Abstract
While a variety of genetic mutations have been shown to be associated with renal cyst formation, mechanisms of renal cyst formation are largely unknown. In prior communications we described alterations in E-cadherin assembly in cultured cystic epithelial cells (Charron AJ, Nakamura S, Bacallao R, Wandinger-Ness A. J Cell Biol 149: 111–124, 2000). Using the same cell line we assayed cadherin expression by RT-PCR using primer pairs that anneal to highly conserved sequences of cadherin genes but flank informative regions of cadherins. Using this approach we found that autosomal dominant polycystic kidney disease (ADPKD) cells express cadherin 8, a neuronal cadherin with limited expression in the kidney. Immunohistochemistry confirmed cadherin 8 expression in cystic epithelia. To test the functional significance of cadherin 8 expression in renal epithelial cells, we adapted a three-dimensional collagen culture method in which HK-2 cells form tubule structures and microinjected adenovirus into the matrix space surrounding tubule structures. Adenovirus expressing cadherin 8 under the control of a tet promoter caused cyst structures to grow out of the tubules when coinjected with adenovirus expressing a tet transactivator. Microinjection of single adenovirus expressing either tet transactivator or cadherin 8 failed to cause cyst formation. When doxycycline was added to the culture, following coinjection of adenovirus, there was a dose-response reduction in cadherin 8 expression and cyst formation. Similarly, HK-2 cells transfected with Flag-tagged cadherin 8 form cysts in addition to tubular structures. HK-2 cells transfected with Flag-tagged N-cadherin do not form cysts. These data suggest that ectopic expression of cadherin 8 in renal epithelial cells is sufficient to cause the morphogenic pattern of cyst formation.
Keywords: HK-2 cells, three-dimensional collagen culture, adenovirus, cysts, autosomal dominant polycystic kidney disease
polycystic kidney disease is a heterogeneous group of genetic disorders associated with a variety of phenotypes ranging from situs inversus, hereditary blindness, obesity, mental retardation, hydrocephalus, and cystic changes in kidneys, liver, and pancreas. While animal models and genomic approaches have greatly expanded our identification of the genes associated with polycystic kidney disease, our understanding of the mechanistic underpinnings of renal cyst formation is largely incomplete. Several leading hypothesis have driven our understanding of cyst formation. The two-hit hypothesis was advanced to account for the focal nature of renal cyst formation (25, 26, 34), while the cilia signaling hypothesis has linked mechano-transduction of flow to gene transcription and terminal differentiation (1, 8, 10, 12). Lastly, defects in planar cell polarity genes have been shown to be cystogenic. Particularly instructive in this regard has been the finding that FAT4 knockout, an atypical cadherin that participates in planar cell polarity, causes renal cysts (3, 5, 15, 29, 32).
In prior work, we demonstrated that cultured renal epithelial cells derived from kidney cysts fail to assemble E-cadherin in the lateral membrane. E-cadherin is made in equivalent amounts in cystic epithelial cells as compared with age-matched control renal epithelial cells but fails to stably integrate into the lateral membrane (9). Since the cells form functional tight junctions, we examined what other cadherins could provide lateral membrane signaling necessary to permit tight junction assembly. We found that N-cadherin was expressed in autosomal dominant polycystic kidney disease (ADPKD) cells by immunostaining and N-cadherin served to stabilize β-catenin at the cell membrane (28). However, this approach did not provide a full evaluation of cadherin family member expression in the ADPKD cells. In this communication we demonstrate that ADPKD cells express cadherin 8, a type II cadherin expressed predominantly in neuronal cells. This expression is found in polycystic kidney, and that ectopic expression of cadherin 8 by HK-2 cells in three-dimensional (3D) culture will drive formation of cysts arising from tubules. This finding suggests that altered cadherin expression as part of a transdifferentiation event may be a final pathway for renal cyst formation.
MATERIALS AND METHODS
Cell lines and culture.
Primary cultured renal tubule epithelial cells were derived from human polycystic kidneys and normal kidneys discarded for transplantation, as previously described (9). Cell lines were maintained up to six passages at which time the cell lines go into senescence. All studies were performed in cell lines at passage 3 or less. Cells were grown in REGM media (Lonza, Basel, Switzerland) in a 5% CO2 environment at 37°C. HK-2 cells were purchased from American Type Culture Collection and grown in DMEM media supplemented with 5% fetal calf serum. The Institutional Review Board at Indiana University reviewed and approved the acquisition of human tissue used in this study.
Antibodies and reagents.
Anti-cadherin 8 was supplied by Santa Cruz Biotechnology (Santa Cruz, CA) and OriGene Technologies (Rockville, MD). Taq polymerase and reverse transcriptase were purchased from Invitrogen. TOPO cloning kits were obtained from Invitrogen (Carlsbad, CA) and used according to the manufacturer's directions. All buffers and chemicals were purchased from Thermo Fisher Scientific (Waltham, MA) and were of reagent-grade quality. Rhodamine phalloidin and Hoescht 33342 were purchased from Invitrogen. PCR primmer pairs using sequences described by Suzuki et al. (33) were ordered from Eurofins MWG Operon (Huntsville, AL). All fluorescence-conjugated secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PA). Plasmids encoding full-length Flag-tagged cadherin 2 (N-cadherin, NM_001792) and cadherin 8 (NM_001796.2) were purchased from OriGene Technologies.
RT-PCR and SSCP gel analysis.
RNA was isolated from ADPKD and age-matched normal kidney cells grown to confluence in 100-mm plastic dishes using Ambion RNA isolation kits (Ambion, Austin, TX) according to the manufacturer's directions. RNA purity was confirmed by measuring A280/260 ratios and running RNA on a 1% agarose gel. RT-PCR reactions were performed using 1 μg total RNA using conditions described by Sambrook et al. (30). PCR products were analyzed by SSCP gels using a GenoMed gel apparatus (GenoMed, St. Louis, MO). PCR products were visualized by running PCR reactions with 33P-labeled dNTPs and exposing the SSCP gels on X-ray file (XOMAT, Kodak, Rochester, NY). PCR products were cloned into TOPO plasmid and sequenced by the Molecular Biology Core facility at Indiana University.
Immunohistochemistry.
ADPKD kidneys were cyrofixed in isopentane cooled with dry ice. Fixed tissue was embedded in OCT (Polysciences, Warrington, PA) and sectioned in a cryomicrotome. Tissue sections were placed on ProbeON slides (Thermo Fisher Scientific) and fixed with 2% paraformaldehyde dissolved in phosphate-buffered saline (PBS) for 30 min. Chemical fixation reactions were quenched with 100 mM NH4Cl dissolved in PBS. Samples prepared for immunohistochemistry labeling were incubated in PBS with 0.3% hydrogen peroxide for 30 min to inactivate endogenous peroxidase. All samples were incubated with tissue block solution consisting of 1% bovine albumin and 0.1% Triton X-100 dissolved in PBS. Ten-micrometer sections of tissue were incubated with anti-cadherin 8 added to tissue block solution and Hoescht 33342. After incubation with primary antibody, samples were washed with PBS and then incubated with horseradish peroxidase-conjugated donkey anti-goat IgG (Vector Laboratories, Burlingame, CA). Samples were developed with VectorStain ABC kit according to the manufacturer's directions and then counterstained with hematoxylin (Vector Laboratories). Alternatively, after incubation with primary antibody, samples were washed with PBS and then incubated with Alexa 568-conjugated donkey anti-goat IgG (Jackson ImmunoResearch). After a second set of washings, the samples were fixed with 2% paraformaldehyde dissolved in PBS for 30 min and mounted in Mowoil.
3D collagen culture.
Cell matrix (Wako Chemicals, Richmond, VA) was mixed with growth media in equal proportions, and 1 ml was layered onto 60-mm Petri dishes with a glass coverslip mounted on the bottom (MatTek, Ashland, MA). Collagen matrix was allowed to set overnight and then HK-2 cells were plated at a density of 100,000 cells/cm2 and incubated overnight. After allowing the cells to attach, excess medium was removed and the cells were overlayered with cell matrix and maintained in a 5% CO2, 37°C culture conditions. Cultures were kept in culture for a maximum of 14 days.
Adenovirus production.
Human cadherin 8 was obtained from Invitrogen and subcloned into pAdTet (11). Cadherin 8 bearing pAdTet was cotransfected into Cre8 293 cells (generously supplied by Josh Lipshutz, University of Pennsylvania, Philadelphia) with Ψ5 Ad backbone (11). Adenovirus expressing tet trans-activator (TTA) was supplied by Josh Lipshutz. Multiplicity of infection (MOI) was determined by performing serial dilutions and assaying cadherin 8 expression by immunohistochemistry in Madin-Darby canine kidney (MDCK) TTA-expressing cells (Clontech, Mountain View, CA).
Cyst formation assay.
HK-2 cells grown in 3D collagen matrix form tubules within 24 h after collagen matrix overlay. Once tubules were formed, three different experimental conditions were evaluated. Adenovirus expressing cadherin 8, adenovirus expressing TTA, or both cadherin 8 adenovirus and TTA adenovirus were microinjected into matrix near the tubules. Typically, 1E6 MOI virus was microinjected in a region. In addition, some experiments were performed in which cadherin 8 and TTA adenovirus were microinjected and the cells were incubated in media supplemented with either 0, 0.5, or 1 μg/ml doxycycline. After microinjection, cultures were incubated for 48 h and then fixed with 2% paraformaldehyde dissolved in PBS. After fixation, samples were labeled with rhodamine phalloidin and Hoescht 33342 (Invitrogen). In an alternative approach, HK-2 cells were transfected with either plasmids bearing Flag-tagged cadherin 8 or N-cadherin using XFect (Sigma, St. Louis, MO) according to the manufacturer's directions. After transfection, cells were grown in regular media for 24 h, passaged with trypsin-EDTA, and mixed with an equal number of untransfected HK-2 cells and plated on collagen matrix at a density of 50,000 cells/cm2. Four hours after plating, the cells were overlaid with collagen and maintained in culture for 24 to 96 h before processing for light microscopy.
Fixation and staining of 3D collagen samples.
Collagen matrix samples were washed three times with PBS supplemented with 0.5 mM MgCl2 and CaCl2. Matrix cultures were treated with 1,000 U/ml collagenase II (Worthington Biochemical, Freehold, NJ) for 5 min. After three washes with ice-cold PBS supplemented with 0.5 mM MgCl2 and CaCl2, samples were fixed for 30 min with 2% or 4% paraformaldehyde for 30 min. Fixation reactions were quenched with 100 mM NH4Cl dissolved in BPS.
To stain samples, collagen matrix plugs were washed with PBS with 0.1% Triton X-100 (Thermo Fisher Scientific) supplemented with 5% normal donkey serum (Jackson ImmunoResearch). Samples were labeled with monoclonal anti-Flag (OriGene), Texas Red-conjugated phalloidin (Invitrogen), and Hoescht 33342 (Invitrogen). After labeling and washes, all samples were postfixed in paraformaldehyde and mounted with Fluorsave (EMD4Biosciences, Darmstadt, Germany).
Light microscopy and image processing.
Images were collected by two photon confocal microscopy using an Olympus Fluor View confocal microscope. Image processing was performed using Metamorph software (version 7.6.5.0; Danaher Medical Technologies, Washington, DC) running on a Dell Optiplex computer (Austin, TX). All image processing was performed using equivalent setting between control and experimental samples.
RESULTS
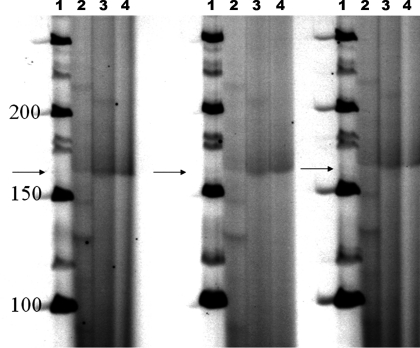
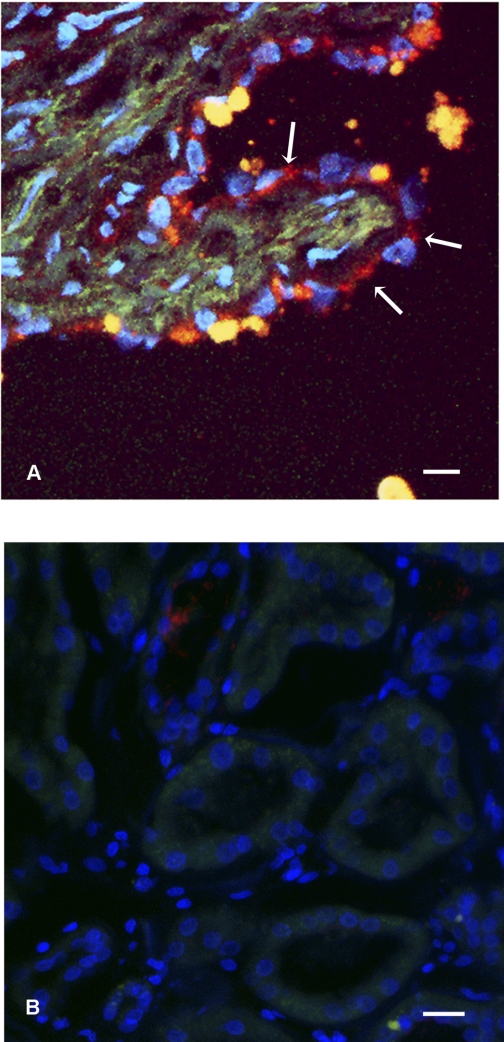
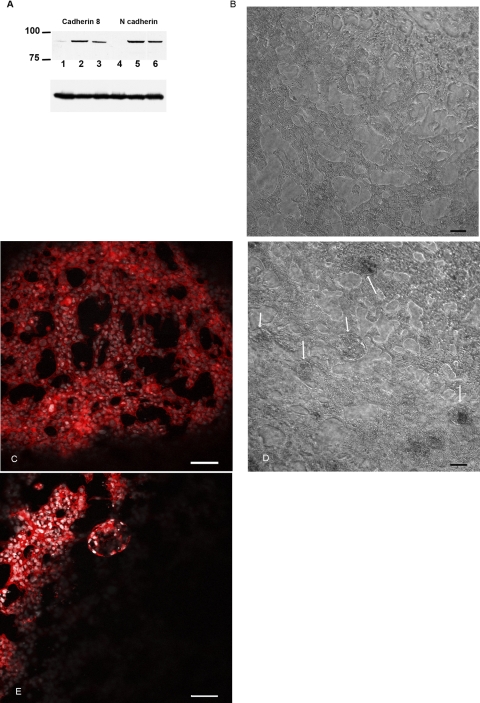
To determine the full profile of cadherin expression in the primary cell lines derived from renal cysts, we performed RT-PCR using primer pairs first described by Suzuki et al. (33). These primers anneal to highly conserved regions of cadherin but span highly informative regions of cadherins which permits unambiguous identification of expressed cadherins (33). To assess the results of the RT-PCR reactions, SSCP gels were run. As shown in Fig. 1, the reaction products obtained from normal kidney cells and PKD cells showed differing migration patterns, suggesting that there were differences in the PCR products. On the basis of this result we cloned the resultant PCR products and screened the plasmid inserts by sequencing. Sequence analysis identified K- and N-cadherin in the normal kidney cells. In contrast, cystic epithelia expressed K- and N-cadherin and cadherin 8 (data not shown). Since cultured cells undergo significant changes in differentiation profile during adaptation to cell culture conditions, we confirmed cadherin 8 by immunostaining for cadherin 8 in ADPKD tissue sections. Cadherin 8 was observed in epithelial cells lining the cysts in a majority of cysts observed (Fig. 2A). We also found that, in agreement with published results, cadherin 8 is not expressed in normal adult kidneys (Fig. 2B) (6). Taken together, our data suggest that cadherin 8 is expressed in renal cystic epithelial cells both in vivo and ex vivo.
Fig. 1.
SSCP gel analysis of cadherin-focused RT-PCR reactions. RT-PCR reactions were run as described in materials and methods. 33P-radiolabeled PCR products were run on a 4–10% polyacrylamide gradient gel, and results were developed on X-ray film. Lane 1, molecular weight markers; lane 2, PCR products from reaction using RNA from Madin-Darby canine kidney (MDCK) cells; lane 3, PCR products resultant from RNA isolated from normal kidney (NK) cells; lane 4, PCR products using RNA isolated from ADPKD cyst epithelial cells. Arrows point to regions on the gels where predominat RT-PCR products are identified. Note that the bands are less defined and smeared in lanes 3 and 4 as compared with lane 2. This suggests that there are more diverse PCR products obtained from NK and ADPKD cells as compared with MDCK cells.
Fig. 2.
Cadherin 8 expression in autosomal dominant polycystic kidney disease (ADPKD) and normal kidney. A: tissue section from an ADPKD kidney, labeled with anti-cadherin 8 (red, arrows) and Hoescht 33342 (blue) to label nuclei. Matrix staining in green is the result of an autofluorescent signal. Note that the cyst-lining epithelial cells express cadherin 8 predominantly in a cytoplasmic distribution (arrows). Cadherin 8 may have redistributed owing to prolonged cross-clamp time required during the nephrectomy. Bar, 20 μm. B: tissue section from a normal human kidney labeled with anti-cadherin 8 (red) and Hoescht 33342 (blue) to label nuclei. Green channel is the result of an auto fluorescent signal. No cellular staining is noted. Bar, 20 μm.
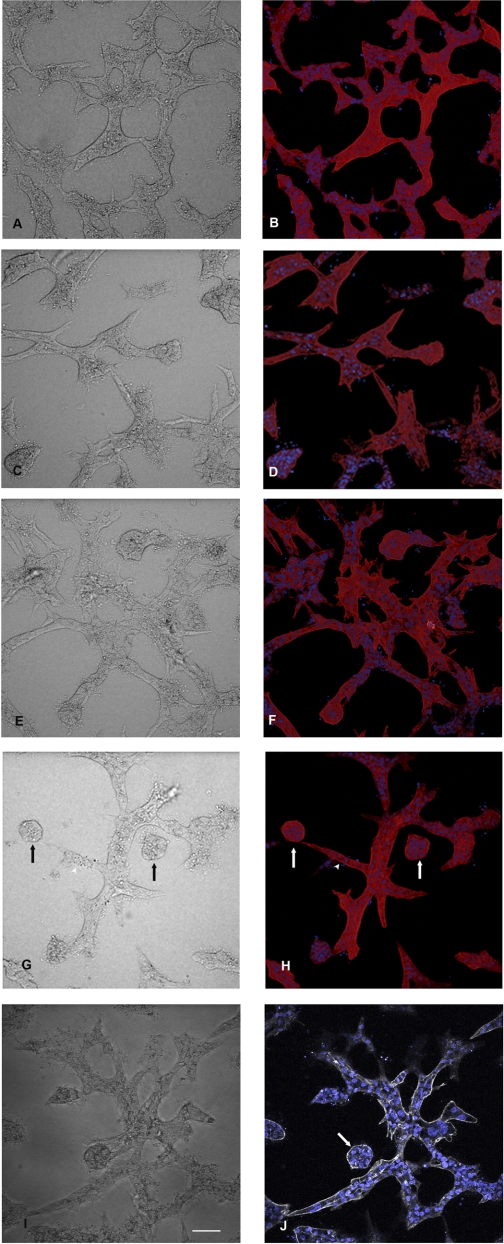
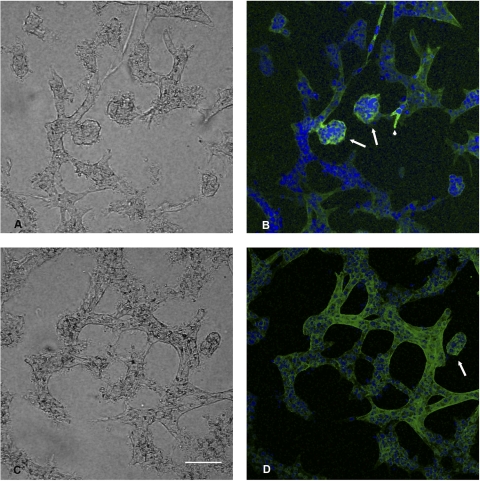
To determine the functional significance of cadherin 8 expression, we developed a novel assay for cystogenesis. This assay takes advantage of the fact that matrix overlay allows HK-2 cells to spontaneously form tubule structures within 24 h (Fig. 3A). When maintained in culture for up to 14 days, the tubule arrays extend and arborize (data not shown). When adenovirus expressing either TTA or cadherin 8 was microinjected into the matrix space surrounding the tubule sections, no changes in tubule structures were observed (Fig. 3, C–F). When the two adenoviruses were microinjected together, stalk like extensions were observed within 24 h and by 48 h independent cysts were observed (Fig. 3, G and H). Immunostaining confirmed that the cysts and stalks were cadherin 8 positive (Fig. 4, A and B). Tubule structures were N-cadherin positive (Fig. 4, C and D) in accordance with the normal cadherin profile of HK cells (2, 23). When doxycycline was added to the cell cultures following microinjection of TTA and cadherin 8 adenoviruses, there was a dose-dependent relationship between the cyst numbers observed in culture and the doxycycline dose (Table 1).
Fig. 3.
Three-dimensional (3D) matrix culture of HK-2 cells. A: HK-2 cells plated at a density of 100,000 cells/cm2 were overlaid with collagen matrix and grown up to 14 days after overlay. Phase-contrast images are presented on the left (A, C, E, G, and I). Composite extended focus images of fluorescent image set labeled for actin and nuclei are on the right (B, D, F, and H). A single confocal fluorescent image plane is shown in J. A and B: phase-contrast image of the tubule structures and phalloidin staining of F-actin (red) with nuclei staining (Hoescht, blue), to show tubule structures and cell organization within tubules in control untreated cells. C and D: 3D culture at 48 h after adenovirus microinjection with adenovirus expressing the tet transactivator only. Rare knoblike extension similar to that seen in control 3D cultures were seen. E and F: adenovirus expressing cadherin 8 was microinjected and the cell culture was fixed 48 h later. No cystic structures were observed, but again some rare knoblike structures were found. G and H: adenovirus expressing cadherin 8 and tet transactivator were coinjected at a multiplicity of infection (MOI) of 1E6. Free cysts and stalk structures were observed in the phase-contrast image (arrow and arrowhead, respectively). I and J: phase-contrast image and a single image plane from the data set. The arrow points to a cyst with a small lumen. Actin staining with phalloidin is shown using gray scale instead of a red scale. Bar, 135 μm.
Fig. 4.
Immune fluorescence images of cysts staining for cadherin 8 or N-cadherin. 3D matrix culture was fixed with paraformaldehyde 48 h after adenovirus microinjection with both cadherin 8 and tet trans-activating adenovirus. A: phase-contrast image of B. B: extended focus image of image stack in which the sample was stained with anti human cadherin 8. Note the cystic outgrowths (arrows) and a small early stalk (arrowhead) expressing cadherin 8 (FITC label-green). Hoescht 33342 (blue) staining of nuclei is also shown. C: phase-contrast image of D. D: N-cadherin staining (FITC label-green) is observed in tubule structures and a cyst structure (arrow). Hoescht 33342 (blue) staining of nuclei is also shown. Bar, 135 μm.
Table 1.
Mean cyst number observed in three-dimensional cultures versus doxycycline dose
| Doxycycline Dose, μg/μl | Mean Cyst Number (n = 3 experiments) | P Value |
|---|---|---|
| 0 | 54 | P < 0.01 |
| 0.5 | 22 | P < 0.01 |
| 1 | 4 |
One potential interpretation of the results is that any cadherin when overexpressed can induce cyst formation. To evaluate this possibility, we transfected plasmids bearing Flag-tagged cadherin 8 or N-cadherin into HK-2 cells and evaluated the structural morphology 48 h after overlay in collagen matrix. N-cadherin was chosen since HK-2 cells normally express N-cadherin (24). In Fig. 5, immunoblot analysis demonstrates that equivalent levels of cadherin 8 and N-cadherin are expressed in transfected HK-2 cells (Fig. 5A). Cells transfected with N-cadherin formed tubule arrays and chords but no cysts were observed (Fig. 5, B and C). In contrast, cadherin 8 transfected cells did form cysts (Fig. 5, D and E). This result confirms that ectopic expression of cadherin 8 is sufficient to drive cystogenic morphology and that simply overexpressing an endogenously expressed cadherin may not cause cyst formation.
Fig. 5.
Transfected N-cadherin fails to form cysts in 3D culture while cadherin 8 does. HK-2 cells were transfected with Flag-tagged N-cadherin or cadherin 8 for 1 day and then put into a 3D collagen matrix. A: immunoblot of Flag-tagged N-cadherin and cadherin 8. Lysates were made from HK-2 cells 24 and 48 h posttransfection. Blot was developed using anti-Flag tag antibody. Lanes 1 and 4, mock-transfected cells; lanes 2 and 4: lysates made from cells 24 h posttransfection; lanes 3 and 6: lysates made from cells 48 h posttransfection. At both time points, 24 and 48 h after transfection, significant amounts of Flag-tagged cadherin transgene are expressed. A, bottom: blot was stripped and probed with anti-actin. B: phase-contrast image of cells transfected with flag tagged N-cadherin grown in 3D culture 48 h postplating. Extensive cell chords and tubules were observed. Bar, 100 μm. C: volume rendering of 3D culture labeled with Hoescht 33342 (gray) and phalloidin (Texas red, red). Cells were transfected with N-cadherin. D: phase-contrast image of cells transfected with Flag-tagged cadherin 8. In addition to chords and tubules, cysts were evident in the 3D culture (arrows). Bar, 100 μm. E: volume rendering of 3D culture labeled with Hoescht 33342 (gray) and phalloidin (Texas red, red). Cells were transfected with cadherin 8. Bar, 100 μm.
DISCUSSION
Role of cadherins in tissue morphology.
In vivo, renal epithelial cells express three cadherins that have been identified at various nephron segments. K-cadherin is expressed in the proximal tubule along with N-cadherin. E-cadherin is expressed more strongly in distal tubule segments (21, 22). During nephrogenesis, N-cadherin and E-cadherin predominate in expression (20). Cadherin 8 has been noted in early nephrogenesis, however, its expression is rapidly downregulated and is absent in adult nephrons (6). Cadherin 8 expression has also been noted in renal cell carcinomas but the significance of its expression is unknown (6). Normally, cadherin 8 is observed in posterior horn cells of the spine, and mouse knockouts of cadherin 8 exhibited defects in response to pain induced by heat. Neurons isolated from the posterior horn of the cadherin 8 knockout also demonstrated changes in TrpMa receptors and attenuated responses to menthol (13). No obvious kidney phenotype was observed in the animal knockout line (13).
Cadherins are transmembrane proteins with a large extracellular domain divided into EC1 domains and EC2 domains arranged in tandem arrays. They have a single transmembrane domain and on the cytoplasmic side there are domains that mediate binding to β- and α-catenin, scribble, and p120 (7, 16–19, 27, 36). Currently, there are 57 known cadherins in the family and they are divided into type I, type II, and atypical cadherins. Cadherin 8 is a type II cadherin with significant homology in its extracellular domain to canonical cadherins such as N- and E-cadherins (19). However, cadherin 8 lacks the HEV sequence found in the EC1 domain of E-cadherin (19). The cytoplasmic domain of cadherin 8 is more highly conserved to cytoplasmic domain of type I cadherins than the extra-cytoplasmic domain and has putative β-catenin and actin-binding sites (19). Since homotypic and heterotypic interactions of cadherins are closely linked to subtle differences in structural features of each family member, it was important to characterize the effect of ectopic cadherin 8 expression in renal epithelia.
To examine the effect of ectopic cadherin 8 expression, we wanted to mimic the stages of renal cyst formation first identified by Baert and Steg (4). Using microdissection techniques, Baert and Steg showed that cysts arise from fully formed tubules and can arise from any nephron segment (4). MDCK cell cysts grown in collagen culture do not form tubules, hence this model of kidney cysts may lack important aspects of cystogenesis that occur in vivo (14). In the model developed in this communication, tubular-like structures are formed prior to experimentally interventions that may lead to cyst formation. We show that ectopic expression of cadherin 8 in HK-2 cells is sufficient to cause the cystic morphogenic pattern to arise from preformed tubules. However, the nature of the cysts observed do not entirely mimic that which is seen in vivo. The lumen size is contracted and a single layer of epithelial cells lining the cyst is not seen. This suggests that this model does not completely model cyst formation and that other aspects of cystic transformation have to be added to fully recapitulate cyst formation in vivo. For example, the addition of bromo-cyclic AMP may increase the lumen size in our model. Alternatively, further derangements in lumen size control may be necessary to increase the cyst lumen. So experiments aimed at increasing activity of AMOT, Crumbs complex, or the PARS3 complex may be needed to increase cyst lumen size (31, 35). Further experiments will be needed to fully characterize this experimental system; however, this 3D culture model offers the opportunity to examine all stages of cyst formation, from initial outgrowth to sealing off the cyst, in detail.
How can the results in this communication be reconciled with current paradigms of cystogenesis? We propose that one downstream component of kidney cell differentiation, under control of integrated planar cell polarity and cilia signaling systems, is cadherin selection. Under conditions where PKD-associated genes are mutated, cadherin selection at a genome level is altered, resulting in focal and clonal changes in cadherins expressed by the affected cells. This change is sufficient to permit changes in migratory potential and tubule morphogenic patterning of affected cells thus resulting in a kidney cyst.
In summary, our study demonstrates that cadherin 8, a type II neuronal cadherin, is expressed in cyst epithelia both in vivo and in vitro. In 3D collagen matrix culture, driving cadherin 8 expression in HK-2 cells with adenovirus is sufficient to cause a cyst-forming growth pattern arising from tubule structures.
GRANTS
This work was supported by National Institutes of Health Grants R21DK067246, R01DK050141, P30DK079312 (to R. L. Bacallao), and R01DK050141 (to A. Wandinger-Ness). The authors thank the Bloch family for continuous support.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Josh Lipshutz for advice and reagents. The authors also thank Bruce Molitoris and Kenn Dunn for helpful suggestions. Timely advice was offered by Martin ter Beest (University of Chicago, Chicago, IL).
REFERENCES
- 1. Adams M, Smith UM, Logan CV, Johnson CA. Recent advances in the molecular pathology, cell biology and genetics of ciliopathies. J Med Genet 45: 257–267, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Aribillaga L, Azqueta A, Ezpeleta O, Lopez de Cerain A. Oxidative DNA damage induced by Ochratoxin A in the HK-2 human kidney cell line: evidence of the relationship with cytotoxicity. Mutagenesis 22: 35–42, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Bacallao RL, McNeill H. Cystic kidney diseases and planar cell polarity signaling. Clin Genet 75: 107–117, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Baert L, Steg A. On the pathogenesis of simple renal cysts in the adult. A microdissection study. Urol Res 5: 103–108, 1977 [DOI] [PubMed] [Google Scholar]
- 5. Benzing T, Simons M, Walz G. Wnt signaling in polycystic kidney disease. J Am Soc Nephrol 18: 1389–1398, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Blaschke S, Mueller CA, Markovic-Lipkovski J, Puch S, Miosge N, Becker V, Mueller GA, Klein G. Expression of cadherin-8 in renal cell carcinoma and fetal kidney. Int J Cancer 101: 327–334, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Brunton VG, MacPherson IRJ, Frame MC. Cell adhesion receptors, tyrosine kinases and actin modulators: a complex three-way circuitry. Biochim Biophys Acta 1692: 121–144, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Calvet JP. New insights into ciliary function: kidney cysts and photoreceptors. Proc Natl Acad Sci USA 100: 5583–5585, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charron AJ, Nakamura S, Bacallao R, Wandinger-Ness A. Compromised cytoarchitecture and polarized trafficking in autosomal dominant polycystic kidney disease cells. J Cell Biol 149: 111–124, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Germino GG. Linking cilia to Wnts. Nat Genet 37: 455–457, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps ML. Construction of adenovirus vectors through Cre-lox recombination. J Virol 71: 1842–1849, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ibraghimov-Beskrovnaya O, Bukanov N. Polycystic kidney diseases: from molecular discoveries to targeted therapeutic strategies. Cell Mol Life Sci 65: 605–619, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Korematsu K, Nishi T, Okamura A, Goto S, Morioka M, Hamada J, Ushio Y. Cadherin-8 protein expression in gray matter structures and nerve fibers of the neonatal and adult mouse brain. Neuroscience 87: 303–315, 1998 [DOI] [PubMed] [Google Scholar]
- 14. McAteer JA, Evan AP, Gardner KD. Morphogenetic clonal growth of kidney epithelial cell line MDCK. Anat Rec 217: 229–239, 1987 [DOI] [PubMed] [Google Scholar]
- 15. McNeill H. Planar cell polarity and the kidney. J Am Soc Nephrol 20: 2104–2111, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Navarro C, Nola S, Audebert S, Santoni MJ, Arsanto JP, Ginestier C, Marchetto S, Jacquemier J, Isnardon D, Le Bivic A, Birnbaum D, Borg JP. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene 24: 4330–4339, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Nejsum LN, Nelson WJ. Epithelial cell surface polarity: the early steps. Front Biosci 14: 1088–1098, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans 36: 149–155, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nollet F, Kools P, van Roy F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol 299: 551–572, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Piepenhagen PA, Nelson WJ. Biogenesis of polarized epithelial cells during kidney development in situ: roles of E-cadherin-mediated cell-cell adhesion and membrane cytoskeleton organization. Mol Biol Cell 9: 3161–3177, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Piepenhagen PA, Nelson WJ. Differential expression of cell-cell and cell-substratum adhesion proteins along the kidney nephron. Am J Physiol Cell Physiol 269: C1433–C1449, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Piepenhagen PA, Peters LL, Lux SE, Nelson WJ. Differential expression of Na+-K+-ATPase, ankyrin, fodrin, and E-cadherin along the kidney nephron. Am J Physiol Cell Physiol 269: C1417–C1432, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Pollack V, Sarkozi R, Banki Z, Feifel E, Wehn S, Gstraunthaler G, Stoiber H, Mayer G, Montesano R, Strutz F, Schramek H. Oncostatin M-induced effects on EMT in human proximal tubular cells: differential role of ERK signaling. Am J Physiol Renal Physiol 293: F1714–F1726, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Prozialeck WC, Edwards JR, Lamar PC, Smith CS. Epithelial barrier characteristics and expression of cell adhesion molecules in proximal tubule-derived cell lines commonly used for in vitro toxicity studies. Toxicol In Vitro 20: 942–953, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Qian F, Germino GG. “Mistakes happen”: somatic mutation and disease. Am J Hum Genet 61: 1000–1005, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qian F, Watnick TJ, Onuchic LF, Germino GG. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell 87: 979–987, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Qin Y, Capaldo C, Gumbiner BM, Macara IG. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol 171: 1061–1071, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roitbak T, Ward CJ, Harris PC, Bacallao R, Ness SA, Wandinger-Ness A. A polycystin-1 multiprotein complex is disrupted in polycystic kidney disease cells. Mol Biol Cell 15: 1334–1346, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 40: 1010–1015, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Laboratory Press, NY, 1989 [Google Scholar]
- 31. Schluter MA, Margolis B. Apical lumen formation in renal epithelia. J Am Soc Nephrol 20: 1444–1452, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G, Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet 37: 537–543, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suzuki S, Sano K, Tanihara H. Diversity of the cadherin family: evidence for eight new cadherins in nervous tissue. Cell Regul 2: 261–270, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Watnick TJ, Torres VE, Gandolph MA, Qian F, Onuchic LF, Klinger KW, Landes G, Germino GG. Somatic mutation in individual liver cysts supports a two-hit model of cystogenesis in autosomal dominant polycystic kidney disease. Mol Cell 2: 247–251, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Wells CD, Fawcett JP, Traweger A, Yamanaka Y, Goudreault M, Elder K, Kulkarni S, Gish G, Virag C, Lim C, Colwill K, Starostine A, Metalnikov P, Pawson T. A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell 125: 535–548, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol 19: 207–235, 2003 [DOI] [PubMed] [Google Scholar]