Abstract
Many fast-spiking inhibitory interneurons, including cerebellar stellate cells, fire brief action potentials and express α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)-type glutamate receptors (AMPAR) that are permeable to Ca2+ and do not contain the GluR2 subunit. In a recent study, we found that increasing action potential duration promotes GluR2 gene transcription in stellate cells. We have now tested the prediction that activation of potassium channels that control the duration of action potentials can suppress the expression of GluR2-containing AMPARs at stellate cell synapses. We find that large-conductance Ca2+-activated potassium (BK) channels mediate a large proportion of the depolarization-evoked noninactivating potassium current in stellate cells. Pharmacological blockade of BK channels prolonged the action potential duration in postsynaptic stellate cells and altered synaptic AMPAR subtype from GluR2-lacking to GluR2-containing Ca2+-impermeable AMPARs. An L-type channel blocker abolished an increase in Ca2+ entry that was associated with spike broadening and also prevented the BK channel blocker-induced switch in AMPAR phenotype. Thus blocking BK potassium channels prolongs the action potential duration and increases the expression of GluR2-containing receptors at the synapse by enhancing Ca2+ entry in cerebellar stellate cells.
Keywords: α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors, cerebellum, action potentials, Ca2+ currents, interneurons
α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors (AMPARs) mediate the vast majority of excitatory synaptic transmission in the central nervous system (CNS). Of the four AMPAR subunits, GluR2 subunits are critical in determining a number of biophysical properties of AMPARs. Incorporation of GluR2 subunits reduces the Ca2+ permeability and channel conductance of AMPARs and prolongs the decay time of synaptic currents (Cull-Candy et al. 2006; Liu and Zukin 2007). The abundance of GluR2 mRNA varies considerably between cell types. Whereas pyramidal cells in the hippocampus and cortex and Purkinje cells in the cerebellum have a high GluR2 mRNA content, many GABAergic interneurons express low levels of GluR2 mRNA and have synaptic AMPARs that lack GluR2 subunits. For example, a rapid synaptic current through GluR2-lacking AMPARs in hippocampal GABAergic interneurons is functionally important for the long-range synchrony of gamma oscillation (Fuchs et al. 2001). We have recently shown that a prolonged decay time of synaptic currents due to enhanced GluR2 gene expression in cerebellar GABAergic interneurons markedly increases the ability of synaptic potentials to evoke an action potential (AP) (Savtchouk and Liu 2011). Thus controlling GluR2 gene expression in GABAergic interneurons can have a profound impact on the neuronal network activity.
In a recent study, we have shown that noradrenaline increases GluR2 gene transcription and promotes the incorporation of GluR2-containing receptors at synapses in cerebellar stellate cells (Liu et al. 2010). This leads to a switch in synaptic AMPAR phenotype from GluR2-lacking, Ca2+-permeable to GluR2-containing receptors. Noradrenaline also prolongs the AP duration in cerebellar stellate cells by enhancing an h-current (Liu et al. 2010; Saitow and Konishi 2000). Since a subset of K+ channels are known to reduce the duration of APs, this raises the possibility that K+ currents via these channels may also modulate the expression of postsynaptic AMPARs by controlling AP repolarization.
Several types of GABAergic interneurons are known to express high levels of potassium channels, including Kv3 and large-conductance Ca2+-activated potassium (BK) channels (Bartos et al. 2007; Perney et al. 1992; Rudy and McBain 2001; Sugino et al. 2006). These channels display a characteristic high activation threshold and generate noninactivating currents, which accelerate the repolarization of APs. These properties reduce the AP duration and allow neurons to fire APs at high frequency (Salkoff et al. 2006; Wang et al. 1998). One intriguing possibility is that the brief duration of such APs suppresses the expression of GluR2 subunits in these neurons. Here, we have tested this hypothesis using cerebellar stellate cells. These inhibitory interneurons express GluR2-lacking AMPARs at the parallel fiber synapse and fire spontaneous APs that are of a brief duration.
Using pharmacological inhibitors, we find that BK channels mediate a large fraction of the noninactivating potassium current in stellate cells. Application of BK channel blockers prolongs the AP duration and promotes the incorporation of GluR2-containing AMPARs at stellate cell synapses, changing the synaptic AMPARs from GluR2-lacking, Ca2+-permeable to GluR2-containing, Ca2+-impermeable receptors. This change is triggered by an enhanced Ca2+ influx through L-type voltage-gated Ca2+ channels during an AP. Our results suggest that reducing AP duration in cerebellar GABAergic interneurons is a novel mechanism that suppresses synaptic GluR2-containing receptor expression.
METHODS
All procedures were approved by the Animal Care and Use Committee of Louisiana State University Health Sciences Center. Cerebellar slices (250 μm) were obtained from 18- to 22-day-old C57BL/6J mice as described in Liu et al. (2010). Slices were maintained in artificial cerebrospinal fluid (ACSF; in mM: 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, and 25 glucose, saturated with 95% O2-5% CO2, pH 7.3) for 60 min before recordings.
Electrophysiology.
Whole cell voltage- and current-clamp recordings were made with an Axopatch 200B and MultiClamp 700B amplifier (Axon Instruments, Foster City, CA) in ACSF bubbled with 95% O2-5% CO2. Stellate cells were identified by their location in the outer two-thirds of the molecular layer in a cerebellar slice and by their ability to fire spontaneous APs in the cell-attached configuration. Synaptic currents, Ca2+ currents, K+ currents, and APs were filtered at 2–5 kHz and digitized at 20 kHz. The pipette resistance was 5–7 MΩ. All recordings were performed at room temperature.
K+ currents were recorded using a pipette solution containing (in mM) 135 KCl, 4.6 MgCl2, 0.1 CaCl2, 10 HEPES, 1 EGTA, 4 ATP-Na, 0.4 GTP-Na, pH 7.3 (75–95% compensation; series resistance = 20.3 ± 1.5 MΩ, n = 11). Amphotericin B (∼0.6 mg/ml) was added to the potassium-based pipette solution in perforated patch recordings (typical series resistance = 25 ± 3 MΩ, n = 4). The bath solution contained 300 nM TTX, 20 μM ZD7288, 1 mM kynurenic acid (KYNA), and 100 μM picrotoxin (PTX) to block Na+ channels, h-currents, ionotropic glutamate receptors, and inhibitory transmission, respectively.
Spontaneous APs were recorded using a whole cell patch configuration in ACSF that contained 1 mM KYNA and 100 μM PTX. The pipette solution contained (in mM) 115 KMeSO3, 2 MgCl2, 0.16 CaCl2, 0.5 EGTA, 10 HEPES, 4 ATP-Na, 0.4 GTP-Na, 14 Tris2-creatine phosphate (0.6 mg/ml amphotericin B for perforated patch recordings), pH 7.3. The frequency of spontaneous APs was recorded extracellularly in the presence of 100 μM PTX and 1 mM KYNA using a cell-attached configuration with a glass electrode filled with ACSF.
Ca2+ currents were measured using a voltage-clamp protocol that mimicked the AP waveform. The waveforms of a control AP (control-AP) and an AP in the presence of 1 mM tetraethylammonium (TEA; TEA-AP) were recorded in current clamp from a stellate cell and had an AP half-width of 1.5 and 2.3 ms and an afterhyperpolarization of −30 and −9 mV, respectively. They were therefore used as voltage commands. The pipette solution contained (in mM): 119 CsCl, 9 EGTA, 10 HEPES, 1.8 MgCl2, 14 Tris2-creatine phosphate, 4 ATP-Mg, 0.4 GTP-Na, 10 TEA, 1 QX-314, pH 7.3. The external solution included 10 mM TEA, 300 nM TTX, 10 μM ZD7288, 1 mM KYNA, and 100 μM PTX to block potassium, sodium, h-currents, and synaptic currents, respectively. Cd2+ (100 μM) was used to block Ca2+ channels. The Ca2+ current was monitored as the difference current (I − ICd).
Adequacy of the space clamp during AP waveforms is an issue. However, stellate cells are electrically compact with an average cell capacitance of 6.0 ± 0.3 pF (n = 16) and input resistance of 2.0 ± 0.5 GΩ. Mean series resistance was 24.6 ± 1.1 MΩ. AP waveforms evoked small Ca2+ currents (108 ± 15 pA, n = 16), and the expected voltage error is <2.5 mV. As an experimental test, we determined the time delay between the peak of the AP waveform and the notch in the rising phase of the Ca2+ current (that correlates with the peak of membrane depolarization) and found a short delay with a latency of 0.20 ± 0.04 ms (n = 8), which appears similar to other studies (Yang and Wang 2006). Also, if the stellate cells were not adequately clamped during APs due to a voltage error, then reducing Ca2+ current would be expected to result in more rapid decay kinetics of AP-evoked calcium currents. Although the amplitude of Ca2+ currents decreased by half as the extracellular Ca2+ concentration decreased from 2 to 1 mM, we observed no significant difference in the decay kinetics of the AP-evoked calcium currents (1.13 ± 0.09 ms in 2 mM Ca2+ and 1.03 ± 0.09 ms in 1 mM Ca2+). These results indicate that stellate cells were adequately voltage-clamped in these experiments.
Cerebellar slices were incubated with 100 nM iberiotoxin or 1 mM TEA for 3 h in the presence of 1 (or 5) mM KYNA and 100 μM PTX at room temperature. As a control, cerebellar slices were incubated in ACSF that contained 1 mM KYNA and 100 μM PTX (“control solution”). In one experiment, slices were treated with 100 nM iberiotoxin (+ KYNA + PTX) for 1 h followed by 2 h in control solution. KYNA and TEA were washed out 15 min before recordings. Spontaneous excitatory postsynaptic currents (sEPSCs) were recorded from stellate cells using a cesium-based pipette solution (in mM: 135 CsCl, 10 HEPES, 10 EGTA, 2 NaCl, 4 ATP-Mg, 5 TEA, 1 QX-314, 0.1 spermine, pH 7.3) in ACSF containing 100 μM PTX. Synaptic events that did not have a smooth rise and decay phase were rejected. Average sEPSCs at each holding potential (typically average of 50–100 events over 10–15 min) were measured using N version 4.0 (written by Steve Traynelis, Emory University) as described previously (Liu and Cull-Candy 2000). The rectification index of sEPSC current-voltage (I-V) relationship was defined as the ratio of the current amplitude at +40 mV to the predicted linear value at +40 mV (extrapolated from linear fitting of the currents at negative potentials). The decay time constant of individual synaptic events was determined by fitting the decay phase of an EPSC with a single exponential function, since the weighted decay time constants calculated from a double exponential decay fit are similar to those obtained with a single exponential fit (Savtchouk and Liu 2011).
All values are expressed as means ± SE. Statistical significance was assessed by a two-tailed Student's t-test. TTX, iberiotoxin, and ZD7288 were obtained from Tocris Bioscience. Water-soluble amphotericin B was obtained from Sigma.
RESULTS
Cerebellar stellate cells express large-conductance Ca2+-activated potassium channels.
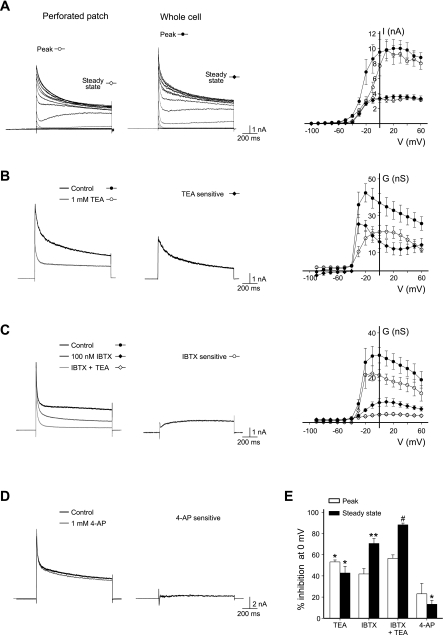
Cerebellar stellate cells fire brief APs (Liu et al. 2010). To identify the K+ channels that control the AP duration in stellate cells, cells were voltage-clamped at −100 mV and stepped from −90 to +60 mV in 10-mV increments. Using whole cell recordings, we found that depolarization beyond −40 mV elicited a transient outward current followed by a sustained steady-state current (Fig. 1A). The conductance of this current reached its maximum at −10 mV and declined as the membrane potential further depolarized (Fig. 1B). To test whether cell dialysis altered the amplitude of total potassium currents, we performed perforated patch recordings. The potassium current waveform and I-V relationship of the transient current and the noninactivating current were indistinguishable from those obtained using whole cell recordings (Fig. 1A). Therefore, further characterization of potassium currents were conducted using whole cell patch recordings.
Fig. 1.
Large-conductance Ca2+-activated potassium (BK) channels mediate a large portion of voltage-gated potassium currents in stellate cells. A: K+ current traces recorded from a stellate cell using perforated and whole cell patch-clamp recordings. Cells were voltage-clamped at −100 mV and stepped from −90 to +60 mV in 10-mV increments. Current-voltage relationship of the peak and noninactivating currents (perforated patch, n = 4; whole cell patch, n = 10) is shown. B and C: depolarization from −100 to 0 mV elicited an outward current. Tetraethylammonium (TEA; 1 mM, n = 4) and iberiotoxin (IBTX; 100 nM, n = 4) inhibited a large portion of the potassium current. Noninactivating conductance (G)-voltage (V) relation shows that TEA- and IBTX-sensitive current (Icontrol − ITEA or IIBTX) are activated at −30 and −20 mV, respectively. D: 4-aminopyridine (4-AP; 1 mM) moderately reduced potassium currents. E: summary of K+ current inhibition at 0 mV by TEA, IBTX, and 4-AP. *P < 0.05; **P < 0.005, current amplitude, control vs. inhibitor; #P < 0.05, IBTX vs. IBTX + TEA.
Several types of potassium channels, including Kv3.1 and BK channels, are known to conduct a high threshold noninactivating K+ current with rapid activation kinetics, which are ideally suited for generating short duration APs. We therefore tested whether these channels contribute to the net K+ current in stellate cells. A low concentration of TEA is known to block preferentially a subset of K+ channels, including Kv3 and BK channels (Rudy and McBain 2001; Wang et al. 1998). We found that bath application of 1 mM TEA reduced the outward transient current at 0 mV by 53.2 ± 1.8% and the noninactivating current by 42.8 ± 6.2% (n = 4; P < 0.05; Fig. 1B). To distinguish between Kv3 and BK channels, we used a pharmacological approach. Kv3, but not BK, channels are sensitive to 1 mM 4-aminopyridine (4-AP) (Coetzee et al. 1999). Since 4-AP inhibited the noninactivating current by 13.3 ± 3.6% (n = 5; P < 0.05; Fig. 1, D and E), Kv3 channels may marginally contribute to the depolarization-evoked outward current. In contrast, a specific BK channel blocker, 100 nM iberiotoxin, markedly inhibited the sustained outward current (70.4 ± 4.8%; n = 4; P < 0.005; Fig. 1C). Iberiotoxin-sensitive currents were activated at −20 mV and exhibited little inactivation (Fig. 1C). Therefore, noninactivating potassium currents in stellate cells are predominantly mediated by BK channels. In the presence of iberiotoxin, application of TEA produced a small reduction in K+ currents (Fig. 1E), and thus BK channels mainly contribute to TEA-sensitive currents.
Inhibition of BK channels prolongs AP duration.
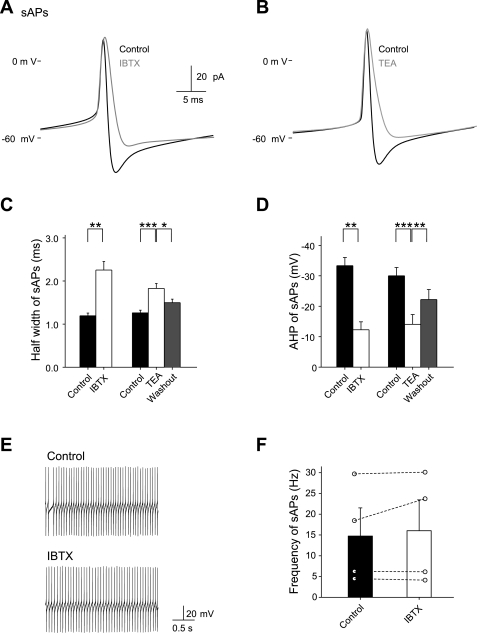
Cerebellar stellate cells fire APs spontaneously in the absence of synaptic input. We next determined the impact of BK channel blockade on the AP waveform in stellate cells. Using the whole cell current-clamp technique, we recorded spontaneous APs in stellate cells in the presence of 1 mM KYNA and 100 μM PTX. Spontaneous APs were of a brief duration (Fig. 2, A and C). Iberiotoxin (100 nM) prolonged AP duration (from 1.2 ± 0.1 to 2.3 ± 0.2 ms, n = 5; P < 0.001) and reduced the afterhyperpolarization (control, −33.3 ± 2.7 mV; iberiotoxin, −12.3 ± 2.6 mV; P < 0.001; Fig. 2, A, C, and D) but did not alter the AP frequency (control 14.7 ± 6.8 Hz; iberiotoxin 16.0 ± 7.4 Hz, n = 4; P > 0.05; Fig. 2, E and F). TEA (1 mM) also significantly increased the AP duration and reduced the afterhyperpolarization without altering the amplitude of APs (Fig. 2, B–D). Changes in AP waveform elicited by iberiotoxin were indistinguishable from those elicited by TEA. Therefore, BK channels exert a strong control over the AP waveform by accelerating repolarization.
Fig. 2.
Inhibition of BK channels increases the spontaneous action potential (sAP) duration in stellate cells. A and B: application of IBTX (100 nM) and TEA (1 mM) altered the AP waveform. C and D: IBTX (n = 5) and TEA (n = 10) prolonged the duration and reduced the afterhyperpolarization of spontaneous APs. E and F: IBTX did not alter the spontaneous AP frequency in stellate cells. Spontaneous APs were recorded in a cell-attached configuration. *P < 0.05; **P < 0.005; ***P < 0.0005.
Blocking BK channels increases the expression of synaptic GluR2-containing AMPARs.
Under basal conditions, cerebellar stellate cells express primarily GluR2-lacking, Ca2+-permeable AMPARs (Liu and Cull-Candy 2000). Does the brief AP waveform in stellate cells suppress the expression of GluR2 subunits and thus promote GluR2-lacking AMPARs at stellate cell synapse? To examine the modulatory effects of postsynaptic APs, cerebellar slices were incubated for 1 h with iberiotoxin (100 nM) followed by further incubation (2 h) in control solution. We have previously shown that repetitive activation of Ca2+-permeable AMPARs enhances synaptic incorporation of GluR2-containing receptors (Liu and Cull-Candy 2000). To avoid any effect arising from altered presynaptic release, KYNA and PTX were present during all control and BK channel inhibitor treatments. Incubation with KYNA and PTX for 3 h did not alter synaptic currents [rectification index: 0.29 ± 0.03, n = 5; vs. without treatment, 0.34 ± 0.03 (Liu et al. 2010); P > 0.05] and therefore was used as control. KYNA was washed out for 15 min before recording.
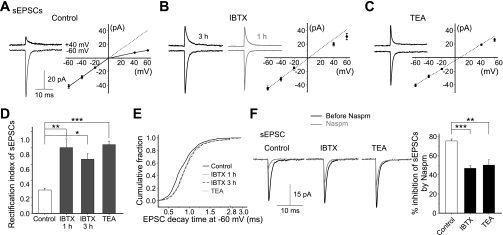
Two pharmacological approaches were used to monitor the synaptic AMPAR subunit composition in these cells. First, inclusion of spermine in the pipette solution is known to confer a voltage-dependent block of AMPARs that lack GluR2 subunits and to produce a characteristic inwardly rectifying I-V relationship (Cull-Candy et al. 2006). sEPSCs were measured at various holding potentials. In control cells, the amplitude of sEPSCs was reduced at positive membrane potentials and displayed an I-V relationship with pronounced inward rectification, indicating that the EPSCs were mediated mainly by GluR2-lacking, Ca2+-permeable AMPARs (Fig. 3A, control). In contrast, in iberiotoxin-treated cells, the I-V relationship of the sEPSC was nearly linear (Fig. 3B), indicating that GluR2-containing AMPARs mediated the synaptic currents. Iberiotoxin treatment altered the rectification index from control, 0.29 ± 0.02 (n = 8), to iberiotoxin, 0.80 ± 0.11 (n = 5; P < 0.005; Fig. 3D). Iberiotoxin increased the sEPSC amplitude at +40 mV from 7.7 ± 0.4 pA under control conditions to 18.5 ± 1.4 pA following iberiotoxin treatment (n = 5; P < 0.001). At +40 mV, the synaptic current is mediated mainly by AMPARs containing GluR2 subunits, and the decay time constant of sEPSCs at −60 mV was prolonged (Kolmogorov-Smirnov test, P < 0.000005; Fig. 3E). Incubation with iberiotoxin (100 nM) for 3 h produced similar effects on the rectification index and decay time of sEPSCs (Fig. 3, B, D, and E). Thus this result is consistent with the synaptic incorporation of GluR2-containing receptors. Iberiotoxin application did not alter the amplitude and frequency of sEPSCs at −60 mV [amplitude: control, −41.8 ± 3.0 pA; iberiotoxin, −44.3 ± 4.1 pA, not significant (NS); frequency: control, 0.12 ± 0.02 Hz; iberiotoxin, 0.15 ± 0.02 Hz, n = 5, NS] and therefore has minimal presynaptic effects.
Fig. 3.
Blocking BK channels in stellate cells induces a change in rectification of the I-V relationship and the 1-naphthyl acetyl spermine (Naspm)-dependent inhibition of spontaneous excitatory postsynaptic currents (sEPSCs). Cerebellar slices were incubated with kynurenic acid (KYNA; 1 mM) and picrotoxin (PTX; 100 μM) in the absence (control) or presence of IBTX [100 nM, 1 and then 2 h in artificial cerebrospinal fluid (ACSF) that contained KYNA and PTX, or IBTX for 3 h] or TEA (1 mM, 3 h) in the presence of KYNA and PTX. sEPSCs were recorded when spermine was included in the pipette. A: average sEPSCs displayed an inwardly rectifying I-V relationship in control, indicating the presence of Ca2+-permeable, GluR2-lacking α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors (AMPARs; n = 8). B and C: following IBTX (1 + 2 h in control solution, n = 5; 3 h, n = 3) and TEA (n = 14) treatment, the synaptic current in stellate cells showed a near linear I-V relationship, indicating that it was mediated mainly by GluR2-containing AMPARs. D: summary of rectification index of EPSCs. E: cumulative distribution of decay time constant of individual EPSCs at −60 mV from 3 to 14 cells under each condition (Kolmogorov-Smirnov test, P < 0.0001). F: application of Naspm (500 nM) produced a greater inhibition of sEPSCs in control cells (n = 4) than in IBTX (3 h, n = 4)- and TEA-treated cells (3 h, n = 5). *P < 0.05; **P < 0.005; ***P < 0.0005.
To confirm that this change in rectification reflects an increase in synaptic GluR2-containing AMPARs, we used a selective Ca2+-permeable AMPAR blocker, 1-naphthyl acetyl spermine (Naspm). Naspm (500 nM) reduced the EPSC amplitude of control cells by ∼80% (n = 6) at −60 mV, a larger reduction than that observed for iberiotoxin-treated cells (3 h, 47 ± 3%; n = 4; P < 0.0002; Fig. 3F). Thus BK channel blockade enhances synaptic incorporation of GluR2-containing AMPARs.
Consistent with the idea that spike broadening promotes the insertion of synaptic GluR2 receptors, the I-V relationship of sEPSCs became more linear (Fig. 3C) and Naspm inhibition decreased (Fig. 3F) following TEA (1 mM) treatment for 3 h (Liu et al. 2010). These findings indicate that the synaptic AMPAR phenotype in cerebellar stellate cells can be altered by the activity of BK channels.
Blockade of BK channels enhances Ca2+ entry during an AP and alters synaptic AMPAR phenotype.
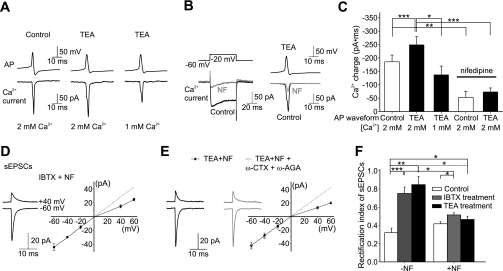
We hypothesized that prolonged APs may enhance Ca2+ entry that triggers an alteration in synaptic AMPAR composition. To examine the effect of AP waveform on Ca2+ influx, we measured the Ca2+ currents in stellate cells under voltage-clamp using AP waveforms that mimicked the control AP (control-AP) or following TEA application (TEA-AP). The TEA-AP waveform with a half-width (2.3 ms) and afterhyperpolarization (−9 mV) is comparable with the average iberiotoxin-AP (Fig. 2, C and D) and therefore would also represent the AP waveform when BK channels were blocked by iberiotoxin. The control-AP evoked an inward current, and TEA-AP increased the duration of the Ca2+ currents. The Ca2+ entry occurring during a TEA-AP (defined as the current integrated over time) was ∼40% higher than that entering during the control-AP waveform (Fig. 4, A–C; n = 15; P < 0.001 vs. control, by a paired t-test). Lowering the extracellular Ca2+ concentration from 2 to 1 mM reduced the Ca2+ entry during a TEA-AP by ∼40% (n = 8; P < 0.05; Fig. 4), primarily due to a decrease in the peak amplitude of the Ca2+ current. Thus increasing the AP duration by inhibiting BK channels significantly enhanced Ca2+ entry.
Fig. 4.
Ca2+ influx through L-type Ca2+ channels is required to trigger K+ channel blockade-induced change in sEPSC rectification. A: Ca2+ currents during a control-AP and a TEA-AP. B: nifedipine (NF; 20 μM) blocked most of the Ca2+ current using step depolarization and TEA-AP as the voltage commands. C: summary of Ca2+ charge. D and E: NF blocked the IBTX (n = 4)- and TEA (n = 7)-induced change in I-V relationship. Inclusion of ω-conotoxin GVIA (ω-CTX; 500 nM) and ω-agatoxin IVA (ω-AGA; 500 nM) together with NF (20 μM) during TEA treatment did not further reduce the rectification index of sEPSCs (n = 4). F: summary of rectification index of EPSCs. *P < 0.05; **P < 0.005; ***P < 0.0005.
Nifedipine (20 μM), an L-type voltage-gated Ca2+ channel blocker, blocked 72.2 ± 3.0% (n = 4) of the Ca2+ current evoked by a TEA-AP, indicating that L-type channels were activated during an AP (Fig. 4, B and C). To determine whether enhanced Ca2+ entry via voltage-gated Ca2+ channels is required for synaptic incorporation of Ca2+-impermeable AMPARs, we included 20 μM nifedipine during the iberiotoxin treatment (3 h). Following this treatment, sEPSCs displayed an inwardly rectifying I-V relationship (Fig. 4D). Thus nifedipine blocked the iberiotoxin-induced increase in rectification index (nifedipine + iberiotoxin, 0.52 ± 0.02, n = 4; P < 0.05 vs. iberiotoxin; Fig. 4), indicating that nifedipine prevented insertion of new GluR2-containing AMPARs. As a control, cerebellar slices were incubated in ACSF containing 20 μM nifedipine (without iberiotoxin) for 3 h. Under these conditions, the rectification index of sEPSCs was greater than that without nifedipine (rectification index = 0.42 ± 0.02, n = 4) but remained inwardly rectifying (P < 0.05 vs. iberiotoxin; Fig. 4F). Nifedipine alone prolonged the AP duration (Supplemental Fig. S1, available in the data supplement online at the Journal of Neurophysiology web site), presumably by reducing the activity of Ca2+-activated potassium channels. Although inclusion of nifedipine together with TEA did not further prolong AP duration, nifedipine also blocked the TEA-induced switch in sEPSC I-V relationship from inwardly rectifying to near linearity (P < 0.05 vs. TEA; Fig. 4E). Although nifedipine can prolong cardiac APs by blocking Kv1.5, this channel does not appear to be expressed in cerebellar stellate cells (Chung et al. 2001; Lin et al. 2001). Thus enhanced Ca2+ entry via L-type Ca2+ channels is most likely required for synaptic incorporation of GluR2-containing receptors. To test whether activation of N- and P-type Ca2+ channels also contributes to the TEA-induced change in synaptic receptors, we included ω-conotoxin GVIA (500 nM) and ω-agatoxin IVA (500 nM) together with nifedipine (20 μM) during the TEA treatment. This combination did not further reduce the rectification index of sEPSCs compared with nifedipine and TEA treatment (Fig. 4E).
DISCUSSION
It is well-established that the number and properties of postsynaptic AMPARs can be regulated by presynaptic activity. However, whether AP firing in postsynaptic neurons can also alter the synaptic AMPARs remains unclear. In the present study, we tested the hypothesis that activation of potassium channels (that control the duration of APs) reduces the level of synaptic GluR2-containing receptors. BK channels mediate substantial fraction of the potassium currents in cerebellar stellate cells, and activation of these channels gives rise to brief APs. We found that pharmacological blockade of BK channels enhances Ca2+ entry during an AP and promotes the incorporation of GluR2 receptors at cerebellar synapses. This switch in AMPAR subtype requires Ca2+ influx through L-type voltage-gated Ca2+ channels. These results indicate that activation of BK channels shortens AP duration and thereby reduces in the expression of synaptic GluR2-containing AMPARs. Therefore, K+ channels in postsynaptic neurons can influence synaptic AMPAR subtype by controlling membrane excitability and AP waveform.
Kv3 potassium channels are expressed at a high level in a subset of GABAergic neurons and in auditory neurons (Perney et al. 1992). Like BK channels, activation of Kv3 channels can shorten AP duration. Intriguingly, a study that examined the gene expression profile of populations of CNS neurons revealed an inverse relationship between the levels of GluR2 mRNA and that of Kv3.1 with many GABAergic interneurons expressing high levels of Kv3 and low levels of GluR2 (Sugino et al. 2006). In this study, we show that blockade of BK channels can increase the expression of GluR2-containing receptors in GABAergic interneurons in the cerebellum. Our results indicate that a brief AP duration limits the amount of Ca2+ that enters the cell through voltage-gated Ca2+ channels, which in turn reduces the expression of synaptic GluR2-containing receptors. We have recently shown that increasing AP duration by TEA promotes GluR2 gene expression in stellate cells (Liu et al. 2010). Therefore, the presence of these K+ channels may be one of the determinants that control GluR2 gene expression. However, other factors that affect Ca2+ entry are also likely to influence GluR2 gene transcription and the postsynaptic AMPAR phenotype. Thus the expression of BK channels by itself does not necessarily lead to a low level of GluR2 gene expression as seen in Purkinje cells and hippocampal pyramidal neurons (Sailer et al. 2006; Womack and Khodakhah 2002).
Potassium currents in cerebellar basket and stellate cells have been previously characterized (Molineux et al. 2005; Southan and Robertson 1998). Basket cells are located in the lower one-third of the molecular layer, whereas stellate cells are found in the upper two-thirds of the molecular layer. 4-AP at 1 mM inhibits 15% of the net K+ current in stellate cells (present study) but does not have any inhibitory effects in basket cells (Southan and Robertson 1998). Thus 4-AP-sensitive channels (Kv3 and Kv1) are unlikely to mediate a large portion of the potassium currents in either stellate or basket cells. Although our results show that iberiotoxin produced a marked inhibition of potassium currents in stellate cells, charybdotoxin, a BK channel blocker, failed to reduce the potassium current in basket cells (Southan and Robertson 1998). This could be due to the presence of β-subunits that reduce the inhibitory potency of charybdotoxin (Behrens et al. 2000) or the higher EGTA (10 mM) concentration of the pipette solution used in their study that suppresses a Ca2+ rise and thus the activity of BK channels. The transient and sustained K+ currents play a distinct role in controlling AP firing in stellate cells. Low-threshold and inactivating K+ currents in stellate cells prolong spike latency (Molineux et al. 2005), whereas the high-threshold noninactivating currents controls the duration of APs (present study).
We and others have previously shown that synaptic activity increases GluR2-containing AMPARs at the parallel fiber to stellate cell synapse. This requires activation of either synaptic Ca2+-permeable AMPARs or extrasynaptic N-methyl-d-aspartate receptors (NMDARs) (Gardner et al. 2005; Liu and Cull-Candy 2000, 2005; Sun and Liu 2007). Activation of metabotropic receptors can also alter synaptic AMPAR subtype in a protein synthesis-dependent manner (Kelly et al. 2009). In a recent study, we have shown that stress upregulates GluR2 mRNA abundance in stellate cells and induces a lasting increase in synaptic GluR2-containing AMPARs (Liu et al. 2010). The stress-induced increase in GluR2 mRNA expression is mediated by β-adrenergic receptors. Noradrenaline increases h-currents, giving rise to membrane depolarization (Saitow and Konishi 2000) and prolongation of AP duration in stellate cells (Liu et al. 2010). However, in the present study, we show that increasing AP duration via a different mechanism, blockade of BK channels, also promotes incorporation of GluR2-containing receptors to the synapse. Although adrenergic receptors can be coupled to a number of signaling pathways, both noradrenaline application and the BK channel blockade-induced switch in AMPAR phenotype requires Ca2+ influx via L-type channels. Furthermore, activation of MAPK and gene transcription are necessary for the incorporation of GluR2-containing receptors triggered by noradrenaline and by TEA (Liu et al. 2010). Together, these results support the idea that noradrenaline increases synaptic GluR2-containing AMPARs, at least in part by increasing AP duration in stellate cells.
Our results suggest that intrinsic membrane excitability in postsynaptic cells can regulate the AMPAR phenotype. An alteration in membrane excitability, such as silencing APs or membrane depolarization, is known to modulate excitatory synaptic transmission. Prolonged blockade of AP firing has been shown to alter the amplitude of synaptic currents and AMPAR phenotype (Sutton et al. 2006; Thiagarajan et al. 2005; Turrigiano and Nelson 2000), a homeostatic plasticity mechanism that stabilizes the activity of neuronal networks. In contrast, membrane depolarization induces a rapid change in synaptic AMPAR from GluR2-lacking to GluR2-containing receptors in immature hippocampal CA3 pyramidal cells (Ho et al. 2007). Recent studies show that activation of Kv4 voltage-gated potassium channels in the postsynaptic hippocampal pyramidal neuron elevates the threshold for long-term potentiation and modifies the subunit composition of synaptic NMDARs (Chen et al. 2006; Jung et al. 2008; Kim et al. 2007). Our results reveal that BK/Kv3 channels modulate synaptic AMPAR phenotype by controlling AP repolarization in the postsynaptic neuron. Whereas 1-h TEA treatment is sufficient to trigger the effect, a change in the expression of synaptic AMPARs requires >1 h (Liu et al. 2010). Thus the activity of K+ channels could potentially shape the synaptic inputs by influencing the expression of synaptic receptors.
Enhanced Ca2+ entry via L-type Ca2+ channels during an AP is required for the TEA/iberiotoxin-induced increase in synaptic GluR2 expression. Inhibition of N- and P-type Ca2+ channels in addition to nifedipine did not further attenuate the TEA-induced enhancement of GluR2 expression, indicating that these Ca2+ channels are unlikely to make an additional contribution. APs can also evoke Ca2+ release from intracellular stores in cerebellar interneurons. Whereas 10 μM ryanodine causes Ca2+ release from intracellular stores and increases spontaneous calcium transients at basket cell terminals, ryanodine at a higher concentration (100 μM) reduces the AP-evoked rise in intracellular Ca2+ (Conti et al. 2004; Llano et al. 2000). We incubated cerebellar slices with 100 μM ryanodine for 3 h in the presence of KYNA and PTX. Following this treatment, the rectification index of sEPSCs increased from 0.29 ± 0.02 (control; n = 8) to 0.72 ± 0.07 (n = 4; P < 0.005), which is not significantly different from that after iberiotoxin and TEA treatment (Supplemental Fig. S2). This could result from an increase in spontaneous calcium transients as the intracellular ryanodine concentration increases during the incubation, and the rise in intracellular Ca2+ then facilitates the insertion of GluR2 receptors at stellate cell synapses (Liu and Cull-Candy 2000). Therefore, we cannot rule out the possibility that a Ca2+-induced Ca2+ release may contribute to the iberiotoxin/TEA-induced increase in GluR2 expression.
Our results show that blocking BK channels prolongs AP duration and increases Ca2+ influx through voltage-gated Ca2+ channels, promoting the expression of GluR2-containing AMPARs at synapses. This reduces the Ca2+ permeability of synaptic AMPARs and may provide a feedback mechanism for Ca2+ homeostasis in cerebellar stellate cells. Incorporation of GluR2-containing AMPARs also slows the decay time of synaptic currents without any alteration in the current amplitude (Fig. 3). This can lead to a marked increase in AP firing probability in response to synaptic activation (Savtchouk and Liu 2011). Thus the expression of BK channels in cerebellar stellate cells can control the kinetics of APs and synaptic AMPAR phenotype and thereby the waveform of synaptic currents in stellate cells.
GRANTS
This work was supported by grants from the National Science Foundation (IBN-0344559) and the National Institutes of Health (NS-58867).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Michael V. L. Bennett and Matthew Whim for helpful discussions and comments on the manuscript.
Present address of Y. Liu: The Solomon H. Snyder Dept. of Neuroscience, Johns Hopkins Univ., School of Medicine.
Present address of S. Acharjee: Univ. of Alberta, Canada.
REFERENCES
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8: 45–56, 2007 [DOI] [PubMed] [Google Scholar]
- Behrens R, Nolting A, Reimann F, Schwarz M, Waldschütz R, Pongs O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett 474: 99–106, 2000 [DOI] [PubMed] [Google Scholar]
- Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci 26: 12143–12151, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YH, Shin C, Kim MJ, Lee BK, Cha CI. Immunohistochemical study on the distribution of six members of the Kv1 channel subunits in the rat cerebellum. Brain Res 895: 173–177, 2001 [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann NY Acad Sci 868: 233–285, 1999 [DOI] [PubMed] [Google Scholar]
- Conti R, Tan YP, Llano I. Action potential-evoked and ryanodine-sensitive spontaneous Ca2+ transients at the presynaptic terminal of developing CNS inhibitory synapse. J Neurosci 24: 6946–6957, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol 16: 288–297, 2006 [DOI] [PubMed] [Google Scholar]
- Du J, Zhang L, Weiser M, Rudy B, McBain CJ. Developmental expression and functional characterization of the potassium-channel subunit Kv3.1b in parvalbumin-containing interneurons of the rat hippocampus. J Neurosci 16: 506–518, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs EC, Doheny H, Faulkner H, Caputi A, Traub RD, Bibbig A, Kopell N, Whittington MA, Monyer H. Genetically altered AMPA-type glutamate receptor kinetics in interneurons disrupt long-range synchrony of gamma oscillation. Proc Natl Acad Sci USA 98: 3571–3576, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, Huganir RL. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron 45: 903–915, 2005 [DOI] [PubMed] [Google Scholar]
- Ho MT, Pelkey KA, Topolnik L, Petralia RS, Takamiya K, Xia J, Huganir RL, Lacaille JC, McBain CJ. Developmental expression of Ca2+-permeable AMPA receptors underlies depolarization-induced long-term depression at mossy fiber CA3 pyramid synapses. J Neurosci 27: 11651–11662, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SC, Kim J, Hoffman DA. Rapid, bidirectional remodeling of synaptic NMDA receptor subunit composition by A-type K+ channel activity in hippocampal CA1 pyramidal neurons. Neuron 60: 657–671, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly L, Farrant M, Cull-Candy SG. Synaptic mGluR activation drives plasticity of calcium-permeable AMPA receptors. Nat Neurosci 12: 593–601, 2009 [DOI] [PubMed] [Google Scholar]
- Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron 54: 933–947, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Wang Z, Fedida D. Influence of permeating ions on Kv1.5 channel block by nifedipine. Am J Physiol Heart Circ Physiol 280: H1160–H1172, 2001 [DOI] [PubMed] [Google Scholar]
- Liu SJ, Cull-Candy SG. Subunit interaction with PICK and GRIP controls Ca2+ permeability of AMPARs at cerebellar synapses. Nat Neurosci 8: 768–775, 2005 [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci 30: 126–134, 2007 [DOI] [PubMed] [Google Scholar]
- Liu SQ, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature 405: 454–458, 2000 [DOI] [PubMed] [Google Scholar]
- Liu Y, Formisano L, Savtchouk I, Takayasu Y, Szabó G, Zukin RS, Liu SJ. A single fear-inducing stimulus induces a transcription-dependent switch in synaptic AMPAR phenotype. Nat Neurosci 13: 223–231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci 3: 1256–1265, 2000 [DOI] [PubMed] [Google Scholar]
- Molineux ML, Fernandez FR, Mehaffey WH, Turner RW. A-type and T-type currents interact to produce a novel spike latency-voltage relationship in cerebellar stellate cells. J Neurosci 25: 10863–10873, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perney TM, Marshall J, Martin KA, Hockfield S, Kaczmarek LK. Expression of the mRNAs for the Kv3.1 potassium channel gene in the adult and developing rat brain. J Neurophysiol 68: 756–766, 1992 [DOI] [PubMed] [Google Scholar]
- Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci 24: 517–526, 2001 [DOI] [PubMed] [Google Scholar]
- Sailer CA, Kaufmann WA, Kogler M, Chen L, Sausbier U, Ottersen OP, Ruth P, Shipston MJ, Knaus HG. Immunolocalization of BK channels in hippocampal pyramidal neurons. Eur J Neurosci 24: 442–454, 2006 [DOI] [PubMed] [Google Scholar]
- Saitow F, Konishi S. Excitability increase induced by β-adrenergic receptor-mediated activation of hyperpolarization-activated cation channels in rat cerebellar basket cells. J Neurophysiol 84: 2026–2034, 2000 [DOI] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci 7: 921–931, 2006 [DOI] [PubMed] [Google Scholar]
- Savtchouk I, Liu SJ. Remodeling of synaptic AMPA receptor subtype alters the probability and pattern of action potential firing. J Neurosci 31: 501–511, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan AP, Robertson B. Patch-clamp recordings from cerebellar basket cell bodies and their presynaptic terminals reveal an asymmetric distribution of voltage-gated potassium channels. J Neurosci 18: 948–955, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C, Huang ZJ, Nelson SB. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci 9: 99–107, 2006 [DOI] [PubMed] [Google Scholar]
- Sun L, Liu SJ. Activation of extrasynaptic NMDA receptors induces a PKC-dependent switch in AMPA receptor subtypes in mouse cerebellar stellate cells. J Physiol 583: 537–553, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell 125: 785–799, 2006 [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron 47: 725–737, 2005 [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol 10: 358–364, 2000 [DOI] [PubMed] [Google Scholar]
- Wang LY, Gan L, Forsythe ID, Kaczmarek LK. Contribution of the Kv3.1 potassium channel to high-frequency firing in mouse auditory neurones. J Physiol 509: 183–194, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Characterization of large conductance Ca2+-activated K+ channels in cerebellar Purkinje neurons. Eur J Neurosci 16: 1214–1222, 2002 [DOI] [PubMed] [Google Scholar]
- Yang TM, Wang LY. Amplitude and kinetics of action potential-evoked Ca2+ current and its efficacy in triggering transmitter release at the developing calyx of held synapse. J Neurosci 26: 5698–5708, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.