Abstract
The capabilities of any sensory system are ultimately constrained by the properties of the sensory neurons: the ability to detect and represent stimuli is limited by noise due to spontaneous activity, and optimal decoding in downstream circuitry must be matched to the nature of the encoding performed at the input. Here, we investigated the firing properties of sensory neurons in the accessory olfactory system, a distinct sensory system specialized for detection of socially relevant odors. Using multielectrode array recording, we observed that sensory neurons are spontaneously active and highly variable across time and trials and that this spontaneous activity limits the ability to distinguish sensory responses from noise. Sensory neuron activity tended to consist of bursts that maintained remarkably consistent statistics during both spontaneous activity and in response to stimulation with sulfated steroids. This, combined with pharmacological and genetic intervention in the signal transduction cascade, indicates that sensory transduction plays a role in shaping overall spontaneous activity. These findings indicate that as-yet unexplored characteristics of the sensory transduction cascade significantly constrain the representation of sensory information by vomeronasal neurons.
Keywords: accessory olfactory system, bursting, signal transduction
a central goal of sensory neurobiology is to reveal how neurons encode information about the environment. This goal has many facets, including efforts to understand the nature of the stimulus [e.g., reviewed in Knutsen and Ahissar (2009) and Maler (2007)], the mechanisms of sensory transduction (Gillespie and Muller 2009; Tsunozaki and Bautista 2009; Yau and Hardie 2009; Singer et al. 2009; Touhara and Vosshall 2009; Ishimaru 2009), and the representation of stimulus information in patterns of action potentials (Rieke et al. 1997; Berry et al. 1997; Shadlen and Newsome 1998). The ability to decode information about the natural environment is heavily influenced by neuronal “noise”; depending on the particular neurons and circuits, responses can be reliable at the level of single action potentials (Berry et al. 1997) or show sufficient variability that many spikes and/or large populations of neurons are required for accurate decoding (Shadlen and Newsome 1998).
The accessory olfactory system (AOS) is a distinct chemical sense found in most tetrapods. This system is specialized to detect nonvolatile compounds used in social communication. Chemical stimuli are pumped into the vomeronasal organ (VNO), where vomeronasal sensory neurons (VSNs) that express individual receptor proteins from large families (Touhara and Vosshall 2009) encode the identity and concentration of ligands via their spiking activity. In the mouse, the AOS is an attractive system for exploring sensory coding and its functional relevance, in part because of the modest number of processing stages between sensory input and behavioral output (Halpern 1987). While physiological investigation of this sensory system is quite recent, there has been progress in identifying VSNs ligands and key components of the sensory transduction cascade, in characterizing the electrical properties of the sensory neurons, and in understanding how large-scale properties of the stimulus (such as the sex or strain of a conspecific) determine VSN responses (Touhara and Vosshall 2009; Tirindelli et al. 2009).
While these studies give insight into the sensory responses of VSNs, there has been little work bridging the gap from ligand to the fundamental unit of neuronal signaling, the single action potential. To obtain a clearer understanding of how sensory transduction is coupled to firing, we performed multielectrode array (MEA) recordings of the intact vomeronasal epithelium in response to a battery of sulfated steroids over a range of concentrations. Observing a significant level of spontaneous activity, we explored the implications this noise has on decoding sensory responses. We then investigated the structure of this activity as well as stimulus-driven activity to better understand what features of a response may communicate sensory information. We observed that VSNs predominantly fired bursts of spikes; while stimulus delivery increased the frequency of bursting, the characteristics of individual bursts were remarkably unchanged by the presence of stimulus. This led us to investigate the root of burst structural stability. By blocking or eliminating part of the signal transduction machinery, we altered the structure of spontaneous activity, suggesting that the signal transduction cascade is involved in both spontaneous and stimulus-driven activity. These results provide a first look at how sensory transduction and the firing properties of VSNs impact the encoding scheme of primary sensory neurons in the accessory olfactory system.
MATERIALS AND METHODS
Solutions and Stimuli
All sulfated steroids were purchased from Steraloids (Newport, RI), and all other chemicals were obtained from Sigma (St. Louis, MO), unless otherwise indicated. Stock solutions of steroids were dissolved in either methanol or deionized water. The following steroids, referred to by their Steraloid catalog number, were used: A0225, A6940, A7010, A7864, P3817, P2135, P3865, P8168, P8200, Q1570, Q2525, Q3910, and Q5545; the exact molecular identities of these compounds are as documented previously (Nodari et al. 2008; Meeks et al. 2010). Ringer solution contained 115 mM sodium chloride, 5 mM potassium chloride, 2 mM calcium chloride, 2 mM magnesium chloride, 25 mM sodium bicarbonate, 10 mM HEPES, and 10 mM d-(+)-glucose and was equlibrated by bubbling with 95% O2-5% CO2. High potassium Ringer solution substituted 50 mM potassium for equimolar sodium. Urine was collected from 3-mo-old BALB/c female mice and flash frozen in liquid nitrogen as previously described (Nodari et al. 2008). The phospolipase C (PLC) inhibitor U-73122 and the inactive analog U-73343 were presented at 10 μM and dissolved in DMSO for a final DMSO concentration of 0.67%. In all recordings involving these drugs, a vehicle containing 0.67% DMSO in Ringer solution was used.
Electrophysiological Recording
Adult male mice 6 to 21 wk of age of the B6D2F1 strain (Jackson Laboratory, Bar Harbor, ME) were used in all recordings except the TrpC2−/− experiments. Those recordings used a line of mice in which the TrpC2 channel is genetically ablated (Stowers et al. 2002). Adult males of the same age range were used. All experimental protocols followed the United States Animal Welfare Acts and National Institutes of Health guidelines and were approved by the Washington University Animal Studies Committee.
Dissection and recording procedures were performed as previously described (Holy et al. 2000; Nodari et al. 2008; Arnson et al. 2010) unless otherwise indicated; briefly, intact vomeronasal epithelia were isolated and mounted on a multielectrode array. The vomeronasal epithelium was removed from the bony capsule, the neuroepithelium was mechanically dissected as an intact sheet from the basal lamina; it was then held in place on the electrode array using a nylon mesh. Urine and sulfated steroids were diluted with Ringer immediately before the dissection and recording session. Sulfated steroids were used at a maximum of 11 concentrations, ranging from 1 nM to 100 μM, in 3-fold increasing intervals, and urine was diluted 100-fold. Final methanol concentration in the stimulus solution was never higher than 0.1%. All experiments included a minimum of two negative control (Ringer) stimuli as well as a vehicle control containing 0.1% methanol in Ringer. Stimuli were dispensed using an HPLC pump (Gilson 307; Gilson, Middleton, WI) and a robotic liquid handler (Gilson 215) capable of taking samples from prepared tubes and injecting them in a HPLC valve (Gilson 819 injection module). This robot was controlled by the Gilson 735 software. Continuously bubbled Ringer solution alternated with stimuli to produce continuous flow over the epithelium; the flow was heated to a temperature of 35°C and aimed directly at the epithelium. The timing of stimulus delivery (valve opening and closing for the Automate apparatus; HPLC valve switch for the Gilson apparatus) was monitored electrically and fed back to the acquisition software. Stimuli were presented for 10 s in a block-randomized order. Delivery was repeated in a newly randomized order at ≥5 times.
Extracellular recording was performed using multielectrode planar arrays (ALA Scientific Instruments, Westbury, NY; 10-μm flat titanium nitride electrodes isolated with silicon nitride) where electrodes were 30 μm apart, in two fields of 6 × 5 electrodes each. Electrical signals were amplified with a MEA 1060 amplifier (ALA Scientific Instruments), acquired at 10 KHz with a data acquisition card (National Instruments, Austin, TX) and saved to disk. We used custom data acquisition and data analysis software based on COMEDI (http://www.comedi.org) and Matlab (MathWorks, Natick, MA).
Data Analysis
Spike sorting.
This and all subsequent analyses were performed in Matlab (MathWorks). Following acquisition, single units were isolated using custom software. Spikes were sorted based on waveform shape across all electrodes using methods similar to those described previously (Segev et al. 2004). All single units had clear refractory periods of ∼25 ms or more.
In response to the highest concentrations of sulfated steroids, the spike amplitude in a few (5/36) of the responsive cells progressively decreased during the response such that we were unable to record their activity using extracellular electrodes for up to a few seconds (Meeks et al. 2010). Our choice of firing rate metric (see Characterizing a stimulus response) compensates for this phenomenon. At the highest stimulus concentrations, burst measurements made at the individual spike level may be biased by this phenomenon; however, of the 73 stimulus responses considered, only 6 were affected by spike amplitude decrement.
Characterizing a stimulus response.
Unless otherwise indicated, Δr was calculated using a Δrmonotonic, a metric that takes into account the observation that the peak response following stimulus delivery occurs with equal or shorter latency with increasing concentration. The peristimulus response was measured using an automated algorithm that finds the global maximum cumulative firing rate across all trials for each stimulus concentration such that the time window is of equal or shorter length with increasing concentration (see below for details). A baseline firing rate was computed using a time window equal to that used following stimulation.
For stimuli presented for a single concentration such as diluted female mouse urine and Ringer solution, the response was averaged over a 10-s time window following stimulus onset. A baseline firing rate was computed using a time window equal to that used following stimulation.
A response was determined to be statistically significant if it differed from the Ringer control using a rank sum test with a P value threshold of 0.05 with a minimum Δr of 2 Hz. To control for methanol and Ringer artifacts, Δr was computed for the vehicle and Ringer solution stimuli. No cells in this data set were found to have a significant Ringer response.
Optimal monotonic time windows for computing Δrmonotonic.
This metric optimizes the time window over which to integrate firing rate, subject to the constraint that the length of the time window varies monotonically, if at all, with stimulus concentration. This does not imply that the firing rate itself is monotonic with concentration. Let the index i refer to all trials using a particular stimulus identity at a given concentration and suppose that we have N different concentrations of the same ligand. The i = 1 corresponds to the highest concentration at which this ligand is applied, and i = N corresponds to the lowest, with all others arranged in order of decreasing concentration. Let Ri(t) be the firing rate, averaged over both trials and time, starting from the beginning of the trial (offset some time after valve opening) until time t. To compute Δrmonotonic, we seek a set of window ending times ti such that the sum
| (1) |
is maximized (S* will denote the maximum), subject to the constraint that the time windows are monotonic in i, that is, ti + 1 ≤ ti with all ti satisfying 0 ≤ ti ≤ T for some maximum trial duration T. This is a global optimization problem, and so it would seem to require a comprehensive search in an N-dimensional space, which even for N ≈11 (as here) is a prohibitively expensive computational problem. However, the problem can be solved much more efficiently using a dynamic programming approach. Consider the (partial) sum over a restricted time window T′ ≤ T and just the n highest concentrations,
| (2) |
Suppose we know the global optimum for these n concentrations at any arbitrarily chosen time T′ ≤ T; then, we can construct the solution for the first n + 1 concentrations for any time T″ satisfying T′ ≤ T″ ≤ T by realizing that
| (3) |
Hence the optimal solution Sn+1* (T″) can be found by considering all possible pairs (T′, tn + 1) satisfying T′ ≤ tn + 1 ≤ T″. To make this practical, we divide time into K discrete bins (we used 20 bins in all analyses). Once the solution for n + 1 is found, the process can be repeated until the solution for N is obtained.
Thus, with this dynamic programming (i.e., inductively over n) approach, a problem that seemed to be O(KN) in time and O(N) in storage can be solved in O(N K2) time and O(N K) storage. Matlab code implementing this global optimization can be downloaded from http://holylab.wustl.edu.
Characterization of VSN firing properties.
All firing property analyses were performed on sorted single units. When indicated, firing was divided into a nonstimulus period and a stimulus-response period. All nonstimulus periods included activity during the 30 s before stimulus presentation, which corresponds to 30–60 s following the previous stimulus for all stimuli. Stimulus-evoked activity corresponds to the 30 s following stimulus onset (using sulfated steroid or 1:100 diluted female mouse urine) if the change in firing rate, or Δr, was judged statistically significant.
INTERSPIKE INTERVAL.
Interspike interval (ISI) was calculated by taking the difference in spike times between adjacent action potentials. All ISI frequency histograms represent the probability of observing that particular ISI for the given cell. This was calculated according to the method outlined by Reich et al. (2000). To accommodate the wide range of relevant time scales in the ISI distribution, we used bin sizes that span logarithmically increasing intervals for a total of 200 time bins ranging from 1 ms to 14.5 s in width. Histogram frequency was calculated by dividing the bin count by the total number of ISIs. All population firing property figures represent the means ± SE of the histograms across the population, unless otherwise indicated.
DETERMINATION OF A BURST.
We define a burst as three or more spikes fired with an ISI < 100 ms. This ISI criteria is based on the ISI distribution (see Fig. 5). A dominant peak was observed at ∼40 ms, corresponding to bursting activity, and a second smaller peak was present at ∼1 s. One-hundred milliseconds correspond to the end of the first peak. As this pattern was consistently observed across cells, this burst definition was used for all cells.
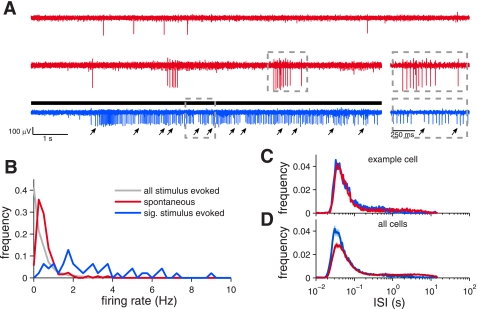
Fig. 5.
Firing properties of VSNs. A: 3 modes of activity (from top to bottom): tonic firing, spontaneous bursting, and responsive bursting. We did not observe a response to stimulus delivery from any tonically firing cells. Middle right inset: 1-s close-up of the spontaneous burst. Arrows at bottom indicate the start time of each individual burst that make up the protracted period of activity. Bottom right inset: 1-s close-up of the stimulus-driven activity. Two bursts can be observed at this magnification, with arrows indicating the starting spike of each. Note the similarity between each stimulus-driven burst and the spontaneous burst. Voltage off of 3 different electrodes from three different preparations is shown. Bar indicates when the stimulus (Q3910 30 μM) was delivered. B: distribution of spontaneous (126 cells; red) and stimulus-evoked (36 cells; blue) firing rates. Number of spikes in the 10 s before stimulus delivery (red) or number of spikes in the 10 s following stimulation (blue) was counted and averaged across trials to obtain the rate. Only responsive stimuli were used to compute stimulus-evoked values while the period before all stimuli were used to compute spontaneous activity. Gray line represents the activity of all 126 cells following all stimulus presentations (40–42 stimuli per cell, 126 cells). Stimulus-driven increases in firing rates were rare. C: normalized ISI histogram of a single cell during spontaneous activity (red) and stimulus-evoked activity (blue). A detailed look at the activity of this example cell is shown in Fig. 1E. D: ISI histogram across the all single units. Dark lines indicate the mean and the lighter lines indicate the SE. Note the similarity of the red and blue distributions in C and D.
BURST MEASUREMENTS.
Interburst interval (IBI) is defined as the time between the last spike of a burst and the first spike of the following burst. The burst duration corresponds to the time between the first and last action potential within a burst. The within-burst ISI was calculated by computing the ISI of each spike within the burst, while keeping track of the spike position within the burst. Spike position relative to the end of the burst was also computed where the last spike was counted as 1, the second last as 2, and so on. The within-burst ISI was normalized to the maximum within-burst ISI on a cell-by-cell basis.
Role of repeats in stimulus reliability.
FANO FACTOR.
To determine the variability of cells, we calculated the Fano factor, or the variance divided by the mean, as a function of time according to the method described by Rieke et al. (1997). The continuous spike train from each cell was divided into a series of time bins ranging in width from 1 ms to 100 s. The spike count in each time bin was computed. The variance and mean of the spike counts across time bins were computed to give the Fano factor.
RECEIVER OPERATING CHARACTERISTICS.
We explored the reliability of single and small numbers of trials using analysis based on receiver operating characteristics (ROC) (Green and Swets 1966; Britten et al. 1992) as described by Dayan et al. (2001). This allows for the computation of performance; if an ideal observer is presented with the Δr value calculated based on measurements from n repeats of the stimulus presentation, the performance indicates the percentage of times the observer would correctly guess a true positive response or a true negative response using a given threshold. A sulfated steroid at 100 μM was used for a “true positive” stimulus, using only cells that clearly responded to this stimulus at that concentration with a Δr >2 Hz and >2 SD above zero. The “true negative” stimulus corresponded to Ringer solution stimuli and steroids that did not elicit a response at any concentration. Each were presented five times to the cell.
Each ROC was calculated using Δr values computed from a set number of repeats (from 1 to all 5) for the “true positive” sulfated steroid stimulus and the “true negative” negative stimulus. To simulate the relative sparseness of ligand responses across VSNs, all negative stimuli were pooled. The same number of repeats were drawn from this pool as were drawn from the responsive pool.
A range of thresholds (z), from the minimum Δr to the maximum Δr value, changing by 1 spike/s were used. At each threshold point, we computed the false alarm rate α: p(Δr ≥ z| −) and the true positive rate β: p(Δr ≥ z|+)using the following equations:
| (4) |
where n is the number of resampled Δr values. This resulted in an α- and β-value for each threshold z. The α- and β-values are plotted as shown in results (see Fig. 3). A separate curve is generated for each number of repeats used. This was done 100 times using 100 blocks of n repeats randomly drawn from the observed Δr values, and the α- and β-values for each Δr were averaged across realizations. The resulting normalized area under the curve (AUC) is equal to the performance of an ideal observer in a two alternative forced choice task (Green and Swets 1966; Dayan et al. 2001) similar to the presented task (either the stimulus evokes a response or it does not). We computed the AUC using the TRAPZ function in Matlab.
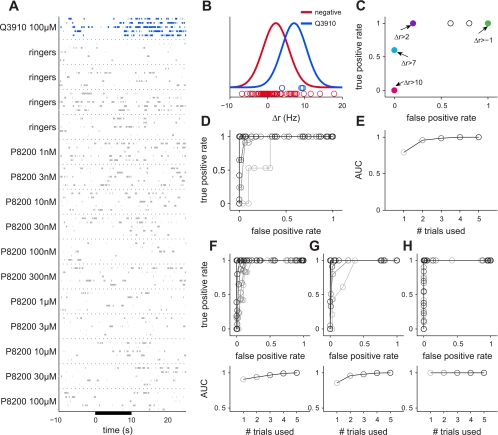
Fig. 3.
Variability of VSNs limits the reliability of detection. A: raster plot of the activity of a representative cell in response to an active sulfated steroid (blue), Ringer controls (gray), and a sulfated steroid that does not activate this cell (gray). Each stimulus was presented during the time indicated by the black bar for 5 separate interleaved trials. This cell was used in A–E. B: mean firing rate change upon stimulation. Circles represent the firing rate change, Δr, calculated on each trial of active stimulation (blue) or inactive stimulation (red). Distribution of firing rates is represented by a Gaussian distribution based on the means ± SD of Δr across trials. While the mean response to negative controls is near zero, spontaneous activity leads to multiple negative trials with high firing rates. C: results of classification of responses based on Δr as a function of threshold (ROC analysis). Each dot corresponds to the rate of true and false positives that would be observed using the indicated criterion value for this cell using 5 responsive trials and 5 nonresponsive trials, drawn randomly from the distribution. For example, when a threshold of 2 Hz was used (purple), 100% of true positives were correctly classified; however, there was a 20% false positive rate. Even with the use of 5 trials, for no value of threshold could one distinguish the 2 categories with perfect reliability. D: ROC analysis using 1–5 repeats. False positive and true positive classification rates were computed using 1 (light gray) to 5 (black) trials of both responsive and nonresponsive stimuli randomly drawn from the distribution shown in B and averaged over different combinations. E: area under the curve (AUC) summarizes the reliability of detection. AUC values using 1–5 repeats computed from D are shown. Reliability increases with the use of more trials. F–H : ROC analyses for the 3 additional responsive cells.
Correlations in VSN activity.
To determine whether or not correlations existed across VSNs, we looked at the spike times of all cells recorded on a single preparation (n = 14 preparations). Using 10-ms time bins, we measured the distribution of time differences between all spikes fired by all pairs of cells within a 10-s window both before and after the spike.
RESULTS
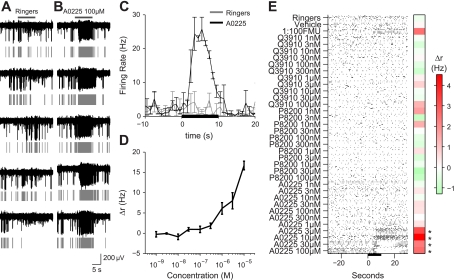
We set out to explore the impact of sensory transduction and firing properties of VSNs on coding in the AOS using MEA recordings of the intact vomeronasal epithelium. We probed VSN function by stimulating with dilute female mouse urine and a concentration series of 13 synthetic sulfated steroids ranging from 1 nM to 100 μM (see Solutions and Stimuli). We sorted spikes from individual VSNs (Fig. 1, A and B, gray rasters) and could follow the spontaneous and stimulated activity of these neurons across multiple trials (Fig. 1, A–C). As has previously been reported (Nodari et al. 2008; Meeks et al. 2010; He et al. 2010; Arnson and Holy, unpublished observations), VSNs increased their firing rate in response to sulfated steroids with a monotonic dependence on concentration (Fig. 1D). We noted high levels of activity, both stimulated and spontaneous, consistent with previous reports (Holy et al. 2000; Nodari et al. 2008).
Fig. 1.
Multielectrode array (MEA) recordings of vomeronasal sensory neurons (VSNs). A: 30 s of activity recorded on a single electrode across 5 repeats during stimulation with Ringer solution. These repeats were interleaved with presentations of other stimuli, not shown, for a total recording time of ∼200 min. Dark gray bar indicates the 10-s stimulus window. Activity of 1 single unit is indicated by the gray rasters below each electrode trace. Note the high level of spontaneous activity before and during stimulation with a negative control. B: same cell as shown in A during stimulation with 100 μM A0225. Note the increase in firing that is consistent across trials. C: peristimulus time histogram of the average activity for this cell in response to Ringer solution (gray) and 100 μM A0225 (black). Error bars reflect the SE across trials at each time point (bin size 1 s). D: response of a different cell to increasing concentrations of A0225. Change in firing rate, Δr, is plotted as a function of delivered concentration. Error bars reflect the SE across trials. E: Activity of an example cell. Each stimulus was presented 5 times for 10 s, as indicated by the black bar on the x-axis. Red-green colored bar to the right of the raster plot indicates the Δr values for that particular stimulus averaged across the 5 trials. *P < 0.05, stimulus elicited a statistically significant response by t-test. Firing rate values are indicated by the scale bar to the right.
High levels of spontaneous firing lead to noise in the signal, potentially making it more difficult to decode. Therefore, we set out to examine the role that this spontaneous activity has on coding in the AOS.
Spontaneous Activity of VSNs Leads to High Variability in Firing
In some cases, tonic spontaneous neural activity is thought to add to the reliability of signal detection by downstream decoders (Rieke et al. 1997). However, sporadic spontaneous activity may also present hurdles to reliable signal detection. For example, if a spontaneous increase in activity coincided with the stimulus period, a trial could be classified as falsely responding to a stimulus (e.g., Fig. 1A, trial 3). If activity coincided with the baseline period preceding stimulus delivery, then one may record a false negative. Therefore, we set out to explore how this noise affects the fidelity of response identification. To do so, we measured the effect of pooling activity across multiple trials, thus reducing the effect of noise, on the variability of stimulus-response detection using ROC analysis (Green and Swets 1966). Across 6 preparations, a total of 61 cleanly isolated cells were tested with 100 μM Q3910, of which 4 were classified as responsive to this ligand (Fig. 2). Each cell was also stimulated with 4 individual Ringer solution controls and 2 additional sulfated steroids each at 11 different concentrations. If a cell did not respond to the additional sulfated steroids at any concentration, those trials were also counted as negative trials (e.g., Fig. 3A). This gave us a distribution of 5 responsive trials and anywhere from 20 (4 Ringer solution controls, 5 repeats) to 130 (2 negative steroids at 11 concentrations plus 4 Ringer) nonresponsive trials. Having a large number of negative trials mimics, in an interpretable fashion, the more common task of identifying sparse actual responses from frequent negative responses. Using the observed distributions of firing rates (Fig. 3B), we calculated the frequency at which an ideal observer would correctly classify responses using a range of different thresholds. By comparing this classification with the known classification, we measured the frequency of true positive and false positive classification rate (Fig. 3C).
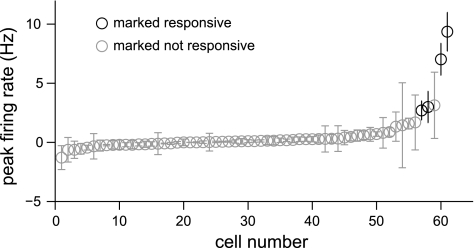
Fig. 2.
Firing rates used to determine a “true” response in receiver operating characteristics (ROC) analysis. Change in firing rate in each cell is plotted with SE across trials. Cells are plotted in order of increasing firing rate. Gray bars represent cells that did not pass our significance criteria of a maximum firing rate >2 Hz and firing that is ≥2 SE above baseline activity. Black bars did meet our criteria.
The activity of an example cell is shown in Fig. 3A. Depending on the number of trials used and choice of firing rate (Δr) threshold, responses to the active stimulus (“true positives”) may be confounded with apparent responses to the negative stimuli (“false positives”) (Fig. 3C). The ROC analyses for all 4 responsive cells are shown in Fig. 3, D and F–H, top. The AUC represents the probability that an ideal observer would correctly identify a response as positive or negative and therefore corresponds to the reliability of the data (Dayan et al. 2001). The AUC using one to five repeats for each example cell is shown in Fig. 3, E and F-H, bottom. In the first example cell, comparing one active trial to one inactive trial resulted in correctly classifying the response 79% of the time. Functionally, this translates to incorrectly identifying a ligand as active or missing a truly active ligand ∼20% of the time; if 30 ligands were tested on this cell using only 1 trial, we would be wrong about our assessment of activity for 6 of the ligands. With the use of as few as four repeats, the likelihood of correct classification climbed to 100%. Reliability varied across cells; two other cells (Fig. 3, F and G, respectively) showed similar rates of misclassification using few trials and high reliability using five trials while the final cell (Fig. 3H) was reliable using only one trial. For nonresponsive cells, the same analysis yielded detection at chance levels (data not shown), indicating (as expected) that the steroid and Ringer solutions were indistinguishable. The majority of responsive cells were not reliable when using single trials, suggesting that VSNs are noisy neurons to the point where pooling of information is required.
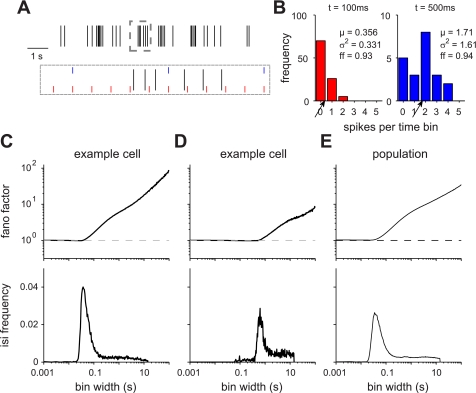
This analysis emphasizes the utility of pooling information, either across trials or across neurons. However, it does not address any questions about the underlying causes of VSN noise. This led us to look in more detail at the variability of VSN spiking statistics across time scales, in terms of the ratio of the variance to the mean, or Fano factor (Rieke et al. 1997). A Fano factor of 1 indicates that the spiking follows Poisson statistics; each spike is independent, not influenced by the prior history of the spike train (Rieke et al. 1997). A Fano factor <1 indicates that there is less variance than predicted by a Poisson model, while a larger value indicates greater variance. To calculate this, we divided the spike train of all cells into time bins of various widths (Fig. 4A) and computed the mean and variance of the number of spikes within each bin (Fig. 4B). Figure 4, C–E, plots the Fano factor as a function of bin width with corresponding ISI histograms of individual cells (C and D) or of the population (E). We found that across all cells the Fano factor hovered near 1 for smaller time bins until increasing drastically for larger time bins. Therefore, if one were to investigate a response, for example, over 10 s of activity (see materials and methods), the average Fano factor is ∼100 (Fig. 4C), or 2 orders of magnitude larger than that of a Poisson process, indicating considerable variability associated with assessing a sensory response across the given timescale. This implies that spikes fired by VSNs were not independent of each other and that some structure in activity may exist.
Fig. 4.
Variability of VSNs. A: 10-s interval of activity of an example cell (same as in C). Each line represents an action potential. Boxed area is shown on a different time scale on the bottom. Red and blue lines indicate example time bin divisions used when calculating the Fano factor (ff): 100 ms (red) and 500 ms (blue). B: spike count for each time bin using 10 s of activity was calculated and the mean and variance across all time bins was computed for both 100 ms (red) and 500 ms (blue) time bins. Ratio of variance over mean gives the Fano factor, shown in the text in B. Arrow points to the mean spike count value. C: variance divided by the mean (Fano factor) is plotted for a representative cell as a function of time bin (top). Corresponding interspike interval (ISI) frequency histogram for that cell is plotted at bottom. Fano factor increased when measured at time bins larger than the peak of the ISI histogram, ∼40 ms. D: cell with lower levels of activity is shown. Fano factor still increased at time bins larger than the peak ISI. E: average Fano factor and ISI histogram are shown. Back lines correspond to the mean ± SE.
To gain some insight into the mechanism underlying this noise, we explored the relationship between the Fano factor time scale and the ISI distribution of the cell. We found that this point of inflection corresponded to the peak in the ISI histogram, most frequently at ∼40 ms. The increase in Fano factor at times longer than a single ISI indicates that successive spikes are not independent, as, for example, might be observed with burst firing.
VSNs Tend to Fire in Bursts
The Fano factor analysis suggested that some structure exists in the spike trains of VSNs, beginning at time scales coinciding with a peak in the ISI histogram. Therefore, we investigated the detailed firing properties of VSNs, both during spontaneous activity (the 10 s before stimulus delivery) and during stimulus-evoked activity (P < 0.05 rank sum test and Δr > 2 Hz). Taking a closer look at the firing patterns of VSNs, we observed that spontaneous activity occurred in two distinct patterns. Most cells (108/126) fired repeated trains of action potentials in rapid succession (Fig. 5A, middle) while the remaining cells fired action potentials with longer ISIs (Fig. 5A, top). Across the population, the spontaneous firing rate was 0.7 Hz with an SD of 0.6 Hz and a range of 0.2–3.8 Hz (n = 126 cells; Fig. 5B). Excluding the slow firing minority group, the average spontaneous firing rate was also 0.7 Hz with a slightly smaller SD of 0.5 Hz and a range of 0.2 to 3.45 Hz. We also counted the number of spikes during a significant response to chemical stimuli (n = 36 cells). The mean firing rate was 3 Hz with an SD of 2 Hz and a range of 0.5–9.0 Hz. While spontaneous activity was lower than stimulus-evoked activity, the firing rate distributions overlapped (Fig. 5B).
We looked across all clearly sorted single units and found that rapidly firing neurons had several consistent features. Their ISI histograms, shown in Fig. 5, C and D, showed a dominant peak at ∼40 ms during both spontaneous and stimulated activity. This consistency, across both the population and under different stimulus conditions, allowed us to use a uniform definition of “bursting,” as three or more spikes fired with ISIs of <100 ms (Fig. 5, C and D; see materials and methods). The responsive neurons had a slightly higher probability of being in the bursting mode, as demonstrated by a higher “burst peak” in the population ISI histogram, while the spontaneous ISIs showed a large peak in the bursting mode with a second smaller peak at ∼1 s. The ISI histogram suggested that while responsive VSNs showed a tendency to spend more time bursting, the peak burst ISI of 40 ms was maintained in both conditions. Therefore, the observed increase in firing rate during a stimulus response was not due to a sizable shift in the overall ISI distribution.
Since we recorded many cells simultaneously (ranging from 3–31 cells in each preparation), we explored whether activity, and more specifically, bursting across cells were correlated. We found no evidence of correlations across cells (data not shown), suggesting that the origin of activity is independent across cells.
Similarities in Bursting Properties During Stimulation and Spontaneous Activity
Our previous analyses suggested that bursting, both spontaneously and during stimulation, underlies the activity of VSNs. We wondered whether fine details of the burst structure might provide valuable clues in distinguishing sensory responses from noise, so we investigated the timing of spikes between and within bursts.
The distribution of burst durations for a single example cell is shown in Fig. 6A. The mean burst duration of this cell was 246.6 ± 6.2 ms (means ± SE; n = 29,581 bursts) and 247.7 ± 9.7 ms (n = 3155 bursts) during spontaneous and stimulus-evoked activity, respectively. The length of bursts was found not to be statistically different between the two conditions (P = 0.94, t-test). Of the 36 cells that were significantly responsive, burst duration during spontaneous and stimulus evoked firing differed significantly in only 11 cells (P < 0.05, t-test). Even in these cells, the average difference in burst length between spontaneous and stimulus evoked conditions was 80 ms, on the order of only one or two ISI. Overall, the cells with stronger responses (Δr > 4 Hz) showed slightly longer bursts during stimulation on the order of a single spike while cells with weaker responses did not (data not shown). Burst length across the population is presented in Fig. 6B. Averaging across the population, the difference in burst duration between stimulus-evoked (327.8 ± 5.5 ms) and spontaneous (279.7 ± 1.4 ms) activity was statistically significant (P < 0.01); however, the difference between the two conditions was ∼50 ms, on the order of only a single ISI.
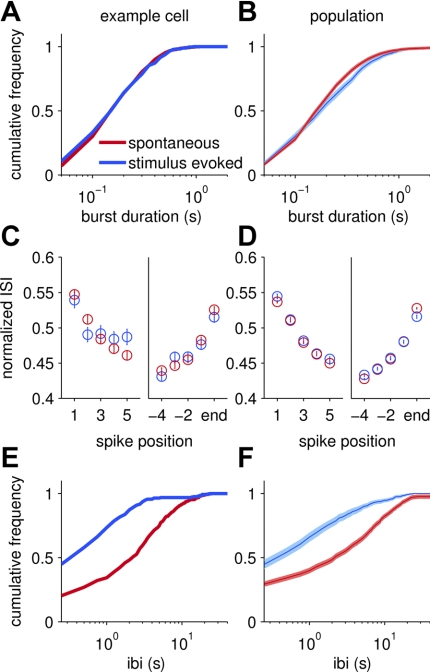
Fig. 6.
Bursting properties of VSNs. A, C, and E were calculated using the same cell (see Fig. 1E), and B, D, and F were calculated using all cells. Spontaneous activity of all cells is indicated by the red line, and stimulus-evoked activity from responsive cells is shown in blue. Burst duration was calculated for an example cell (A) and across the entire population (B), as measured by the time between the first spike in a burst and the last spike in that same burst. The center line of the cumulative probability histogram represents the mean and the lighter lines represent the standard error. Note the similarity between burst duration during spontaneous and stimulus-evoked activity. C and D: relative timing of the first and last 5 spikes within a burst. Circles represent the mean with error bars indicating SE. In both the single cell example (C) and across all cells (D), there was an initial acceleration in firing followed by a slowdown in firing at the end of the burst. E: time between consecutive bursts for a single cell and for the population (F). Interburst interval (IBI) was significantly shorter during a stimulus response than it was during spontaneous activity.
As the burst duration differed minimally between stimulus evoked and spontaneous activity, we next investigated the spike timing within bursts to see if there was evidence of sensory adaptation (Spehr et al. 2009) or other similar phenomenon during a response. A slowdown in firing was frequently observed towards the end of a burst. In Fig. 6C, we plotted the normalized ISI vs. spike position within the burst for the first five spikes and last five spikes for the representative cell. We found that across all bursts the first five spikes slightly accelerated their timing to reach a plateau and the final five spikes demonstrated an increased ISI, indicating a slowdown in firing. In both stimulus-evoked and spontaneous conditions, the same trend of acceleration and slight deceleration was observed for this cell (P > 0.05, t-test at each point). Across all single units, the first five spikes showed an initial decrease in the ISI followed by an eventual increase in the ISI during both spontaneous and stimulus-evoked bursting, as shown in Fig. 6D. Only the first spike of each of the two metrics (first 5 and last 5 spikes) was significantly distinguishable between stimulus-evoked and spontaneous conditions (P < 0.05, t-test), despite the fact that the timing of >3,000 ISIs were compared (n = 3,285 ISIs). This suggests that there is minimal difference in spike timing within bursts during stimulation and spontaneous activity.
Time Between Bursts Decreases During a Response
Thus far we observed limited differences in firing statistics between spontaneous and stimulus-evoked activity, differences too small to explain the increase in firing during a response. We next investigated the time between bursts or the IBI. This metric is related to the burst frequency and was investigated by measuring the interval between the last spike in one burst and the first spike in the following burst. Figure 6E shows the cumulative frequency histogram of the IBI for a representative cell, both during spontaneous and stimulus-evoked activity. During spontaneous activity, the average between-burst interval was 3.8 ± 0.2 s while it was 1.45 ± 0.2 s in response to a stimulus (P < 0.01, t-test). This increase in burst frequency is illustrated in Fig. 6E by the leftward shift of the responsive curve. In 21/36 responsive cells, stimulus caused a decrease in the IBI compared with spontaneous activity (P < 0.05, t-test). Of the 15 cells that did not display a significant difference in IBI, 10 similarly had decreased IBI (and 4 had increased IBI) during stimulus-driven activity, but these changes did not meet our significance criteria largely due to the sparseness of these cells' responses. Across the population, the mean burst spacing was significantly different between conditions (P < 0.01, t-test) at 4.15 ± 0.04 and 1.58 ± 0.05 s for spontaneous and stimulus-evoked activity, respectively.
Overall, the firing properties of VSNs demonstrated a tendency towards low rates of spontaneous activity (almost 1 Hz), consisting of both bursts and single spikes. While the probability of bursting increased during a stimulus response, the properties of burst duration and timing within bursts remained similar to that observed during spontaneous activity. The most prominent difference observed was that the time between bursts decreased during sensory responses. These results suggest that the primary feature in stimulus encoding involves the burst frequency or timing between bursts rather than a change in spike timing or longer burst duration.
Signal Transduction Pathway Is Involved in Shaping Spontaneous Activity
The similarity of bursts during both spontaneous activity and stimulus responses suggests that they are generated by a common mechanism. One possibility is that initiation of bursting is controlled by the membrane properties of the soma (Krahe and Gabbiani 2004; Bean 2007); another possibility is that bursts are triggered by the signal transduction cascade and that this cascade is sometimes activated spontaneously.
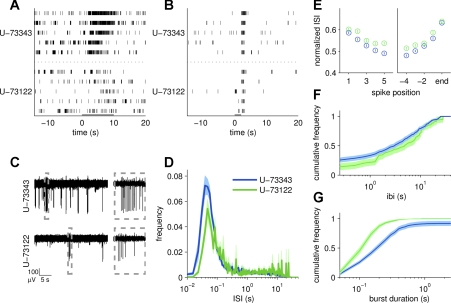
To distinguish between these possibilities, we first exploited the fact that vomeronasal sensory transduction is PLC dependent (Holy et al. 2000; Touhara and Vosshall 2009). We treated vomeronasal tissue with the PLC inhibitor U-73122 (10 μM) or its inactive structural analog U-73343 (10 μM) while stimulating with sulfated steroids and 50 mM K+ Ringer solution. Consistent with previous work (Holy et al. 2000), the PLC inhibitor successfully eliminated receptor-dependent responses in 14/16 responsive cells (n = 49 cells; Fig. 7A) and responses were greatly reduced in the other two cells. Activity due to K+-induced depolarization is thought to bypass any signal transduction machinery, which is consistent with the maintenance of K+ Ringer solution response during treatment with U-73122 in all but two cells that were active throughout the recordings (Fig. 7B). Cells still maintained some degree of spontaneous activity in the absence of PLC activity (Fig. 7C), but the average firing rate dropped from 0.8 to 0.3 Hz. The reduced firing rate is reflected by a smaller amplitude peak in the population ISI histogram (Fig. 7D). While bursting was maintained during PLC block, the overall structure appeared qualitatively different (Fig. 7C). We found that U-73122 slowed the rate of firing within bursts (Fig. 7E) and increased the time between bursts (Fig. 7F). However, the most striking feature was that bursts were significantly reduced in length from 240.7 to 122.4 ms (Fig. 7E). One interpretation of these results is that signal transduction machinery plays a role in shaping spontaneous, as well as stimulus driven, activity in VSNs, potentially both in terms of the total amount of firing as well as sculpting the dynamics of individual bursts.
Fig. 7.
Blocking the signal transduction cascade affects spontaneous VSN activity. A: a raster plot showing the response of a cell to 10 μM A0225 while being treated with the inactive U-73343 (top) and the active U-73122 (bottom). The gray bar indicates the time over which stimulus was delivered. Stimulus-driven, not spontaneous, activity was eliminated in the bottom condition. B: a raster plot showing the activity of a different cell in response to 50 mM K+ Ringer solution. The response was maintained in both conditions. C: activity on a single electrode during Ringer solution stimulation in the presence of U-73343 (top) and U-73122 (bottom). Activity was greatly reduced at bottom, and the bursts were shorter, as illustrated by the boxed off region showing 1 s of activity from each trace. D: ISI histogram for the population of 49 cells that were treated with U-73343 (blue) and U-73122 (green). There is a slight rightward shift following PLC inhibition, suggesting a slowdown in activity. Here, and in subsequent panels, use activity was measured in the absence of stimulus. E: relative timing of the first and last 5 spikes within a burst. Time between spikes was slightly increased following drug treatment. F: cumulative frequency histogram of the IBI across the population. There was more time between bursts during PLC inhibition. G: cumulative frequency histogram of burst duration across the population during treatment with U-73343 (blue) and U-73122 (green). PLC inhibitor treatment led to a reduction in burst duration.
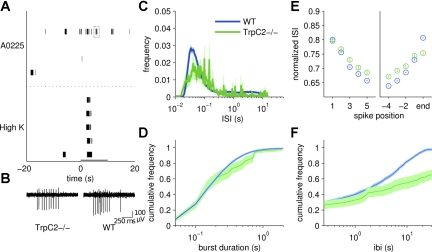
However, pharmacological intervention may have effects on machinery other than the intended target. Therefore, we also used a line of mice in which TrpC2, the ion channel previously demonstrated to be required for ligand-driven responses in VSNs (Stowers et al. 2002), is knocked out. This affects the signal transduction cascade at a point downstream of PLC. Consistent with previous reports (Stowers et al. 2002; Nodari et al. 2008), these TrpC2−/− mice no longer displayed stimulus-driven responses yet did respond to high K+ Ringer solution (Fig. 8A; n = 28 cells). As observed during PLC inhibition, spontaneous activity was reduced in the mutant mice compared with wild-type mice yet bursting persisted (Fig. 8B). The ISI histogram indicated a reduced peak at the shortest firing latencies (Fig. 8C). In contrast with the results from U-73122, the burst duration in TrpC2−/− VSNs was remarkably similar to that found in the wild-type VSNs (Fig. 8D). The phenomena of acceleration at the beginning and deceleration at the end were also similar, with slightly slower firing in the mutant mice (Fig. 8E). The time between bursts was greatly reduced in the absence of functional TrpC2 channels (Fig. 8F), consistent with the overall decrease in activity.
Fig. 8.
TrpC2 channel is involved in shaping spontaneous activity. A: stimulus-driven activity was abolished in the mutant vomeronasal organs (VNOs; top). Activity in response to 50 mM K+ Ringer solution persisted (bottom). Spontaneous activity was also reduced. Gray bar indicates the duration of stimulus delivery. Gray box around a burst of activity (top) is blown up in B (left). B: close-up of bursting activity in TrpC2−/− (left) and wild type (WT; right) animals. WT activity is taken from Fig. 5A, middle inset. C: ISI histogram of spontaneous activity from 28 cells from mutant animals (green) and 126 cells from WT animals (blue). WT histogram is the same as in Fig. 5C. D: cumulative frequency histogram of burst duration in mutant (green) and wild type (blue) VSNs. In this, and in E and F, the WT spontaneous activity is taken from Fig. 6. Overall burst duration in the mutant VNO was similar to that in WT VNO. E: ISI of spikes within a burst. Spikes within TrpC2−/− VSN bursts (green) were slightly slower overall than spikes from WT animals (blue). F: timing between bursts was greatly increased in the mutant animals (green) compared with the WT animals (blue).
DISCUSSION
The murine accessory olfactory system has been subject to a number of recent studies spanning both sensory neurons and downstream circuits (Meeks et al. 2010; Ben-Shaul et al. 2010; Hendrickson et al. 2008; He et al. 2008, 2010; Nodari et al. 2008); however, the question of how information is being represented at the level of individual spikes has not been explored. In this study, we aimed to elucidate the physiological properties of VSNs that relate to how stimulus information is encoded and to explore the implications of these properties for decoding sensory responses.
VSNs Use a “Burst Rate” Code for Representing Sensory Information
A large body of literature in neuroscience has been devoted to exploring mechanisms by which neurons encode sensory information, such as through spike rates or spike timing (Rieke et al. 1997). Bursts have been reported to encode sensory information in numerous systems including in olfactory receptor neurons (Eyherabide et al. 2008; Rospars et al. 2000). In various brain regions, the utility of bursts has been attributed to encoding information by burst duration (Kepecs et al. 2002; DeBusk et al. 1997; Martinez-Conde et al. 2002), number of spikes within the burst (Eyherabide et al. 2008), or within-burst ISI (Oswald et al. 2007).
We first explored the within-burst ISIs and the burst duration and observed only minimal differences in these characteristics between stimulus-response and spontaneous conditions, suggesting that VSNs were not encoding stimulus characteristics using intraburst differences. In our preparation, we also found little evidence to suggest that the precise timing of individual spikes was important. VSNs fired both in bursting and nonbursting modes, with very high variability (Fig. 4), which implies that codes depending on fine details of spike timing may be insufficiently robust. Moreover, the timing of spikes within bursts is nearly independent of sensory stimulation (Fig. 5). However, a caveat of this (and all other published investigations of VSN activity) is the ex vivo preparation. In vivo detection of stimuli is elicited by active pumping of the VNO (Meredith 1994), and it remains possible that pumping imposes a more detailed temporal structure on sensory encoding in the VNO. It is also possible that the natural kinetics of VSNs are upset by the ex vivo preparation. While great care was taken to maintain the integrity of the vomeronasal epithelium, it is possible that the ion concentration of our Ringer solution differs from that of the VNO lumen (currently unknown).
Overall, we observed that an increase in burst frequency signaled a sensory response. Therefore, we propose that VSNs encode sensory information via a burst rate code, in which bursts are the unit, rather than individual spikes. In this scheme, a burst code is approximately equal to a rate code, as the spike count and duration of bursts was approximately fixed (Fig. 5).
If bursts are not contributing additional coding information, that begs the question as to the purpose of bursts. It has been proposed that bursts increase the fidelity of synaptic transmission (Eggermont et al. 1993; Krahe and Gabbiani 2004). It is possible that there is low coupling between VSN spikes and mitral cells within a glomerulus and that the presence of bursts may increase the probability that a stimulus will elicit a response in the accessory olfactory bulb (AOB). Mechanistically, a possibility explored below is that bursts are a by-product of sensory transduction or the membrane properties of VSNs.
Implications of VSN Properties on the Coding and Measuring of Olfactory Information
We observed that variability in VSN spike trains increased dramatically at time bins greater than the peak in the ISI histogram (typically ∼40 ms; Fig. 5). Therefore, when a sensory response was measured, which is typically done over seconds with VSNs (Meeks et al. 2010; Nodari et al. 2008), variability in the signal was large. This potentially can lead to misclassification (Fig. 3) of ligand responses.
While this variability affects us as experimenters, it presumably also plays a role in how the AOS processes information. A common role by which systems can increase the reliability of highly variable systems is by pooling activity across a population (Rieke et al. 1997). The ∼150,000 neurons of the VNO (Leinders-Zufall et al. 2009) express ∼300 different receptor types; consequently, many cells will be expressing the same receptor type in any given VNO. In the AOS, multiple VSNs expressing the same receptor type project to a limited number of glomeruli in the AOB (Belluscio et al. 1999; Rodriguez et al. 1999; Del Punta et al. 2002; Wagner et al. 2006), meaning that there exists significant pooling of similar inputs. This pooling may allow the system to obtain a more reliable signal by averaging across redundant information, as indeed has been observed when comparing VSN responses to those of AOB neurons (Meeks et al. 2010). Given the capacity for pooling across many cells, it is indeed possible that the unreliability of individual neurons may be an acceptable price to pay for mechanisms of sensory transduction or firing that might increase sensitivity to weak stimuli.
Physiological recordings are typically blind to the molecular identity of the cells. As a proxy for pooling of common signals, we therefore use multiple repeats of the same stimulus on a given cell to explore the implications of variability on signal detection (Fig. 3). By pooling across multiple repeats, the reliability of the signal increased. These results support the idea that stimuli are represented by population activity in vivo and emphasize the importance of performing multiple repeats of stimuli.
Implications for Chemosensory Transduction
For many neurons, an increase in firing rate corresponds to a decrease in the ISI. Surprisingly, VSNs fired in bursts that maintained similar spike timing properties regardless of stimulation status. This poses an interesting question as to the nature of the signal transduction cascade: how can ligands trigger bursting without substantively modifying the membrane properties during a burst?
One possible mechanism is that intrinsic membrane properties alone drive bursting. However, this interpretation appears to be in conflict with the observation that burst frequency was significantly reduced by altering the sensory transduction cascade (Figs. 7 and 8). An alternative hypothesis is that activation of the G protein is effectively quantal, where a single temporally isolated quantum corresponds to a single burst. If the signal transduction cascade is occasionally activated spontaneously, this mechanism would be predicted to produce stereotyped bursting even in the absence of stimulation. This interpretation appears to be consistent with an overall decrease in spontaneous activity (Figs. 7 and 8) and decrease in burst length when PLC is inhibited (Fig. 7). However, while the rate of initiating bursts was greatly reduced in TrpC2−/− VSNs, the burst length was not significantly altered, although there was a slight slowdown of spikes within the bursts (Fig. 8). Overall, the uniformity of burst structure and the change in spontaneous activity following intervention in various components of the signal transduction cascade argue that signal transduction machinery is involved in triggering spontaneous activity, while membrane properties may play the major role in the burst structure.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grant R01-DC-005964 (to T. E. Holy) and NSF IGERT 0548890 (to H. A. Arnson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Julian Meeks, Richard Roberts, Dennis Barbour, Ralf Wessel, Illya Tolokh, and Zhiguang Xu for comments on the manuscript.
REFERENCES
- Arnson HA, Fu X, Holy TE. Multielectrode array recordings of the vomeronasal epithelium. J Vis Exp 37: 1845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci 8: 451–465, 2007 [DOI] [PubMed] [Google Scholar]
- Belluscio L, Koentges G, Axel R, Dulac AC. A map of pheromone receptor activation in the mammalian brain. Cell 97: 209–220, 1999 [DOI] [PubMed] [Google Scholar]
- Ben-Shaul Y, Katz LC, Mooney R, Dulac C. In vivo vomeronasal stimulation reveals sensory encoding of conspecific and allospecific cues by the mouse accessory olfactory bulb. Proc Natl Acad Sci USA 107: 5172–5177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MJ, Warland DK, Meister M. The structure and precision of retinal spike trains. Proc Natl Acad Sci USA 94: 5411–5416, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten K, Shadlen M, Newsome W, Movshon J. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci 12: 4745, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, Abbott L, Abbott L. Theoretical Neuroscience: Computational and Mathematical Modeling of Neural Systems. Cambridge, MA: MIT Press, 2001 [Google Scholar]
- DeBusk BC, DeBruyn EJ, Snider RK, Kabara JF, Bonds AB. Stimulus-dependent modulation of spike burst length in cat striate cortical cells. J Neurophysiol 78: 199–213, 1997 [DOI] [PubMed] [Google Scholar]
- Del Punta K, Puche A, Adams NC, Rodriguez I, Mombaerts P. A divergent pattern of sensory axonal projections is rendered convergent by second-order neurons in the accessory olfactory bulb. Neuron 35: 1057–1066, 2002 [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Smith GM, Bowman D. Spontaneous burst firing in cat primary auditory cortex: age and depth dependence and its effect on neural interaction measures. J Neurophysiol 69: 1292–1313, 1993 [DOI] [PubMed] [Google Scholar]
- Eyherabide HG, Rokem A, Herz AVM, Samengo I. Burst firing is a neural code in an insect auditory system. Front Comput Neurosci 2: 3, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie PG, Muller U. Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell 139: 33–44, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D, Swets J. Signal Detection Theory and Psychophysics. New York: Wiley, 1966 [Google Scholar]
- Halpern M. The organization and function of the vomeronasal system. Annu Rev Neurosci 10: 325–362, 1987 [DOI] [PubMed] [Google Scholar]
- He J, Ma L, Kim S, Nakai J, Yu CR. Encoding gender and individual information in the mouse vomeronasal organ. Science 320: 535–538, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Ma L, Kim S, Schwartz J, Santilli M, Wood C, Durnin MH, Yu CR. Distinct signals conveyed by pheromone concentrations to the mouse vomeronasal organ. J Neurosci 30: 7473–7483, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson RC, Krauthamer S, Essenberg JM, Holy TE. Inhibition shapes sex selectivity in the mouse accessory olfactory bulb. J Neurosci 28: 12523–12534, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holy TE, Dulac C, Meister M. Responses of vomeronasal neurons to natural stimuli. Science 289: 1569–1572, 2000 [DOI] [PubMed] [Google Scholar]
- Ishimaru Y. Molecular mechanisms of taste transduction in vertebrates. Odontology 97: 1–7, 2009 [DOI] [PubMed] [Google Scholar]
- Kepecs A, Wang XJ, Lisman J. Bursting neurons signal input slope. J Neurosci 22: 9053–9062, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsen PM, Ahissar E. Orthogonal coding of object location. Trends Neurosci 32: 101–109, 2009 [DOI] [PubMed] [Google Scholar]
- Krahe R, Gabbiani F. Burst firing in sensory systems. Nat Rev Neurosci 5: 13–23, 2005 [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Ishii T, Mombaerts P, Zufall F, Boehm T. Structural requirements for the activation of vomeronasal sensory neurons by mhc peptides. Nat Neurosci 12: 1551–1558, 2009 [DOI] [PubMed] [Google Scholar]
- Maler L. Neural strategies for optimal processing of sensory signals. Prog Brain Res 165: 135–154, 2007 [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel DH. The function of bursts of spikes during visual fixation in the awake primate lateral geniculate nucleus and primary visual cortex. Proc Natl Acad Sci USA 99: 13920–13925, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks JP, Arnson HA, Holy TE. Representation and transformation of sensory information in the mouse accessory olfactory system. Nat Neurosci 13: 723–730, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith M. Chronic recording of vomeronasal pump activation in awake behaving hamsters. Physiol Behav 56: 345–354, 1994 [DOI] [PubMed] [Google Scholar]
- Nodari F, Hsu FF, Fu X, Holekamp TF, Kao LF, Turk J, Holy TE. Sulfated steroids as natural ligands of mouse pheromone-sensing neurons. J Neurosci 28: 6407–6418, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald AMM, Doiron B, Maler L. Interval coding. I. Burst interspike intervals as indicators of stimulus intensity. J Neurophysiol 97: 2731–2743, 2007 [DOI] [PubMed] [Google Scholar]
- Reich D, Mechler F, Purpura K, Victor J. Interspike intervals, receptive fields, and information encoding in primary visual cortex. J Neurosci 20: 1964, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, Warland D, van Steninck RR, Bialek W. Spikes-Exporing the Neural Codes. Cambridge, MA: MIT Press, 1997 [Google Scholar]
- Rodriguez I, Feinstein P, Mombaerts P. Variable patterns of axonal projections of sensory neurons in the mouse vomeronasal system. Cell 97: 199–208, 1999 [DOI] [PubMed] [Google Scholar]
- Rospars JP, Lánský P, Duchamp-Viret P, Duchamp A. Spiking frequency versus odorant concentration in olfactory receptor neurons. Biosystems 58: 133–141, 2000 [DOI] [PubMed] [Google Scholar]
- Segev R, Goodhouse J, Puchalla J, Berry MJ. Recording spikes from a large fraction of the ganglion cells in a retinal patch. Nat Neurosci 7: 1154–1161, 2004 [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosci 18: 3870–3896, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Glowatzki E, Moser T, Strowbridge BW, Bhandawat V, Sampath AP. Functional properties of synaptic transmission in primary sense organs. J Neurosci 29: 12802–12806, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spehr J, Hagendorf S, Weiss J, Spehr M, Leinders-Zufall T, Zufall F. Ca2+-calmodulin feedback mediates sensory adaptation, and inhibits pheromone-sensitive ion channels in the vomeronasal organ. J Neurosci 29: 2125–2135, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination, and male-male aggression in mice deficient for trp2. Science 295: 1493–500, 2002 [DOI] [PubMed] [Google Scholar]
- Tirindelli R, Dibattista M, Pifferi S, Menini A. From pheromones to behavior. Physiol Rev 89: 921–956, 2009 [DOI] [PubMed] [Google Scholar]
- Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol 71: 307–332, 2009 [DOI] [PubMed] [Google Scholar]
- Tsunozaki M, Bautista DM. Mammalian somatosensory mechanotransduction. Curr Opin Neurobiol 19: 362–369, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Gresser AL, Torello AT, Dulac AC. multireceptor genetic approach uncovers an ordered integration of vno sensory inputs in the accessory olfactory bulb. Neuron 50: 697–709, 2006 [DOI] [PubMed] [Google Scholar]
- Yau KW, Hardie RC. Phototransduction motifs and variations. Cell 139: 246–264, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]