Abstract
Motor control is critical in daily life as well as in artistic and athletic performance and thus is the subject of intense interest in neuroscience. Mouse models of movement disorders have proven valuable for many aspects of investigation, but adequate methods for analyzing complex motor control in mouse models have not been fully established. Here, we report the development of a novel running-wheel system that can be used to evoke simple and complex stepping patterns in mice. The stepping patterns are controlled by spatially organized pegs, which serve as footholds that can be arranged in adjustable, ladder-like configurations. The mice run as they drink water from a spout, providing reward, while the wheel turns at a constant speed. The stepping patterns of the mice can thus be controlled not only spatially, but also temporally. A voltage sensor to detect paw touches is attached to each peg, allowing precise registration of footfalls. We show that this device can be used to analyze patterns of complex motor coordination in mice. We further demonstrate that it is possible to measure patterns of neural activity with chronically implanted tetrodes as the mice engage in vigorous running bouts. We suggest that this instrumented multipeg running wheel (which we name the Step-Wheel System) can serve as an important tool in analyzing motor control and motor learning in mice.
Keywords: motor control, locomotion, sequences of actions, timing and rhythm
motor control typically involves not only the performance of particular movements, but also coordination of the timing of these movements. Disturbance of such timing control often appears in neurological disorders (Freeman et al. 1993, 1996; Ivry 1996; Keele 1968; Nagasaki et al. 1978; Nakamura et al. 1978; Schubotz et al. 2000). Animal models of such disorders, and, in particular, mouse genetic models, have increasingly been developed to study the neurobiology underlying motor control (Hansson et al. 1999; Kahle et al. 2000). A number of systems have been developed to quantify the motor status of such mouse models, including the rotarod and balance-beam tests (e.g., Carter et al. 1999; Chapillon et al. 1998; Jones and Roberts 1968; Sango et al. 1995, 1996) for analyzing balance and motor coordination, and gait analysis methods, such as the “Catwalk” system (e.g., Masocha and Pavarthy 2009) and other footprint analysis methods (e.g., Barlow et al. 1996; Carter et al. 1999; Crawley and Paylor 1997), and the horizontal ladder paradigm (Seeds et al. 2003; Van Der Giessen et al. 2008). The study of motor timing and its neural basis in these models has been challenging, however, because of paucity of motor tasks that permit recording of continuous motor activity with precise timing measurements combined with recordings of ongoing neural activity. Our goal in this work was to address this problem by developing a sensitive tool for analyzing locomotor patterns in the mouse.
To achieve this end, we designed a running wheel with a configurable running surface made of multiple pegs fitted with instrumentation to monitor the individual footfalls of the mice. By using a water reward obtainable by running at the speed of the motor-driven wheel, we were able to control the running rate and position of the mouse. By introducing flexible ladder-like floor patterns, we were able to impose patterns of gait. Finally, by designing the system to be compatible with long-term recording with electrodes chronically implanted in the brain, we were able to combine the analysis of motor activity with the analysis of concurrent neural activity. This flexible Step-Wheel System allows detailed analysis of the locomotor coordination of mice and opens the way to analyses of motor learning.
METHODS
Animals.
All procedures were approved by the Committee on Animal Care of the Massachusetts Institute of Technology, the Committee on Animal Care of Frontier Biosciences of the Osaka University, and the Institutional Animal Care and Use Committee of the National Institute for Basic Biology and performed in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals. ICR and C57BL/6 mice (10–15 wk old) were studied. These strains were selected because we conducted the initial test of the Step-Wheel System with the strain most often used for genetically engineered mouse models of neurodegenerative diseases (C57BL/6) and the one with a large body size (ICR). We used these two mouse strains with different body sizes to test whether the size of the Step-Wheel and distances between pegs on which they locomote are suitable for both strains. The mice were housed individually in their home cages to control their daily water intake and allowed to access freely to dry pellet food. The mice were on a water restriction schedule from a day before the initial training session to the end of the experimental periods. During this period, they were allowed to drink water in the wheel during and after the training session. Additional water was given in their home cages as needed, so that they received 3 ml of water each day. They were given free access to water for an entire day every 1–2 wk during training. Ad libitum water was also given if their weight fell to <80% of its initial weight.
For the duration of water restriction, health condition of all mice was monitored closely by the experimenter and animal care staff. Daily records were kept for skin tent duration, food intake, fur condition, body temperature, and spontaneous home-cage behaviors. These measures did not show any indication of excessive dehydration or abnormal stress associated with water deprivation. Despite being on water deprivation, none of the mice exhibited behaviors related to high levels of stress.
The Step-Wheel System configuration.
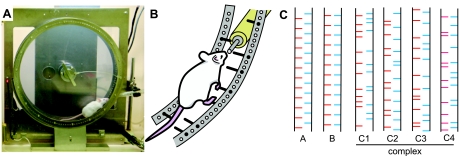
The Step-Wheel was fabricated by O'Hara (Tokyo, Japan). The wheel is formed by two parallel plastic circular discs, 30 cm in diameter, 5.5 cm apart (Fig. 1A). Stainless steel pegs inserted into holes along the circumference of each disc serve as footholds for the mouse. An external motor drives the wheel so that the mouse has to run on the pegs at roughly the speed chosen by the experimenter. The turning speed of the wheel was chosen so that the mouse could drink water while running (see Training procedures). Figure 1, A and B, illustrates the typical position of a mouse running in the Step-Wheel. The pegs (2-mm diameter) extend inward ∼2.5 cm from the wheel walls (Fig. 1, B and C). Each peg is connected to a voltage generator. A spout, from which the mouse could drink water, is inserted into the wheel through the space between the left and right pegs so as not to interfere with the turning of wheel (Fig. 1B). Water is delivered naturally by gravity when the mouse contacts the spout with its mouth. The water-deprived mice learned to run so as to drink nearly continuously by licking the spout.
Fig. 1.
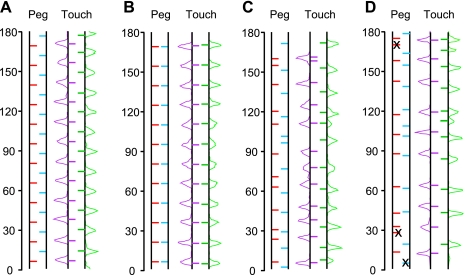
The wheel and peg pattern. A: photograph of the running wheel with a mouse actively running to obtain water at the water spout protruding between the right and left pegs. The wheel was turning clockwise, driven by a motor set behind the wheel. The mouse was drinking water while running. The photograph was taken with the touch sensor detached from the wheel to allow viewing of the mouse (see Fig. 3D for an illustration of the wheel with the sensor attached). B: schematic drawing showing a running mouse drinking water from the spout. C: peg patterns used in this study.
Altogether 144 peg holes were available on each disc, allowing pegs to be inserted in different configurations to evoke different footfall patterns during running. We organized the footholds by constructing a series of peg patterns composed of 24 pegs, 12 on the right and 12 on the left. This configuration allowed a single peg pattern to be repeated twice in 1 turn of the Step-Wheel. Running through all 24 pegs in a pattern was considered as 1 trial. Patterns could be maintained over days or changed across daily sessions. Figure 1C illustrates examples of the peg patterns used.
Training procedures.
Mice were pretrained by placing them in the stationary Step-Wheel for ∼20 min. During this period, the mice could find the waterspout and drink water ad libitum. After an average of 2 days of this habituation, training sessions were given with the Step-Wheel turning at speeds low enough (e.g., 1 turn in 3 min) to allow the mice to drink water in more than half of the sessions, which lasted 5–20 min depending on the speed. Wheel rotation was immediately stopped either manually when the mouse was detached from the water spout during the 1st wk of training or automatically when the mouse moved away from the water spout and broke the photobeam b during the rest of training (see Behavioral monitoring). The speed was gradually increased over a period of 3–5 wk, ensuring that the mice could drink water also as the speed of the wheel rotation was increased up to 6–10 turns/min. Nearly all mice (>90%) learned and performed the Step-Wheel task consistently over the course of training.
Behavioral monitoring.
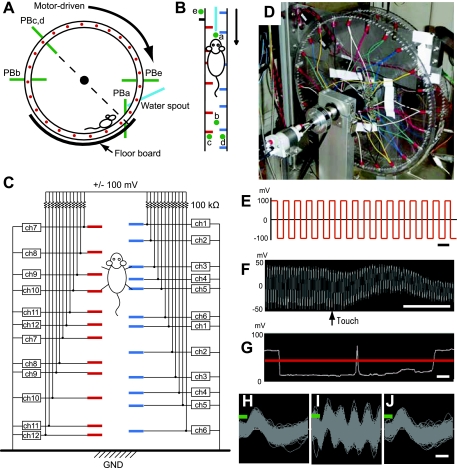
To analyze the movements of the mice more thoroughly, we monitored both the position of the mouse in the wheel and the time of each footfall on each peg touched. To monitor the location of the mouse and pegs, we used five sets of infrared photobeam units (Fig. 3, A and B). Photobeam a detected the mouse when it was at the spout. Photobeam b detected the mouse when it was at the intersection point of the horizontal diameter of the wheel; if this photobeam was interrupted, the wheel automatically stopped for the safety of the mouse.
Fig. 3.
Instrumentation for monitoring behavior and peg patterns. A and B: scheme showing infrared photobeam system. Photobeams are shown in pink. Photobeam a (PBa) was used for detecting a mouse in close vicinity of the spout; photobeam b (PBb) for detecting the mouse if it was on the other side of the wheel; photobeams c and d (PBc and PBd) for detecting pegs on the left and right, respectively; and photobeam e (PBe) for detecting turn markers, which were used as markers of the initiation of a peg pattern. C: diagram illustrating the voltage sensor. Pegs were independently connected to the voltage sensor. ch, Channel; GND, ground. D: photograph of the wheel with the sensor attached. E: applied voltage schedule with 20-kHz square waves of ±100 mV loaded onto each peg. Scale bar, 50 μs. F: an example of recorded voltage. When the peg was touched, the amplitude was reduced. Scale bar, 5 ms. G: the voltage amplitude is shown as a white trace. A drop in amplitude below the chosen threshold (red line) was counted as a touch. Two touches were counted in this trace. Scale bar, 50 ms. H–J: waveforms of recorded voltage signals that exceeded the threshold for spike detection (green line) after being filtered to remove oscillations above 6 kHz, recorded while the touch sensor was turned off (I), turned on at 5 kHz (J), and turned on at 20 kHz (K). Scale bar, 200 μs.
Photobeams c and d monitored, respectively, the passage of the right and left floor pegs (Fig. 3, A and B). Finally, photobeam e, which was placed outside of the wheel and was interrupted by two rods protruding from the wheel, monitored wheel rotation. The rods were set 180° apart from each other on the wheel. The interruption of photobeam e by a turn marker, a small rod attached outside of the wheel, was used to time stamp the onset of a given peg pattern, because each single peg pattern appeared twice along the 360° circumference of the wheel.
The voltage sensor was controlled and monitored through a computer board, PXI-6229 (National Instruments), by custom software developed with LabVIEW (National Instruments). The photobeam sensors were monitored with the same board and software as the voltage sensor. Time stamps for these events were stored in a computer and could be coordinated with the simultaneously recorded neural data. All paw-touch analyses were conducted with custom programs made with MATLAB (MathWorks, Natick, MA).
For gait analysis, video images affording a bottom-up view of the running mice (n = 4) were captured with a charge-coupled device (CCD) camera (LCL-660A; Watec America) located under the spout at 30 frames/s and were monitored with a computer through a computer board, PXI-1409 (National Instruments). When video images were to be captured, a transparent floorboard was used (Fig. 3A). Otherwise, a nontransparent steel floorboard was used. The captured images were analyzed with a custom program made with LabVIEW and MATLAB.
Electrophysiology.
Recordings were made in ICR mice. Before the experiment, a headstage containing four or six tetrodes was implanted on the mouse. The headstage was specifically designed for rodents, as described elsewhere (Jog et al. 2002; Kubota et al. 2009). The fabrication of the headstage and methods for data collection were as described in Kubota et al. (2009). Briefly, tetrodes were made of twisted 10-μm NiCr wire and loaded in the headstage that allowed independent advancement of each tetrode.
For implantation of the tetrode headstage, mice were deeply anesthetized with either sodium pentobarbital (50 mg/kg) or ketamine hydrochloride (100 mg/kg) and xylazine (20 mg/kg). The skin overlying the skull was incised, and a small (∼1 mm in diameter) opening in the skull was made under stereotaxic guidance with a dental drill at coordinates corresponding to the left primary motor cortex (anteroposterior = +0.2 mm, mediolateral = +1.2 mm), and the dura mater was incised to allow penetration of the tetrodes. Dental acrylic was used to affix the headstage to the skull, into which jeweler's screws were placed for stabilization. Tetrodes were lowered gradually into the neocortex (0.5–1 mm below the brain surface) during the first postoperative week. On each day, tetrodes were advanced in small steps as neuronal activity was continuously monitored. This procedure was followed to ensure high-quality unit activity could be recorded during the course of long-term behavioral training.
A 16- or 32-channel preamplifier (Neuralynx, Bozeman, MT) was connected to the headstage during neuronal recording. The data were sent to 8-channel programmable amplifiers (gain: 10,000, filter: 0.6–6 kHz) and were displayed and stored with a Cheetah Data Acquisition System (Neuralynx). To coordinate time stamps of neural data with data from the photobeams and voltage sensors, the transistor-transistor logic signals for start and stop of acquisition of sensor data were sent to the Cheetah Data Acquisition System. Recorded unit activity containing the spikes of multiple neurons was sorted manually, offline, to obtain putative single units according to spike parameters such as peak height and valley depth, using the SpikeSort3D (Neuralynx).
RESULTS
Behavioral performance in the Step-Wheel.
We found that within 20 min, a water-deprived mouse placed in the stationary wheel with a given peg pattern could find the water spout and begin to drink. Mice were trained to run with incremental increases in wheel speed. Final speeds of 6–10 turns/min were reached in 3–5 wk, after which the speed of the wheel was maintained. After the final speed was reached, the mice were trained >5 days before the 1st change of peg patterns. All data reported here were obtained from mice trained for more than a week with a given peg pattern.
Video files of side and bottom views of mice running in the Step-Wheel are available in the Supplemental Video (in the data supplement online at the Journal of Neurophysiology web site).
Video analysis.
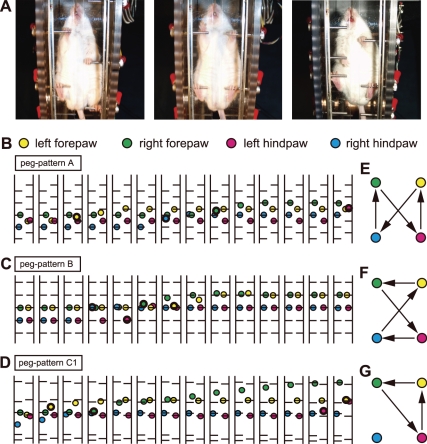
To monitor the pattern of locomotion in the Step-Wheel, bottom-view video images of 4 running mice were captured, and frame-by-frame tracings of paw position were made. Figure 2 shows examples of bottom-view images (Fig. 2A) and the paw positions (Fig. 2, B–D) of mice running on the peg patterns A, B, and C1 shown in Fig. 1C. When a paw was on a peg in a given video frame and was not on the peg in the next frame, the paw was considered to have been lifted between the frame times, and the position of the paw in the 2nd frame was shown as circles with thick contours (Fig. 2, B–D). The sequences of paw detachment from pegs in Fig. 2, B–D, are illustrated in Fig. 2, E–G. The timing of detachment was not always determined exactly in each cycle because of the nature of the bottom view, in which the contact face between a peg and a paw was always on the side opposite to the camera, and also because of a slow frame rate of the video (>1 paw often detached in a frame, and consequently the order of the detachments was not always determined). Nevertheless, 100 continuous cycles of the 4 limbs in peg patterns A and B were traced, and the sequences of detachments of the 4 paws were analyzed.
Fig. 2.
Video and gait analyses of locomotor patterns observed during running in the Step-Wheel. A: bottom-view photographs of mice running in the wheel while drinking. The mouse is shown running, from left to right, on peg patterns A, B, and C1 shown in Fig. 1C. B–D: example of paw positions of 14 consecutive frames in peg pattern A (B), peg pattern B (C), and complex peg pattern C1 (D). The positions of left forepaw, right forepaw, left hindpaw, and right hindpaw are shown in yellow, green, red, and blue circles, respectively. The circles with thick contour represent the positions of paws just detached from a peg (the 1st frame in a swing phase). E–G: representative gait patterns during performance of peg pattern A (E) and peg pattern B (F) and 1 of the gait patterns observed in complex peg pattern C1 (G). The sequence in which the paws departed from pegs is indicated by arrows.
In peg pattern A, the sequence of paw lifts in all cycles in which the sequence of detachments could be exactly analyzed was that shown in Fig. 2E, which is the typical gait sequence for the walk pattern. In peg pattern B, the sequence of detachments in all cycles in which the sequence of detachments could be exactly analyzed was that shown in Fig. 2F, which is typical gait pattern for the gallop. The paw sequence observed in a complex pattern (Fig. 2D) was different from the typical gait patterns shown in Fig. 2, E and F. The gait patterns for the complex patterns were mixtures of the walk, gallop, and other nontypical gait patterns. Although other nontypical gait patterns were observed in mice running in complex patterns, precise gait analysis of complex patterns should await future video analysis with a higher frame rate camera.
Sensors.
To monitor individual footfalls, we applied a 20-kHz square-wave voltage with an amplitude of ±100 mV to each peg (Fig. 3, C–E) and used a voltage sensor to detect changes in amplitude of the applied voltage produced by each footfall (Fig. 3, F and G). The amplitude was reduced when the mouse touched the peg (Fig. 3, F and G). The frequency of the square-wave voltage was determined as 20 kHz because any potential effect of this square-wave signal on neuronal recording signals could be cut off by using the high-cut filter (6 kHz) in the Neuralynx amplifier. We found that the spike waveforms were not affected by the 20-kHz voltage signal, although a 5-kHz test-wave voltage signal at the same amplitude caused extensive noise (Fig. 3, H–J). Pilot experiments indicated that the applied voltage did not lead to aversive behavioral responses. Mice presented in their home cages with the choice of obtaining water from a spout with applied voltage or another without the applied voltage responded to the two equally.
The voltage data were monitored and recorded with a custom program. Twelve independent sensor lines were used to monitor the 48 pegs on the wheel, so that each sensor line was assigned to 4 pegs (Fig. 3C). No 2 pegs connected to the same line could ever be touched by a mouse at the same time. Because the positions of the pegs were monitored with the infrared sensors, and touches were counted only when mice were close to the spout, the pegs being touched could be identified unambiguously. For analysis of the locomotor patterns, an amplitude threshold was set to detect the paw touches (red line in Fig. 3G). A voltage drop across the threshold was counted as a paw touch. The 1st peg touch in each trial was extracted and used for analysis (Fig. 4, D–F) with custom software developed in MATLAB. Complex peg patterns were generated by choosing holes to insert pegs using a pseudorandom function that deleted peg patterns that were impossible for the mice to run (spatial gaps larger than the reach of the running mouse).
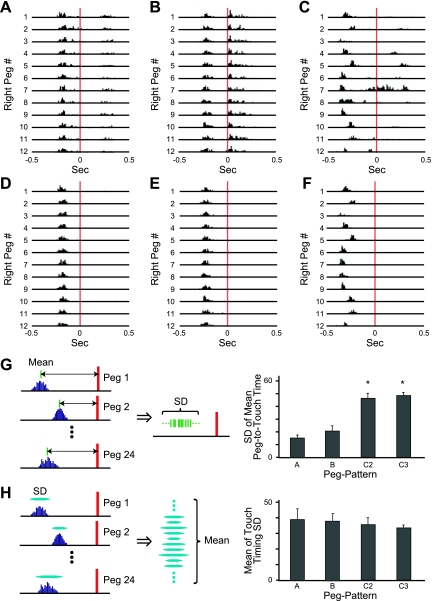
Fig. 4.
Touch analysis. A–C: all touches on each of 12 right pegs counted in a session with peg patterns A (A), B (B), and C2 (C) are shown in histograms. The touches are aligned to the time at which the corresponding peg arrived at the photobeam a location (red lines at time 0). D–F: the 1st touches in each trial of the same session as shown in A–C. G: times of peg touches, relative to peg arrival times, were averaged for all individual pegs. Then, the standard deviation of the averages over all pegs was calculated. Bottom shows standard deviations for peg patterns A, B, C2, and C3. *P < 0.001, A vs. C2, A vs. C3, B vs. C2, B vs. C3 (ANOVA and post hoc Tukey-Kramer test). H: the standard deviations of touches to a peg were calculated for all individual pegs. Then, the standard deviations were averaged over all pegs. These average standard deviations are shown for peg patterns A, B, C2, and C3.
Touch analysis.
We constructed touch histograms by monitoring the suprathreshold voltage deviations for each peg on both sides of the wheel (red horizontal line in Fig. 3G) and aligning them to the time each given peg reached the level of the photobeam a. Figure 4, A–C, illustrates typical histograms for all right touches made by mice that ran for 60 trials in, respectively, peg patterns A, B, and C2.
In most instances, the sensor detected two or three closely spaced touches (Fig. 4, A–C), presumably because the mouse ran with its hindpaw touching a peg only slightly after its forepaw touched that peg. Figure 2A, right, illustrates an instance in which the mouse grabbed a peg with its forepaw first and then grabbed the same peg with its hindpaw. From our analysis, we judge that the second peaks in such histograms probably were touches by hindpaws. Occasionally, third peaks were recorded. These might have reflected increased pressure on the pegs made by the hindlimbs just before they released the pegs, so that the area of paw touches increased, reducing the voltage amplitude.
Although the sensor thus could detect touches by both forelimb and hindlimb, we were unable to distinguish touches made by forelimb or hindlimb routinely in this system. We noted that with some peg patterns, although the hindpaw touched the peg after the forepaw, it would touch a peg while the forepaw was still on the peg. In such cases, it was impossible to detect the timing of the hindpaw touch. For these reasons, we focused on the first touch in each trial, accepting them as forepaw touches, by excluding the resting touches in the same trial. Figure 4, D–F, illustrates a typical first-touch set of detected footfalls extracted from the histogram of all touches shown in Fig. 4, A–C, respectively.
In peg patterns A and B, the timing of first touches to pegs was relatively constant for all pegs, whereas the timing of the touches varied among pegs in the more complex peg pattern, C2 (Fig. 4, D–F). The standard deviations among mean touch times for individual pegs, relative to the peg detection times (i.e., the variability of touch times among pegs), were smaller for peg patterns A and B than those for complex peg patterns C2 and C3 (F3,19 = 27.49, P < 0.001; ANOVA and post hoc Tukey-Kramer test; Fig. 4G). This result indicates that the timing of touches on individual pegs varied significantly among pegs in the complex peg patterns and that the touch timing was controlled by the positions of the pegs. Nevertheless, the average of the standard deviations of touch timing for each single peg (i.e., the variability of touch times within individual pegs) was not different across peg patterns (F3,19 = 0.35, P = 0.78; ANOVA; Fig. 4H), indicating that the timing of touches was controlled in the complex peg pattern performance as well as in performance with the simple peg patterns.
Next, the times of the touches were aligned by the turn marker to compare the pattern of touches in a trial with the peg pattern being run. The median of touch times for a given peg were calculated, and such medians for all 24 pegs were aligned by the turn marker, which was designated as the touch pattern. Figure 5 illustrates, as examples, peg patterns A, B, C2, and C3 and the corresponding touch patterns. Histograms of the touch times for the right (blue) and left (red) pegs are shown to the right and left sides of the touch patterns in green and purple, respectively. Some pegs were skipped in the complex peg pattern, C3, and such pegs are marked with an x in Fig. 5D. Because the Step-Wheel turns at a constant speed, the position of touches can be determined by the timing of touches. Thus the touch pattern can be considered as the median of paw positions at the time at which a given paw touched pegs. The peg patterns and the touch patterns were mostly identical in the simple peg patterns A and B. However, the touch patterns in complex peg patterns were not identical to their peg patterns, indicating that mice did not always follow the peg positions while they ran but instead moved their paws forward or backward to touch the pegs. The amount of the dissociation between the peg position and touch position was different from peg to peg. The mechanisms underlying how such touch positions were chosen by the mice is not clear, but this fact indicates that the positions of touches were controlled according to the peg patterns in the Step-Wheel. Therefore, the movement of paws of mice running in the Step-Wheel could be controlled both temporally and spatially by the peg pattern.
Fig. 5.
Relation between peg patterns and touch patterns. The touch patterns for peg patterns A, B, C2, and C3 are illustrated in A, B, C, and D, respectively. Peg positions and touch positions were calculated according to the time from the time of the nearest preceding turn marker. The medians of the timings of right peg, left peg, right touch, and left touch are shown as bars in blue, red, green, and purple, respectively. The span of a pattern corresponds to 180° of the wheel because a single peg pattern was repeated twice in 1 turn of the wheel. The touch pattern consists of the median touch timing to the corresponding pegs. Histograms of touch timing for the right and left pegs are illustrated, respectively, at the right and left sides of the touch patterns in green and purple traces. x, Skipped pegs.
Electrophysiological recording of spike activity during wheel running.
A critical requirement for the success of the Step-Wheel System was the ability to record neural activity during the runs. We did this by connecting lightweight cables to the preamplifier fixed to the implant on the head of the mouse and passing these cables through the space between the right and left pegs at the top of the wheel.
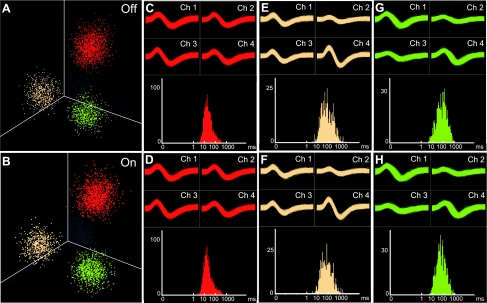
To test whether we could reliably detect neuronal activity related to task performance, we chronically implanted multiple tetrodes into the primary motor cortex, where we expected to find neurons with stepping-related activity profiles. These recordings were done in the left hemisphere in all mice so that ipsilateral and contralateral activities could be easily illustrated. Figure 6 shows examples of neural activity recorded on one of the tetrodes as a mouse ran in the Step-Wheel. We compared the neural activity recorded with the voltage sensor off (Fig. 6, A, C, E, and G) and on (Fig. 6, B, D, F, and H) to determine whether the voltage sensor interfered with our ability to record the neural signals. Recorded spike activity was sorted into putative single units (clusters) according to spike parameters such as peak height and valley depth, with manual guidance. The shapes of clusters for these putative single units were virtually identical whether the sensor was on or off (Fig. 6, A and B). The waveforms of the recordings with and without the voltage sensor active were essentially indistinguishable, demonstrating the feasibility of the in-run recordings with the paw-touch monitoring device in operation (Fig. 6, C–H, top). The interspike intervals (ISIs) of the units showed clear absolute refractory periods (Fig. 6, C–H, bottom), suggesting that contamination of spikes with noise was rare even with the voltage sensor on. This result demonstrates that potential noise produced by the voltage applied to the pegs (20 kHz) could be removed with the high-cutoff filter used for capturing the neural activity (6 kHz).
Fig. 6.
Recording of neural activity during performance in the running wheel. A, C, E, and G: unit activity recorded from 1 of the tetrodes implanted in the primary motor cortex of a mouse running in the wheel with the voltage sensor turned off. Spikes of putative single units (clusters) are shown in red, yellow, and green, and remaining spikes that were not included in these clusters are indicated by gray (A). Clusters of spikes are plotted based on spike height on channels 1–3. Spike waveforms recorded on each of the 4 tetrode channels (Ch 1–4; top) and interspike interval plots showing absolute refractory periods (bottom) are shown for the red (C), yellow (E), and green (G) units. B, D, F, and H: activity of units recorded from the same tetrode with the voltage sensor on, shown as in A, C, E, and G.
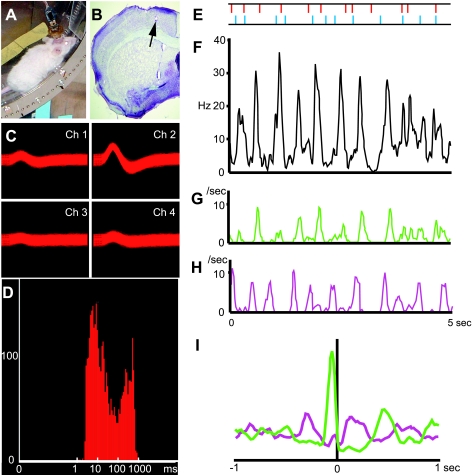
Given the sampling times that we used, touch and spike data could be obtained in the Step-Wheel System with submillisecond accuracy for the spikes and with <5-ms accuracy for the touches, allowing the analysis of the relationship between touches and spikes. Figure 7 gives an example of the spike histogram (Fig. 7F) and touch timing to right and left pegs (Fig. 7, G and H) obtained from a mouse running on the complex peg pattern C4 (Fig. 7, A and E). The spike activity was recorded from a tetrode located in the primary motor cortex of the left hemisphere (Fig. 7B). Figure 7, C and D, shows spike waveforms and ISI histogram for this putative single unit, which was one of two units identified in the activity recorded from the tetrode.
Fig. 7.
Simultaneous recordings of neuronal spike activity and paw touches from a mouse running in the Step-Wheel. A: mouse running in the wheel with a preamplifier connected to a 4-tetrode headstage. B: Nissl-stained brain section illustrating a tetrode track and its tip (arrow) in the primary motor cortex. C and D: spike waveforms (C) and interspike interval plot (D) for a putative single unit recorded. E: the peg pattern on which the mouse ran. The horizontal axes in F–H correspond to the span of this peg pattern. The right and left pegs are shown in blue and red, respectively. F: histogram of the spike activity of the unit shown in C and D. G and H: histogram of the timing of right (G) and left (H) peg touches. I: cross-correlograms of the timing of right (green) and left (purple) touches relative to all spike occurrences.
We aligned right and left paw touches to occurrences of spikes and found a prominent peak for the right paw touches ∼50 ms before spikes, indicating that this unit had a high probability to fire just after the right paw touches (Fig. 7I). These results demonstrate the feasibility of combining neural recording with precise monitoring of the timing of footfalls and detecting precise timing relationships between behavioral performance and neural activity.
DISCUSSION
There is a pressing need to obtain accurate information about motor timing in relation to neural activity in rodents, the mammals most widely used for genetic studies. To meet this need, we have developed a novel running wheel, the Step-Wheel System, which permits the simultaneous recording of locomotor patterns and neural activity in mice running on configurable patterned arrays of footholds in a running wheel. We demonstrate the properties of the wheel, the sensitivity of footfall monitoring, and the feasibility of using a voltage sensor device to record the footfalls. We outline potential uses of the Step-Wheel System for analyzing motor control patterns in the mouse. These include measurements of limb coordination during locomotion and running, locomotor adaptation to different running speeds imposed by wheel rotation, and locomotor adaptation to different patterns imposed by the pegs that provide the footholds in the wheel. Together, these features of the Step-Wheel suggest that it can be a powerful tool to analyze mouse behavior, including the behavior of mice genetically engineered for studying neurological disorders.
We point out that the system, in this first iteration, does not have a video-monitoring capability built in. This weakness can be readily overcome in future iterations of the Step-Wheel. Another potential issue is that the task requires mice to be on a water-deprivation schedule. This protocol is necessary to make mice run at a constant location in the wheel so that the running speed and timing of each footfall can be controlled. We monitored the mice carefully throughout training and did not find any indication of adverse effects on health condition or behavior of these mice. We note that we tested using sugar water as an alternative way to motivate drinking in mice without water-deprivation, but this method failed to induce running behavior consistently.
Horizontal tracks with ladder-like arrays have also been used to study the acquisition of locomotor tasks in rodents (Seeds et al. 1995, 2003; Van Der Giessen et al. 2008; Watson and McElligott 1983). Other systems are also available to analyze stepping patterns and general motor coordination of the mouse (Barlow et al. 1996; Carter et al. 1999; Chapillon et al. 1998; Crawley and Paylor 1997; Jones and Roberts 1968; Masocha and Pavarthy 2009; Sango et al. 1995, 1996). The design of the Step-Wheel that we introduce here has the advantage of accuracy of monitoring footfalls in combination with the capability of allowing ready variation of the imposed running speeds and peg-imposed sequences of footfall patterns required for successful performance of the Step-Wheel task.
A further advantage of the Step-Wheel System is proved by the water-spout arrangement. Although this feature does mean that the mice need to be water-deprived, it not only provides a motivating feature favoring extended performance by the mice, but also motivates the mice to adjust their steps with timing appropriate to contact the spout as they run. We note that the Step-Wheel System could be scaled up for use with larger animals, such as rats, also increasingly being used in genetic and circuit-manipulation studies. These characteristics allow the Step-Wheel System to have broad applicability as a novel way to study action timing in continuous sequential behaviors and open up the opportunity to investigate neural mechanisms underlying timing and rhythm in motor control and motor learning.
Theories of how motor timing is achieved have been put forth, and much evidence suggests that multiple brain regions are implicated (Buhusi and Meck 2005; Coull et al. 2011; Gibbon et al. 1997; Ivry 1996; Mauk and Buonomano 2004; Meck et al. 2008). The ability to make detailed multielectrode recordings in different sites during running in the Step-Wheel System, with the possibility of relating the recorded spike and local field potential oscillatory activity, should allow new contributions to the field. Furthermore, the ability to track the neural behavior accompanying acquisition of new gait patterns related to experimenter-chosen peg patterns in the Step-Wheel should open the possibility to study the acquisition of new neural timing patterns in relation to behavioral learning. Such combined behavioral and electrophysiological recordings should yield valuable information about motor control and motor learning in normal rodents and in rodent models of clinical disorders and should provide a new means for studying how the brain controls timing and rhythmicity in the motor system.
GRANTS
This work was supported by the Precursory Research for Embryonic Science and Technology (PRESTO) from Japan Science and Technology (JST), a Grant-in-Aid for challenging Exploratory Research from the Ministry of Education, Science, Sports, and Culture of Japan (JSPS), a Japan-U.S. Brain Research Cooperative Program Grant to T. Kitsukawa, a JSPS Grant-in-Aid for Scientific Research on Innovative Areas to T. Kitsukawa and T. Yamamori, a Grant-in-Aid for Scientific Research on Priority Areas from Ministry of Education, Culture, Sports, Science and Technology of Japan to T. Yamamori, and the National Institutes of Health Grant R01-MH-060379 to A. M. Graybiel.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Tetsuya Yagi for valuable ideas about the touch sensor and Henry Hall and Dr. Dan Hu for their help in developing the neural recording capability in combination with the Step-Wheel.
Present address of R. Tomioka: Center for Brain Research, Medical Univ. of Vienna, 1090 Vienna, Austria.
REFERENCES
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86: 159–171, 1996 [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci 6: 755–765, 2005 [DOI] [PubMed] [Google Scholar]
- Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J Neurosci 19: 3248–3257, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapillon P, Lalonde R, Jones N, Caston J. Early development of synchronized walking on the rotorod in rats: effects of training and handling. Behav Brain Res 93: 77–81, 1998 [DOI] [PubMed] [Google Scholar]
- Coull JT, Cheng RK, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology 36: 3–25, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm Behav 31: 197–211, 1997 [DOI] [PubMed] [Google Scholar]
- Freeman JS, Cody FW, O'Boyle DJ, Craufurd D, Neary D, Snowden JS. Abnormalities of motor timing in Huntington's disease. Parkinsonism Relat Disord 2: 81–93, 1996 [DOI] [PubMed] [Google Scholar]
- Freeman JS, Cody FW, Schady W. The influence of external timing cues upon the rhythm of voluntary movements in Parkinson's disease. J Neurol Neurosurg Psychiatry 56: 1078–1084, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon J, Malapani C, Dale CL, Gallistel CR. Toward a neurobiology of temporal cognition: advances and challenges. Curr Opin Neurobiol 7: 170–184, 1997 [DOI] [PubMed] [Google Scholar]
- Hansson O, Petersen A, Leist M, Nicotera P, Castilho RF, Brundin P. Transgenic mice expressing a Huntington's disease mutation are resistant to quinolinic acid-induced striatal excitotoxicity. Proc Natl Acad Sci USA 96: 8727–8732, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry RB. The representation of temporal information in perception and motor control. Curr Opin Neurobiol 6: 851–857, 1996 [DOI] [PubMed] [Google Scholar]
- Jog MS, Connolly CI, Kubota Y, Iyengar DR, Garrido L, Harlan R, Graybiel AM. Tetrode technology: advances in implantable hardware, neuroimaging, and data analysis techniques. J Neurosci Methods 117: 141–152, 2002 [DOI] [PubMed] [Google Scholar]
- Jones BJ, Roberts DJ. The quantitative measurement of motor incoordination in naive mice using an accelerating rotarod. J Pharm Pharmacol 20: 302–304, 1968 [DOI] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, Okochi M, Leimer U, van der Putten H, Probst A, Kremmer E, Kretzschmar KA, Haass C. Subcellular localization of wild-type and Parkinson's disease-associated mutant alpha-Synuclein in human and transgenic mouse brain. J Neurosci 20: 6365–6373, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele SW. Movement control in skilled motor performance. Psychol Bull 70: 387–403, 1968 [Google Scholar]
- Kubota Y, Liu J, Hu D, DeCoteau WE, Eden UT, Smith AC, Graybiel AM. Stable encoding of task structure coexists with flexible coding of task events in sensorimotor striatum. J Neurophysiol 102: 2142–2160, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masocha W, Pavarthy SS. Assessment of weight bearing changes and pharmacological antinociception in mice with LPS-induced monoarthritis using the Catwalk gait analysis system. Life Sci 85: 462–469, 2009 [DOI] [PubMed] [Google Scholar]
- Mauk MD, Buonomano DV. The neural basis of temporal processing. Annu Rev Neurosci 27: 307–340, 2004 [DOI] [PubMed] [Google Scholar]
- Meck WH, Penney TB, Pouthas V. Cortico-striatal representation of time in animals and humans. Curr Opin Neurobiol 18: 145–152, 2008 [DOI] [PubMed] [Google Scholar]
- Nagasaki H, Nakamura R, Taniguchi R. Disturbances of rhythm formation in patients with Parkinson's disease: part II. A forced oscillation model. Percept Mot Skills 46: 79–87, 1978 [DOI] [PubMed] [Google Scholar]
- Nakamura R, Nagasaki H, Narabayashi H. Disturbances of rhythm formation in patients with Parkinson's disease: part I. Characteristics of tapping response to the periodic signals. Percept Mot Skills 46: 63–75, 1978 [DOI] [PubMed] [Google Scholar]
- Sango K, McDonald MP, Crawley JN, Mack ML, Tifft CJ, Skop E, Starr CM, Hoffmann A, Sandhoff K, Suzuki K, Proia RL. Mice lacking both subunits of lysosomal beta-hexosaminidase display gangliosidosis and mucopolysaccharidosis. Nat Genet 14: 348–352, 1996 [DOI] [PubMed] [Google Scholar]
- Sango K, Yamanaka S, Hoffmann A, Okuda Y, Grinberg A, Westphal H, McDonald MP, Crawley JN, Sandhoff K, Suzuki K, Proia RL. Mouse models of Tay-Sachs and Sandhoff diseases differ in neurologic phenotype and ganglioside metabolism. Nat Genet 11: 170–176, 1995 [DOI] [PubMed] [Google Scholar]
- Schubotz RI, Friederici AD, Yves von Cramon D. Time perception and motor timing: a common cortical and subcortical basis revealed by fMRI. Neuroimage 11: 1–12, 2000 [DOI] [PubMed] [Google Scholar]
- Seeds NW, Basham ME, Ferguson JE. Absence of tissue plasminogen activator gene or activity impairs mouse cerebellar motor learning. J Neurosci 23: 7368–7375, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds NW, Williams BL, Bickford PC. Tissue plasminogen activator induction in Purkinje neurons after cerebellar motor learning. Science 270: 1992–1994, 1995 [DOI] [PubMed] [Google Scholar]
- Van Der Giessen RS, Koekkoek SK, van Dorp S, De Gruijl JR, Cupido A, Khosrovani S, Dortland B, Wellershaus K, Degen J, Deuchars J, Fuchs EC, Monyer H, Willecke K, DeJeu MT, De Zeeuw CI. Role of olivary electrical coupling in cerebellar motor learning. Neuron 58: 599–612, 2008 [DOI] [PubMed] [Google Scholar]
- Watson M, McElligott JG. 6-OHDA induced effects upon the acquisition and performance of specific locomotor tasks in rats. Pharmacol Biochem Behav 18: 927–934, 1983 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.