Abstract
Rats sense the environment through rhythmic vibrissa protractions, called active whisking, which can be simulated in anesthetized rats by electrically stimulating the facial motor nerve. Using this method, we investigated barrel cortex field potential and superior colliculus single-unit responses during passive touch, whisking movement, active touch, and texture discrimination. Similar to passive touch, whisking movement is signaled during the onset of the whisker protraction by short-latency responses in barrel cortex that drive corticotectal responses in superior colliculus, and all these responses show robust adaptation with increases in whisking frequency. Active touch and texture are signaled by longer latency responses, first in superior colliculus during the rising phase of the protraction, likely driven by trigeminotectal inputs, and later in barrel cortex by the falling phase of the protraction. Thus, superior colliculus is part of a broader vibrissa neural network that can decode whisking movement, active touch, and texture.
Keywords: sensory processing, whisker, movement, vibrissa, thalamus, barrel cortex
behaving rats use rhythmic vibrissa movements (active whisking) to sense the environment (Carvell and Simons 1990; Gao et al. 2001; Kleinfeld et al. 2006). A similar movement pattern, called artificial whisking, can be produced in anesthetized rats by electrically stimulating the facial motor nerve (Brown and Waite 1974; Szwed et al. 2003; Zucker and Welker 1969). Artificial whisking is a very useful model to investigate active whisking under highly controlled conditions that enable sophisticated experimental procedures that are technically difficult in freely moving animals.
Vibrissa sensory information travels via trigeminal ganglion neurons to the trigeminal complex in the brain stem, from where two main pathways ascend toward the contralateral midbrain and forebrain. One reaches the thalamus in the forebrain (trigeminothalamic), and the other reaches the superior colliculus in the midbrain (trigeminotectal). During active whisking, the brain receives a motor whisking signal because some trigeminal ganglion cells discharge during artificial whisking in air (without object contact) (Szwed et al. 2003), although more dispersed and less phase-locked ganglion cell discharges appear to occur during natural whisking in air in behaving animals (Khatri et al. 2009). In addition, some thalamic and cortical neurons are driven by artificial whisking in air (Derdikman et al. 2006; Yu et al. 2006). However, it is not known if superior colliculus cells sense whisking movement.
Cells in the intermediate layers of the superior colliculus are highly responsive to passive touch of vibrissa (i.e., deflection of stationary whiskers) (Cohen and Castro-Alamancos 2007, 2010b; Cohen et al. 2008; Grunwerg and Krauthamer 1990; Hemelt and Keller 2007; McHaffie et al. 1989). Superior colliculus passive touch responses are driven by direct inputs from the trigeminal complex (trigeminotectal; peak1) followed by activity returning from the barrel cortex (corticotectal; peak2) in both anesthetized (Cohen et al. 2008) and freely behaving rats (Cohen and Castro-Alamancos 2010a). However, the superior colliculus responses produced by active touch (i.e., whisking on objects) are unknown.
By whisking on objects, rats can obtain information about object position and properties. Among object properties, rats are known to discriminate surface texture (Carvell and Simons 1990; Guic-Robles et al. 1989), and there is considerable debate about the pattern of surface-induced whisker micromotions that enable texture discrimination (coding) by the brain (Andermann et al. 2004; Arabzadeh et al. 2005; Diamond et al. 2008a, 2008b; Jadhav and Feldman 2010; Jadhav et al. 2009; Ritt et al. 2008; von Heimendahl et al. 2007; Wolfe et al. 2008). However, it is not known if whisker-sensitive superior colliculus cells code surface texture.
In urethane-anesthetized rats, we recorded single-unit responses in the intermediate layers of the superior colliculus and field potential (FP) responses in the barrel cortex during air-puff stimulation of stationary whiskers (passive touch), artificial whisking in air (whisking movement), and artificial whisking on three different surfaces varying in texture (active touch) as if the rat was brushing its whiskers on an adjacent wall.
METHODS
Thirty-two adult Sprague-Dawley rats (300–350 g) were used in this study and cared for in accordance with National Institutes of Health guidelines for laboratory animal welfare. All experiments were approved by the Drexel University Institutional Animal Care and Use Committee. Rats were anesthetized with urethane (1.5 g/kg ip) and placed in a stereotaxic frame. All skin incisions and frame contacts with the skin were injected with lidocaine (2%). Small craniotomies and small incisions of the dura were made over the target structures as necessary. Body temperature was automatically maintained constant with a heating pad at 37°C. The level of anesthesia was monitored with FP recordings and limb withdrawal reflexes and kept constant at about stage III/3 (i.e., slow large-amplitude FP cortical oscillations, absence of pinch withdrawal reflex, absence of whisker movements) using supplemental doses of urethane.
Electrophysiology.
In every case, a tungsten electrode was lowered into the depth of the barrel cortex (0.6–1 mm) to record FP activity. A second electrode or set of electrodes was lowered into the superior colliculus to perform single-unit recordings from cells located within the following coordinates: 2–2.5 mm from lambda, 1.5–2.5 mm lateral from midline, and 3.6–5.3 mm in depth. These coordinates routinely yield whisker-responsive cells (Cohen et al. 2008). Single-unit recordings were obtained from quartz-insulated platinum/tungsten electrodes (80-μm shaft diameter) pulled and ground to a fine tip (3–7 MΩ) and independently moved with a seven-channel Eckhorn system (Thomas Recording, Giessen, Germany). On occasion, we recorded several cells simultaneously (2–4 cells), each on a different electrode.
Whisker stimulation protocols.
Once a single unit was isolated in superior colliculus, a hand-held probe was used to determine the whiskers that activated the cell (receptive field). This led to a classification of cells based on receptive field types as described in the results. All the cells presented in this study responded to passive whisker stimulation with the use of a hand-held probe and an air-puff (puff) stimulus.
After identification of the receptive field with the hand-held probe, puff stimulation was delivered by aiming a 2.5-mml:diameter tube 15–25 mm away from the receptive field (slightly elevated and facing down with a slight angle from the front toward the whiskers) so that whiskers were pushed backwards by a 50-ms duration puff of pressurized air (40 psi). Stimulus trials consisted of a 2-s period of no stimulation (1.5 s used to measure spontaneous firing) followed by 35 puffs at 0.5, 2, 5, or 10 Hz. Peristimulus time histograms (PSTHs) and barrel cortex FP responses were obtained by averaging the responses to the last 30 stimuli in each trial. The PSTHs and FP responses were corrected by subtracting the time it took for the air to reach the whiskers, which was determined using a sensing piezoelectric device placed in the location of the whiskers.
Puff stimulation was followed by artificial whisking in air. To trigger artificial whisking, we cut and positioned a pair of stainless steel wires in the buccal branch of the facial nerve (0.7–1 mm apart) and delivered a train of 5 pulses (40-μs duration) at 100 Hz. The stimulus intensity was adjusted (range 0.04–0.17 mA) to produce a protraction with a 10–35° angle. In a few cases, we used synchronous video monitoring (500 frames per second; Motionscope, Red Lake Imaging, Morgan Hill, CA) to track whisker movement frame by frame (Fig. 1A). After tracking of each frame, the movements measured during each of the last 30 trains were averaged. As shown in Fig. 1A, each train produced a whisker protraction (rising phase) that crested at around 50 ms from the train onset, and the whiskers returned back to the starting point (falling phase) within the next 30 ms. As per puff stimulation, each whisking in air trial consisted of a 2-s period of no stimulation (1.5 s used to measure spontaneous firing) followed by 35 trains at 0.5, 2, 5, or 10 Hz. PSTHs and barrel cortex FP responses were obtained by averaging the responses to the last 30 trains in each trial. Stimulus artifacts created by the nerve stimulation were very distinct and easily removed.
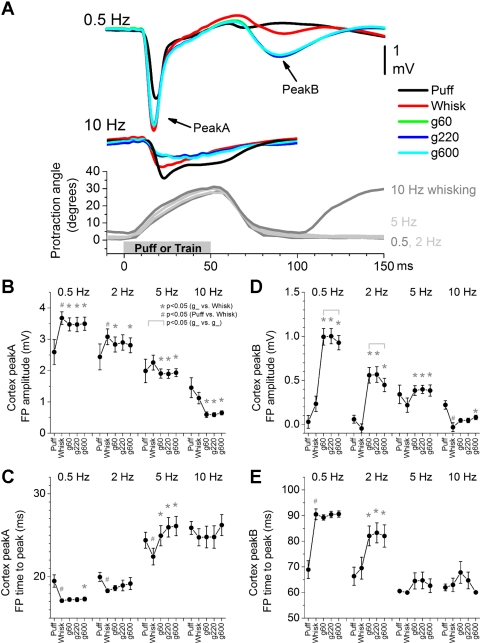
Fig. 1.
Population data showing the effect of air-puff stimulation (puff), whisking in air (whisk), and whisking on surfaces (g60, g220, and g600 sandpaper) on field potentials (FP) in the barrel cortex. A: overlaid mean traces of FP responses evoked by the different stimuli at 0.5 and 10 Hz (top). Bottom: whisker movement (angle in degrees) measured with video tracking during artificial whisking at 0.5, 2, 5, and 10 Hz (positive values indicate that the whiskers protracted). The maximum negative amplitude (B) and the time to peak (C) of short-latency (peakA) responses were measured during a 5- to 30-ms time window. The maximum negative amplitude (D) and the time to peak (E) of long-latency (peakB) responses were measured during a 50- to 100-ms time window. Statistical tests for data in B, C, D, and E compared the puff and whisking in air responses (#P < 0.05), the whisking in air and each of the whisking on surfaces responses (*P < 0.05), and the whisking on surfaces responses between each other (marked by brackets, P < 0.05). Values are means ± SE.
Whisking in air was followed by active touch consisting of whisking on three different surfaces. The surface was placed parallel to the rat's midline to mimic a wall that the whiskers brush against in the rostrocaudal direction. The distance from the wall to the whisker pad was adjusted to ensure that most whiskers (except rostral microvibrissa, which are too short) made contact. In some cases (n = 15 cells), we compared the responses produced by three different distances (2.5, 3.2, and 3.9 cm from the midline) but did not find a significant effect; thus we routinely used the intermediate distance. We used three different sandpaper surfaces that varied in coarseness based on grit size (g60, g220, and g600, coarse to smooth) and were placed on each of three sides of a rotating cube; rotating the cube 90° led to the presentation of a different surface. During whisking on surfaces, the electrical stimulus and the trial setup were identical to whisking in air but the whiskers contacted the surface. As described below (Fig. 1), the movement evoked by whisking in air did not differ between the different whisking frequencies (0.5, 2, 5, or 10 Hz). However, the movement of the whiskers was not monitored during whisking on surfaces because the whiskers are pushed against the texture wall, which makes it difficult to image them. Therefore, we cannot rule out the possibility that the movement evoked by whisking on surfaces differed between the different whisking frequencies; one possibility is that as the whisking frequency increases, the whiskers become more obstructed and move a shorter distance.
Once the whisking on surfaces trials ended, all stimulus trials were repeated several times (2–5), and the data from different trials were averaged together unless otherwise stated. Time zero for all PSTHs and FP responses corresponds to the onset of the stimulus (electrical or puff).
Analysis.
Spontaneous activity was computed from a 1.5-s period before each trial for different stimulus types (i.e., puff, whisking in air, and whisking on surfaces). In the superior colliculus, we measured the spontaneous firing rate (Hz) of single units. In the barrel cortex, we used the fast-Fourier transform (FFT) to derive a power spectrum of the spontaneous FP activity. The 0.1–10 Hz FFT power range is normalized as a percentage of the total power (range 0.1–50 Hz). This frequency range is used because it is very sensitive to changes in state and informs about the level of forebrain activation (becoming smaller during forebrain activation).
In the barrel cortex, we measured the peak amplitude and the time to peak of the first (peakA; 5–30 ms) and second (peakB; > 50 ms) negative peaks in the FP response. In the superior colliculus, we measured spike probability during a long time window (2–80 ms poststimulus) or several short time windows. Peak1 (2–10 ms), peak2 (11–20 ms), and peak3 (21–80 ms) time window responses were calculated because they reflect responses of different origins and with different sensitivities to behavior (Cohen and Castro-Alamancos 2010a, 2010b, 2010c; Cohen et al. 2008). All data are expressed as means ± SE unless otherwise stated.
The contact index (c-index) for each cell was determined using the long time window by calculating the difference between the whisking in air response and the mean whisking on surfaces response (mean of 3 surfaces) and dividing it by the sum of these two values so that the c-index varied between ±1. The c-index calculation is equivalent to the “touch index (TI index)” used by Szwed et al. (2003), but we changed the term because of clear differences with our study; in that study a few whiskers touched a small object (vertical bar) during the protraction, while in our study most whiskers brushed against a wall.
The texture index (t-index) for each cell was determined using the long time window by calculating the difference between the whisking on smooth surface and the whisking on rough surface response and dividing it by the sum of these two values so that the t-index varied between ±1.
Statistical analyses consisted, for the most part, of paired comparisons of responses evoked in the same cells by different stimulus types (puff, whisking in air, and whisking on surfaces). In some cases, independent comparisons between different cell groups were also performed. If the data were considered normally distributed, according to the Shapiro-Wilk normality test, we used parametric statistics. For two groups, we used the t-test (paired or independent). For more than two groups, we tested for a significant main effect using the repeated-measures ANOVA followed by comparisons with Tukey's test. If the data were considered not normally distributed, we used nonparametric statistics consisting of the Wilcoxon signed ranks (paired comparisons) and the Mann-Whitney (independent comparisons) tests.
Histology.
At the end of the experiments, animals were euthanized with an overdose of pentobarbital, and the brain was rapidly removed and placed in fixative (4% paraformaldehyde). Coronal sections (100 μm) of the superior colliculus were obtained using a vibratome, and these were stained with cresyl violet. Based on coordinates, electrode tracks, and lesions made to mark the recording sites (0.02-mA constant current, 10-s duration, twice), the cells were located in the intermediate layers of the superior colliculus.
RESULTS
Whisking responses in barrel cortex.
Superior colliculus responses driven by whisker stimulation are partly dependent on corticotectal inputs returning from the barrel cortex (Cohen et al. 2008). Therefore, we obtained simultaneous FP recordings from the barrel cortex during recordings of superior colliculus cells. FP and single-unit responses were first evoked by an air-puff (puff) stimulus delivered at 0.5, 2, 5, and 10 Hz. This was followed by artificial whisking in air (whisk) at 0.5, 2, 5, and 10 Hz and by artificial whisking on three surfaces of sandpaper with different grit size (g60, g220, g600) at 0.5, 2, 5, and 10 Hz. The surfaces varied in coarseness and consisted of a rough surface (g60), a smooth surface (g600), and a surface in between (g220). As shown in Fig. 1A, bottom, artificial whisking consisted of a protraction with a rising phase and a falling phase. The rising phase crested at ∼50 ms, after which the protraction returned to baseline within the next ∼30 ms.
In this section, we describe the barrel cortex FP responses (n = 15 experiments). FP responses in barrel cortex driven by mechanical whisker deflections lag the stimulus by ∼5 ms and are well correlated with intracellular postsynaptic potentials (Hirata and Castro-Alamancos 2008). Stimulation of the receptive field with a puff stimulus produced a fast and large-amplitude FP response in barrel cortex similar to that observed during mechanical whisker deflections. Figure 1A overlays average FP responses recorded in the barrel cortex during puff (black traces), whisking in air (red traces), and whisking on surfaces (green, blue, and cyan traces) at 0.5 and 10 Hz. Low-frequency puff stimulation produced a short-latency FP response (peakA) in barrel cortex that peaked at ∼19 ms. Low-frequency whisking in air produced a significantly larger and faster (∼17 ms) peakA response driven by the onset of the rising phase of the protraction. Furthermore, low-frequency whisking on surfaces produced a similar peakA response to whisking in air but a very different long-latency (peakB) response. The peakB response rose with the falling phase of the protraction, was mostly absent during whisking in air or puff stimulation, and tended to be larger during whisking on rough surfaces than on smooth surfaces.
Since the average time to peakA of FP responses evoked by whisking in air is ∼17 ms and the time to peak of FP responses evoked, under identical conditions, by a direct mechanical stimulator is ∼12 ms (Hirata and Castro-Alamancos 2008), we estimate that the delay between the motor nerve electrical stimulus and the onset of the whisker protraction is ∼5 ms (i.e., the difference between direct passive movement and movement driven by the nerve). Indeed, this was verified with high-speed video equipment (500 fps), so that the first frame after stimulus onset showing whisker movement is frame number 3, which corresponds to 4–6 ms; these estimates are similar to those of previous studies (Szwed et al. 2003).
Figure 1 shows population amplitude and time to peak measurements of peakA and peakB responses. Regarding peakA (Fig. 1, B and C), we found that puff and whisking frequency resulted in significant response adaptation, so that responses at 10 Hz were significantly smaller and slower (P < 0.01) than responses at 0.5 Hz for all stimulus types (puff, whisk, g60, g220, g600). PeakA responses evoked by low-frequency (0.5–2 Hz) whisking in air were significantly larger and faster than those evoked by low-frequency puff stimulation (P < 0.01). Also, peakA responses evoked by 0.5–10 Hz whisking on surfaces were significantly smaller and tended to be slower than those evoked by whisking in air (P < 0.01). There were no significant differences in peakA amplitudes or times between different textures at any whisking frequency. These results indicate that whisking on surfaces (active touch) produces stronger adaptation of short-latency FP responses in barrel cortex than whisking in air or passive touch.
A long-latency FP response, termed peakB (Fig. 1, D and E), developed in barrel cortex during active touch. PeakB was largest for low-frequency (0.5–2 Hz) whisking on surfaces, started at ∼65 ms during the onset of the falling phase of the protraction, rose with the falling phase of the protraction (Fig. 1E), and was significantly larger for rough surfaces. Thus, peakB responses are driven by touch as the whiskers return from the protraction.
In conclusion, FP responses in barrel cortex are highly sensitive to whisking movement, active touch, and texture. Whisking movement is signaled by a short-latency response at the onset of the protraction that resembles the response to passive touch. During low-frequency whisking, active touch is signaled by the appearance of a long-latency FP response that codes texture and is driven by the falling phase of the protraction. During high-frequency whisking, active touch is signaled by stronger adaptation (suppression) of short-latency FP responses that are evoked by the onset of the protraction.
Whisking responses in superior colliculus.
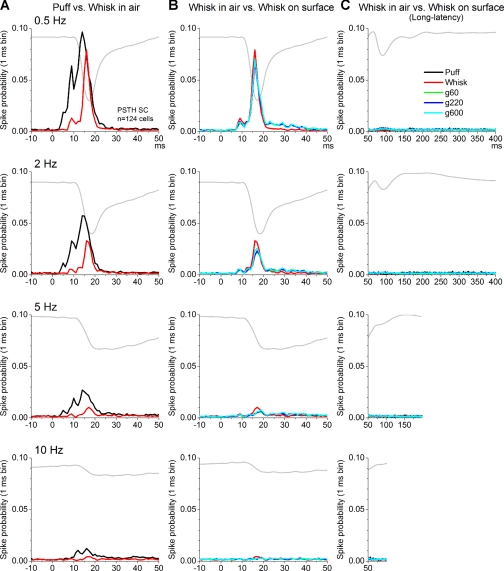
Whisker-sensitive single units (n = 124) were recorded from the intermediate layers of the superior colliculus. These cells were determined to be responsive to whisker stimulation by a hand-held probe and by an air-puff stimulus delivered at low frequency (0.5 Hz). The puff was aimed at the receptive field (i.e., whiskers that were determined to drive the cell using a hand-held probe) and was delivered at 0.5, 2, 5, and 10 Hz. Puff stimulation was followed by whisking in air (whisk) and whisking on three surfaces (g60, g220, g600) at 0.5, 2, 5, and 10 Hz. Figure 2 shows population PSTHs of cells (n = 124) recorded in the superior colliculus. Figure 2A overlays responses during puff and whisking in air at different frequencies (0.5–10 Hz), while Fig. 2B overlays responses during whisking in air and whisking on different surfaces. Figure 2C overlays long-latency responses (>50 ms) for all the conditions. The barrel cortex FP response evoked by whisking on rough surfaces (g60) is shown for reference (gray trace).
Fig. 2.
Mean responses showing the effects of puff, whisking in air, and whisking on surfaces on single units in the superior colliculus. A: population peristimulus time histograms (PSTHs) of superior colliculus cells evoked by puff and whisking in air at 0.5, 2, 5, and 10 Hz. SC, superior colliculus. B: population PSTHs evoked by whisking in air and whisking on 3 surfaces (g60, g220, and g600) that differ in texture (rough, intermediate, and smooth) at 0.5, 2, 5, and 10 Hz. C: overlay of all previous responses to display long-latency responses (>50 ms). For comparison, all histograms display the FP response (gray) evoked in barrel cortex by whisking on rough surfaces (g60).
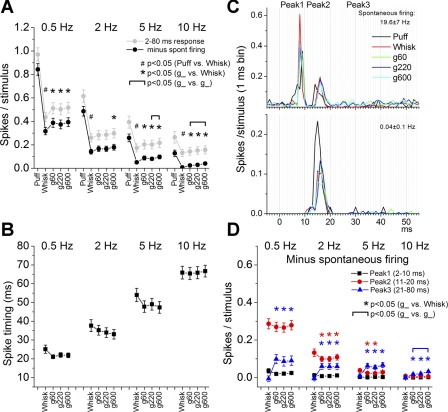
Figure 3A shows population (all cells considered together) spike probabilities for superior colliculus cells measured during a long (2–80 ms) window poststimulus uncorrected (gray) and corrected (black) by subtracting the spontaneous firing. Note that statistical analyses refer to responses corrected by spontaneous firing, and all subsequent figures show responses corrected by spontaneous firing. Puff and whisking frequency resulted in significant response adaptation, so that responses at 10 Hz were significantly smaller (P < 0.01) than responses at 0.5 Hz for all stimulus types (puff, whisk, g60, g220, g600). Regarding passive touch, we found that responses evoked by the puff stimulus were significantly larger than those evoked by whisking in air (P < 0.01) at all frequencies (0.5–10 Hz, P < 0.01). Regarding active touch, we found that responses evoked by whisking on surfaces were significantly larger than those evoked by whisking in air for most frequencies. For example, during whisking in air at 10 Hz, responses were virtually inexistent, but during whisking on surfaces at 10 Hz, responses were significantly larger (P < 0.01), indicating that superior colliculus spike probability is enhanced by active touch. Regarding texture, responses were significantly different during 10-Hz whisking on rough and smooth surfaces (P < 0.01), indicating superior colliculus responses code texture.
Fig. 3.
Population data showing the effect of puff, whisking in air, and whisking on surfaces on superior colliculus single-unit responses. A: spike probability measured during a long time window between 2 and 80 ms, uncorrected (gray) and corrected (black) by the spontaneous firing. B: spike timing measured during a long time window. C: example PSTHs from 2 superior colliculus cells recorded simultaneously during puff, whisking in air, and whisking on surfaces. The spontaneous firing rate is indicated at top right. D: spike probability measured during 3 different time windows corresponding to peak1 (2–10 ms; black squares), peak2 (11–20 ms; red circles), and peak3 (21–80 ms; blue triangles) corrected by the spontaneous firing. Statistical tests for A, B, and D compared the puff and whisking in air responses (#P < 0.05), the whisking in air and each of the whisking on surfaces responses (*P < 0.05), and the whisking on surfaces responses between each other (marked by brackets, P < 0.05). In A, statistical comparisons were similar for the corrected and uncorrected data. In D, the color of the symbols indicates the response time window that is significant. Values are means ± SE.
In order to calculate how the spike timing was affected by the different stimuli (Fig. 3B), we measured for each cell the time from stimulus onset at which 30% of the spikes comprising the response time window (2–100 ms) occur. Since this measure and subsequent measures can be affected by slight differences in the location (e.g., distance to whiskers on different parts of the snout) of the puff stimulus, we did not make additional comparisons between puff responses and whisking responses. We found that responses during 10-Hz whisking were significantly slower (P < 0.01) than responses during 0.5-Hz whisking for all stimulus types (whisk, g60, g220, g600). Responses evoked by whisking in air tended to be slower than those evoked by whisking on surfaces, particularly at low frequencies, but these effects were not significant (P ∼ 0.05). Finally, responses were not significantly different in spike timing during whisking on rough and smooth surfaces.
Percentage of whisking-sensitive cells in superior colliculus.
The previous results indicate that passive touch-sensitive cells in superior colliculus are also sensitive to whisking in air (whisking movement) and whisking on surfaces (active touch). Using the spike probability data (corrected by spontaneous firing) and an arbitrary threshold (0.1 spikes per stimulus), we determined the percentage of cells that were sensitive (excited or inhibited) to low-frequency (0.5 Hz) and high-frequency (10 Hz) whisking movement (whisking in air response was >0.1 or less than −0.1) and active touch (the mean of the 3 whisking on surfaces response minus whisking in air was >0.1 or less than −0.1). Thus, whisking- and active touch-sensitive cells fell in three main categories, which are similar to the categories used by (Szwed et al. 2003): 1) cells that are only sensitive to whisking movement were previously called “whisking cells”; 2) cells that are only sensitive to active touch were previously called “touch cells,” and we refer to them in this study as “active touch cells” to differentiate them from “passive touch cells” (i.e., cells that respond to passive whisker deflections but not to active touch); and 3) cells that are sensitive to both whisking movement and active touch were previously called “whisking/touch cells” (see Table 1).
Table 1.
Percentage of whisking-sensitive cells in superior colliculus
| Cells Sensitive to: | Cells Code: | Cells During Whisking, % |
|
|---|---|---|---|
| 0.5 Hz | 10 Hz | ||
| Whisking in air only | Whisking movement | 32.2 | 4.8 |
| Whisking on surfaces only | Active touch | 17.7 | 4 |
| Both of the above | Both of the above | 36.3 | 5.7 |
| None of the above | Passive touch only | 13.7 | 90 |
During 0.5-Hz whisking, a small group of cells, 13.7%, were sensitive to neither whisking in air nor active touch, but this increased to 90% during 10-Hz whisking. In fact, during 10-Hz puff stimulation, 33% of cells were no longer responsive. Most cells, 68.5% of passive touch-sensitive cells, were sensitive to whisking in air at 0.5 Hz, but this was reduced to 10.5% at 10 Hz. About half of these cells (32.2% at 0.5 Hz and 4.8% at 10 Hz) were selectively responsive to whisking in air, unaffected by active touch (i.e., response was similar during whisking in air and on surfaces). This indicates that a sizeable population of cells in the superior colliculus signals whisking movement.
In addition, about half (54%) of passive touch-sensitive cells were sensitive to active touch at 0.5 Hz, but this was reduced to 9.7% at 10 Hz. Only a portion of these cells (17.7% at 0.5 Hz and 4% at 10 Hz) were selectively responsive to active touch (i.e., response to whisking in air was nil). Despite strong frequency adaptation, the whisking movement and active touch signals were present during high-frequency whisking in ∼10% of cells. Thus, the superior colliculus decodes whisking movement and active touch signals.
Superior colliculus whisking responses measured during specific time windows.
So far the analyses measured superior colliculus responses using a long time window (2–80 ms). In a previous study (Cohen et al. 2008), we found that whisker responses evoked by passive touch (mechanical deflection) produce two stereotyped peak responses in superior colliculus that occur at ∼2–10 ms (peak1) and 11–20 ms (peak2). Peak1 is caused by direct trigeminotectal input, while peak2 is caused by whisker-evoked activity returning from the barrel cortex (corticotectal input), and both of these responses adapt (depress) robustly with frequency. Figure 3C shows PSTHs obtained from a pair of simultaneously recorded cells. The cell represented in Fig. 3C, top, had high spontaneous firing and responded to puff, whisking in air, and whisking on surfaces with two stereotyped peaks that fell within the peak1 and peak2 time windows. The cell at bottom had nil spontaneous firing and responded only with a peak2 response and some longer latency (>20 ms) activity. Interestingly, recordings in freely behaving animals have also revealed a third peak at 21–80 ms (peak3) that is very sensitive to the behavioral state of the animal, becoming larger during activated states (Cohen and Castro-Alamancos 2010a). Moreover, superior colliculus whisking on surfaces responses differ from whisking in air mostly in the peak3 region (between 20 and 50 ms; see Fig. 2B), which coincides with the rising phase of the protraction, and there is little activity after the falling phase of the protraction (>70 ms; see Fig. 2C).
Using the spike probability data for each peak (corrected by spontaneous firing) and an arbitrary threshold (0.1 spikes per stimulus; as described above), we determined the percentage of cells that responded to low-frequency (0.5 Hz) and high-frequency (10 Hz) whisking movement (whisking in air) and active touch (whisking on surfaces minus whisking in air) with a change in peak1, peak2, and peak3. Most cells have nil peak1 responses during both whisking in air and whisking on surfaces (92% at 0.5 Hz and 99.2% at 10 Hz). Whisking in air affects peak2 responses in most cells (62.1% at 0.5 Hz and 2.4% at 10 Hz), peak3 responses in some cells (17% at 0.5 Hz and 6.4% at 10 Hz), and peak1 responses in very few cells (7.2% at 0.5 Hz and 0.8% at 10 Hz). Whisking on surfaces affects peak2 (39.5% at 0.5 Hz and 4% at 10 Hz) and peak3 responses in some cells (29% at 0.5 Hz and 8% at 10 Hz) and peak1 responses in virtually none (1% at 0.5 Hz and 0% at 10 Hz). These results indicate that mainly peak2 and peak3, not peak1, decode whisking movement and active touch in superior colliculus.
Figure 3D shows population spike probabilities during the peak1, peak2, and peak3 time windows for all the cells in our study. Regarding spike probability for peak1 responses, whisking frequency resulted in significant response adaptation, so that responses at 10 Hz were significantly smaller (P < 0.01) than responses at 0.5 Hz. Peak1 responses evoked by whisking in air were not significantly different than those evoked by whisking on surfaces at any frequency, indicating that peak1 responses do not signal active touch. Peak1 responses were not significantly different during whisking on rough and smooth surfaces, indicating that peak1 population responses do not code texture.
Regarding spike probability for peak2 responses, whisking frequency resulted in significant response adaptation, so that responses at 10 Hz were significantly smaller (P < 0.01) than responses at 0.5 Hz. Peak2 responses evoked by whisking in air were significantly larger than those evoked by whisking on surfaces but only for 2–5 Hz whisking (P < 0.05), indicating that suppression of peak2 responses signals active touch during 2–5 Hz whisking. Peak2 responses were not significantly different during whisking on rough and smooth surfaces, indicating that peak2 population responses do not code texture.
Regarding spike probability for peak3 responses, we found that during whisking in air peak3 responses do not adapt with frequency, while during whisking on surfaces peak3 responses adapt significantly. Thus, peak3 responses at 10 Hz were significantly smaller (P < 0.01) than responses at 0.5 Hz during whisking on surfaces (g60, g220, g600) but not during whisking in air. In contrast to peak1 and peak2 responses, peak3 responses evoked during whisking on surfaces were significantly larger than those evoked during whisking in air for all frequencies (P < 0.01), indicating that peak3 responses signal active touch. For the most part, peak3 responses were not significantly different during whisking on rough and smooth surfaces, with one notable exception. During 10-Hz whisking, peak3 responses evoked by the smooth surface (g600) were significantly larger than those evoked by the rough surface (g60) (P < 0.01), indicating that during high-frequency whisking, peak3 population responses code texture. The suppression of peak2 population responses and enhancement of peak3 population responses during 2-Hz whisking on surfaces explains why some responses (g60 and g220) measured during the long time window (which includes peak1, peak2, and peak3) were not significantly affected by active touch at 2 Hz (see Fig. 3A).
In conclusion, considering together the responses of all the cells as a population, we found that during whisking at any tested frequency, peak1 (trigeminotectal) population responses do not signal whisking movements, active touch, or texture. Peak2 (corticotectal) population responses are driven by whisking movements and, during whisking at 2–5 Hz, suppression of peak2 population responses signals active touch but not texture. Finally, during whisking at all tested frequencies, enhancement of peak3 population responses signals active touch. Moreover, during whisking at 10 Hz, suppression of peak3 population responses by rough surfaces codes texture. The previous population analyses combined all cells in our sample; below, we differentiate cells based on spontaneous firing, receptive field, and sensitivity to surface contact and texture.
Spontaneous cell firing and whisking responses.
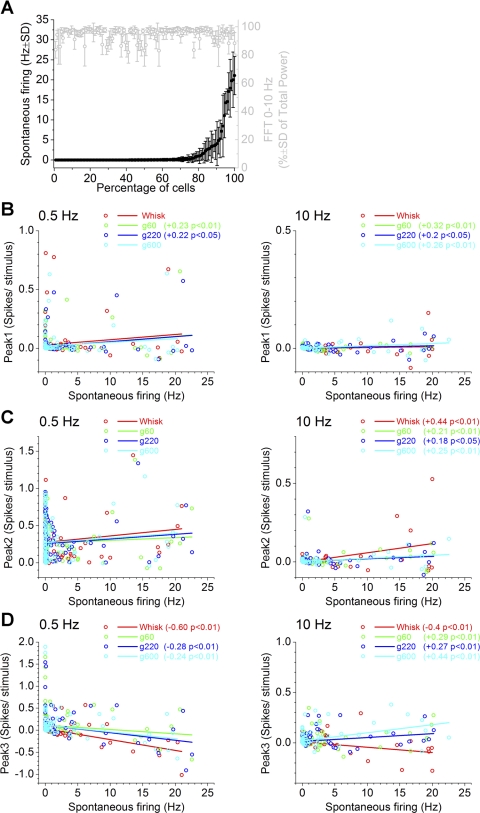
Figure 4A shows the mean ± SD spontaneous firing of each superior colliculus cell (n = 124) measured across all stimulus trials. The majority of cells (65.3%) had around nil spontaneous firing (low firing, <0.1 Hz), and a second group of cells (26.6%) had significant spontaneous firing (medium firing, <6 Hz), while a small group of cells (8.1%) had robust spontaneous firing (high firing, >6 Hz). Although the spontaneous firing of superior colliculus cells can change with the state of the animal (Cohen and Castro-Alamancos 2010a), the different groups of cells we found could not be explained by the level of activation based on two facts. First, the FFT power spectrum in the range of 0.1–10 Hz (normalized by the total power) measured simultaneously from the barrel cortex (Fig. 4A, gray) did not explain the different cell types, because there was no significant correlation (P > 0.5) between the cell spontaneous firing and the FFT power. Thus, cells with different firing rates are not recorded during different states of forebrain activation. Second, as already shown in Fig. 3C, we routinely recorded simultaneously from pairs of cells that had very distinct spontaneous firing, indicating that these distinct firing rates are intrinsic and not caused by the state of the animal.
Fig. 4.
Relation between spontaneous cell firing and whisking responses in superior colliculus. A: spontaneous firing (black) for each superior colliculus cell (n = 124) plotted in increasing order. Also plotted (gray) is the fast-Fourier transform (FFT) power measured in barrel cortex during the same periods, when spontaneous firing was calculated, to show that there is no direct relation between spontaneous firing and forebrain activation. Values are means ± SD. B–D: linear fit between spontaneous cell firing and peak1 (B), peak2 (C), and peak3 (D) responses during 0.5- and 10-Hz whisking. Only significant Pearson correlations are indicated.
To determine if spontaneous firing cell types were related to responsiveness during whisking, we conducted two analyses. In the first analysis (Fig. 4, B–D), we tested if the spontaneous firing of each cell was correlated with peak1, peak2, and peak3 spike probability evoked by whisking in air and whisking on surfaces. Thus, Fig. 4, B–D, plots a linear fit of the spontaneous firing of each cell against the peak1, peak2, and peak3 spike probability (corrected by the spontaneous firing) for low-frequency (0.5 Hz) and high-frequency (10 Hz) whisking. In the second analysis (Fig. 5, A–C), we show the peak1, peak2, and peak3 spike probabilities (corrected by the spontaneous firing) for each class of spontaneous firing cell (low, medium, and high firing).
Fig. 5.
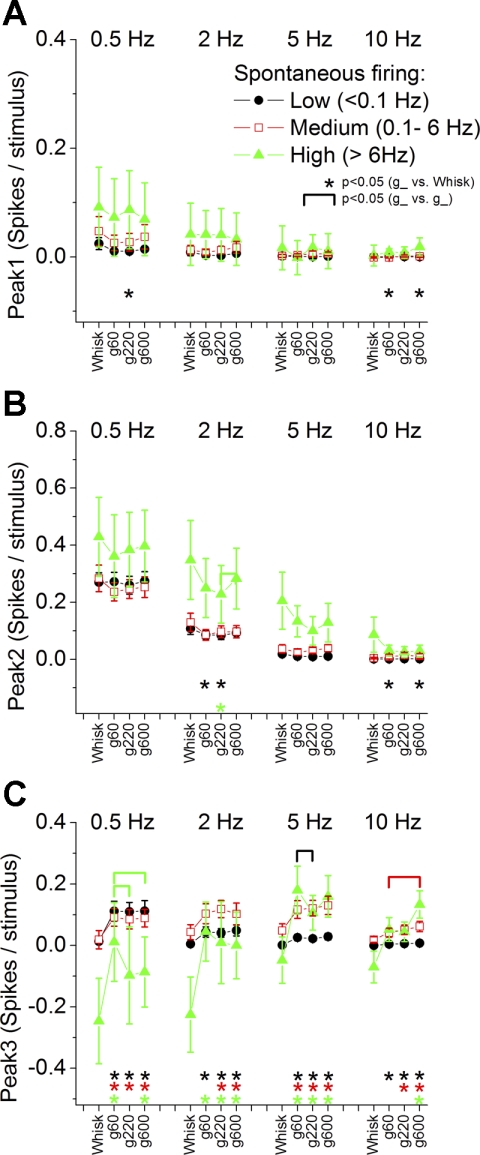
Superior colliculus responses for 3 groups of cells classified according to spontaneous firing. Peak1 (A), peak2 (B), and peak3 (C) responses evoked by whisking in air (whisk) and whisking on surfaces of different textures (g60, g220, and g600) at different frequencies (0.5, 2, 5, and 10 Hz) are shown for cells with low (black circles), medium (red open squares), and high spontaneous firing (green triangles). Statistical tests for A, B, and C compare the whisking in air and each of the whisking on surfaces responses (*P < 0.05) and the whisking on surfaces responses between each other (marked by brackets, P < 0.05). The color of the symbols indicates the cell group that is significant. Values are means ± SE.
Regarding peak1 responses evoked by low frequencies, we found no significant correlation between whisking in air and spontaneous firing (Fig. 4B). However, whisking on rough surfaces (g60 and 220), but not on smooth surfaces (g600), was positively correlated with spontaneous firing. Similar results were obtained for responses evoked by high frequencies, but at 10 Hz both responses to rough and smooth surfaces are positively correlated with spontaneous firing. However, as shown in Fig. 5A, peak1 responses were very small in all three groups of spontaneous firing cells, and high-firing cells tended to have the largest peak1 responses.
Regarding peak2 responses evoked by low frequencies, we found no significant correlation between whisking in air or whisking on any surface and spontaneous firing (Fig. 4C). However, there was a significant positive correlation between spontaneous firing and peak2 responses evoked by high-frequency whisking. This relation was strongest for whisking in air and was dampened by whisking on surfaces. This indicates that cells with higher spontaneous firing tend to have larger peak2 responses during high-frequency whisking. As shown in Fig. 5B, peak2 responses were larger in high-firing cells but did not consistently code active touch or texture.
Regarding peak3 responses evoked by low frequencies, we found that whisking in air responses showed a negative correlation with spontaneous firing, as also did whisking on smooth and intermediate (g220 and g600) surfaces, but not on rough (g60) surfaces (Fig. 4D). This indicates that peak3 responses of cells with high firing is inhibited by low-frequency whisking in air and whisking on smooth surfaces, but not by low-frequency whisking on rough surfaces. During high-frequency whisking in air, peak3 responses were also negatively correlated with spontaneous firing, but this relation became positive during whisking on surfaces. This is consistent with the interpretation that cells with high spontaneous firing are inhibited by high-frequency whisking in air but are excited by high-frequency whisking on surfaces. Figure 5C confirms these results and shows that low-, medium-, and high-firing cells had consistently larger peak3 responses to whisking on surfaces than to whisking in air for all frequencies. Regarding texture, high-firing cells coded texture by responding more robustly to rough surfaces during low-frequency whisking, while medium-firing cells coded texture by responding more robustly to smooth surfaces during high-frequency whisking.
In conclusion, high-firing cells tend to have the largest peak1 and peak2 responses. Peak3 responses in high-firing cells are inhibited by whisking movement. Regardless of spontaneous firing type, peak3 responses are consistently larger during whisking on surfaces than during whisking in air. Thus, peak3 codes active touch in all spontaneous firing cell types.
Receptive fields and whisking responses.
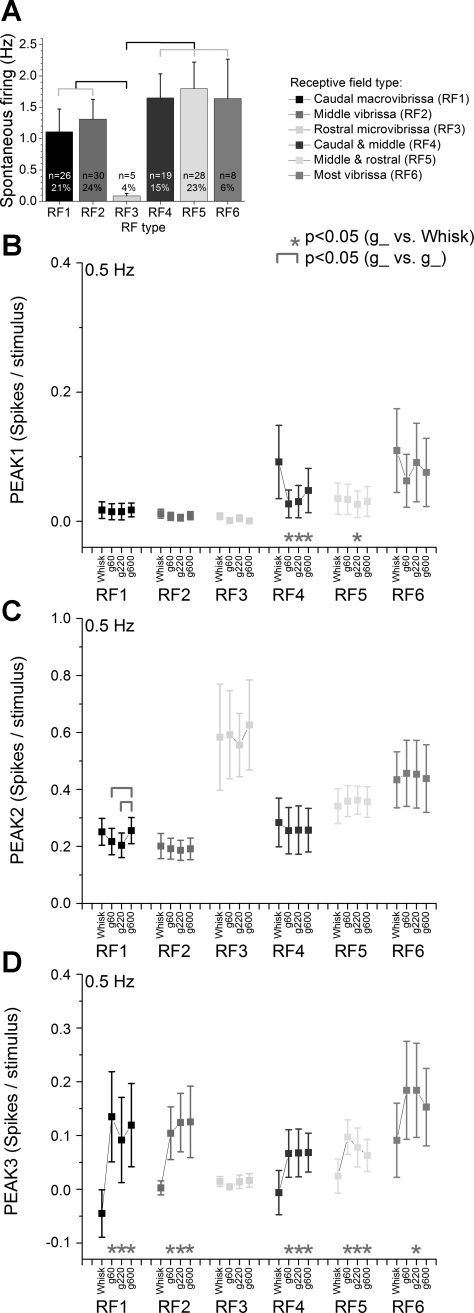
All cells, except a small group (7% were not mapped), were classified according to the size and location of the receptive field defined using a hand-held probe (Fig. 6A). Based on this mapping, half of the cells (49%) had small receptive fields (1–4 vibrissa) that were selectively localized within the caudal macrovibrissa (21% of cells; straddler whiskers and arc 1; RF1), the middle vibrissa (24% of cells; arcs 2–3; RF2), or the rostral microvibrissa (4% of cells; arcs 4–7; RF3). The other cells (38%) had much larger receptive fields (5–10 vibrissa), which were localized in both the caudal and middle vibrissa (15% of cells; straddler whiskers and arcs 1–3; RF4) or the middle and rostral vibrissa (23% of cells; arcs 2–7; RF5). Finally, a small group of cells (6%; RF6) had very large receptive fields (>10 vibrissa) that were spread out. The small population of cells that responded to rostral microvibrissa (RF3) had significantly lower spontaneous firing than the cells in the other receptive field groups (P < 0.01; Fig. 6A). Thus, about half of the cells had fairly localized multiwhisker receptive fields, while the other half had much larger receptive fields. Next we determined if responses evoked by whisking at 0.5 Hz differed among the RF groups.
Fig. 6.
Superior colliculus responses for 6 groups of cells classified according to receptive field (RF). A: spontaneous firing for cells that responded to caudal macrovibrissa (RF1), middle vibrissa (RF2), rostral microvibrissa (RF3), both caudal and middle vibrissa (RF4), both middle and rostral vibrissa (RF5), or most vibrissa (RF6). The total and percentage of cells per group are indicated. Brackets indicate significant differences between the groups (P < 0.05). Peak1 (B), peak2 (C), and peak3 (D) responses evoked by whisking in air (whisk) and whisking on surfaces of different textures (g60, g220 and g600) at different frequencies (0.5, 2, 5, and 10 Hz) are shown for cells classified as in A. Statistical tests for B, C, and D compare the whisking in air and each of the whisking on surfaces responses (*P < 0.05) and the whisking on surfaces responses between each other (marked by brackets, P < 0.05). Values are means ± SE.
In most RF groups, peak1 responses evoked by low-frequency whisking responses were usually nil in cells with small receptive fields (RF1–RF3; see Fig. 6B; note that only 0.5-Hz responses are shown in Fig. 6, B–D). Peak1 responses were inhibited by whisking on any surface in RF4 cells (P < 0.01). Regarding peak2 responses (Fig. 6C), the group of cells that responded solely to the rostral microvibrissa (RF3) tended to have the largest peak2 responses across all stimulus types. This is significant in light of the fact that these cells had nil peak1 responses during whisking and generally low spontaneous firing rates. Peak2 responses were not affected by whisking on surfaces in any of the RF groups. Regarding peak3 responses (Fig. 6D), we found that RF3 cells had close to nil responses across stimulus types, which emphasizes the selective responsiveness of these cells within the peak2 time window. Apart from this cell group, the other group of cells had larger responses during whisking on surfaces than during whisking in air. Thus, peak3 is particularly sensitive to active touch for all cell types, except rostral microvibrissa, which are too short to contact the surface. Regarding texture, none of the cell groups coded texture with peak1 and peak3 responses. However, RF1 cells coded texture with peak2 responses that were significantly larger during whisking on smooth textures than on rough or intermediate textures (P < 0.05).
In conclusion, rostral microvibrissa cells are characterized by the largest peak2 (corticotectal) responses during whisking movement, nil peak1 and peak3 responses, low spontaneous firing rates, and lack of sensitivity to active touch. All other RF types were sensitive to whisking movement during the peak2 time window and signaled active touch during the peak3 time window.
Active touch-sensitive cells.
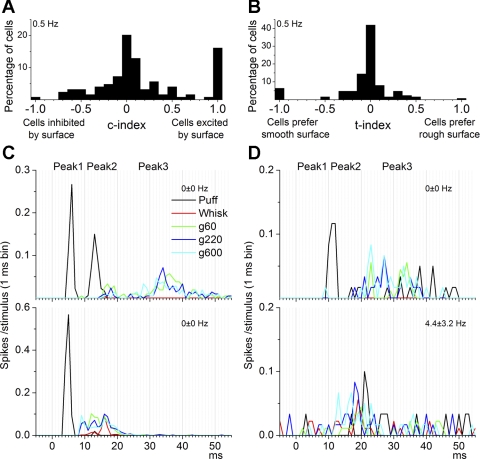
In the previous analyses, all cells in our study were either taken together or separated according to spontaneous firing and receptive field type. This section considers separately cells that were sensitive to active touch. Thus, we calculated a c-index that indicates if the cell responds to active touch by comparing responses during whisking in air and during whisking on surfaces (see methods). A positive value indicates that the cell responded better to whisking on a surface than to whisking in air (i.e., cell was excited by surface contact). A negative value indicates that the cell responded better to whisking in air than to whisking on a surface (i.e., cell was inhibited by surface contact). A value around zero indicates that the cell responded equally to whisking in air and whisking on a surface, and thus the effect of surface contact was nil (none). If the cell did not respond at all (zero spike probability) to whisking (in air and on surfaces), it was considered undefined. In the following analyses we focused on 0.5-Hz whisking because responses during higher frequency whisking were too suppressed.
Figure 7A shows the percentage of cells that had different c-index values (0.1 interval) during whisking at 0.5 Hz. Figure 7, C and D, shows the responses of two pairs of cells (each pair was recorded simultaneously). The cells represented at top were unresponsive to whisking in air (red trace) but responded to whisking on surfaces with a peak3 response (g60, green; g220, blue; g600, magenta). Thus, these cells are sensitive to active touch and are excited by surfaces. Moreover, the cell represented in Fig. 7D, top, was more sensitive to smooth surfaces than to rough surfaces. The cell represented in Fig. 7C, bottom, responded mostly with a peak2 to both whisking in air and whisking on surfaces, although the active touch response was stronger. In contrast to the other cells, the cell represented in Fig. 7D, bottom, had significant spontaneous firing and was mostly unresponsive.
Fig. 7.
Contact- and texture-sensitive cells. A: percentage of cells for each c-index value (0.1 interval). The c-index measures the sensitivity of cells to surface contact during 0.5-Hz whisking. Insensitive cells have a value around 0, cells excited by surface have a value close to 1, and cells inhibited by surface have a value close to −1. B: percentage of cells for each t-index value (0.1 interval). The t-index measures the sensitivity of cells to surface texture during 0.5-Hz whisking. Insensitive cells have a value around 0, cells that prefer rough surfaces have a value close to 1, and cells that prefer smooth surfaces surface have a value close to −1. C: example PSTHs from 2 superior colliculus cells recorded simultaneously during puff, whisking in air, and whisking on surfaces. The cell represented at top was insensitive to whisking in air (red trace) but sensitive to whisking on surfaces during the peak3 time window and had similar responses to smooth (magenta trace) and rough surfaces (blue trace). The cell at bottom was sensitive to whisking in air and more so to whisking on surfaces during the peak2 time window. Both cells responded to puff stimulation (black trace). The spontaneous firing rate is indicated at top right. D: example PSTHs from 2 superior colliculus cells recorded simultaneously during puff, whisking in air, and whisking on surfaces. The cell represented at top was insensitive to whisking in air (red trace) but sensitive to whisking on surfaces during the peak3 time window and had larger responses to smooth (magenta trace) compared to rough surfaces (blue trace). The cell represented at bottom had significant spontaneous firing but did not respond significantly to whisking or puff.
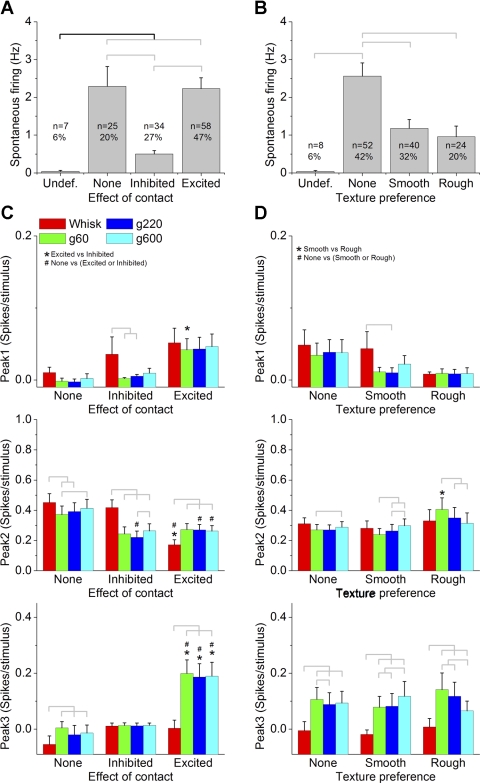
To select touch-sensitive cells, we determined a c-index threshold by comparing whisking in air and on surface responses (paired comparison) for cells that fell within each c-index value (0.1 interval). We found that cells with a c-index different than zero (greater than or equal to +0.1 or less than or equal to −0.1) had significantly different responses (P < 0.01) for this comparison, and thus we set the c-index threshold at ±0.1. Note that this is an arbitrary threshold and does not imply that we are establishing perfectly separate cell classes (sensitive and insensitive cells). Using the c-index values measured during whisking at 0.5 Hz, Fig. 8A shows the mean spontaneous firing and the percentage of cells that were excited by contact (c-index greater than or equal to +0.1), inhibited by contact (c-index less than or equal to −0.1), unaffected by contact (c-index = 0; None), or undefined. Using this threshold, we found that about three-quarters (74%) of all the cells were affected (excited or inhibited) by contact, and most (47%) of these were excited by contact. Moreover, cells that were inhibited by contact had significantly lower firing rates than cells that were excited or unaffected by contact (P < 0.01). We also calculated correlations between the c-index values (positive and negative c-index values calculated separately and each including zero) and the spontaneous firing of the cells. We found a significant correlation in both cases that was negative for positive c-index values (cells excited by contact; −0.24, P < 0.01) and positive for negative c-index values (cells inhibited by contact; 0.2, P < 0.01). This suggests that the more sensitive (regardless of whether they are excited or inhibited) cells are to touch, the lower their spontaneous firing. Regarding c-index and receptive fields, we found that only cells in the RF2 group had an average c-index (+0.3 ± 0.1) that was significantly different from zero (greater than +0.1 or less than −0.1), indicating that cells driven by middle vibrissa (arcs 2–3) are likely to be excited by active touch.
Fig. 8.
Superior colliculus responses for cells classified according to their sensitivity to surface contact and surface texture. A: spontaneous firing for cells that were classified according to the effect of surface contact measured using the c-index. The effect of surface contact was, undefined for unresponsive cells (undef.), none for cells that did not differ between whisking in air and whisking on surfaces (c-index <0.1 and greater than −0.1), inhibited (c-index less than or equal to −0.1), or excited (c-index ≥0.1). The total and percentage of cells per group are indicated. Brackets indicate significant differences between the groups (P < 0.05). B: spontaneous firing for cells that were classified according to the effect of surface texture measured using the t-index. The effect of surface texture was undefined for unresponsive cells, none for cells that did not differ between whisking on rough and smooth (t-index <0.1 and greater than −0.1), smooth for cells that responded better to g600 (t-index less than or equal to −0.1), or rough for cells that responded better to g60 (t-index ≥0.1). The total and percentage of cells per group are indicated. Brackets indicate significant differences between the groups (P < 0.05). C: peak1, peak2, and peak3 responses evoked by whisking in air (whisk) and whisking on surfaces of different textures (g60, g220, and g600) at 0.5 Hz for cells classified as in A. D: peak1, peak2, and peak3 responses evoked by whisking in air and whisking on surfaces of different textures at 0.5 Hz for cells classified as in B. Statistical tests for C and D compare the whisking in air and each of the whisking on surfaces responses (marked by brackets, P < 0.05), the whisking on surfaces responses between each other (marked by brackets, P < 0.05), the sensitive cells to the insensitive cells (#P < 0.05), and the 2 groups of sensitive cells between each other (*P < 0.05).
The c-index measure considers together the full response time window, but since different peak responses have different origins, next we determined if active touch-sensitive and -insensitive cells varied in their peak1 (trigeminotectal), peak2 (corticotectal), and peak3 responses to the different stimuli (Fig. 8C). In cells excited by contact, it was peak2 and, especially, peak3 that were enhanced by active touch. In cells inhibited by contact, it was peak2 (not peak3) that was suppressed by active touch. Peak3 responses of cells excited by contact were significantly larger than peak3 responses of cells inhibited or insensitive to active touch. Thus, it is peak3 responses that stick out during whisking on surfaces in active touch-sensitive cells. There are some additional results that this analysis yielded (see Fig. 8C). For example, cells excited by contact had smaller peak2 responses to whisking in air than cells inhibited or insensitive to contact.
We also calculated correlations between the c-index values (positive and negative c-index values calculated separately and each including zero) and the peak1, peak2, and peak3 responses to the different stimuli. We found a significant correlation between c-index and peak 2 for the three whisking on surface stimuli that was negative for positive c-index values (cells excited by contact; g60 = −0.42, P < 0.01; g220 = −0.47, P < 0.01; g600 = −0.48, P < 0.01) and positive for negative c-index values (cells inhibited by contact; g60 = 0.46, P < 0.01; g220 = 0.48, P < 0.01; g600 = 0.45, P < 0.01). This suggests that the more sensitive (regardless of whether they are excited or inhibited) cells are to touch, the smaller their peak2 responses. This same correlation was found for whisking in air responses, but only for positive c-index values (cells excited by contact; whisk = −0.58, P < 0.01), indicating that the more cells are excited by touch, the smaller their peak2 responses to whisking in air. There was also a significant positive correlation between c-index and peak 3, but only for the three whisking on surface stimuli (not for whisking in air) and only for positive c-index values (cells excited by contact; g60 = 0.22, P < 0.05; g220 = 0.26, P < 0.05; g600 = 0.28, P < 0.01). This suggests that the more cells are excited by touch, the larger their peak3 responses.
In conclusion, about three-quarters of passive touch-responsive cells in superior colliculus are sensitive to active touch, and these have significantly lower spontaneous firing rates than active touch-insensitive cells. While both peak2 (corticotectal) and peak3 responses code active touch, it is only peak3 that is enhanced in cells excited by contact compared to all other cells. Thus, we conclude that peak3 activity in the intermediate layers of the superior colliculus driven by the rising phase of the protraction codes active touch.
Texture-sensitive cells.
We calculated a t-index that reflects if the cell codes texture by responding differently to whisking on a smooth versus on a rough surface. A positive value indicates that the cell responds better to whisking on a rough surface, while a negative value indicates that the cell responds better to whisking on a smooth surface. A value around zero indicates that the cell responded equally to whisking on rough or smooth surfaces, and thus the texture preference was nil (none). If the cell did not respond to whisking on surfaces, it was considered undefined.
Figure 7B shows the percentage of cells that had different t-index values (0.1 interval) during whisking at 0.5 Hz. To select texture-sensitive cells, we used a similar strategy as that used to select active touch-sensitive cells. Thus, we determined a t-index threshold by comparing responses for whisking on smooth surfaces and on rough surfaces (paired comparison) for cells at each t-index value (0.1 interval). We found that cells with a t-index different than zero (greater than or equal to +0.1 or less than or equal to −0.1) had significantly different responses (P < 0.01) for this comparison, and thus we set the t-index threshold at ±0.1. Using the t-index values measured during whisking at 0.5 Hz, Fig. 8B shows the mean spontaneous firing and the percentage of cells that preferred rough texture (t-index greater than or equal to +0.1), preferred smooth textures (t-index less than or equal to −0.1), were unaffected by texture (t-index = 0; none), or were undefined. Using this threshold, we found that 52% of all the cells were affected by texture; 32% preferred smooth textures while 20% preferred rough textures. Moreover, cells that were affected by texture had significantly lower firing rates than cells that were unaffected by texture (P < 0.01). We also calculated correlations between the t-index values (positive and negative c-index values calculated separately and each including zero) and the spontaneous firing of the cells. We found a significant correlation in both cases that was negative for positive c-index values (cells excited by contact; −0.14, P < 0.01) and positive for negative c-index values (cells inhibited by contact; 0.16, P < 0.01). This suggests that the more sensitive (regardless of whether they prefer smooth or rough surfaces) cells are to texture, the lower their spontaneous firing.
The t-index measure considers together the full response time window, but since different peak responses have different origins, next we determined if texture-sensitive and -insensitive cells varied in their peak1 (trigeminotectal), peak2 (corticotectal), and peak3 responses to the different stimuli (Fig. 8D). In cells sensitive to texture, it was peak3 (not peak1 and peak2) that coded texture (i.e., responded better to the preferred texture and more than to whisking in air). Thus, like for active touch-sensitive cells, it is peak3 responses that stick out during texture coding. We also calculated correlations between the t-index values (positive and negative t-index values calculated separately and each including zero) and the peak1, peak2, and peak3 responses to the different stimuli. We only found a significant positive correlation between t-index and peak 2 for the two rougher whisking on surface stimuli (g60 and g220) and for whisking in air, but only for negative t-index values (cells that prefer smooth surfaces; g60 = 0.22, P < 0.05; g220 = 0.26, P < 0.05; g600 = 0.28, P < 0.01). This suggests that the more cells prefer smooth surfaces, the smaller their peak2 responses.
In conclusion, about half of the passive touch-responsive cells in superior colliculus are sensitive to texture, and these have significantly lower spontaneous firing rates than texture-insensitive cells. We conclude that peak3 activity in the intermediate layers of the superior colliculus driven by the rising phase of the protraction codes texture.
DISCUSSION
Population responses in barrel cortex signal whisking movement and active touch.
Although the current study is focused on the superior colliculus, we also studied population FP responses in barrel cortex for two reasons. First, this provides a means for direct comparison with future studies that use this measure. Second, peak2 responses in superior colliculus are driven by barrel cortex activity. The results revealed that FP responses in barrel cortex are highly sensitive to active touch, whisking movement, and texture. The ability of cortical responses to code texture agrees with previous studies (Diamond et al. 2008b; Jadhav and Feldman 2010; Johnson and Hsiao 1992; von Heimendahl et al. 2007).
Rats move their whiskers in different ways to detect and discriminate object features. For example, there is 5–12 Hz exploratory whisking, higher frequency “foveal” whisking, and low-frequency brushing of whiskers across objects using head movements (Carvell and Simons 1990; Harvey et al. 2001; Kleinfeld et al. 2006). Whisking movement is robustly signaled in barrel cortex by a short-latency (peakA) population response that was similar to that evoked by passive touch. This short-latency response, that signals passive touch and whisking movement, is suppressed by active touch, and the suppression is stronger during high-frequency (10 Hz) whisking, which indicates that active touch enhances frequency adaptation. Stronger adaptation of sensory responses occurs in behaving animals during alertness and processing of significant sensory stimuli in behavioral contexts (Castro-Alamancos 2004a), can be caused by enhanced thalamocortical firing due to depression of thalamocortical synapses and/or stronger recruitment of cortical inhibition, and serves to focus sensory stimuli in neocortex (Castro-Alamancos 2002c, 2004b; Castro-Alamancos and Oldford 2002; Moore 2004). We speculate that during high-frequency whisking, touch is signaled in barrel cortex by suppressing whisking movement responses as a consequence of thalamocortical synaptic depression and/or recruitment of cortical inhibition. Considering the relation between cortical and superior colliculus sensory responses (Cohen et al. 2008), we would expect peak2 responses in superior colliculus to be suppressed by active touch because these responses are driven by short-latency barrel cortex responses. As discussed below, this was the case; peak2 responses tend to be smaller during whisking on surfaces than during whisking in air.
Particularly intriguing was the selective appearance of a long-latency (peakB) response in barrel cortex (60–90 ms) during low-frequency active touch. This response coincides with the onset of the falling phase of the protraction and was absent during passive touch, whisking movement, and high-frequency active touch. Thus, it specifically signals low-frequency active touch in barrel cortex. Since the long-latency barrel cortex response only appears if the whiskers make contact during low-frequency whisking, this signal may be useful to indicate the presence of an object during the onset of high-frequency whisking bouts, during single bouts of whisker movements, or during slow head movements that brush the whiskers across objects.
Superior colliculus cells signal whisking movement.
Whisker motion-sensitive cells are found in trigeminal ganglion, whisker thalamus, and barrel cortex (Derdikman et al. 2006; Szwed et al. 2003; Yu et al. 2006). In superior colliculus, about two-thirds of passive touch-sensitive cells are sensitive to whisking in air, and about half of these are insensitive to active touch. Thus, a sizeable population of cells in the superior colliculus signals whisking movement. In superior colliculus, whisking movement was generally signaled by peak 2, and not by peak3. Peak1 signaled whisking movement in a few large receptive field cells (RF4–RF6), but these responses were small compared to peak2 responses. The fact that short-latency (peakA) responses in barrel cortex, on which peak2 depends, were very sensitive to whisker movement (Fig. 1) explains the corresponding large peak2 responses in superior colliculus (Fig. 3D). Moreover, the largest peak2 whisking responses occurred in cells that are responsive to rostral microvibrissa (RF3), which are known to be immobile during whisking movement (Kleinfeld et al. 2006). Hence, the signal to rostral microvibrissa cells during whisking movement must be coming from the barrel cortex. Taken together, these results indicate that the superior colliculus robustly signals whisking movement driven by corticotectal inputs from the barrel cortex.
In the monkey visual system, the superior colliculus signals eye movements, and this corollary discharge is then used to stabilize visual perception (Sommer and Wurtz 2008). A similar function is likely to take place during active whisking and touch (Curtis and Kleinfeld 2009). Also, electrical stimulation of the superior colliculus produces both eye and whisker movements (McHaffie and Stein 1982). The sustained whisker protractions driven by superior colliculus originate from cells that are usually unresponsive to whisker stimulation (Hemelt and Keller 2008; McHaffie and Stein 1982). Thus, the superior colliculus appears capable of both driving and decoding whisker movements.
Superior colliculus cells signal active touch.
A sizeable population of cells in the superior colliculus signals active touch. In superior colliculus, active touch was signaled by peak3, and not by peak1. Peak2 responses were inhibited by active touch in most cells. In contrast, cells sensitive to active touch had the largest peak3 responses, and these were only excited by active touch. Thus, peak3 responses in superior colliculus signal active touch. Since the superior colliculus contains a population of cells that selectively signal active touch and others that selectively signal whisking movement, this indicates that it can decode touch from whisking. The active touch signal in superior colliculus cannot be driven by the long-latency (peakB) population signal we observed in barrel cortex because this happened much later. Therefore, direct trigeminotectal inputs driven by the rising phase of the protraction are likely responsible for the peak3 activity in superior colliculus.
Interestingly, as the whisking frequency increases, all signals in the superior colliculus including passive touch (Cohen et al. 2008), whisking movement, and active touch show rapid adaptation. This is a feature shared by other ascending sensory pathways, such as the trigeminothalamic pathway, and is known to be highly sensitive to behavioral state and neuromodulator influences (Castro-Alamancos 2002a, 2002b, 2004b). In fact, recent work has shown that whisker-sensitive cells in the superior colliculus are highly sensitive to changes in behavioral state (Cohen and Castro-Alamancos 2010a). Moreover, neuromodulators, such as acetylcholine, norepinephrine, and serotonin, are released in the superior colliculus during specific states and are likely to affect whisking and active touch signals. Future work is needed to determine how these factors affect whisking and active touch responses in superior colliculus and barrel cortex.
Superior colliculus cells code texture.
Texture-sensitive cells tend to have low spontaneous firing, indicating that they are selectively driven by active touch. Among these, roughly half prefer rough textures while the other half prefer smooth textures. Only peak3 (>20 ms) responses code texture and are significantly larger than whisking in air responses. Thus, long-latency activity driven by the rising phase of the protraction codes texture in the intermediate layers of the superior colliculus. Since cortical cells are well known to code texture (Arabzadeh et al. 2005; Diamond et al. 2008a, 2008b; Jadhav and Feldman 2010; Johnson and Hsiao 1992; Kleinfeld et al. 2006; von Heimendahl et al. 2007), it is possible that corticotectal activity is driving part of the texture signal. However, active touch and texture were signaled in barrel cortex by the peakB FP response, which occurs during the falling phase of the protraction (i.e., later than the superior colliculus active touch and texture signals). This indicates that touch-sensitive cells that code texture in superior colliculus are directly driven by trigeminotectal inputs. Therefore, whisker micromotions responsible for texture discriminations (Andermann et al. 2004; Arabzadeh et al. 2004; Jadhav and Feldman 2010; Ritt et al. 2008; Wolfe et al. 2008) during the rising phase of the protraction may be coded by superior colliculus cells.
Conclusions.
Our results indicate that whisker-sensitive cells in the intermediate layers of the superior colliculus carry signals for whisking, active touch, and texture. The whisking movement signal is likely caused by corticotectal inputs that follow a short-latency barrel cortex response driven by the onset of the whisker protraction. The active touch signal has a longer latency but occurs earlier than the cortical active touch signal, indicating that it may reflect direct trigeminotectal inputs driven by the rising phase of the whisker protraction. Texture coding occurs in a small percentage of cells that may be sensitive to trigeminotectal inputs driven by vibrissa micromotions during the rising phase of the protraction.
GRANTS
This work was supported by the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Andermann ML, Ritt J, Neimark MA, Moore CI. Neural correlates of vibrissa resonance; band-pass and somatotopic representation of high-frequency stimuli. Neuron 42: 451–463, 2004 [DOI] [PubMed] [Google Scholar]
- Arabzadeh E, Panzeri S, Diamond ME. Whisker vibration information carried by rat barrel cortex neurons. J Neurosci 24: 6011–6020, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabzadeh E, Zorzin E, Diamond ME. Neuronal encoding of texture in the whisker sensory pathway. PLoS Biol 3: e17, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AW, Waite PM. Responses in the rat thalamus to whisker movements produced by motor nerve stimulation. J Physiol 238: 387–401, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ. Biometric analyses of vibrissal tactile discrimination in the rat. J Neurosci 10: 2638–2648, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Absence of rapid sensory adaptation in neocortex during information processing states. Neuron 41: 455–464, 2004a [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Different temporal processing of sensory inputs in the rat thalamus during quiescent and information processing states in vivo. J Physiol 539: 567–578, 2002a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Dynamics of sensory thalamocortical synaptic networks during information processing states. Prog Neurobiol 74: 213–247, 2004b [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Properties of primary sensory (lemniscal) synapses in the ventrobasal thalamus and the relay of high-frequency sensory inputs. J Neurophysiol 87: 946–953, 2002b [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Role of thalamocortical sensory suppression during arousal: focusing sensory inputs in neocortex. J Neurosci 22: 9651–9655, 2002c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Oldford E. Cortical sensory suppression during arousal is due to the activity-dependent depression of thalamocortical synapses. J Physiol 541: 319–331, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Castro-Alamancos MA. Behavioral state dependency of neural activity and sensory (whisker) responses in superior colliculus. J Neurophysiol 104: 1661–1672, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Castro-Alamancos MA. Detection of low salience whisker stimuli requires synergy of tectal and thalamic sensory relays. J Neurosci 30: 2245–2256, 2010b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Castro-Alamancos MA. Early sensory pathways for detection of fearful conditioned stimuli: tectal and thalamic relays. J Neurosci 27: 7762–7776, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Castro-Alamancos MA. Neural correlates of active avoidance behavior in superior colliculus. J Neurosci 30: 8502–8511, 2010c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Hirata A, Castro-Alamancos MA. Vibrissa sensation in superior colliculus: wide-field sensitivity and state-dependent cortical feedback. J Neurosci 28: 11205–11220, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis JC, Kleinfeld D. Phase-to-rate transformations encode touch in cortical neurons of a scanning sensorimotor system. Nat Neurosci 12: 492–501, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdikman D, Yu C, Haidarliu S, Bagdasarian K, Arieli A, Ahissar E. Layer-specific touch-dependent facilitation and depression in the somatosensory cortex during active whisking. J Neurosci 26: 9538–9547, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME, von Heimendahl M, Arabzadeh E. Whisker-mediated texture discrimination. PLoS Biol 6: e220, 2008a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME, von Heimendahl M, Knutsen PM, Kleinfeld D, Ahissar E. ‘Where’ and ‘what’ in the whisker sensorimotor system. Nat Rev Neurosci 9: 601–612, 2008b [DOI] [PubMed] [Google Scholar]
- Gao P, Bermejo R, Zeigler HP. Whisker deafferentation and rodent whisking patterns: behavioral evidence for a central pattern generator. J Neurosci 21: 5374–5380, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwerg BS, Krauthamer GM. Vibrissa-responsive neurons of the superior colliculus that project to the intralaminar thalamus of the rat. Neurosci Lett 111: 23–27, 1990 [DOI] [PubMed] [Google Scholar]
- Guic-Robles E, Valdivieso C, Guajardo G. Rats can learn a roughness discrimination using only their vibrissal system. Behav Brain Res 31: 285–289, 1989 [DOI] [PubMed] [Google Scholar]
- Harvey MA, Bermejo R, Zeigler HP. Discriminative whisking in the head-fixed rat: optoelectronic monitoring during tactile detection and discrimination tasks. Somatosens Mot Res 18: 211–222, 2001 [DOI] [PubMed] [Google Scholar]
- Hemelt ME, Keller A. Superior colliculus control of vibrissa movements. J Neurophysiol 100: 1245–1254, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemelt ME, Keller A. Superior sensation: superior colliculus participation in rat vibrissa system. BMC Neurosci 8: 12, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Castro-Alamancos MA. Cortical transformation of wide-field (multiwhisker) sensory responses. J Neurophysiol 100: 358–370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Feldman DE. Texture coding in the whisker system. Curr Opin Neurobiol 20: 313–318, 2010 [DOI] [PubMed] [Google Scholar]
- Jadhav SP, Wolfe J, Feldman DE. Sparse temporal coding of elementary tactile features during active whisker sensation. Nat Neurosci 12: 792–800, 2009 [DOI] [PubMed] [Google Scholar]
- Johnson KO, Hsiao SS. Neural mechanisms of tactual form and texture perception. Annu Rev Neurosci 15: 227–250, 1992 [DOI] [PubMed] [Google Scholar]
- Khatri V, Bermejo R, Brumberg JC, Keller A, Zeigler HP. Whisking in air: encoding of kinematics by trigeminal ganglion neurons in awake rats. J Neurophysiol 101: 1836–1846, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D, Ahissar E, Diamond ME. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr Opin Neurobiol 16: 435–444, 2006 [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Kao CQ, Stein BE. Nociceptive neurons in rat superior colliculus: response properties, topography, and functional implications. J Neurophysiol 62: 510–525, 1989 [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Stein BE. Eye movements evoked by electrical stimulation in the superior colliculus of rats and hamsters. Brain Res 247: 243–253, 1982 [DOI] [PubMed] [Google Scholar]
- Moore CI. Frequency-dependent processing in the vibrissa sensory system. J Neurophysiol 91: 2390–2399, 2004 [DOI] [PubMed] [Google Scholar]
- Ritt JT, Andermann ML, Moore CI. Embodied information processing: vibrissa mechanics and texture features shape micromotions in actively sensing rats. Neuron 57: 599–613, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Brain circuits for the internal monitoring of movements. Annu Rev Neurosci 31: 317–338, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwed M, Bagdasarian K, Ahissar E. Encoding of vibrissal active touch. Neuron 40: 621–630, 2003 [DOI] [PubMed] [Google Scholar]
- von Heimendahl M, Itskov PM, Arabzadeh E, Diamond ME. Neuronal activity in rat barrel cortex underlying texture discrimination. PLoS Biol 5: e305, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe J, Hill DN, Pahlavan S, Drew PJ, Kleinfeld D, Feldman DE. Texture coding in the rat whisker system: slip-stick versus differential resonance. PLoS Biol 6: e215, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Derdikman D, Haidarliu S, Ahissar E. Parallel thalamic pathways for whisking and touch signals in the rat. PLoS Biol 4: e124, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker E, Welker WI. Coding of somatic sensory input by vibrissae neurons in the rat's trigeminal ganglion. Brain Res 12: 138–156, 1969 [DOI] [PubMed] [Google Scholar]