0.72 |
| Overt nephropathy | −0.78 (0.35) | 0.03 |
| Proliferative Retinopathy | 0.10 (0.33) | 0.76 |
| Symptomatic autonomic neuropathy | −1.41 (0.61) | 0.02 |
| Distal symmetrical polyneuropathy | −0.55 (0.34) | 0.11 |
| Lower extremity arterial disease | −0.53 (0.55) | 0.34 |
| Intensive insulin therapy: | ||
| Incident during follow-up vs Never | 0.77 (0.37) | 0.04 |
| Family history of T2D | 0.39 (0.43) | 0.36 |
| Annual Household Income <$20,000 | 0.34 (0.34) | 0.31 |
| Some college | 0.006 (0.33) | 0.98 |
| Current smoker | 0.08 (0.36) | 0.83 |
| Alcohol consumption: 3+gl/wk | −0.36 (0.36) | 0.31 |
Weight change was represented as the residuals of baseline BMI regressed on BMI at last follow-up. BMI units=kg/m2
Natural-logarithmically transformed before analysis.
Table 3.
Predictors of Weight Change in Adults with Type 1 Diabetes: Multivariable Adjusted Linear Regression Model.
| Standardized regression coefficient (SE) | P-value | |
|---|---|---|
| Baseline HbA1c, % | 0.43 (0.18) | 0.02 |
| Initiating intensive insulin therapy after baseline | 0.77 (0.42) | 0.07 |
| Baseline symptomatic autonomic neuropathy | −1.49 (0.67) | 0.03 |
| Baseline BMI | 1.01 (0.06) | <0.0001 |
| Follow-up time | 0.16 (0.04) | 0.0006 |
Weight change was represented as the residuals of baseline BMI regressed on BMI at last follow-up.
Stepwise selection also allowed for age, insulin dose/kg of body weight, and overt nephropathy.
Baseline predictors of a 5 kg/m2 gain or a 2 kg/m2 loss are presented in Appendix Tables 3 and 4. After multivariable adjustment, independent predictors of a 5 kg/m2 gain were low annual household income (HR=1.63, 1.08–2.47) and alcohol consumption (HR=0.53, 0.31–0.91). Independent predictors of a 2 kg/m2 loss were female sex (HR=3.57, 1.57–8.14), HbA1c (HR=1.62, 1.13–2.32), overt nephropathy (HR=3.27, 1.58–6.75), symptomatic autonomic neuropathy (HR=5.80, 2.13–15.78) and current smoker (HR=2.79, 1.28–6.06).
Discussion
Several important findings emerge from these analyses of long-term temporal patterns in weight change in type 1 diabetes. First, the prevalence of being overweight or obese is increasing in type 1 diabetes. Second, we have shown that the percentage on intensive insulin therapy increased nearly 10 fold during the course of follow-up, an increase which parallels the increase of overweight or obesity. Finally, we have shown that traditional predictors of weight change, that is, smoking, and socioeconomic status, do not appear to have as strong of an impact on weight change in type 1 diabetes while the major complications of diabetes have a impact on weight change in this population.
We have previously shown a lower prevalence of overweight/obesity in the EDC study relative to the general population, although the incidence (12%) in both populations was similar after a mean of 7 years of follow-up [4]. After 18 years of follow-up, the prevalence of overweight in type 1 diabetes appears to have increased at a faster pace than in the general population. At baseline (1986–1988), the combined prevalence of overweight and obesity in our population was much lower than the general population (31.9 vs. 55.9) [10]; by 2004–2007 there was no difference in the two populations (64.6 vs. 66.3, EDC vs. NHANES 2003–2004). Similarly, the prevalence of obesity in our population increased 7-fold, from 3.3 % to 22.7%. While these comparisons may be biased since the NHANES population was older than ours and in the general population the prevalence of overweight and obesity tends to increase with age, the rise in overweight and obesity in EDC appears to be unrelated to aging itself as our data have demonstrated similar increases in overweight and obesity in age-specific strata (Table 1). In the DCCT, weight gain was also apparent, with mean BMI rising from approximately 23 kg/m2 to approximately 26 kg/m2 over nine years of follow-up [11].
There are several possible reasons for the rising prevalence of overweight and obesity observed in our population. First of all, there is in the total cohort a healthy survivor effect. By the end of follow-up, 24% of the population had died. Thus, as overt nephropathy and symptomatic autonomic neuropathy are associated with weight loss, the survivors are biased toward weight gain. We have recently reported that in adults aged eighteen years and older at baseline in this population, each increasing tertile of weight change during the first ten years of follow-up was associated with an approximately 33% reduction in the subsequent ten years [8]. It is likely that those remaining (n=379) in 2004–2007 represent a substantially survival biased cohort.
Insulin itself promotes weight gain in that it stimulates lipogenesis, inhibits protein catabolism, and slows down basal metabolism. This, in combination with the abnormal physiological route of insulin via its peripheral insulin administration in those with T1D, which is also associated with a reduced energy metabolism [12–13], is likely to relate to the well known weight gain with the advent of intensive insulin therapy. Type 1 diabetes and insulin therapy induce a state of insulin resistance which is selective for carbohydrate metabolism. Thus, increasing insulin doses appears often necessary to maintain glycemic control but insulin maintains its role in lipogenesis and protein metabolism and therefore promoting fat mass and lean body mass gain. This seems to be evidenced in our data by the marked increase in obesity after 1996–1998 (Figure 1) reflecting a dramatic increase in intensive insulin therapy post DCCT results. At baseline, only 7.2 % of our participants were on intensive insulin therapy, but by 2004–2007 82% were on intensive insulin therapy (Figure 2). The United Kingdom Prospective Diabetes Study (UKPDS), aimed at assessing the benefits of lowering glycemia in type 2 diabetes, also demonstrated that those in the intensive treatment arm gained an average of 3 kg more than those in the conventional treatment arm during the ten years of follow-up [14].
Weight gain associated with insulin therapy in type 1 diabetes might be traditionally viewed as a normalization of weight, i.e. the correction of glucosuria, diuresis, and/or catabolism (wasting) with the initiation of insulin therapy. Consistent with this, we found that a higher baseline HbA1c was predictive of a 2kg/m2 weight loss, independent of complications associated with wasting and poor glycemic control, namely overt nephropathy and symptomatic autonomic neuropathy. However, higher HbA1c in our population was also a positive predictor of weight gain, a finding consistent with the DCCT [5], and is suggestive of subsequent intensification of insulin therapy and tighter glucose control. We have also previously shown that those with the worst glycemic control at baseline showed the greatest glycemic improvement after an average of 7 years. This group also gained the greatest amount of weight [4], indicating a reversal of catabolism. Nevertheless, the anabolic role of insulin itself appears to be quite strong as others have reported an association between weight gain and daily insulin dosage [15].
Income, education, physical activity, and smoking, all inverse risk factors for obesity in the general population [16–18], were not associated with absolute weight change from baseline to last follow-up, although low income was predictive of major weight gain, i.e. gaining 5 kg/m2 during follow-up. In type 1 diabetes, the advantages that income and education offer in reducing the risk of overweight and obesity may be offset by the increased access they provide to insulin and intensive insulin therapy. Likewise, the same may be operant in the relationship of smoking with weight change in type 1 diabetes. Smokers may be more likely to be of a lower socioeconomic status and therefore affected by the same competing influences on weight change. Although smoking was not predictive of absolute weight change from baseline to last follow-up, it was predictive of a 2kg/m2 weight loss. Physical activity, however, was protective against a 2kg/m2 weight loss during follow-up. In type 1 diabetes, physical activity may reflect both health and morbidity. That is, it may reflect increased leisure time activity in sports or other types of exercise, and thus protective against weight gain. Conversely, it may reflect increased morbidity from complications that are part of its natural history, suggested by its inverse relationship with a 2 kg/m2 weight loss, i.e. sicker individuals are less mobile and also are more likely to lose weight. Finally, alcohol consumption, though not associated with absolute weight change from baseline to last follow-up, was protective against excessive (5 kg/m2) weight gain. In the general population as well, inconsistent associations between alcohol consumption and weight gain are observed [19–21].
A limitation of these analyses was the inclusion of reported height and weight data in the determination of adiposity temporal patterns during the last ten years of follow-up. Although the R2 for the reported and measured weight during that time period was very high in this population, BMI was underreported by about a half of a BMI unit. Therefore, the prevalence of overweight and obesity for this time period was likely underestimated. However, in the determination of weight change, only measured BMI was used. Another major limitation of this study is the substantial survival bias in the population during the last two examination periods, a time associated with a marked increase in the prevalence of overweight and obesity and the widespread adaptation of intensive insulin therapy. However, as type 1 diabetes is characterized by a very early mortality, 24% of this population died, with a mean age of 45 years at death, this could not be avoided and age specific analyses confirmed a major weight gain.
Taken as a whole, the results of this study may appear quite alarming as we have demonstrated a marked increase in the prevalence of overweight and obesity in type 1 diabetes. However, weight change in type 1 diabetes is complex. The increasing prevalence of overweight and obesity may reflect both the anabolic effects of insulin and the “normalization” of the population that intensive insulin therapy allows, i.e. allowing it to follow the temporal trend occurring in the background population. As overweight and obesity increased with time, overweight and obesity may also be a marker of survival. The effect of adiposity and weight gain on mortality in this population suggests that the overweight state may not be harmful and that weight gain is actually protective [8]. Thus despite the rise of overweight and obesity in type 1 diabetes, caution should be used in admonishing patients to lose weight or maintain an ideal body weight (BMI <25), particularly in light of the role that insulin plays in weight gain and the protective association of weight gain with mortality in this population.
Supplementary Material
Acknowledgments
Funding
This work was supported by National Institutes of Health [grant number DK34818].
This data was presented in part at the 68th Annual American Diabetes Association Scientific Sessions, June 2008. We would like to think the participants of the Epidemiology of Diabetes Complications study for their continued dedication and cooperation.
Footnotes
Declaration of Competing Interests
We, the authors, have no competing interests to declare.
Contributor Information
Baqiyyah Conway, Vanderbilt University, Division of Epidemiology, 2525 West End Ave, 8th Fl, Nashville, TN 37203-1738.
Rachel G Miller, The University of Pittsburgh, Department of Epidemiology, 3512 Fifth Ave, 2nd Fl, Pittsburgh, PA 15213.
Tina Costacou, The University of Pittsburgh, Department of Epidemiology, 3512 Fifth Ave, 2nd Fl, Pittsburgh, PA 15213.
Linda Fried, VA Pittsburgh Healthcare System, University Drive Division, Mailstop 111F-U, Pittsburgh, PA 15240.
Sheryl Kelsey, The University of Pittsburgh, Department of Epidemiology, A525 Crabtree Hall, 130 DeSoto St, Pittsburgh, PA 15261.
Rhohert Evans, The University of Pittsburgh, Department of Epidemiology, 502 Parran Hall, 130 DeSoto St, Pittsburgh, PA 15213.
Trevor Orchard, The University of Pittsburgh, 3512 Fifth Ave, 2nd Fl, Pittsburgh, PA 15217.
References
- 1.Ogden C, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Flegal K, et al. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad A, et al. The spread of the obesity epidemic in the United States, 1991–1998. JAMA. 1999;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 4.Williams K, et al. Improved glycemic control reduces the impact of weight gain on cardiovascular risk factors in type 1 diabetes. The Epidemiology of Diabetes Complications Study. Diabetes Care. 1999;22:1084–1091. doi: 10.2337/diacare.22.7.1084. [DOI] [PubMed] [Google Scholar]
- 5.Purnell J, et al. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure. JAMA. 1998;280:140–146. doi: 10.1001/jama.280.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Block CE, Leeuw IH, Van Gaal LF. Impact of overweight on chronic microvascular complications in type 1 diabetic patients. Diabetes Care. 2005;28:1649–1655. doi: 10.2337/diacare.28.7.1649. [DOI] [PubMed] [Google Scholar]
- 7.Orchard TJ, et al. Factors associated with the avoidance of severe complications after 25 years of insulin-dependent diabetes mellitus: Pittsburgh Epidemiology of Diabetes Complications I. Diabetes Care. 1990;13:741–747. doi: 10.2337/diacare.13.7.741. [DOI] [PubMed] [Google Scholar]
- 8.Conway B, MR, Costacou T, Fried L, Kelsey S, Evans RW, Orchard TJ. Adiposity and Mortality in Type 1 Diabetes. International Journal of Obesity. 2009;33(7):796–805. doi: 10.1038/ijo.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleinbaum D, KL, Muller K, Nizam A. Applied Regression Analysis and Other Multivariable Methods. 3. Pacific Grove: International Thompson Publishing Inc; 1998. [Google Scholar]
- 10.Ogden C, et al. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2012. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 11.DCCT Research Group. Weight gain is associated with intensive therapy in the Diabetes Control and Complications Trial. Diabetes Care. 2001;241:1711–1721. doi: 10.2337/diacare.11.7.567. [DOI] [PubMed] [Google Scholar]
- 12.Nair K, Hallisay D, Garrow J. Increased energy expenditure in poorly controlled type 1 (insulin-dependent) diabetic patients. Diabetologia. 1984;27:13–16. doi: 10.1007/BF00253494. [DOI] [PubMed] [Google Scholar]
- 13.Charlton M, Nair K. Role of hyperglucagonemia in catabolism associated with type 1 diabetes. Effects of leucine metabolism and the resting metabolic rate. Diabetes. 1998;47:1748–1756. doi: 10.2337/diabetes.47.11.1748. [DOI] [PubMed] [Google Scholar]
- 14.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose contol with sulphoylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPSD 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 15.Ferriss J, et al. Weight gain is associated with improved glycaemic control but with adverse changes in plasma lipids and blood pressure in type 1 diabetes. Diabet Med. 2006;23:557–564. doi: 10.1111/j.1464-5491.2006.01847.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Beydoun M. The obesity epidemic in the United States-gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 17.Erlichman J, Kerbey A, James W. Physical activity and its impact on health outcomes. Paper 2: prevention of unhealthy weight gain and obesity by physical activity: an analysis of the evidence. Obes Rev. 2002;3:273–288. doi: 10.1046/j.1467-789x.2002.00078.x. [DOI] [PubMed] [Google Scholar]
- 18.Fogelholm M, Kukkonen-Harjula K. Does physical activity prevent weight gain-a systematic review. Obes Rev. 2000;1:95–112. doi: 10.1046/j.1467-789x.2000.00016.x. [DOI] [PubMed] [Google Scholar]
- 19.Lewis C, et al. Seven-year trends in body weight and associations with lifestyle and behavioural characteristics in black and white young adults: the CARDIA STUDY. Am J Pub Health. 1997;87:635–642. doi: 10.2105/ajph.87.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wannamethee S, Shaper A. Alcohol, body weight and weight gain in middle-aged men. Am J Clin Nutr. 2003;77:1312–1317. doi: 10.1093/ajcn/77.5.1312. [DOI] [PubMed] [Google Scholar]
- 21.Gruchow HSK, Barboriak J, Scheller B. Alcohol consumption, nutrient intake and relative body weight among US adults. Am J Clin Nutr. 1985;42:289–295. doi: 10.1093/ajcn/42.2.289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.