Abstract
A 40-year-old lady presented with severe endothelial cell loss in both eyes 14 years after angle-supported phakic intraocular lens (AS PIOL) implantation. The left eye had severe corneal edema with bullous keratopathy. The right eye had markedly reduced endothelial cell count (655 cells/mm2) although the cornea was clear. She underwent simultaneous bilensectomy (AS PIOL explantation and phacoemulsification) and Descemet's stripping and endothelial keratoplasty (DSEK) in the left eye. Explanted AS PIOL was identified as ZSAL-4 (Morcher, Stuttgart, Germany) model. Corneal edema cleared completely in 2 months with a best corrected visual acuity (-2.25 D sph) of 20/60. No intervention was done in the right eye. The present case illustrates that AS PIOL-induced endothelial decompensation can be effectively managed by simultaneous bilensectomy and endothelial keratoplasty.
Keywords: Endothelial cell loss, endothelial keratoplasty, phakic phakic intraocular lens
Phakic intraocular lens (IOL) implantation is a surgical approach to correct refractive error, which permits the optical correction while maintaining accommodation.[1,2] Most important complication reported with angle-supported phakic intraocular lens (AS PIOL) implantation is early or late endothelial cell loss.[3–5] We describe a technique of one-step bilensectomy (AS IOL explantation and phacoemulsification) and endothelial keratoplasty as a treatment modality for AS PIOL-induced endothelial decompensation.
Case Report
A 40-year-old lady was referred to cornea services of Sanjivni Eye Care, Ambala, India, for dimness of vision in the left eye associated with recurrent pain, redness, and watering since 3 months. She had undergone angle supported phakic intraocular lens (AS PIOL) implantation for high myopia (−11 diopter (D) in the right eye (RE) and –18 D in the left eye (LE) elsewhere in 1995. Apart from her recent complaints in LE, she was comfortable with vision all these years. Her old records mentioned that she had uneventful surgery. As per her records, her intraocular pressure (IOP) measurements in the early postoperative period were between 14 and 18 mmHg and there was no record of unusual or delayed uveitis in either eye. The model and design of AS PIOL were not mentioned in the records. She denied frequent rubbing of either of her eyes. The patient did not report any night glare or haloes. The preoperative endothelial cell count and anterior chamber depth (ACD) were not mentioned in her records.
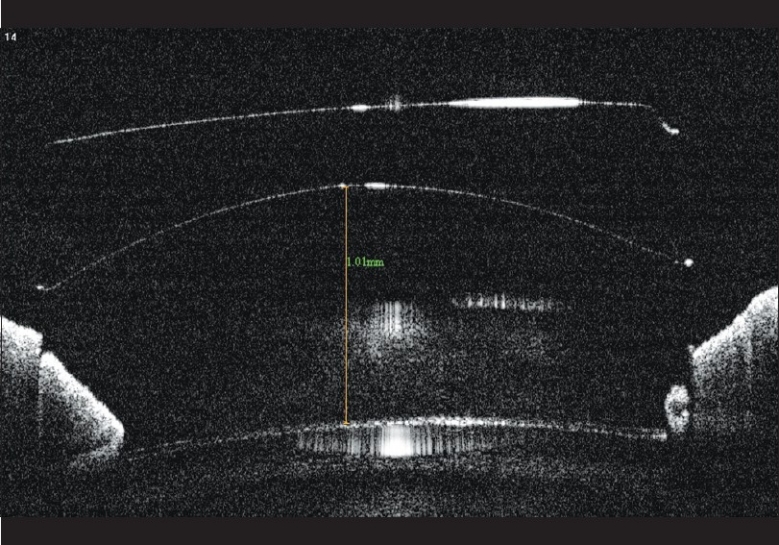
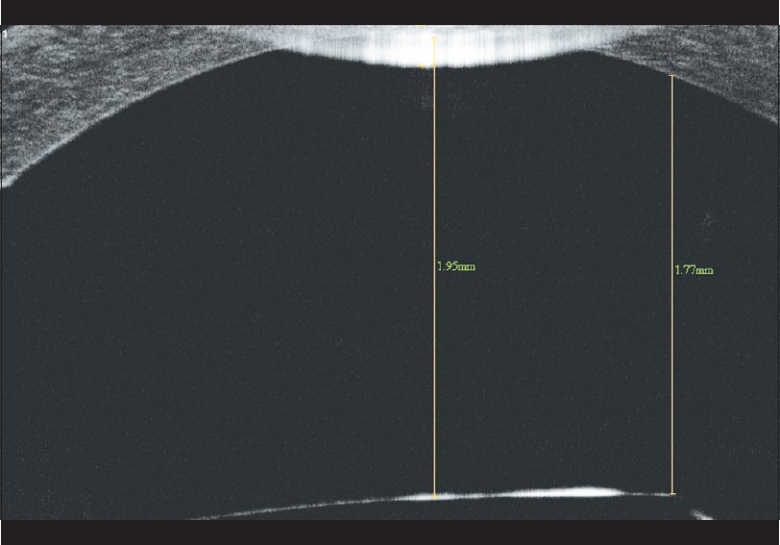
On examination, best corrected visual acuity (BCVA) was counting fingers at 1 m in LE and 20/60 (–1.5 D sph/–0.5 D cyl at 50°) in RE. Slit lamp examination showed circumciliary congestion, diffuse stromal corneal edema, and bullous keratopathy in LE [Fig. 1]. RE had clear cornea with no evidence of guttae changes or dispersed pigments on endothelium [Fig. 2]. AS PIOL was present in both eyes which was stable and well placed. Pupil was slightly oval in the meridian of haptics in both eyes. There was no peripheral iridectomy in either eye. Crystalline lens was clear in RE and was hazily seen in LE. Fundus examination showed myopic retinal degeneration in RE and it was not visible due to corneal edema in LE. IOP measured by Applanation tonometry was 14 and 16 mmHg in RE and LE, respectively. Gonioscopic examination in RE showed well-positioned haptics of AS PIOL at the iridocorneal angle. [Fig. 3] Due to hazy view, gonioscopy could not be performed in the left eye. Central endothelial cell density (ECD) as measured by a noncontact specular microscope (Topcon) in RE was 655 cells/mm2. Coefficient of variation in cell size was 29. Specular image acquisition was not possible in LE due to marked corneal edema. Central ultrasonic pachymetry was 532 μm in RE and 810 μm in LE. White-to-white diameter as measured by Orbscan was 11.7 mm in both eyes. Anterior segment optical coherence tomography (OCT) (Optovue, IOC) of the RE showed planoconcave AS PIOL placed in front of the crystalline lens [Fig. 4]. The distance measured by anterior segment OCT between the phakic intraocular lens edge and peripheral endothelium was 1.77 mm [Fig. 5]. The ACD in RE (3.73 mm) was calculated by adding the distance between the anterior surface of the cornea to the anterior surface of AS PIOL (2.4 mm), thickness of AS PIOL (0.32 mm) and distance between posterior AS PIOL surface to anterior surface of crystalline lens (1.01 mm), all measured by anterior segment OCT. OCT image of the anterior segment was not clear in LE.
Figure 1.
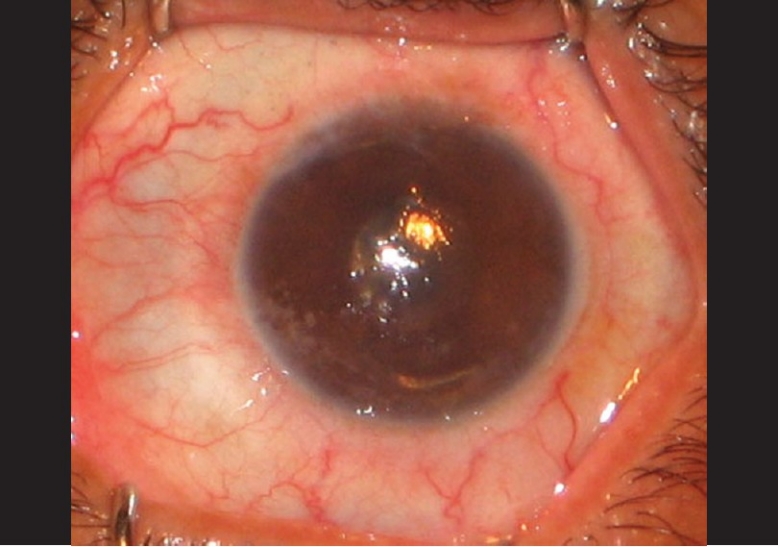
Left eye: angle-supported phakic intraocular lens-induced total corneal decompensation
Figure 2.
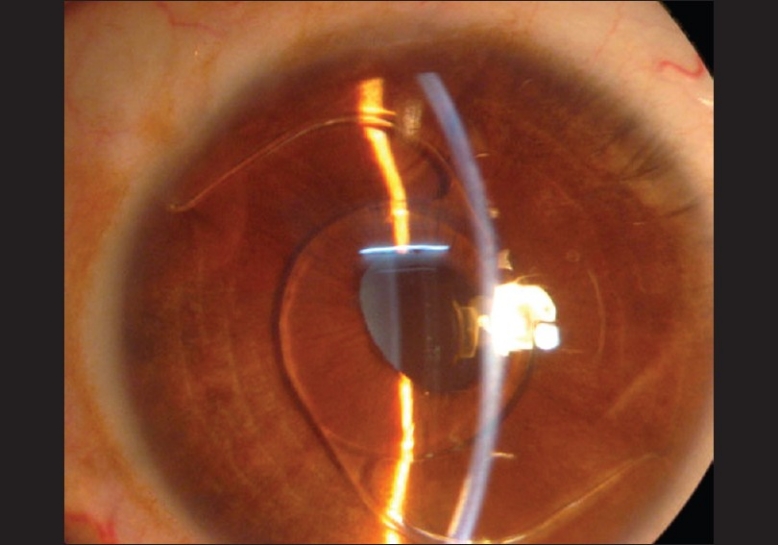
Right eye: clear cornea with well-placed angle-supported phakic intraocular lens
Figure 3.

Gonioscopic picture of right eye showing well positioned haptics in iridocorneal angle
Figure 4.
Anterior segment optical coherence tomography picture of the right eye showing the planoconcave phakic intraocular lens in front of the crystalline lens
Figure 5.
Anterior segment optical coherence tomography picture of the right eye showing the distance between the edge of optic and peripheral endothelium
The patient underwent bilensectomy (AS PIOL explantation and phacoemulsification of the crystalline lens) with posterior chamber intraocular lens (PCIOL) implantation and Descemet's stripping and endothelial keratoplasty (DSEK) in LE. No intervention was done in RE.
The axial length as measured by ultrasound biometry in the pseudophakic mode was 29.50 mm in RE and 29.71 mm in LE. Keratometry was 43.50/44.50 in both eyes. IOL power was calculated using the SRK-T formula. In view of simultaneous endothelial keratoplasty, the planned postoperative refraction was –2 diopters.
Surgery was performed under peribulbar anesthesia. Thick and edematous corneal epithelium was removed to improve the visibility. A 5.5-mm superotemporal scleral tunnel was used for the surgery. A dispersive ophthalmic viscosurgical device (OVD) was injected over and below AS PIOL. The optic was gently grasped with a McPherson forceps and removed from the eye without being cut or enlarging the incision. After explantation of AS PIOL, the clear crystalline lens was removed using coaxial phacoemulsification from superonasal clear corneal incision and a posterior chamber acrylic IOL (Aurolab, Madurai, India) was implanted in the capsular bag. Trypan blue (0.06%) solution was injected in the AC to increase the visibility of Descemet's membrane (DM). The AC was continuously formed by an AC maintainer attached to the balanced salt solution. DM was scored and separated in a circular pattern in the peripheral cornea with the help of a reverse Sinskey hook (Ankur Metal Works, Kolkotta, India) and removed from the AC with the forceps.
A 39-year-old phakic, clinically “very good” donor corneal tissue was used for endothelial keratoplasty. Donor tissue was dissected into anterior two-thirds and posterior one-third of stroma before surgery using artificial anterior chamber (Madhu Instruments, New Delhi, India) and blunt dissectors (Ankur Metal Works, Kolkotta, India). After dissection, donor tissue was trephinated using 8.5-mm corneal trephine (Madhu Instruments, New Delhi, India). Posterior stromal donor tissue with healthy endothelium was separated from the anterior layer and loaded onto the Busin glide (Moria, France) and pulled into the AC by a blunt forceps from the clear corneal side port at the opposite end. Donor tissue was secured in position by filling the AC with air which was replaced with the balanced salt solution after 1 h.
AS PIOL explanted was a planoconcave lens with Z-shaped haptics. It had an optical diameter of 5.5 mm and an overall diameter of 13 mm. This was identified as ZSAL-4 AS PIOL (Morcher, Stuttgart, Germany), a fourth-generation angle-supported phakic IOL[6] [Fig. 6].
Figure 6.
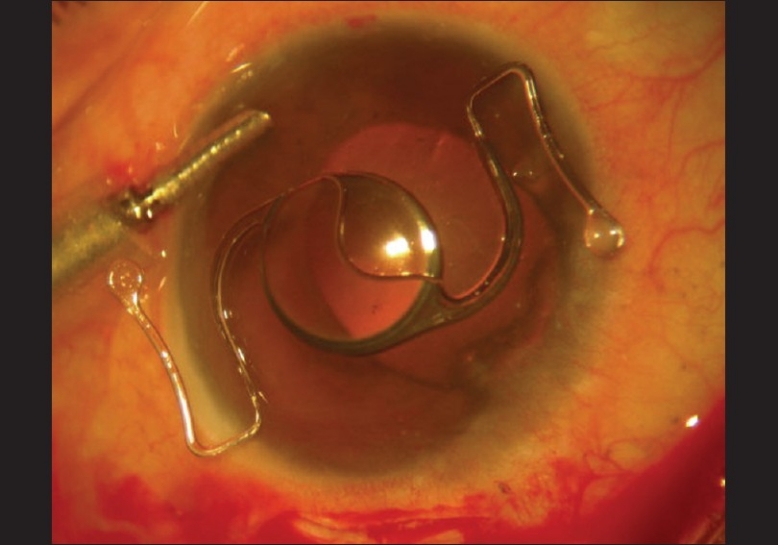
Explanted angle-supported phakic intraocular lens (ZSAL-4) from the left eye
Postoperatively, corneal edema cleared completely in 2 months with BCVA (−2.25 DSph) of 20/60 which was maintained till the last follow-up (9 months) [Fig. 7]. The central endothelial cell count at 3 months was 1378 cells/mm2 with a coefficient of variation in cell size of 39. Central ultrasonic pachymetry was 596 μm. Gonioscopic examination of the left eye showed open angles and diffuse iris pigments. Intraocular pressure as measured by applanation tonometry was 18 mmHg. Fundus examination showed myopic macular degeneration.
Figure 7.
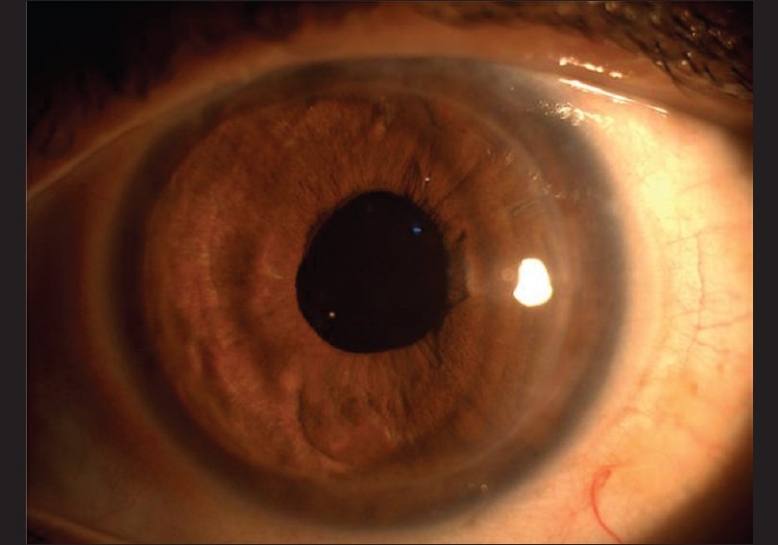
Left eye: 2-month postoperative clinical picture showing total resolution of corneal edema
Discussion
AS PIOL implantation has been reported to be associated with endothelial cell loss.[3–5] The reported endothelial cell damage was more with initial AS PIOL designs like Baikoff ZB (Domilens, Lyon, France) and less with modified designs like ZB5M (Domilens) and ZSAL-4 (Morcher).[6–9] We describe a case of bilateral endothelial cell loss 14 years after the implantation of an AS PIOL of a modified design (ZSAL-4).
We tried to look for the cause of endothelial cell loss in our patient. Previous studies on AS PIOLs describe the distance between the optic edge and peripheral endothelium as the most important parameter to determine endothelial cell loss.[3,10] For example, the distance between the edge of the optic and the peripheral endothelium of ZB lens was quite less (approximately 1.16 mm) and was associated with high endothelial cell loss.[7,8] This distance was raised in further designs like ZB5M (1.56 mm) and ZSAL-4 (1.54 mm) and these lenses were found to be endothelium friendly.[6,9–11] In our case, the distance between the optic edge of the AS PIOL and endothelium was 1.77 mm which is enough to avoid endothelial touch. Second important factor to determine endothelial cell loss is the length of AS PIOL.[3,5] In most of the studies on AS PIOL (ZB5M and ZSAL-4), the authors calculated the size of AS PIOL by adding 1 mm to white-to-white corneal diameter in largest meridian.[6,10] Our patient had a white-to-white corneal diameter of 11.7 mm and an overall length of AS PIOL 13 mm which is the correct size and hence should be stable. Other less important factors include early postoperative elevated IOP and chronic inflammation of the anterior chamber, none of which were mentioned in the records of our patient.[5] It is possible that apart from the visible risk factors, there is subtle endothelial damage even with the best design of AS PIOL which gets expressed only after cumulative loss over a long term.
The management of AS PIOL-associated endothelial cell loss includes AS PIOL explantation as the primary procedure.[3] The associated cataract or endothelial decompensation is managed with simultaneous cataract surgery or corneal transplantation, respectively.[3] Coullet et al. reported about three eyes with endothelial cell loss after angle-supported foldable AS PIOL (I-Care, Corneal Laboratories) implantation.[5] Two of these three eyes underwent AS PIOL explantation alone in which ECD remained stable after the surgery (640 cells/mm2and 919 cells/mm2).
The term “bilensectomy” stands for simultaneous removal of AS PIOL and crystalline lens.[3–5] Alio et al. performed bilensectomy in 18 out of their 24 cases of endothelial cell loss after AS PIOL implantation.[3] Apart from associated cataract, patient's age (more than 45 years) was their indication for simultaneous phacoemulsification.
If the endothelial cell loss is extreme and has caused corneal edema, associated corneal transplantation (penetrating keratoplasty [PKP] or endothelial keratoplasty) is required for visual rehabilitation. Endothelial keratoplasty has several advantages over PKP like early rehabilitation, better quality of vision, absence of expulsive hemorrhage, and no suture-related complications.[12] Patel et al. managed two (three eyes) of three patients of hyperopic AS PIOL-induced corneal decompensation with simultaneous bilensectomy and PKP.[4] Of the three eyes that received combined procedures, two eyes achieved good BCVA (20/40 and 20/25). The third eye had an expulsive choroidal hemorrhage, and the final BCVA was hand motions. Due to closed-chamber surgery, this complication is virtually absent in endothelial keratoplasty. One patient of Coullet et al. who underwent bilensectomy for endothelial cell loss after AS PIOL implantation developed pseudophakic bullous keratopathy after the procedure which was later managed by Descemet's stripping and automated endothelial keratoplasty (DSAEK).[5] Our patient had endothelial decompensation after implantation of AS PIOL and was managed by simultaneous bilensectomy and Descemet's stripping and endothelial keratoplasty with satisfactory results. Another option in our case was AS PIOL exchanged with posterior chamber phakic intraocular lens or AS PIOL explantation and endothelial keratoplasty.[3] But in view of age and need for long-term topical steroids, she underwent phacoemulsification in the same sitting.
Since our patient had endothelial cell loss in the right eye as well, she was advised bilensectomy to stabilize the damage but she refused any intervention in that eye for the time being.
The present case illustrates that endothelial decompensation is a possible long-term complication after AS PIOL implantation which can be effectively managed by combined bilensectomy and endothelial keratoplasty.
Acknowledgments
We thank Prof Merab Dvali, Tbilisi State Medical University, Tbilisi, Georgia for reviewing the manuscript and Ms Banu, Librarian, LV Prasad Eye Institute, Hyderabad, India for literature support.
References
- 1.Dvali ML. Intraocular correction of high myopia. Vestn Oftalmol. 1986;102:29–31. [PubMed] [Google Scholar]
- 2.Budo C, Hessloehl JC, Izak M, Luyten GP, Menezo JL, Sener BA, et al. Multicenter study of the Artisan phakic intraocular lens. J Cataract Refract Surg. 2000;26:1163–71. doi: 10.1016/s0886-3350(00)00545-9. [DOI] [PubMed] [Google Scholar]
- 3.Alió JL, Abdelrahman AM, Javaloy J, Iradier MT, Ortuño V. Angle-supported anterior chamber phakic intraocular lens explantation: Causes and outcome. Ophthalmology. 2006;113:2213–20. doi: 10.1016/j.ophtha.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 4.Patel SR, Chu DS, Ayres BD, Hersh PS. Corneal edema and penetrating keratoplasty after anterior chamber phakic intraocular lens implantation. J Cataract Refract Surg. 2005;31:2212–5. doi: 10.1016/j.jcrs.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 5.Coullet J, Mahieu L, Malecaze F, Fournié P, Leparmentier A, Moalic S, et al. Severe endothelial cell loss following uneventful angle-supported phakic intraocular lens implantation for high myopia. J Cataract Refract Surg. 2007;33:1477–81. doi: 10.1016/j.jcrs.2007.03.061. [DOI] [PubMed] [Google Scholar]
- 6.Alió JL, de la Hoz F, Pérez-Santonja JJ, Ruiz-Moreno JM, Quesada JA. Phakic anterior chamber lenses for the correction of myopia; a 7-year cumulative analysis of complications in 263 cases. Ophthalmology. 1999;106:458–66. doi: 10.1016/S0161-6420(99)90103-3. [DOI] [PubMed] [Google Scholar]
- 7.Mimouni F, Colin J, Koffi V, Bonnet P. Damage to the corneal endothelium from anterior chamber intraocular lenses in phakic myopic eyes. Refract Corneal Surg. 1991;7:277–81. [PubMed] [Google Scholar]
- 8.Saragoussi JJ, Cotinat J, Renard G, Savoldelli M, Abenhaim A, Pouliquen Y. Damage to the corneal endothelium by minus power anterior chamber intraocular lenses. Refract Corneal Surg. 1991;7:282–5. [PubMed] [Google Scholar]
- 9.Javaloy J, Alió JL, Iradier MT, Abdelrahman AM, Javaloy T, Borrás F. Outcomes of ZB5M angle-supported anterior chamber phakic intraocular lenses at 12 years. J Refract Surg. 2007;23:147–58. doi: 10.3928/1081-597X-20070201-07. [DOI] [PubMed] [Google Scholar]
- 10.Pérez-Santonja JJ, Alió JL, Jiménez-Alfaro I, Zato MA. Surgical correction of severe myopia with an angle-supported phakic intraocular lens. J Cataract Refract Surg. 2000;26:1288–302. doi: 10.1016/s0886-3350(00)00543-5. [DOI] [PubMed] [Google Scholar]
- 11.Saragoussi JJ, Puech M, Assouline M, Berges O, Renard G, Pouliquen YJ. Ultrasound biomicroscopy of Baikoff anterior chamber phakic intraocular lenses. J Refract Surg. 1997;13:135–41. doi: 10.3928/1081-597X-19970301-09. [DOI] [PubMed] [Google Scholar]
- 12.Basak SK. Descemet's stripping and endothelial keratoplasty in endothelial dysfunctions: Three-month results in 75 eyes. Indian J Ophthalmol. 2008;56:291–6. doi: 10.4103/0301-4738.41412. [DOI] [PMC free article] [PubMed] [Google Scholar]


