Abstract
The frontal pursuit area (FPA) lies posterior to the frontal eye fields in the frontal cortex and contains neurons that are directionally selective for pursuit eye movements. Lesions of the FPA (alternately called “FEFsem”) cause deficits in pursuit acceleration and velocity, which are largest for movements directed toward the lesioned side. Conversely, stimulation of the FPA evokes pursuit from fixation and increases the gain of the pursuit response. On the basis of these properties, it has been hypothesized that the FPA could underlie the selection of pursuit direction. To test this possibility, we manipulated FPA activity and measured the effect on target selection behavior in rhesus monkeys. First, we unilaterally inactivated the FPA with the GABA agonist muscimol. We then measured the monkeys' performance on a pursuit-choice task. Second, we applied microstimulation unilaterally to the FPA during pursuit initiation while monkeys performed the same pursuit-choice task. Both of these manipulations produced significant effects on pursuit metrics; the inactivation decreased pursuit velocity and acceleration, and microstimulation evoked pursuit directly. Despite these changes, both manipulations failed to significantly alter choice behavior. These results show that FPA activity is not necessary for pursuit target selection.
Keywords: smooth pursuit, frontal eye fields, decision making, muscimol, eye movement
the frontal pursuit area (FPA) plays an important role in controlling the metrics of smooth pursuit. The FPA is a cortical structure adjacent and posterior to the frontal eye fields (FEF) on the anterior bank of the arcuate sulcus; consequently, it also is often referred to as “FEFsem.” Neurons in this area exhibit directionally selective activity during pursuit (MacAvoy et al. 1991; Tanaka and Lisberger 2002b). FPA neurons show a range of activity patterns during pursuit in the preferred direction, ranging from phasic bursts of activity at pursuit onset to slowly developing, tonic activity that is sustained throughout the pursuit response (Ono and Mustari 2009). Many FPA neurons also receive vestibular inputs (Akao et al. 2007, 2009; Fukushima et al. 2000) that may be important for coordinating smooth eye movements during whole body movements. FPA neurons with different activity patterns tend to project to different precerebellar nuclei in the pons (Ono and Mustari 2009), implying that the descending output form the FPA controls multiple components of the pursuit response. The FPA also has dense reciprocal connections to both cortical areas involved in processing visual motion, such as the medial superior temporal area (MST) (Dürsteler and Wurtz 1988; Newsome et al. 1988), and areas involved in cognitive aspects of pursuit control, such as the supplementary eye fields (SEF) (Heinen 1995; Tian and Lynch 1995, 1996). Thus the FPA is in a special position among cortical areas to mediate between the visual motion inputs for pursuit and the construction of the pursuit motor commands.
Artificially decreasing activity in the FPA worsens several aspects of pursuit behavior in monkeys. FPA lesions severely impair pursuit velocity and acceleration and cause permanent impairments in predictive and anticipatory pursuit (Keating 1991, 1993; Keating et al. 1996; Lynch 1987; MacAvoy et al. 1991); similar deficits in pursuit metrics occur with reversible inactivation (Shi et al. 1998). The deficits to pursuit metrics and predictive pursuit are observed for pursuit in all directions but are most strongly expressed for pursuit ipsiversive to the lesion. Similar results have recently been reproduced in humans: transcranial magnetic stimulation of the area during ongoing pursuit reduces eye velocity (Gagnon et al. 2006).
Electrical stimulation has further clarified the role of the FPA. Unlike any other structure in the cortex, stimulation of the FPA during fixation evokes smooth pursuit, which is typically directed ipsiversive to the stimulated side (Gottlieb et al. 1993; Tanaka and Lisberger 2002a). In addition to eliciting pursuit-like changes in eye velocity, stimulation of the FPA also appears to change the gain of the pursuit system. During ongoing pursuit, stimulation increases the gain of pursuit regardless of pursuit direction (Tanaka and Lisberger 2001, 2002a). Stimulation during pursuit or fixation also increases the eye velocity response to perturbations of the tracked or fixated target stimulus (Tanaka and Lisberger 2001). These effects are analogous to the increase in the gain of the response to target perturbations during ongoing pursuit (Schwartz and Lisberger 1994) and demonstrate that activity in the FPA exerts a powerful influence over the gain of eye movement responses to visual motion inputs.
Several experiments have suggested that there is a tight link between target selection and gain control. Lisberger (1998) demonstrated that smooth pursuit velocity is enhanced in the wake of initial saccades that accompany pursuit. Gardner and Lisberger (2001, 2002) extended this observation by showing that saccades toward a pursuit target accompany an increase in the selectivity of the pursuit response, suggesting that saccades could help set the gain for pursuit. Relying on this link between target selection and gain control, several experimenters have hypothesized that the FPA, because of its established role in gain control, could contribute to the selection of a pursuit target (Srihasam et al. 2009; Tanaka and Lisberger 2001).
In this study, we have explicitly tested the role of the FPA in smooth pursuit target selection. In separate experiments, we used chemical inactivation and electrical stimulation to manipulate activity in the FPA while monkeys performed a two-alternative forced-choice pursuit selection task. Our manipulations produced significant, directionally asymmetric changes in pursuit metrics. However, both manipulations failed to bias the monkeys' pursuit choice behavior. These results confirm the role of the FPA in preparing and executing the motor plan for smooth pursuit but cast doubt on the possibility that it underlies the selection of pursuit direction.
METHODS
Subjects and Data Acquisition
We collected data from two (J and T) adult rhesus monkeys (Macaca mulatta). Each monkey had a titanium chamber placed over the left FEF and FPA. The chamber location was placed stereotaxically at coordinates determined by locating the arcuate sulcus in a high-resolution structural MRI; the chamber was centered 25 mm anterior and 18 mm lateral of stereotaxic zero, with an angle of 40°. All experimental protocols for the monkeys were approved by the Institutional Animal Care and Use Committee and complied with U.S. Public Health Service policy on the humane care and use of laboratory animals.
The experiments were controlled by a computer using the Tempo software package (Reflective Computing), and a second computer running the Psychophysics Toolbox (Brainard 1997; Pelli 1997) in MATLAB (The MathWorks) acted as a server device for presenting the visual stimuli. Stimuli were presented with a video monitor (75 Hz; ∼20 pixels/°) at a viewing distance of 41 cm. Eye movements were recorded using scleral search coils (Judge et al. 1980) and the electromagnetic induction technique (Fuchs and Robinson 1966) with standard phase detector circuits (Riverbend Instruments). All data and events related to the onset of stimuli were stored on disk during the experiment (1-kHz sampling rate) for additional analysis.
Behavioral Tasks
We presented subjects with a display consisting of a small white spot (82 cd/m2) against a uniform gray background (7 cd/m2). Once they fixated this spot, an experimental trial initiated.
Target selection task.
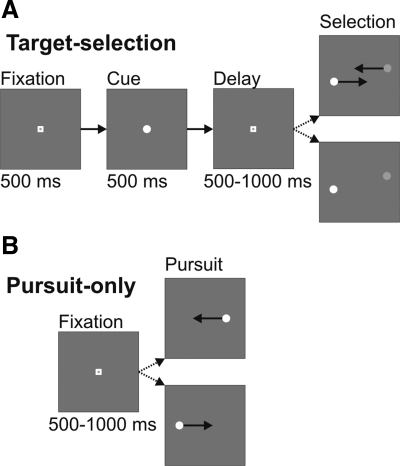
The primary behavioral manipulation in all of our experiments was a target selection task (Fig. 1A). The task began with the monkey directing its gaze at a central fixation point. After a 500-ms delay, the fixation point was briefly replaced by a luminance cue. The cue was a white (82 cd/m2) or gray (19 cd/m2) circular dot (radius 0.17°) presented for 500 ms and then replaced by the fixation point. After a variable delay (500–1,000 ms), the fixation point was extinguished and the choice stimuli were displayed. At this point, the trial branched into one of two possible conditions.
Fig. 1.
Sequence and timing of trial events. A: target selection task. Trials began with subjects directing gaze at a central fixation point. The fixation point transiently turned into a luminance cue. After a randomized delay, the fixation point was extinguished and the stimuli appeared. If the stimuli were moving, the monkey had to smoothly pursue the target that matched the luminance cue; if they were stationary, the monkey had to make a saccade to the correct target. B: pursuit-only task. Trials began with subjects directing gaze to a central fixation point. After a randomized delay, a single target appeared and moved in a step-ramp fashion toward the fixation point. The monkey was rewarded for smoothly pursuing the target.
The first condition was smooth pursuit target selection and comprised 75% of the total target selection trials. In this condition, monkeys were shown a pair of stimuli (1 gray and 1 white) and were rewarded for tracking the stimulus that matched the luminance of the cue. The initial stimulus locations were displaced horizontally by ∼3° to eliminate the need for initial saccades (Rashbass 1961). A small vertical offset (0.20°) was also added so that the two stimuli did not occlude each other as they passed through the center of the display. The dots moved in parallel but opposite directions toward the center of the screen. The axis of motion was always along the horizontal meridian. The speed of both stimuli was 16°/s. All possible combinations of the stimuli (i.e., direction of motion, target luminance) were presented with equal probability. The monkeys were required to keep their eyes directed within a 2° position window centered on either of the two choice stimuli for the duration of the trial and were given a grace period of 300 ms from stimulus onset to enter a stimulus window. If the monkey left this window, or if he made a saccade during 0–300 ms after target motion onset, the trial was aborted and the monkey was given a timeout of 3 s. If the monkey successfully remained in the window that matched the target stimulus, he was given a juice reward at the end of the trial.
The second possible condition was saccade target selection and comprised 25% of the total target selection trials. In this condition, monkeys were shown a pair of stationary stimuli (1 gray and 1 white) and were rewarded for making a saccade to the stimulus that matched the luminance of the cue. Identically to the previous condition, the stimulus locations were horizontally offset by ∼3° and vertically offset by 0.20°. The monkeys were required to make a saccade to one of the two choice stimuli within 500 ms. The trial was aborted if the monkey failed to move his eyes into a 2° window around one of the stimuli or failed to fixate the chosen stimulus for 500 ms. These trials were included to help prevent anticipation of pursuit responses.
Pursuit-only task.
In this task (Fig. 1B), the monkey engaged exclusively in single-target step-ramp pursuit (Rashbass 1961). The axis of motion was always along the horizontal meridian. The horizontal step size was ∼2° and was adjusted to eliminate initial saccades. There was no vertical offset in this task, the stimulus speed was 16°/s, and the direction of the stimulus (left or right) was chosen pseudorandomly on each trial. The monkeys were required to stay within a 2° position window centered on the target for the duration of the trial after an initial 300-ms grace period. The stimulus was white, with a luminance of 82 cd/m2 and a radius of 0.17°.
Inactivation
We transiently inactivated the FPA with the GABA agonist muscimol (Chen et al. 2001; Hikosaka and Wurtz 1985). We had two criteria that were met for every inactivation. First, the day before the inactivation, we were able to evoke smooth pursuit eye movements at the target site with currents less than 50 μA (tungsten electrodes, impedances 900 kΩ to 3.0 MΩ). Second, on the day of the inactivation itself, we were able to evoke smooth pursuit eye movements at our intended depth with our custom-made injectrode (Chen et al. 2001; Hafed et al. 2008). The sites targeted for inactivation were the same locations at which we had previously recorded pursuit-related neuronal activity, as described in detail previously (Mahaffy and Krauzlis 2011).
During each inactivation experiment, we first collected preinjection baseline data on the target selection task. We then lowered the injectrode into the FPA and confirmed our target site with microstimulation before injecting muscimol. We injected between 0.5 and 1.0 μl of muscimol (5 μg/μl), typically over ∼40 min (Table 1). After injection, we waited an additional 15 min before collecting postinjection behavioral data. We first tested the monkeys on the pursuit-only task, to confirm and document the effects of FPA inactivation on pursuit metrics, and then tested them on the target selection task. Finally, we tested them on the pursuit-only task again to confirm that the effects on pursuit metrics were still present.
Table 1.
Parameters for all inactivation experiments
| Inactivation No. | Monkey | Site | Evoked Direction | Volume, μl |
|---|---|---|---|---|
| 1 | J | (5.0, 2.0) | Left | 0.5 |
| 2 | J | (4.7, 2.0) | Left | 0.8 |
| 3 | J | (4.2, 1.8) | Right/down-right | 1.0 |
| 4 | J | (4.1, 2.2) | Left | 1.0 |
| 5 | J | (5.0, 2.5) | Right/down-right | 0.8 |
| 6 | J | (5.0, 1.5) | Left/up-right | 0.5 |
| 7 | J | (4.5, 3.0) | Left/up-left | 0.8 |
| 8 | T | (−3, 2.5) | Right/up-right | 1.0 |
| 9* | T | (−3.5, 2.0) | Left/up-right | 1.0 |
| 10 | T | (−4.0, 2.0) | Left/up-left | 1.0 |
| 11 | T | (−2.5, 2.0) | Up/up-left | 0.5 |
| 12 | T | (−3.5, 3.0) | Right/down-right | 1.0 |
| 13* | T | (−3.0, 3.0) | Right/down-right | 1.0 |
| 14 | T | (−3.5, 2.5) | Up/up-right | 1.0 |
| 15 | T | (−3.0, 2.0) | Left/up | 1.0 |
| 16 | T | (−3.0, 1.5) | Up/up-right | 1.0 |
Sixteen inactivation experiments were conducted using 2 monkeys (J or T). The sites indicated were targeted for inactivation, and the directions of pursuit evoked by stimulation on the day of the inactivation are shown, as well as the volume of muscimol injected at the site.
Experiments 9 and 13 showed no effect of inactivation and were not included in the analysis.
The day following each injection, we tested the subjects on the same behavioral tasks to ensure that they had fully recovered. We also performed a control injection with saline, following the same protocol described above.
Stimulation
In separate experiments, we microstimulated the FPA during the target selection task. We used the same criteria as described above, the ability to evoke pursuit from fixation with currents less than 50 μA, for identifying the FPA. Monkeys performed the target selection task (Fig. 1A), and on half of the trials we applied stimulation (biphasic pulses, 40 μA, 500 Hz) for 400 ms beginning at stimulus onset. Stimulation trials were pseudorandomly interleaved with no-stimulation trials. Stimulation evoked pursuit, and if the evoked pursuit was large enough to drive the eyes out of the fixation window, we expanded the window just enough to accommodate the induced change in eye position.
Data Analysis
Eye movement analysis.
We detected saccades using velocity and acceleration thresholds (Krauzlis and Miles 1996). During tracking, these thresholds were applied relative to the average eye velocity and acceleration to avoid erroneously flagging periods of smooth tracking with nonzero velocity as saccades (de Brouwer et al. 2002). All detected saccades were visually verified, and saccades during ongoing pursuit were excised from velocity traces.
We measured latency with two methods. For data from the inactivation experiments, we marked pursuit latency on each trial individually by fitting a “hinge” to the velocity data (Adler et al. 2002). The hinge is a combination of two conjoined lines, one horizontal and the other angled to fit the initial portion (first ∼50 ms) of the pursuit response. We allowed the angle and placement of the hinge to vary to minimize the mean squared error of the model's fit to the data. The placement of the hinge was confined to a window 50–350 ms from motion onset. We visually verified the results from every trial. In particular, we confirmed that the fit of the posthinge portion of the model was not contaminated by changes in pursuit direction on error trials.
The hinge model technique is not suitable for marking pursuit latency on stimulation trials, because the stimulation-evoked eye velocity cannot be readily distinguished from voluntary smooth pursuit. To address this problem, we used a second method based on signal detection theory that involved comparing eye velocity on trials where the correct target moved to the left vs. to the right (Green and Swets 1966). We constructed receiver-operating characteristic (ROC) curves at each millisecond based on the distribution of eye velocities and calculated 95% confidence intervals for the ROC area by performing a bootstrap permutation analysis (Britten et al. 1996; Horwitz and Newsome 2001). We then identified pursuit latency as the millisecond at which the ROC area crossed the 95% confidence intervals and then remained above it for 100 ms. Finally, we calculated confidence intervals on the measured latencies using a bootstrap analysis.
Pursuit choice analysis.
For the inactivation experiment, we calculated percent correct directly by measuring the average direction of eye velocity from 0 to 50 ms after pursuit onset. This was not practical with the stimulation experiment, because we were not able to reliably identify the onset of voluntary pursuit on individual trials. We therefore devised a two-part method for assessing pursuit choices that measured instantaneous percent correct over time. First, we measured the subjects' baseline velocity in a 10-ms interval beginning 20 ms before average pursuit onset; pursuit onset was identified with an ROC-based analysis of control eye velocity. Second, at each millisecond, we tallied the percentage of trials with velocities greater than that baseline, relative to the target velocity. The percentage of trials with velocities shifted toward the target velocity from the baseline at any moment in time was defined as the instantaneous percent correct. For this analysis, saccades were excised from the data and those portions of each trial were not counted. We are also confident that the analysis is robust for a number of different baseline choices. For example, we conducted our analysis with a fixed baseline interval from 130 to 140 ms after stimulus onset with similar results.
Bias was calculated in units of d′ assuming a constant criterion, based on methods from signal detection theory (Macmillan and Creelman 1991). Correct selection of a contralateral target was scored as a “hit”, and incorrect selection of a contralateral distracter was scored as a “false alarm.” Bias (c) was defined as the sum of the z-transformed hit (H) and false alarm rates (FA), divided by −2: c = −0.5[z(H) + z(FA)].
Sensitivity was also measured in units of d′ and defined as the difference of the z-transformed hit and false alarm rates, divided by the square root of 2: d′ = [z(H) − z(FA)][1/].
Statistical tests.
We used a two-factor ANOVA to test for significant effects of the inactivation and interactions between the inactivation and pursuit direction. We used a binomial test to evaluate the significance of changes of percent correct and instantaneous percent correct. We used a bootstrap procedure to generate confidence intervals for ROC-measured pursuit latencies. All error bars are 95% confidence intervals. Where error bars would obscure the data, filled and open symbols are used to indicate significant and nonsignificant data points, respectively.
RESULTS
Inactivation
We conducted a total of 16 inactivations in 2 monkeys. Fourteen of the 16 inactivations produced significant pursuit deficits and contributed to the data in this study. We first describe the effects of the inactivation on single-dot pursuit metrics, which served as a positive control to confirm that the injected muscimol suppressed FPA activity. Second, we present the results of inactivation on pursuit choice behavior on a target selection task. We report that inactivation of the FPA asymmetrically impaired ipsiversive pursuit velocity and acceleration but failed to bias pursuit selection.
Single-Dot Pursuit
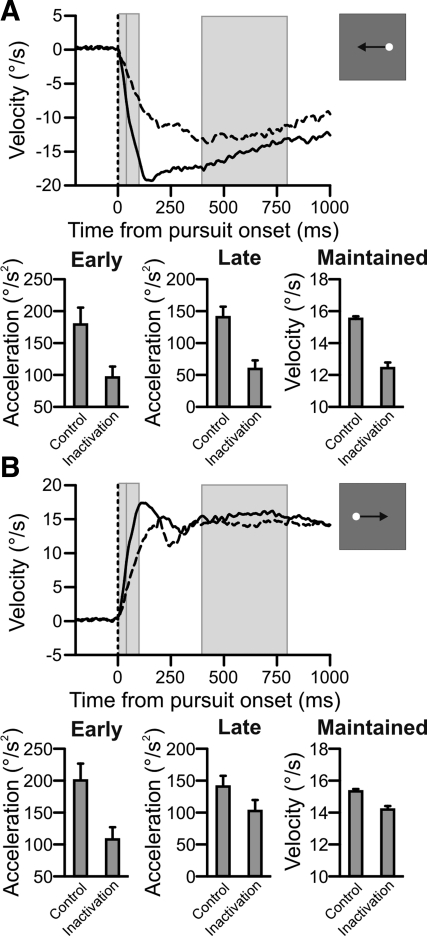
To assess the magnitude and directionality of the inactivation-induced deficit, we tested the subjects with single-dot pursuit (Fig. 1B). The inactivation affected several aspects of the subject's pursuit performance for trials directed ipsiversive to the inactivation, as illustrated by results from a typical experiment (Fig. 2A). The first effect was a decrease in pursuit acceleration. Previous work (Krauzlis and Lisberger 1994b; Lisberger and Westbrook 1985) demonstrated that initial pursuit acceleration (0–40 ms after pursuit onset) is less dependent on motion velocity than later intervals (41–100 ms). Following these conventions, for ipsiversive pursuit (i.e., toward the site of inactivation), we observed a significant decrease of 82°/s2 in early acceleration (Fig. 2A, left bar plot) and a significant decrease of 81°/s2 in late acceleration (Fig. 2A, middle bar plot) compared with preinjection pursuit. Also, we observed a significant decrease of 3.1°/s in maintained velocity, defined as average eye velocity in the interval 400–800 ms after pursuit onset (Fig. 2A, right bar plot). The decrease in ipsiversive velocity was the most consistent effect of FPA inactivation and was our primary means of confirming that the muscimol injection was successful.
Fig. 2.
Inactivation impaired single-dot pursuit metrics: example data. A: horizontal velocity traces and measurements for control (solid line) and inactivation (dashed line) leftward pursuit trials, ipsiversive to the inactivation. The 3 gray vertical bars span 0–40, 41–100, and 400–800 ms after pursuit onset and correspond to early acceleration, late acceleration, and maintained velocity measurement intervals, respectively. Error bars are 95% confidence intervals. B: horizontal velocity traces and measurements for rightward pursuit trials, contraversive to the inactivation. Plotting conventions are identical to those in A.
The inactivation also impaired pursuit contraversive, or away, from the site of inactivation (Fig. 2B). Again, there were effects on both acceleration and maintained velocity. The decrease in early acceleration of 92°/s2 was significantly different from preinjection but not significantly different from the reduction in early acceleration in the ipsiversive direction. In contrast, the decrease in late acceleration of 38°/s2 was significantly smaller for contraversive pursuit than for ipsiversive pursuit. Also, the maintained velocity for contraversive pursuit following the inactivation decreased by 1°/s, which was also significantly smaller than the change we observed for ipsiversive pursuit. Thus, consistent with previous studies (e.g., Keating 1991; Lynch 1987; Shi et al. 1998), our FPA inactivations produced significant directional defects for late acceleration and steady-state velocity for ipsiversive pursuit.
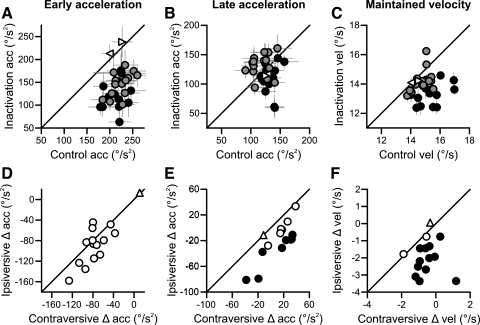
The results from our 14 inactivation experiments showed consistent pursuit velocity and acceleration deficits following inactivation (Fig. 3), and the results in the two monkeys were qualitatively similar. First, early accelerations (0–40 ms after pursuit onset) were severely impaired (Fig. 3A). The average change was a reduction of 90°/s2 for pursuit ipsiversive to the inactivation and 80°/s2 for pursuit contraversive to the inactivation. Twelve of 14 inactivations showed a significant decrease in ipsiversive early acceleration, and 13 of 14 inactivations showed a significant decrease in contraversive early acceleration. A two-way ANOVA revealed a significant effect of the inactivation on early accelerations [F(1,52) = 154.6, P < 0.001]. There was, however, no significant interaction between the inactivation and pursuit direction (Fig. 3D). The changes fell largely on the line of unity; none of the changes in contraversive vs. ipsiversive early acceleration were significantly different from one another. The reduction of early acceleration seemed to be omnidirectional.
Fig. 3.
Inactivation impaired single-dot pursuit metrics: population data. A–C: measurements of accelerations from 0 to 40 ms after pursuit onset (early), from 41 to 100 ms after pursuit onset (late), and velocity measurements from 400 to 800 ms after pursuit onset (maintained). Filled black and gray circles represent ipsiversive and contraversive trials, respectively. Each point is a separate inactivation. Open triangles indicate saline injections; right- and left-pointing triangles indicate ipsiversive and contraversive trials, respectively. Error bars are 95% confidence intervals. D–F: changes (Δ) in early and late acceleration and maintained velocity for every inactivation. Filled black circles show a significant difference between the ipsiversive and contraversive change. Open triangles indicate metrics changes for saline injections. acc, Acceleration; vel, velocity.
Second, we found that the late phase of acceleration was asymmetrically changed by FPA inactivation (Fig. 3B). For ipsiversive pursuit, 5 of the 14 inactivations showed significant reductions in late acceleration. For contraversive pursuit, only 1 inactivation resulted in a significant impairment of late contraversive acceleration, and 4 of 14 inactivations showed significant increases in late acceleration. Overall, there was no significant main effect of the inactivation on late acceleration. However, there was a significant interaction between direction and inactivation [F(1,52) = 9.82, P < 0.01]. The inactivation tended to decrease ipsiversive acceleration and increase contraversive acceleration (Fig. 3E). For 8 of 14 inactivations, the change in late acceleration was significantly lower for pursuit ipsiversive to the inactivation; the other 6 inactivations tended to show a trend in this same direction. On average, the inactivation decreased late ipsiversive acceleration by 21°/s2 and increased late contraversive acceleration by 12°/s2.
Finally, we found that FPA inactivation impaired maintained velocity (Fig. 3C). Every inactivation led to a significant decrease in ipsiversive pursuit velocity, and most (11/14) also significantly reduced contraversive velocity. The average reductions in ipsiversive and contraversive pursuit velocity following inactivation were 2.1 and 0.5°/s, respectively. Twelve of 14 inactivations showed a significantly stronger reduction in ipsiversive than contraversive pursuit velocity (Fig. 3F). As a population, the inactivation significantly decreased maintained pursuit velocity [F(1,52) = 51.81, P < 0.001], and a 2-way ANOVA revealed a significant interaction between inactivation and pursuit direction [F(1,52) = 16.29, P < 0.001].
We also measured pursuit latency on each trial, and the changes after FPA inactivation were quite small and variable (not shown). Four of 14 inactivations showed an increase in ipsiversive pursuit latency, 2 of 14 inactivations showed an increase in contraversive latency, and 1 inactivation showed a significant decrease in latencies ipsiversive to the inactivation. On average, the inactivation increased latencies by 3.2 and 2.6 ms for ipsiversive and contraversive trials, respectively, although a two-way ANOVA failed to reveal any significant effect of FPA inactivation on latency or any interaction between inactivation and direction.
The effects we observed on pursuit velocity and acceleration from the single-dot pursuit task provide evidence that our muscimol injections successfully inactivated neurons in the FPA. The saline injection showed none of these effects (Fig. 3, open triangles), ruling out the possibility that the effects were due to mechanical damage.
Target Selection
We used a two-alternative forced-choice target selection paradigm that had been used previously to probe selection behavior (Carello and Krauzlis 2004; Ferrera and Lisberger 1997a; Krauzlis and Dill 2002). Briefly, the monkeys were shown a white or gray cue and asked to smoothly pursue the stimulus that matched the luminance of the cue (Fig. 1A). There was no significant effect of target luminance (i.e., white or gray) on either monkey's performance (ANOVA, P > 0.10); consequently, the data were pooled across the two types of stimulus conditions.
Pursuit Choices Following FPA Inactivation
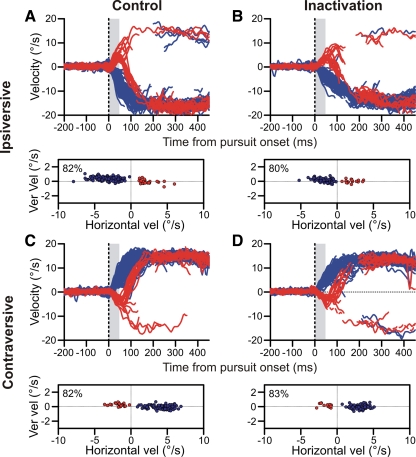
Despite impairing pursuit metrics, FPA inactivation failed to alter target selection behavior, as illustrated by a sample experiment (Fig. 4). Before the inactivation, for trials ipsiversive to the inactivated direction, the monkey performed at 82% correct (Fig. 4A). Correct trials (blue) were classified based on the monkey's initial pursuit direction (as described in methods). On the majority of incorrect trials, the monkey eventually corrected his choice (red). If the FPA were responsible for choosing a pursuit direction, inactivating the area should have decreased choices in the ipsiversive direction. However, following the inactivation, despite a change in initial velocity, the monkey's performance remained statistically unchanged at 80% correct (Fig. 4B).
Fig. 4.
Inactivation failed to affect pursuit choice behavior: example data. A: horizontal velocity for all control trials in an example experiment where the correct target was moving ipsiversive to the inactivated side. Red and blue traces are incorrect and correct trials, respectively. Saccades are excised from the plots. Vertical gray bar is from 0 to 50 ms after pursuit onset. The scatter plot (bottom) shows the mean velocities for correct (blue) and incorrect (red) trials from this window. Percent correct is noted. B: horizontal velocity for ipsiversive trials following inactivation. C and D: horizontal velocity for contraversive trials before and after the inactivation, respectively. Plotting conventions are the same as in A and B. Ver vel, vertical velocity.
Pursuit performance was similarly unaffected for trials contraversive to the inactivation (Fig. 4, C and D). If the FPA were responsible for pursuit selection, inactivation should improve performance contraversive to the inactivation. This did not happen; the inactivation did not produce a significant change in choice behavior for our example experiment.
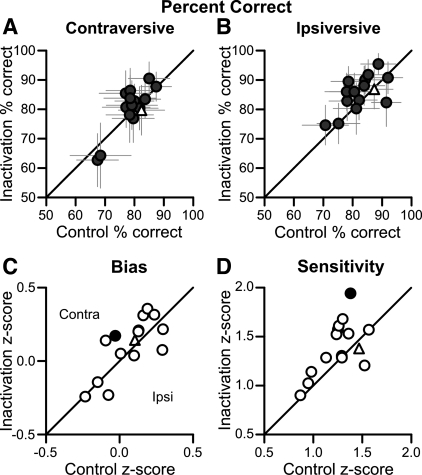
A similar pattern of results was found across our FPA inactivation experiments (Fig. 5). For contraversive pursuit, none of the 14 inactivations significantly altered percent correct (Fig. 5A); the mean change in performance was a 1.4% increase in percent correct. For ipsiversive pursuit, 1 of the 14 inactivations significantly increased performance in the contraversive direction (Fig. 5B); the mean change in performance for this direction was a 2.5% elevation in percent correct. A two-way ANOVA on the population did not show any significant effect of inactivation or any significant interaction between direction and inactivation. We also measured the change in the subjects' bias for every experiment (Fig. 5C). By our convention, positive values indicate a contraversive bias so that we would expect a positive change in bias if FPA inactivation impaired ipsiversive pursuit choices. Only 1 of 14 inactivations resulted in a significant change in bias. Sensitivity also was largely unchanged by the inactivation; only 1 of 14 inactivations produced a significant increase in sensitivity (Fig. 5D). In summary, the inactivations had very little effect on the subjects' pursuit choice behavior.
Fig. 5.
Inactivation failed to affect pursuit choice behavior: population data. A: percent correct before and after the inactivation for trials contraversive to the inactivation. Error bars are 95% confidence intervals. Open triangle indicates saline injection. B: same plotting conventions as in A for trials ipsiversive to the inactivation. C: bias before and after inactivation. Positive values indicate a greater bias for contraversive pursuit, and negative values indicate a bias for ipsiversive pursuit. Filled black circle shows a point at which inactivation significantly changed bias. Open triangle indicates saline injection. D: sensitivity before and after inactivation. Plotting conventions are the same as in C.
An Alternate Method for Assessing Choice Behavior
In the previous analysis, we classified pursuit choice on the basis of its initial direction. This is a common technique in the field for judging pursuit behavior (e.g., Adler et al. 2002; Carello and Krauzlis 2004). However, it has some drawbacks. First, it relies on a somewhat subjective assessment of pursuit initiation. Second, the standard technique only identifies pursuit choice at a single moment in time and ignores any changes that might occur later in the trial. To address these problems and to provide a more objective assessment of pursuit choice, we developed a new analysis that gauges choice behavior over time. We first established a baseline of pursuit velocity and then measured, at each moment in time, the percentage of trials that exceeded the baseline velocity in the direction of the correct target. The baseline we used was the mean eye velocity across control trials of both directions from 20 to 10 ms before pursuit onset. Average pursuit latency was identified by measuring the discriminability of pursuit velocity on trials of opposite directions with an ROC analysis. In practice, for the inactivation experiment, the range of baselines was 0 ± 1°/s. We tested two other methods of establishing the baseline: fixing it at 0°/s and setting it to the mean velocity in a fixed interval relative to stimulus onset. Both of these procedures produced similar results for the inactivation experiment.
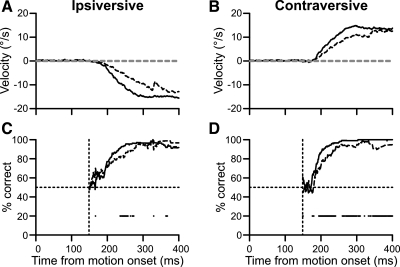
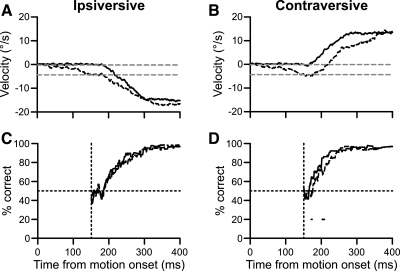
Applying this method to our sample experiment revealed that FPA inactivation delayed the increase in percent correct slightly for both directions (Fig. 6). For trials ipsiversive to the inactivation, the inactivation decreased the median horizontal velocity (Fig. 6A). The baseline velocities for this example were near 0°/s (gray dashed lines). We measured the percentage of trials, at each millisecond, that exceeded these baselines and found that the inactivation slightly reduced the instantaneous percent correct (Fig. 6C). The data are plotted from the start of the baseline period; before that time, the data were unsuitable for analysis. Significant bins (black dots) were determined with a binomial test. If the decrease in instantaneous percent correct was due to a change in choice behavior, we should see an opposite trend for the other direction, but this was not the case. To the contrary, instantaneous percent correct was also impaired for trials in which the correct target moved contraversive to the inactivation (Fig. 6D). Since performance was not improved for trials contraversive to the inactivation, the deficit was probably due to a global change in pursuit metrics and not a change in choice behavior. Also, although this graph shows a maintained choice deficit, this was not always present in the results from other inactivations.
Fig. 6.
Example data showing alternate method of estimating choice over time. A: control (solid trace) and inactivation (dashed traces) horizontal pursuit velocities for trials where the correct target moved ipsiversive to the inactivation. Gray dashed lines are baseline velocity measurements for control and inactivation trials. B: same plotting conventions as A for contraversive trials. C: instantaneous percent correct for control (solid trace) and inactivation (dashed trace) trials. Dots below traces are bins where the 2 traces are significantly different. Data are plotted from start of baseline interval: 149 ms after pursuit onset in this example. D: same plotting conventions as C for trials contraversive to the inactivation.
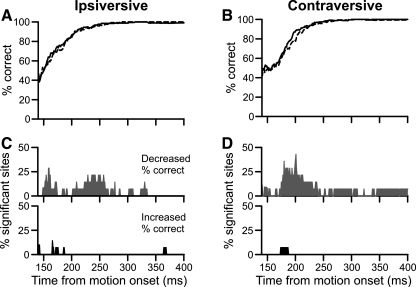
The population results showed a small, symmetric decrease in initial percent correct following FPA inactivation (Fig. 7). All plots begin 140 ms after target motion onset, at which time the baseline period had begun for 9 of the 14 experiments. The data preceding this interval were unreliable due to the lower number of contributing data sets. We averaged the instantaneous percent correct together for the 14 experiments and found that it decreased slightly, or perhaps was slightly delayed, in both directions (Fig. 7, A and B). A change in choice behavior would have produced an asymmetric, larger deficit for trials ipsiversive to the inactivation. To the contrary, we found that percent correct for contraversive trials (Fig. 7B) actually showed a slightly greater reduction than ipsiversive trials (Fig. 7A).
Fig. 7.
Population data showing alternate method of estimating choice over time. A: grand averages over time of instantaneous percent correct for control (solid trace) and inactivation (dashed trace) trials ipsiversive to the inactivation. Data are plotted from 140 ms after motion onset, at which time more than one-half of the inactivations were contributing data. B: same plotting conventions as in A for contraversive trials. C: percentage of sites with a significant change in percent correct following inactivation. Gray trace (top) indicates the inactivation decreased percent correct; black trace (bottom) indicates that the inactivation increased percent correct. Note the y-axes only extend to 50%. D: same plotting conventions as in C for trials contraversive to the inactivation.
We quantified the results from this analysis by counting the number of inactivations that led to significant changes in percent correct at each millisecond. We sorted these significant differences based on whether the inactivation caused significant increases or decreases to percent correct. For ipsiversive trials, we found that no more than 21% (3/14) of inactivations significantly decreased instantaneous percent correct (Fig. 7C, top). The changes caused by the inactivation were minimal and transitory (Fig. 7C, bottom). Similarly, for trials contraversive to the inactivation, no more than 40% (6/14) of inactivations decreased percent correct (Fig. 7D, top). The inactivations failed to produce sustained increases in percent correct (Fig. 7D, bottom); both directions of pursuit showed a modest decrease in instantaneous percent correct near pursuit onset.
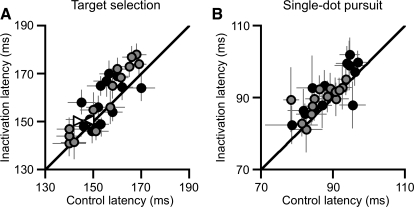
This pattern of results suggested that the changes in instantaneous percent correct were due to changes in pursuit metrics or latency, rather than to deficits in pursuit choice behavior. We documented the changes in pursuit acceleration that accompanied inactivation (Fig. 3), which could have delayed the increase in instantaneous percent correct. We also measured pursuit latencies on each trial and found small changes (Fig. 8A). Five of 14 inactivations led to a significant increase in ipsiversive pursuit latency, and 8 of 14 inactivations led to a significant increase in contraversive pursuit latency. The mean increases in pursuit latency following inactivation were 3.5 and 5 ms for ipsiversive and contraversive pursuit, respectively. These modest changes in latency could have contributed to the global reduction in choices with the pursuit target that we observed in our time series analysis. The slightly larger change in contraversive latency is also consistent with the larger change in contraversive instantaneous percent correct. Similar, but smaller, changes in latency also occurred in the single-dot pursuit-only trials (Fig. 8B): 2 of 14 inactivations led to a significant increase in ipsiversive pursuit latency, and 2 of 14 inactivations led to a significant increase in contraversive pursuit latency. The mean increases in single-dot pursuit latency were 3 and 2 ms for ipsiversive and contraversive pursuit, respectively. These increases in single-dot pursuit latency are consistent with the interpretation that the changes in latency of pursuit target selection are motor related, rather than delays in choice.
Fig. 8.
Inactivation caused mild changes in pursuit latency. A: latency during target selection trials. B: latency during single-dot pursuit-only trials. Filled black and gray circles correspond to trials in the ipsiversive and contraversive direction, respectively. Right- and left-pointing open triangles indicate contraversive and ipsiversive trials for saline injection, respectively. Error bars are 95% confidence intervals. These measurements were made by fitting a hinge model to the velocity on each trial.
Saccadic Choices Following FPA Inactivation
Overall, there was little change in saccade target selection behavior after FPA inactivation. A two-way ANOVA on the population results showed no significant effect on overall choice or any interaction between direction and choice. However, there were significant changes in saccade choice behavior in a few individual experiments. In 2 of 14 experiments, inactivation of the left FPA caused a significant increase in percent correct for leftward saccades, and in 1 of 14 experiments, inactivation of the left FPA caused a significant decrease in the percent correct for rightward saccades. Both of these effects are what would be expected if the inactivation spread into the left FEF and impaired the ability to select or generate rightward saccades. One of the experiments with significant effects on saccade choice was also the one experiment in which we observed a significant effect on pursuit choice.
Stimulation
We also tested the effects of FPA stimulation on pursuit choice at 37 sites. Subjects again performed the target selection experiment (Fig. 1A), and on half the trials we applied 40-μA stimulation for 400 ms beginning at stimulus onset. These stimulation parameters were strong enough to evoke smooth pursuit eye movements during fixation, consistent with previous studies (Gottlieb et al. 1993; Tanaka and Lisberger 2002a). Stimulation trials were randomly interleaved with control trials, and we analyzed the pursuit responses to determine whether FPA stimulation affected the choice of pursuit targets.
Stimulation of the FPA Does Not Affect Pursuit Choice
The pursuit evoked by FPA stimulation made identifying voluntary pursuit initiation impractical. We considered performing a subtraction of the evoked pursuit during stimulation, but we found that stimulation during pursuit onset typically evoked pursuit with different amplitudes and temporal profiles than did stimulation during passive fixation. Thus we decided against marking pursuit initiation on individual trials. Instead, we used the same method described earlier (Fig. 6) to calculate instantaneous percent correct.
Stimulation failed to significantly alter pursuit choice behavior in our example experiment (Fig. 9). Stimulation at this site evoked a leftward eye movement and enhanced initial pursuit velocity when the monkey pursued toward the stimulated side (Fig. 9A), in agreement with previous findings (Tanaka and Lisberger 2002a). The baseline velocity for the control trials, measured 20–10 ms before average pursuit onset, was near zero (gray dashed line). For the stimulation trials, however, the baseline was approximately −4°/s. This offset baseline velocity corrected for the stimulation-evoked pursuit. Despite eliciting pursuit to the left, the stimulation failed to substantially alter instantaneous percent correct (Fig. 9C): there were no time bins in which these two traces significantly differed. Indeed, once we corrected for the stimulation-induced velocity, the same frequency and time course of pursuit choice behavior was almost identical between the stimulation and control trials.
Fig. 9.
Example data illustrating choices over time for the stimulation experiment. A: control (solid trace) and stimulation (dashed trace) horizontal pursuit velocities for trials where the correct target moved ipsiversive to the stimulation. Gray dashed lines are baseline velocities for control and stimulation trials, where the stimulation baseline is −4.3°/s. B: same plotting conventions as in A for contraversive trials. C: instantaneous percent correct for control (solid trace) and inactivation trials (dashed trace). Data are plotted from start of baseline interval: 151 ms after pursuit onset in this example. D: same plotting conventions as in C for trials contraversive to the inactivation. Dots below the traces indicate milliseconds where the 2 traces are significantly different.
For trials contraversive to the stimulation, pursuit velocity was slightly suppressed throughout the stimulated interval (Fig. 9B), but the monkey was still able to select the correct target. Near pursuit onset, the instantaneous percent correct was slightly lower for the stimulated trials compared with the control trials (Fig. 9D). This difference was significant in several bins near 200 ms after stimulus onset. However, the shapes of the curves were largely identical. In this example, selection of the correct target seemed slightly delayed for stimulated trials. In summary, we did not observe a directionally asymmetric change in percent correct.
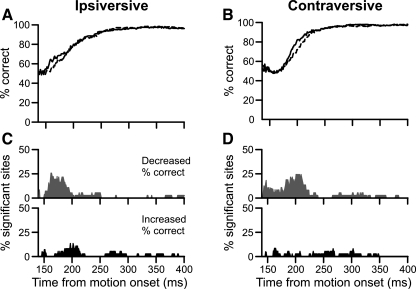
The population data also showed little evidence that FPA stimulation biased choice behavior (Fig. 10). We averaged together our estimates of instantaneous percent correct for all stimulation sites and found that, for trials ipsiversive to the stimulation, choice behavior was slightly reduced with stimulation (Fig. 10A). We quantified this change by tallying the number of significant experiments at each millisecond and found that stimulation decreased ipsiversive percent correct at ∼25% of sites (Fig. 10C, top). Stimulation increased ipsiversive percent correct at only 11% (4/37) of sites (Fig. 10C, bottom).
Fig. 10.
Summary of choice behavior for stimulation experiment. A: grand averages over time of instantaneous percent correct for control (solid trace) and stimulation (dashed trace) trials ipsiversive to the stimulation. Data are plotted from 136 ms after motion onset, at which time more than one-half of the stimulations were contributing data. B: same plotting conventions as in A for contraversive trials. C: percentage of sites with a significant changes in percent correct with stimulation. Gray trace (top) indicates the stimulation decreased percent correct; black trace (bottom) indicates that the stimulation increased percent correct. Note the y-axes only extend to 50%. D: same plotting conventions as in C for trials contraversive to the stimulation.
Pursuit directed contraversive to the site of stimulation also showed a modest decrease in instantaneous percent correct (Fig. 10B). Stimulation decreased instantaneous percent correct at ∼20% of sites in the time interval from 175 to 225 ms after pursuit onset (Fig. 10D, top). Very few sites showed an increase in contraversive percent correct (Fig. 10D, bottom). Thus both directions of pursuit showed a small decrease in percent correct with stimulation.
One possible objection to this analysis is that, because FPA stimulation does not always evoke ipsiversive pursuit, the effect on pursuit choice should likewise not always be ipsiversive. Indeed, 7 of 37 of our stimulation baselines were positive, indicating a velocity deflection directed contraversive to the site of the stimulation. Repeating the analysis after excluding these sites did not change the outcome: there remained a small symmetric decrease in initial instantaneous percent correct.
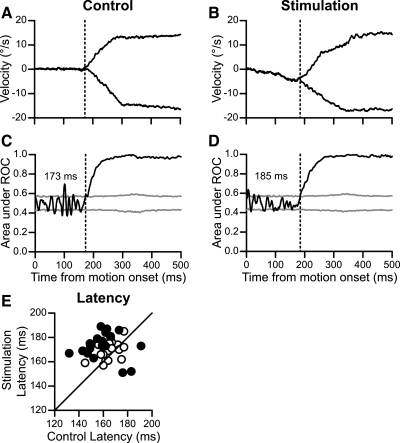
The decrease in instantaneous percent correct could be caused by delays in pursuit onsets with stimulation. We calculated pursuit latency by measuring the discriminability between the velocities for left and right trials with an ROC analysis (Green and Swets 1966) and constructed 95% confidence intervals for the ROC area by performing a bootstrapped permutation analysis on the eye velocity data. We marked pursuit latency at the millisecond where the ROC area crossed the confidence intervals and then remained above it for 100 ms. For our example stimulation experiment, we found that the ROC-assessed pursuit latency was 173 ms for control trials (Fig. 11C) and 185 ms for stimulation trials (Fig. 11D); this difference was significant by bootstrap analysis.
Fig. 11.
Stimulation increased pursuit latencies. A: horizontal eye velocity for control trials, example data. Trials directed to the right and left are plotted separately. Vertical dashed line corresponds to the latency of 173 ms, which is calculated below. B: same plotting conventions as in A for eye velocity during stimulation. Stimulation lasted for 400 ms from motion onset. C: receiver-operating characteristic (ROC) analysis identifying pursuit latency for control trials. Black line is the area under the ROC curve comparing trials to the right and left. Gray lines are 95% confidence intervals generated with a shuffled bootstrap analysis. Latency is marked when the ROC area crosses the confidence interval and remains above it for 100 ms. D: same plotting conventions as in C for stimulation data. E: stimulation and control latencies for all experiments. Filled black circles show significant changes with stimulation. Confidence intervals for these data (not shown) were generated with bootstrap analysis and used to determine significance.
Use of this same analysis technique for each of our experiments confirmed that stimulation tended to delay pursuit onset (Fig. 11E). Stimulation yielded significant increases of pursuit latency at 16 of the 37 sites tested and significant decreases at an additional 3 sites. On average, stimulation increased pursuit latency by 9 ms. A one-factor ANOVA revealed a significant effect of stimulation on pursuit latency [F(1,72) = 13.72, P < 0.001]. For comparison, we conducted this same analysis on our inactivation data, for which we had found that five inactivations yielded bidirectional increases in pursuit latency, using conventional methods for measuring pursuit latency. Three of those five inactivations also showed significant changes in pursuit latency with this ROC latency technique, providing some verification that this technique was reliable, if conservative, at identifying changes in pursuit latency.
Changes in Saccade Choices With FPA Stimulation
Stimulation of the FPA also had some effects on saccade choices, but these may have been a secondary consequence of the smooth eye movements caused by microstimulation. Stimulation of the left FPA increased the percent correct for leftward saccades on 17 of 37 experiments and decreased the percent correct for rightward saccades on 2 of 37 experiments. Overall, a two-factor ANOVA showed a significant improvement in saccade performance for leftward targets and a significant interaction between saccade direction and stimulation condition. However, the direction of the effect is not what would be expected from facilitating activity in the left FEF, since this would be expected to facilitate the generation of rightward, not leftward, saccades. Instead, the changes in saccade choice could be a secondary effect caused by the smooth eye movements induced by FPA stimulation: by moving the eyes closer to the left target and further from the right target, these stimulation-induced smooth movements could have introduced a behavioral bias in favor of the nearer target.
DISCUSSION
We have performed chemical inactivation and electrical stimulation of the FPA. These manipulations modulated pursuit metrics including acceleration, velocity, and latency but failed to alter pursuit choice behavior. These observations lead us to conclude that the FPA does not participate in the selection of targets, but rather is involved in the motor execution of smooth pursuit.
Inactivation Failed to Impair Selection
Lesions and inactivation of the FPA decrease maintained pursuit gain, decrease pursuit acceleration, and impair predictive pursuit (Keating 1991, 1993; Keating et al. 1996; Lynch 1987; MacAvoy et al. 1991), especially pursuit ipsiversive to the side of the lesion. These effects show that the FPA is involved in the circuitry that produces smooth pursuit. However, it is not clear whether the role of the FPA is limited to the motor production of pursuit or also includes the visual selection of the target direction. The demonstrated role of the FPA in gain control for pursuit (Tanaka and Lisberger 2001, 2002a) suggests that it could be important for pursuit target selection.
The reversible inactivations described in this report provide evidence that the FPA's role does not extend to visual selection of the target. In each of our inactivation experiments, we observed significant motor deficits that more strongly affected pursuit ipsiversive to the inactivated side, as expected for unilateral reduction of FPA activity (Lynch 1987; MacAvoy et al. 1991; Shi et al. 1998). However, we found no evidence of a deficit or bias in the capacity to choose a direction of motion in a pursuit-choice task. None of the inactivation experiments produced the pattern of results that would indicate biased performance: a significant decrease in ipsiversive choices and a significant increase in contraversive choices.
To confirm our findings, we devised a new method of assessing pursuit choices that measures the instantaneous percent correct over time. There are several advantages to this method: it is less biased since it does not rely on experimenter-marked pursuit latencies, it provides information about choice behavior throughout the trial, and it works even in the presence of artificially induced velocity signals, such as those caused by FPA stimulation. The disadvantage of this new method is that its output is sensitive to changes in acceleration and latency as well as choice behavior. We overcame these limitations by directly measuring acceleration and latency, and also by comparing instantaneous percent correct between directions to see if the changes were asymmetrical.
Applying this method to our data confirmed our findings that inactivation does not change choice behavior. We observed a transient dip in instantaneous percent correct following inactivation for both directions of pursuit. Since the changes in performance were symmetrical, they were not likely due to a change in choice behavior. Rather, the instantaneous percent correct was probably altered because of changes in acceleration and latency.
Stimulation Failed to Enhance Selection
Electrical stimulation of the FPA evokes smooth pursuit and enhances the gain of the smooth pursuit system. This latter property has been demonstrated in several ways: stimulating during ongoing pursuit increases gain omnidirectionally, stimulating during pursuit initiation facilitates acceleration ipsiversive to the stimulation, and stimulating during pursuit or fixation increases the gain of the response of the pursuit system to a transient target perturbation (Gottlieb et al. 1993; Tanaka and Lisberger 2001, 2002a). Gardner and Lisberger (2001, 2002) demonstrated that saccades to a moving target were accompanied by an increase in the gain of pursuit for the motion at the saccade's endpoint, which suggests that gain and target selection are interconnected. By implication, facilitating pursuit gain by stimulating the FPA could influence target selection.
This does not seem to be the case. We have applied electrical stimulation to the FPA at 37 sites in 2 monkeys. To assess the effect of stimulation on target selection, we measured the instantaneous percent correct with and without FPA stimulation. If stimulation drove pursuit choices in the stimulated direction, we would have expected to see an increase in the percentage of ipsiversive choices. Instead, we found that stimulation caused only a mild initial dip in percent correct for choices in both directions. The cause of this dip did not seem to be any change in the fraction of choices, but rather an ∼10-ms delay in pursuit initiation during stimulation. This result complements our inactivation data and suggests that activity in the FPA is neither necessary nor sufficient for the selection of a pursuit target.
Implications for Target Selection
Several behavioral results have shown that information about the direction of motion can be a strong cue for facilitating target selection for pursuit (Adler et al. 2002; Garbutt and Lisberger 2006; Krauzlis and Adler 2001). Our results suggest that the directional selection for pursuit does not occur in the FPA. This raises several issues. First, which other structures might mediate directional selection? Several experimenters have tested the possibility that the motion processing areas of middle temporal (MT)/MST could serve this function. Ferrera and Lisberger (1997b) found that most MT and MST neurons were not modulated by directional choice in a target selection task and concluded that the activity they observed probably could not account for pursuit selectivity. Recanzone and Wurtz (1999, 2000) found correlates of pursuit selectivity in the activity of MT/MST neurons, but these modulations were largely confined to the situation where both targets were in a single receptive field and thus could not explain the full range of selection behavior of which primates are capable, in particular, the types of selection tasks typically used in behavioral studies of pursuit choice behavior (e.g., Ferrera and Lisberger 1997a; Krauzlis and Dill 2002). One possible area involved in directional selection is the SEF, which plays a role in anticipatory pursuit (Missal and Heinen 2001, 2004). Recently, Shichinohe et al. (2009) demonstrated that inactivation of the supplementary eye fields hinders performance on a memory-based pursuit-choice task, which is consistent with the hypothesis that the area assists in the selection of a pursuit direction. Other candidate cortical areas include the ventral intraparietal area (VIP), lateral intraparietal area (LIP), area 7a, and the fundus of the superior temporal sulcus (FST), all of which contain directionally selective activity during pursuit (Bremmer et al. 1997; Erickson and Dow 1989; Schlack et al. 2003) but have not yet been tested in target selection paradigms.
Could pursuit selection be purely spatial? Several pieces of evidence point to the superior colliculus (Carello and Krauzlis 2004; Horwitz and Newsome 1999; Krauzlis and Dill 2002; McPeek and Keller 2002, 2004) and the FEF (Hanes and Schall 1996; Schall et al. 1995) as sites of target selection for saccadic eye movements. The colliculus also contributes to visual selection for smooth pursuit tasks (Carello and Krauzlis 2004; Krauzlis and Dill 2002), suggesting a generalized role for selecting targets in space. Subjects could spatially select targets from distracters regardless of the eventual motor output. Indeed, behavioral results in human subjects shows that spatial cues have larger effects on pursuit latency than either motion or color cues (Adler et al. 2002). The main objection to this possibility is that the smooth pursuit system operates on velocity inputs (Krauzlis and Lisberger 1994a; Lisberger et al. 1987; Rashbass 1961; Robinson et al. 1986), and it remains unclear how the spatial selection of a target could be transformed into a velocity command. However, the presence of pursuit selection signals in the colliculus and the absence of such signals in the FPA provide some support for the possibility that selection is accomplished in a retinotopic reference frame, rather than between possible directions of motion.
What is the relationship between gain and target selection? There is very strong evidence that the FPA is involved in setting the online gain of pursuit (Tanaka and Lisberger 2001, 2002a). Nevertheless, the results from the experiments in this study refute the hypothesis that the FPA contributes to target selection. Consistent with this finding, we recorded from FPA neurons during a target selection task and found that they failed to discriminate target from distracter early enough to affect pursuit directional selection (Mahaffy and Krauzlis 2011). Although saccadic target selection may enhance pursuit gain (Gardner and Lisberger 2001, 2002), our results suggest that this relationship is not reciprocal: target selection enhances pursuit gain, but enhancing pursuit gain does not affect target selection. Together, these results imply that the brain processes that select pursuit targets precede the FPA in the stream of visuomotor processing.
Implications for the FPA
The FPA is not necessary for the selection of a pursuit target, but rather is involved in the motor preparation and execution of the smooth pursuit response. The FPA is part of a cortical network of sensorimotor structures including MST and SEF (Tian and Lynch 1996), and it has downstream projections to eye movement structures in the pons (Distler et al. 2002), which in turn project to eye movement-related regions of the cerebellum. The FPA is therefore well-positioned to transform motion signals into eye movement commands. Indeed, the FPA contains both cells that fire transiently at pursuit onset and cells that maintain their activity throughout the pursuit response (Ono and Mustari 2009). This makes the FPA suitable to influence every phrase of the smooth pursuit response, consistent with our inactivation results.
Our inactivations caused significant changes in pursuit acceleration. These changes could be divided into effects on early and late acceleration. The early acceleration response, which lasts for the first 40 ms of pursuit, was impaired equally in both directions we tested. This effect is consistent with single-unit recording results: Tanaka and Fukushima (1998) first reported that FPA cells display pursuit-related buildup activity before the onset of target motion, and we have found that the level of this build-up activity correlates with the early acceleration response (Mahaffy and Krauzlis 2011). Together, these results suggest that the FPA could be involved in preparing the monkey to pursue generally, and decreasing activity in the area initially impairs pursuit in all directions. This is consistent with the observation that stimulation of the FPA during ongoing pursuit boosts the gain of pursuit regardless of its direction (Tanaka and Lisberger 2002a).
The late portion of acceleration, from 41 to 100 ms after pursuit onset, as well as the maintained velocity output, were asymmetrically impaired, with ipsiversive pursuit more strongly affected. This is consistent with previous reports of lesions and inactivations in the FPA, which consistently report asymmetric deficits. Forty milliseconds after pursuit onset, the vast majority of FPA cells have begun to respond selectively to pursuit in the preferred direction, which could be the neural basis for the directionally selective impairment. It is important to note, though, that even the contraversive direction of pursuit is significantly impaired. This is unsurprising given the abundance of cells in the FPA that have preferred directions contraversive to their side of the brain.
Our results suggest that the FPA is essentially a motor structure. It seems to be centrally involved in ensuring that pursuit tracks the intended target with high gain as soon as possible, but it is not actually involved in choosing that target. The FPA is downstream, or at least separated from, the target selection process; even if the eyes are artificially induced to track the incorrect stimulus, the subject still displays the same fundamental pattern of choice behavior. This dissociation is reminiscent of previous findings that FPA lesions disrupt anticipatory pursuit by causing motor impairments, not by disrupting the ability to predict target motions (Keating 1991, 1993). Together, these results point toward a role for the FPA in response selection, namely, promoting and implementing a pursuit eye movement response, rather than selecting or predicting the target for the movements.
GRANTS
This research was supported by National Eye Institute Grant R01 EY-012212 and by a National Science Foundation Graduate Research Fellowship to S. Mahaffy.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Natalie Dill and Eileen Boehle for outstanding technical assistance and Mary Garcia for administrative help.
REFERENCES
- Adler SA, Bala J, Krauzlis RJ. Primacy of spatial information in guiding target selection for pursuit and saccades. J Vis 2: 627–644, 2002 [DOI] [PubMed] [Google Scholar]
- Akao T, Saito H, Fukushima J, Kurkin S, Fukushima K. Latency of vestibular responses of pursuit neurons in the caudal frontal eye fields to whole body rotation. Exp Brain Res 177: 400–410, 2007 [DOI] [PubMed] [Google Scholar]
- Akao T, Kurkin S, Fukushima J, Fukushima K. Otolith inputs to pursuit neurons in the frontal eye fields of alert monkeys. Exp Brain Res 193: 455–466, 2009 [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997 [PubMed] [Google Scholar]
- Bremmer F, Distler C, Hoffmann KP. Eye position effects in monkey cortex. II. Pursuit- and fixation-related activity in posterior parietal areas LIP and 7A. J Neurophysiol 77: 962–977, 1997 [DOI] [PubMed] [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci 13: 87–100, 1996 [DOI] [PubMed] [Google Scholar]
- Carello CD, Krauzlis RJ. Manipulating intent: evidence for a causal role of the superior colliculus in target selection. Neuron 43: 575–583, 2004 [DOI] [PubMed] [Google Scholar]
- Chen LL, Goffart L, Sparks DL. A simple method for constructing microinjectrodes for reversible inactivation in behaving monkeys. J Neurosci Methods 107: 81–85, 2001 [DOI] [PubMed] [Google Scholar]
- de Brouwer S, Missal M, Barnes G, Lefevre P. Quantitative analysis of catch-up saccades during sustained pursuit. J Neurophysiol 87: 1772–1780, 2002 [DOI] [PubMed] [Google Scholar]
- Distler C, Mustari MJ, Hoffmann KP. Cortical projections to the nucleus of the optic tract and dorsal terminal nucleus and to the dorsolateral pontine nucleus in macaques: a dual retrograde tracing study. J Comp Neurol 444: 144–158, 2002 [DOI] [PubMed] [Google Scholar]
- Dürsteler MR, Wurtz RH. Pursuit and optokinetic deficits following chemical lesions of cortical areas MT and MST. J Neurophysiol 60: 940–965, 1988 [DOI] [PubMed] [Google Scholar]
- Erickson RG, Dow BM. Foveal tracking cells in the superior temporal sulcus of the macaque monkey. Exp Brain Res 78: 113–131, 1989 [DOI] [PubMed] [Google Scholar]
- Ferrera VP, Lisberger SG. The effect of a moving distractor on the initiation of smooth-pursuit eye movements. Vis Neurosci 14: 323–338, 1997a [DOI] [PubMed] [Google Scholar]
- Ferrera VP, Lisberger SG. Neuronal responses in visual areas MT and MST during smooth pursuit target selection. J Neurophysiol 78: 1433–1446, 1997b [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol 21: 1068–1070, 1966 [DOI] [PubMed] [Google Scholar]
- Fukushima K, Sato T, Fukushima J, Shinmei Y, Kaneko CR. Activity of smooth pursuit-related neurons in the monkey periarcuate cortex during pursuit and passive whole-body rotation. J Neurophysiol 83: 563–587, 2000 [DOI] [PubMed] [Google Scholar]
- Gagnon D, Paus T, Grosbras MH, Pike GB, O'Driscoll GA. Transcranial magnetic stimulation of frontal oculomotor regions during smooth pursuit. J Neurosci 26: 458–466, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt S, Lisberger SG. Directional cuing of target choice in human smooth pursuit eye movements. J Neurosci 26: 12479–12486, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JL, Lisberger SG. Linked target selection for saccadic and smooth pursuit eye movements. J Neurosci 21: 2075–2084, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JL, Lisberger SG. Serial linkage of target selection for orienting and tracking eye movements. Nat Neurosci 5: 892–899, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb JP, Bruce CJ, MacAvoy MG. Smooth eye movements elicited by microstimulation in the primate frontal eye field. J Neurophysiol 69: 786–799, 1993 [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley, 1966 [Google Scholar]
- Hafed ZM, Goffart L, Krauzlis RJ. Superior colliculus inactivation causes stable offsets in eye position during tracking. J Neurosci 28: 8124–8137, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science 274: 427–430, 1996 [DOI] [PubMed] [Google Scholar]
- Heinen SJ. Single neuron activity in the dorsomedial frontal cortex during smooth pursuit eye movements. Exp Brain Res 104: 357–361, 1995 [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol 53: 266–291, 1985 [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Separate signals for target selection and movement specification in the superior colliculus. Science 284: 1158–1161, 1999 [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Target selection for saccadic eye movements: prelude activity in the superior colliculus during a direction-discrimination task. J Neurophysiol 86: 2543–2558, 2001 [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980 [DOI] [PubMed] [Google Scholar]
- Keating EG. Frontal eye field lesions impair predictive and visually-guided pursuit eye movements. Exp Brain Res 86: 311–323, 1991 [DOI] [PubMed] [Google Scholar]
- Keating EG. Lesions of the frontal eye field impair pursuit eye movements, but preserve the predictions driving them. Behav Brain Res 53: 91–104, 1993 [DOI] [PubMed] [Google Scholar]
- Keating EG, Pierre A, Chopra S. Ablation of the pursuit area in the frontal cortex of the primate degrades foveal but not optokinetic smooth eye movements. J Neurophysiol 76: 637–641, 1996 [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Adler SA. Effects of directional expectations on motion perception and pursuit eye movements. Vis Neurosci 18: 365–376, 2001 [DOI] [PubMed] [Google Scholar]
- Krauzlis R, Dill N. Neural correlates of target choice for pursuit and saccades in the primate superior colliculus. Neuron 35: 355–363, 2002 [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Lisberger SG. A model of visually-guided smooth pursuit eye movements based on behavioral observations. J Comput Neurosci 1: 265–283, 1994a [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Lisberger SG. Temporal properties of visual motion signals for the initiation of smooth pursuit eye movements in monkeys. J Neurophysiol 72: 150–162, 1994b [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Miles FA. Release of fixation for pursuit and saccades in humans: evidence for shared inputs acting on different neural substrates. J Neurophysiol 76: 2822–2833, 1996 [DOI] [PubMed] [Google Scholar]
- Lisberger SG. Postsaccadic enhancement of initiation of smooth pursuit eye movements in monkeys. J Neurophysiol 79: 1918–1930, 1998 [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Morris EJ, Tychsen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu Rev Neurosci 10: 97–129, 1987 [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Westbrook LE. Properties of visual inputs that initiate horizontal smooth pursuit eye movements in monkeys. J Neurosci 5: 1662–1673, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JC. Frontal eye field lesions in monkeys disrupt visual pursuit. Exp Brain Res 68: 437–441, 1987 [DOI] [PubMed] [Google Scholar]
- MacAvoy MG, Gottlieb JP, Bruce CJ. Smooth-pursuit eye movement representation in the primate frontal eye field. Cereb Cortex 1: 95–102, 1991 [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A User's Guide. New York: Cambridge University Press, 1991 [Google Scholar]
- Mahaffy S, Krauzlis RJ. Neural activity in the frontal pursuit area does not underlie pursuit target selection. Vision Res 51: 853–866, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci 7: 757–763, 2004 [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol 88: 2019–2034, 2002 [DOI] [PubMed] [Google Scholar]
- Missal M, Heinen SJ. Facilitation of smooth pursuit initiation by electrical stimulation in the supplementary eye fields. J Neurophysiol 86: 2413–2425, 2001 [DOI] [PubMed] [Google Scholar]
- Missal M, Heinen SJ. Supplementary eye fields stimulation facilitates anticipatory pursuit. J Neurophysiol 92: 1257–1262, 2004 [DOI] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J Neurophysiol 60: 604–620, 1988 [DOI] [PubMed] [Google Scholar]
- Ono S, Mustari MJ. Smooth pursuit-related information processing in frontal eye field neurons that project to the NRTP. Cereb Cortex 19: 1186–1197, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997 [PubMed] [Google Scholar]
- Rashbass C. The relationship between saccadic and smooth tracking eye movements. J Physiol 159: 326–338, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Wurtz RH. Effects of attention on MT and MST neuronal activity during pursuit initiation. J Neurophysiol 83: 777–790, 2000 [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Wurtz RH. Shift in smooth pursuit initiation and MT and MST neuronal activity under different stimulus conditions. J Neurophysiol 82: 1710–1727, 1999 [DOI] [PubMed] [Google Scholar]
- Robinson DA, Gordon JL, Gordon SE. A model of the smooth pursuit eye movement system. Biol Cybern 55: 43–57, 1986 [DOI] [PubMed] [Google Scholar]
- Schall JD, Hanes DP, Thompson KG, King DJ. Saccade target selection in frontal eye field of macaque. I. Visual and premovement activation. J Neurosci 15: 6905–6918, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlack A, Hoffmann KP, Bremmer F. Selectivity of macaque ventral intraparietal area (area VIP) for smooth pursuit eye movements. J Physiol 551: 551–561, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JD, Lisberger SG. Initial tracking conditions modulate the gain of visuo-motor transmission for smooth pursuit eye movements in monkeys. Vis Neurosci 11: 411–424, 1994 [DOI] [PubMed] [Google Scholar]
- Shi D, Friedman HR, Bruce CJ. Deficits in smooth-pursuit eye movements after muscimol inactivation within the primate's frontal eye field. J Neurophysiol 80: 458–464, 1998 [DOI] [PubMed] [Google Scholar]
- Shichinohe N, Akao T, Kurkin S, Fukushima J, Kaneko CR, Fukushima K. Memory and decision making in the frontal cortex during visual motion processing for smooth pursuit eye movements. Neuron 62: 717–732, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srihasam K, Bullock D, Grossberg S. Target selection by the frontal cortex during coordinated saccadic and smooth pursuit eye movements. J Cogn Neurosci 21: 1611–1627, 2009 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Fukushima K. Neuronal responses related to smooth pursuit eye movements in the periarcuate cortical area of monkeys. J Neurophysiol 80: 28–47, 1998 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG. Regulation of the gain of visually guided smooth-pursuit eye movements by frontal cortex. Nature 409: 191–194, 2001 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG. Enhancement of multiple components of pursuit eye movement by microstimulation in the arcuate frontal pursuit area in monkeys. J Neurophysiol 87: 802–818, 2002a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG. Role of arcuate frontal cortex of monkeys in smooth pursuit eye movements. I. Basic response properties to retinal image motion and position. J Neurophysiol 87: 2684–2699, 2002b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian JR, Lynch JC. Corticocortical input to the smooth and saccadic eye movement subregions of the frontal eye field in Cebus monkeys. J Neurophysiol 76: 2754–2771, 1996 [DOI] [PubMed] [Google Scholar]
- Tian JR, Lynch JC. Slow and saccadic eye movements evoked by microstimulation in the supplementary eye field of the cebus monkey. J Neurophysiol 74: 2204–2210, 1995 [DOI] [PubMed] [Google Scholar]