Abstract
Subthreshold ionic currents, which activate below the firing threshold and shape the cell's firing properties, play important roles in shaping neural network activity. We examined the distribution and synaptic roles of the hyperpolarization-activated inward current (Ih) in the pyloric network of the lobster stomatogastric ganglion (STG). Ih channels are expressed throughout the STG in a patchy distribution and are highly expressed in the fine neuropil, an area that is rich in synaptic contacts. We performed double labeling for Ih protein and for the presynaptic marker synaptotagmin. The large majority of labeling in the fine neuropil was adjacent but nonoverlapping, suggesting that Ih is localized in close proximity to synapses but not in the presynaptic terminals. We compared the pattern of Ih localization with Shal transient potassium channels, whose expression is coregulated with Ih in many STG neurons. Unlike Ih, we found significant levels of Shal protein in the soma membrane and the primary neurite. Both proteins were found in the synaptic fine neuropil, but with little evidence of colocalization in individual neurites. We performed electrophysiological experiments to study a potential role for Ih in regulating synaptic transmission. At a synapse between two identified pyloric neurons, the amplitude of inhibitory postsynaptic potentials (IPSPs) decreased with increasing postsynaptic activation of Ih. Pharmacological block of Ih restored IPSP amplitudes to levels seen when Ih was not activated. These experiments suggest that modulation of postsynaptic Ih might play an important role in the control of synaptic strength in this rhythmogenic neural network.
Keywords: hyperpolarization-activated inward current, synaptic integration, Shal, transient potassium current, central pattern generator, Crustacea, stomatogastric ganglion
cell-specific differences in ion channel expression help to create a neuron's unique firing properties and function within a network (Baro et al. 1997; Schulz et al. 2006, 2007). Based on these differences, neuromodulation of subthreshold currents can change the unique input-output properties of individual neurons and alter the resulting network output. One such current is the hyperpolarization-activated inward current (Ih). Ih is an inward current carried mostly by Na+ and K+ ions that is slowly activated by subthreshold hyperpolarizations and only slowly deactivates on repolarization. It is well known for its contribution to the depolarizing pacemaker current in the heart (Baruscotti and Difrancesco 2004; DiFrancesco 2006; DiFrancesco and Ojeda 1980), but Ih is also found in the central and peripheral nervous systems, where it plays a number of roles (Debanne et al. 2006; Harris-Warrick 2002; Johnston et al. 2000; Kaupp and Seifert 2002; Magee 1999; Pape 1996; Robinson and Siegelbaum 2003; Wahl-Schott and Biel 2009). Ih helps to set the resting potential and contributes to postinhibitory rebound (Harris-Warrick et al. 1995; Pape 1996; Robinson and Siegelbaum 2003), thus playing a supporting role in the generation of plateau potentials and bistability (Kiehn and Harris-Warrick 1992; Robinson and Siegelbaum 2003). In some neurons, Ih also shapes dendritic integration. In layer V pyramidal cells of the somatosensory cortex, Ih activation disconnects somatic and dendritic spike initiation zones and may prevent initiation of dendritic calcium action potentials in the absence of proximal input (Berger et al. 2003). Ih is also involved in the regulation of synaptic transmission, long-term facilitation, and integration of synaptic events through shaping temporal summation as well as spatial normalization of distant synaptic events (Beaumont and Zucker 2000; Berger et al. 2003; Genlain et al. 2007; Harris-Warrick et al. 1995; Magee 1998, 1999; Migliore et al. 2005; Williams and Stuart 2000).
In the pyloric network of the crustacean stomatogastric ganglion, Ih is present in all six identified neuron types; it has different voltage-dependence and kinetic properties in the different neurons and is subject to neuron-specific monoamine modulation (Harris-Warrick et al. 1995; Peck et al. 2006). Ih has also been measured in pyloric neuron axons, where it is strongly modulated by dopamine (Ballo and Bucher 2009; Ballo et al. 2010). The gene for the Ih channel protein PIIH has been cloned from the spiny lobster, Panulirus interruptus (Ouyang et al. 2007). Although there are four isoforms of the homolog mammalian HCN channel genes, only one invertebrate isoform exists, which undergoes a large amount of alternative splicing, generating variants that encode currents with very different voltage-dependence and kinetic properties and cAMP sensitivity. Soma recordings of Ih in pyloric neurons show that the activation and deactivation of this current is most likely too slow to directly impact firing properties on a cycle-by-cycle basis in the pyloric network, and it may act as a slowly changing leak current. However, the large degree of Ih modulation in the stomatogastric ganglion (STG) suggests a functional role for Ih,, which may vary in a state-dependent way.
In earlier work, we showed that neuronal upregulation of Ih could help to compensate for the artificial overexpression of a transient potassium current (IA) and might be involved in homeostatic regulation of neuronal firing properties (MacLean et al. 2003, 2005; Zhang et al. 2003). Correlations in the normal expression levels of Ih and IA mRNA have been documented for many neurons in the STG (Schulz et al. 2007) and in the cardiac ganglion of crabs (Tobin et al. 2009). Blocking Ih during ongoing pyloric network activity did not dramatically alter the motor pattern, but this blockade had a more significant effect during application of dopamine, which enhances Ih in selected neurons (Peck et al. 2006).
In this article, we further study the possible roles of Ih in the pyloric network. We describe the anatomic distribution of Ih channels in the STG, with a concentration in the fine neuropil where synaptic interactions occur. In addition, we have used electrophysiological experiments to demonstrate that Ih can act to limit the amplitudes of inhibitory synaptic interactions within this rhythmogenic network.
METHODS
Preparation.
Adult California spiny lobsters, P. interruptus, were obtained from Don Tomlinson Commercial Fishing (San Diego, CA) and maintained in artificial seawater at 16°C until use. All procedures were in accordance with the guidelines established by the National Institutes of Health and, where applicable, were approved by the Institutional Animal Care and Use Committees at Cornell University. Lobsters were anesthetized on ice for 30 min before dissection. The STG, along with its motor nerves and associated commissural and esophageal ganglia, was dissected and pinned in a silicone elastomer (Sylgard)-coated dish, as described by Mulloney and Selverston (1974). The physiological saline solution consisted of (in mM) 479 NaCl, 12.8 KCl, 13.7 CaCl2, 3.9 Na2SO4, 10.0 MgSO4, 2 glucose, and 11.1 Tris base, pH 7.4 (Mulloney and Selverston 1974). Neurons were identified during intracellular recordings (3 M KCl or 0.6 M K2SO4 + 20 mM KCl, 10–25 MΩ) by their typical membrane potential oscillation shapes and synaptic inputs (Kloppenburg et al. 1999) and by coincidence of action potentials recorded intracellularly with extracellular recordings using suction or pin electrodes on the respective motor nerves.
Immunocytochemistry.
Identified neurons were labeled with 4% neurobiotin (NB) in 50 mM Tris and 0.5 M KCl. For NB injection, tips of low-resistance electrodes were backfilled with the NB solution for 10 min. The shaft was then filled with 2 M KCl, leaving a 1-cm gap between the NB in the tip and the KCl in the shaft to avoid mixing. The resistance of the filled electrode was 25–90 MΩ. NB was injected for about 40 min with 500-ms, +5-nA pulses at 1 Hz. Lucifer yellow (LY) was injected for 10–40 min with 500-ms, −1- to −14-nA pulses at 1 Hz. Preparations were left for 1 h for individual neuron staining.
STGs were fixed in 2 or 3.5% paraformaldehyde in phosphate-buffered saline (PBS) for 50–90 min at room temperature. The fix was washed out with 8 changes of PBST (PBS + 0.3–1% Triton X-100) over 2–8 h. The tissue was then blocked for 3 h with 5% normal goat serum and 1% BSA in PBST at room temperature and incubated overnight in a rabbit anti-Shal (1:2,000) (Baro et al. 2000), rabbit anti-synaptotagmin (1:1,000; generated by the laboratory of Dr. N. Reist), or mouse anti-penta-His (1:20–1,000; Abcam) primary antibody in PBST + 5% normal goat serum and 0.1% BSA. The primary antibody was washed out with PBST for 2 h. The tissue was then incubated for 2 h with the respective Alexa Fluor-conjugated secondary antibodies (Molecular Probes) at 1:500 dilution in PBST + 5% normal goat serum and 0.1% BSA. The secondary antibody was washed out with PBS for 2 h. All incubations were performed at room temperature with constant shaking. NB was visualized by addition of Alexa Fluor 568-conjugated StrepAvidin (1:250: Molecular Probes) during secondary antibody incubation. The LY signal was amplified with a rabbit anti-LY antibody (1:500; Molecular Probes) and a secondary Alexa Fluor 488-conjugated anti-rabbit antibody. The STG was mounted and cleared on a slide with Vectashield mounting medium (Vector Laboratories). For measurements on sectioned ganglia, fixed ganglia were imbedded in 4% low-melting point agarose (Sigma) in Panulirus saline. Slices (40–70 μm) were made with a vibrating microtome (Leica Microsystem; speed setting 4, frequency setting 9) and transferred to PBST-filled wells. Antibody treatment was performed on the floating agarose sections or individual ganglion slices on a slide. Antibody staining in images of x-y planes or series of z-stacks was visualized and collected with a Leica TCS SP2 confocal system. For multiple staining, sequential imaging and narrow emission settings were used to prevent bleed-through effects. Image analysis and three-dimensional (3-D) reconstructions were performed with Volocity visualization and classification software. The “Colocalization” feature in Volocity was used to evaluate colocalization as described by Manders et al. (1993), using automatic thresholding (Costes et al. 2004) to generate colocalization channels. To improve visibility of colocalization in transparency projections, the brightness and opacity of the filled neuron and the colocalization channel were adjusted (see Fig. 2, B–E, Fig. 4B, and Fig. 5, B and C). Gamma values of the channels were not changed. For one preparation (see Fig. 2), confocal stacks were acquired as tiles at ×63 magnification and subsequently aligned and stitched with a GUI-based MATLAB tool, written by Ted Brookings.
Fig. 2.
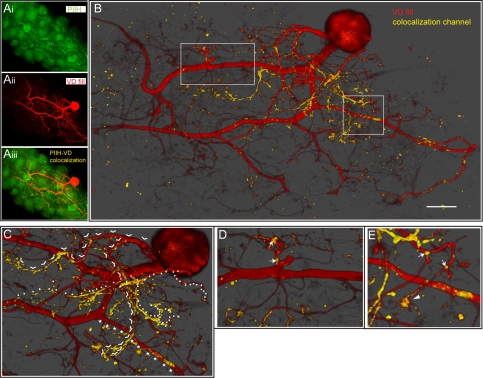
PIIH-like immunolabeling on a ventricular dilator (VD) neuron. A: extended focus projection of PIIH-like immunoreactivity (Ai) and a dye-filled VD neuron (Aii) in a whole mount STG (z-stack of 89 0.7-μm-thick optical sections, adjusted to show an ∼35-μm-thick midsection of stack). A colocalization channel (yellow) was generated and overlaid with both channels (Aiii). The brightness and density of the colocalization channel were enhanced to reveal PIIH-like immunolabeling relative to the VD neuron. B: 3-dimensionally (3-D) rendered transparency projection of z-stack of 89 0.7-μm-thick optical sections. A colocalization channel was generated to emphasize PIIH-like immunolabeling in or in contact with the VD neuron. Nonuniform and patchy distribution of PIIH-like immunolabeling along the VD neuron (yellow) was visualized. Opacity was adjusted to show an ∼35-μm-thick midsection of stack. Most PIIH-like immunoreactivity was found on and around medium-size and small branches. The larger diameter processes in the coarse neuropil, which consist of the primary neurite and the 2 axon precursors (left), show very little PIIH. See D and E for details of boxed regions. Scale bar, 50 μm. C: most of the smaller PIIH-like immunolabeled processes originated from a few single branches, which split off the primary neurite just below the soma. Continuous branches are individually marked with arrowheads and stars to help with visual separation. D: although most of the primary neurite is PIIH negative, a short, stubby structure that branches into several very thin processes from the primary neurite has patches of PIIH-like immunolabeling (arrows). E: patchy appearance of PIIH-like immunolabeling on branch points in the fine neuropil (arrows) and on flat paddle- or handlike structures (arrowhead), which are often found at or toward the end of very thin, long processes.
Fig. 4.
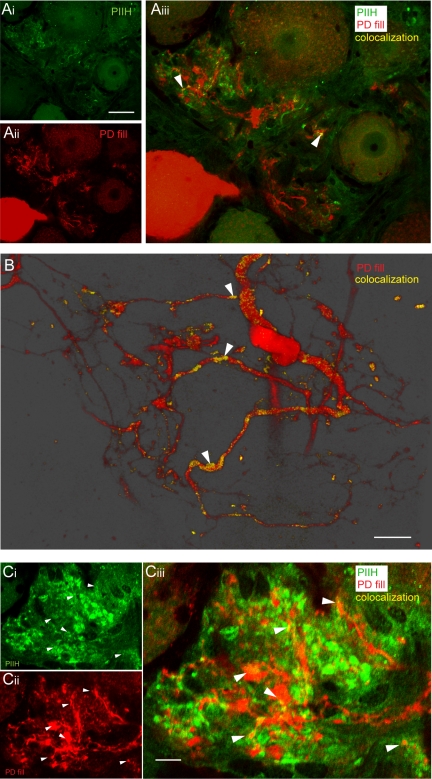
PIIH-like immunoreactivity in the pyloric dilator (PD) fine neuropil. A: overlay of PIIH-like immunolabeling in the fine neuropil of a sectioned ganglion (Ai) with a Lucifer yellow-labeled PD neuron (Aii) revealed relatively sparse PIIH-like immunolabeling of the PD neuropil (arrowheads in Aiii). Scale bar, 50 μm. B: a minority of branches of the PD neuron showed more extensive PIIH-like immunolabeling, as shown in a 3-D rendered transparency projection from 25 1-μm-thick optical slices from a different preparation. Scale bar, 30 μm. C: clusters of PIIH-like immunolabeling in the fine neuropil (Ci) occurred in close proximity to the dye-labeled PD neuropil (Cii), with occasional overlap visible (filled arrowheads in Ciii). Single optical section (0.45 μm) of a sectioned ganglion. Scale bar, 10 μm.
Fig. 5.
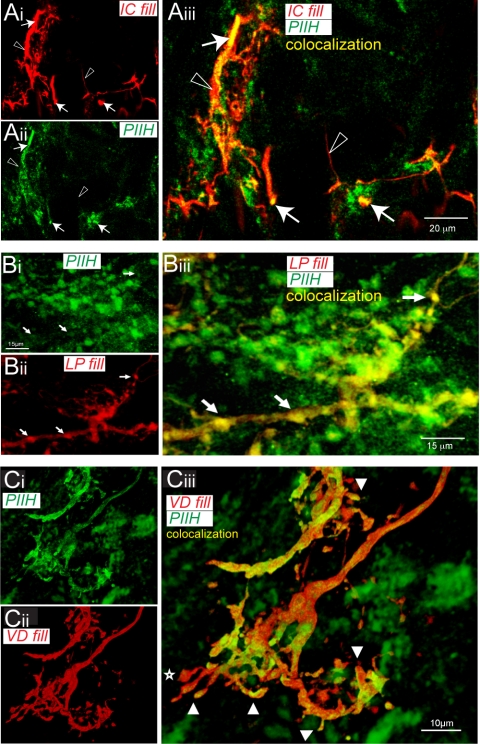
PIIH-like and synaptotagmin immunoreactivity in the STG. A: anti-PIIH (Ai) and anti-synaptotagmin (Aii) both labeled in the fine neuropil. A colocalization channel was generated and overlaid with the PIIH-like and synaptotagmin-labeled channels to reveal overlap in a single optical section of a sectioned STG (filled arrowheads in Aiii). Notice that the majority of PIIH-like immunolabeled patches did not colocalize with synaptotagmin immunoreactivity (open arrowheads in Aiii); also, many synaptotagmin-positive spots did not overlap with PIIH-like immunolabeling (stars in Aiii). Scale bar, 50 μm. B: PIIH-like and synaptotagmin immunoreactivity on a NB-labeled IC neuron, visualized as 3-D rendered transparency projection from 17 1.3-μm optical slices. Colocalization channels were generated to show only immunolabeling in or on the surface of the filled cell. The yellow colocalization channel (Bi) was generated from intensity correlations between the IC neuropil and PIIH-like immunolabeling, and the blue colocalization channel (Bii) was generated from intensity correlations between the IC neuropil and synaptotagmin immunoreactivity. Overlay of the IC neuropil fill and both colocalization channels reveals PIIH-like immunolabeled branches in yellow (open arrowheads in Biii), patches of colocalization with synaptotagmin immunolabeling in blue (stars in Biii), and triple labeling of PIIH-like and synaptotagmin immunolabel colocalization in white (filled arrowheads in Biii). Scale bar, 40 μm. C: enhanced zoom of boxed area in Biii shows a PIIH-like labeled branch of the IC neuropil with a distinct patch of synaptotagmin colocalization (filled arrowhead in Ci). Overlay of the unprocessed channels (Cii), which also reveals protein outside of the IC neuropil, shows that the PIIH-like signal at this location appears to follow along the IC branch (open arrowhead in Cii) and therefore is likely to be postsynaptic, whereas the synaptotagmin signal shows along a different process (open arrows in Ciii), which forms a close contact with the IC branch, without any indication of presynaptic PIIH-like labeling in this presynaptic process. Scale bar, 13 μm.
Synaptic transmission measurements.
The pyloric dilator (PD) and lateral pyloric (LP) neurons were impaled with two electrodes each to allow independent current injection and voltage recording in each cell (Fig. 1A). Action potentials and transient potassium currents [INa(V) and IA] were blocked with 0.1 μM tetrodotoxin and 4 mM 4-aminopyridine to isolate Ih. Although only little IA should be active in the voltage ranges that were tested, the long hyperpolarization of the PD neuron causes a significant removal of IA inactivation, which could interfere with the inhibitory postsynaptic potential (IPSP) measurements, especially at or near physiological membrane potentials. Ideally, we would have preferred to block all outward currents; however, the drug of choice (tetraethylammonium) can block graded synaptic transmission and therefore was not used. Synaptic measurements were recorded under current-clamp conditions with step depolarizations of the presynaptic LP neuron to evoke graded IPSPs in the PD cell (see Fig. 7, A and B). LP-evoked IPSPs were recorded while the PD was depolarized or hyperpolarized to activate or deactivate Ih to differing extents (see results). Current injection protocols (see Fig. 7B) were generated by Clampex software (Molecular Devices, Sunnyvale, CA). The PD membrane potential was changed by a series of 8-s current injecting steps in 0.5- to 2-nA increments at 1-min intervals to allow full recovery of Ih. If necessary, a bias current was injected into the PD cell to hold the membrane potential at −58 mV, the average PD membrane potential after blocker application. IPSPs were elicited by 200-ms depolarizing steps to −30 mV in the LP cell at the beginning or the end (after 7.8 s) of the PD polarization. The LP cell was held at −58 to −60 mV between steps. To avoid Cl− loading during the current steps, which would alter the reversal potential of the PD IPSP, we used relatively high-resistance (20 MΩ or higher) electrodes filled with 0.6 M K2SO4 + 20 mM KCl. IPSP amplitudes were measured and plotted against the membrane potential immediately before the IPSP.
Fig. 1.
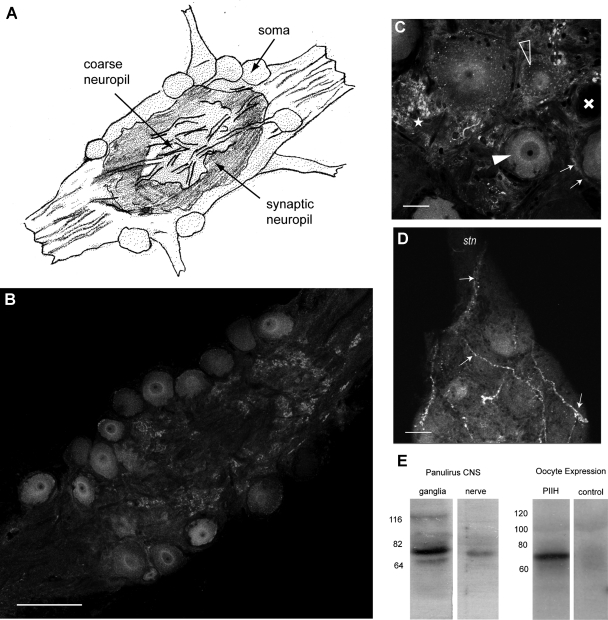
Panulirus interruptus hyperpolarization-activated inward current (Ih) protein (PIIH)-like immunolabeling is located in the somata and in the neuropil regions throughout the stomatogastric ganglion (STG). A: general anatomy of the STG. The STG contains about 30 motor and interneurons. The somata are situated on the outer surface of the ganglion and send large neurites toward the coarse neuropil in the middle, from where smaller processes branch into the layer of the fine (or synaptic) neuropil, located between the somata and the coarse neuropil. B: PIIH-like immunolabeling is found in STG somata and in the fine neuropil, whereas the larger processes of the coarse neuropil in the middle of the ganglion are much less brightly stained. Single 1-μm-thick optical section of the confocal stack. Scale bar, 150 μm. C: variable PIIH-like immunolabeling in and around the STG somata. Most neurons show strong perinuclear (filled arrowhead) or punctate staining in the soma (open arrowheads), whereas some are apparently lacking PIIH-like immunolabeling (cross). Dense PIIH-like immunolabeling is present in tissue surrounding the somata in the form of strong punctate staining (star) and halo-like layers (small arrows) around cell bodies, which may be glia or connective tissue. Single 1-μm-thick optical section of the confocal stack. Scale bar, 30 μm. D: PIIH-like immunolabeling reveals large fibrous structures (arrows) of unknown origin, which enter the STG from the stomatogatric nerve (stn) and dorsal ventricular nerve and branch within the ganglion. Maximum intensity projection of 7 consecutive confocal sections. Scale bar, 30 μm. E: specificity of the PIIH antibody was determined with Western blots of Panulirus central nervous system (CNS) tissue and from Xenopus oocyte, expressing PIIH RNA. The antibody labeled a strong (∼76 kDa) band in protein extracted from ganglia and from stomatogastric nervous system nerves (left). A larger band in the range of 116 kDa was found in Panulirus brain tissue and may be phosphorylated, glycosylated, or oligomeric forms, whereas the faint smaller band (∼64 kDa) may be the result of protein breakdown or smaller splice variants. Comparison of protein extracted from Xenopus oocytes without (control) and with injection of PIIH RNA shows a strong PIIH-positive band of similar weight in the PIIH-expressing oocytes. The Panulirus tissue and the oocyte extracts were run separately with different molecular mass ladders.
Fig. 7.
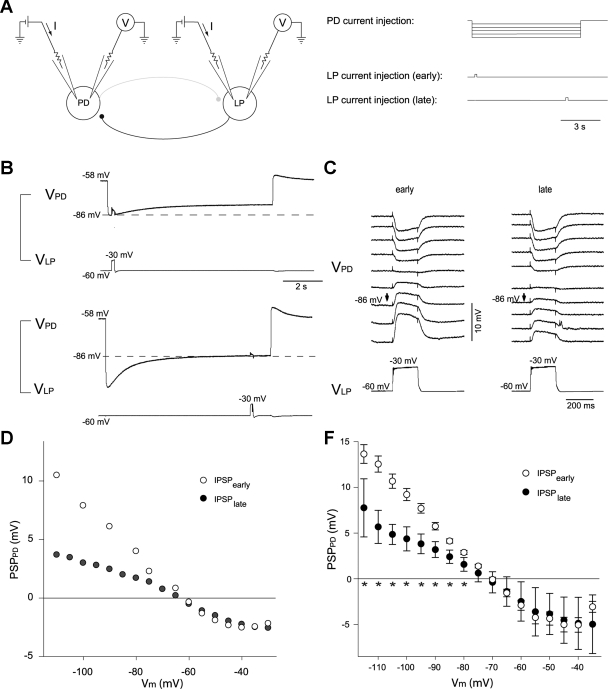
Amplitude of evoked LP inhibitory postsynaptic potentials (IPSPs) in the PD cell correlates with activation of Ih. A: recording configuration (left) and current injection paradigm (right). The PD and LP neurons were impaled with 2 electrodes each to allow independent current injection and voltage recording in each cell. Amplitudes are shown at the beginning (B, top, and C, left) of the hyperpolarizing voltage step in the PD cell, when very little Ih had been activated, and at the end of the PD hyperpolarization (B, bottom, and C, right), when Ih was maximally activated for that voltage, as shown by the depolarizing sag in the PD voltage. Arrows in C point to traces at equal membrane potential of the PD neuron (VPD; −86 mV) at the time of LP depolarization. VLP, membrane potential of LP neuron. D: IPSP amplitude at different membrane potentials (Vm) of 1 individual PD cell (PSPPD) at the beginning of PD polarizing current injection, when Ih was only weakly activated (IPSPearly) or at the end, when Ih was fully activated by the PD hyperpolarization (IPSPlate). E: summarized data from 11 experiments. ★P < 0.05.
Statistics.
All values are means ± SD. Statistical significances were determined using ANOVA and Student's t-test after testing for normality (P < 0.05) and using the Mann-Whitney rank sum test when the normality test failed. Regression lines were plotted, and R values were determined using SigmaPlot and SigmaStat 10.0 (Systat Software).
RESULTS
Immunocytochemical detection of Ih channels.
In our first attempt to map the distribution of Ih channels in the STG of P. interruptus, we used a polyclonal antibody raised against PAIH, the Ih protein in the related species P. argus, kindly provided to us by Dr. B. Ache. This antibody has been successfully used in P. argus to localize Ih channel protein to the transduction compartment of olfactory receptor neurons (Gisselmann et al. 2005). Unfortunately, the specificity of this antibody was not adequate for use in our species, because the strongest band in Western blots was of lower molecular mass (∼60 kDa) than what would be expected for PIIH, based on the sequences of previously identified splice variants (77–82 kDa). Furthermore, incubation of the Western membrane with preimmune serum still produced bands. Thus we could not rule out the possibility of nonspecific binding of this PAIH antibody to a highly abundant unidentified protein. Instead, we took advantage of the fact that the carboxy-terminal region of the PIIH gene from P. interruptus has an unusual string of eight continuous histidine residues. This sequence could be specifically recognized by a commercially available monoclonal anti-penta-His antibody, which is usually used to help purify proteins that have been artificially tagged with a sequence of five histidines. We confirmed the anti-penta-His recognition of the Ih protein using Western blots of proteins extracted from PIIH-expressing and control noninjected Xenopus oocytes. Figure 1E shows a clear band at ∼76 kDa, near the predicted range of 77–82 kDa, as calculated from the sequences, showing that the anti-penta-His antibody does recognize the PIIH protein. A major band of the same size was labeled in protein extracts from P. interruptus brain and nervous tissue (Fig. 1E). A minor band in the range of 116 kDa was found in Panulirus brain tissue and may be phosphorylated, glycosylated, or oligomeric forms, whereas a faint smaller band (∼64 kDa) may be a consequence of protein breakdown or smaller splice variants. However, we emphasize that these bands may also represent other proteins recognized by the antibody. A National Center for Biotechnology Information BLASTP search of arthropod sequences restricted to Crustacea (search for: short and nearly exact matches; expect threshold: 200,000; Word size: 2; SEG filters: off; Score matrix: PAM30) found only 10 other proteins with 5 or more repeated histidines (5+His), 7 of which can be excluded by their molecular mass, which do not match any bands on the Western blot. E75, a nuclear receptor found in Daphnia pulex and D. magna with a molecular mass of 104 and 102 kDa, respectively, could be of concern with regard to our 116-kDa band. However, the repetitive histidine sequence in this receptor is not evolutionarily conserved: it is not found in any of its arthropod homologs. It therefore seems unlikely, although not entirely dismissible, that the Cancer borealis E75 receptor would also contain this 5+His repeat and be recognized by the antibody. In addition, this nuclear protein would be unlikely to be highly expressed in distal neuropil (see below). The other two proteins of concern are doublesex-Mab related 99B (BAG12873.1) and a transcription cofactor vestigial protein (BAJ05330.1), both from D. magna, with calculated molecular masses of 63.9 and 59.5 kDa, similar to the smaller 64-kDa band on our Western blot. Since a band of this size was also recognized by the polyclonal anti-PAIH antibody, we feel it unlikely to be caused by homologs of these proteins. Thus we used the anti-penta-His antibody to study the distribution of PIIH protein in the STG.
Ih protein is expressed in the soma and neuropil regions of STG neurons.
In the lobster STG, the majority of the 30 neuronal somata are located on the ventral surface, surrounded by glial cells and neuropil (Fig. 1A); some of the neurons are on the dorsal surface. These unipolar neurons send a primary neurite into the central core of the ganglion. This region, called the coarse neuropil, contains primarily large processes of the STG neurons as well as the axons of neurons in other ganglia and sensory neurons; there are no synaptic contacts in this region. The primary neurite then divides repeatedly into smaller branches, which in turn ramify in a more superficial area under and in between the somata called the fine or synaptic neuropil. This is where all the synapses in the ganglion are located (Fig. 1A).
In the superficial soma compartment, we found immunolabeling consistent with Ih protein expression in the somata of pyloric neurons (Fig. 1B). In this article, we refer to this labeling as PIIH-like immunoreactivity. The density of soma labeling varied between different neurons of the same ganglion, with some somata very strongly labeled, whereas others showed weak or no label (Fig. 1C). Among the pyloric neurons, the somata of the anterior burster, ventricular dilator (VD), and inferior cardiac (IC) neurons were usually more strongly labeled, whereas the PD neurons often exhibited weaker staining; however, due to the variability in staining between ganglia, there was not a statistically significant correlation between neuron type and somatic PIIH-like labeling intensity. The membranes of the somata did not show stronger PIIH-like staining than the cytosolic label; however, often a concentric pattern of higher intensity was located intracellularly around the nucleus (Fig. 1C), likely arising from intracellular protein still bound in the Golgi apparatus or endoplasmic reticulum (ER). There was significant labeling in between the somata, reflecting intense labeling in the synaptic fine neuropil (see below) as well as putative glial cells and connective tissue located there (Fig. 1C). There was also sometimes PIIH-like labeling in large fibrous structures of unknown origin, which enter the STG from the stomatogastric nerve and dorsal ventricular nerve and branch on the surface and within the neuropil of the ganglion (Fig. 1D).
Figure 2 shows a double-label experiment of a VD neuron filled with LY (Aii) and stained for PIIH-like immunoreactivity (Ai). A colocalization channel was generated to emphasize PIIH-like staining in or in contact with the VD neuron, generated from 89 0.7-μm optical sections (Fig. 2, A and B). A nonuniform and patchy distribution of PIIH-like immunoreactivity along the VD neuron (yellow) was visualized using a 3-D rendered transparency projection of the colocalization channel and the VD fill. Most of the PIIH-like immunolabeling was found in a patchy distribution on and around the medium-size and small branches, often close to branch points (arrows in Fig. 2, D and E). Most of the smaller PIIH-positive processes originated from a few single branches, which split off the primary neurite just below the soma (Fig. 2C). The large central neurites in the coarse neuropil and the two processes that lead to axons showed very little PIIH-like immunoreactivity; one exception was a short, stubby structure off the primary neurite, which gave rise to several very thin processes and showed patches of PIIH-like staining (Fig. 2D). A distinguishing feature of PIIH-like labeling was its localization on branch points in the fine neuropil and on flat paddle- or handlike structures, which were often found at or toward the end of very thin, long processes (Fig. 2E).
Similar PIIH-like neuropil labeling was seen in double stains of other pyloric neurons. Consistently, the strongest PIIH-like immunoreactivity was observed in the fine neuropil, where it often appeared in clouds at the ends of very fine branches, in bulbous or fingerlike structures (Fig. 3). The fine neuropil of an IC neuron was labeled nonuniformly with patches of PIIH-like staining; not all branches were labeled (Fig. 3A). The fine neuropil of the LP neuron showed bulbous varicosities that were strongly positive for PIIH-like immunoreactivity (Fig. 3B). In a different, sectioned VD neuron, which had been filled with NB, many spiny and bulbous processes showed overlap with PIIH-like staining (Fig. 3C). The physiological properties of Ih have previously been studied in detail using voltage clamp in the pyloric pacemaker neurons, specifically the PD neurons. After filling a PD neuron with LY (a dye that does not cross gap junctions in the STG of P. interruptus), we sectioned the ganglion into 40-μm slices and labeled for PIIH-like immunoreactivity. Overall, PIIH-like labeling of the PD neuropil was somewhat sparser than in the other neurons. However, we found a number of branches that showed PIIH-like immunolabeling (Fig. 4, A and B). Clusters of strong PIIH-like signal were found in close proximity to the dye-labeled PD fine neuropil, with many overlapping regions (Fig. 4C).
Fig. 3.
PIIH-like immunolabeling in fine neuropil structures in different pyloric neurons. A: PIIH-like labeling on fine branches of an inferior cardiac (IC) neuron. Anti-PIIH-like (Ai) staining labeled fine processes in the neuropil of a neurobiotin (NB)-filled IC neuron (Aii). Double labeling showed nonuniform distribution of strong PIIH-like labeling within IC neuropil branches (arrows in Aiii); however, not all branches showed PIIH-like immunoreactivity (open arrowheads in Aiii). Scale bar, 20 μm. B: PIIH-like immunoreactivity in NB-labeled lateral pyloric (LP) fine neuropil. Overlay of the PIIH-like immunolabeling (Bi) and NB signal (Bii) revealed overlap in bulbous varicosities of the LP neuropil (arrows in Biii). Scale bar, 15 μm. C: PIIH-like immunolabeling on a fine branch of the VD neuropil in a sectioned ganglion (Ci) stained small branchlike processes of the VD neuropil. NB double labeling (Cii) confirmed that some of the signal was located in the processes of the NB-filled neuron. Notice spiny and bulbous processes on the neuron often showed strong PIIH-like labeling (arrow heads in Ciii), but not always (star). Scale bar, 10 μm.
Ih expression in the synaptic neuropil.
The fine neuropil has been shown by electron microscopy to be the site of synaptic interactions within the STG (King 1976), raising the question of whether Ih channels are selectively located at or near synapses. To answer this question, we performed double-labeling experiments for PIIH and a Drosophila anti-synaptotagmin antibody, which labels both synaptic vesicles and dense-core vesicles in Crustacea (Skiebe and Wollenschlaeger 2002). Anti-synaptotagmin labeling was seen in large clusters with punctate staining throughout the fine neuropil; several areas showed concentrated staining in the ganglion (Fig. 5A). Costaining for PIIH-like immunoreactivity revealed an increased density of Ih protein in areas of strong synaptotagmin labeling. A colocalization channel was generated and overlaid with the PIIH-like and synaptotagmin channels to reveal overlap in a single 1-μm optical section of a sectioned STG (Fig. 5Aiii). We found some spots of overlap between PIIH-like staining and synaptotagmin immunoreactivity; however, the majority of PIIH-like patches did not colocalize with synaptotagmin immunoreactivity, and many synaptotagmin-positive spots did not overlap with PIIH-like staining. To better understand PIIH-like and synaptotagmin immunoreactivity distribution, we performed triple labeling of a NB-filled IC neuron with both antibodies. Again, colocalization channels were generated and manual thresholding was used to show only protein in or within immediate proximity to the surface of the filled cell (Fig. 5B). Overlay of the IC neuropil fill, the PIIH-like colocalization channel, and the synaptotagmin colocalization channel revealed fine PIIH-like labeled branches (yellow; open arrowheads in Biii), patches of synaptotagmin colocalization with the IC neuropil (blue; stars in Biii), and triple labeling of PIIH-like and synaptotagmin immunoreactivity colocalization on the IC neurites (white; filled arrowheads in Biii). High magnification of such a spot in Fig. 5C shows an example of a PIIH-like positive branch of the IC neuropil with a distinct patch of apparent synaptotagmin colocalization (filled arrowhead in Ci). Overlay of the unprocessed channels (Cii), which also revealed PIIH-like and synaptotagmin immunoreactivity outside of the IC neuropil, showed that the PIIH-like signal at this location appeared to follow along the IC branch (open arrow in Cii) and therefore was likely to be postsynaptic, whereas the synaptotagmin signal at this location followed a crossing (non-IC) process. This synaptotagmin-positive process appeared to form a close contact with the IC branch, but without any indication of presynaptic PIIH-like staining in the presynaptic process.
In general, we often observed patches of strong PIIH-like labeling on very small processes of NB-filled neurons in close vicinity to synaptotagmin labeling, but not overlapping enough to show the double label at the single-pixel level. The virtual absence of membrane-bound double labeling for PIIH-like and synaptotagmin immunoreactivity, despite the large occurrence of synaptotagmin-labeled structures (Fig. 5B), might indicate a primarily postsynaptic distribution of PIIH. However, without the higher gain analysis using electron microscopy, we cannot determine with certainty whether Ih channels are localized at pre- or postsynaptic sites.
Ih and IA protein localization.
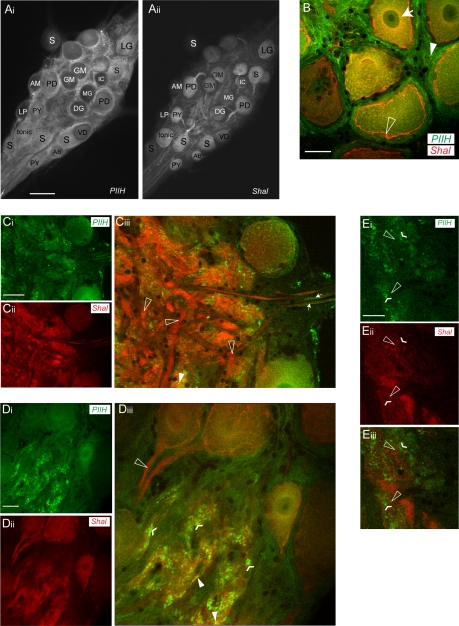
We previously described a homeostatic interaction between Ih and A-type potassium channels such that artificial upregulation of IA by Shal RNA injection led to a compensatory neuronal upregulation of Ih to retain normal firing activity (MacLean et al. 2003, 2005). The molecular mechanisms for this coregulation remain unknown. One possibility is that the proteins are physically coupled and are trafficked together to the cell surface. G protein receptors and ion channels have previously been shown to physically interact with scaffold proteins and other regulatory proteins in multiprotein complexes (Cooper 2003; Ma and Jan 2002; Mathie et al. 1998). If this were so, one might expect to find overlapping patterns of PIIH-like and Shal immunoreactivity localization. We therefore used a rabbit polyclonal antibody to lobster Shal, which encodes IA in the STG, that was previously designed and tested in our laboratory (Baro et al. 2000). Costaining of Shal and PIIH-like immunoreactivity revealed distinctly different patterns of labeling of the STG (Fig. 6). Whereas PIIH-like immunolabeling was found throughout the STG in neurons and the surrounding tissue, Shal immunolabeling was primarily concentrated in neurons, with a high level of immunostaining in the primary neurites (Fig. 6A).
Fig. 6.
PIIH-like and Shal immunoreactivity in the STG. A: overview of PIIH-like (Ai) and Shal immunoreactivity (Aii) in the same STG whole mount shows a distinctly different labeling pattern. Whereas PIIH-like immunoreactivity was found throughout the STG in neurons and the surrounding tissue, Shal immunoreactivity was concentrated in neurons with high concentrations in the primary neurites. Scale bar, 150 μm. AB, anterior burster neuron; AM, anterior median neuron; DG, dorsal gastric neuron; GM, gastric mill neuron; LG, lateral gastric neuron; MG, median gastric neuron; PY, pyloric neuron; S, STG. B: PIIH-like and Shal immunolabeling overlap in the soma of STG neurons. Both show a perinuclear distribution of labeling (arrow), but only Shal was highly concentrated in the soma membranes (open arrowhead), and only PIIH-like immunolabeling was found in the tissue surrounding the soma (filled arrowhead). Scale bar, 30 μm. C: PIIH-like (Ci) and Shal immunolabeling (Cii) in the coarse neuropil. Transparency projection of 7 1.3-μm optical slices reveals cloudy and patchy PIIH-like immunoreactivity throughout the coarse neuropil (Ci), whereas anti-Shal distinctly labeled large processes of the primary neurites (Cii). Overlay shows that large processes in the coarse neuropil generally lack PIIH-like immunoreactivity while showing strong Shal immunolabeling (open arrowheads in Cii). Some PIIH-like immunolabel was also found in or in close vicinity of axons, shown for anterior lateral nerve (arrows, right) on leaving the STG. Scale bar, 70 μm. D: single optical section shows area of the fine neuropil, which shows intense PIIH-like (Di) and Shal labeling (Dii). At a superficial level, both signals occurred within the same regions of the fine neuropil (Diii). Note the complete absence of PIIH-like immunoreactivity in the first stretch of the primary neurites, where the most intense Shal immunolabeling was found (open arrowhead). In the neuropil, PIIH-like staining is punctate (flat arrowheads) and only occasionally appears to overlap with Shal immunolabeling (filled arrowheads). Scale bar, 30 μm. E: at high magnification, PIIH-like labeling (Ei) and Shal immunolabeling (Eii) are frequently found in close apposition throughout the fine neuropil but rarely are overlapping and colocalizing. At this level of magnification, PIIH-like immunolabeling still appears concentrated in a patchy or punctate pattern (flattened arrowheads in Eiii), which is found in the close vicinity of Shal-positive structures (open arrowheads in Eiii), but the overlay shows that both signals rarely overlap at the pixel range (Eiii). Scale bar, 20 μm.
As previously described, Shal protein is trafficked to the cell surface of STG somata and their primary neurites; this was seen as bright ringlike staining in cross sections of the soma. However, such ringlike staining was not observed with the PIIH antibody (Fig. 6, A and B). Both antibodies showed strong intracellular perinuclear immunolabeling in the soma of STG motoneurons (Fig. 6B). In addition, anti-Shal strongly labeled the primary neurites arising from the somata, whereas PIIH-like immunolabeling did not (Fig. 6, A, C, D). Strong Shal immunoreactivity was also seen in the coarse neuropil (Fig. 6C) (Baro et al. 2000), whereas PIIH-like labeling was patchy and sparse in this region (Fig. 6C). PIIH-like immunoreactivity was also found in or in close vicinity to axon precursors (Fig. 6C), but we did not find convincing PIIH-like immunolabeling in the axons of the nerves (not shown).
In the fine neuropil, PIIH-like and Shal immunolabeling revealed similar distribution patterns at a superficial level, with high concentrations in the densest part of the synaptic neuropil (Fig. 6D). However, at high magnifications it was apparent that the PIIH-like and Shal-immunoreactive structures in the fine neuropil were not identical but in close apposition (Fig. 6E). Shal immunolabeling in the membranes of the fine branches was usually more homogenous than PIIH-like immunolabeling, which, as stated above, often showed distinct punctate staining patterns.
Ih activation reduces the strength of synaptic transmission.
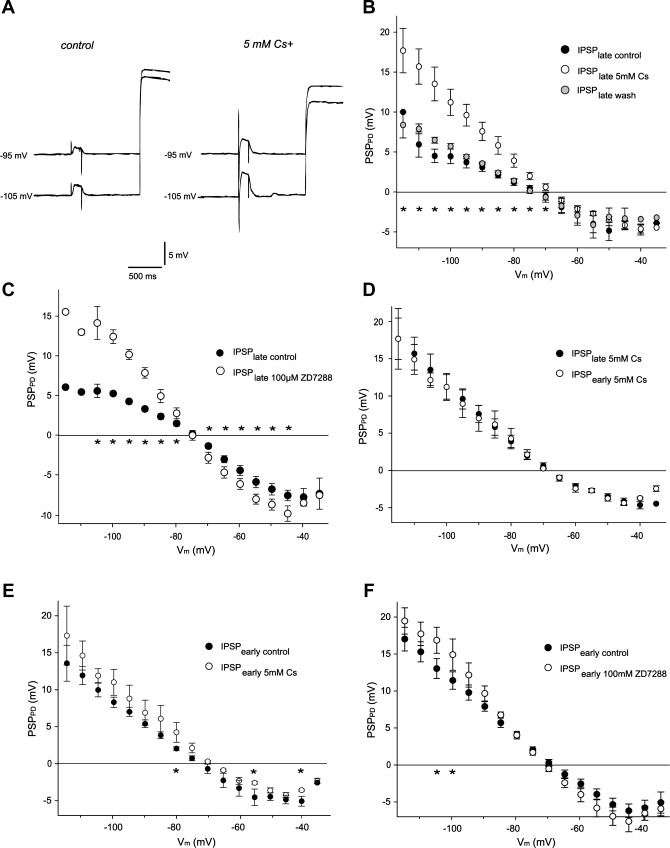
The strong PIIH-like immunolabeling in the synaptic fine neuropil suggested that this channel might function to regulate synaptic transmission in the pyloric network. To test this, we studied the effect of Ih activation on IPSP amplitude at the glutamatergic LP → PD synapse. For this purpose, we used the two-electrode current-clamp technique to control the membrane potential of the presynaptic LP cell and recorded LP-evoked graded IPSPs in the PD neuron while it was stepped to different membrane potentials (Fig. 7A). A range of 8-s hyperpolarizing and depolarizing current steps were injected into the PD neuron to activate or deactivate Ih to differing extents. Ih activation was monitored by the expression of a slow depolarizing sag potential in the PD neuron (Fig. 7B). At the beginning of a PD hyperpolarization, the Ih channels have only just begun to open, so Ih activation is low. We compared LP-evoked IPSP amplitudes at this point with those recorded after 7.8 s of PD hyperpolarization, when Ih channels were more completely activated to their steady-state level for that voltage (Fig. 7, B and C). Comparisons were made at the same voltages early and late in the current step (Fig. 7, B and C); thus larger initial steps had to be made to correct for the depolarizing sag to obtain comparable voltages at the end of the current step as at the beginning.
We consistently found that the amplitude of IPSPs at the same voltage after Ih activation at the end of PD hyperpolarization was smaller than that at the beginning, before Ih was significantly activated. This effect was strong for all voltages hyperpolarized below −75 mV (Fig. 7, B–E) and could be quite large. For example, when the PD neuron was hyperpolarized to −110 mV at the beginning of the step, the IPSP amplitude was 12.3 ± 1.3 mV, whereas when the PD was at −110 mV at the end of the step,the IPSP amplitude was only 5.6 ± 1.8 mV (62% decrease, n = 6, P < 0.01). Overall, the difference between IPSPs before and after activation of Ih was statistically significant at all PD membrane potentials of −80 mV and below (see stars below x-axis in Fig. 7E). With depolarizing current steps, when little Ih was activated by the step, there was not a large difference between IPSP amplitude measured at the beginning and the end of the step. At the most depolarized membrane potentials between −40 and −35 mV, the IPSPs began to decline in amplitude, most likely due to shunting by other voltage-activated currents. With these depolarizing current steps, the IPSP amplitude at the beginning of the PD polarization tended to be smaller than at the end, although this difference did not reach statistical significance. This could be a consequence of slow inactivation of voltage-dependent currents (including K+ and Ca2+ currents) or deactivation of resting Ih itself (see below).
IPSP amplitude during block of Ih channels.
If activation of Ih channels during hyperpolarizing voltage steps were shunting synaptic input, then Ih blockers should reduce or eliminate the difference between the IPSP amplitudes at the beginning and at the end of the PD current steps. To block Ih, we used 5 mM CsCl or 100 μM ZD7288, both of which cause comparable block of Ih in this system (Peck at al. 2006). Blockade of Ih dramatically changed the amplitudes of LP-evoked IPSPs in the PD neuron. In the presence of either 5 mM CsCl (Fig. 8, A and B) or 100 μM ZD7288 (Fig. 8C), the late IPSP elicited at the end of a PD hyperpolarization was significantly increased in amplitude at all membrane potentials below −65 mV. This effect partially reversed for CsCl after 30–45 min of washout (Fig. 8B), but not for ZD7288 (data not shown). There was also a trend with both blockers toward larger late IPSP amplitudes at more depolarized potentials between −65 and −45 mV, although it did not reach statistical significance for CsCl (Fig. 8, B and C).
Fig. 8.
Ih block by 5 mM CsCl or 100 μm ZD7288 increases LP-evoked IPSP amplitude in the PD cell. A: PSP amplitude at the end of PD polarization under control conditions (left) and in the presence of the Ih blocker 5 mM CsCl (right). B and C: IPSP amplitudes at the end of the PD current injection under control conditions (IPSPlate control) and increased IPSP amplitudes during Ih blockade with 5 mM Cs2+ (IPSPlate 5mM Cs) or ZD7288 (IPSPlate 100μM Z7288). For Cs2+, this effect was partially reversible through washout (IPSPlate wash). ★P < 0.05, control vs. blocker. D: during block of Ih with Cs2+, almost no difference was seen between IPSP amplitudes at the beginning (IPSPearly 5mM Cs) and at the end (IPSPlate 5mM Cs) of PD cell polarization. E and F: Ih blockade with Cs2+ (E) or ZD7288 (F) caused a small increase of IPSP amplitudes at the beginning of polarizing current injection, indicating that a small fraction of Ih channels were open. IPSPearly control, early IPSPs under control condition; IPSPearly 5mM CS, early IPSPs during Ih block with Cs2+; IPSPearly 100μM ZD7288, early IPSPs during Ih block with ZD7288. ★P < 0.05.
If the late IPSP attenuation after long hyperpolarizing steps under control conditions were entirely due to activation of Ih, the difference between early and late IPSP amplitudes should be eliminated during Ih block. Indeed, after application of 5 mM CsCl, IPSPs at the beginning of the PD polarization were not significantly larger than those at the end of the pulse (n = 5, P > 0.05, Fig. 8D).
A fraction of Ih channels might be open at the resting potential. To test this hypothesis, we blocked Ih by bath application of either 5 mM CsCl or 100 μM ZD7288 for at least 20 min. Both drugs slightly but significantly hyperpolarized the resting potentials of the PD and LP neurons. On average, bath application of 5 mM CsCl caused the PD neurons to hyperpolarize by 4.1 ± 1.4 mV (from −56.2 to −60.3 mV, P = 0.04, n = 7). Similarly, 100 μM ZD7288 hyperpolarized PD neurons by 2.2 ± 1.6 mV (from −55.6 to −59.0 mV, P = 0.03, n = 5).
To further study whether some Ih was active at more depolarized membrane potentials and could affect synaptic transmission within the normal voltage range of the PD neurons, we compared IPSPs at the beginning of the PD voltage steps under control conditions and after blockade of Ih. We found that blockade of Ih by either 5 mM CsCl (Fig. 8E) or 100 μM ZD7288 (Fig. 8F) increased the early IPSP amplitude at most membrane potentials, which in the case of ZD7288 was noticeable (up to 2-fold in individual experiments) even in the physiological range of −60 to −45 mV (Fig. 8F). At these potentials the increase approached significance (P < 0.2), but it was only statistically significant (P < 0.05, n = 5) at more hyperpolarized PD voltages (Fig. 8F). CsCl had a similar though smaller effect (Fig. 8E), perhaps due to the voltage dependence of Ih block by CsCl (DiFrancesco 1982). It should be emphasized that these experiments were performed in the absence of modulatory inputs, which can shift the voltage dependence of Ih and alter its contribution to the resting potential (Peck et al. 2006).
DISCUSSION
We have demonstrated that PIIH-like immunoreactivity, consistent with expression of the protein encoding Ih, is present in the lobster STG. Although only low membrane labeling is detected in the somata or coarse neuropil, areas in the fine neuropil, which is the site of synaptic interactions, are strongly labeled. This led us to test whether Ih can regulate synaptic strength; our results suggest that it can shunt inhibitory synaptic events when it is activated.
Distribution of Ih protein in the STG.
The distribution of Ih channels is not uniform in STG neurons. The somata of individual neurons revealed a wide range of PIIH-like immunoreactivity; this varied between different ganglia, and overall, there was not a statistically significant correlation between neuron type and somatic PIIH-like immunolabeling intensity. In the somata, the majority of PIIH-like immunoreactivity was seen in a concentric ring around the nucleus, probably reflecting protein still bound in the ER/Golgi system and trafficking vesicles. We saw no strong rings of label around the somata or parallel “railroad track” labeling of the membranes of neurites, as were previously seen for membrane-bound potassium channels (Fig. 6) (Baro et al. 2000; French et al. 2004). Tissue in between and around the somata on the dorsal and ventral surfaces of the ganglion usually revealed strong PIIH-like immunoreactivity. This could arise in part from the superficial fine neuropil (see below) but could also reflect the presence of PIIH in connective tissue or glial cells. Glial cells express a number of voltage-sensitive channels encoding potassium and sodium currents, where they can affect the glial resting potential and, indirectly, the activity of nearby neurons (Janigro 1997; Kang et al. 1998; Yamazaki et al. 2005).
In the neuropil, we found sparse and patchy PIIH-like immunolabeling on larger primary and secondary neurites of the coarse neuropil; this contrasts with the relative smooth distribution of Shal potassium channel immunolabeling on these neurites (Baro et al. 2000). The fine neuropil had the most intense, cloudy PIIH-like immunolabeling with localized punctate staining at higher magnifications. Labeling often occurred close to branching points or on very thin, long branches. Thus a significant amount of PIIH protein appears to be localized to the fine neuropil, where synaptic interactions are localized.
Synaptotagmin, a vesicular protein thought to be the calcium sensor that triggers exocytosis, is associated with release sites for both clear and dense-core vesicles in Crustacea (Skiebe and Wollenschläger 2002). Our double-labeling experiments for PIIH-like and synaptotagmin immunoreactivity showed a strong proximity of PIIH-like and synaptotagmin labeling in the branches of the fine neuropil; however, the two labels typically were not close enough to show pixel-to-pixel colocalization at the light microscopic level. This suggests that Ih channels are not primarily localized at the presynaptic terminal itself and provides some evidence for a postsynaptic localization of the channel. However, more detailed electron microscopy studies are needed to verify this. The close proximity of PIIH-like immunolabeling to synapses suggests that Ih activation may act to modulate synaptic strength, which we discuss below.
Ih and IA protein localization.
We earlier showed that artificial upregulation of IA (by Shal RNA injection) evoked a homeostatic compensatory upregulation of Ih to maintain the normal firing properties of the neuron (MacLean et al. 2003, 2005); even under normal conditions, PD neurons maintain a fixed ratio of expression of IA and Ih (MacLean et al. 2005; Schulz 2006, 2007). One possible explanation for this coupled coregulation could be a coordinated surface expression of IA and Ih channels, perhaps mediated by joint binding to surface scaffold proteins. We tested this with double-labeling studies to look for colocalization of Shal and PIIH-like immunoreactivity. These studies revealed similarities and differences in their immunolabeling patterns. Shal immunoreactivity showed a smooth distribution of labeling along the membranes of the cell bodies and primary neurites, which showed much less and more patchy PIIH-like labeling, which was mostly intracellular in these compartments. The fine neuropil exhibited equally strong labeling for PIIH-like and Shal immunoreactivity. However, at high magnifications, the sites of the most intense staining in the fine neuropil usually showed Shal and PIIH-like immunoreactivity adjacent to each other but rarely overlapping. This lack of colabeling makes direct interactions of the channels in a multiprotein complex a less attractive hypothesis to explain the homeostatic response. It appears that the compensatory response can occur even though the proteins are physically separate, although both are highly enriched in the synaptic neuropil. However, our light-level study cannot conclusively rule out the possibility of colocalization of a subset of channels, which would lead to some overlap and some differential staining.
Ih activation reduces the size of synaptic input.
Since Ih channels are strongly expressed in the synaptic regions of the fine neuropil, we tested whether Ih activation can affect synaptic strength. Our experimental design at the graded LP → PD synapse measured the postsynaptic effects of Ih activation by comparing the LP-evoked IPSP amplitude at varying PD voltages under conditions where Ih was either weakly or strongly activated. We found that activation of Ih dramatically decreased the size of the postsynaptic responses. This effect could be abolished by the Ih blockers ZD7288 and CsCl, confirming that our voltage-step protocols were monitoring the selective effect of activating Ih. It should be noted that both CsCl and ZD7288 can have nonspecific effects. CsCl blocks other potassium currents at higher concentrations (Llinás 1988), and ZD7288 has been known to affect synaptic transmission in a non-Ih-dependent way (Chevaleyre and Castillo 2002). The fact that both blockers had approximately the same effect on the membrane potential-IPSP amplitude curves, despite their very different nonspecific effects, supports our argument that this change is indeed due to the block of Ih.
Studies in other systems have suggested that Ih can both directly and indirectly increase synaptic transmission (Beaumont and Zucker 2000, 2002; Boyes et al. 2007; Genlain et al. 2007), but it can also have indirect effects to reduce postsynaptic integration (Maccaferrie et al. 1996; Magee 1998, 1999; McCormick and Pape 1990). Activation of Ih channels will reduce the neuron's membrane resistance and thus shunt synaptic events by reducing the effective length constant (Berger et al. 2003; Magee 1998, 1999; Williams and Stuart 2000). Migliore et al. (2004) showed that Ih could also selectively block temporal summation of unsynchronized input. We propose that an increase in Ih in the postsynaptic PD neuron significantly reduces the synaptic amplitude both by reducing the postsynaptic input resistance near the synapse and by providing an inward countercurrent to shunt the inhibitory IPSPs.
In the lobster, the voltage range for Ih activation is relatively hyperpolarized (V1/2 of the various splice forms ranges from −83 to −107 mV; Ouyang et al. 2007). Thus the shunting effects of Ih activation were most predominant at rather hyperpolarized potentials, where Ih activation is high. However, when we compared the amplitude of PD IPSPs at the beginning of the PD polarization (with low Ih activation) before and after Ih block with 100 μM ZD7288, we observed an increase in IPSP amplitude at all membrane potentials, including large effects (up to 2-fold) in the physiological range of −70 to −35 mV. In addition, both ZD7288 and low concentrations of CsCl, which also blocks Ih in the STG, caused a slight hyperpolarization of the resting potential. These data indicate that a small fraction of Ih channels are open at physiological membrane potentials and can regulate normal synaptic transmission.
Two possible mechanisms could make Ih an important regulatory current in the pyloric network, despite its hyperpolarized voltage dependence of activation (Ouyang et al. 2007). First, the hyperpolarizing inhibitory synaptic responses in distal parts of the neuron will be larger than when they reach the soma, which would enhance the distal activation of Ih and thus its role in regulating synaptic strength. Second, and more importantly, Ih activation kinetics and voltage dependence are significantly affected by cAMP binding, which can be changed by neuromodulatory inputs (Ballo et al. 2010; Ouyang et al. 2007; Robinson and Siegelbaum 2003; Rosenkranz and Johnston 2006; Santoro et al. 2004). In the absence of additional modulatory inputs, the contribution of open Ih channels to pyloric activity may be small, because we previously showed that blockade of Ih with CsCl had only subtle effects on the ongoing rhythmic pyloric motor pattern (Peck et al. 2006). However, cAMP binding at the CN-binding site depolarizes the voltage dependence of activation, which would increase the resting active Ih in the physiologically relevant voltage range. Consistent with this possibility, in the STG, dopamine shifts the activation curve of Ih in the AB cell by 10–15 mV in the depolarizing direction (Peck et al. 2006). Using optical methods to monitor cAMP levels, Hempel et al. (1996) showed that different neuromodulators caused unique and local elevations of cAMP transients in the fine neuropil of STG neurons. Thus dendritic Ih channels near synaptic sites are a likely target for cAMP-dependent modulation, which would regulate synaptic strength in a cell- and possibly even compartment-specific manner. Modulatory activation of postsynaptic Ih channels close to synaptic inputs could effectively shunt these synapses. This modulation could provide a potentially important and powerful mechanism to regulate the strength of synaptic input and thus modulate the rhythmic activity of the entire circuit in a context-dependent manner.
We examined the functional implications of Ih specifically for the LP → PD synapse. All the neurons within this network receive inhibitory input, most of them with very similar timing, and therefore the role of Ih in modulating or shunting the IPSP could be similar in the other pyloric neurons. This might be specifically important during different behavioral states in the presence of different modulators, which can dramatically change the output of this seemingly rigid network (Harris-Warrick and Marder 1991). On the other hand, it is certainly possible that this is not the only function of Ih and that its function may vary among the different neurons of the STG networks.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01-NS17323 to R. Harris-Warrick.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Ted Brookings for providing the GUI-based MATLAB alignment and stitching tool, Carol Bayles for technical assistance during confocal image acquisition, and Pat Rivlin for gifting the synaptotagmin antibody.
REFERENCES
- Ballo AW, Bucher D. Complex intrinsic membrane properties and dopamine shape spiking activity in a motor axon. J Neurosci 29: 5062–5074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballo AW, Keene JC, Troy PJ, Goeritz ML, Nadim F, Bucher D. Dopamine modulates Ih in a motor axon. J Neurosci 30: 8425–8434, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baro DJ, Ayali A, French L, Scholz NL, Labenia J, Lanning CC, Graubard K, Harris-Warrick RM. Molecular underpinnings of motor pattern generation: differential targeting of shal and shaker in the pyloric motor system. J Neurosci 20: 6619–6630, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baro DJ, Levini RM, Kim MT, Willms AR, Lanning CC, Rodriguez HE, Harris-Warrick RM. Quantitative single-cell-reverse transcription-PCR demonstrates that A-current magnitude varies as a linear function of shal gene expression in identified stomatogastric neurons. J Neurosci 17: 6597–6610, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruscotti M, DiFrancesco D. Pacemaker channels. Ann NY Acad Sci 1015: 111–121, 2004 [DOI] [PubMed] [Google Scholar]
- Beaumont V, Zhong N, Froemke RC, Ball RW, Zucker RS. Temporal synaptic tagging by Ih activation and actin: involvement in long-term facilitation and cAMP-induced synaptic enhancement. Neuron 33: 601–613, 2002 [DOI] [PubMed] [Google Scholar]
- Beaumont V, Zucker RS. Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nat Neurosci 3: 133–141, 2000 [DOI] [PubMed] [Google Scholar]
- Berger T, Senn W, Lüscher HR. Hyperpolarization-activated current Ih disconnects somatic and dendritic spike initiation zones in layer V pyramidal neurons. J Neurophysiol 90: 2428–2437, 2003 [DOI] [PubMed] [Google Scholar]
- Boyes J, Bolam JP, Shigemoto R, Stanford IM. Functional presynaptic HCN channels in the rat globus pallidus. Eur J Neurosci 325: 2081–2092, 2007 [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Assessing the role of Ih channels in synaptic transmission and mossy fiber LTP. Proc Natl Acad Sci USA 99: 9538–9543, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DM. Regulation and organization of adenylyl cyclases and cAMP. Biochem J 375: 517–529, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J 86: 3993–4003, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Gastrein P, Campance E. Queer channels in hippocampal basket cells: h-current without sag. J Physiol 574: 2, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. Block and activation of the pace-maker channel in calf purkinje fibres: effects of potassium, caesium and rubidium. J Physiol 329: 485–507, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. Funny channels in the control of cardiac rhythm and mode of action of selective blockers. Pharmacol Res 53: 399–406, 2006 [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Ojeda C. Properties of the current if in the sino-atrial node of the rabbit compared with those of the current iK, in Purkinje fibres. J Physiol 308: 353–367, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French LB, Lanning CC, Matly M, Harris-Warrick RM. Cellular localization of Shab and Shaw potassium channels in the lobster stomatogastric ganglion. Neuroscience 123: 919–930, 2004 [DOI] [PubMed] [Google Scholar]
- Genlain M, Godaux E, Ris L. Involvement of hyperpolarization-activated cation channels in synaptic modulation. Neuroreport 18: 1231–1235, 2007 [DOI] [PubMed] [Google Scholar]
- Gisselmann G, Marx T, Bobkov Y, Wetzel CH, Neuhaus EM, Ache BW, Hatt H. Molecular and functional characterization of an Ih-channel from lobster olfactory receptor neurons. Eur J Neurosci 21: 1635–1647, 2005 [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM. Voltage-sensitive ion channels in rhythmic motor systems. Curr Opin Neurobiol 12: 646–651, 2002 [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM, Coniglio LM, Levini RM, Gueron S, Guckenheimer J. Dopamine modulation of two subthreshold currents produces phase shifts in activity of an identified motoneuron. J Neurophysiol 74: 1404–1420, 1995 [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM, Marder E. Modulation of neural networks for behavior. Annu Rev Neurosci 14: 39–57, 1991 [DOI] [PubMed] [Google Scholar]
- Hempel CM, Vincent P, Adams SR, Tsien RY, Selverston AI. Spatio-temporal dynamics of cyclic AMP signals in an intact neural circuitum. Nature 384: 166–169, 1996 [DOI] [PubMed] [Google Scholar]
- Janigro D, Gasparini S, D'Ambrosio R, McKhann G, DiFrancesco D. Reduction of K+ uptake in glia prevents long-term depression maintenance and causes epileptiform activity. J Neurosci 17: 2813–2824, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Hoffman DA, Magee JC, Poolos NP, Watanabe S, Colbert CM, Migliore M. Dendritic potassium channels in hippocampal pyramidal neurons. J Physiol 525: 75–81, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci 1: 683–692, 1998 [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev 82: 769–824, 2002 [DOI] [PubMed] [Google Scholar]
- Kiehn O, Harris-Warrick RM. 5-HT modulation of hyperpolarization-activated inward current and calcium-dependent outward current in a crustacean motor neuron. J Neurophysiol 68: 496–508, 1992 [DOI] [PubMed] [Google Scholar]
- King DG. Organization of crustacean neuropil. I. Patterns of synaptic connections in lobster stomatogastric ganglion. J Neurocytol 5: 207–237, 1976 [DOI] [PubMed] [Google Scholar]
- Kloppenburg P, Levini RM, Harris-Warrick RM. Dopamine modulates two potassium currents and inhibits the intrinsic firing properties of an identified motor neuron in a central pattern generator network. J Neurophysiol 81: 29–38, 1999 [DOI] [PubMed] [Google Scholar]
- Llinás RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science 242: 1654–1664, 1988 [DOI] [PubMed] [Google Scholar]
- Ma D, Jan LY. ER transport signals and trafficking of potassium channels and receptors. Curr Opin Neurobiol 12: 287–292, 2002 [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J Physiol 497: 119–130, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JN, Zhang Y, Goeritz ML, Casey R, Oliva R, Guckenheimer J, Harris-Warrick RM. Activity-independent coregulation of IA and Ih in rhythmically active neurons. J Neurophysiol 94: 3601–3617, 2005 [DOI] [PubMed] [Google Scholar]
- MacLean JN, Zhang Y, Johnson BR, Harris-Warrick RM. Activity-independent homeostasis in rhythmically active neurons. Neuron 32: 109–120, 2003 [DOI] [PubMed] [Google Scholar]
- Magee J. Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci 2: 508–514, 1999 [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci 18: 7613–7624, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manders EEM, Verbeek FJ, Aten JA. Measurement of co-localization of objects in dual-colour confocal images. J Microscopy 169: 375–382, 1993 [DOI] [PubMed] [Google Scholar]
- Mathie A, Wooltorton JR, Watkins CS. Voltage-activated potassium channels in mammalian neurons and their block by novel pharmacological agents. Gen Pharmacol 30: 13–24, 1998 [DOI] [PubMed] [Google Scholar]
- McCormick D, Pape H. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol 431: 291–318, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore M, Ferrante M, Ascoli GA. Signal propagation in oblique dendrites of CA1 pyramidal cells. J Neurophysiol 94: 4145–4155, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore M, Messineo L, Ferrante M. Dendritic Ih selectively blocks temporal summation of unsynchronized distal inputs in CA1 pyramidal neurons. J Comput Neurosci 16: 5–13, 2004 [DOI] [PubMed] [Google Scholar]
- Mulloney B, Selverston A. Antidromic action potentials fail to demonstrate known interactions between neurons. Science 177: 69–72, 1972 [DOI] [PubMed] [Google Scholar]
- Mulloney B, Selverston AI. Organization of the stomatogastric ganglion in the spiny lobster. I. Neurons driving the lateral teeth. J Comp Physiol 91: 1–32, 1974 [Google Scholar]
- Ouyang Q, Goeritz ML, Harris-Warrick RM. Panulirus interruptus Ih-channel gene PIIH: modification of channel properties by alternative splicing and role in rhythmic activity. J Neurophysiol 97: 3880–3892, 2007 [DOI] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol 58: 299–327, 1996 [DOI] [PubMed] [Google Scholar]
- Peck JH, Gaier E, Stevens E, Repicky S, Harris-Warrick RM. Amine modulation of Ih in a small neural network. J Neurophysiol 96: 2931–2940, 2006 [DOI] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol 65: 453–480, 2003 [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Johnston D. Dopaminergic regulation of neuronal excitability through modulation of Ih in layer V entorhinal cortex. J Neurosci 26: 3229–3244, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Wainger BJ, Siegelbaum SA. Regulation of HCN channel surface expression by a novel C-terminal protein-protein interaction. J Neurosci 24: 10750–10762, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz DJ, Goaillard JM, Marder EE. Variable channel expression in identified single and electrically coupled neurons in different animals. Nat Neurosci 104: 356–362, 2006 [DOI] [PubMed] [Google Scholar]
- Schulz DJ, Goaillard JM, Marder EE. Quantitative expression profiling of identified neurons reveals cell-specific constraints on highly variable levels of gene expression. Proc Natl Acad Sci USA 9: 13187–13191, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiebe P, Wollenschläger T. Putative neurohemal release zones in the stomatogastric nervous system of decapod crustaceans. J Comp Neurol 453: 280–291, 2002 [DOI] [PubMed] [Google Scholar]
- Tobin AE, Cruz-Bermúdez ND, Marder EE, Schulz DJ. Correlations in ion channel mRNA in rhythmically active neurons. PLoS One 4: e6742, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl-Schott C, Biel M. HCN channels: structure, cellular regulation and physiological function. Cell Mol Life Sci 66: 470–94, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Backpropagation of physiological spike trains in neocortical pyramidal neurons: implications for temporal coding in dendrites. J Neurosci 20: 8238–8246, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Hozumi Y, Kaneko K, Li J, Fujii S, Miyakawa H, Kudo Y, Kato H. Direct evidence for mutual interactions between perineuronal astrocytes and interneurons in the CA1 region of the rat hippocampus. Neuroscience 134: 791–802, 2005 [DOI] [PubMed] [Google Scholar]
- Zang Y, MacLean JN, An WF, Lanning CC, Harris-Warrick RM. KChIP1 and frequenin modify shal-evoked potassium currents in pyloric neurons in the lobster stomatogastric ganglion. J Neurophysiol 89: 1902–9, 2003 [DOI] [PubMed] [Google Scholar]