Abstract
Presynaptic modulation of Ia afferents converging onto the motor neuron pool of the extensor carpi radialis (ECR) was compared during contractions (20% of maximal force) sustained to failure as subjects controlled either the angular position of the wrist while supporting an inertial load (position task) or exerted an equivalent force against a rigid restraint (force task). Test Hoffmann (H) reflexes were evoked in the ECR by stimulating the radial nerve above the elbow. Conditioned H reflexes were obtained by stimulating either the median nerve above the elbow or at the wrist (palmar branch) to assess presynaptic inhibition of homonymous (D1 inhibition) and heteronymous Ia afferents (heteronymous Ia facilitation), respectively. The position task was briefer than the force task (P = 0.001), although the maximal voluntary force and electromyograph for ECR declined similarly at failure for both tasks. Changes in the amplitude of the conditioned H reflex were positively correlated between the two conditioning methods (P = 0.02) and differed between the two tasks (P < 0.05). The amplitude of the conditioned H reflex during the position task first increased (129 ± 20.5% of the initial value, P < 0.001) before returning to its initial value (P = 0.22), whereas it increased progressively during the force task to reach 122 ± 17.4% of the initial value at failure (P < 0.001). Moreover, changes in conditioned H reflexes were associated with the time to task failure and force fluctuations. The results suggest a task- and time-dependent modulation of presynaptic inhibition of Ia afferents during fatiguing contractions.
Keywords: muscle fatigue, H reflexes, electromyograph, presynaptic inhibition, group I afferents
previous work has reported an increased reflex size, evoked either by mechanical stimuli applied to the muscle or electrical pulses delivered to homonymous group I afferents [Hoffmann (H) reflex], during tasks involving the control of limb position (position task) compared with tasks that require the control of muscle force (force task) (Akazawa et al. 1983; Baudry et al. 2009a; Doemges and Rack 1992; Maluf et al. 2007). Changes in reflex size are mediated by pre- and postsynaptic mechanisms (Pierrot-Deseilligny and Mazevet 2000), including presynaptic inhibition of group I afferents, which reduce the release of neurotransmitters from the afferent fibers due to their depolarization by primary afferent depolarization (PAD) interneurons (Rudomin and Schmidt 1999). Indirect evidence has suggested that the greater reflex size during the position task is attributable to lower levels of presynaptic inhibition of Ia afferents (Baudry and Enoka 2009; Baudry et al. 2010a).
Time to failure, which occurs when the participant is no longer able to match the task criterion, is briefer for the position task than for the force task (Baudry et al. 2009b; Hunter et al. 2002; Maluf et al. 2005; Rudroff et al. 2007). Moreover, previous work has indicated that the synaptic input received by motor neurons differs during force and position tasks (Baudry et al. 2009b; Mottram et al. 2005; Rudroff et al. 2010) and suggests a more rapid increase in presynaptic inhibition of Ia afferents during the position task when sustained to failure (Klass et al. 2008). As a decrease in Ia afferent input can reduce the discharge rate of motor units (Macefield et al. 1993), a disfacilitation that may hasten the recruitment of the motor unit pool during a sustained contraction. Consistent with this interpretation, Mottram et al. (2006) found that vibration-induced reductions in Ia afferent input decreased the time to failure of the position task. These results suggest that the modulation of Ia afferents at a presynaptic level may contribute to the difference in time to failure between the force and position tasks.
The purpose of the present study was to compare the modulation of presynaptic inhibition of Ia afferents during force and position tasks sustained to failure with the wrist extensor muscles. Two independent methods based on H reflex conditioning were used to estimate the change in presynaptic inhibition of Ia afferents converging onto the motor neurons that innervate the extensor carpi radialis muscle (ECR). The modulation of presynaptic inhibition was expected to differ across time and task with a greater level of presynaptic inhibition at the end of the position task relative to the force task. Some of these data have been previously presented in abstract form (Baudry et al. 2010b).
METHODS
Seven young adults (3 women and 4 men, age: 19–35 yr) volunteered to participate in the study after written informed consent was obtained. None of the subjects reported any neurological disorders. Participants reported to the laboratory for four sessions that were separated by at least 72 h and were asked to refrain from exercising the arm muscles for 24 h before testing. The Human Research Committee of the University of Colorado (Boulder, CO) approved the protocol.
Experimental Setup
Subjects were seated in a chair with the left arm abducted by ∼1.3 rad, the elbow flexed to 1.57 rad, and the forearm relaxed and pronated. The left wrist joint was aligned with the shaft of a servo-controlled torque motor (PMA44Q, Pacific Scientific, Rockford, IL, and PCI-7352, National Instruments, Austin, TX). The hand exerted an upward (extension) force against a padded metal plate (2 × 10 cm) located over the middle of the metacarpals and attached to the servo-controlled torque motor (see Fig. 1 in Baudry et al. 2010). The torque motor used a Labview Real Time system (2 PCs using a PCI-6029 and PCI-6021, National Instruments) to simulate an inertial load in a gravitational field, which was required for the position task. Signals from the servo-controlled torque motor were analog to digital sampled at 200 Hz (Power 1401, 16-bit resolution, Cambridge Electronic Design, Cambridge, UK) and stored on a computer for subsequent analysis.
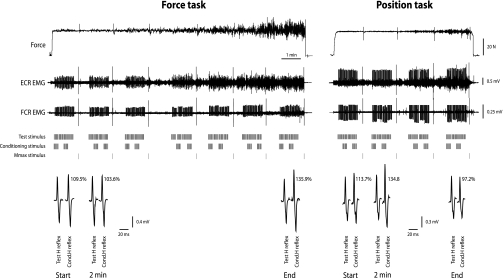
Fig. 1.
Force and position tasks with heteronymous Ia facilitation of the Hoffmann (H) reflex for one subject. Top: representative traces for force and electromyographic (EMG) activity from the extensor carpi radialis (ECR) and flexor carpi radialis (FCR) muscles are shown. The tic marks in the middle indicate the timing of the stimuli applied to the radial nerve to evoke an H reflex in the ECR (test stimulus), palmar branch of the median nerve to condition the H reflex (conditioning stimulus), and single supramaximal stimulus applied to the radial nerve to evoke a maximal M wave (Mmax) stimulus. Bottom: averages of 20 test H reflexes and 20 conditioned (Cond.) H reflexes at the start, at 2 min, and at the end of the two sustained contractions. Percentages indicate the amplitude of the conditioned H reflex relative to the associated test H reflex.
Visual feedback of the force exerted by the hand (force task) and wrist angle (position task) was provided on a monitor at a gain equal to 3%/cm of the maximal performance range, operationally defined as maximal voluntary contraction (MVC) force for the force task and full range of motion about the wrist joint for the position task. These visual gains matched the sizes of the fluctuations in the displayed feedback signal during the two tasks.
Electromyographic Recordings
Electromyographic (EMG) signals were recorded from the ECR, flexor carpi radialis (FCR), abductor pollicis brevis (APB), and brachioradialis (BRD) muscles with surface electrodes (Ag/AgCl electrodes, 4-mm diameter, Coulbourn Instruments, Allentown, PA) placed in a bipolar configuration. EMG signals were amplified (×500–5,000) and bandpass filtered (13–1,000 Hz) before being sampled at 2 kHz (Coulbourn Instruments) and stored on a computer. Voluntary contractions in the directions of wrist extension, wrist flexion, wrist abduction, and thumb abduction were used to verify the correct placement of the surface electrodes.
MVC Force
The wrist extension MVC involved a gradual increase in force from zero to maximum over 3 s and sustaining the maximal force for an additional ∼3 s. Subjects were instructed to use only the wrist extensor muscles and to avoid contracting the elbow flexors. At least two trials were performed, with subjects resting for 90–120 s between trials to minimize fatigue. If the peak forces were within 5% of each other, the greater value was taken as the maximum and used as a reference for subsequent submaximal contractions. Otherwise, additional trials were performed until the 5% criterion was achieved. Additionally, a single MVC was performed with the wrist flexors, elbow flexors, and thumb abductors for EMG normalization of FCR, BRD, and APB activity.
Electrical Stimulation
Electrical stimulation (Grass S88K, Astra-Med, West Warwick, RI, 1-ms rectangular pulse) was used to elicit test H reflexes and to provide conditioning stimuli that produced either D1 inhibition (Aimonetti et al. 1999) or heteronymous Ia facilitation (Baudry and Enoka 2009; Baudry et al. 2010). Stimuli were delivered via a constant-current unit (model CCU1, Astra-Med) that was connected to adhesive surface electrodes (Conmed, Utica, NY) placed in a bipolar configuration. The radial nerve (test H reflex) was stimulated from the lateral side of the upper arm between the brachialis and triceps brachii muscles. The median nerve (D1 inhibition) was stimulated from the medial side of the upper arm just proximal to the insertion of biceps brachii. The palmar branch of the median nerve (heteronymous Ia facilitation) was stimulated from the anterior surface of the forearm at the level of the wrist. Stimulation locations were determined at rest (median nerve and palmar branch of the median nerve) and during a contraction at 5% MVC force (radial nerve).
The motor threshold for stimulating the median nerve in the arm and the palmar branch at the wrist was determined by checking for evoked responses in the form of an M wave and a twitch response in the FCR and APB during voluntary contractions of the wrist extensors performed at 20% MVC force.
Test H Reflexes and Conditioned H Reflexes
Test H reflexes.
The recruitment curve for the H reflex and M wave for ECR was recorded as subjects exerted a wrist extension force (20% MVC force) against a rigid restraint. Series of five stimuli were delivered with an interval of ∼3 s between each stimulus intensity. The recruitment curve was used to identify the ascending phase of the relation between the H reflex amplitude and intensity of the electrical stimuli delivered to the radial nerve and to determine the stimulus intensity required to evoke a maximal M wave (Mmax). Electrical stimuli were delivered to the radial nerve during the sustained contraction in trains of 10 pulses with a pseudorandom interval of 1.75 ± 0.25 s between pulses. One potential concern with this protocol is that such stimulation intervals may have induced homosynaptic postactivation depression, a reduction in neurotransmitter release from Ia terminals due to repeated activation (Hultborn et al. 1996), and thereby reduced the size of the successive H reflexes. The influence of this mechanism, however, is minimized during voluntary contractions for stimulation rates up to 4 Hz (Burke et al. 1989; Stein and Thompson 2006; Stein et al. 2007). Moreover, when the size of each H reflex within a train was compared with that of the first H reflex of the train, no trend indicating a significant involvement of homosynaptic postactivation depression was noted. For example, the average size (across subjects) of the last H reflex in a train delivered at the beginning of the task relative to the size of the first H reflex of the same train was 99.6% for the force task and 97.8% for the position task.
The EMG activity in response to each stimulus within the train was monitored on an oscilloscope and stored on a computer for subsequent analysis. The initial intensity was set below the H reflex threshold and gradually increased until Mmax was evoked. The stimulus intensity was then adjusted to elicit a test H reflex with a size of 10–20% of Mmax and located on the ascending phase of the H reflex recruitment curve.
D1 inhibition.
The test H reflex was conditioned by a stimulus applied to the median nerve to activate PAD interneurons responsible for presynaptic inhibition of the Ia afferents from the ECR (Aimonetti et al. 1999; Berardelli et al. 1987). The delay between median (conditioning stimulus) and radial (test stimulus) nerve stimuli that produced the greatest depression of the H reflex amplitude was determined for each participant by 5-ms increments from 5 to 30 ms. The amplitudes of the test H reflex and conditioned H reflex were obtained by averaging at least 10 reflexes. The intensity of the conditioning stimulus applied to the median nerve was set at 1.0 × motor threshold. One limitation of this method is that the reflex gain may change during the voluntary contraction and thereby alter the amount of H reflex depression without changing the level of Ia presynaptic inhibition (Pierrot-Deseilligny and Mazevet 2000). As other issues associated with the D1 inhibition method can also confound the interpretation (Pierrot-Deseilligny and Mazevet 2000), the results obtained with D1 inhibition were compared with those obtained by an independent method involving heteronymous Ia facilitation.
Heteronymous Ia facilitation.
An electrical stimulus was applied to the palmar branch of the median nerve at the level of the wrist to evoke monosynaptic (heteronymous) facilitation of the motor neuron pool of the ECR (Baudry and Enoka 2009). As only the first 0.5 ms of the heteronymous facilitation arises from monosynaptic Ia afferent inputs (Aimonetti et al. 1999), the onset of heteronymous Ia facilitation of the H reflex in the ECR in response to stimulation of the palmar branch of the median nerve (1.0 × motor threshold) was established from an initial interval of 4 ms between the conditioning and test stimuli that was changed in 1-ms increments and then 0.2-ms increments. An increase of 5–10% in the amplitude of the conditioned H reflex relative to the test H reflex was used to identify the onset of the facilitation. Several trials were used to ensure the onset of facilitation. The delay was then set 0.2 ms later than the observed onset of facilitation for the rest of the experiment to ensure adequate activation of heteronymous Ia afferents while remaining within the 0.5-ms monosynaptic window (Baudry and Enoka 2009; Baudry et al. 2010). Under these conditions, the reflex facilitation was assumed to depend only on the size of the excitatory postsynaptic potential elicited by the monosynaptic input from heteronymous Ia afferents.
Experimental Procedures
Subjects participated in four experimental sessions to assess Ia presynaptic inhibition during the force and position tasks: the D1 inhibition method was used in two sessions (force and position tasks) and the heteronymous Ia facilitation method was used in two other sessions (force and position tasks). The force task required the subject to control the force exerted against a rigid restraint, whereas the position task involved controlling the angular position of the wrist while supporting an equivalent inertial mass. Therefore, load compliance and the type of feedback displayed on the monitor differed for the force and position tasks (for a review, see Enoka et al. 2011).
The order of the sessions was counterbalanced across subjects. Each experimental session began with the subject performing MVCs in the directions of wrist extension and wrist flexion. After the MVCs, the location of the stimulating electrodes for the radial nerve (test H reflex) was determined, and the recruitment curve for the H reflex and M wave for ECR was recorded. Next, the location of the electrodes for either the median nerve (D1 inhibition) or the palmar branch of the median nerve (heteronymous Ia facilitation) was determined, and the intensity and delay between the test and conditioning stimuli were established as subjects performed the force task at 20% of MVC force.
The main part of the protocol involved evoking test H reflexes and conditioned H reflexes as subjects sustained the force or position task to failure at 20% of MVC force. Test and conditioned H reflexes were alternated in a counterbalanced order during each sustained contraction with a stimulus sequence that comprised 4 sets of 10 reflexes, for a total of 20 test H reflexes and 20 conditioned H reflexes per sequence (Fig. 1). The sequence was repeated every 2 min. At the end of each stimulus sequence, an Mmax was evoked in the ECR through the same electrodes used for the test H reflex but triggered from another stimulator with the appropriate intensity. The first sequence began ∼15 s after the start of the sustained contraction. At the end of the sustained contraction, subjects performed an MVC. The criteria for terminating the fatiguing contraction were an inability to sustain the force within 5% of the target value for >3 s (force task) or an inability to maintain the wrist angle within 0.2 rad of the target for >3 s (position task).
Subjects were asked to exert wrist extension force against the padded metal plate while minimizing the contributions of other muscles. Moreover, the posture of the subjects and the position of their arms were continuously checked by visual inspection, and, when required, corrective instructions were given to help the subject maintaining the correct posture.
Data Analysis
The averaged and rectified EMG (aEMG) during the sustained contractions was measured over 2-s epochs that preceded each stimulation sequence. The EMG activity recorded from each muscle was normalized to the maximal value measured during 0.5 s of the relevant MVC. The coactivation ratio was calculated such that the aEMG amplitude for FCR corresponded to a percentage of aEMG for ECR. The coefficient of variation (equal to SD/mean × 100) for force was calculated over 2-s epochs that preceded each stimulation sequence.
H reflex responses were characterized from interference EMG traces by 1) latency (time from the stimulus artifact to the beginning of the EMG response), 2) duration (time between the onset of EMG activity and the return to the mean background EMG level), and 3) peak-to-peak amplitude (difference between positive and negative peaks). The amplitude of the test and conditioned H reflexes was expressed relative to the amplitude of the Mmax evoked in the same sequence of stimulations to control for changes in muscle geometry and to ensure that the H reflex amplitude remained within the linear range of the input-output relation of the motor neuron pool.
Statistics
Before comparing each dependent variable, the normality of the data was confirmed with a Kolmogorov-Smirnov test. The time to failure and MVC force and associated EMG activity before the sustained contraction were analyzed with two-way ANOVA (conditioning method × task) with repeated measures of both factors. Changes in the MVC force and EMG (expressed as a percentage of the values recorded before the sustained contractions) were analyzed with three-way ANOVA (conditioning method × task × time) with repeated measures on the three factors. The coefficient for variation for force was analyzed with similar procedures.
The latency, duration, and amplitude of the test H reflex (normalized to Mmax) and Mmax were compared at the beginning of the two sustained contractions with two-way ANOVA (task × conditioning method) with repeated measures for both factors to ensure similar values were obtained in the different conditions.
As the duration of the sustained contractions differed between the force and position tasks and the stimulation sequences for the H reflexes were elicited every 2 min, only three time points were used to analyze data obtained during the sustained contractions to ensure similar time points across subjects: at the beginning of the contraction, after 2 min, and just before failure. The data collected at the beginning of the sustained contraction are referred to as initial values. As the D1 inhibition and heteronymous facilitation methods represent independent assessments of spinal pathways, the change in the conditioned H reflex (percentage of the initial value) was compared using two-way ANOVA (task × time) with repeated measures within each conditioning method. When a significant main effect was found with ANOVA, a Tukey post hoc test was used to identify the significant differences among selected means.
Due to the relatively few subjects completing in the study, a post hoc power analysis examined the task × time interaction for each of the conditioned H methods. The effect size was calculated based on the partial η2, which corresponds to the sum of squaresbetween/(sum of squarestotal + sum of squareserror). Partial η2 was 0.32 and 0.48 for heteronymous facilitation and D1 inhibition, respectively, and the associated power (1-β error probability) was 0.99 for both methods. Thus, the statistical analysis was valid despite the small number of subjects.
The coefficient of determination (r2) extracted from Pearson product-moment correlations was calculated for 1) the durations of the two tasks when paired for the same subject and for the same conditioning method, 2) the results obtained from the D1 inhibition and heteronymous Ia facilitation methods, 3) the changes in the amplitude of the conditioned H reflex and the coefficient of variation for force, and 4) the changes in the amplitude of the conditioned H reflex and the time to task failure.
The level of statistical significance was set at P < 0.05 for all comparisons. Values are expressed as means ± SD in the text and means ± SE in the figures.
RESULTS
Seven subjects completed the four sessions for assessing changes in heteronymous Ia facilitation (2 sessions) and D1 inhibition (2 sessions), which resulted in 14 sustained contractions being performed until failure for each task. All subjects had briefer times to failure for the position task with mean ± SD values of 495 ± 119 s for the position task and 728 ± 140 s for the force task (task main effect, P < 0.0001). The intraclass correlation coefficient for the time to task failure in the same task performed in two different sessions (heteronymous Ia facilitation and D1 inhibition) was 0.94 (95% confidence interval: 0.81–0.98), indicating a high reliability in the time to task failure between sessions. Moreover, the durations of the two tasks were linearly associated (r = 0.74, P = 0.004), indicating that individuals who performed long contractions did so for both tasks.
MVC force (123 ± 41 and 124 ± 46 N, task × conditioning method, P = 0.40) and aEMG for the ECR (274 ± 187 and 292 ± 275 μV, task × conditioning method, P = 0.44) were similar before the sustained contraction for the force and position tasks, respectively. At task failure, the declines in MVC force decreased to 74 ± 19% and 71 ± 18% of the initial value (time main effect, P < 0.001), and aEMG for the ECR was reduced to 80 ± 17% and 79 ± 12% of the initial value (time main effect, P = 0.008).
aEMG Activity During Force and Position Tasks
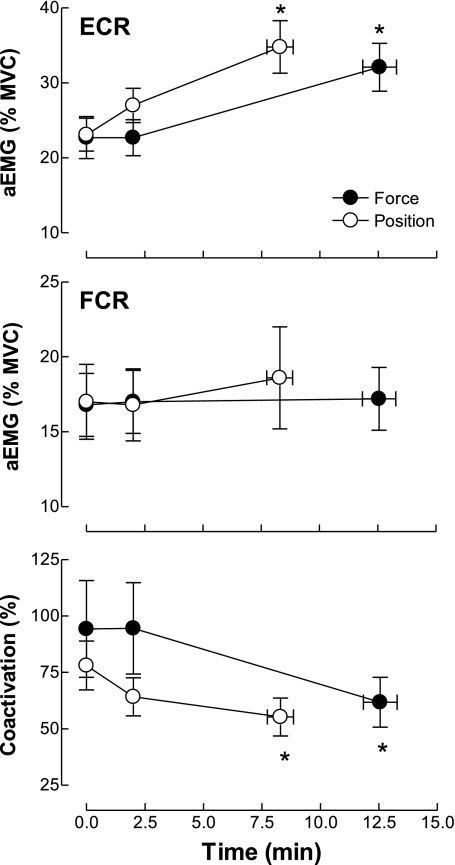
At the beginning of the sustained contraction, the aEMG for ECR was 22.7 ± 10.3% MVC and 23.1 ± 8.4% MVC for the force and position tasks, respectively, and did not differ significantly between tasks (task × time, P = 0.31; Fig. 2). The aEMG for the ECR at task failure increased to 32 ± 12% MVC and 35 ± 13% MVC for the force and position tasks, respectively (time main effect, P < 0.001; Tukey post hoc test for time, P < 0.001). In contrast, the aEMG activity for an antagonist muscle (FCR) did not change significantly during either sustained contraction (task × time, P = 0.34; Fig. 2). The coactivation ratio [(aEMG for the FCR/aEMG for the ECR) × 100] was similar (time × task, P = 0.12) at the beginning of the two sustained contractions (94 ± 80% and 78 ± 40% for the force and position control, respectively) and declined by a similar amount for both tasks at failure (time main effect, P < 0.001; Tukey post hoc test, P = 0.001; Fig. 2). The aEMG activity for the BRD increased similarly between the force and position tasks (time main effect, P = 0.009) to reach 144 ± 24% and 157 ± 39% of the initial value at task failure, respectively (Tukey post hoc test, P = 0.001).
Fig. 2.
Changes in averaged and rectified EMG (aEMG) activity and coactivation ratio during the force and position tasks. aEMGs for the ECR (top) and FCR (middle) were normalized to maximal voluntary contraction (MVC) values (means ± SE). Bottom: the coactivation ratio [(FCR aEMG/ECR aEMG) × 100] was determined from values averaged over 1-s intervals before the stimulation sequence elicited at the start of the contraction, at 2 min, and just before failure. *P < 0.005 compared with initial values.
Force Fluctuations
The coefficient of variation for force exerted by the hand did not differ at the beginning of the force and position tasks (0.78 ± 0.21% and 0.77 ± 0.36%, respectively, conditioning method × task × time, P = 0.98). The coefficient of variation for force increased during the two sustained contractions (task × time, P = 0.004) to reach 480 ± 307% (Tukey post hoc test, P = 0.002) and 241 ± 143% of the initial value (Tukey post hoc test, P = 0.002) at task failure for the force and position tasks, respectively. The relative increase at failure was greater in the force task than in the position task (Tukey post hoc test, P < 0.001).
Test H reflexes and Mmax
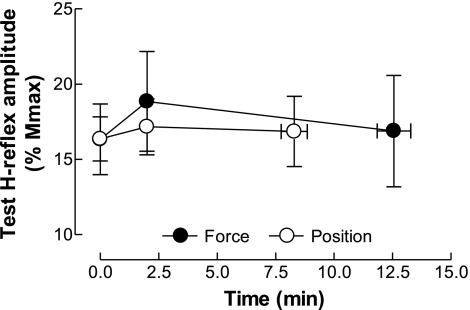
The latency of the H reflexes recorded in the ECR in response to radial nerve stimulation was similar for the force and position tasks (17.4 ± 0.8 and 16.7 ± 1.4 ms, respectively) at the beginning of the sustained contractions and did not change significantly during the sustained contractions (task × time × conditioning method, P = 0.31). Similarly, the duration of the H reflexes did not differ between tasks (10.0 ± 1.3 and 10.2 ± 1.9 ms for the force and position tasks, respectively) and throughout the sustained contractions (task × time, P = 0.51). The amplitude of the test H reflexes was 15.8 ± 9.0% Mmax and 16.8 ± 7.0% Mmax for the force and position tasks (when pooled across conditioning method), respectively, and did not change throughout the sustained contractions (task × time, P = 0.68; Fig. 3). At the beginning of the sustained contractions, the amplitude of the Mmax was 6.5 ± 2.8 and 8.0 ± 1.1 mV for the force and position tasks, respectively, and did not change during the two sustained contractions (conditioning method × task × time, P = 0.43).
Fig. 3.
Amplitude of the test H reflex during the force and position tasks. Amplitudes of the test H reflex (percentage of Mmax) recorded from D1 inhibition and heteronymous Ia facilitation when data were collapsed across the two methods for the force and position tasks are shown.
Although the amplitude of the test H reflexes did not change significantly during the two tasks, the size of the H reflex remained within the range where it is highly sensitive to excitatory and inhibitory inputs (Crone et al. 1990), and small variations in its amplitude may have elicited large changes in the conditioned H reflex. Nonetheless, there was no association (r2 = 0.014, P = 0.29) between the size of the test H reflex and the amplitude of the conditioned H reflex (expressed as a percentage of the test H reflex), indicating that the changes observed in the conditioned H reflex were not influenced by small variations in the size of the test H reflex.
D1 Inhibition
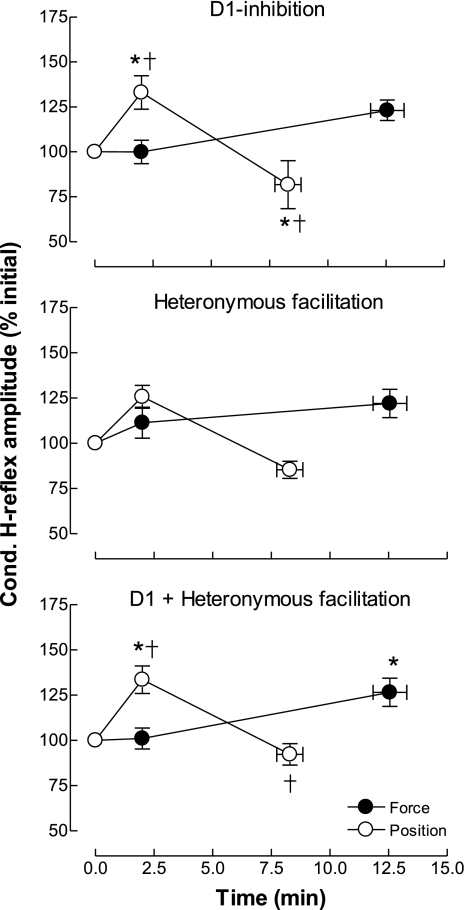
The delay between the conditioning stimulus (median nerve stimulation) and the test stimulus was 19 ± 2 ms (range: 15–20 ms). The size of the conditioned H reflex (percentage of the test H reflex) at the beginning of the sustained contractions was 64 ± 12% and 65 ± 9% for the force and position tasks, respectively (t-test, P = 0.26). The time courses of the conditioned H reflexes differed for the two tasks (force vs. position, task × time, P < 0.001). The size of the conditioned H reflex reached 123 ± 15% of its initial value at the end of the force task (Fig. 4, top) without being statistically significant (Tukey post hoc test, P = 0.28). In contrast, the size of the conditioned H reflex increased significantly 2 min after the beginning of the position task to 133 ± 24% of its initial value (Tukey post hoc test, P = 0.042) and decreased thereafter back to its initial value at the end of the sustained contraction (Tukey post hoc test, P = 0.44; Fig. 4, top). The change in the size of the conditioned H reflex with D1 inhibition differed significantly between the two tasks at 2 min (Tukey post hoc test, P = 0.042) and at task failure (Tukey post hoc test, P = 0.01).
Fig. 4.
Changes in H reflex amplitude after conditioning by the D1 inhibition and heteronymous Ia facilitation methods during the force and position tasks. Amplitudes of the conditioned H reflex (percentage of the initial value) recorded from D1 inhibition (top), heteronymous Ia facilitation (middle), and collapsed across the two methods (bottom) for the force and position tasks are shown. *P < 0.05 compared with initial values; †P < 0.05 compared with force task at the same time point.
Heteronymous Ia Facilitation
The delay between the conditioning stimulus for heteronymous Ia facilitation and the test stimulus was 5.6 ± 0.4 ms (range: 5.2–6.4 ms). The size of the conditioned H reflex (percentage of the test H reflex) at the beginning of the sustained contractions was 103 ± 7% and 114 ± 7% for the force and position tasks, respectively (t-test, P = 0.24). As observed with D1 inhibition, the time course of the change in the size of the conditioned H reflex differed during the two tasks (task × time, P = 0.02; Fig. 4, middle). The size of the conditioned H reflex reached 122 ± 21% at the end of the force task (Tukey post hoc test, P = 0.73). In contrast, the size of the heteronymous conditioned H reflex was 126 ± 17% of its initial value after 2 min (Tukey post hoc test, P = 0.15) and decreased thereafter (Fig. 4). At task failure, the change in size of the conditioned H reflex for the position task was statistically different from that at 2 min (Tukey post hoc test, P = 0.014) and from that at task failure during the force task (Tukey post hoc test, P = 0.049).
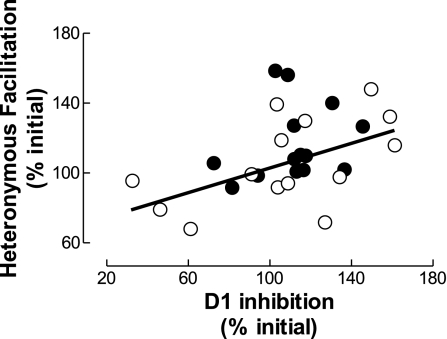
Association Between D1 Inhibition and Heteronymous Ia Facilitation
The two conditioning methods used in the present study are assumed to assess changes in the same subset of spinal PAD interneurons (Aymard et al. 2001; Baudry and Enoka, 2009). Therefore, changes in the conditioned H reflex obtained with D1 inhibition should parallel changes obtained with heteronymous Ia facilitation. The data for the two conditioning methods were significantly correlated (r = 0.45, P = 0.02; Fig. 5). Based on this association, the data for H reflexes conditioned with the two methods were combined (Fig. 4, bottom). The size of the combined conditioned H reflex increased gradually during the force task to reach 122 ± 17% of the initial value at task failure (task × time, P < 0.001; Tukey post hoc test, P = 0.035). In contrast, the size of the conditioned H reflex during the position task was increased at 2 min after the beginning of the contraction (Tukey post hoc test, P < 0.001) before returning to its initial level at the end of the contraction (Tukey post hoc test, P = 0.22). The size of the conditioned H reflex differed significantly between the two tasks at 2 min after the beginning of the sustained contraction (Tukey post hoc test, P = 0.027) and at task failure (Tukey post hoc test, P < 0.001).
Fig. 5.
Relation between the changes in the conditioned H reflex obtained with the two conditioning methods. Changes in the amplitude of the conditioned H reflexes at 2 min and at failure obtained with the heteronymous Ia facilitation and D1 methods for each subject in the force (●) and position (○) tasks are shown. The coefficient of determination (r2) was 0.20 (P = 0.02).
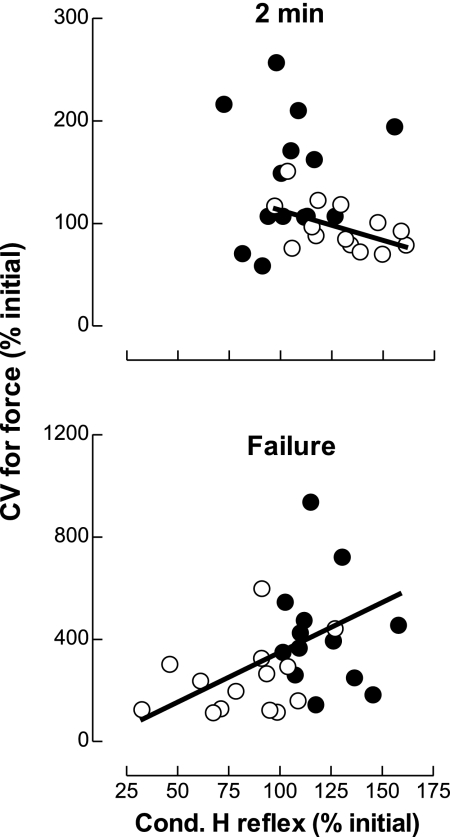
Associations Between Changes in the Amplitude of the Conditioned H Reflex and the Coefficient of Variation for Force
As the conditioned H reflexes from the two methods did not change similarly for the force and position tasks, correlations were first calculated for each task and then for the pooled data (force and position tasks). The latter approach was performed to underscore the effect of time on the association between the coefficient of variation for force and the modulation of Ia afferents within each task and across time. The change in the coefficient of variation for force was not associated with the change in the amplitude of the conditioned H reflex at 2 min after the beginning for the force task (r2 = 0.016, P = 0.67). In contrast, there was a trend for a negative association at the same time point during the position task (r2 = 0.27, P = 0.057) with a greater change in the amplitude of the conditioned H reflex being related to a smaller increase in the coefficient of variation for force (Fig. 6, top). At failure, however, a positive association (r2 = 0.19, P = 0.022) was found (pooled force and position tasks) between the change in the conditioned H reflex and the coefficient of variation for force (Fig. 6, bottom).
Fig. 6.
Relation between the change in the coefficient of variation (CV) for the force exerted by the hand and the change in the amplitude of the conditioned H reflex. Top: the change in the amplitude of the conditioned H reflex (D1 inhibition and heteronymous facilitation pooled) was negatively associated with the change in the CV for force after 2 min (r2 = 0.27, P = 0.0472) for the position task (○) but not for the force task (●). Bottom: at failure, the change in the conditioned H reflex was positively associated (r2 = 0.19, P = 0.022) with the change in the CV for force (force and position tasks pooled together).
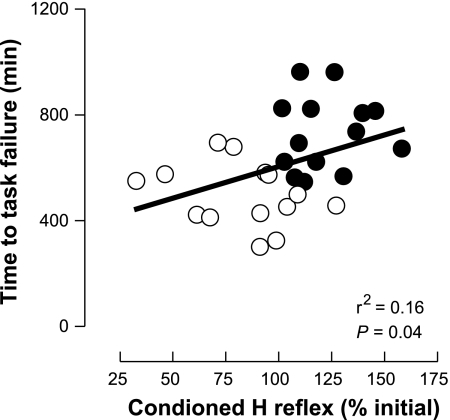
When the percent change in the conditioned H reflex (pooled D1 inhibition and heteronymous Ia facilitation) was plotted against the time to task failure (pooled force and position tasks), there was a moderate but significant relation (r2 = 0.16, P = 0.035), indicating that longer times to task failure were associated with greater increases in the amplitude of the conditioned H reflex (Fig. 7).
Fig. 7.
Relation between the time to task failure and the change in the amplitude of the conditioned H reflex. The time to task failure in the force (●) and position (○) tasks was modestly associated with the extent of change in the amplitude of the conditioned H reflex.
DISCUSSION
The key findings of the study were that the time course of the change in the amplitude of the conditioned H reflex differed during the two fatiguing contractions and was related to both the time to task failure and changes in force fluctuations. The results extend previous work on the influence of the task on presynaptic inhibition of group I afferents by demonstrating differences in the modulation of Ia afferent input across time between two types of fatiguing contractions.
Methodological Considerations
The goal of this study was to estimate the modulation of presynaptic inhibition of Ia afferents converging onto the ECR motor neuron pool when young adults performed fatiguing contractions with the wrist extensor muscles. The modulation was compared when participants either pushed against a rigid restraint to match a target force or maintained a constant wrist angle while supporting an inertial load equivalent to the force exerted during the force task. The change in presynaptic inhibition of group I afferents was assessed using two methods based on conditioning of H reflexes with the assumption that a change in the level of presynaptic inhibition should induce similar changes in the amplitude of the conditioned H reflexes recorded with the two methods (Aymard et al. 2001; Baudry and Enoka 2009; Baudry et al. 2010). The similar changes obtained with the two methods during the two tasks suggests a similar mechanism was responsible for the changes, namely, presynaptic inhibition of Ia afferents. Nevertheless, it is not possible to exclude a potential contribution from the Ib afferent pathway when assessing changes in presynaptic inhibition with the compound H reflex. Further work is needed to investigate modulation of Ib afferents during the force and position tasks.
In addition, there are at least three methodological issues that could confound the interpretation of the present results. First, the sensitivity of the monosynaptic reflex to excitatory and inhibitory inputs, which depends on the size of the reflex (Crone et al. 1990), was controlled by adjusting the intensity of the stimulation to standardize the amplitude of the test H reflex (relative to its corresponding Mmax) at the beginning of the two fatiguing contractions. Second, weak stimulation of cutaneous afferents from the hand depresses the amount of presynaptic inhibition exerted on the Ia afferents converging onto the ECR motor neuron pool (Aimonetti et al. 1999). In our experimental conditions, only the back of the hand was in contact with the device, and this was similar contact during the force and position tasks. It is unlikely, therefore, that the activation of the cutaneous differed between the two tasks. Moreover, the electrical pulses used to evoke H reflexes and conditioning stimulations were within the same range of intensities for the two tasks, minimizing the potential influence of those stimulations on the difference observed between the two tasks due to cutaneous afferent activation. Third, the absence of a change in the amplitude of the H reflex throughout the tasks despite an increase in ECR EMG activity suggests a change in the recruitment gain of the reflex that would have influenced the input-output relation of the motor neuron pool (Kernell and Hultborn 1990). Such an effect, however, would produce opposing changes in the amplitudes of the two conditioned H reflexes during the same task (Pierrot-Deseilligny 1997) rather than the similar changes observed in the present study. Although the methods used in the present study do have limitations (Pierrot-Deseilligny 1997), the similar changes in the amplitude of the conditioned H reflexes with the two conditioning methods likely reflect differences in the modulation of Ia presynaptic inhibition during the force and position tasks.
Time to Task Failure
The difference in time to failure between the two tasks (∼34%) was in the range of those reported for other upper limb muscles: the first dorsal interosseus [∼40% (Maluf et al. 2005)] and elbow flexor muscles [∼22–40% (Baudry et al. 2009; Klass et al. 2008; Rudroff et al. 2007)]. Furthermore, the similar decrease in MVC force at task failure (−26% and −29% for the force and position task, respectively) was also within the range of values observed for the first dorsal interosseus [∼40% (Maluf et al. 2005)], elbow flexor muscles [∼22–40% (Rudroff et al. 2007; Klass et al. 2008)], and ankle dorsiflexors [∼30% (Yoon et al. 2009)]. The briefer time to failure for the position task, however, suggests that the mechanisms underlying the decrease in force capacity developed more rapidly in this task compared with the force task. Moreover, the positive linear relation between the times to failure for the two tasks indicates that the neural adjustments underlying the briefer time to task failure for the position task may represent a general feature of the neural control of limb position during sustained efforts, independent of the endurance capacity of individuals.
Test H Reflexes During Force and Position Tasks
The H reflex has been used for several decades to investigate the input-output relations of the reflex loop when individuals perform various motor tasks (Schieppati 1987). The amplitude of the H reflex depends on 1) the Ia excitatory input onto motor neurons that can be altered at a presynaptic level by the PAD interneuron network or postactivation depression of Ia afferents, 2) the excitability of the motor neuron pool that can be influenced by descending drive as well as postsynaptic excitatory and inhibitory spinal inputs, and 3) the integrity of the neuromuscular junction (for s review, see Misiaszek 2003). The H reflex amplitude during sustained contractions of various muscles has been shown to increase (Löscher et al. 1996; Patikas et al. 2006), decrease (Klass et al. 2008), or remain unchanged (Cresswell and Löscher 2000; Martin et al. 2007). These various changes in H reflex amplitude are likely the consequence of different adjustments due to varying experimental conditions (muscle studied, amplitude of the initial H reflex, and intensity of the contraction), although Ia presynaptic inhibition has often been suggested to be responsible for such modulation of the H reflex amplitude.
In the present study, the amplitude of the H reflex did not change during the force and position tasks when they were sustained to failure. The absence of a change in the amplitude of the associated Mmax rules out any depression or potentiation at the neuromuscular junction, unless both occurred concurrently. In contrast, the absence of a change in amplitude of the test H reflex while the conditioned H reflex varied differently for the two tasks suggests that the nervous system adjusted the descending and peripheral inputs in a task- and time-dependent manner to control the net synaptic input received by the motor neuron pool. This interpretation is consistent with previous studies showing different motor unit discharge rates and recruitment thresholds during the two tasks (Baudry et al. 2009b; Mottram et al. 2005; Rudroff et al. 2010).
One potential source of modulation for spinal pathways involving the upper arm is a propriospinal pathway. Although its existence in humans is uncertain, the cervical propriospinal system is presumed to provide an oligosynaptic relay conveying descending and peripheral inputs to the motor neuron pools of upper limb muscles (Burke and Pierrot-Deseilligny 2005). As the cervical propriospinal system can modulate the size of the H reflex (Burke et al. 1992), some of the H reflex modulation in the present study may have involved changes in the activation of propriospinal neurons. Moreover, changes in presynaptic inhibition of group I afferents converging onto propriospinal interneurons may have influenced the contribution of these interneurons in the net synaptic input received by the motor neurons (Burke et al. 1992).
Task- and Time-Dependent Modulation of Presynaptic Inhibition
The relative changes in Ia afferent presynaptic inhibition at task failure differed between the two tasks. As the net muscle force and EMG activity were similar at failure for the two tasks, the different changes in presynaptic inhibition indicate a qualitative difference in the synaptic input received by the motor neuron pool during the two tasks. The gradual reduction in presynaptic inhibition during the force task may have contributed to the greater time to task failure for this task compared with the position task. This interpretation is consistent with the observation that decreasing the peripheral input from large diameter afferent fibers reduced the time to task failure for the position task (Mottram et al. 2006) and the observed positive relation between time to task failure and the amplitude of the conditioned H reflex found in the present study. Moreover, reductions in Ia afferent input decrease the motor unit discharge rate (Macefield et al. 1993) and may be responsible for the faster recruitment of the motor unit pool during the position task (Baudry et al. 2009b; Mottram et al. 2005). Furthermore, the absence of a difference in the time to failure for the two tasks when the target force exceeded the upper limit of motor unit recruitment (Maluf et al. 2005; Rudroff et al. 2010) suggests that the capacity to modulate the rate of motor unit recruitment contributes to differences in the time to failure for these types of tasks. Nonetheless, the strength of these associations between changes in presynaptic inhibition and the time to task failure observed in the present study suggests that other mechanisms also contribute to the difference in the time to failure for the two tasks.
Given that a reduction in Ia presynaptic inhibition likely delays task failure, it is curious why such a strategy is not used during the position task. A possible explanation involves the management of the fluctuations in motor output to meet the criterion of maintaining a constant limb position. The coefficient of variation for force was used as an index of the fluctuations in motor output during both tasks. At 2 min into the position task, but not the force task, there was a trend for lower coefficients of variation for force to be associated with greater increases in the amplitude of the conditioned H reflex (Fig. 6, top), which suggests that greater Ia input improved the control of the fluctuations in motor output at the beginning of the position task. By task failure, however, lower coefficients of variation for force were associated with decreases in the amplitude of the conditioned H reflex (Fig. 6, bottom). In the more compliant position task, such a depression of Ia input would have reduced reflex-mediated joint oscillations (Stein and Oguztoreli 1976) that would have induced joint angular fluctuations larger than the criterion used for task failure but would have hastened task failure. Consistent with this interpretation, muscle tremor was reduced during voluntary contractions when input from large-fiber afferents was suppressed (Cresswell and Löscher 2000). The change in the relation between the amplitude of the conditioned H reflex and mechanical fluctuations from 2 min to task failure suggests that the strategy used to correct deviations from the target position changed from short-latency responses to corrective reactions (Matthews and Stein 1969). By comparing the modulation of short- and long-latency responses during the two fatiguing contractions it would be possible to assess this hypothesis.
In conclusion, the present findings are the first to demonstrate that Ia presynaptic inhibition is modulated during sustained contractions and that the time course of the modulation differs with load compliance as a strategy to manage the fluctuations in mechanical output. The greater modulation with the more compliant load, however, reduces the duration that the fatiguing contraction could be sustained.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-043275 (to R. M. Enoka).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Aimonetti JM, Schmied A, Vedel JP, Pagni S. Ia presynaptic inhibition in human wrist extensor muscles: effects of motor task and cutaneous afferent activity. J Physiol 93: 395–401, 1999 [DOI] [PubMed] [Google Scholar]
- Akazawa K, Milner TE, Stein RB. Modulation of reflex EMG and stiffness in response to stretch of human finger muscle. J Neurophysiol 49: 16–27, 1983 [DOI] [PubMed] [Google Scholar]
- Aymard C, Baret M, Katz R, Lafitte C, Pénicaud A, Raoul S. Modulation of presynaptic inhibition of la afferents during voluntary wrist flexion and extension in man. Exp Brain Res 137: 127–131, 2001 [DOI] [PubMed] [Google Scholar]
- Baudry S, Enoka RM. Influence of load type on presynaptic modulation of Ia afferent input onto two synergist muscles. Exp Brain Res 199: 83–88, 2009 [DOI] [PubMed] [Google Scholar]
- Baudry S, Jordan K, Enoka RM. Heteronymous reflex responses in a hand muscle when maintaining constant finger force or position at different contraction intensities. Clin Neurophysiol 120: 210–217, 2009 [DOI] [PubMed] [Google Scholar]
- Baudry S, Rudroff T, Pierpont LA, Enoka RM. Load type influences motor unit recruitment in biceps brachii during a sustained contraction. J Neurophysiol 102: 1725–1735, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry S, Maerz AH, Enoka RM. Presynaptic modulation of Ia afferents in young and old adults when performing force and position control. J Neurophysiol 103: 623–631, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry S, Maerz AH, Gould JR, Enoka RM. Control of Limb Position During a Fatiguing Contraction Involves a Unique Modulation of Ia Presynaptic Inhibition. Paris: Motoneuron Meeting, 2010 [Google Scholar]
- Berardelli A, Day BL, Marsden CD, Rothwell JC. Evidence favouring presynaptic inhibition between antagonist muscle afferents in the human forearm. J Physiol 391: 71–83, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Adams RW, Skuse NF. The effect of voluntary contraction on the H reflex of various muscles. Brain 112: 417–433, 1989 [DOI] [PubMed] [Google Scholar]
- Burke D, Gracies JM, Mazevet D, Pierrot-Deseilligny E. Convergence of descending and various peripheral inputs onto common propriospinal-like neurons in man. J Physiol 449: 655–671, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell AG, Löscher WN. Significance of peripheral afferent input to the alpha-motoneurone pool for enhancement of tremor during an isometric fatiguing contraction. Eur J Appl Physiol 82: 129–136, 2000 [DOI] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Mazières L, Morin C, Nielsen J, Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res 81: 35–45, 1990 [DOI] [PubMed] [Google Scholar]
- Doemges F, Rack PM. Task-dependent changes in the response of human wrist joints to mechanical disturbance. J Physiol 447: 575–585, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, Baudry S, Rudroff T, Farina D, Klass M, Duchateau J. Unraveling the neurophysiology of muscle fatigue. J Electromyogr Kinesiol 21: 208–219, 2011 [DOI] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res 108: 450–462, 1996 [DOI] [PubMed] [Google Scholar]
- Hunter SK, Ryan DL, Ortega JD, Enoka RM. Task differences with the same load torque alter the endurance time of submaximal fatiguing contractions in humans. J Neurophysiol 88: 3087–3096, 2002 [DOI] [PubMed] [Google Scholar]
- Kernell D, Hultborn H. Synaptic effects on recruitment gain: a mechanism of importance for the input-output relations of motoneurone pools? Brain Res 507: 176–179, 1990 [DOI] [PubMed] [Google Scholar]
- Klass M, Lévénez M, Enoka RM, Duchateau J. Spinal mechanisms contribute to differences in the time to failure of submaximal fatiguing contractions. J Neurophysiol 99: 1096–1104, 2008 [DOI] [PubMed] [Google Scholar]
- Löscher WN, Cresswell AG, Thorstensson A. Excitatory drive to the α-motoneuron pool during a fatiguing submaximal contraction in man. J Physiol 491: 271–280, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Gandevia SC, Bigland-Ritchie B, Gorman RB, Burke D. The firing rates of human motoneurones voluntarily activated in the absence of muscle afferent feedback. J Physiol 471: 411–427, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maluf KS, Shinohara M, Stephenson JL, Enoka RM. Muscle activation and time to task failure differ with load type and contraction intensity for a human hand muscle. Exp Brain Res 167: 1–13, 2005 [DOI] [PubMed] [Google Scholar]
- Maluf KS, Barry BK, Riley ZA, Enoka RM. Reflex responsiveness of a human hand muscle when controlling isometric force and joint position. Clin Neurophysiol 18: 2063–2071, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Gandevia SC, Taylor JL. Muscle fatigue changes cutaneous suppression of propriospinal drive to human upper limb muscles. J Physiol 580: 211–223, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiaszek JE. The H-reflex as a tool in neurophysiology: its limitations and uses inunderstanding nervous system function. Muscle Nerve 28: 144–160, 2003 [DOI] [PubMed] [Google Scholar]
- Mottram CJ, Hunter SK, Rochette L, Anderson MK, Enoka RM. Time to task failure varies with the gain of the feedback signal for women, but not for men. Exp Brain Res 174: 575–587, 2006 [DOI] [PubMed] [Google Scholar]
- Mottram CJ, Jakobi JM, Semmler JG, Enoka RM. Motor-unit activity differs with load type during a fatiguing contraction. J Neurophysiol 93: 1381–1392, 2005 [DOI] [PubMed] [Google Scholar]
- Patikas DA, Bassa H, Kotzamanidis C. Changes in the reflex excitability during and after a sustained, low-intensity muscle contraction. Int J Sports Med 27: 124–130, 2006 [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of Ia afferents during movement in humans. J Neurosci Methods 74: 189–199, 1997 [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Mazevet D. The monosynaptic reflex: a tool to investigate motor control in humans. Interest and limits. Neurophysiol Clin 30: 67–80, 2000 [DOI] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res 129: 1–37, 1999 [DOI] [PubMed] [Google Scholar]
- Rudroff T, Barry BK, Stone AL, Barry CJ, Enoka RM. Accessory muscle activity contributes to the variation in time to task failure for different arm postures and loads. J Appl Physiol 102: 1000–1006, 2007 [DOI] [PubMed] [Google Scholar]
- Rudroff T, Jordan K, Enoka JA, Matthews SD, Baudry S, Enoka RM. Discharge of biceps brachii motor units is modulated by load compliance and forearm posture. Exp Brain Res 202: 111–120, 2010 [DOI] [PubMed] [Google Scholar]
- Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol 28: 345–376, 1987 [DOI] [PubMed] [Google Scholar]
- Stein RB, Thompson AK. Muscle reflexes in motion: how, what, and why? Exerc Sport Sci Rev 34:145–153, 2006 [DOI] [PubMed] [Google Scholar]
- Stein RB, Estabrooks KL, McGie S, Roth MJ, Jones KE. Quantifying the effects of voluntary contraction and inter-stimulus interval on the human soleus H-reflex. Exp Brain Res 182: 309–319, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RB, Og̃uztöreli MN. Tremor and other oscillations in neuromuscular systems. Biol Cybern 22: 147–157, 1976 [DOI] [PubMed] [Google Scholar]
- Yoon T, Hawe R, Hunter SK. Variation in limb support influences the time to task failure for a postural contraction. J Mot Behav 41: 393–395, 2009 [DOI] [PubMed] [Google Scholar]