Highlights
▸ Equal levels of distress following exclusion in children with and without ASD. ▸ ASD participants had hypoactivation to exclusion in ventral ACC and right insula. ▸ ASD participants had hyperactivation to rule violation in right insula and dPFC. ▸ In ASD, right insula was hypoactive to exclusion and hyperactive to rule violation.
Keywords: Social exclusion, Rule violation, Autism Spectrum Disorder, Right insula, Functional magnetic resonance imaging
Abstract
The present study aimed to explore the neural correlates of two characteristic deficits in Autism Spectrum Disorders (ASDs): social impairment and restricted, repetitive behavior patterns. To this end, we used comparable experiences of social exclusion and rule violation to probe potentially atypical neural networks in ASD. In children and adolescents with and without ASD, we used the interactive ball-toss game (Cyberball) to elicit social exclusion and a comparable game (Cybershape) to elicit a non-exclusive rule violation. Using functional magnetic resonance imaging (fMRI), we identified group differences in brain responses to social exclusion and rule violation. Though both groups reported equal distress following exclusion, the right insula and ventral anterior cingulate cortex were hypoactive during exclusion in children with ASD. In rule violation, right insula and dorsal prefrontal cortex were hyperactive in ASD. Right insula showed a dissociation in activation; it was hypoactive to social exclusion and hyperactive to rule violation in the ASD group. Further probed, different regions of right insula were modulated in each game, highlighting differences in regional specificity for which subsequent analyses revealed differences in patterns of functional connectivity. These results demonstrate neurobiological differences in processing social exclusion and rule violation in children with ASD.
1. Introduction
Autism Spectrum Disorder (ASD) is characterized by a triad of deficits: social impairment, restricted and repetitive patterns of behavior, and delayed or absent communicative skills (APA, 2000). Social deficits in individuals with ASD often result in exclusion from social interactions (Ochs et al., 2001, Symes and Humphrey, 2010); a pattern that likely interferes with social learning and exacerbates interpersonal vulnerabilities. Thus, the importance of elucidating neural correlates of social exclusion in children and adolescents with ASD is of specific interest given the behavioral manifestation of the disorder. To accomplish this end, the present study used Cyberball, an interactive computerized ball-toss game (Williams et al., 2000), to elicit feelings of social exclusion in children and adolescents with and without ASD. Building from a host of behavioral work, Cyberball has been used in functional magnetic resonance imaging (fMRI) studies to examine brain responses to social exclusion in typically developing adolescents and adults (Bolling et al., 2011, Eisenberger et al., 2003, Masten et al., 2009, Onoda et al., 2009, Onoda et al., 2010, Sebastian et al., in press). These studies have identified brain regions involved in processing social exclusion including ventral anterior cingulate cortex (vACC), dorsal anterior cingulate cortex (dACC), and insula, all of which have been linked to the emotional response to exclusion; as well as ventrolateral prefrontal cortex (vlPFC), which has a hypothesized role in emotion regulation; and posterior cingulate cortex.
The Cyberball paradigm has been used in adolescents with ASD (Sebastian et al., 2009) as well as adults with Asperger Syndrome (AS; Andari et al., 2010). Sebastian and colleagues reported that mood was significantly modulated by exclusion in typically developing adolescents, but not in adolescents with ASD. In contrast, both groups of participants in the study showed comparable decreases in state anxiety following inclusion that did not persist after exclusion. Andari et al. found that atypical emotional and behavioral responses to exclusion in AS participants were normalized with intranasal oxytocin administration. From the adolescent work, it remains unclear whether similarities in self-reported experience reflect comparable neural substrates, or even whether individuals with ASD experience the game of Cyberball in the same way. Alternatively, non-social factors might be responsible for the experience of Cyberball distress in ASD. This is highly relevant, as social exclusion also involves an element of expectancy violation, in that one naturally expects to be included in social interactions. Given the rigid cognitive style that characterizes ASD, it is possible that the experience of social exclusion is distressing specifically because of the violation of an implicit rule governing social inclusion.
The current study used two interactive ball-toss games to extricate behavioral correlates and neurobiological substrates of social exclusion and rule (expectancy) violation in children with ASD and typically developing peers. An adaptation of the conventional Cyberball paradigm was employed to assess brain regions active during the experience of social exclusion. We used a second ball-toss game, Cybershape (Bolling et al., 2011, Crowley et al., 2011), to elicit rule violation in a comparable social context in the absence of social exclusion. In this game, a shape-matching rule dictated to whom the ball should be thrown. The rule was periodically violated by one of the online players, although the participant was never excluded from the game. Because some abnormal psychological responses to exclusion have been demonstrated in ASD, we hypothesized that brain mechanisms for processing social exclusion may underlie these differences. Specifically, as suggested by past work elucidating the neural correlates of social exclusion, we predicted group differences in brain responses to exclusion in vACC and right insula, two regions demonstrated to correlate with distress during exclusion in typical adolescents (Masten et al., 2009). Further, the preservation of anxiety reactions to social exclusion in adolescents with ASD suggests that some level of response to exclusion is preserved in autism, and we hypothesize that it may be the expectancy violation inherent in exclusion to which individuals with ASD are sensitive. We hypothesize that, in contrast to social exclusion, children with ASD would show increased behavioral and neural sensitivity to rule violation.
Examining social exclusion and rule violation in children with and without ASD allows for the investigation of two distinct domains of deficits characteristic of ASD. The present study aimed to identify brain mechanisms associated with differential functioning during social exclusion and rule violation in children with ASD as compared to their typically developing counterparts.
2. Participants
Data for this study were collected from 27 typically developing (TD) children and 30 children with Autism Spectrum Disorder (ASD). Following the implementation of our various exclusion criteria, participants included in analyses were 24 typically developing (TD) children and adolescents (17 males, mean age = 12.83 ± 2.59 years, range = 9.42–17.58 years) and 24 children and adolescents with an Autism Spectrum Disorder (ASD; 15 males, mean age = 12.81 ± 3.69 years, range = 7–17.92 years). Children with ASD were diagnosed using the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000) and the Autism Diagnostic Interview-Revised (ADI-R; Lord et al., 1994), as well as experienced clinical judgment (Table 1). In both groups, participants were excluded from analysis for head motion deviation from initial position greater than 4.5 mm or degrees in any of the 3 translational and 3 rotational directions at any point throughout the 5 min scan, for excessive shape-matching errors in Cybershape (>6 errors), and for previously playing any versions of Cyberball. One TD and one ASD participant were analyzed on only a portion of the Cyberball paradigm (8 and 5 blocks, respectively) due to excessive motion only at the end of the scan. Following these exclusions, 21 TD participants (15 males, 12.90 ± 2.59 years) and 16 ASD participants (10 males, 12.36 ± 4.06 years) were included in the Cyberball study. The mean amount of motion between two successive volume acquisitions during Cyberball was 0.89 ± 0.76 mm and 0.96 ± 1.03 mm in TD and ASD participants, respectively. Nineteen TD participants (14 males, 13.19 ± 2.24 years) and 21 ASD participants (15 males, 12.81 ± 3.52 years) were included in the Cybershape study. The mean amount of motion between two successive volume acquisitions during Cybershape was 0.59 ± 0.60 mm and 0.86 ± 67 mm in TD and ASD participants, respectively. IQ, age, and volume-to-volume motion in each game did not differ between participant groups (p > 0.05). Order of game presentation was counterbalanced across participants for both groups. Sixteen TD participants and 13 ASD participants were analyzed in both games. Fifteen participants included in the analyses believed that they might have been playing against real people (9 ASD mean age 13 years, 6 TD mean age 11.6 years; no significant age difference between groups, p > 0.05). While the remaining participants suspected that the online players were not real, past behavioral work has found that even when participants knew the fictional nature of their opponents, Cyberball exclusion still had negative effects on mood and need threat (Zadro et al., 2004). The average number of participant errors (shape thrown to the wrong player by the participant) in Cybershape was 1.25 (±1.6; maximum: 6), with 18 of the participants making no errors (10 ASD, 8 TD). Written informed consent was obtained from each participant according to a protocol approved by the Yale University Human Investigation Committee.
Table 1.
Demographic information.
| Measure | Group |
|
|---|---|---|
| TD | ASD | |
| IQ | ||
| n | 24 | 23 |
| Verbal | 103.54 (±17.12) | 102.61 (±21.91) |
| Nonverbal | 98.17 (±14.84) | 97.70 (±18.74) |
| Overall | 101.17 (±16.96) | 100.87 (±18.81) |
| ADI-R | ||
| N | 17 | |
| Social | 21.06 (±4.44) | |
| Communication and language | 16.76 (±4.04) | |
| Restricted and repetitive behaviors | 4.29 (±2.69) | |
| ADOS (module 3) | ||
| N | 19 | |
| Social and communication | 9.26 (±2.94) | |
| Stereotyped repetitive behaviors | 2.58 (±1.17) | |
| SRS | ||
| N | 21 | 18 |
| Raw total score* | 24.52 (±23.59) | 95.44 (±32.53) |
IQ data are as measured by the Developmental Abilities Scale (DAS). All autism assessment measures met the minimum cutoff for autism. Abbreviations: Autism Diagnostic Interview-Revised (ADI-R), Autism Diagnostic Observation Schedule (ADOS), Social Responsiveness Scale (SRS).
ASD > TD, p < 0.001.
2.1. Cyberball
A game of Cyberball began with a sham Google® search engine screen where a link to “Cyberball” was clicked by the experimenter, followed by a “loading” screen. During the loading period, the participants were told that they, along with the other online players connecting to the game through the Internet, were being logged into the game by the experimenter. The participants were given two button boxes, one in each hand, allowing them to throw the virtual ball to either the right or left. Prior to starting game play, instructions were delivered visually and auditorily, and the participants practiced playing the game for 16 throws, after which the scan commenced. Participants continued to play the Cyberball game for 5 min in 10 continuous, alternating blocks of fair play and exclusion. The paradigm used was identical to the one previously described by Bolling et al. (2011). The computer players’ pictures were matched on gender and ethnicity to each participant to intensify feelings of exclusion (Wirth and Williams, 2009). Immediately following the completion of the Cyberball game, a ten-item questionnaire was given to 20 of the 21 TD participants and 14 of the 16 ASD participants analyzed in Cyberball to assess exclusion-related distress. The ten items of the questionnaire were administered visually and auditorily to the participant while still in the scanner; though no functional data acquisition occurred during this period. The participant could communicate with the experimenter during this period if clarification was needed on any of the items on the questionnaire. This questionnaire was an abbreviated version of the Needs Threat Scale (van Beest and Williams, 2006). Please refer to Bolling et al. (2011) for a complete list of the items on this questionnaire.
2.2. Cybershape
Cybershape (Bolling et al., 2011) was used to explore brain activation to social expectancy violation in the absence of social exclusion. Cybershape began in the same manner as Cyberball with the experimenter choosing a link to “Cybershape” from a mock Google® screen, again followed by a loading screen during which participants were told that they were being logged into the game with other online players. Identities of computer players varied across the two cyber games. A visual and auditory explanation of the shape matching rule, in which participants were asked to throw the shape in their glove to the player with the matching shape next to their picture, was followed by a period of practice during which the participant's understanding of the game was confirmed. Once the scan began, participants played Cybershape for 5 min in 10 continuous, alternating blocks of fair play (rule consistent) and rule violation. The paradigm used was identical to the one previously described by Bolling et al. (2011). In fair play, participants received the shape one-third of the time, and the shape rule was never broken by the online players. In rule violation, participants still received the shape one-third of the time, but one of the virtual players consistently threw the shape to the wrong person. The rule violations occurred both in favor of the participant (getting the ball when it was not his or her shape) and in disfavor of the participant (not getting the ball when it was his or her shape). As with our previous work, a ten-item questionnaire to assess rule violation-related distress was given to 17 of the 19 TD participants and 17 of the 21 ASD participants analyzed in Cybershape immediately following the completion of the Cybershape game. The ten items of the questionnaire were administered visually and auditorily to the participant while still in the scanner; though no functional data acquisition occurred during this period. The participant could communicate with the experimenter during this period if clarification was needed on any of the items on the questionnaire. Refer to Bolling et al. (2011) for a complete list of the items on this questionnaire.
3. Imaging protocol
Images were collected on a Siemens 3T Tim Trio scanner located in the Yale University Magnetic Resonance Research Center. Whole-brain T1-weighted anatomical images were acquired using an MPRAGE sequence (TR = 1900 ms; TE = 2.96 ms; flip angle = 9°; FOV = 256 mm; image matrix 256 mm2; voxel size = 1 mm × 1 mm × 1 mm; 160 slices; NEX = 1). Whole-brain functional images were acquired using a single-shot, gradient-recalled echo planar pulse sequence (TR = 2000 ms; TE = 25 ms; flip angle = 60°; FOV = 220 mm; image matrix = 64 mm2; voxel size = 3.4 mm × 3.4 mm × 4 mm; 34 slices) sensitive to blood oxygen level dependent (BOLD) contrast.
4. Data analysis
Imaging data were preprocessed and analyzed using the BrainVoyager QX 2.0 software package (Brain Innovation, Maastricht, The Netherlands). Preprocessing of the functional data included slice time correction (using sinc interpolation), three-dimensional rigid-body motion correction (using trilinear-sinc interpolation), spatial smoothing with a FWHM 4-mm Gaussian kernel, and temporal high-pass filtering (fast Fourier transform based with a cutoff of 3 cycles/time course). Functional datasets were coregistered to within-session anatomical images, which were in turn normalized to Talairach space.
Prior to multi-participant analyses, activation from events in which participants were throwing the ball was removed from the dataset for each participant in a regression analysis (Bolling et al., 2011). This regression was performed to eliminate the potential confound of the lack of decision making and motor response in the exclusion blocks. For analytic consistency, the regression was also performed in Cybershape despite the fact that participants threw with equal frequency in fair play and rule violation. In addition, all subsequent analyses were limited to only voxels within the extent of the MNI brain normalized to Talairach space. This whole brain mask consisted of 1,449,746 (1 mm × 1 mm × 1 mm) voxels.
To identify brain regions modulated by exclusion in Cyberball and rule violation in Cybershape, a random-effects multi-participant general linear model (GLM)-based analysis was performed for each game in each participant group separately. Regressors were defined as boxcar functions peaking during each condition, convolved with a double-gamma hemodynamic response function (HRF). To additionally account for motion during each scan, functions of all of the 3 directions and 3 translations of movement from each participant were included in each single-subject GLM-based analysis as predictors of no interest.
For Cyberball a multi-participant GLM analysis was performed for each participant group, and brain activation in the contrast social exclusion > fair play was assessed at an uncorrected statistical threshold of p < 0.05. To correct for multiple comparisons, we used a cluster threshold of 34 contiguous functional (3 mm3) voxels (Forman et al., 1995, Goebel et al., 2006, Xiong et al., 1995). This cluster threshold was calculated to correspond to a corrected threshold of α < .05 using a BrainVoyager QX Cluster-level Statistical Threshold Estimator plug-in. This plug-in utilizes a Monte Carlo simulation (with 1000 iterations) to determine the relative frequency of each cluster size based on characteristics of the dataset, with an α < .05 indicating that all clusters of activation reported had a <5% probability of occurring by chance. For Cybershape, two multi-participant GLM analyses were performed, and the contrast of rule violation > fair play was assessed at the same statistical threshold of p < 0.05, corrected to α < .05 with an estimated cluster threshold of 34 functional voxels.
Following GLM analyses of Cyberball and Cybershape within each participant group, we performed secondary 2 × 2 whole brain ANCOVA within each game to identify regions exhibiting a significant Condition (fair play versus social exclusion or rule violation) × Group (TD versus ASD) interaction. This analysis allowed us to identify regions that showed different patterns of activation between participant groups which may not have exhibited a main effect of experimental condition in each group individually. This analysis was assessed in each game at an uncorrected threshold of p < 0.05, corrected with a cluster threshold of 20 contiguous functional voxels. A slightly lower cluster threshold than implemented in the within group analyses was used here to account for the lower statistical power for identifying interaction effects, and to ensure that potentially meaningful regions of interaction were not wrongfully excluded in this secondary analysis. Findings from this analysis were subsequently validated in more specific ROI analyses.
More specific region of interest (ROI) analyses were used to explore two regions that have been repeatedly implicated in the processing of social exclusion in typically developing populations: right insula and ventral anterior cingulate cortex (vACC) (Bolling et al., 2011, Eisenberger et al., 2003, Krill and Platek, 2009, Masten et al., 2009, Onoda et al., 2009, Sebastian et al., in press). While vlPFC has also been repeatedly implicated in emotion regulation during social exclusion, concern about group differences in emotional responses to exclusion influencing potential differences in neural indices of emotion regulation led to the decision not to explore a structural vlPFC ROI. The vACC region was modified from the ACC region defined by the Talairach database (Lancaster et al., 1997, Lancaster et al., 2000). The modification excluded all voxels above the plane z = 9, corresponding to the tip of the cingulate genu. The right insula region was defined by drawing insular gray matter on the MNI brain, defined as the region enclosed by the anterior, superior, and inferior periinsular sulci. Mean contrast beta values of activation in Cyberball (social exclusion–fair play) and Cybershape (rule violation–fair play) were calculated for each participant group in both structurally defined ROIs. Group (TD versus ASD) × Game (social exclusion–fair play versus rule violation–fair play) interactions were calculated with 2 × 2 ANOVAs for each of the two structural ROIs.
A specific examination of the interaction between group and game in the right insula revealed two distinct regions of insula that were active in each participant group. TD children showed activation in right middle and posterior insula (PI) during social exclusion, while children with ASD showed activation in right anterior insula (AI) during rule violation. A psychophysiological interaction analysis was utilized in these two conditions to attempt to compare the functional brain networks associated with these two non-overlapping regions of right insula. The PPI analyses allowed us to identify regions that showed increased connectivity with right PI during social exclusion in TD children, and regions that showed increased connectivity to right AI during rule violation in children with ASD. Prior to the connectivity analyses, the global mean (average signal across voxels) was removed from each volume, as a surrogate method for physiological artifact removal (Fox et al., 2005). Using functionally defined regions of activation from the contrasts of social exclusion > fair play (in TD children) and rule violation > fair play (in children with ASD), PPI regressors for each game were created by multiplying the difference of the two task regressors (convolved with a double-gamma HRF) by the preprocessed, normalized right insula ROI time course for each participant. This PPI function along with the task regressors and right insula time course were used as regressors in 2 multi-participant random-effects GLM analyses. The results were assessed at a statistical threshold of p < 0.05, corrected with a cluster threshold of 34 functional voxels to α < .05.
In Cyberball, we examined the relationship between brain activation during social exclusion and a social responsiveness measure administered to both TD and ASD participants. We used raw scores on the Social Responsiveness Scale (SRS; Constantino and Todd, 2003) as a continuous measure of social functioning which we subsequently used in a whole brain voxel-wise covariate analysis. Raw scores were used because scores higher than 116 do not have a corresponding t-score, disallowing correlation analyses including participants with these scores. We looked for brain regions in the contrast of social exclusion–fair play that correlated with raw SRS scores in a combined group of 19 TD and 12 ASD participants. Results from this analysis were initially assessed at a p < 0.05, corrected to α < .05 with a calculated cluster threshold of 100 contiguous functional voxels. Because the contiguous regions of activation at p < 0.05 were so expansive, results were reported at a p < 0.01 (with a cluster threshold of 4 contiguous functional voxels) to discern local maxima of significant activation. All regions present in the p < 0.01 results were also significant in the alpha-corrected p < 0.05 results.
5. Results
The post-game measures confirmed that participants felt distress following the experiences of social exclusion and rule violation, and also identified group differences in distress levels. In TD children, the average total score on the social exclusion distress questionnaire (SED-Q) was 25.70 (±7.68, n = 20; a score of 10 indicates no distress, while a score of 50 indicates extreme distress), while the average total score on the rule violation distress questionnaire (RVD-Q) was 22.17 (±4.76, n = 17, a score of 10 indicates no distress, while a score of 50 indicates extreme distress). In children with ASD, the average total score on the SED-Q was 29.57 (±7.76, n = 14), and the average total score on the RVD-Q was 25.59 (±4.37, n = 17). Scores on the SED-Q did not differ between groups (p > 0.05). However, the ASD group reported significantly higher distress than the TD group on the RVD-Q (t = −2.10, p = 0.04).
To investigate brain regions modulated by the experience of social exclusion in each group, random-effects multi-participant-GLM analyses comparing social exclusion > fair play were performed in TD children and children with ASD separately (Fig. 1). Peak coordinates, statistical values, size, and anatomical labels for the regions of differential activation in Cyberball are displayed in Table 2. TD children showed significant activation in regions previously implicated in processing exclusion in adults, including bilateral posterior insula, bilateral posterior cingulate cortex (PCC), and ventral anterior cingulate cortex (vACC). In addition, left vlPFC, left anterior superior temporal sulcus, and left parahippocampal gyrus (PHG) were also more active to social exclusion. Regions that showed preferential activation to fair play in Cyberball included left cerebellum, parietal cortex, and right precentral gyrus.
Fig. 1.
Top: whole-brain comparison of social exclusion and fair play in TD (n = 21; left) and ASD participants (n = 16; right). Regions in orange showed greater activation in social exclusion compared to fair play. Regions in blue showed greater activation in fair play compared to social exclusion (p < 0.05, k = 34). Bottom: whole-brain comparison of rule violation and fair play in TD (n = 19; left) and ASD participants (n = 21; right). Regions in orange showed greater activation in rule violation compared to fair play. Regions in blue showed greater activation in fair play compared to rule violation (p < 0.05, k = 34). Activations are displayed on a Talairach-transformed template brain in radiological orientation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Table 2.
Areas of activation emerging from the comparison of social exclusion and fair play during Cyberball.
| Brain region | X | Y | Z | Size | t | p |
|---|---|---|---|---|---|---|
| Social exclusion > fair play | ||||||
| TD | ||||||
| Right posterior insula | 42 | −13 | 10 | 8846 | 4.59 | 0.000181 |
| Right PCC | 9 | −52 | 7 | 2202 | 4.27 | 0.000371 |
| Left PCC/PHG | −9 | −58 | 7 | 10,005 | 5.01 | 0.000066 |
| Ventral ACC | −12 | 41 | 7 | 1841 | 3.33 | 0.003328 |
| Left vlPFC | −51 | 18 | −8 | 2040 | 3.66 | 0.001573 |
| Left anterior STS | −45 | −7 | −8 | 2807 | 4.79 | 0.000111 |
| Left posterior insula | −45 | −22 | 13 | 8275 | 4.90 | 0.000086 |
| ASD | ||||||
| Left retrosplenial cortex | −9 | −34 | 4 | 2701 | 5.45 | 0.000068 |
| Left superior precentral gyrus | −54 | −13 | 40 | 1430 | 3.58 | 0.002731 |
| Left inferior precentral gyrus | −57 | −7 | 10 | 1387 | 3.65 | 0.002392 |
| Fair play > social exclusion | ||||||
| TD | ||||||
| Left cerebellum | −42 | −40 | −38 | 1939 | −3.49 | 0.002282 |
| Parietal cortex | 27 | −58 | 58 | 26,296 | −4.96 | 0.000075 |
| Right precentral gyrus | 39 | −1 | 61 | 5739 | −3.82 | 0.00108 |
| ASD | ||||||
| Right parietal cortex | 36 | −46 | 49 | 10,895 | −5.62 | 0.000049 |
| Right MFG | 33 | 29 | 28 | 5069 | −4.59 | 0.000356 |
| Right anterior insula | 33 | 23 | 7 | 1475 | −4.22 | 0.000742 |
| Right vlPFC | 36 | 44 | 25 | 1108 | −3.04 | 0.008249 |
| Right precentral gyrus | 21 | 8 | 61 | 1289 | −4.74 | 0.000262 |
| Right dmPFC | 15 | 53 | 25 | 1156 | −3.82 | 0.001665 |
| Left vlPFC | −21 | 53 | 4 | 2266 | −3.51 | 0.003131 |
| Left parietal cortex | −33 | −49 | 28 | 2648 | −3.75 | 0.001941 |
| Left cerebellum | −39 | −37 | −35 | 1903 | −3.96 | 0.001265 |
Regions identified in a full brain contrast of social exclusion to fair play within each group (TD and ASD). Talairach coordinates and statistics refer to the voxel with the maximum signal change in each region of interest. Abbreviations: posterior cingulate cortex (PCC), parahippocampal gyrus (PHG), anterior cingulate cortex (ACC), ventrolateral prefrontal cortex (vlPFC), middle frontal gyrus (MFG), dorsomedial prefrontal cortex (dmPFC), superior temporal sulcus (STS).
In children with ASD, only left retrosplenial cortex and left precentral gyrus showed increased activation to social exclusion during Cyberball. Regions that were more active during fair play included bilateral parietal cortex, bilateral ventrolateral prefrontal cortex (vlPFC), right anterior insula, right middle frontal gyrus (MFG), right dorsomedial prefrontal cortex (dmPFC), left cerebellum, and right precentral gyrus.
In Cybershape, both groups showed significant brain differences in the contrast of rule violation versus fair play (Fig. 1, Table 3). TD children showed increased activation to rule violation in orbitofrontal cortex, parietal cortex, bilateral dorsolateral prefrontal cortex (dlPFC), dmPFC, and right inferior temporal gyrus. In contrast, this group showed increased activation during Cybershape fair play in bilateral insula, bilateral hippocampus, bilateral paracentral lobule, right cerebellum and cerebellar vermis, right posterior cingulate cortex, left precentral gyrus, dorsal anterior cingulate cortex (dACC), left retrosplenial cortex and cuneus, and pons.
Table 3.
Areas of activation emerging from the comparison of rule violation and fair play during Cybershape.
| Brain region | X | Y | Z | Size | t | p |
|---|---|---|---|---|---|---|
| Rule violation > fair play | ||||||
| TD | ||||||
| Right MTG | 60 | −46 | −2 | 3947 | 4.96 | 0.000102 |
| Right parietal cortex | 39 | −46 | 52 | 6501 | 5.01 | 0.000091 |
| Left parietal cortex | −39 | −70 | 40 | 3984 | 4.92 | 0.000111 |
| Bilateral orbitofrontal cortex | −18 | 59 | 1 | 23,543 | 5.25 | 0.000055 |
| Right anterior ITS | 48 | −1 | −26 | 1700 | 3.71 | 0.001587 |
| Right dlPFC | 33 | 11 | 46 | 2258 | 3.61 | 0.001985 |
| Left dlPFC | −30 | 14 | 40 | 3540 | 4.49 | 0.000285 |
| Dorsomedial PFC | 3 | 29 | 46 | 7204 | 4.22 | 0.000512 |
| ASD | ||||||
| Right supramarginal gyrus | 45 | −43 | 52 | 10,622 | 4.87 | 0.000092 |
| Right STS | 57 | −19 | −5 | 1303 | 3.61 | 0.001741 |
| Right cerebellum | 33 | −76 | −35 | 6292 | 4.48 | 0.000229 |
| Right MOG | 39 | −82 | 16 | 1278 | 3.76 | 0.001235 |
| Right caudate | 12 | 2 | 7 | 2017 | 4.88 | 0.000091 |
| Cuneus | −6 | −55 | 37 | 4866 | 4.49 | 0.000224 |
| Left caudate | −15 | −4 | 7 | 1900 | 4.13 | 0.000516 |
| Left SMG/STS | −45 | −43 | 28 | 22,574 | 5.16 | 0.000048 |
| Right insula | 39 | 20 | 13 | 7117 | 4.44 | 0.000252 |
| Left insula | −36 | 20 | −5 | 7409 | 5.40 | 0.000027 |
| Dorsomedial and dorsolateral PFC | 36 | 8 | 49 | 98,004 | 6.76 | 0.000001 |
| Fair play > rule violation | ||||||
| TD | ||||||
| Right cerebellum | 21 | −22 | −23 | 1671 | −4.58 | 0.000234 |
| Right posterior insula | 33 | −16 | 25 | 1633 | −4.19 | 0.000545 |
| Right paracentral lobule | 18 | −46 | 52 | 1307 | −3.68 | 0.001706 |
| Cerebellar vermis | 3 | −49 | −11 | 3927 | −5.50 | 0.000032 |
| Pons | −9 | −16 | −35 | 2126 | −4.37 | 0.000366 |
| Left cuneus | −15 | −76 | 13 | 1790 | −3.81 | 0.001294 |
| Left precentral gyrus | −36 | −25 | 52 | 2596 | −3.68 | 0.001726 |
| Right insula | 39 | −4 | 4 | 3306 | −4.46 | 0.000304 |
| Right PCC | 21 | −46 | 13 | 3147 | −4.54 | 0.000253 |
| Right hippocampus | 30 | −31 | 10 | 3830 | −4.02 | 0.000806 |
| Left insula | −27 | −4 | 19 | 5147 | −4.00 | 0.000839 |
| Left hippocampus | −33 | −34 | −5 | 4922 | −5.04 | 0.000085 |
| Left retrosplenial cortex | −15 | −43 | 4 | 3820 | −4.9792 | 0.000097 |
| Dorsal ACC/left paracentral lobule | −15 | −16 | 28 | 2913 | −4.00 | 0.00084 |
| ASD | ||||||
| Subgenual ACC | 3 | 14 | 1 | 2262 | −4.30 | 0.00035 |
| Ventral ACC | −6 | 38 | −2 | 1618 | −3.44 | 0.002613 |
| Left anterior MTG | −63 | −7 | −11 | 1046 | −4.06 | 0.000614 |
| Bilateral paracentral lobule | 9 | −25 | 58 | 8697 | −5.94 | 0.000008 |
| Right posterior insula | 39 | −10 | 22 | 1233 | −3.50 | 0.002251 |
| Right PCC/Hp | 18 | −37 | 13 | 7664 | −6.24 | 0.000004 |
| Left Hp | −36 | −25 | −8 | 6060 | −5.33 | 0.000033 |
| Left PCC | −24 | −31 | 7 | 5816 | −4.70 | 0.000136 |
Regions identified in a full brain contrast of rule violation to fair play within each group (TD and ASD). Talairach coordinates and statistics refer to the voxel with the maximum signal change in each region of interest. Abbreviations: posterior cingulate cortex (PCC), anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (dlPFC), prefrontal cortex (PFC), middle temporal gyrus (MTG), inferior temporal sulcus (ITS), superior temporal sulcus (STS), middle occipital gyrus (MOG), supramarginal gyrus (SMG), hippocampus (Hp).
Children with ASD showed increased activation to rule violation in dorsomedial and lateral prefrontal cortex, bilateral insula, bilateral superior temporal sulcus (STS) and supramarginal gyrus, bilateral caudate, right cerebellum, middle occipital gyrus (MOG), and cuneus. This group showed increased activation during Cybershape fair play in subgenual ACC, vACC, left anterior middle temporal gyrus, bilateral paracentral lobule, bilateral hippocampus, bilateral PCC, and right posterior insula.
A 2 × 2 whole-brain GLM analysis in Cyberball identified regions showing a Group × Condition interaction (Fig. 2, Table 4). Regions of interaction driven by significant modulation during Cyberball in only the TD group included right posterior insula, left PCC, right hippocampus, precuneus, and left vACC. Regions showing an interaction driven by significant modulation during Cyberball in only the children with ASD included right postcentral gyrus, right anterior insula, right MFG, and left SFG. Left MOG was modulated by the experimental manipulation during Cyberball in both groups. All of these regions except for precuneus showed greater activation to social exclusion in TD children compared to children with ASD.
Fig. 2.
Regions showing significant Group × Condition interactions in Cyberball (top) and Cybershape (bottom; p < 0.05, k = 20). Bar graphs depict beta value differences (social exclusion–fair play or rule violation–fair play) in regions of interaction. Regions described are not inclusive of all regions showing a significant interaction. * Denotes similar patterns of interaction bilaterally. Activations are displayed on a Talairach-transformed template brain in radiological orientation.
Table 4.
Regions showing significant Group × Condition interaction in Cyberball and Cybershape.
| Brain region | X | Y | Z | Size | F | p |
|---|---|---|---|---|---|---|
| Cyberball | ||||||
| Right posterior insula | 45 | −16 | 22 | 1314 | 11.80 | 0.001544 |
| Right postcentral gyrus | 42 | −25 | 43 | 2735 | 10.37 | 0.00276 |
| Right anterior insula | 33 | 14 | 1 | 850 | 13.00 | 0.000961 |
| Right hippocampus | 30 | −34 | −8 | 586 | 9.52 | 0.003957 |
| Right MFG | 30 | 32 | 22 | 558 | 8.61 | 0.005868 |
| Precuneus | −3 | −73 | 46 | 585 | 8.93 | 0.005092 |
| Left ventral ACC | 0 | 26 | 10 | 660 | 9.58 | 0.003859 |
| Left SFG | −12 | 14 | 49 | 556 | 8.38 | 0.006491 |
| Left PCC | −15 | −58 | 13 | 1017 | 8.74 | 0.005552 |
| Left middle occipital gyrus | −33 | −64 | 13 | 763 | 12.75 | 0.00106 |
| Cybershape | ||||||
| Right supramarginal gyrus | 54 | −31 | 28 | 584 | 13.79 | 0.000653 |
| Left supramarginal gyrus | −57 | −37 | 19 | 5107 | 18.01 | 0.000136 |
| Right anterior insula | 39 | 20 | 13 | 6115 | 16.26 | 0.000256 |
| Left anterior insula | −51 | 8 | 7 | 4802 | 14.82 | 0.00044 |
| Right dlPFC | 30 | 38 | 34 | 36,111 | 14.68 | 0.000464 |
| Left dlPFC | −42 | 35 | 31 | 5599 | 16.23 | 0.000259 |
| Right vlPFC | 36 | 41 | −9 | 722 | 8.028 | 0.007333 |
| Left vlPFC | −42 | 38 | −8 | 1070 | 11.11 | 0.001919 |
| Right precentral gyrus | 45 | −1 | 40 | 1907 | 14.35 | 0.000527 |
| Left precentral gyrus | −42 | −7 | 43 | 4105 | 11.57 | 0.001592 |
| Left cerebellum | 30 | −43 | −26 | 845 | 18.25 | 0.000125 |
| Cerebellar vermis | 6 | −49 | −11 | 1121 | 21.88 | 0.000036 |
| Subgenual ACC | −15 | 26 | −12 | 1523 | 22.55 | 0.000029 |
| Left SFG | −6 | 5 | 40 | 2482 | 9.39 | 0.003997 |
| Left dorsal ACC | −9 | 14 | 34 | 1888 | 14.16 | 0.000566 |
| Left precuneus | −12 | −73 | 13 | 752 | 12.18 | 0.001239 |
Talairach coordinates and statistics refer to the voxel with the maximum signal change in each region of interest. Abbreviations: anterior cingulate cortex (ACC), superior frontal gyrus (SFG), posterior cingulate cortex (PCC), dorsolateral prefrontal cortex (dlPFC), ventrolateral prefrontal cortex (vlPFC).
In an identical 2 × 2 whole-brain GLM analysis in Cybershape (Fig. 2, Table 4), regions showing a Group × Condition interaction driven by significant modulation during Cybershape in only the TD group included bilateral vlPFC, cerebellar vermis, and left precuneus. Regions of interaction driven by significant modulation during Cybershape in only the children with ASD included bilateral dlPFC, right precentral gyrus, bilateral supramarginal gyrus, dorsal ACC, left SFG, and left cerebellum. Regions that were modulated by the experimental manipulation in Cybershape in both groups included bilateral anterior insula and left precentral gyrus (both more active to rule violation in the ASD group), as well as subgenual ACC (more active to rule violation in the TD group).
Group × Game interaction analyses were performed in structurally defined regions of vACC and right insula, chosen a priori to be of interest in processing social exclusion (Fig. 3). These analyses revealed a significant Group (TD versus ASD) × Game (social exclusion–fair play versus rule violation–fair play) interaction in a structurally defined region of right insula (F = 7.78, p = 0.007) but not in vACC (p > 0.05).
Fig. 3.
Group × Game interaction in a structurally defined region of right insula (left panel). Beta value differences in each group were graphed for Cyberball (social exclusion–fair play; middle panel) and Cybershape (rule violation–fair play; right panel). This Group × Game interaction was significant at a p = 0.007.
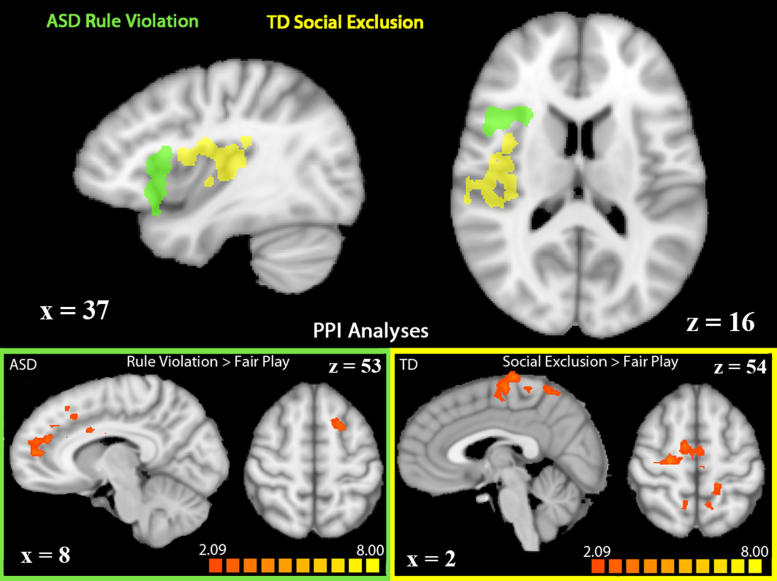
Further probing of the right insula guided by the finding that this region showed a Group × Game interaction revealed that the area of right insula active in TD children during social exclusion is regionally distinct from (posterior to) the region of right insula that is active in children with ASD during rule violation. An exploration of the task-dependent functional connectivity of these regions using PPI analyses revealed two distinct brain networks associated with right insula activation in each game (Table 5, Fig. 4). The area showing greater functional connectivity during social exclusion (compared to fair play) to the posterior region of right insula active in TD children was bilateral paracentral lobule. Regions showing greater functional connectivity during rule violation (compared to fair play) to the anterior region of right insula active in children with ASD included dorsal ACC, medial prefrontal cortex, and mid-cingulate cortex, as well as left SFG, bilateral IFG, right precentral gyrus, and left anterior insula.
Table 5.
Summary of activations identified in the PPI analyses of functional connectivity with right insula.
| Brain region | X | Y | Z | Size | t | p |
|---|---|---|---|---|---|---|
| Connectivity with right posterior insula in TD group | ||||||
| Social exclusion > fair play | ||||||
| Paracentral lobule/somatosensory cortex | 21 | −19 | 55 | 10,559 | 5.02 | 0.000066 |
| Connectivity with right anterior insula in ASD group | ||||||
| Rule violation > fair play | ||||||
| Right IFG | 58 | 17 | 22 | 4877 | 4.25 | 0.000388 |
| Right precentral gyrus | 21 | −16 | 46 | 1705 | 3.81 | 0.001109 |
| Dorsal ACC/dorsomedial PFC | 36 | −1 | 31 | 3028 | 5.34 | 0.000032 |
| Medial PFC | 12 | 47 | 22 | 1849 | 4.65 | 0.000154 |
| MidCC | 3 | −7 | 28 | 935 | 4.51 | 0.000214 |
| Left SFG | −18 | −1 | 43 | 1517 | 4.11 | 0.000545 |
| Left IFG/left AI | −30 | 20 | 7 | 1214 | 3.70 | 0.001417 |
The TD right insula region was defined by greater activation to social exclusion versus fair play, to which functional connectivity was subsequently assessed with a PPI analysis in the same Cyberball contrast. The ASD right insula region was defined by greater activation to rule violation versus fair play, to which functional connectivity was subsequently assessed with a PPI analysis in the same Cybershape contrast. Talairach coordinates and statistics refer to the voxel with the maximum signal change in each region of interest. Abbreviations: inferior frontal gyrus (IFG), anterior cingulate cortex (ACC), middle cingulate cortex (midCC), superior frontal gyrus (SFG), anterior insula (AI).
Fig. 4.
PPI analyses in regions showing significant activation in TD children during social exclusion (shown in yellow) or in ASD children during rule violation (green). Seed regions for the PPI analyses are depicted in the top panel. Regions showing increased functional connectivity to seed regions during social exclusion (right) or rule violation (left) are depicted in the bottom panel (p < 0.05, k = 34). Activations are displayed on a Talairach-transformed template brain in radiological orientation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
A whole-brain voxel-wise covariate analysis including all participants analyzed in the Cyberball contrast (social exclusion–fair play) using raw SRS score as a covariate, revealed several regions that showed a negative correlation between activation to social exclusion and SRS score included right posterior superior temporal sulcus (STS), right anterior insula, left anterior STS, left cerebellum, and right postcentral gyrus (Table 6, Fig. 5). This negative correlation indicates that the more socially responsive the participant (lower SRS score), the more activation during social exclusion in the identified regions. No regions showed a positive correlation with SRS scores.
Table 6.
Correlation between SRS score and activation in social exclusion versus fair play.
| Brain region | X | Y | Z | Size | r | p |
|---|---|---|---|---|---|---|
| Social exclusion > fair play | ||||||
| Right postcentral gyrus | 54 | −16 | 28 | 443 | −0.60 | 0.000474 |
| Right posterior STS | 45 | −37 | 16 | 111 | −0.53 | 0.002743 |
| Right anterior insula | 36 | 2 | 16 | 642 | −0.63 | 0.000221 |
| Left cerebellum | −12 | −49 | −20 | 437 | −0.55 | 0.001658 |
| Left anterior STS | −57 | 2 | −11 | 137 | −0.58 | 0.000843 |
This analysis was inclusive of all participants. Talairach coordinates and statistics refer to the voxel with the maximum signal change in each region of interest. Abbreviations: superior temporal sulcus (STS).
Fig. 5.
Whole brain correlation analysis in Cyberball with all participants. Regions in blue showed a negative correlation with Social Responsiveness Scale (SRS) scores in the contrast social exclusion > fair play. These regions were more active, the more socially responsive the participant (p < 0.01, k = 4). Activations are displayed on a Talairach-transformed template brain in radiological orientation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
6. Discussion
The current study identified brain differences in processing social exclusion and rule violation between children with and without ASD. While both participant groups reported comparable levels of distress following the experience of social exclusion, each showed fundamentally different patterns of brain activation during this exclusion. In an analogous social task where a non-exclusive rule violation occurred, participants with ASD also showed divergent brain activation from their TD counterparts. Unlike social exclusion, self-reported distress following rule violation differed between groups. Thus, the current study identified brain differences underlying processing in two domains of deficits characteristic of autism—social impairment and restricted, repetitive behavior patterns.
Self-report measures confirmed that both groups experienced distress following social exclusion and rule violation. Average item scores on the exclusion questionnaire were 2.57 and 2.97 for TD and ASD children, respectively. An item score of 1 corresponds to no distress, while a score of 5 corresponds to extreme distress. These scores were comparable to past work using a similar version of the NTS in adolescents which reported a mean item score of 2.90 (±0.73; Masten et al., 2009). This distress did not differ as a function of group membership; children with ASD were equally distressed by exclusion as their TD counterparts. In contrast, we did find group differences in distress following rule violation, with ASD participants reporting significantly greater distress following Cybershape than TD participants. This difference may reflect the inflexible adherence to routines or rituals representing rigid, restricted behavior in ASD (APA, 2000). Somewhat surprisingly, post hoc analyses did not reveal any correlations between self-reported distress and brain activation to exclusion or rule violation (versus fair play) in the a priori ROIs in either participant group.
Exploring the main effects of the experimental manipulations in each cyber game separately by group, we demonstrated that in both participant groups BOLD responses were sensitive to the experiences of social exclusion and rule violation compared to fair play (Table 2, Table 3, Fig. 1). TD participants showed differential activation during Cyberball in regions previously implicated in processing exclusion in typical adults and adolescents, including right insula, ventral ACC, and posterior cingulate cortex (PCC; Bolling et al., 2011, Eisenberger et al., 2003, Krill and Platek, 2009, Masten et al., 2009, Onoda et al., 2009, Sebastian et al., in press). Participants with ASD showed activation to social exclusion only in retrosplenial cortex and left precentral gyrus. In Cybershape, TD participants showed activation to rule violation in several regions also shown to be active in typical adults during this task (Bolling et al., 2011). These include bilateral parietal cortex, dorsolateral prefrontal cortex, and dorsomedial prefrontal cortex. In addition, TD children showed activation to rule violation in orbitofrontal cortex. Participants with ASD showed similar activation to TD peers in dorsomedial and lateral prefrontal cortex and right parietal cortices. In addition, ASD participants showed activation to rule violation in bilateral caudate, superior temporal sulcus, and anterior insula.
To examine group-dependent brain differences in processing social exclusion, we conducted a Group × Condition interaction analysis, and identified regions that differed significantly by group in their differential responses to exclusion and fair play (Table 4, Fig. 2). Ten regions met this criteria: right anterior and posterior insula, right hippocampus, right middle frontal gyrus, left ventral ACC, right postcentral gyrus, left superior frontal gyrus, left PCC, left middle occipital gyrus, and precuneus. All of these regions except precuneus showed greater activation to exclusion in TD compared to ASD participants. This pattern of differences describes a deficit in neural processing of social exclusion in ASD which is in line with the notion that autism is fundamentally a disorder of social impairment (Kanner, 1943, Wing and Gould, 1979). The unique, opposite pattern seen in precuneus is interesting, given this region's central role in default mode network (DMN) processing. This network of brain regions shows greater connectivity during task-independent processing and self-reflection (Buckner et al., 2008, Fox et al., 2005). While speculative, one might attribute the increased precuneus activation in ASD participants to task disengagement during social exclusion. Further work investigating DMN function in ASD is necessary to support this hypothesis.
To examine group differences in processing rule violation, we conducted a similar Group × Condition interaction analysis, and identified brain regions that differed significantly by group in their differential responses to rule violation and fair play (Table 4, Fig. 2). This analysis identified 16 distinct regions of interaction. Regions with greater activation to rule violation in the ASD group (compared to the TD group) included bilateral anterior insula, bilateral dorsolateral prefrontal cortex, bilateral precentral gyrus, bilateral supramarginal gyrus, dorsal ACC, cerebellum, left superior frontal gyrus, and left cuneus. Regions with greater activation to rule violation in the TD group included ventral ACC and bilateral ventrolateral prefrontal cortex. The identification of regions which were hyperactive to rule violation in ASD participants is concordant with our behavioral finding of higher self-reported levels of distress following rule violation.
The group dissociation found in dorsal versus ventral anterior cingulate cortex and lateral prefrontal cortex during Cybershape may reflect a difference in the mode of processing employed by each group during rule violation. A distinction in ACC functioning has been suggested such that dorsal and ventral regions function in cognitive and emotional tasks, respectively (Bush et al., 2000, Devinsky et al., 1995, Steele and Lawrie, 2004). Activation in dorsal ACC and dorsolateral PFC in the ASD group during rule violation suggests that this group processed the distressing rule violation in a more cognitive manner. The same experience in TD children that activated ventral ACC and ventrolateral PFC may be processed in a more emotional manner due to the social context of the game.
A Group × Game interaction analysis of activation in a structurally defined region of right insula revealed that, compared to TD participants, children with ASD showed hypoactivation in this region during social exclusion and hyperactivation during rule violation (Fig. 3). Given this region's established role in typical processing of social exclusion, more specific analyses were performed to assess the activation underlying this significant interaction. A comparison of right insula regions showing a main effect of manipulation in each game revealed that the region active during social exclusion in TD children was posterior to and non-overlapping with the region active during rule violation in ASD children. Past work exploring functional differences in insula subregions describes the more posterior region as having an interoceptive function (Craig, 2002, Craig, 2009, Dupont et al., 2003), with functional connections to somatosensory and motor cortices (Deen et al., in press). In contrast, the anterior insula is described as subserving a host of functions, including subjective awareness (Craig, 2009) and cognitive control (Critchley et al., 2001, Dosenbach et al., 2007, Grinband et al., 2006, Thielscher and Pessoa, 2007), with functional connections to the middle and dorsal ACC (Deen et al., in press, Taylor et al., 2009). Anterior insula has been linked to subjective experiences of physical pain (Casey et al., 1996, Coghill et al., 1994, Craig et al., 2000, Craig, 2009, Dupont et al., 2003, Derbyshire et al., 1997, Peyron et al., 2000, Brooks et al., 2002, Kong et al., 2006) as well as emotion processing (Critchley et al., 2005, Hennenlotter et al., 2005, Jabbi et al., 2007) and conscious awareness (Klein et al., 2007, Critchley et al., 2004). The finding that posterior insula responds to exclusion in TD participants may reflect a more visceral response to social exclusion, while the activation of anterior insula to rule violation in ASD participants may reflect a more cognitive, conscious emotional response.
PPI analyses to assess task-related functional connectivity in each of these right insula regions in their respective participant groups further illuminated differences in the group-divergent insular processing of social exclusion and rule violation. The right posterior insula region active during social exclusion in TD children showed increased functional connectivity during exclusion (versus fair play) to regions of motor and somatosensory cortices. The right anterior insula region active during rule violation in ASD children showed increased connectivity during rule violation (versus fair play) to dorsal ACC, medial PFC, and left anterior insula. This is in line with work describing dACC and anterior insula as a “salience network” (Seeley et al., 2007). The anterior insula activation and connectivity may relate to the increased salience of rule violation in ASD reflected in their higher self-reported distress levels following Cybershape. Anterior insula has been previously found to show disordered functioning in individuals with ASD during social tasks (Dapretto et al., 2006, Di Martino et al., 2009). The finding that this region was highly sensitive to a social game in which rule violation occurs suggests dysfunction in anterior insula might be better described as differential contextual specificity in ASD (Fig. 4).
In an attempt to elucidate behavioral factors in the brain processing of social exclusion in both participant groups, we included all participants in a whole-brain correlation analysis using scores on the SRS as a covariate. Regions showing a negative correlation between SRS score and activation to social exclusion > fair play describe areas which were more active to exclusion the more socially responsive the participant. One region showing this pattern which we found of specific importance was right posterior superior temporal sulcus (pSTS). Past work has shown that pSTS gray matter volume varied linearly with autistic traits in typical adults (von dem Hagen et al., 2011), and pSTS activation was decreased during social processing in autism (Kaiser et al., 2010, Herrington et al., 2007, Freitag et al., 2008). The absence of a Group × Condition interaction in this region supports the theory that pSTS activation to social exclusion indexes a general behavioral trait of social responsiveness. This finding has the potential to inform future work on individual differences in processing social exclusion in children with and without ASD, providing support for the use of pSTS as a brain mechanism by which social responsiveness might be indexed in autism. It is worth noting that the SRS correlation analysis was held to a more stringent threshold than the other analyses reported here (p < 0.01 versus p < 0.05). This was due to the fact that the results of the SRS correlation were more robust than those of both the within group and between group analyses. While interesting, this may be a result of the fact that the SRS correlation collapsed across participant groups, increasing the statistical power of the analysis (Table 6).
While illuminating in its identification of atypical neural responses to both social exclusion and rule violation in children and adolescents with ASD, this study incurred several limitations. First, many participants suspected that the online players were not real, although past behavioral results suggest that exclusion by computer opponents is significantly distressing (Zadro et al., 2004). Second, the modification of the original behavioral Cyberball paradigm to an alternating block design introduced several factors that may have affected brain responses to exclusion. The original paradigm which had one long block of inclusion followed by a long block of exclusion was susceptible to order effects, as well as confounding factors of scanner drift and participant motion and fatigue differentially affecting the later portions of the task. In addition, a long period of exclusion was not ideal for young participants, as there was concern that this design would result in task disengagement. The current study's alternating design with shorter periods of exclusion is ideal for eliminating order effects and keeping children engaged while still eliciting feelings of exclusion, as evidenced by our self-report measures. A result of using the alternating design, however, is that the distress questionnaire could only be administered after exclusion. A better comparison would be distress scores following inclusion and exclusion separately, but the nature of the design did not allow for this.
7. Conclusions
The present study used fMRI techniques to inform our understanding of the experiences of social exclusion and rule violation in children with ASD. It also provided evidence for the ability of future studies to use neuroimaging to draw conclusions about the experience of social interactions in children with ASD that might not be apparent from behavioral or observational measures alone. Further, this work lays foundations for future investigation of brain mechanisms that may underlie the sensitivity to rule violations characteristic of autism. Finally, in demonstrating hyperactivation of anterior insula during rule violation, we provide an example suggesting that “deficits” in activation of social brain regions in autism may be contextually specific.
Acknowledgements
The research presented herein was supported by grants from the National Institute of Mental Health, the John Merck Scholars Fund, the Bial Foundation (M.J.C.), and the Simons Foundation. Kevin Pelphrey was supported by a Career Development Award from the National Institutes of Health (NIMH Grant MH071284). Linda Mayes was also supported by a Career Development Award (NIDA K05 DA020091). Naomi Pitskel was supported by a grant from the Doris Duke Charitable Foundation to Yale University. James McPartland was supported by a Career Development Award (NIMH K23 MH086785).
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV-TR) American Psychiatric Association; Washington, DC: 2000. Pervasive developmental disorders. [Google Scholar]
- Andari E., Duhamel J.-R., Zalla T., Herbrecht E., Leboyer M., Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc. Natl. Acad. Sci. U.S.A. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling D.Z., Pitskel N.B., Deen B., Crowley M.J., McPartland J.C., Mayes L.C., Pelphrey K.A. Dissociable brain mechanisms for processing social exclusion and rule violation. NeuroImage. 2011;54:2426–2471. doi: 10.1016/j.neuroimage.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J.C.W., Nurmikko T.J., Bimson W.E., Singh K.D., Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. NeuroImage. 2002;15:293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schachter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N.Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulated cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Casey K.L., Minosima S., Morrow T.J., Koeppe R.A. Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain. J. Neurophysiol. 1996;76:571–581. doi: 10.1152/jn.1996.76.1.571. [DOI] [PubMed] [Google Scholar]
- Coghill R.C., Talbot J.D., Evans A.C., Meyer E., Gjedde A., Bushnell M.C., Duncan G.H. Distributed processing of pain and vibration by the human brain. J. Neurosci. 1994;14:4095–4106. doi: 10.1523/JNEUROSCI.14-07-04095.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino J.N., Todd R.D. Autistic traits in the general population: a twin study. Arch. Gen. Psychiatry. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel—now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig A.D., Chen K., Bandy D., Reiman E.M. Thermosensory activation of insular cortex. Nat. Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Mathias C.J., Dolan R.J. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29:537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Rotshtein P., Nagai Y., O’Doherty J., Mathias C.J., Dolan R.J. Activity in the human brain prediction differential heart rate responses to emotional facial expressions. NeuroImage. 2005;24:751–762. doi: 10.1016/j.neuroimage.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Wiens S., Rotshtein P., Ohman A., Dolan R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Crowley, M.J., Bolling, D.Z., Wu, J., Pelphrey, K.A., Mayes, L.C., 2011. Cybershape: An experimental paradigm to assess neural response to rule violation. Social Affective Neuroscience and Development Laboratory (SANDL). Yale Child Study Center.
- Dapretto M., Davies M.S., Pfeifer J.H., Scott A.A., Sigman M., Bookheimer S.Y., Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen, B., Pitskel, N.B., Pelphrey, K.A., Three systems of insular functional connectivity identified with cluster analysis. Cereb. Cortex, in press. [DOI] [PMC free article] [PubMed]
- Derbyshire S.W.G., Jones A.K.P., Gyulai F., Clark S., Townshend D., Firestone L.L. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- Devinsky O., Morrell M.J., Vogt B.A. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Di Martino A., Ross K., Uddin L.Q., Sklar A.B., Castellanos F.X., Milham M.P. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol. Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A.T., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., Petersen S.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U.S.A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S., Boiuilleret V., Hasboun D., Semah F. Functional anatomy of the insula: new insights from imaging. Surg. Radiol. Anat. 2003;25:113–119. doi: 10.1007/s00276-003-0103-4. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D., Williams K.D. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag C.M., Konrad C., Haberlen M., Kleser C., von Gontard A., Reith W., Troje N.F., Krick C. Perception of biological motion in autism spectrum disorders. Neuropsychologia. 2008;46:1480–1494. doi: 10.1016/j.neuropsychologia.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Goebel R., Esposito F., Formisano E. Analysis of functional image analysis contest (FIAC) data with BrainVoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum. Brain Mapp. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinband J., Hirsch J., Ferrera V.P. A neural representation of categorization uncertainty in the human brain. Neuron. 2006;49:757–763. doi: 10.1016/j.neuron.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Hennenlotter A., Schroeder U., Erhard P., Castrop F., Haslinger B., Stoecker D., Lange K.W., Ceballos-Baumann A.O. A common neural basis for receptive and expressive communication of pleasant facial affect. NeuroImage. 2005;26:581–591. doi: 10.1016/j.neuroimage.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Herrington J.D., Baron-Cohen S., Wheelwright S.J., Singh K.D., Bullmore E.T., Brammer M., Williams S.C.R. The role of MT+/V5 during biological motion perception in Asperger syndrome: an fMRI study. Res. Autism Spectr. Disord. 2007;1:14–27. [Google Scholar]
- Jabbi M., Swart M., Keysers C. Empathy for positive and negative emotions in the gustatory cortex. NeuroImage. 2007;34:1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Kaiser M.D., Hudac C.M., Shultz S., Lee S.M., Cheung C., Berken A.M., Deen B., Pitskel N.B., Sugrue D.R., Voos A.C., Saulnier C.A., Ventola P., Wolf J.M., Klin A., Vander Wyk B.C., Pelphrey K.A. Neural signatures of autism. Proc. Natl. Acad. Sci. U.S.A. 2010;107:21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbance of affective contact. Nerv. Child. 1943;2:217–250. [Google Scholar]
- Klein T.A., Endrass T., Kathmann N., Neumann J., Yves von Cramon D., Ullsperger M. Neural correlates of error awareness. NeuroImage. 2007;34:1774–1781. doi: 10.1016/j.neuroimage.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Kong J., White N.S., Kwong K.K., Vangel M.G., Rosman I.S., Gracely R.H., Gollub R.L. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum. Brain Mapp. 2006;27:715–721. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krill A., Platek S.M. In-group and out-group membership mediates anterior cingulated activation to social exclusion. Front. Evol. Neurosci. 2009;1:1–7. doi: 10.3389/neuro.18.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Rainey L.H., Summerlin J.L., Freitas C.S., Fox P.T., Evans A.C., Toga A.W., Mazziotta J.C. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum. Brain Mapp. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Woldorff M.G., Parsons L.M., Liotti M., Freitas C.S., Rainey L., Kotchunov P.V., Nickerson D., Mikiten S.A., Fox P.T. Automated Talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E.H., Jr., Leventhal B.L., DiLavore P.C., Pickles A., Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Masten C.L., Eisenberger N.I., Borofsky L.A., Pfeifer J.H., McNealy K., Mazziotta J.C., Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc. Cogn. Affect. Neurosci. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs E., Kremer-Sadlik T., Solomon O., Sirota K.G. Inclusion and social practice: views of children with autism. Soc. Dev. 2001;10:399–419. [Google Scholar]
- Onoda K., Okamoto Y., Nakashima K., Nittono H., Ura M., Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Soc. Neurosci. 2009;4:443–454. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Onoda K., Okamoto Y., Nakashima K., Nittono H., Yoshimura S., Yamawaki S., Yamaguchi S., Ura M. Does low self-esteem enhance social pain? The relationship between trait self-esteem and anterior cingulate cortex activation induced by ostracism. Soc. Cogn. Affect. Neurosci. 2010;5:385–391. doi: 10.1093/scan/nsq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron R., Laurent B., Garcia-Laurrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Clin. Neurophysiol. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Sebastian C., Blakemore S.-J., Charman T. Reactions to ostracism in adolescents with autism spectrum conditions. J. Autism Dev. Disord. 2009;39:1122–1130. doi: 10.1007/s10803-009-0725-4. [DOI] [PubMed] [Google Scholar]
- Sebastian, C.L., Tan, G.C.Y., Roiser, J.P., Viding, E., Dumontheil, I., Blakemore S.-J., Developmental influences on the neural bases of responses to social rejection: implications of social neuroscience for education. NeuroImage, in press. [DOI] [PubMed]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele J.D., Lawrie S.M. Segregation of cognitive and emotional function in the prefrontal cortex: a stereotactic meta-analysis. NeuroImage. 2004;21:868–875. doi: 10.1016/j.neuroimage.2003.09.066. [DOI] [PubMed] [Google Scholar]
- Symes W., Humphrey N. Peer-group indicators of social inclusion among pupils with autistic spectrum disorders (ASD) in mainstream secondary schools: a comparative study. School Psychol. Int. 2010;31:478–494. [Google Scholar]
- Taylor K.S., Seminowicz D.A., David K.D. Two systems of resting state connectivity between insula and cingulate cortex. Hum. Brain Mapp. 2009;30:2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielscher A., Pessoa L. Neural correlates of perceptual choice and decision making during fear–disgust discrimination. J. Neurosci. 2007;27:2908–2917. doi: 10.1523/JNEUROSCI.3024-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beest I., Williams K.D. When inclusion costs and ostracism pays, ostracism still hurts. J. Pers. Soc. Psychol. 2006;91:918–928. doi: 10.1037/0022-3514.91.5.918. [DOI] [PubMed] [Google Scholar]
- von dem Hagen E.A.H., Nummenmaa L., Yu R., Engell A.D., Ewbank M.P., Calder A.J. Autism spectrum traits in the typical population predict structure and function in the posterior superior temporal sulcus. Cereb. Cortex. 2011;21:493–500. doi: 10.1093/cercor/bhq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K.D., Cheung C.K.T., Choi W. Cyberostracism: effects of being ignored over the Internet. J. Pers. Soc. Psychol. 2000;79:748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Wing L., Gould J. Severe impairments of social interaction and associated abnormalities in children: epidemiology and classification. J. Autism Dev. Disord. 1979;9:11–29. doi: 10.1007/BF01531288. [DOI] [PubMed] [Google Scholar]
- Wirth J.H., Williams K.D. ‘They don't like our kind’: consequences of being ostracized while possessing a group membership. Group Process. Intergroup Relat. 2009;12:111–127. [Google Scholar]
- Xiong J., Gao J.H., Lancaster J.L., Fox P.T. Clustered pixels analysis for functional MRI activation studies of the human brain. Hum. Brain Mapp. 1995;3:287–301. [Google Scholar]
- Zadro L., Williams K.D., Richardson R. How low can you go? Ostracism by a computer is sufficient to lower self-reported levels of belonging, control, self-esteem, and meaningful existence. J. Exp. Soc. Psychol. 2004;40:560–567. [Google Scholar]