Abstract
Noninvasive detection and in vivo imaging of apoptosis plays a critical role in the development of therapeutics in many different fields including cancer. We have developed an apoptosis biosensor by fusing green fluorescent protein (GFP) to the N-terminus of the naturally secreted Gaussia luciferase separated by a caspase-3 cleavage peptide consisting of aspartic acid (D), glutamic acid (E), valine (V), and aspartic acid (D) or DEVD. We showed that this fusion is retained in the cytoplasm of cells in an inactive form. Upon apoptosis, the DEVD peptide is cleaved in response to caspase-3 activation, freeing ssGluc, which can now enter the secretory pathway where it is folded properly and is released from the cells and can be detected in the conditioned medium in culture or in blood of live animals ex vivo over time. Because Gluc is secreted from cells via conventional pathway through the endoplasmic reticulum (ER), Golgi and vesicles, we showed that the presence of Gluc in these compartments in response to apoptosis can be visualized in vivo using bioluminescence imaging. This reporter provides a valuable tool for imaging and real-time monitoring of apoptosis and is compatible with high-throughput functional screening application in cultured cells and animal models.
Introduction
Programmed cell death or apoptosis is an important cellular event that plays a critical role in the treatment of different disorders. Two common pathways of apoptosis are known:1,2,3 (i) an intrinsic pathway which relies on mitochondrial dysfunction followed by cytochrome c release leading to activation of series of caspases including caspase-3, -6 and -7; (ii) an extrinsic pathway through binding of toxic molecules to a death-receptor on the cell surface initiating a chain of events including activation of the BH3 interacting domain death agonist (Bid) and caspase-8 leading to activation of caspase-3 followed by cell death. In cancer, many therapeutic modalities rely on the activation of apoptosis specifically in tumor cells.4,5,6 For instance, the alkylating agent temozolomide methylates the DNA at a specific guanine residue leading to intrinsic activation of apoptosis in tumor cells.7,8 On the other hand, the tumor necrosis factor-related apoptosis- inducing ligand (TRAIL) binds to death receptors present specifically on tumor cells leading to activation of the extrinsic apoptosis pathway.9,10
Development of methods to monitor apoptosis noninvasively can help advance novel therapeutics for different diseases. Many techniques have been established for detection of apoptosis in vivo. Since caspase-3 activation is the end product of both the intrinsic and extrinsic pathways, the most commonly used apoptosis reporters are based on a small peptide, consisting of aspartic acid (D), glutamic acid (E), valine (V), and aspartic acid (D) or DEVD, which is recognized and cleaved by this protease. In one study, firefly luciferase (Fluc) was cloned between two modified mouse estrogen receptor regulatory domains separated by DEVD (ER-DEVD-Fluc-DEVD-ER).11 The presence of these regulatory domains silences the activity of Fluc. During apoptosis, active caspase-3 cleaves the DEVD sequence, freeing the Fluc which is then detected using in vivo bioluminescence imaging after injection of its substrate, -luciferin. In another study, multimodal imaging of apoptosis was achieved by fusing the monomeric red fluorescent protein (mRFP) to HSV1 thymidine kinase (TK) and Fluc all separated by DEVD sequence (mRFP-DEVD-TK-DEVD-Fluc).12 Fusing these three reporters together leads to a dramatic decrease in their activity. Upon caspase-3 activation and DEVD cleavage, each reporter will be free which is then detected using the corresponding imaging modality: fluorescence for mRFP, positron emission tomography for TK and bioluminescence for Fluc. Recently, a fluorescently labeled activity-based probes using an acyloxymethyl ketone which covalently label active caspases has been developed and successfully used for detection of apoptosis in vivo.13 Although these methods have been shown to be useful for in vivo imaging, kinetic analysis of caspase-3 activation in real-time was not easily achieved. Furthermore, the sensitivity of these reporters remains an issue because there was only a moderate increase (twofold) in most of these reporters activity in response to apoptosis. Most of these techniques are not well suited for high-throughput functional screening applications and does not allow kinetic analysis over time.
Recently, we characterized a luciferase from the marine copepod Gaussia princeps (Gluc) which is >2,000-fold more sensitive than commonly used luciferases such as Fluc when expressed in mammalian cells.14 Since Gluc is naturally secreted, we showed that in vivo processes can be monitored noninvasively and in real-time by assaying few microliters of blood for this luciferase activity.15,16 We also showed that secreted Gluc is well suited for high-throughput functional screening applications and allows study of drug kinetics because its activity in the medium can be measured at different time points from a single well, keeping the viable cells intact for conformational analysis.17,18 In this study, we show that Gaussia luciferase requires processing through the secretory pathway for proper folding and full activity and that Gluc looses >90% of its activity once it is modified to be retained in the cytoplasm. We fused Gluc including its signal sequence (ssGluc) to the C-terminus of green fluorescent protein (GFP) separated by DEVD, the caspase-3 cleavage peptide (GFP-DEVD-ssGluc). The presence of GFP in this fusion protein forces the Gluc to reside in the cytoplasm in an inactive form. Upon caspase-3 activation during apoptosis, DEVD is cleaved, therefore freeing ssGluc which can then enter the secretory pathway where it is folded properly and is released from the cells and can be detected in the conditioned medium in culture or in blood of live animals ex vivo over time. Furthermore, since the Gluc secretes from cells through the conventional cell secretory pathway, i.e., rough endoplasmic reticulum (ER), Golgi and vesicles,19 the presence of this reporter in these compartments in response to apoptosis can be imaged in vivo using Gluc bioluminescence imaging. This reporter provides a valuable tool for imaging and real-time monitoring of apoptosis which is compatible with high-throughput functional screening application in cultured cells and animal models.
Results
Construction of the apoptosis biosensor
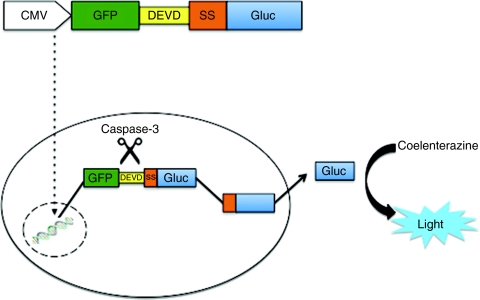
We have developed a fusion protein which upon cleavage by caspase-3, it releases a secreted luciferase into the conditioned medium of cultured cells or into blood of mice bearing cells expressing it in vivo. We fused GFP to the N-terminal region of the Gaussia luciferase (including its signal sequence, ssGluc) separated by a DEVD, the caspase-3 cleavage peptide (GFP-DEVD-ssGluc; Figure 1). This fusion protein resides in the cytoplasm. Upon caspase-3 activation during apoptosis, the DEVD sequence is cleaved, freeing ssGluc, which can then enter the ER and be secreted outside of the cell (Figure 1). We cloned the expression cassette for this fusion into a lentivirus vector under the control of CMV promoter.
Figure 1.
Apoptosis biosensor. Schematic overview of the GFP-DEVD-ssGluc system. ptGFP lacking stop codon is fused to the N-terminal region of the Gaussia luciferase (including its signal sequence, ssGluc) separated by a DEVD, the caspase-3 cleavage peptide (GFP-DEVD-ssGluc). This fusion protein resides in the cytoplasm. Upon caspase-3 activation during apoptosis, the DEVD sequence is cleaved, freeing ssGluc, which can then enter the endoplasmic reticulum (ER) and be secreted into the conditioned medium of cultured cells or into blood of mice bearing cells expressing it in vivo. GFP, green fluorescent protein; ptGFP, Ptilosarcus GFP.
Gluc is not active when retained in the cytoplasm
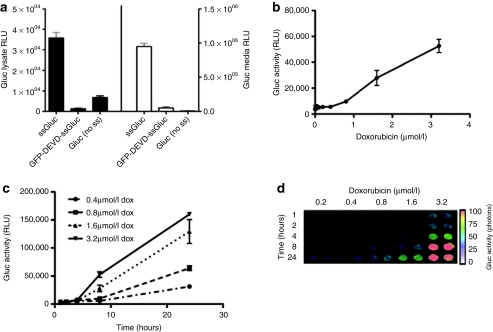
We compared the intracellular and the extracellular (conditioned medium) activity of the secreted Gluc, a cytoplasmic version of Gluc (Gluc cDNA cloned without the signal sequence; no ss) as well as the apoptosis biosensor GFP-DEVD-ssGluc. 293T human fibroblast cells were transfected with plasmid expressing either of these reporters under the CMV promoter. Forty-eight hours later, cell lysates or conditioned medium were assayed for Gluc activity. As expected, since the GFP-DEVD-ssGluc and Gluc without the ss (no ss) reside in the cytoplasm, their activities in the conditioned medium were >100× lower as compared to wild-type Gluc (Figure 2a). On the other hand, the intracellular activity of GFP-DEVD-ssGluc was 40-fold lower as compared to ssGluc showing that Gluc requires to enter the secretory pathway to be folded properly by different chaperone proteins and therefore to be active. Similarly, the activity of Gluc without the signal sequence was eightfold lower compare to native ssGluc (Figure 2a).
Figure 2.
Real-time monitoring of apoptosis in culture. (a) Gluc is inactive when it resides in the cytoplasm. 293T human fibroblast cells were transfected in triplicates with plasmid expressing either native secreted Gluc (ssGluc), GFP-DEVD-ssGluc, or Gluc without the signal sequence (no ss). Forty-eight hours later, cell lysates or conditioned medium were assayed in triplicates for the Gluc activity. (b,c) Gli36 human glioma cells stably expressing GFP-DEVD-ssGluc were plated in a 6-well plate and treated with different amounts of doxorubicin in triplicates. (b) Twenty-four hours later, or at (c) different time points, 50 µl aliquots of the conditioned medium was collected in triplicates and assayed for Gluc activity using a luminometer. (d) Aliquots (in duplicates) of conditioned medium from (c) were imaged for Gluc activity using a CCD camera. For Gluc activity assay, data presented as mean ± SD for the three biological and three experimental replicates. GFP, green fluorescent protein.
GFP-DEVD-ssGluc as a sensitive apoptosis reporter
To determine the usefulness of the GFP-DEVD-ssGluc in detecting apoptosis, Gli36 human glioma cells stably expressing this reporter were plated in a 6-well plate and treated with different amounts of doxorubicin. Twenty-four hours later, the conditioned medium was collected and assayed for Gluc activity. An increase in Gluc level in the medium with increasing doxorubicin concentration was observed. At the 3.2 µmol/l doxorubicin dose, a more than tenfold increase in Gluc level as compared to the untreated sample was observed showing the sensitivity of our reporter in detecting apoptosis (Figure 2b).
To check the efficacy of this biosensor in monitoring apoptosis in real-time, Gli36 cells expressing the GFP-DEVD-ssGluc were treated with different concentrations of doxorubicin. At different time points, 50 µl aliquots of the conditioned medium were collected and assayed for Gluc activity using the luminometer and imaged using a CCD camera (Figure 2c,d). An increase in Gluc level in the conditioned medium was observed over time confirming that apoptosis can be monitored in real-time from the same well, keeping the cells viable for validation analysis.
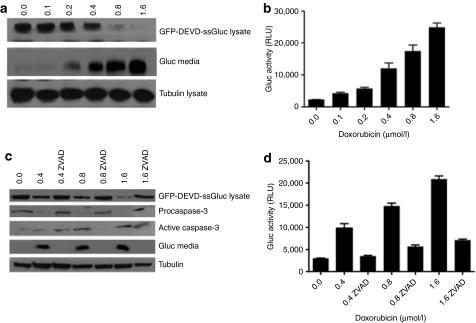
To corroborate these results using a different assay, Gli36 cells-expressing GFP-DEVD-ssGluc were treated with different concentration of doxorubicin. Sixteen hours later, 50 µl aliquots of conditioned medium were assayed for Gluc activity. At the same time, cell lysates as well as conditioned medium were analyzed by western blotting using an antibody against Gluc. As expected, upon increasing dose of doxorubicin, the GFP-DEVD-ssGluc fusion in the cell lysate was decreased due to cleavage of the DEVD peptide, leading to an increase in the free Gluc level in the medium in a dose-dependent manner, which correlated with an increase in the Gluc activity using the luminometer (Figure 3a,b).
Figure 3.
GFP-DEVD-ssGluc is cleaved specifically in response to apoptosis. (a,b) Gli36 cells-expressing GFP-DEVD-ssGluc were treated with different concentration of doxorubicin in triplicates. Sixteen hours later, cell lysates as well as conditioned medium were analyzed by western blotting using an antibody against (a) Gluc and 50 µl aliquots of conditioned medium were assayed for (b) Gluc activity. (c,d) Gli36 cells-expressing GFP-DEVD-ssGluc were treated with either different amounts of doxorubicin in triplicates, or a combination of both doxorubicin and ZVAD-fmk, a caspase inhibitor. Sixteen hours later, cell lysates and conditioned medium were analyzed by western blotting for Gluc as well as caspase-3 using (c) specific antibodies and conditioned medium was assayed for (d) Gluc activity. Western blots shown are representative from a single well of different groups. For Gluc assay, data presented as mean ± SD for three biological and three experimental replicates.
To confirm that the activity of released Gluc in the conditioned medium is strictly due to caspase-3 activation and therefore apoptosis, Gli36 cells-expressing GFP-DEVD-ssGluc were treated with either different amounts of doxorubicin, or a combination of both doxorubicin and ZVAD-fmk, a caspase inhibitor. Sixteen hours later, cell lysates were analyzed by western blotting for Gluc as well as caspase-3 using specific antibodies. Upon treatment with doxorubicin alone, pro-caspase-3 level in the cell lysates decreased as the cleaved active caspase-3 increased which correlated with an increase in the Gluc level in the medium (Figure 3c,d). As expected, upon inhibiting caspase-3 with ZVAD-fmk, the active caspase-3, and the Gluc levels in the medium were comparable to their levels in the nontreated control sample (Figure 3c). These results were also confirmed by the Gluc assay in the conditioned medium which showed similar pattern of Gluc levels observed by western blot analysis (Figure 3d).
Monitoring of extrinsic apoptosis pathway
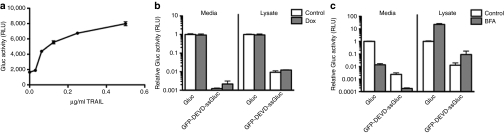
Since doxorubicin induces the intrinsic apoptosis pathway, we tested whether our sensor can also be used to detect the extrinsic pathway. Gli36 cells-expressing GFP-DEVD-ssGluc were plated in a 96-well plate and treated with different amounts of the TRAIL. This protein is known to bind to death receptors on tumor cells initiating series of cascades leading to caspase-3 activation. Sixteen hours post-treatment, conditioned medium was assayed for Gluc activity. As expected, an increase in Gluc level in the medium correlated with an increase in the TRAIL dose showing that this sensor can detect both intrinsic and extrinsic apoptosis pathways (Figure 4a).
Figure 4.
Monitoring of extrinsic apoptosis and validation of Gluc secretion. (a) GFP-DEVD-ssGluc as a biosensor for extrinsic apoptosis. Gli36 cells-expressing GFP-DEVD-ssGluc were plated in a 96-well plate and treated with different concentrations of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in triplicates. Sixteen hours later, aliquots of conditioned medium (in triplicates) were assayed for Gluc activity. (b–c) Cleaved ssGluc is secreted from cells via conventional secretory pathway. Gli36 cells-expressing GFP-DEVD-ssGluc or native Gluc were treated with phosphate-buffered saline (PBS) or brefeldin A (BFA; 5 µg/ml) for 2 hours. Cells were then treated with either PBS or doxorubicin (0.8 µmol/l) in triplicates and, 8 hours later, conditioned media and lysates were assayed for Gluc activity. Data presented as mean ± SD for relative Gluc activity in which the signal obtained from control native ssGluc treated with PBS only in b or treated with dox only (no BFA) in c is set to 1.
Active Gluc released upon apoptosis, like native Gluc, is secreted from cells via the conventional secretory pathway
Recently, we showed that native Gluc leaves the cells via the conventional secretory pathway, i.e., ER, Golgi and vesicles.19 In order to show that upon apoptosis, GFP-DEVD-ssGluc is cleaved, freeing ssGluc which then enters the ER and secretes from cells in a similar manner to native Gluc, Gli36 cells-expressing GFP-DEVD-ssGluc or native Gluc were treated with phosphate-buffered saline (PBS) or brefeldin A (5 µg/ml) for 2 hours. This drug inhibits anterograde ER export to the Golgi, but allows retrograde Golgi–ER transport, resulting in a fusion of the ER and the Golgi and blocking of secretion. Cells were then treated with PBS or doxorubicin (0.8 µmol/l) and, 8 hours later, conditioned media and lysates were assayed for Gluc activity. As expected, conditioned medium from GFP-DEVD-ssGluc cells treated with doxorubicin alone showed around twofold increase in Gluc activity whereas the wild-type Gluc cells did not show any change (Figure 4b). Signals from conditioned medium of either native Gluc or GFP-DEVD-ssGluc cells treated with brefeldin A and doxorubicin decreased significantly (80-fold for Gluc versus 15-fold for GFP-DEVD-ssGluc; Figure 4c). On the opposite, Gluc activity in both lysates increased (20-fold for Gluc and 8-fold for GFP-DEVD-ssGluc) proving that cleaved Gluc enters the ER and leaves the cells via the conventional secretory pathway similar to native Gluc.
Noninvasive imaging and ex vivo blood monitoring of apoptosis in animal models
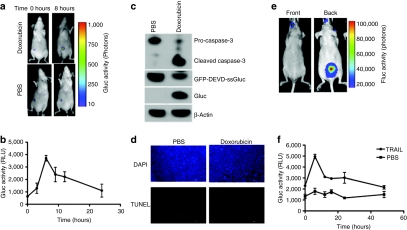
In order to show that the apoptosis biosensor developed here is useful for in vivo imaging and real-time monitoring of apoptosis noninvasively, Gli36 human glioma cells-expressing GFP-DEVD-ssGluc were implanted subcutaneously in nude mice. Three weeks later, tumors were either injected with PBS (control) or with 10 µg doxorubicin (n = 5 per group). Immediately before and 8 hours postinjection, mice were intravenously injected with coelenterazine and imaged using a CCD camera. Around fivefold increase in the Gluc tumor level was observed in the doxorubicin treated-tumors with no change in PBS-treated controls showing that this reporter is sensitive in imaging apoptosis in vivo (Figure 5a). In the same experiment, 5 µl blood (in triplicates) were withdrawn from a tail cut at different time points and assayed for the Gluc activity upon addition of coelenterazine using a luminometer. A significant increase in Gluc blood level over time was observed, as compared to time zero, reaching a maximum at 8 hours (P < 0.001 as calculated by Student's t-test) showing that this reporter is sensitive for real-time monitoring of apoptosis ex vivo (Figure 5b). To confirm these findings, a set of tumors from each group were removed 8 hours post-treatment and assayed either by western blotting for Gluc cleavage and caspase-3 activation or TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling) staining for DNA fragmentation, a typical feature of apoptosis. Doxorubicin-treated tumors versus control tumors showed lower levels of GFP-DEVD-ssGluc fusion, increased levels of both free Gluc and cleaved caspase-3 as well as increased TUNEL positive cells confirming cleavage of the biosensor through apoptosis induction (Figure 5c,d). To corroborate these findings in a clinically relevant in vivo model, nude mice were injected into the left ventricles with MDA-MB-231BR breast cancer cells engineered to stably express Fluc. Four weeks postinjection, small foci in different tissues were observed with Fluc bioluminescence imaging showing that these cells have metastasized to different tissues (Figure 5e). Mice were then injected with either PBS or TRAIL (0.5 mg/kg body weight; n = 6 per group). Immediately before and at different time points post-treatment, 5 µl blood (in triplicates) were withdrawn and assayed for Gluc activity. A significant increase in Gluc activity in blood was observed 6 hours postinjection as compared to time zero (P < 0.001; Student's t-test) in the TRAIL-treated mice and not control showing that this reporter can be used to monitor apoptosis in systemic metastasis model (Figure 5f). The Gluc blood levels slowly decayed to basal level over 48 hours. The same mice were also imaged with Gluc bioluminescence before and 8 hours post-treatment, however, no Gluc activity was detected showing that this reporter is not sensitive enough to image apoptosis in systemic metastasis or small foci in deep tissues (data not shown).
Figure 5.
In vivo imaging and ex vivo monitoring of apoptosis. (a) Gli36 cells-expressing GFP-DEVD-ssGluc were implanted subcutaneously in 10 nude mice. Two weeks later, tumors were injected with either PBS (control) or 10 µg of doxorubicin (n = 5 per group). Immediately before and after 8 hours injection, mice were intravenously (i.v.) injected with coelenterazine and imaged using a CCD camera. (b) In the same experiment, 5 µl blood (in triplicates) was withdrawn at different time points and assayed for Gluc activity using a luminometer. (c) A set of tumors from each group in a were removed 8 hours post-treatment and assayed by western blotting using antibodies against Gluc and caspase-3. (d) A set of mice from (a) were perfused with 4% paraformaldehyde in PBS, tumors were removed, fresh frozen, sectioned into 10-µm sections and analyzed using TUNEL (red) as well as DAPI (blue) staining. Bar = 100 µm. (e,f) MDA-MB-231BR breast cancer cells were injected into the left ventricle of female nude mice. Four weeks later, mice were imaged with Fluc bioluminescence after intraperitoneal (i.p.) injection of -luciferin. A representative mouse imaged front and back showing that these cells did metastasize (e). Mice were then i.p. injected with either PBS or TRAIL (n = 6 per group) and at different time points, 5 µl blood (in triplicates) was withdrawn at different time points and assayed for Gluc activity using a luminometer (f). Data shown as mean ± SD.
Discussion
Noninvasive imaging and real-time monitoring of apoptosis play a crucial role in the development and testing the efficiency of novel therapeutics in different fields including cancer. We have developed a multimodal apoptosis biosensor by fusing GFP to the N-terminus of the naturally secreted Gaussia luciferase separated by DEVD, the caspase-3 cleavage peptide. We showed that this reporter allows simultaneous real-time monitoring of apoptosis by assaying an aliquot of the conditioned medium in culture or few microliters of blood ex vivo for the Gluc activity at different time points. Moreover, since the native Gluc is secreted from cells via the conventional secretory pathway, i.e., ER, Golgi and vesicles, the presence of Gluc in these compartments in response to apoptosis can be imaged using in vivo bioluminescence. This reporter has several advantages over other commonly used bioluminescent-based apoptosis reporter: (i) Gluc is over 2,000-fold more sensitive than Fluc or Renilla luciferase and >20,000-fold than the secreted alkaline phosphatase when expressed in mammalian cells;14,19 (ii) Gluc is naturally secreted, therefore its activity can be monitored over time by assaying an aliquot of conditioned medium at different time points, keeping the cells intact for conformational analysis; (iii) Gluc assay is compatible with functional screening and allows the study of apoptosis kinetics from the same well in a high-throughput format;17,18 (iv) In vivo, Gluc level in the blood can be used to monitor biological processes in real-time;15,16 (v) Gluc has a half-life of <10 minutes in circulation16 and therefore its level/activity in the blood is a true estimation of caspase-3 activation at a given time point.
One of the major limitations of bioluminescence imaging is light absorption by pigmented molecules such as hemoglobin and scattering by mammalian tissues, thereby limiting the usefulness of this technology in deep tissues. When we applied our biosensor to image apoptosis in a systemic metastasis model, we could not obtain significant Gluc activity in any tissue upon caspase activation. However, this limitation was overcome by the sensitivity of the Gluc blood assay to monitor apoptosis which reports not only from small tumor foci in deep tissues, but also from circulating tumor cells, giving a marker for the overall apoptotic events.
An apoptosis reporter based on Fluc was previously developed and used to monitor apoptosis in glioma cells implanted subcutaneously in mice.11 Around threefold mean increase in bioluminescent signal was observed upon caspase activation in response to TRAIL. This study was limited to a 75-minute period without taking into consideration the longer in vivo half-life of Fluc. In another study, a multimodal imaging of apoptosis was established based on three reporters, RFP, Fluc, and HSV1-TK.12 In this study, normalization to cell viability using Renilla luciferase was required in order to achieve statistically significant results with moderate twofold increase in Fluc or TK activity, 24 hours post-apoptosis induction. Neither of these reporters had the ability to monitor apoptosis kinetics in vivo. Our GFP-DEVD-ssGluc reporter allows imaging of apoptosis in tumors with a fivefold increase in Gluc signal in doxorubicin-treated tumors versus control, 8 hours post-treatment. Furthermore, this reporter showed to be useful in real-time monitoring of caspase-3 activation kinetics ex vivo by assaying few microliters of blood for the Gluc activity at different time points. Still, the triple reporter has the advantage of being imaged in vivo with optical and micro-positron emission tomography with the ladder being compatible with clinical applications.
Recently, a fluorescently labeled activity-based probes based on acyloxymethyl ketone which covalently label active caspases has been developed and successfully used for imaging apoptosis in vivo.13 This study showed around threefold increase in fluorescent signal in tumors treated with the monoclonal antibody Apomab as compared to controls. Unlike our apoptosis bioluminescent sensor, kinetic analysis of apoptosis using activity-based probes was not achieved noninvasively due to the slow clearance of these probes with and without the Tat peptide. Also, fluorescence imaging is generally less sensitive than bioluminescence imaging. Furthermore, the specificity of activity-based probes to caspases remains an issue due to crossreactivity of these probes with legumain. However, this caspase-targeted probe has the advantage over our system because no gene-transfer of the reporter is required.
The biosensor developed here allows multimodal and simultaneous detection of apoptosis in the conditioned medium of cultured cells, in the blood of animals ex vivo, as well as in vivo using bioluminescence imaging. It allows the acquisition of timed datasets which provides a high degree of validation and internal control. This caspase reporter can also be used to monitor certain cases of necrosis in which caspase activation has been shown to play a critical role.20,21 Despite the incompatibility of our system with the use in human, the apoptosis biosensor could be useful for preclinical analysis and can be easily applied for high-throughput screening to study drug kinetics over time and, more importantly, the same system can be used for validation analysis in vivo.
Materials and Methods
Expression constructs. GFP-DEVD-ssGluc fusion was cloned and provided by Dr Rampyari Walia (Targeting Systems, El Cajon, CA). Briefly, the humanized Ptilosarcus GFP cDNA (PtGFP) lacking the stop codon was amplified by PCR from pCMV-PtGFP (Nanolight, Pinetop, AZ) introducing NheI site at the 5′ end and BamHI at the 3′ end. The humanized Gaussia luciferase cDNA (Gluc) including the signal sequence was also amplified by PCR using pCMV-Gluc (Targeting Systems) using an upstream primer which introduced a BamHI site followed by a DEVD coding sequence (gacgaagtggac) at the 5′ end and NheI site at the 3′ end. PtGFP and Gluc were fused together in frame using the BamHI site and cloned in pCMV mammalian expression plasmid (Targeting Systems). The GFP-DEVD-ssGluc fusion was then subcloned into the NheI site of CSCW2, a self-inactivating lentivirus vector under the control of CMV promoter,22 producing lenti-GFP-DEVD-ssGluc. The integrity of all constructs was verified by sequencing. All lentivirus vectors were produced and tittered as transducing units/ml as previously described.22
Cell culture. Gli36 human glioma cells were obtained from Dr Anthony Capanogni (UCLA, CA). MDA-MB-231BR breast cancer cells were provided by Dr Patricia S. Steeg (National Cancer Institute, Bethesda, MD).23 293T human embryonic kidney fibroblasts were obtained from Dr Maria Calos (Stanford University School of Medicine, Stanford, CA). All cells were cultured in Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum (Sigma, St Louis, MO); 100 U penicillin, and 0.1 mg streptomycin (Sigma) per ml at 37 °C, in a 5% CO2 humidified incubator. Stable cell lines were generated by infecting cells in a 6-well plate (70–80% confluent) using a multiplicity of infection of 30 transducing units/cell in the presence of 8 µg/ml polybrene as described.16
In vitro monitoring of apoptosis. Three-hundred thousand Gli36 cells-expressing GFP-DEVD-ssGluc were plated in 6-well plates. Twenty-four hours later, cells were treated in triplicates with different concentration of doxorubicin, a chemotherapeutic agent knowing to induce apoptosis in tumor cells, or the tumor necrosis factor-related apoptosis-inducing ligand. At different time points, aliquots of the conditioned medium were assayed for Gluc activity.
Gluc assay. For determination of Gluc activity in culture, 50 µl aliquots of the cell-free conditioned medium were collected in triplicates at different time points and transferred into a white 96-well plate. Gluc activity was assayed by injecting 50 µl 40 µmol/l coelenterazine, the Gluc substrate (Nanolight) diluted in PBS, or GAR-2 reagent (Targeting Systems) and acquiring photon counts for 10 seconds using a plate luminometer (Dynex, Richfield, MN). For the Gluc blood assay in vivo, 5 µl of blood in triplicates was collected over time by making a small nick in the mouse tail which was mixed immediately with 1 µl 20 mmol/l EDTA. Gluc activity was then measured using the luminometer after injecting 100 µl 100 µmol/l coelenterazine and acquiring signal over 10 seconds.
Western blot analysis. Gli36 cells-expressing GFP-DEVD-ssGluc were plated in 6-well plates and treated with different concentration of doxorubicin and/or 20 µnol/l of ZVAD-fmk (carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]- fluoromethylketone; Calbiochem, Gibbstown, NJ), the caspase inhibitor. Twenty-four hours post-treatment, the conditioned medium was collected and cells were washed with PBS and lysed using 50 µl of RIPA buffer containing 150 mmol/l NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS in 50 mmol/l Tris–HCl (pH 8) with 5 µl protease inhibitors (PI Complete; Boehringer Mannheim, Mannheim, Germany). The protein concentration in lysates was measured using the Bradford reagent (Bio-Rad, Hercules, CA). The conditioned medium was concentrated using microcon3 (cut-off 3 kDa). Protein (25 µg) as well 25 µl of concentrated medium were analyzed by electrophoresis on 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad). The blots were stained with ponceau to verify even protein loading. Membranes were blocked overnight using 10% nonfat milk powder in TBS-T (150 mmol/l NaCl, 50 mmol/l Tris, pH 7.9, 0.5% Tween) at 4 °C. The membranes were then probed using antibodies against Gluc (1:500 in TBS-T for 1 hour; Massachusetts General Hospital, Charlestown, MA, antibody production core),19 Caspase-3 (1:200 for 1 hour; Biolegend, San Diego, CA), or β-tubulin (1:1,000 for 1 hour; Sigma). Membranes were then incubated with horseradish peroxidase conjugated to secondary antibodies: sheep anti-mouse immunoglobulin G-horseradish peroxidase (1:10,000) (Amersham Pharmacia Biotech, Piscataway, NJ). Proteins were detected using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL). For western blotting from tumor tissues, tumors were removed 8 hours post-treatment and cells were first dissociated using trypsin, then lysed and analyzed as above.
Ex vivo monitoring of apoptosis. All animal studies were approved by the Massachusetts General Hospital Review Board. For subcutaneous tumor model, one million Gli36 cells-expressing GFP-DEVD-ssGluc were resuspended in 50 µl PBS, mixed with an equal volume of Matrigel (BD Bioscience, Franklin Lakes, NJ) and immediately implanted subcutaneously in the flanks of nude mice. Three weeks later, 10 µg of doxorubicin or PBS (control) were injected into each tumor (n = 5 per group). Before doxorubicin injection and at different time points after injection, 5 µl blood (in triplicates) was withdrawn and assayed for the Gluc activity using a luminometer (see above).
For the breast cancer metastasis model, MDA-MB-231BR cells stably expressing Fluc24 were infected with lentivirus vector expression GFP-DEVD-ssGluc. Six-to-seven weeks old female nude mice were injected with 250,000 of these cells into the left ventricles as described.24 Four weeks later, mice were imaged with Fluc bioluminescence imaging (see below) and were intraperitoneally injected with either PBS (control) or TRAIL (0.5 mg/kg body weight; n = 6 per group). At different time points, 5 µl aliquots (in triplicates) of blood were assayed for Gluc activity as above. The Gluc activity at different time points was compared to basal level at time zero. Statistical analysis (P value) was calculated using Student's t-test.
In vivo bioluminescence imaging. Mice were anesthetized with intraperitoneal injection of ketamine (100 mg/kg) and xylazine (5 mg/kg) and Gluc imaging was performed immediately after intraocular injection (intravenous) of 150 µl coelenterazine (4 mg/kg body weight) and recording photon counts over 5 minutes using a cooled CCD camera with no illumination.14 Fluc imaging in the MDA-MB-231BR breast cancer metastasis model was performed 10 minutes after intraperitoneal injection of 150 µl -luciferin (150 mg/kg body weight). A light image of the animal was taken in the chamber using dim polychromatic illumination. Following data acquisition, postprocessing and visualization was performed using CMIR-Image, a program developed by the Center for Molecular Imaging Research using image display and analysis suite developed in IDL (Research Systems, Boulder, CO). Regions of interest were defined using an automatic intensity contour procedure to identify bioluminescence signals with intensities significantly greater than the background. The mean, s.d., and sum of the photon counts in these regions were calculated as a measurement of Gluc activity. For visualization purposes, bioluminescence images were fused with the corresponding white light surface images in a transparent pseudocolor overlay, permitting correlation of areas of bioluminescent activity with anatomy.
TUNEL staining. Eight hours post-treatment, animals were sacrificed by transcardial perfusion with 4% paraformaldehyde in PBS under deep anesthesia. Tumors were removed, fresh frozen using tissue freezing medium and sectioned into 10 µm sections. Sections were mounted on slides and analyzed using TUNEL staining as described in the manufacturer's instructions (Promega, Madison, WI). DNA/Nucleus was also stained using DAPI. Stained sections were evaluated using fluorescent microscopy.
Acknowledgments
This work was supported partly by grants from NIH/NCI P50CA86355, NIH/NINDS 1R21NS061051, 1R01NS064983 and P30NS045776. We thank Dr Ralph Weissleder, Director of the Center for Molecular Imaging Research-MGH for the use of the CCD cameral; Dr Rampyari Walia (Targeting Systems) for cloning the GFP-DEVD-Gluc fusion in pCMV plasmid; Dr Xandra Breakefield for scientific discussions; Ms Lee-Ann Tjon-Kon-Fat for technical assistance; and Dr Euiheon Chung for assistance with the breast cancer metastasis model.
REFERENCES
- Budihardjo I, Oliver H, Lutter M, Luo X., and, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- Moffitt KL, Martin SL., and, Walker B. Proteases implicated in apoptosis: old and new. J Pharm Pharmacol. 2010;62:563–576. doi: 10.1211/jpp.62.05.0002. [DOI] [PubMed] [Google Scholar]
- Blankenberg FG. Apoptosis imaging: anti-cancer agents in medicinal chemistry. Anticancer Agents Med Chem. 2009;9:944–951. doi: 10.2174/187152009789377727. [DOI] [PubMed] [Google Scholar]
- Fulda S., and, Debatin KM. Signaling through death receptors in cancer therapy. Curr Opin Pharmacol. 2004;4:327–332. doi: 10.1016/j.coph.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Meng XW, Lee SH., and, Kaufmann SH. Apoptosis in the treatment of cancer: a promise kept. Curr Opin Cell Biol. 2006;18:668–676. doi: 10.1016/j.ceb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- D'Atri S, Tentori L, Lacal PM, Graziani G, Pagani E, Benincasa E.et al. (1998Involvement of the mismatch repair system in temozolomide-induced apoptosis Mol Pharmacol 54334–341. [DOI] [PubMed] [Google Scholar]
- Günther W, Pawlak E, Damasceno R, Arnold H., and, Terzis AJ. Temozolomide induces apoptosis and senescence in glioma cells cultured as multicellular spheroids. Br J Cancer. 2003;88:463–469. doi: 10.1038/sj.bjc.6600711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehrer S, Nowak D, Hoelzer D, Mitrou PS., and, Chow KU. The molecular biology of TRAIL-mediated signaling and its potential therapeutic exploitation in hematopoietic malignancies. Curr Med Chem. 2006;13:2091–2100. doi: 10.2174/092986706777935294. [DOI] [PubMed] [Google Scholar]
- Fulda S., and, Debatin KM. Modulation of TRAIL signaling for cancer therapy. Vitam Horm. 2004;67:275–290. doi: 10.1016/S0083-6729(04)67015-4. [DOI] [PubMed] [Google Scholar]
- Laxman B, Hall DE, Bhojani MS, Hamstra DA, Chenevert TL, Ross BD.et al. (2002Noninvasive real-time imaging of apoptosis Proc Natl Acad Sci USA 9916551–16555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P, De A, Patel M., and, Gambhir SS. Monitoring caspase-3 activation with a multimodality imaging sensor in living subjects. Clin Cancer Res. 2008;14:5801–5809. doi: 10.1158/1078-0432.CCR-07-5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgington LE, Berger AB, Blum G, Albrow VE, Paulick MG, Lineberry N.et al. (2009Noninvasive optical imaging of apoptosis by caspase-targeted activity-based probes Nat Med 15967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannous BA, Kim DE, Fernandez JL, Weissleder R., and, Breakefield XO. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol Ther. 2005;11:435–443. doi: 10.1016/j.ymthe.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Tannous BA. Gaussia luciferase reporter assay for monitoring biological processes in culture and in vivo. Nat Protoc. 2009;4:582–591. doi: 10.1038/nprot.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurdinger T, Badr C, Pike L, de Kleine R, Weissleder R, Breakefield XO.et al. (2008A secreted luciferase for ex vivo monitoring of in vivo processes Nat Methods 5171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire CA, Deliolanis NC, Pike L, Niers JM, Tjon-Kon-Fat LA, Sena-Esteves M.et al. (2009Gaussia luciferase variant for high-throughput functional screening applications Anal Chem 817102–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr CE, Wurdinger T., and, Tannous BA. Functional Drug Screening Assay Reveals Potential Glioma Therapeutics. Assay Drug Dev Technol (epub ahead of print) 2010. [DOI] [PMC free article] [PubMed]
- Badr CE, Hewett JW, Breakefield XO., and, Tannous BA. A highly sensitive assay for monitoring the secretory pathway and ER stress. PLoS ONE. 2007;2:e571. doi: 10.1371/journal.pone.0000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Honda T, Proske RJ., and, Yeh ET. Regulation of reactive oxygen species-induced apoptosis and necrosis by caspase 3-like proteases. Oncogene. 1998;17:2753–2760. doi: 10.1038/sj.onc.1202211. [DOI] [PubMed] [Google Scholar]
- Niquet J, Allen SG, Baldwin RA., and, Wasterlain CG. Evidence of caspase-3 activation in hyposmotic stress-induced necrosis. Neurosci Lett. 2004;356:225–227. doi: 10.1016/j.neulet.2003.11.063. [DOI] [PubMed] [Google Scholar]
- Sena-Esteves M, Tebbets JC, Steffens S, Crombleholme T., and, Flake AW. Optimized large-scale production of high titer lentivirus vector pseudotypes. J Virol Methods. 2004;122:131–139. doi: 10.1016/j.jviromet.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM.et al. (2007Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain Cancer Res 674190–4198. [DOI] [PubMed] [Google Scholar]
- Chung E, Yamashita H, Au P, Tannous BA, Fukumura D., and, Jain RK. Secreted Gaussia luciferase as a biomarker for monitoring tumor progression and treatment response of systemic metastases. PLoS ONE. 2009;4:e8316. doi: 10.1371/journal.pone.0008316. [DOI] [PMC free article] [PubMed] [Google Scholar]