Abstract
For the last several decades, the development of antitumor immune-based strategies and the engineering and testing of oncolytic viruses (OVs) has occurred largely in parallel tracks. Indeed, the immune system is often thought of as an impediment to successful oncolytic virus delivery and efficacy. More recently, however, both preclinical and clinical results have revealed potential synergy between these two promising therapeutic strategies. Here, we summarize some of the evidence that supports combining OVs with immuno-therapeutics and suggest new ways to mount a multipronged biological attack against cancers.
Introduction
Advanced metastatic cancers are largely incurable and the last several decades of research into the biology of cancer has made it clear just why this is. Cancers have found multiple different ways to usurp signaling pathways to gain a growth advantage, making it unlikely that pharmacological attack on a single molecular target will significantly impact the long-term progression of the malignancy.1 Furthermore, tumor cells become very heterogeneous (genetically and phenotypically) as they evolve under the selective pressure of their microenvironment.2 The question becomes “how to deal with the chameleon-like behavior of evolving malignancies” that allows them to escape therapeutic intervention. We argue that what is required is a therapeutic strategy that can match the heterogeneity of the tumor and utilize the same activated pathways that drive tumor cell growth. Our immune systems have the capacity to rapidly respond and evolve to deal with a vast array of complex invading microorganisms and certainly have the potential to recognize the antigenic variations presented by malignant cells.3 Viruses, on the other hand, have evolved to take advantage of many of the same pathways that cancer cells activate during their malignant progression and inherently activate both innate and adaptive immune responses.4,5 Recent clinical and preclinical studies argue that there is significant interplay between viral and immune therapy approaches to cancer and that thoughtful partnering of these strategies could turn the tide on cancer.
Stimulating Antitumor Immunity: Harnessing Both Innate and Adaptive Responses
When tumor-associated antigens (TAAs), and the cytotoxic T cells (CTL) capable of recognizing them were identified and isolated toward the end of the last century, it seemed it would only be a matter of time before clinical strategies to activate specific, adaptive antitumor immunity would be improving patient outcomes.6,7 Various approaches to present TAA to the immune system in an immuno-stimulatory context have been successfully piloted in preclinical animal models and early clinical trials; whole cells, cell lysates, proteins, single/multiple/long peptides, DNA and RNA were given with adjuvants or immune effector cells [particularly dendritic cells (DCs)], and shown to elicit CTL.8 However, the final translational steps of proof of clinical benefit and adoption into routine clinical practice have proved elusive to date. For some time, the identification of TAA arguably led to a disproportionate focus on the adaptive arm of the antitumor immune response, to the exclusion of therapeutic strategies addressing nonspecific innate immune activation, despite its critical role in the early stages of adaptive priming. Significantly, clinical data show a correlation between improved outcome and infiltration into tumors of both innate natural killer and adaptive T cells, for example, in colorectal cancer,9,10 and one of the few cancer immunotherapies in widespread clinical use—the intravesical administration of Bacillus Calmette–Guerin for superficial bladder cancer—is clearly innate and nonspecific in its action, utilizing antimicrobe immunity for antitumor effects.11
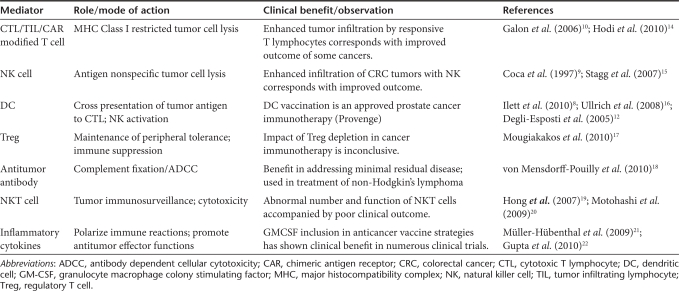
As the mechanisms underlying successful cancer immunotherapy were shown to include linked innate and adaptive effectors (for example cross-activation between natural killer cells and DC12,13), the importance of nonspecific as well as specific immune activation has become increasingly recognized, and both arms of the immune response have recently taken significant steps forward in the clinical arena (Table 1).8,9,10,12,14,15,16,17,18,19,20,21,22 From a TAA-specific, adaptive perspective, the US Food and Drug Administration approval of sipuleucel-T (Provenge—a DC-based treatment for prostate cancer23) is encouraging, while the demonstration that ipilimumab (a nonspecific innate immunomodulatory antibody which blocks inhibitory CTLA-4) improves survival of patients with metastatic melanoma,14,24 shows that specificity is not a prerequisite for therapeutic success. Hence, separate clinical progress with both specific (adaptive) and nonspecific (innate) cancer immunotherapy is now a reality; it would be ideal if the two could be harnessed together.
Table 1. The armory: antitumor immune mediators.
Oncolytic Viruses: Creating an Immune Storm Within Tumors
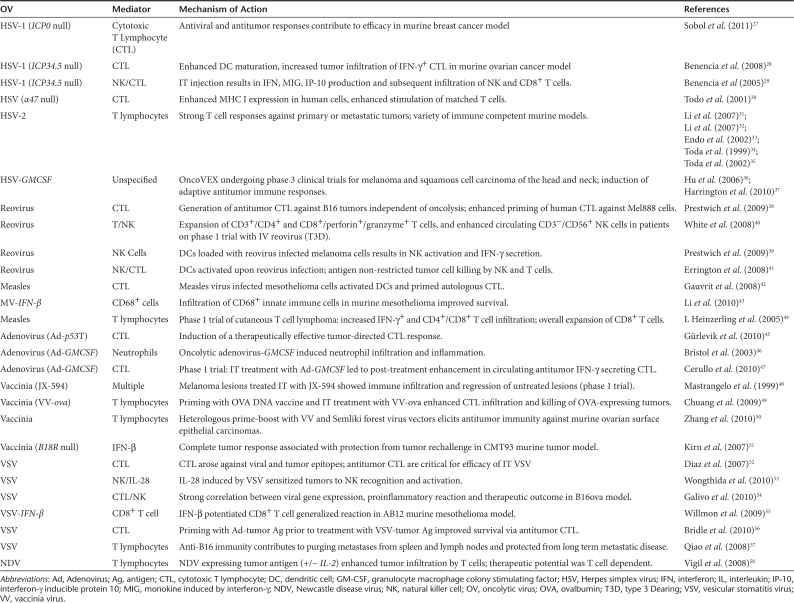
Oncolytic virus (OV) therapeutics are designed to rapidly and specifically grow in tumors with the primary objective of directly lysing cancer cells. However, it is becoming clear that their targeted infection of the tumor has the potential to create an “inflammatory storm” that arouses the innate and adaptive immune responses against tumors (Figure 1b). Indeed it appears that in some instances, during natural virus infections, an immune response can be generated that may protect from the onset of certain kinds of cancers.25,26 Perhaps the transient expression of “neo-antigens” during normal tissue repair in the context of a severe inflammatory reaction leads to the generation of an immune response with cancer surveillance properties.25 There is increasing evidence that therapeutic virus mediated destruction or damage of tumors can lead to an antitumor immune response (Figure 1b and Table 2).27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54, 55,56,57,58 For instance, over a decade ago, Mastrangelo and his colleagues demonstrated that in advanced melanoma patients it was possible to use intralesional “vaccination” with an oncolytic vaccinia virus expressing granulocyte macrophage colony stimulating factor (GMCSF) (trade-name JX-59459) to generate significant clinical responses that correlated with antitumor immune responses. Injected tumors became inflamed and infiltrated with a variety of immune cell types. Significantly, tumors that were not injected with JX-594 responded, suggesting that systemic antitumor immune responses had evolved during therapy.48,60
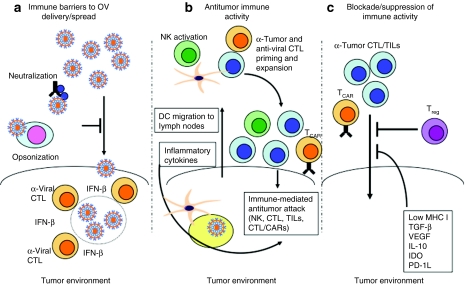
Figure 1.
It takes two to tango: striking the balance between antitumor activity, and antivector immunity. (a) Immune barriers to oncolytic virus (OV) delivery/spread. (b) Antitumor immune activity. (c) Blockade/suppression of immune activity. CAR, chimeric antigen receptor; CTL, cytotoxic T lymphocyte; DC, dendritic cell; IDO, indoleamine 2,3-dioxygenase; IFN, interferon; IL, interleukin; MHC, major histocompatibility complex; NK, natural killer cell; TGF-β, transforming growth factor-β TIL, tumor infiltrating lymphocyte; VEGF, vascular endothelial growth factor.
Table 2. The dream team: antitumor immune responses elicited by OVs.
It has since become increasingly clear with other oncolytic virus platforms that the immune responses triggered by oncolytic virus infection is a critical component of the clinical benefit of these therapeutics. Reovirus, a naturally occurring, unmodified virus that has already completed significant clinical testing (as Reolysin), and has just entered phase 3 for head and neck cancer, can elicit antitumor immune activation.61 In some models, reoviral replication and direct oncolysis are not necessarily required for therapy,38 although the clinically relevant contribution of direct tumor killing and antitumor immune activation for any OV remains to be elucidated in patients. The antitumor immune effects of reovirus can be enhanced with the addition of interleukin-2 (IL-2),62 and are associated with adaptive priming against TAA in tumor-draining lymph nodes,61 illustrating that both innate and adaptive arms of the immune response can be exploited to improve therapy. This murine data is consistent with human in vitro systems, which show that reovirus activates DCs41 to both stimulate natural killer cells and prime specific antitumor CTL. For viruses that can readily be genetically modified, the potential of antitumor immune activation after OV treatment has been further exploited to improve therapy. A range of genes has been incorporated into a number of viruses, although immuno-stimulatory modification of a virus does not inevitably enhance antitumor therapy. A vesicular stomatitis virus (VSV) encoding CD40L was no better than its unmodified equivalent on intratumoral injection, and indeed was less effective than a nonreplicating adenoviral vector expressing CD40L.54 In this case, early nonspecific T-cell activation initiated by replicating VSV-CD40L distracted the immune response away from TAA, illustrating how important it is to compare, select, and optimize different viral and gene platforms in the context of innate and adaptive OV-associated antitumor immunity.
To date, GMCSF is the immune gene inserted most successfully into clinically advanced OV. This preference for GMCSF derives from its potent ability to generate systemic adaptive antitumor immunity in vivo after expression in tumor cells,63 which is associated with the recruitment and differentiation of activating DC in the tumor microenvironment. As alluded to above, a replicating vaccinia virus expressing GMCSF (JX-594) has shown promise in preclinical64,65 and clinical studies,64 and is rapidly progressing toward phase 3 testing. A replicating herpes simplex virus type-1 expressing GMCSF caused tumor regression in mice,66 a finding reproduced in a phase 2 trial in melanoma.67 Currently, this virus (Oncovex) is being tested in a phase 3 clinical study, which is recruiting in both the United States and Europe. The other immunomodulatory gene which has been inserted most often into OV to date is interferon-β (IFN-β), although these viruses have not yet progressed as far in clinical testing as those expressing GMCSF. Interestingly, the initial aim of IFN-β expression by OV was to restrict viral replication in normal tissue, thus increasing direct oncolysis and the therapeutic index. However, IFN-β, despite its role in innate antiviral immune responses, can also support activation of antitumor immunity when expressed in vaccinia,51 VSV,55 and measles43. Hence genetic modifications which support both adaptive (GMCSF) and innate (IFN-β) antitumor immunity have been applied to improve OV therapy. Various other immunomodulatory molecules (including IL-12, IL-24, IL-4, RANTES, CD80, IL-18, and IFN-α), which impact on immunity via a range of effector pathways, have also been proposed for expression by OV.68
How can oncolytic virotherapy and cancer immunotherapy most effectively unite to improve potential treatment for patients? One key issue is how OV are delivered and access the cancer. In the largest, most promising published clinical trials to date, oncolytic viruses have been injected directly into the tumor, to initiate both local and distant regression.64,67 Intratumoral delivery avoids the concern of virus neutralization by circulating antibodies (Figure 1a) and suits the paradigm whereby the mere presence of a virus within a tumor can act as a “danger signal” to alert and activate the immune system.69 However, despite the acceptance of the intratumoral route used in the current phase 3 trial of Oncovex in melanoma, systemic intravenous delivery, if effective, is always likely to be more popular with clinicians. Moreover, there is currently no clinical evidence that the antiviral immune response to systemic OV impairs therapy in patients; indeed relatively late tumor regression can occur at a time when neutralizing antibody levels are known to be high. Indeed, in some preclinical models, the anticancer activity of oncolytic vaccinia was actually enhanced when animals were preimmunized against the virus.70 More clinical experience will be required to determine the optimal mode of virus delivery to malignancies but, as discussed below with some viruses, systemic administration may be critical to maximize the immune boosting effects of some platforms.71 In the meantime, as early OV clinical experience slowly accumulates, there is also a growing realization that apparently unrelated novel strategies to stimulate antitumor immunity, as well as the optimal application of traditional prime-boost immune vaccine sequencing, may have enormous potential in relation to OV, and it is to these that we turn next.
Immunotherapy to Complement Oncolytic Virotherapy: Activating Cellular Assassins to Kill Tumors
One of the exciting new strategies in immunotherapy is the adoptive T-cell therapy protocol developed by Rosenberg's group at the National Cancer Institute wherein tumor infiltrating lymphocytes (TILs) are isolated and expanded ex vivo before reinfusion back into the patient.72,73 The successful application of this approach requires significant in vivo expansion of the infused cell product and this only occurs if the patient first undergoes chemotherapeutic or radiotherapeutic lymphodepletion.74,75 While the response rates with this approach are breathtaking (objective tumor responses in up to 70% of cases75) patients experience sometimes lethal virus reactivation and other side effects of cytotoxic chemotherapy that reduce patients' quality of life.
Autologous T cells specific for Epstein–Barr virus (EBV) derived proteins have produced complete remission of disease in over 60% of patients with multiply relapsed or refractory EBV-associated lymphoma76 while ex vivo expanded TILs have produced complete remissions in patients with melanoma.73 However, the extension of these successes to a broader range of tumors will require strategies to overcome many different mechanisms of immune evasion used by tumors to avoid immune elimination.77 Perhaps, foremost of these mechanisms is poor presentation of tumor antigens to effector T cells. Not only do tumors downregulate molecules such as peptide transporter molecules,78 endoplasmic reticulum aminopeptidases79 and HLA class I molecules that are essential for antigen processing and presentation, but also they inhibit the maturation of local professional antigen-presenting cells by secreting IL-10 and transforming growth factor-β.80,81 This inhibits their expression of costimulatory molecules, like CD80, CD86, and 41BB-ligand that are essential for the expansion of T cells activated by recognition of antigen through their T-cell receptor. Tumors also directly inhibit T cells and instead of costimulatory molecules, many tumors express coinhibitory molecules like PD-L1 and Caecam1 that signal through SHP1/2 phosphatases to dephosphorylate the kinases induced by T-cell receptor ligation and costimulation. Some tumors may not themselves express inhibitory molecules, but recruit inhibitory cell types that do. T-regulatory cells, myeloid suppressor cells, and tumor stroma secrete IL-10, vascular endothelial growth factor, and transforming growth factor-β and express arginase and indoleamine 2,3-dioxygenase that deplete amino acids from the tumor environment and induce metabolic stress in T cells (Figure 1c). Several clinical trials have indicated that in vivo expansion of adoptively transferred T cells is an absolute requirement for tumor-specific T-cell efficacy, so that ensuring T-cell expansion after infusion has emerged as the holy grail of T-cell immunotherapy.77,82
T-cell numbers in the body are maintained at a homeostatic steady state unless disturbed by infection or lymphopenia. Inflammatory responses to most pathogens result from the recognition of pathogen-associated molecular patterns by receptors on innate immune system cells like dendritic cells and natural killer cells. For example, toll-like receptors recognize structures unique to pathogens such as bacterial lipopolysaccharides, flagellins or double stranded RNAs, and toll-like receptor ligation signals the production of cytokines and chemokines that recruit and induce expansion of T cells specific for the infecting pathogen. Once the pathogen is eliminated the innate immune responses becomes quiescent and T-cell numbers return to their steady state. Unfortunately, even if tumor cells present tumor-specific antigens (TAs), they do not express pathogen-associated molecular patterns and therefore fail to activate the innate immune system. However, vaccines may be used to increase T-cell numbers and oncolytic viruses may encode several toll-like receptor ligands that effectively activate innate immunity.83,84,85,86
Another strategy that not only targets tumor antigens, but also enhances T-cell expansion in cases where tumor antigens are weak or unidentified, investigators have developed multifunctional CARs (chimeric antigen receptors) that can be expressed as transgenes in T cells and redirect T cells to tumor antigens, regardless of their native T-cell receptor specificity. CAR expressing T cells therefore can recognize and kill both tumor targets through their CAR and the natural target through their T-cell receptor. Each CAR is composed of single chain antibody variable regions that recognize whole antigens on a tumor cell surface, linked to the zeta ζ-chain of the T-cell receptor to trigger killing and to the intracellular endodomains of costimulatory molecules to trigger proliferation. Such receptors eliminate the requirement for antigen processing and presentation on HLA molecules and provide signals that induce T-cell cytotoxicity and proliferation upon antigen-receptor engagement, in principle eliminating the requirement for professional antigen presentation. In clinical practice, this strategy has yet to be optimized to produce antitumor effects without toxicity. The incorporation of a CD28 endodomain alone has so far been insufficient to induce extensive in vivo proliferation of transduced T cells, although a complete response of follicular lymphoma to a T cells expressing a CD19CAR encoding CD28 and zeta chain signaling domains infused after non-myeloablative conditioning has been described.87 The addition of a 41BB endodomain to the CD28 endodomain onto a HER2-directed CAR to enhance T-cell proliferation produced a massive and fatal inflammatory response in a patient with metastatic colon cancer, who received a large dose (1010) of cells after non-myeloablative chemotherapy.88 Therefore, a strategy that balances in vivo proliferation and antitumor activity without toxicity is needed.
Marrying Adoptive Cell Therapy with OVs: TIL(s) Death Do Us Part?
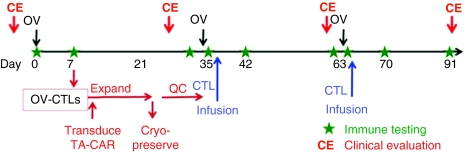
Our group has evaluated the use of EBV-specific T cells as cellular hosts for CARs, with the idea that the in vivo presentation of EBV antigens by persistently infected B cells would ensure the correct stimulation of gene-modified EBV-specific T cells. We redirected EBV-specific T cells to the disialoganglioside, GD2 expressed by neuroblastoma using a GD2-specific CAR. Transduced EBV-specific T cells persisted for longer than similarly transduced CD3-activated T cells in an intra patient comparison in which three complete tumor remissions in 11 patients with relapsed disease were observed as well as tumor responses in 50%.89 While EBV can produce potent antigenic stimulation in vivo, infused T cells compete with endogenous EBV-specific T cells that circulate with high frequency, and the degree of in vivo stimulation by EBV is uncontrollable. However, if T cells specific for oncolytic viruses could be produced from patients receiving virotherapy, then the T cells could be expanded, at will, using the OV as a vaccine. If the OV-specific T cells were modified to express a tumor-specific CAR, then virotherapy could be consolidated with tumor directed T-cell infusions (see Figure 2). The virotherapy would reduce the bulk of the tumor and modulate the immunosuppressive environment by activation of toll-like receptors and expression of transgenic immune enhancing cytokine like GM-CSF, while the T cells would eliminate residual and metastatic tumor cells that may be resistant to viral lysis. Additional modification of tumor cells with molecules that protect them from inhibitory ligands like transforming growth factor-β, may increase the potency of this approach.90 Importantly, this strategy should have little toxicity, and should not require cytotoxic lymphodepletion.
Figure 2.
Combining oncolytic virotherapy with tumor-specific T cells. Oncolytic virus (OV)-specific T cells could be expanded ex vivo after the second vaccination. If the individual was already exposed to the OV by vaccination or prior infection, then the T cells could be manufactured earlier. After activation, the T cells could be transduced with a retroviral vector expressing a tumor-specific chimeric antigen receptor (CAR) to redirect their specificity to a tumor antigen. The transduced cytotoxic T lymphocytes (CTLs) could then be infused after virotherapy when the tumor load would be reduced. The OV could then be used as a vaccine to induce T-cell expansion and maintain function.
OVs may also provide a solution to the problem of tumor antigen-specific T-cell anergy. While stimulation of peripheral blood T cells with viral antigens to which the donor has been exposed can reactivate polyclonal CD4+ and CD8+ T cells with specificity for multiple HLA class I and II epitopes in multiple viral antigens, this is rarely true for T cells specific for nonviral “self” antigens, which are frequently tolerized during development and hence are weak and anergic to in vitro reactivation and expansion for use as T-cell therapy. While it is known that TILs have been successfully expanded from melanoma patients and retain their antitumor specificity, not all tumors have TILs and not all TILs can be successfully expanded in vitro. Enhanced reactivation of TA-specific T cells in patients who received an oncolytic adenovirus encoding human GM-CSF has been reported.47 This characteristic of OVs may be exploited by the transgenic expression of tumor-encoded antigens, so that OV may be used not only to eliminate tumors, but to facilitate the ex vivo reactivation and expansion of TA-specific T cells that could subsequently be gene modified and infused as described above and further induced to expand by additional OV treatment.
Choreographing the Dance Between OVs and the Immune System—Getting the Most Out of Priming and Boosting
As discussed above, the ability of OVs to induce and express payloads of immune stimulating cytokines locally and to high levels within the tumor beds provides significant improvements in therapy both in animal models and in humans. Another strategy that is gaining support from several groups is to engineer OVs to encode and express TAAs. This has the advantage of expressing a relevant target antigen exactly at the time and site of an inflammatory reaction. Furthermore, the OV is likely to spread from the tumor bed and express the TAA in relevant immune organs (e.g., draining lymph nodes, spleen). Key to the success of this approach is selecting the correct/optimum tumor antigen. As one can imagine there are multiple parameters that could be considered in choosing a therapeutic target antigen and Cheever and colleagues have extensively reviewed a compendium of factors to be considered.3
Vigil and colleagues58 have engineered an oncolytic NDV to express an artificial tumor antigen (β-galactosidase) and demonstrated that repeat intralesional administration of this virus into mice bearing tumors expressing the antigen was much more effective therapeutically. This approach would be especially useful in a situation where the tumor expresses a “foreign antigen” such as a viral protein (e.g., human papillomavirus) or a somatically mutated cellular protein. In a variation of this approach Chuang and colleagues vaccinated animals with a foreign antigen (ovalbumin or OVA) and then subsequently treated intratumorally with a vaccinia virus engineered to express OVA. This “prime boost” scenario is designed to educate or prime the immune system to recognize OVA and then locally boost this response by virus directed expression of the antigen at high levels within the tumor bed.49 The observed increase in efficacy in this setting may reflect an epitope-spreading event within the tumor wherein new immune reactions against the tumor are generated. In principle by encoding OVA within the virus so that it is only expressed upon productive infection would allow systemic administration of the virus. This study demonstrated that it might be possible to design OVs to express antigens that the general population is already immunized against (e.g., diphtheria toxin) and then “boost” an already established immune response locally within the tumor through an oncolytic virus infection. Another prime boost strategy involves sequential treatment with two antigenically distinct oncolytic viruses expressing a common tumor associated antigen. Zhang and colleagues showed this is in principle possible by treating sequentially with oncolytic Semliki Forest Virus and Vaccinia Virus both encoding OVA.50
Bridle et al. have created a novel system that combines tumor-associated antigen immune stimulation with systemic oncolytic virus administration and may be the prime boost “pièce de résistance.”56,71 These authors reasoned that: (i) oncolytic destruction of tumors stimulates antitumor immunity, (ii) systemic administration of an OV is more likely to be effective against metastatic disease, (iii) OVs expressing tumor antigens increase immune response in infected tumors, (iv) prime:boost with heterologous expression systems is more likely to focus immunity on the tumor and not the vector.
To test their approach, they used a very aggressive and challenging tumor model which involved implanting the rapidly growing murine melanoma tumor (B16) in the brains of C57 mice.91 The B16 tumor expresses an endogenous cellular antigen, dopachrome tautomerase (DCT) and so the authors engineered an oncolytic version of VSV that overexpresses DCT upon productive infection. To take advantage of the prime:boost strategy Bridle and colleagues vaccinated tumor bearing animals with an adenovirus vaccine vector expressing DCT. The Ad-DCT vaccine provided only modest improvement in animal survival although it did successfully generate a cellular anti-DCT response within the animals. They then showed that their replicating VSV-DCT oncolytic virus on its own could target brain tumors following intravenous administration and indeed demonstrated the virus caused substantive tumor destruction, but very limited impact on animal survival. What happens when a systemic oncolytic prime boost is used in this model? The results were quite remarkable: (i) ~40% of the circulating T cells in treated animals were now directed against DCT; (ii) there was substantive immunity generated against additional tumor antigens (epitope spreading); (iii) the immune response to VSV antigens was actually dampened (compared to treatment with VSV alone); and (iv) most importantly, some durable cures were observed. These results are striking considering the rapid growth of this tumor and its location within the brain. Furthermore the authors had broken tolerance to an endogenously expressed cellular antigen. So what are the critical factors that lead to the impressive therapeutic outcomes observed by Bridle and colleagues. Sequential treatment with VSV-DCT (as both prime and boost) did not generate the impressive immune responses or improve animal survival arguing that a heterologous prime is required. Second, intravenous injection of the boosting vector is essential; intratumoral or subcutaneous VSV-DCT was ineffective. Perhaps a component of the activity requires that the VSV vector infects and expresses its TAA payload in a cell compartment that is only efficiently targeted by systemic administration. Other priming strategies are also effective with the oncolytic VSV-DCT boost suggesting that it may be effective with a number of vaccine platforms or perhaps in patients that have natural pre-existing anti-TAA immune responses that may just need a “jump-start”.
What's Next?
The interplay between OVs and the immune system is at times a love–hate relationship. The ability to deliver OVs to tumors by systemic administration is a huge value of the platform but of course may be curtailed by the evolution of the immune response against the vector itself. When given appropriately in animal models, OVs are clearly capable of harnessing the innate and adaptive arms of the immune response they elicit, potentially bringing together both aspects of human cancer immunotherapy recently endorsed by the clinical success of sipuleucel-T92 and ipilimumab.93 The current consensus from the available preclinical data is that the immune response to OV is neither pure hindrance nor pure help, but something of both. The challenge is how best to manipulate the system to maximize benefit for clinical application. Additional factors which impact on the interface between oncolytic virotherapy and cancer immunotherapy are multiple and include the method as well as route of delivery (“neat” OV versus cell delivered94), co-treatment with other modalities (biotherapeutics,95 small molecules,96,97 chemotherapy,98 and radiotherapy99 and tumor-associated factors such as the vasculature and interstitial pressure.100,101,102
Old-fashioned vaccinology, as well as complementary advances in cancer immunotherapy which were not initially developed with OVs in mind, are now suggesting further rational strategies for improved viro-immunotherapy. Using OVs as vaccines to expand T cells which can be genetically modified with CAR, and protocols based on classic prime-boost immune priming are two examples in a field of united immune- and viral-therapies which is already blossoming in the laboratory. We believe that it is time to move toward more clinical testing of the ideas presented in this review, including extensive monitoring of the immune response against both virus and tumor in patients, to provide as much translational data as possible for continued iterative testing and optimization between laboratory and clinic.
REFERENCES
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P.et al. (2008Core signaling pathways in human pancreatic cancers revealed by global genomic analyses Science 3211801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subarsky P., and, Hill RP. The hypoxic tumour microenvironment and metastatic progression. Clin Exp Metastasis. 2003;20:237–250. doi: 10.1023/a:1022939318102. [DOI] [PubMed] [Google Scholar]
- Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT.et al. (2009The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research Clin Cancer Res 155323–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Williamson CT, Prudhomme J, Bebb DG, Riabowol K, Lee PW.et al. (2010The viral tropism of two distinct oncolytic viruses, reovirus and myxoma virus, is modulated by cellular tumor suppressor gene status Oncogene 293990–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez M, García-Castro J., and, Alemany R. Oncolytic virotherapy for neuroblastoma. Discov Med. 2010;10:387–393. [PubMed] [Google Scholar]
- Boon T., and, Old LJ. Cancer Tumor antigens. Curr Opin Immunol. 1997;9:681–683. doi: 10.1016/s0952-7915(97)80049-0. [DOI] [PubMed] [Google Scholar]
- Boon T, Coulie PG., and, Van den Eynde B. Tumor antigens recognized by T cells. Immunol Today. 1997;18:267–268. doi: 10.1016/s0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- Ilett EJ, Prestwich RJ., and, Melcher AA. The evolving role of dendritic cells in cancer therapy. Expert Opin Biol Ther. 2010;10:369–379. doi: 10.1517/14712590903559830. [DOI] [PubMed] [Google Scholar]
- Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C.et al. (1997The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma Cancer 792320–2328. [DOI] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C.et al. (2006Type, density, and location of immune cells within human colorectal tumors predict clinical outcome Science 3131960–1964. [DOI] [PubMed] [Google Scholar]
- Alexandroff AB, Jackson AM, O'Donnell MA., and, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353:1689–1694. doi: 10.1016/S0140-6736(98)07422-4. [DOI] [PubMed] [Google Scholar]
- Degli-Esposti MA., and, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5:112–124. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- Andrews DM, Andoniou CE, Scalzo AA, van Dommelen SL, Wallace ME, Smyth MJ.et al. (2005Cross-talk between dendritic cells and natural killer cells in viral infection Mol Immunol 42547–555. [DOI] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB.et al. (2010Improved survival with ipilimumab in patients with metastatic melanoma N Engl J Med 363711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg J., and, Smyth MJ. NK cell-based cancer immunotherapy. Drug News Perspect. 2007;20:155–163. doi: 10.1358/dnp.2007.20.3.1092096. [DOI] [PubMed] [Google Scholar]
- Ullrich E, Ménard C, Flament C, Terme M, Mignot G, Bonmort M.et al. (2008Dendritic cells and innate defense against tumor cells Cytokine Growth Factor Rev 1979–92. [DOI] [PubMed] [Google Scholar]
- Mougiakakos D, Choudhury A, Lladser A, Kiessling R., and, Johansson CC. Regulatory T cells in cancer. Adv Cancer Res. 2010;107:57–117. doi: 10.1016/S0065-230X(10)07003-X. [DOI] [PubMed] [Google Scholar]
- von Mensdorff-Pouilly S. Vaccine-induced antibody responses in patients with carcinoma. Expert Rev Vaccines. 2010;9:579–594. doi: 10.1586/erv.10.51. [DOI] [PubMed] [Google Scholar]
- Hong C., and, Park SH. Application of natural killer T cells in antitumor immunotherapy. Crit Rev Immunol. 2007;27:511–525. doi: 10.1615/critrevimmunol.v27.i6.20. [DOI] [PubMed] [Google Scholar]
- Motohashi S., and, Nakayama T. Invariant natural killer T cell-based immunotherapy for cancer. Immunotherapy. 2009;1:73–82. doi: 10.2217/1750743X.1.1.73. [DOI] [PubMed] [Google Scholar]
- Müller-Hübenthal B, Azemar M, Lorenzen D, Huber M, Freudenberg MA, Galanos C.et al. (2009Tumour Biology: tumour-associated inflammation versus antitumor immunity Anticancer Res 294795–4805. [PubMed] [Google Scholar]
- Gupta R., and, Emens LA. GM-CSF-secreting vaccines for solid tumors: moving forward. Discov Med. 2010;10:52–60. [PMC free article] [PubMed] [Google Scholar]
- Harzstark AL., and, Small EJ. Immunotherapy for prostate cancer using antigen-loaded antigen-presenting cells: APC8015 (Provenge) Expert Opin Biol Ther. 2007;7:1275–1280. doi: 10.1517/14712598.7.8.1275. [DOI] [PubMed] [Google Scholar]
- Hodi FS. Overcoming immunological tolerance to melanoma: Targeting CTLA-4. Asia Pac J Clin Oncol. 2010;6 Suppl 1:S16–S23. doi: 10.1111/j.1743-7563.2010.01271.x. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Vitonis AF, Pinheiro SP, McKolanis JR, Fichorova RN, Brown KE.et al. (2010Mumps and ovarian cancer: modern interpretation of an historic association Cancer Causes Control 211193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölmel KF, Grange JM, Krone B, Mastrangelo G, Rossi CR, Henz BM.et al. (2005Prior immunisation of patients with malignant melanoma with vaccinia or BCG is associated with better survival. An European Organization for Research and Treatment of Cancer cohort study on 542 patients Eur J Cancer 41118–125. [DOI] [PubMed] [Google Scholar]
- Sobol PT, Boudreau JE, Stephenson K, Wan Y, Lichty BD., and, Mossman KL. Adaptive antiviral immunity is a determinant of the therapeutic success of oncolytic virotherapy. Mol Ther. 2011;19:335–344. doi: 10.1038/mt.2010.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benencia F, Courrèges MC, Fraser NW., and, Coukos G. Herpes virus oncolytic therapy reverses tumor immune dysfunction and facilitates tumor antigen presentation. Cancer Biol Ther. 2008;7:1194–1205. doi: 10.4161/cbt.7.8.6216. [DOI] [PubMed] [Google Scholar]
- Benencia F, Courrèges MC, Conejo-García JR, Mohamed-Hadley A, Zhang L, Buckanovich RJ.et al. (2005HSV oncolytic therapy upregulates interferon-inducible chemokines and recruits immune effector cells in ovarian cancer Mol Ther 12789–802. [DOI] [PubMed] [Google Scholar]
- Todo T, Martuza RL, Rabkin SD., and, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci USA. 2001;98:6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Dutuor A, Tao L, Fu X., and, Zhang X. Virotherapy with a type 2 herpes simplex virus-derived oncolytic virus induces potent antitumor immunity against neuroblastoma. Clin Cancer Res. 2007;13:316–322. doi: 10.1158/1078-0432.CCR-06-1625. [DOI] [PubMed] [Google Scholar]
- Li H, Dutuor A, Fu X., and, Zhang X. Induction of strong antitumor immunity by an HSV-2-based oncolytic virus in a murine mammary tumor model. J Gene Med. 2007;9:161–169. doi: 10.1002/jgm.1005. [DOI] [PubMed] [Google Scholar]
- Endo T, Toda M, Watanabe M, Iizuka Y, Kubota T, Kitajima M.et al. (2002In situ cancer vaccination with a replication-conditional HSV for the treatment of liver metastasis of colon cancer Cancer Gene Ther 9142–148. [DOI] [PubMed] [Google Scholar]
- Toda M, Rabkin SD, Kojima H., and, Martuza RL. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum Gene Ther. 1999;10:385–393. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- Toda M, Iizuka Y, Kawase T, Uyemura K., and, Kawakami Y. Immuno-viral therapy of brain tumors by combination of viral therapy with cancer vaccination using a replication-conditional HSV. Cancer Gene Ther. 2002;9:356–364. doi: 10.1038/sj.cgt.7700446. [DOI] [PubMed] [Google Scholar]
- Hu JC, Coffin RS, Davis CJ, Graham NJ, Groves N, Guest PJ.et al. (2006A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor Clin Cancer Res 126737–6747. [DOI] [PubMed] [Google Scholar]
- Harrington KJ, Hingorani M, Tanay MA, Hickey J, Bhide SA, Clarke PM.et al. (2010Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck Clin Cancer Res 164005–4015. [DOI] [PubMed] [Google Scholar]
- Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T.et al. (2009Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication Clin Cancer Res 154374–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich RJ, Errington F, Steele LP, Ilett EJ, Morgan RS, Harrington KJ.et al. (2009Reciprocal human dendritic cell-natural killer cell interactions induce antitumor activity following tumor cell infection by oncolytic reovirus J Immunol 1834312–4321. [DOI] [PubMed] [Google Scholar]
- White CL, Twigger KR, Vidal L, De Bono JS, Coffey M, Heinemann L.et al. (2008Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial Gene Ther 15911–920. [DOI] [PubMed] [Google Scholar]
- Errington F, Steele L, Prestwich R, Harrington KJ, Pandha HS, Vidal L.et al. (2008Reovirus activates human dendritic cells to promote innate antitumor immunity J Immunol 1806018–6026. [DOI] [PubMed] [Google Scholar]
- Gauvrit A, Brandler S, Sapede-Peroz C, Boisgerault N, Tangy F., and, Gregoire M. Measles virus induces oncolysis of mesothelioma cells and allows dendritic cells to cross-prime tumor-specific CD8 response. Cancer Res. 2008;68:4882–4892. doi: 10.1158/0008-5472.CAN-07-6265. [DOI] [PubMed] [Google Scholar]
- Li H, Peng KW, Dingli D, Kratzke RA., and, Russell SJ. Oncolytic measles viruses encoding interferon beta and the thyroidal sodium iodide symporter gene for mesothelioma virotherapy. Cancer Gene Ther. 2010;17:550–558. doi: 10.1038/cgt.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzerling L, Künzi V, Oberholzer PA, Kündig T, Naim H., and, Dummer R. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood. 2005;106:2287–2294. doi: 10.1182/blood-2004-11-4558. [DOI] [PubMed] [Google Scholar]
- Gürlevik E, Woller N, Strüver N, Schache P, Kloos A, Manns MP.et al. (2010Selectivity of oncolytic viral replication prevents antiviral immune response and toxicity, but does not improve antitumoral immunity Mol Ther 181972–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol JA, Zhu M, Ji H, Mina M, Xie Y, Clarke L.et al. (2003In vitro and in vivo activities of an oncolytic adenoviral vector designed to express GM-CSF Mol Ther 7755–764. [DOI] [PubMed] [Google Scholar]
- Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Arstila PT, Ugolini M.et al. (2010Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients Cancer Res 704297–4309. [DOI] [PubMed] [Google Scholar]
- Mastrangelo MJ, Maguire HC, Jr, Eisenlohr LC, Laughlin CE, Monken CE, McCue PA.et al. (1999Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma Cancer Gene Ther 6409–422. [DOI] [PubMed] [Google Scholar]
- Chuang CM, Monie A, Wu A, Pai SI., and, Hung CF. Combination of viral oncolysis and tumor-specific immunity to control established tumors. Clin Cancer Res. 2009;15:4581–4588. doi: 10.1158/1078-0432.CCR-08-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Tsai YC, Monie A, Wu TC., and, Hung CF. Enhancing the therapeutic effect against ovarian cancer through a combination of viral oncolysis and antigen-specific immunotherapy. Mol Ther. 2010;18:692–699. doi: 10.1038/mt.2009.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn DH, Wang Y, Le Boeuf F, Bell J., and, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J.et al. (2007Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus Cancer Res 672840–2848. [DOI] [PubMed] [Google Scholar]
- Wongthida P, Diaz RM, Galivo F, Kottke T, Thompson J, Pulido J.et al. (2010Type III IFN interleukin-28 mediates the antitumor efficacy of oncolytic virus VSV in immune-competent mouse models of cancer Cancer Res 704539–4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galivo F, Diaz RM, Thanarajasingam U, Jevremovic D, Wongthida P, Thompson J.et al. (2010Interference of CD40L-mediated tumor immunotherapy by oncolytic vesicular stomatitis virus Hum Gene Ther 21439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmon CL, Saloura V, Fridlender ZG, Wongthida P, Diaz RM, Thompson J.et al. (2009Expression of IFN-beta enhances both efficacy and safety of oncolytic vesicular stomatitis virus for therapy of mesothelioma Cancer Res 697713–7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridle BW, Hanson S., and, Lichty BD. Combining oncolytic virotherapy and tumour vaccination. Cytokine Growth Factor Rev. 2010;21:143–148. doi: 10.1016/j.cytogfr.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Qiao J, Kottke T, Willmon C, Galivo F, Wongthida P, Diaz RM.et al. (2008Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy Nat Med 1437–44. [DOI] [PubMed] [Google Scholar]
- Vigil A, Martinez O, Chua MA., and, García-Sastre A. Recombinant Newcastle disease virus as a vaccine vector for cancer therapy. Mol Ther. 2008;16:1883–1890. doi: 10.1038/mt.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn DH., and, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- Mastrangelo MJ., and, Lattime EC. Virotherapy clinical trials for regional disease: in situ immune modulation using recombinant poxvirus vectors. Cancer Gene Ther. 2002;9:1013–1021. doi: 10.1038/sj.cgt.7700538. [DOI] [PubMed] [Google Scholar]
- Prestwich RJ, Errington F, Ilett EJ, Morgan RS, Scott KJ, Kottke T.et al. (2008Tumor infection by oncolytic reovirus primes adaptive antitumor immunity Clin Cancer Res 147358–7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottke T, Thompson J, Diaz RM, Pulido J, Willmon C, Coffey M.et al. (2009Improved systemic delivery of oncolytic reovirus to established tumors using preconditioning with cyclophosphamide-mediated Treg modulation and interleukin-2 Clin Cancer Res 15561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K.et al. (1993Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity Proc Natl Acad Sci USA 903539–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC.et al. (2008Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial Lancet Oncol 9533–542. [DOI] [PubMed] [Google Scholar]
- Kim JH, Oh JY, Park BH, Lee DE, Kim JS, Park HE.et al. (2006Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF Mol Ther 14361–370. [DOI] [PubMed] [Google Scholar]
- Liu BL, Robinson M, Han ZQ, Branston RH, English C, Reay P.et al. (2003ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties Gene Ther 10292–303. [DOI] [PubMed] [Google Scholar]
- Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G.et al. (2009Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma J Clin Oncol 275763–5771. [DOI] [PubMed] [Google Scholar]
- Kaur B, Cripe TP., and, Chiocca EA. “Buy one get one free”: armed viruses for the treatment of cancer cells and their microenvironment. Curr Gene Ther. 2009;9:341–355. doi: 10.2174/156652309789753329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallucci S., and, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- Hu W, Davis JJ, Zhu H, Dong F, Guo W, Ang J.et al. (2007Redirecting adaptive immunity against foreign antigens to tumors for cancer therapy Cancer Biol Ther 61773–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridle BW, Stephenson KB, Boudreau JE, Koshy S, Kazdhan N, Pullenayegum E.et al. (2010Potentiating cancer immunotherapy using an oncolytic virus Mol Ther 181430–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff SL, Smith FO, Klapper JA, Sherry R, Wunderlich JR, Steinberg SM.et al. (2010Tumor infiltrating lymphocyte therapy for metastatic melanoma: analysis of tumors resected for TIL J Immunother 33840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Restifo NP, Yang JC, Morgan RA., and, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP.et al. (2005Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma J Clin Oncol 232346–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U.et al. (2008Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens J Clin Oncol 265233–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollard CM, Gottschalk S, Leen AM, Weiss H, Straathof KC, Carrum G.et al. (2007Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer Blood 1102838–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Rooney CM., and, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–265. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- Maeurer MJ, Gollin SM, Storkus WJ, Swaney W, Karbach J, Martin D.et al. (1996Tumor escape from immune recognition: loss of HLA-A2 melanoma cell surface expression is associated with a complex rearrangement of the short arm of chromosome 6 Clin Cancer Res 2641–652. [PubMed] [Google Scholar]
- Fruci D, Ferracuti S, Limongi MZ, Cunsolo V, Giorda E, Fraioli R.et al. (2006Expression of endoplasmic reticulum aminopeptidases in EBV-B cell lines from healthy donors and in leukemia/lymphoma, carcinoma, and melanoma cell lines J Immunol 1764869–4879. [DOI] [PubMed] [Google Scholar]
- Strobl H., and, Knapp W. TGF-beta1 regulation of dendritic cells. Microbes Infect. 1999;1:1283–1290. doi: 10.1016/s1286-4579(99)00256-7. [DOI] [PubMed] [Google Scholar]
- Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW.et al. (1991IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells J Immunol 1463444–3451. [PubMed] [Google Scholar]
- Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y.et al. (1998Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients Blood 921549–1555. [PubMed] [Google Scholar]
- Shi Z, Cai Z, Sanchez A, Zhang T, Wen S, Wang J.et al. (2011A novel toll-like receptor that recognizes vesicular stomatitis virus J Biol Chem 2864517–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M, Mitchell LM, Puckett S, Brzoza-Lewis KL, Lyles DS., and, Hiltbold EM. Vesicular stomatitis virus M protein mutant stimulates maturation of Toll-like receptor 7 (TLR7)-positive dendritic cells through TLR-dependent and -independent mechanisms. J Virol. 2009;83:2962–2975. doi: 10.1128/JVI.02030-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appledorn DM, Aldhamen YA, Godbehere S, Seregin SS., and, Amalfitano A. Sublingual administration of an adenovirus serotype 5 (Ad5)-based vaccine confirms Toll-like receptor agonist activity in the oral cavity and elicits improved mucosal and systemic cell-mediated responses against HIV antigens despite preexisting Ad5 immunity. Clin Vaccine Immunol. 2011;18:150–160. doi: 10.1128/CVI.00341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley M, Martinez J, Huang X., and, Yang Y. A critical role for direct TLR2-MyD88 signaling in CD8 T-cell clonal expansion and memory formation following vaccinia viral infection. Blood. 2009;113:2256–2264. doi: 10.1182/blood-2008-03-148809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA.et al. (2010Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19 Blood 1164099–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM., and, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G.et al. (2008Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma Nat Med 141264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollard CM, Rössig C, Calonge MJ, Huls MH, Wagner HJ, Massague J.et al. (2002Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity Blood 993179–3187. [DOI] [PubMed] [Google Scholar]
- Bridle BW, Li J, Jiang S, Chang R, Lichty BD, Bramson JL.et al. (2010Immunotherapy can reject intracranial tumor cells without damaging the brain despite sharing the target antigen J Immunol 1844269–4275. [DOI] [PubMed] [Google Scholar]
- Lü C, Williams AK, Chalasani V, Martínez CH., and, Chin J. Immunotherapy for metastatic prostate cancer: where are we at with sipuleucel-T. Expert Opin Biol Ther. 2011;11:99–108. doi: 10.1517/14712598.2011.538677. [DOI] [PubMed] [Google Scholar]
- Tarhini A, Lo E., and, Minor DR. Releasing the brake on the immune system: ipilimumab in melanoma and other tumors. Cancer Biother Radiopharm. 2010;25:601–613. doi: 10.1089/cbr.2010.0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima H, Kaur B., and, Chiocca EA. Directing systemic oncolytic viral delivery to tumors via carrier cells. Cytokine Growth Factor Rev. 2010;21:119–126. doi: 10.1016/j.cytogfr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Boeuf F, Diallo JS, McCart JA, Thorne S, Falls T, Stanford M.et al. (2010Synergistic interaction between oncolytic viruses augments tumor killing Mol Ther 18888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo JS, Le Boeuf F, Lai F, Cox J, Vaha-Koskela M, Abdelbary H.et al. (2010A high-throughput pharmacoviral approach identifies novel oncolytic virus sensitizers Mol Ther 181123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyên TL, Abdelbary H, Arguello M, Breitbach C, Leveille S, Diallo JS.et al. (2008Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis Proc Natl Acad Sci USA 10514981–14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandha HS, Heinemann L, Simpson GR, Melcher A, Prestwich R, Errington F.et al. (2009Synergistic effects of oncolytic reovirus and cisplatin chemotherapy in murine malignant melanoma Clin Cancer Res 156158–6166. [DOI] [PubMed] [Google Scholar]
- Dai MH, Zamarin D, Gao SP, Chou TC, Gonzalez L, Lin SF.et al. (2010Synergistic action of oncolytic herpes simplex virus and radiotherapy in pancreatic cancer cell lines Br J Surg 971385–1394. [DOI] [PubMed] [Google Scholar]
- Wojton J., and, Kaur B. Impact of tumor microenvironment on oncolytic viral therapy. Cytokine Growth Factor Rev. 2010;21:127–134. doi: 10.1016/j.cytogfr.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbach CJ, Paterson JM, Lemay CG, Falls TJ, McGuire A, Parato KA.et al. (2007Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow Mol Ther 151686–1693. [DOI] [PubMed] [Google Scholar]
- De Silva N, Atkins H, Kirn DH, Bell JC., and, Breitbach CJ. Double trouble for tumours: exploiting the tumour microenvironment to enhance anticancer effect of oncolytic viruses. Cytokine Growth Factor Rev. 2010;21:135–141. doi: 10.1016/j.cytogfr.2010.02.007. [DOI] [PubMed] [Google Scholar]