Abstract
BACKGROUND
Optimal treatment of right ventricular (RV) dysfunction observed in patients after tetralogy of Fallot (TOF) repair is unclear. Studies of biventricular (BiV) stimulation in patients with congenital heart disease have been retrospective or have included patients with heterogeneous disorders.
OBJECTIVE
The purpose of this study was to determine the effects on cardiac function of stimulating at various cardiac sites in an animal model of RV dysfunction and dyssynchrony and in eight symptomatic adults with repaired TOF.
METHODS
Pulmonary stenosis and regurgitation as well as RV scars were induced in 15 piglets to mimic repaired TOF. The hemodynamic effects of various configurations of RV and BiV stimulation were compared with sinus rhythm (SR) 4 months after surgery. In eight adults with repaired TOF, RV and left ventricular (LV) dP/dtmax were measured invasively during SR, apical RV stimulation, and BiV stimulation.
RESULTS
At 4 months, RV dilation, dysfunction, and dyssynchrony were present in all piglets. RV stimulation caused a decrease in LV function but no change in RV function. In contrast, BiV stimulation significantly improved LV and RV function (P< .05). Echocardiography and epicardial electrical mapping showed activation consistent with right bundle branch block during SR and marked resynchronization during BiV stimulation. In patients with repaired TOF, BiV stimulation increased significantly RV and LV dP/dtmax (P< .05).
CONCLUSION
In this swine model of RV dysfunction and in adults with repaired TOF, BiV stimulation significantly improved RV and LV function by alleviating electromechanical dyssynchrony.
Keywords: Cardiac resynchronization, Congenital heart disease, Right ventricular dysfunction, Tetralogy of Fallot, Ventricular dyssynchrony
Introduction
The population of adults with repaired tetralogy of Fallot (TOF) and other congenital heart diseases is growing rapidly.1,2 In particular, surgical repair of TOF is highly successful but later may be complicated by right ventricular (RV) or biventricular (BiV) dysfunction due to volume and pressure overload.3,4 The incompletely understood mechanisms of these delayed adverse developments may be partially due to surgically induced permanent right bundle branch block (BBB) and ventricular dyssynchrony.5–7 Unlike left ventricular (LV) failure, RV failure is poorly understood and its management remains largely empirical. In contrast to the vast experience with BiV stimulation gathered in adults with acquired LV dysfunction,8–10 studies of the safety and efficacy of cardiac resynchronization in patients with congenital heart disease and RV dysfunction are limited to case reports, retrospective analyses of heterogeneous populations, and small, crossover trials conducted in the immediate postoperative period.11–16 Although preliminary results are encouraging, the applications of resynchronization in patients with repaired TOF and the mechanisms by which it might be therapeutic remain unclear.
The aims of the present study were to (1) develop a reliable and reproducible long-term swine model of RV dysfunction and RV dyssynchrony, (2) study the electrophysiologic and hemodynamic effects of stimulation at different ventricular sites and configurations in this model compared to control animals, and, based on these observations, (3) compare the immediate hemodynamic effects of RV versus BiV stimulation in adults with repaired TOF and RV dysfunction.
Methods
Studies in an animal model of repaired TOF
The experimental protocols were in compliance with the Guiding Principles in the Use and Care of Animals published by the National Institutes of Health (NIH Publication No. 85–23, Revised 1996).
Creation of the swine model
The experimental study included 15 newborn piglets weighing less than 8 kg. The animals were sedated with intramuscular injection of 20 mg/kg ketamine hydrochloride and anesthetized with 10 mg/kg sodium pentobarbital before endotracheal intubation. Anesthesia was maintained with ketamine 500 mg/hour, and prophylactic intravenous antimicrobials were administered. Peripheral oxygen saturation, heart rate, and blood pressure were monitored continuously. Via left thoracotomy, the RV outflow tract was occluded partially with a clamp and incised longitudinally across the pulmonic valve annulus. The operation was designed to cause (1) RV volume overload from valvular regurgitation by excision of two pulmonic valve leaflets, (2) RV pressure overload by a loose tape partially occluding the pulmonary artery, and (3) RV outflow tract scar around the patch placed to close the RV incision. After the procedure was completed, the animals were extubated and received supplemental oxygen and analgesia as needed, before their transfer to a long-term postoperative care facility.
Study of the animal model
The index operation was performed in 15 animals, of which 1 died in the immediate postoperative period and 2 in the late postoperative period. After 4 months of postoperative recovery, we studied the electrophysiology and hemodynamic characteristics of our model of repaired TOF in the first 7 of the 12 long-term survivors. The animals were sedated, intubated, and anesthetized in the catheterization laboratory, as described earlier. A 7Fr catheter was introduced into the internal jugular vein for infusion of pharmaceuticals and fluids. A 7Fr Millar catheter tip micromanometer was placed inside the LV cavity via the left carotid artery, and inside the RV cavity via the right jugular vein, to measure intraventricular pressures and LV and RV dP/dtmax. The heart was exposed via median sternotomy and lateral thoracotomy and suspended in a pericardial cradle. After stabilization for 20 minutes, baseline LV pressure, aortic flow, and surface ECG were recorded. Signals were digitized at 200 Hz and stored on disk for offline analysis.
Echocardiographic cine loops of three cardiac cycles were analyzed offline to confirm the presence of pulmonic valve stenosis. Pulmonic and tricuspid valve insufficiency were assessed using conventional criteria by two-dimensional imaging and color Doppler flow from a Vivid Seven digital ultrasound system (GE Healthcare, London, UK). Aortic ejection and velocity–time integrals were measured using pulsed-wave Doppler imaging. Tissue Doppler imaging of segmental wall motion was used to quantify intra-LV and intra-RV dyssynchrony as previously described.6 Briefly, intra-LV dyssynchrony was defined as the difference between the shortest and longest of four basal LV electromechanical delays (lateral, septal, anterior, inferior). Intra-RV dyssynchrony was defined as the difference between electromechanical delay of septum and RV free wall.
The same experimental protocol was performed and the same measurements were made for comparison in a control group of seven age-matched, previously nonoperated animals.
Study of the hemodynamic effects of BiV stimulation in the animal model
The hemodynamic effects of stimulating at different sites were studied in the seven animals used to characterize the model. Temporary myocardial pacing leads were attached to (1) the roof of the right atrium; (2) the epicardial surface of the RV apical, lateral, and anterior walls; and (3) the epicardial surface of the lateral LV wall. The leads were connected to a four-channel external pulse generator (Medtronic, Inc., Minneapolis, MN, USA) for measurements of capture threshold at each site and stimulation from each electrode, separately or in combination.
Baseline hemodynamic measurements were made during (1) sinus rhythm (SR); (2) stimulation from each of the three RV sites; and (3) BiV stimulation between the LV site and each of the three RV sites, in random order. The pacing mode was VDD (atrial sensing triggering ventricular stimulation), and the atrioventricular delay was programmed between 20 and 40 ms to ensure ventricular capture in all pacing configurations. Measurements were averages of 10 cardiac cycles made after 30 seconds of stimulation. At the end of the experiments, the animals were sacrificed with an intravenous overdose of pentobarbital.
Electroanatomic mapping during SR and BiV stimulation in the animal model
The five remaining animals were similarly operated 4 months after the index procedure. In this subgroup, temporary myocardial pacing leads were attached to the roof of the right atrium and to apical RV and lateral LV epicardial walls. Dyssynchrony was echocardiographically ascertained during SR and during BiV stimulation. BiV epicardial electroanatomic mapping was performed with CARTO navigation system (Biosense Webster, Diamond Bar, CA, USA), as previously described.17 The torso of the animal was covered by three magnetic fields of different frequencies. A location reference was fixed on the back of the pig while a mapping catheter navigated on the epicardium of the animal. The magnetic sensor in the tip of the catheter and the location reference compared the intensities of the three magnetic fields, ensuring that the location of the catheter could be accurately determined. Color-coded, three-dimensional maps of epicardial activation were constructed during SR and after BiV stimulation. Epicardial mapping was limited to the anterior, apical, lateral, and inferior RV and LV segments. Neither the septum nor the posterior wall could be mapped in detail.
Studies in adults with repaired TOF
Informed written consent was obtained from the patients. Measurements of RV and LV function were made in eight men with a history of surgically repaired TOF, in SR with complete right BBB and QRS duration >120 ms, and with echocardiographic signs of RV dysfunction, estimated qualitatively and confirmed by decreased systolic velocity of the lateral RV wall on tissue Doppler imaging, who underwent clinically indicated cardiac catheterization. Pacing leads were advanced transvenously into the right atrium, to the RV apex, and into a lateral LV vein via the coronary sinus. Each patient was studied during SR and during atrial-synchronized VDD RV and BiV stimulation delivered in random order. Measurements of RV and LV pressures and dP/dtmax were made using Mikro-Tip pressure-tip catheters (Millar Instruments, Inc., Houston, TX, USA) advanced into each chamber. Atrioventricular delay was programmed at 70% of the intrinsic PR interval to ensure complete ventricular capture, and the RV and LV were stimulated simultaneously in BiV mode. RV and LV dP/dtmax measured during five consecutive cycles were averaged for each pacing configuration after 2 minutes of stable stimulation. Primary analyses were changes in RV and LV dP/dtmax.
Statistical analysis
Results are expressed as mean ± SEM. Continuous variables were compared using paired Wilcoxon-Mann-Whit-ney test. For comparisons of the effects of stimulation from the various sites, the animals or patients served as their own control. Repeated measures analysis of variance was used. Pearson correlation coefficient was used to examine the presence of correlations between quantitative variables. P< .05 was considered significant.
Results
Animal experiments
Characteristics of the animal model
Table 1 and Figure 1 summarize the main characteristics of the model. Compared to the control group, the seven operated animals developed prominent pulmonic stenosis and regurgitation, RV pressure and volume overload, marked RV enlargement, depressed LV and RV function, bilateral intraventricular dyssynchrony, and increased QRS duration.
Table 1.
Characteristics of operated versus control animals
| Operated (n = 7) | Control (n = 7) | P value | |
|---|---|---|---|
| QRS duration (ms) | 70 ± 10 | 53 ± 2 | <.01 |
| Left ventricular ejection fraction (%) | 50 ± 3 | 66 ± 3 | <.01 |
| Left ventricular dP/dtmax (mmHg/s) | 2,066 ± 554 | 2,836 ± 488 | <.01 |
| Right ventricular dP/dtmax (mmHg/s) | 513 ± 72 | 712 ± 81 | <.01 |
| Right/left ventricular diameter ratio | 1.3 ± 0.1 | 0.6 ± 0.1 | <.01 |
| Right ventricular pressure (mmHg) | |||
| Peak systolic | 42 ± 14 | 18 ± 6 | <.01 |
| End-diastolic | 11 ± 9 | 4 ± 2 | <.05 |
| Pulmonic valve gradient (mmHg) | 19 ± 6 | 4 ± 2 | <.01 |
| Intraventricular dyssynchrony (ms) | |||
| Left | 28 ± 3 | 12 ± 3 | <.01 |
| Right | 27 ± 3 | 13 ± 2 | <.01 |
Values are given as mean ± SEM.
Figure 1.
Echocardiographic characteristics of the animal model. A: Prominent gradient between right ventricle and pulmonary artery. B: Right bundle branch block with activation proceeding from the left ventricular lateral wall, to the septum, and finally to the right ventricular free wall. C: Marked right ventricular dilation.
Effects of RV and BiV stimulation
In these seven animals, no difference was observed in heart rate measured during SR (113 ± 14 bpm) versus during various stimulation configurations. QRS duration was significantly shorter (P< .05) during SR (70 ± 10 ms) than during RV apical (92 ± 4 ms), lateral (93 ± 5 ms), and anterior (91 ± 5 ms) stimulation. QRS duration during SR (70 ± 10 ms) was not significantly different from that measured during BiV stimulation with the RV lead placed at the apex (75 ± 8 ms), lateral (76 ± 9 ms), or anterior wall (76 ± 9 ms).
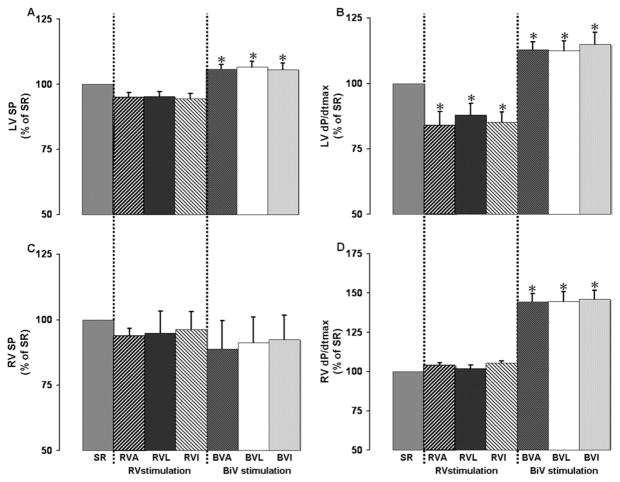
Compared with SR, stimulation from the three RV sites decreased LV dP/dtmax and dP/dtmin significantly, but not RV dP/dtmax and dP/dtmin. In contrast, the three BiV stimulation configurations significantly improved LV and RV function compared to RV stimulation and SR (all P< .05; Figure 2).
Figure 2.
Effects of biventricular stimulation between various right ventricular sites and lateral left ventricular wall on main measurements made in seven operated animals. Baseline measurements during sinus rhythm (SR) were left ventricular (LV) systolic pressure (SP) = 103 ± 10 mmHg, LV dP/dtmax = 2,066 ± 210 mmHg/s, right ventricular (RV) systolic pressure = 42 ± 5 mmHg, and RV dP/dtmax =513 ± 72 mmHg/s. BVA, BVL, BVI = biventricular stimulation between right ventricular apex (RVA), RVL (right ventricular lateral wall), and RVI (right ventricular inferior wall) and LV lateral wall, respectively. *P <.01 vs SR.
Electroanatomic activation during SR and BiV stimulation
In the five operated animals, BiV stimulation was associated with a significant decrease in echocardiographic RV (17 ± 3 ms vs 27 ± 3 ms; P< .05) and LV (18 ± 4 ms vs 28 ± 3 ms; P< .05) dyssynchrony compared with activation during SR. Epicardial electroanatomic maps created during SR showed an activation sequence consistent with right BBB. The first activated area was the basolateral LV, and the last was the RV free wall. BiV stimulation resynchronized the ventricles, with the earliest activated segment near the stimulation site and shortening of LV and RV activation in all animals, along with overall improvements in hemodynamic function.
Results of multisite pacing in adults with repaired TOF
Eight patients (mean age 40 ± 12 years) who had undergone surgical repair of TOF at the age of 6.8 ± 6.2 years were studied. All patients had clinical manifestations of RV dysfunction, including fatigue and ankle edema, and were in New York Heart Association functional class II (n = 4) or III (n = 4). Echocardiography showed severe pulmonic regurgitation in two patients and mild-to-moderate regurgitation in six. Mean systolic velocity of the lateral RV wall on tissue Doppler imaging was 9.0 ± 2.3 cm/s, and mean LV ejection fraction was 56.2% ± 10.1%.
Mean heart rate was similar during SR (70 ± 3 bpm) and during RV (69 ± 3 bpm) and BiV stimulation (70 ± 3 bpm). BiV and RV stimulation (Figure 3) increased RV dP/dtmax significantly (P< .05) compared with SR, and BiV stimulation increased LV dP/dtmax (P< .05). QRS duration was significantly longer (P< .01) during RV stimulation (184 ± 7 ms) than during SR (167 ± 5 ms). In contrast, BiV stimulation (149 ± 5 ms) significantly shortened QRS duration versus SR (P< .05) and RV stimulation (P< .01). Changes in QRS duration were not correlated with changes in RV dP/dtmax but were correlated with changes in LV dP/dtmax (r = 0.69, P< .05).
Figure 3.
Effects of biventricular stimulation (BiV) on right ventricular (RV) and left ventricular (LV) systolic pressure (SP), QRS duration, and RV and LV dP/dtmax in eight adults with repaired tetralogy of Fallot. Baseline measurements during sinus rhythm (SR) were RV SP = 54 ± 11 mmHg; RV dP/dtmax = 43.5 ± 113 mmHg/s; LV SP = 107 ± 10 mmHg; and LV dP/dtmax = 1,279 ± 160 mmHg/s. *P <.01 vs SR; †P <.01 vs RV.
Discussion
This study examined the effects of BiV stimulation on RV and LV function, first in a swine model and then in human adults with repaired TOF. Our animal model of surgically corrected TOF emulated the mechanical and electrophysiologic abnormalities observed in patients. In particular, RV dysfunction was associated with prominent RV mechanical delay, which was significantly mitigated by BiV stimulation. This is the first demonstration of the favorable effects conferred by BiV stimulation in an animal model of chronic right heart dysfunction. These observations prompted our clinical study, which confirmed these effects of resynchronization in patients with RV dysfunction and right BBB after surgical repair of TOF, suggesting potential new applications of cardiac resynchronization therapy, which thus far has been limited to patients with LV dysfunction.
Characterization of the model
As in earlier studies of the effects of BiV resynchronization on LV dysfunction,18,19 we began with an animal model of RV dysfunction, dilation, and dyssynchrony. As previously reported, this model, which combines RV volume and pressure overload, is reliable, reproducible, and associated with low mortality.20 Echocardiography and invasive measurements confirmed the development of prominent RV dilation and dysfunction, as well as moderate LV dysfunction, as observed in humans after surgical repair of TOF and in some patients with long-standing lung disease and RV dysfunction. Furthermore, despite the absence of marked prolongation of the QRS immediately after the index operation, over time the animals developed RV and LV electromechanical dyssynchrony along with marked QRS widening, as observed in humans.21 Echocardiography and epicardial mapping confirmed the presence of typical right BBB, with early electrical and mechanical activation of the lateral LV wall and delayed activation of the RV free wall. Thus, the model reproduced the right heart dysfunction and right BBB observed after surgical repair of TOF.
Effects of BiV stimulation
In both the animal model and in the human study, significant benefits were conferred by BiV rather than by apical RV stimulation. We chose RV and LV dP/dtmax as an index of the functional benefit conferred by cardiac resynchronization. dP/dtmax has been widely validated and probably is the gold standard measure of ventricular contractility. It is independent from ventricular geometry and changes in after-load and was measurable in the animal and human experiments. In the animal model, RV stimulation from three separate sites conferred no functional benefit compared with spontaneous activation during SR and significantly decreased LV (but not RV) contractility. Conversely, BiV stimulation significantly improved the function of both ventricles by invasive measurements and by echocardiography. This improvement was accompanied by shortening of electromechanical activation delay between the septum and lateral LV wall and between the septum and lateral RV wall.
Of note, apical RV stimulation alone improved RV function in patients with repaired TOF and right BBB, suggesting that single-chamber stimulation of a failing ventricle may improve its function. However, it did not improve LV function. Conversely, the increase in RV and LV contractility by BiV resynchronization, compared to apical RV stimulation, was associated with mitigation of BiV dyssynchrony. Echocardiography and electroanatomic mapping showed a considerable decrease in ventricular dyssynchrony by BiV stimulation in the animal model.
Clinical implications
Isolated right heart failure is a relatively common disorder with multiple causes, including congenital heart disease, pulmonary arterial hypertension, RV infarction, and acquired right heart valvular disease. The pathophysiology and clinical course of these diseases are often poorly understood. Our study offers new potential indications for BiV stimulation. As a result of the progress made in the surgical repair of, and survival into adulthood of patients suffering from, congenital heart disease, RV dysfunction is the object of growing interest, although the therapeutic options remain limited. Several echocardiographic studies have found marked BiV dyssynchrony in adults suffering from TOF.5–7 A few case reports and nonrandomized retrospective studies, which included inhomogeneous patient populations suffering from congenital heart disease, suggest that cardiac resynchronization has favorable effects on cardiac function. The hemodynamic benefit conferred by BiV stimulation in this study was significant in both ventricles and was found in all animals and all patients studied. The indications for implantation of a cardioverter-defibrillator as sudden death prophylaxis are increasing in these patients.22–24 Once this decision has been made, whether to add cardiac resynchronization to improve an often precarious hemodynamic status is a question must be addressed. This study may contribute valuable information to this debate.
Study limitations
To preserve the safety and expeditiousness of the surgical procedures, electroanatomic mapping was not performed, and only a single atrioventricular delay was tested in patients. Further studies are needed to optimize BiV stimulation in these patients by defining the optimal RV stimulation site(s) and interventricular and atrioventricular delays. The detection of an optimal fusion between spontaneous and stimulated activation might improve the functional response during RV stimulation.
The lateral wall was the only LV site of stimulation tested in our human and animal experiments. Studies in children and in immature animals have reported improvements in LV function by LV apical stimulation.25,26 Therefore, our results might have been superior with BiV stimulation with the LV lead placed at the apex, or LV apical stimulation alone might have produced similar results with less instrumentation. However, because of the challenge represented by the placement of an endocardial lead at the LV apex, we chose a site of stimulation associated with a high success rate for both our animal and our human experiments. Likewise, in patients, RV stimulation was limited to the RV apex. Therefore, the superiority of BiV versus RV stimulation can only be claimed in reference to the RV apex. Stimulation from the septum or the RV outflow tract might have yielded different results, although this was not observed in two earlier studies.6,13 These uncertainties need to be addressed in future investigations.
The animal experiments were performed with the chest open, which may markedly alter diastolic function. In addition, our electroanatomic mapping was epicardial. Attempts to include the interventricular septum should be made in future studies.
The very short-term observations made in this study do not closely emulate the long-term clinical circumstances of recipients of implantable cardiac resynchronization therapy systems, and our swine model of RV dysfunction and right BBB is not a perfect replica of repaired TOF. Nevertheless, the model closely reproduced the electrophysiologic and mechanical abnormalities found in patients with this disorder, including the delayed development of right BBB. We believe that our observations in patients are sufficiently encouraging to justify the organization of a randomized study, with a view to confirming these observations and validating their clinical application.
Conclusion
This study in a pig model of right heart dysfunction and right BBB and in adult patients presenting with surgically repair TOF yielded concordant results and showed beneficial effects on cardiac function conferred by BiV stimulation. These encouraging observations suggest that this therapy might be applicable to new patient populations. Cardiac resynchronization therapy currently is limited to patients with LV dysfunction. Patients with predominant RV dysfunction and right BBB also might respond favorably to this treatment.
ABBREVIATIONS
- BBB
bundle branch block
- BiV
biventricular
- LV
left ventricular
- RV
right ventricular
- SR
sinus rhythm
- TOF
tetralogy of Fallot
References
- 1.Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. First of two parts. N Engl J Med. 2000;342:256–263. doi: 10.1056/NEJM200001273420407. [DOI] [PubMed] [Google Scholar]
- 2.Nollert G, Fischlein T, Bouterwek S, Böhmer C, Klinner W, Reichart B. Long-term survival in patients with repair of tetralogy of Fallot: 36-year follow-up of 490 survivors of the first year after surgical repair. J Am Coll Cardiol. 1997;30:1374–1383. doi: 10.1016/s0735-1097(97)00318-5. [DOI] [PubMed] [Google Scholar]
- 3.Murphy JG, Gersh BJ, Mair DD, et al. Long-term outcome in patients undergoing surgical repair of tetralogy of Fallot. N Engl J Med. 1993;329:593–599. doi: 10.1056/NEJM199308263290901. [DOI] [PubMed] [Google Scholar]
- 4.Davlouros PA, Kilner PJ, Hornung TS, et al. Right ventricular function in adults with repaired tetralogy of Fallot assessed with cardiovascular magnetic resonance imaging: detrimental role of right ventricular outflow aneurysms or akinesia and adverse right-to-left ventricular interaction. J Am Coll Cardiol. 2002;40:2044–2052. doi: 10.1016/s0735-1097(02)02566-4. [DOI] [PubMed] [Google Scholar]
- 5.Vogel M, Sponring J, Cullen S, Deanfield JE, Redington AN. Regional wall motion and abnormalities of electrical depolarization and repolarization in patients after surgical repair of tetralogy of Fallot. Circulation. 2001;103:1669–1673. doi: 10.1161/01.cir.103.12.1669. [DOI] [PubMed] [Google Scholar]
- 6.Bordachar P, Iriart X, Chabaneix J, et al. Presence of ventricular dyssynchrony and haemodynamic impact of right ventricular pacing in adults with repaired tetralogy of Fallot and right bundle branch block. Europace. 2008;10:967–971. doi: 10.1093/europace/eun178. [DOI] [PubMed] [Google Scholar]
- 7.Abd El Rahman MY, Hui W, Yigitbasi M, et al. Detection of left ventricular asynchrony in patients with right bundle branch block after repair of tetralogy of Fallot using tissue-Doppler imaging-derived strain. J Am Coll Cardiol. 2005;45:915–921. doi: 10.1016/j.jacc.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 8.Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 9.Abraham WT, Fisher WG, Smith AL, Messenger J, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 10.Cleland JG, Daubert JC, Erdmann E, et al. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 11.Janousek J, Tomek V, Chaloupecky V, Gebauer RA. Dilated cardiomyopathy associated with dual-chamber pacing in infants: improvement through either left ventricular cardiac resynchronization or programming the pacemaker off allowing intrinsic normal conduction. J Cardiovasc Electrophysiol. 2004;15:470–474. doi: 10.1046/j.1540-8167.2004.03481.x. [DOI] [PubMed] [Google Scholar]
- 12.Janousek J, Vojtovic P, Hucin B, et al. Resynchronization pacing is a useful adjunct to the management of acute heart failure after surgery for congenital heart defects. Am J Cardiol. 2001;88:145–152. doi: 10.1016/s0002-9149(01)01609-5. [DOI] [PubMed] [Google Scholar]
- 13.Dubin AM, Feinstein JA, Reddy VM, Hanley FL, Van Hare GF, Rosenthal DN. Electrical resynchronization: a novel therapy for the failing right ventricle. Circulation. 2003;107:2287–2289. doi: 10.1161/01.CIR.0000070930.33499.9F. [DOI] [PubMed] [Google Scholar]
- 14.Strieper M, Karpawich P, Frias P, Gooden K, Ketchum D, Fyfe D, Campbell R. Initial experience with cardiac resynchronization therapy for ventricular dysfunction in young patients with surgically operated congenital heart disease. Am J Cardiol. 2004;94:1352–1354. doi: 10.1016/j.amjcard.2004.07.134. [DOI] [PubMed] [Google Scholar]
- 15.Dubin AM, Janousek J, Rhee E, et al. Resynchronization therapy in pediatric and congenital heart disease patients: an international multicenter study. J Am Coll Cardiol. 2005;46:2277–2283. doi: 10.1016/j.jacc.2005.05.096. [DOI] [PubMed] [Google Scholar]
- 16.Janousek J, Tomek V, Chaloupecký VA, et al. Cardiac resynchronization therapy: a novel adjunct to the treatment and prevention of systemic right ventricular failure. J Am Coll Cardiol. 2004;44:1927–1931. doi: 10.1016/j.jacc.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 17.Callans DJ, Ren JF, Michele J, Marchlinski FE, Dillon SM. Electroanatomic left ventricular mapping in the porcine model of healed anterior myocardial infarction. Correlation with intracardiac echocardiography and pathological analysis. Circulation. 1999;100:1744–1750. doi: 10.1161/01.cir.100.16.1744. [DOI] [PubMed] [Google Scholar]
- 18.Peschar M, de Swart H, Michels KJ, Reneman RS, Prinzen FW. Left ventricular septal and apex pacing for optimal pump function in canine hearts. J Am Coll Cardiol. 2003;41:1218–1226. doi: 10.1016/s0735-1097(03)00091-3. [DOI] [PubMed] [Google Scholar]
- 19.Leclercq C, Faris O, Tunin R, et al. Systolic improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with left bundle-branch block. Circulation. 2002;106:1760–1763. doi: 10.1161/01.cir.0000035037.11968.5c. [DOI] [PubMed] [Google Scholar]
- 20.Zeltser I, Gaynor JW, Petko M, et al. The roles of chronic pressure and volume overload states in induction of arrhythmias: an animal model of physiologic sequelae after repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2005;130:1542–1548. doi: 10.1016/j.jtcvs.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 21.Gatzoulis MA, Till JA, Somerville J, Redington AN. Mechanoelectrical interaction in tetralogy of Fallot. QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation. 1995;92:231–237. doi: 10.1161/01.cir.92.2.231. [DOI] [PubMed] [Google Scholar]
- 22.Gatzoulis MA, Balaji S, Webber SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–981. doi: 10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 23.Khairy P, Harris L, Landzberg MJ, et al. Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation. 2008;117:363–370. doi: 10.1161/CIRCULATIONAHA.107.726372. [DOI] [PubMed] [Google Scholar]
- 24.Berul CI, Van Hare GF, Kertesz NJ, et al. Results of a multicenter retrospective implantable cardioverter-defibrillator registry of pediatric and congenital heart disease patients. J Am Coll Cardiol. 2008;51:1685–1691. doi: 10.1016/j.jacc.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 25.Cojoc A, Reeves JG, Schmarkey L, et al. Effects of single-site versus biventricular epicardial pacing on myocardial performance in an immature animal model of atrioventricular block. Cardiovasc Electrophysiol. 2006;17:884–889. doi: 10.1111/j.1540-8167.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 26.Vanagt WY, Prinzen FW, Delhaas T. Reversal of pacing-induced heart failure by left ventricular apical pacing. N Engl J Med. 2007;357:2637–2638. doi: 10.1056/NEJMc072317. [DOI] [PubMed] [Google Scholar]