Abstract
Magnetic resonance spectroscopy-based magnetization transfer techniques (MT) are commonly used to assess the rate of oxidative (i.e., mitochondrial) ATP synthesis in intact tissues. Physiologically appropriate interpretation of MT rate data depends on accurate appraisal of the biochemical events that contribute to a specific MT rate measurement. The relative contributions of the specific enzymatic reactions that can contribute to a MT Pi→ATP rate measurement are tissue dependent; nonrecognition of this fact can bias the interpretation of MT Pi→ATP rate data. The complexities of MT-based measurements of mitochondrial ATP synthesis rates made in striated muscle and other tissues are reviewed, following which, the adverse impacts of erroneous Pi→ATP rate data analyses on the physiological inferences presented in selected published studies of cardiac and skeletal muscle are considered.
Keywords: magnetization transfer, oxidative ATP synthesis, striated muscle
magnetic resonance (MR) magnetization transfer techniques (MT) are commonly used for the in vitro and in vivo estimation of the rates of oxidative ATP synthesis [i.e., the net inorganic phosphate (Pi) →ATP rate of mitochondrial ATP synthase] in heart, skeletal muscle, brain, liver, and other tissues. However, physiologically relevant interpretation of MT data depends on an accurate appraisal of the biochemical events that contribute to a specific MT rate measurement.1
This communication is focused on MT studies of the rate of oxidative ATP synthesis in cardiac and skeletal muscles because this technique has been employed in many studies of the physiology and pathophysiology of these tissues. The complexities of MT-based measurements of mitochondrial ATP synthesis rates made in striated muscle and other tissues are reviewed following which, the adverse impacts of erroneous Pi→ATP rate data analyses on the physiological inferences presented in selected published studies of cardiac and skeletal muscle are considered.
MT As Applied to the Evaluation of Enzyme Rates: Methodological Considerations
In MT studies of enzymatic rates, the nuclear spin of an atom in a reactant of an enzyme catalyzed process is selectively irradiated to disturb its magnetization away from the thermal equilibrium value. This perturbation subsequently migrates to the other reactants as the magnetically disturbed atom within the selectively irradiated reactant is incorporated into the other reactant(s) through chemical conversion. The selective irradiation can be applied so as to “saturate” an atomic moiety; i.e., the bulk magnetization of the saturated moiety becomes zero and this moiety is no longer detected in a MR spectrum. However, the same atom within the other reactants can be detected, albeit perturbed from its original thermal equilibrium signal intensity. Notably, the actual chemical processes (i.e., reactant distribution, chemical equilibrium, reaction rates, etc.) remain unaltered, but the “spin” equilibrium is perturbed. An alternative strategy is a selective irradiation pulse applied briefly to invert the spins of one atom in one reactant. Subsequently, these spins recover toward their thermal spin equilibrium and the recovery kinetics contain information about the intrinsic processes that induce spin relaxation as well as the chemical reaction itself.
In the ATP synthesis/hydrolysis reaction, the terminal phosphate of ATP (ATPγ) becomes Pi when the ATP molecule is cleaved to form ADP and Pi (Fig. 1A). The phosphorus nuclear spin in the Pi and ATPγ moieties have very different resonance frequencies and, thus, can be detected as separate peaks in a 31P MR spectrum. However, when the thermal equilibrium magnetization of the ATPγ resonance (which is directly proportional to the signal intensity in the MR spectrum) is perturbed by selective saturation, this perturbation is reflected in the signal intensity of the Pi resonance (Fig. 1B) because when the perturbed ATPγ is cleaved through ATP hydrolysis, Pi is generated. Once within the Pi moiety, the phosphorus nuclear spins have a different resonance frequency and are no longer subject to the direct influence of the selective saturation of ATPγ; therefore, they relax toward thermal equilibrium with a time constant equal to intrinsic T1 (T1i; i.e., the T1 that would be measured in the absence of any chemical exchange). But if the rate of Pi reincorporation into ATPγ is sufficiently fast compared with intrinsic T1, the Pi atoms reincorporated into ATPγ again experience the selective irradiation. Ultimately, a new spin equilibrium for Pi is reached, reflected directly in the signal intensity of this peak in the MR spectrum (Fig. 1B). This new spin equilibrium is determined by the competition between the relaxation back to thermal equilibrium with the rate constant 1/T1i and the rate of reconversion to ATPγ (i.e., Pi+ADP→ATPγ). Thus, if the T1i of the Pi resonance is known, the unidirectional2 rate of the Pi+ADP→ATP reaction (i.e., the unidirectional rate of ATP synthesis) can be calculated.
Fig. 1.
A: equation for ATP hydrolysis. B: 31P magnetic resonance spectra recorded from a perfused rat heart. B, top: the arrow indicates a (“neutral”) region of the spectrum being irradiated with saturating pulses, and this spectrum does not otherwise differ from an unirradiated spectrum (not shown). B, bottom: the effects of irradiating ATPγ are shown. Reductions in the areas of the phosphocreatine (PCr) and inorganic phosphate (Pi) resonances are obvious. This cartoon is based on data reported previously (20, 21, 43).
For simplicity, we will focus on the compounds directly detected in the magnetization transfer process and indicate this rate as the unidirectional Pi→ATP rate. The necessary parameters to calculate this unidirectional rate can be measured in several different ways. For example, spectra acquired as a function of time after the onset of the selective irradiation to saturate the ATPγ peak can be used to extract both the rate of approach to the new spin equilibrium and the intensity of Pi in this new spin equilibrium. Alternatively, the intensities of the new spin equilibrium can be measured in one spectrum after allowing sufficient time to establish a new steady state under saturation, and in addition, T1 for the Pi resonance in the presence of the selective saturation is separately measured. For the creatine kinase reaction, saturation of ATPγ leads to a perturbation of the phosphate moiety of phosphocreatine (PCr) (shown in Fig. 1B).This measurement then allows the determination of the unidirectional PCr→ATP rate.
An alternative method for determination of the Pi→ATP rate involves selective inversion of one of the reactants (versus saturation), and, as mentioned above, has also been used. Depending on the intrinsic T1 of the inverted or saturated resonance, one method can be more sensitive than the other. For simplicity we will focus on the MT method using saturation rather than inversion since this is the approach most commonly used in the in vivo studies.
Although the MT technique measures unidirectional rates (i.e., the number of molecules converted per unit time), one formulates the process mathematically using unidirectional rate constants (rate per unit molecule). Thus, for the unidirectional rates of A↔B conversion, the apparent unidirectional rate constants are defined so that the unidirectional rates vA→B and vB→A are given by the equations vA→B = kA→Ba[A] and vB→A = kB→Aa[B], respectively. The designation “apparent” is used for the rate constant because, in an enzymatic reaction, the rate constant calculated in this way is a complex function of the reactants and products and does not have the straightforward meaning it would have in a nonenzymatic reaction.
Obviously, measurement of enzyme rates with MT is a powerful analytic technique. However, major interpretive complications are inherent in the method when applied in vivo. In aerobic tissues, the net rate of ATP synthesis by the mitochondrial ATP synthase (i.e., the net rate of ATP synthesis through oxidative phosphorylation) is proportional to the oxygen consumption rate of that tissue by the P/O ratio. Under conditions where ATP concentrations are time independent (i.e., in a steady state), the net rates of ATP synthesis by the oxidative phosphorylation process and by other pathways (e.g., glycolysis) must be equal to the net rate of ATP utilization by all energy-consuming processes, such as muscle contraction and maintenance of ion gradients, etc. Ignoring for the time being possible contributions by all other pathways (like glycolysis) that can contribute to ATP synthesis, and assuming that all ATP synthesis is oxidative, we can consider the problem as composed of two unidirectional rates of the mitochondrial ATP synthase (in the Pi→ATP and ATP→Pi directions) and the rate of ATP utilization by energy-driven cellular processes, which operates unidirectionally in the ATP→Pi direction. In this case, the difference between the two unidirectional rates of the mitochondrial ATP synthase (i.e., the net ATP synthesis rate) must be equal to the rate of ATP utilization under steady-state conditions. In other words,
 |
where FATPsynthesisNET Cell is the net intracellular ATP synthesis rate, and FATPsynthesisNET Mitochondria is defined as [(vPi→ATPMitochondria) − (vATP→PiMitochondria)]. It is FATPsynthesisNET Mitochondria that is always proportional to the oxygen consumption rate by the P/O ratio. In this case, we assumed ATP synthesis to occur only oxidatively so that FATPsynthesisNET Cell = FATPsynthesisNET Mitochondria. The unidirectional rates are represented by v, and vATP→Piutilization represents ATP consumption by the energy-driven processes, such as contraction, which operate only in one direction. In this specific case, the experimentally measured rate by MT where ATPγ is selectively saturated, vPi→ATPMT exp, is equal to vPi→ATPmitochondria. This implies that the experimentally measured rate (vPi→ATPMT exp) is equal to the net rate of oxidative ATP synthesis (FATPsynthesisNET Mitochondria) if and only if the mitochondrial ATP synthase operates far out of equilibrium (i.e., vATP→Pimitochondria ≈ 0). Otherwise, (vPi→ATPMT exp) >> FATPsynthesisNET Mitochondria. Note that the rate calculated by the product of the oxygen consumption rate and the P/O ratio, which we can designate as FOxy ConsP/O, is always equal, by definition, to FATPsynthesisNET Mitochondria. Consequently, FOxy ConsP/O ≈ vPi→ATPMT exp if and only if the mitochondrial ATP synthase operates far out of equilibrium.
However, there is the potential confounding factor of additional contributions to the Pi→ATP rate from enzymes and pathways other than ATP synthase. Thus, we must consider
or
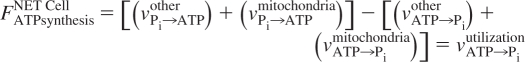 |
In this more general case, the experimentally measured rate by MT, vPi→ATPMT exp, is equal to [(vPi→ATPother) + (vPi→ATPmitochondria)]. Notably, the net rate of ATP synthesis of all other pathways may be negligible compared with net rate of aerobic ATP synthesis, and, hence, insignificant with regard to the energy balance and ATP turnover of that tissue; yet, the unidirectional rates of ATP synthesizing enzymes within that pathway may be very fast and may make a major contribution to the MT-measured unidirectional rate (e.g., Refs. 21 and 43 and references therein). Expressing this mathematically using the rate equations above, FATPsynthesisNET other = [(vPi→ATPother) − (vATP→Piother)] ≈ 0 can be the case in the tissue but vPi→ATPother can be comparable or even larger than vPi→ATPmitochondria so that the experimentally measured rate cannot be equated with vPi→ATPmitochondria alone. Under these circumstances, although the apparent rate constant and unidirectional rate measurements may be accurate, they represent the sum of the unidirectional rates of all of the enzymes generating the MT effect (Fig. 2). As discussed in greater detail later, this is in fact the case in cardiac muscle. In the fortunate case that one of these component unidirectional reactions is much faster than all others, the measured rate can (reasonably) be assigned to that specific reaction. However, it is critically important to realize that this situation cannot, a priori, be assumed to be applicable. Thus, the rate calculated from the oxygen consumption rate multiplied by the P/O ratio is not necessarily equal to the experimentally measured rate by MT when selectively saturating ATPγ. The most general case is that
 |
and thus vPi→ATPMT exp ≥ FOxy ConsP/O. To claim that vPi→ATPMT exp ≅ FOxy ConsP/O requires that all other pathway contributions to the unidirectional Pi→ATP rates must be negligible and the mitochondrial ATPase operates unidirectionally in the Pi→ATP direction. These conditions cannot be assumed to be valid in intact tissues. On the contrary, as will be discussed, this problem constitutes an important complication for the use of MT-measured Pi→ATP rates to determine the net rate of oxidative ATP synthesis by mitochondrial ATP synthase in striated muscle and in most other tissues thus far studied; this complication, however, has been largely ignored despite a few publications calling attention to the large discrepancy between ATP synthesis, calculated from oxygen consumption rate and the P/O ratio, and the MT-determined rates in the Pi→ATP direction. A notable exception where this complication does not exist appears to be the brain. The consequences of this methodological confound in several tissue types are discussed next.
Fig. 2.
This schematic illustrates the point that the magnetization transfer technique (MT)-determined Pi→ATP rate in the perfused rat heart with intact glycolysis is the sum of rates arising from different enzymes, the GAPDH/PGK enzyme couple, and the mitochondrial ATP synthase. The sum of these rates equals the MT-measured rate.
Pi→ATP Rate Measurements in Cardiac Muscle
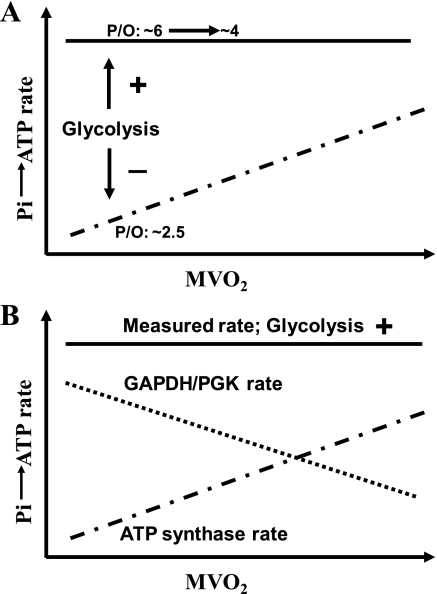
More than 20 years ago, we described the use of MT for the determination of Pi→ATP rate measurements to estimate the P/O (defined as the moles of ATP produced per half-mole of oxygen consumed) in intact myocardium (Langendorff-perfused hearts). Our initial study (Fig. 3A, solid line) yielded an apparent P/O of ∼6 at low cardiac work states (20). Disturbingly, this was twice the then canonical value of P/O (∼3) claimed in many contemporary studies of isolated mitochondria. We noted that the measured Pi→ATP rate did not increase as myocardial mechanical performance and myocardial oxygen consumption rates (MV̇o2) were increased by heart rate elevation and catecholamine infusion. Furthermore, although the apparent P/O fell moderately as MV̇o2 increased, it remained >4 at the highest cardiac work state achieved. One possible explanation was that, in the intact heart, mitochondrial ATP synthase had a significant rate in the ATP→Pi direction. Hence, although the net rate of mitochondrial ATP synthesis must be proportional to MV̇o2 by the P/O ratio, the two unidirectional Pi→ATP and ATP→Pi rates can each be much larger than the net Pi→ATP rate. This speculation was theoretically possible because ATP synthase had long been known to be capable of generating a significant ATP→Pi rate under some experimental conditions and this enzyme had also been considered to be “near equilibrium” in intact myocardium (12). If our early speculation had been correct, our data would have predicted that, as ATP demand increased, the net Pi→ATP rate increased solely as a consequence of a progressively decreasing ATP→Pi rate through ATP synthase.
Fig. 3.
A: in perfused rat hearts with normal glycolysis, the measured Pi→ATP rate did not increase as MV̇o2 was increased and the apparent P/O fell modestly as MV̇o2 increased (solid line). In contrast, in the hearts with inhibited glycolysis (dashed-dotted line), the Pi→ATP rate did increase proportionally in relation to MV̇o2 and the apparent P/O (∼2.5) did not change as MV̇o2 increased. B: this cartoon represents our concept of the alterations in the contributions of the GAPDH/PGK couple (dotted line) and the ATP synthase (dashed-dotted line) rates to the measured Pi→ATP rate (solid line) that occur when MV̇o2 increases in the perfused rat heart in which glycolysis is active (see text for further discussion). These cartoons are based on data reported previously (20, 21, 43).
However, we soon realized that another potential contributor to the measured unidirectional Pi→ATP rate in myocardium was the glyceraldehyde dehydrogenase (GAPDH)/phosphoglycerate kinase (PGK) enzyme couple. In 1987, Brindle and Radda (5) reported data obtained from the study of isolated GAPDH/PGK enzymes (the assay milieu employed mimicked conditions thought to be present in the cytosol of cardiomyocytes) and we (21, 43) also reported new data obtained in the perfused rat heart bearing on this issue. The data in these reports strongly supported the view that the coupled GAPDK/PGK reaction pair operates near equilibrium (i.e., the forward and backward unidirectional rates of this enzyme couple are approximately equal) and both of these unidirectional rates are large and far exceeded the rates of net ATP production by and of carbon flux through the glycolytic pathway. These considerations led us to hypothesize that, in the myocardium, it might be impossible to evaluate the Pi→ATP rate through the mitochondrial ATP synthase in the presence of active glycolysis (i.e., when the GAPDH/PGK couple was active) even though the net ATP production rate by the glycolysis pathway is far overshadowed by the rate of oxidative ATP synthesis in this highly oxidative tissue. In short, we hypothesized that ATP synthase contributed only a fraction of the MT-measured Pi→ATP rate and that another large fraction was contributed by the GAPDH/PGK couple (Fig. 2).
To test this hypothesis, the perfused rat heart experiments were repeated while glycolysis was inhibited (by treatment with iodoacetate or by glycogen depletion induced by a preceding period of carbon substrate-free perfusion) (21, 43). In these studies, the perfusion medium was glucose free and contained pyruvate (in lieu of glucose) to support oxidative metabolism. Under these experimental conditions, the MT-measured unidirectional Pi→ATP rate increased in concert with increasing MV̇o2 and the P/O ratio calculated from the MT data was ∼2.5 during the baseline work state and did not change as MV̇o2 values increased (Fig. 3A, dashed-dotted line). These data indicated that the GAPDH/PGK couple did make a substantial contribution to the MT-measured Pi→ATP rate when glycolysis was active. Hence, when GAPDH/PGK activity was eliminated, the MT-determined Pi→ATP rate was proportional to MV̇o2 by the P/O ratio. As discussed previously, this is possible 1) if and only if no other pathways make a significant contribution to the MT-determined unidirectional Pi→ATP rate, and 2) if and only if the mitochondrial ATP synthase operates essentially unidirectionally (i.e., far out of equilibrium) in the Pi→ATP direction. That a large glycolytic contribution to the MT-measured unidirectional Pi→ATP rates could occur was also shown in studies of Escherichia coli and yeast (6).
Figure 3B shows our concept of how the rates of each of the two subcomponents of the measured Pi→ATP flux change as MV̇o2 increases in the glucose-perfused heart. The oxidative Pi→ATP rate due to mitochondrial ATP synthase at any given level of MV̇o2 is expected to be essentially the same irrespective of whether glycolysis is active or inhibited. Hence, the observation that when glycolysis is active, the measured Pi→ATP rate is high at the lowest work state and does not increase further in conjunction with increasing MV̇o2 (Fig. 3B, solid line) indicates on stoichiometric grounds that the GAPDK/PDK rate contribution to the measured Pi→ATP rate decreases moderately as MV̇o2 increases (Fig. 3B, dotted line). In contrast, the mitochondrial Pi→ATP rate contribution increases (Fig. 3B, dashed-dotted line) just as shown in the studies in which glycolysis was blocked (Fig. 3A, dashed-dotted line). We cannot explain why, in the perfused heart, the absolute contribution of the GAPDH/PGK couple to the measured Pi→ATP flux decreases as MV̇o2 increases; however, as will be seen later, this phenomenon does not appear to occur in autoperfused rat hindlimb muscles.
Using these methods for determining the “true” P/O in an intact tissue, we subsequently directly determined the extent of mitochondrial uncoupling induced by administration of high concentrations of fatty acids or dinitrophenol in perfused rat hearts (22). In another report, we also demonstrated that a period of nonlethal global ischemia sufficient to cause myocardial stunning did not necessarily induce mitochondrial uncoupling (37) as had been previously proposed. Importantly, these MT studies strongly supported the concept that, in intact myocardium operating under conditions of adequate oxygen and carbon substrate availability, there was not a significant reverse rate (i.e., in the ATP→Pi direction) through mitochondrial ATP synthase.
When we extended these studies to in vivo canine hearts (with intact glycolysis), we found that the glycolytic contribution to the measured unidirectional Pi→ATP rate was also substantial as indicated by the presence of an unphysiologically high P/O (36). Portman (33) confirmed our finding that the GAPDH/PGK contribution to the Pi→ATP rate was quite high in in vivo myocardium in a study performed in sheep hearts (with intact glycolysis). In that study, a work jump was employed to increase the rate of ATP utilization. When the Δ(Pi→ATP) was divided by the Δ(MV̇o2) (where Δ = differences between basal and high work state measurements), a physiologically realistic P/O ratio of ∼2.7 was calculated. Collectively, these in vitro and in vivo data indicate that the magnitude of the glycolytic contribution to Pi→ATP rate in the heart (and presumably in any tissue) must be known (or eliminated) if the unidirectional Pi→ATP rate through ATP synthase (i.e., the rate of oxidative phosphorylation) is to be inferred from MT measurements.
Our Cardiac Pi→ATP Rate Data Are Consistent With Findings in Isolated Mitochondria
Using radioactive tracers, LaNoue et al. (24) showed that the ATP→Pi rate was very low in fully energized (isolated) cardiac mitochondria undergoing state 3 respiration. In contrast, under state 4 respiratory conditions, where ADP availability was limited but carbon substrate and oxygen availability were not limiting, ATP in the incubation medium was rapidly hydrolyzed to form ADP and Pi via ATP synthase. Because mitochondria in perfused working hearts appear to be working under “state 3-like” conditions, our data were consistent with the data obtained in isolated mitochondria. This concordance supports our conclusion that, in the perfused heart, the mitochondrial ATP→Pi rate was minimal and that the MT-measured unidirectional rate did reflect the net rate of mitochondrial ATP synthesis so long as glycolysis was inhibited.
How Do These Considerations Relate to the Possible Presence of Significant ATP→Pi Rates in Resting Skeletal Muscle?
Although rate data obtained in skeletal muscle will be discussed in considerable detail later, to our knowledge, no direct measurements of mitochondrial derived ATP→Pi rates have been made in resting skeletal muscles. As pointed out, LaNoue et al. (24) showed that there was a considerable ATP→Pi + ADP rate in isolated mitochondria operating under state 4 conditions and essentially none when state 3 conditions were present. In resting skeletal muscle, ATP synthetic rates are limited by ADP availability because the rate of ATP expenditure is low and carbon substrate and oxygen availabilities are not limiting (7). Hence, mitochondrial function in resting skeletal muscle may be more comparable to that present during state 4 rather than during state 3 respiratory conditions. If so, then a significant ATP→Pi rate for the mitochondrial ATP synthase may be present in resting skeletal muscle.
The physiological implications of this possibility are interesting. If there is a substantial ATP→Pi rate through ATP synthase in resting skeletal muscle, then the MT-measured unidirectional Pi→ATP rate must exceed the net rate of mitochondrial ATP synthesis, even if the potential contribution from the GAPDK/PGK couple or other possible pathways to that measured rate are excluded. This is because, as previously discussed, the net rate of ATP synthesis must equal to the difference between the two unidirectional rates operating in the Pi→ATP and ATP→Pi directions.
Are There Other (Additional to GAPDH/PGK) Potential Enzymatic Sources of the MT-Measured Pi→ATP Rate in Striated Muscle?
In addition to the mitochondrial ATP synthase, we are aware of two enzymes that could potentially contribute to the MT-measured Pi→ATP rate. The reaction catalyzed by pyruvate kinase (PK) is reversible in some tissues (44). In the forward direction, PK supports substrate level phosphorylation of ADP, and in the reverse direction, PK supports several synthetic pathways. However, in skeletal muscle, PK generates a significant reverse rate only when intracellular lactate levels are quite high and lactate is a substrate for gluconeogenesis (44). This situation does not occur in normal cardiac muscle (in which there is not much accumulation of lactate in the cytosol even during high work states) or in resting skeletal muscle unless blood and intracellular lactate levels are high (i.e., following vigorous exercise). Hence, in resting skeletal muscle the Pi→ATP rate of PK should also be quite low (i.e., equal to the net glycolytic rate) when compared with the rate of mitochondrial ATP production and should not make a significant contribution to the MT rate measurements obtained in these tissues.
A second enzyme that catalyzes substrate level phosphorylation of ADP is succinyl CoA synthase, a component of the TCA cycle. Phillips et al. (32) recently showed that Pi allosterically activated this enzyme and that the ATP synthesis catalyzed by this enzyme could make significant contributions to cellular ATP production when mitochondrial matrix Pi levels were high and mitochondria were energy limited, (i.e., during ischemia). However, generation of more than one ATP per turn of the TCA cycle (e.g., by this enzyme) should not occur in normally working myocardium or in resting skeletal muscle in which the aforementioned conditions are absent.
Last, as is well known, many cellular ATPases (i.e., the Na+-K+-ATPase, etc.) are capable of generating unidirectional Pi→ATP rates. However, this phenomenon is present only when experimental conditions are nonphysiological and these conditions are not present in the oxygenated perfused heart or in resting human skeletal muscle.
Tissue Heterogeneity As a Confounding Factor
Our work and that of other investigators in the field have been (implicitly) premised on the concept that metabolic behavior described is homogeneous within the voxel or region sampled. However, as is well known, there is evidence of macroheterogeneity within large skeletal muscles (18) and microheterogeniety within the heart (10). This phenomenon can (to an unknown extent) complicate interpretation of data acquired using MR methods. However, a detailed discussion of this issue exceeds the scope of this review. Nevertheless, in the brain, the observation that MT-measured Pi→ATP rate in the brain does track the rate of oxidative phosphorylation may result from tissue heterogeneity (see discussion further on).
Problematic Interpretation of Pi→ATP Rate Data in a Perfused Heart Study
In a perfused guinea pig heart study examining the response of myocardial high-energy phosphate compound metabolism to nitro-l-arginine methyl ester (l-NAME) inhibition of nitric oxide synthase (NOS) (40), MT-measured Pi→ATP rates and measured MV̇o2 values were used to evaluate the hypothesis that inhibition of NOS decreased energetic efficiency. In that report, efficiency was defined as the ratio of the MT-measured unidirectional Pi→ATP rate to the measured MV̇o2 value. The P/O ratio defines mitochondrial efficiency and uncoupling reduces the efficiency of oxidative phosphorylation and thus the P/O ratio. However, as previously discussed, the ratio of the MT-measured unidirectional Pi→ATP rate to the measured MV̇o2 value can be equal to the P/O ratio 1) if there are no other reactions, like GAPDH/PKG, contributing to the MT measurement and 2) if mitochondrial ATPase operates virtually unidirectionally in the Pi→ATP direction. The first condition is unlikely to have been met in this study because their perfusate carbon substrate was 11 mM glucose. Hence, the GAPDH/PGK couple must have been quite active even if it is assumed that cardiac metabolism was also being partially supported by utilization of endogenous lipids (23).
It was observed that the mean values of the MT-determined Pi→ATP rates were the same in the control and l-NAME groups although MV̇o2 was significantly higher in the latter group. If the Pi→ATP rate measurements actually represented only the rate of oxidative ATP synthesis, then these data would imply the presence of some degree of mitochondrial uncoupling when NOS was inhibited. However, the MV̇o2 values estimated from their Figure 4 [(40); ∼25 and 29 μmol·g dry wt−1·min−1, respectively, for control and l-NAME treated hearts] can be used to calculate rates of oxidative ATP synthesis assuming a P/O of 2.5. Both groups of hearts were studied at heart rate-systolic pressure product (RPP) rates of ∼22,000 mmHg/min and the ATP synthetic rates calculated from the MV̇o2 data are ∼125 and ∼145 μmol·g dry wt−1·min−1 for the control and l-NAME-treated groups, respectively. Yet the means of the MT-measured Pi→ATP rates attributed to oxidative phosphorylation were ∼3.5 and 3.8 mM/s in the control and treatment groups, respectively. When the latter rates are converted to the same units used for our MV̇o2-based ATP synthase rate calculations [assuming cell water to be ∼65% of tissue weight (16)], the measured unidirectional Pi→ATP rates for the two groups were ∼1,050 and 1,140 μmol·g dry wt−1·min−1. These values are substantially higher than the rate of oxidative ATP synthesis estimated from the MV̇o2-based calculations. As is evident, under the described experimental conditions, these unidirectional MT-based Pi→ATP rate data were inappropriately used to infer the presence of mitochondrial uncoupling because the ATP synthase-derived component of the measured Pi→ATP rates was substantially smaller than their GAPDH/PGK-associated Pi→ATP rate components, as we had previously reported (21, 43). Although acute inhibition of NOS could have caused some mitochondrial uncoupling and accounted for the small differences in MV̇o2 between the groups, the Pi→ATP rate data reported cannot be used to support this hypothesis.
MT Studies in Liver and Brain Further Illustrate the Presence of Tissue-Related Variations in the Size of the Glycolytic Contribution to the Measured Pi→ATP Rate
Liver.
A study performed in our laboratory by Thoma and Ugurbil (42) is instructive with regard to the tissue variability of the magnitudes of the glycolytic and mitochondrial contributions to the MT-determined unidirectional Pi→ATP rate. In the perfused liver (in which it is possible to accurately measure the rate of O2 consumption), it was found that MT-measured unidirectional Pi→ATP rate was almost entirely of glycolytic origin. In a similar (but noninvasive) study performed in human subjects, Schmid et al. (39) reported that the MT-measured Pi→ATP rate in the liver was ∼29 mM/min. These authors pointed out, based on published estimates of in vivo human hepatic oxygen consumption and an assumed P/O ratio of 2, that glycolysis accounted for ∼75% of the MT-measured unidirectional Pi→ATP rate.
Brain.
Lei et al. (26) (also from our laboratory) have reported Pi→ATP rate measurements in the human visual cortex. In contrast to observations made in the liver and heart, estimates of the Pi→ATP rates calculated from published measurements of brain oxygen consumption rates were comparable to the MT-measured Pi→ATP rates. These findings were confirmed in a subsequent study in which MT-measured Pi→ATP rates and estimates of the oxidative ATP synthesis rate made from concomitant 13C measurements of the TCA cycle rate were also shown to be concordant in the human brain (11). Hence, the brain may be at the other extreme (as compared with liver) with regard to the degree that MT measurements of Pi→ATP rate reflect the activity of mitochondrial ATP synthase alone.
The actual reason(s) why MT-determined Pi→ATP rates in brain appear to be concordant with non-MT-based rate measurements is unknown. The authors speculated that the presence of two different primary cell types in the brain, i.e., neurons and astrocytes, might alter the results of the MT studies. It has been proposed that the main oxidative carbon substrate utilized by neurons might be lactate that is glycolytically generated by astrocytes (30). If so, then glycolytic activity mediating a Pi→ATP rate might be predominantly sequestered in the astrocytes (and other glial cells of the brain) while the MT-measured Pi→ATP flux measured in the brain might emanate primarily from neurons. The latter would be possible, for example, if the Pi levels were higher in neurons (as compared with glial cells) and the Pi content of glial cells were too low to be detectable in 31P MR spectra of the brain. In this construct, the Pi detected in brain would arise mainly from neurons and the MT-measured Pi→ATP rate would be of neuronal origin while the major glycolysis associated GAPDK/PGK contribution in the Pi→ATP direction would occur in astrocytes (and other glial cells) and be “MR invisible.”
Unidirectional Pi→ATP Rate Measurements in Skeletal Muscle
Animal studies.
One of the earliest (and most frequently cited) studies of MT-determined Pi→ATP rates in skeletal muscle was carried out by Brindle et al. (4). Because the data and analyses presented in this paper serve as the template on which virtually all subsequent MT-based Pi→ATP rate measurement studies of skeletal muscle are based, we analyzed it in some detail. This paper reported the effects of isometric contractions of increasing duration and frequency on MT-measured Pi→ATP rates in the (autoperfused) hindlimb muscles of anesthetized rats. The Pi→ATP rate was found to increase in concert with the tension-time index (an index of contractile energy expenditure; TTI). However, in resting muscle, a substantial (rather than a modest) unidirectional Pi→ATP rate was also present although the TTI was zero.
As already mentioned, Brindle and associates (5) had previously studied (in vitro) the capacity of GAPDH/PGK couple to generate a MT-detectible Pi→ATP rate. Thus, these authors analyzed their data with the possibility in mind that the GAPDH/PGK rate might contribute to their measurements of the Pi→ATP rate. On the basis of calculations using their TTI data and the oxygen consumption data obtained in rat skeletal muscle by Hood et al. (15), they concluded that the observed work-associated increase of measured Pi→ATP rate probably did not contain a major GAPDH/PGK component.
This conclusion is puzzling because the methods section of the Brindle et al. (5) report indicates that the study rats were not fasted before the start of the experiments. Hence, blood fatty acid levels were likely not as high as they would have been in fasted animals and the blood insulin levels were probably high enough to foster significant glucose uptake in resting skeletal muscle. Hence, a significant GAPDH/PGK contribution to the unidirectional Pi→ATP rate measurement would have been expected to be present in both the resting and exercising muscles. Furthermore, because resting rat hindlimb oxygen consumption rates were known to be very low (15), the rate of mitochondrial oxidative ATP synthesis must also have been commensurately low. However, Brindle et al. reported a resting muscle Pi→ATP rate measurement of 0.8 μmol·g dry wt−1·s−1. As will be shown, this value is much higher than ATP synthetic rates calculated from the data of Hood et al. (15), who directly measured oxygen consumption rates in glucose buffer-perfused resting rat hindlimbs at rest and during a progressive sciatic nerve stimulation protocol. The latter group's resting muscle oxygen consumption measurements averaged ∼0.37 μmol·g dry wt−1·min−1. This can be converted into the same rate units used in the Brindle et al. paper by assuming a P/O of 3 and a tissue wet-to-dry ratio of 4.35. This calculation yields a value of 0.16 μmol·g dry wt−1·s−1 of oxidative ATP synthesis as compared with the value of 0.8 μmol·g dry wt−1·s−1 reported by Brindle et al. In other words, the net ATP synthesis rate calculated from published oxygen consumption data approximated ∼20% of the MT-measured Pi→ATP rate reported by Brindle et al. This implies that the rate measurement obtained in the resting muscle in the latter report likely did include a major contribution from the GAPDH/PGK couple and/or the mitochondrial ATPase had a significant unidirectional rate in the ATP→Pi direction.
During the stimulation protocol, the TTI values reported in the Brindle et al. paper ranged from ∼0.05 to ∼0.35 N·s−1·cm−2; i.e., TTI increased sevenfold from the lowest to the highest stimulation induced work state. Over the same TTI range, the reported MT-measured Pi→ATP rate increased by approximately ∼2.8-fold. However, when the estimated resting muscle Pi→ATP rate component that we presume was generated by glycolysis (0.64 μmol·g dry wt−1·s−1; see calculations above) is subtracted from each of the data points obtained during muscle stimulation, the fold increase in Pi→ATP rate over the TTI range increased to ∼5.2-fold. This “corrected” estimate of the magnitude of the Pi→ATP rate increase from the lowest to the highest stimulation rates approximates the expected increase of the rate of oxidative ATP synthesis if one assumes linearity between the TTI and oxygen consumption as do the authors. In contrast, the fold increase calculated from the “uncorrected” data in the report markedly underestimates the increase in the Pi→ATP rate generated by ATP synthase during the stimulation protocol.
Our reanalysis of these data, taken together with the O2 consumption data reported by Hood et al. (15), suggests that, in resting rat skeletal muscle, 1) the measured unidirectional Pi→ATP rate contains a major GAPDH/PGK component and/or mitochondrial ATPase operates with a significant unidirectional rate in the ATP→Pi direction, as reported for isolated mitochondria respiring under state 4 conditions; and 2) net rates of both glycolytic and oxidative ATP synthesis are low. During the muscle stimulation protocol, 1) the glycolytic contribution to the overall unidirectional Pi→ATP rate measurement (unlike the oxidative contribution) and/or a possible unidirectional mitochondrial ATPase rate in the ATP→Pi direction (24) may not have changed much, and 2) the component of the total unidirectional Pi→ATP rate associated with mitochondrial ATPase increased appropriately in concert with the TTI.
In a different study of oxidative ATP synthesis in resting rat skeletal muscle, MT evaluation of the Pi→ATP rate was done in concert with a 13C MR-based measurement of the TCA cycle rate. It was reported that the transition from the fed to fasted state was not associated with mitochondrial uncoupling as evidenced by a lack of change of the ratio of the MT-measured Pi→ATP rate to the TCA cycle rate (17). In another study by this group, the effects of triiodo-l-thyronine or dinitrophenol (the latter a classical mitochondrial uncoupling agent) were examined in resting rat skeletal muscle by using MT and 13C methods (16). In that report, reductions of the ratio of the Pi→ATP rate to the TCA cycle were observed with both interventions and this was claimed to be evidence of the presence of mitochondrial uncoupling. However, in both of the aforementioned reports, our calculations of oxidative ATP synthesis rates (from their measured TCA cycle turnover rates) yielded Pi→ATP rate values that were far lower than those estimated from their MT data. Hence, it is likely that, in these experiments, a significant GAPDH/PGK contribution to the unidirectional Pi→ATP rate and/or a significant unidirectional mitochondrial ATPase rate in the ATP→Pi direction was present. This complication, in our view, invalidates their claimed demonstration of increased mitochondrial uncoupling in these muscles despite the fact that some degree of uncoupling was undoubtedly present following the cited pharmacological interventions.
Additional reports such as those of Yerby et al. (46) have also claimed that MT-based Pi→ATP rates determined in resting rat skeletal muscle reflect the rate of oxidative ATP synthesis. However, their data analysis is also based on that presented by Brindle et al. (4). Notably, the measured Pi→ATP rates in all groups studied in the Yerby et al. report were approximately three to six times higher than oxidative ATP synthesis rates calculated from the resting rat skeletal muscle oxygen consumption data reported by Hood et al. (15). The conclusions presented in the Yerby et al. report were challenged in a Letter to the Editor (19) whose authors pointed out the discrepancies between the MT-determined Pi→ATP rate measurements and previous estimates of oxidative ATP synthesis rate made from either oxygen consumption or 13C MRS TCA cycle turnover rate measurements.
Human Skeletal Muscle
Relevant physiological characteristics of human skeletal muscle.
In resting human skeletal muscle, intracellular Po2 levels are high (34) and carbon substrate availability is nonlimiting; furthermore, as pointed out above, regulation of oxidative ATP synthesis appears to be ADP (and Pi) limited in resting skeletal muscle (7).
Oxygen consumption of resting human skeletal muscle.
Data reported by Richardson and Saltin (35) allowed us to calculate the oxygen consumption rate of resting human quadriceps muscle (i.e., ∼0.39 μmol·g wet wt−1·min−1). In that study, thigh arteriovenous oxygen content differences, muscle blood flows, and muscle volume were measured and it was assumed that almost all blood flow and other data obtained were derived from the quadriceps. However, some of their reported blood flow and oxygen uptake values may have arisen from tissues in the leg other than the quadriceps. Hence, their estimate of quadriceps oxygen uptake may have been somewhat high. From these data (assuming an operational P/O ratio of 3), the oxidative ATP synthetic rate can be estimated to be ∼2.4 μmol·g wet wt−1·min−1 in the resting quadriceps. In another study in which human vastus lateralis muscle blood flow and oxygen uptakes were measured by means of positron emission tomography, the estimated resting muscle oxygen uptake ranged between ∼0.04 and 0.08 μmol·g wet wt−1·min−1 depending on whether the subject was untrained or trained and on which region of the muscle was sampled (18). The oxidative ATP synthetic rates calculated from these oxygen consumption measurements (again assuming a P/O of 3) ranged from ∼0.24 and 0.48 μmol·g wet wt−1·min−1. The true rate of human resting quadriceps muscle oxygen consumption (and the net rate of oxidative ATP synthesis estimated from this value) likely resides somewhere between the values stated in the aforementioned reports. For the purposes of the discussion of the human studies cited below, the resting quadriceps oxygen consumption values calculated from data reported by Richardson and Saltin (the largest literature estimate that we found) will be used for comparison with MT-derived Pi→ATP rate measurements.
MT-Based Pi→ATP Rates in Resting Human Skeletal Muscle
As noted above, the oxidative ATP synthetic rate calculated from the oxygen consumption rate present in resting quadriceps muscle was ∼2.4 μmol·g wet wt−1·min−1. These values are far below the range of the MT-derived ATP synthesis rates (∼ 8–14 μmol·g wet wt−1·min−1) reported by Szendroedi et al. (41) and Petersen et al. (31) in the quadriceps of awake normal human subjects and also in the nondiabetic offspring of type 2 diabetes mellitus (DM2) patients. Because the muscle oxygen consumption rate is the “gold standard” for indirect estimation of the rate of oxidative ATP synthesis (assuming normal mitochondrial coupling), it is clear that, in these reports, the MT Pi→ATP rate values far exceeded the rates calculated from the aforementioned oxygen consumption data.
The view that the uncorrected MT-based Pi→ATP rate measurements in resting skeletal muscle markedly overestimate the true rate of oxidative ATP synthesis is also supported by recent data reported by Befroy et al. (1). In that study, the turnover rates of the TCA cycle (using 13C MR methods) were measured in resting skeletal muscle of both normal subjects and in the nondiabetic offspring of DM2 patients. It was reported that the TCA cycle turnover rates were significantly lower in the offspring of the diabetics than in normal subjects (−60 vs. 96 μmol·g wet wt−1·min−1, respectively), suggesting that the rate of oxidative ATP production was lower in the former. Oxidative ATP synthetic rates of 0.9 and 1.4 μmol·g wet wt−1·min−1, respectively, can be calculated from the TCA cycle rate data cited by assuming a stoichiometry of 15 molecules of ATP synthesized from the reducing equivalents produced during each turn of the TCA cycle. This calculation is actually a modest underestimate of the rate of cytochrome oxidase catalyzed oxygen consumption because both the glucose and the fatty acid metabolic pathways themselves produce reducing equivalents that also contribute to the rates of mitochondrial ATP synthesis rates. However, in the normal subjects (despite the aforementioned simplification of our calculations), the reported TCA cycle based estimates of the rate of oxidative ATP synthesis are near the oxidative ATP synthetic rate calculated from the aforementioned oxygen consumption data (∼2.4 μmol·g wet wt−1·min−1) obtained in resting human skeletal muscle (35). In contrast, the estimates of the Pi→ATP rates from the reported TCA cycle data (1) were markedly lower than the MT-acquired Pi→ATP rate values previously reported in comparable groups of subjects (31, 41). We do not dispute the possibility that the true mitochondrial Pi→ATP rate (i.e., the rate estimated from the TCA cycle data) may be lower in the muscle of insulin insensitive subjects. However, whether “mitochondrial inadequacy” (our term) is the basis of this difference is questionable as will be argued next.
Can Mild to Moderate Reductions of the (Tissue) Mitochondrial Vmax for Oxidative ATP Synthesis Affect the Oxidative Pi→ATP Rate in Resting Skeletal Muscle?
Decreased mitochondrial density in the skeletal muscle of DM2 subjects and their offspring has been reported (28). However, in a study that examined the functional consequences of this finding in more detail, it was found that although the Vmax value for oxidative ATP synthesis was reduced in muscle homogenates obtained from patients with DM2, the Vmax values for ATP synthesis per milligram of mitochondrial protein extracted from the homogenates were normal (3). These data indicate that, although the tissue Vmax for ATP synthesis is modestly reduced in these subjects, the ATP synthetic capacity of individual mitochondria is not reduced (27).
Given the aforementioned data, the question arises whether a modest reduction of the tissue Vmax for oxidative ATP synthesis (i.e., per mg of muscle) could limit the rate of oxidative ATP generation present in either resting or even during submaximally exercising skeletal muscle. On the basis of considerations of enzyme kinetic theory, the answer to this question is likely no. This theory posits that the Vmax of a given quantity of an enzyme (E) is an indication of its maximum turnover rate if this measurement is obtained under conditions where substrate availability is in excess and product inhibition is minimal (i.e., during the initial phase of the reaction). However, in a cuvette, under conditions in which the actual turnover rate of E is constrained because the concentration of a substrate is significantly lower than the enzyme Km for that substrate, then increasing concentration of that substrate will speed up the enzyme turnover rate. Hence, at the markedly submaximal level of ATP demand present in a resting muscle, a mildly reduced mitochondrial content would not limit the rate of ATP synthesis because of kinetic compensatory mechanisms such as 1) increased mitochondrial reducing equivalent (NADH or FADH2) availability, 2) increased cytosolic ADP concentration ([ADP]), and 3) other mechanisms (see Ref. 13 for discussion of this issue). Furthermore, it is also well known that the turnover rates of the TCA cycle and ATP synthase are influenced by the availability of intracellular [Ca2+] and that mean cytosolic [Ca2+] in striated muscle increases with increasing intensity of muscle activity. Hence, in working muscle this could also compensate (at submaximal rates of muscle ATP expenditure) for modest reductions in muscle mitochondrial density. The expected result of these compensatory phenomena would be the maintenance of the balance between ATP synthesis and ATP utilization rates. Hence, in resting skeletal muscle, the main observable “energetic” consequence of modestly reduced tissue mitochondrial density would be expected to be increased [ADP] and [Pi] and reduced [PCr] in the cytosol. As will be seen later, these changes do not appear to be present in human subjects with DM2.
We have reported related energetic compensatory phenomena in studies of perfused rat hearts in which we showed that the relationship between intracellular [ADP] and MV̇o2 (at any observed MV̇o2 value) was inconstant and that it was dependent on the composition of perfusate carbon substrate (13). In that report, it was presumed that the variable relationship between [ADP] and MV̇o2 was due to the differences in the concentrations of mitochondrial reducing equivalents present under the different substrate conditions.
The relevance of these considerations to the specific case of skeletal muscle with decreased insulin sensitivity is that they indicate that a reduction of the (extremely submaximal) rate of oxidative ATP synthesis (see aforementioned TCA cycle rate data) present in resting skeletal muscle cannot be a consequence of a modest reduction of mitochondrial numbers and/or even a modest reduction of the Vmax of individual mitochondria. More likely is that the reduction of the TCA cycle rate observed in insulin-insensitive skeletal muscle indicates that the rate of muscle ATP utilization in these subjects is for some reason decreased.
In additional reports that employed MT measurements of Pi→ATP rates to assess the rate of oxidative ATP synthesis resting in human skeletal muscle, data analysis also appears to be problematic. In one (25), the effects of hyperthyroidism (induced by exogenous thyroid hormone administration) on mitochondrial coupling were examined. In the mildly hyperthyroid experimental subjects, TCA cycle turnover rates in resting muscle were increased by ∼40% as compared with those of control subjects while the MT-determined Pi→ATP rate was comparable in both groups. In consequence, the calculated P/O (indexed by the ratio of the MT-measured Pi→ATP rate to the measured TCA rate in this study) was reduced by ∼50%. Taken at face value, these data would imply that severe mitochondrial uncoupling was present in these mildly hyperthyroid subjects. However, the oxidative ATP synthetic rates we calculated from their TCA cycle data [this calculation assumed that no significant uncoupling was present] were ∼1.1 and 1.8 μmol·g−1·min−1, respectively, in the normal and hyperthyroid subjects while the MT-determined Pi→ATP rates were ∼5 μmol·g−1·min−1 in both groups of subjects. Even if the increased TCA cycle turnover rates in the (resting) skeletal muscle of the hyperthyroid subjects were completely due to uncoupling (as opposed to also being a consequence of increased rates of tissue ATP utilization), this effect could not have been detected by comparing MT Pi→ATP rate measurements to the TCA rate measurements because the Pi→ATP rates were largely generated by the GAPDH/PGK enzyme couple and/or arose because of the presence of significant amount of mitochondrial activity in the Pi→ATP direction.
Similarly, in another study that employed the same analytic methods (2), mitochondrial uncoupling was reported to be increased in healthy, endurance trained (as compared with untrained) human resting skeletal muscle. However, comparison between the rates of oxidative ATP synthesis measured by MT and TCA cycle rates measured with 13C MR techniques yielded disparities similar to those present in the study of the mildly hyperthyroid subjects discussed above. Hence, these MT-derived data cannot be used to impute the presence of uncoupling in trained skeletal muscle. However, the increased TCA cycle turnover rates that were present in resting trained muscle (as compared with levels present in untrained muscle) are of interest. Although, this TCA cycle rate increase could have been due to mitochondrial uncoupling, it seems more likely that it was a consequence of increased mitochondrial density in the trained muscle. It is well known that mitochondrial content increases in the relevant skeletal muscles when untrained subjects undergo endurance training. Under these circumstances, even if the “resting” (i.e., obligatory near state 4 oxygen consumption rate) of individual mitochondria was not altered, the increased mitochondrial numbers present in each gram of trained muscle would be expected to increase the tissue TCA cycle rate because of the obligatory resting state metabolism of the additional mitochondria.
Additional Evidence That Oxidative ATP Synthesis Is Not Significantly Limited in Skeletal Muscle With Reduced Insulin Sensitivity
1) Nair et al. (29) have shown, in muscle biopsy samples obtained from Asian Indians with insulin insensitivity or frank DM2, that tissue mitochondrial DNA copy numbers were elevated as were the oxidative and ATP synthetic capacities of mitochondria isolated from biopsy specimens. Indeed, the mitochondrial oxidative capacities of these DM2 patients were “supranormal” when compared with similar data obtained from normal subjects of North American or European origin. Hence, in this population of DM2 patients, skeletal muscle mitochondrial ATP synthetic capacity was not reduced despite the presence of severe insulin resistance. These observations convincingly demonstrate that there is not an obligatory mechanistic link between the presence of severe skeletal muscle insulin resistance and a decreased mitochondrial capacity for oxidative phosphorylation.
2) Investigators from Maastricht University (9) studied a large group of patients with DM2 in whom in vivo resting skeletal muscle mitochondrial function was assessed with conventional 31P MR spectroscopy. Resting muscle values of [ATP], [PCr], and [ADP] and also the rates of [PCr] and [ADP] recoveries following a brief period of imposed ischemia were measured. It was reported that mitochondrial oxidative capacity was strongly correlated with a marker of overall fitness (i.e., the maximal whole body oxygen uptake rate). Moreover, it was also reported that the relationship between these variables in the DM2 group was quantitatively comparable to that found in a group of nondiabetic subjects. Hence, the presence of insulin insensitivity per se was not associated with variations in the kinetics of oxidative ATP synthesis (assessed by the rate of postischemic recovery). Last, these investigators showed that, in the DM2 group, resting skeletal muscle high-energy phosphate compound levels (including calculated [ADP]) were also comparable to those present in the nondiabetic group. The finding that [ADP] levels were not increased in diabetic skeletal muscles further supports our view (see earlier discussion) that oxidative ATP synthetic capacity is not sufficiently limiting in these resting muscles to warrant invocation of kinetic compensatory mechanisms.
Last, this research group (using the same 31P MR spectroscopic techniques employed in their human study) also reported data from Zucker diabetic fatty rats (a genetic DM2 model). In that animal model, there was no evidence of abnormal mitochondrial oxidative function at any stage of the development of the disease (8).
3) As pointed out by Holloszy (14), in primary mitochondrial diseases in which skeletal muscle oxidative ATP synthetic capacity is known to be reduced, glucose uptake and insulin sensitivity are increased rather than being decreased. These data also contradict the assertion that, in skeletal muscle, limitation of oxidative ATP synthesis per se can cause insulin insensitivity.
Why Are the MT-Determined Pi→ATP Rates Abnormally Low in Insulin-Insensitive Skeletal Muscle?
In resting skeletal muscle, MT-measured Pi→ATP rates substantially exceed oxidative ATP synthesis rates calculated from either oxygen consumption measurements or TCA cycle turnover rate measurements. This is because, as discussed, the component of MT-based Pi→ATP rate measurements originating from the GAPDH/PGK couple and/or mitochondrial ATPase activity in the ATP→Pi direction is substantial.
Thus, we ask what the lower Pi→ATP rates observed in insulin-insensitive muscle might mean in metabolic terms and why the MT-measured Pi→ATP rate increases substantially during a hyperinsulinemic, normoglycemic clamp in normal, but not insulin-insensitive resting skeletal muscle? In normal resting (i.e., insulin-sensitive) muscle, it is not likely that augmentation of glucose uptake (such as occurs during an hyperinsulinemic, normoglycemic clamp) speeds up the rate of oxidative ATP synthesis to the extent that would be predicted by the increases of MT-determined Pi→ATP rates reported (31, 41). Additional data support the view that an increased rate of glucose uptake per se does not affect the rate of oxidative ATP synthesis (i.e., the mitochondrial component of the MT-determined Pi→ATP rate). It has been found that in transgenic mice that overexpressed myocardial glucose transporter 1 (GLUT1; a constitutively active glucose transporter), the rates of glycolysis, lactate export, and the relative contribution of glucose to oxidative ATP synthesis were substantially increased; in contrast, the rate of oxidative phosphorylation itself (calculated from measured MV̇o2 values) was not altered in these hearts (45).
The above biochemical considerations and the findings that higher MT-determined Pi→ATP rates are observed in normal than insulin-sensitive subjects during insulin clamp conditions suggests that the turnover rate of the GAPDH/PGK couple (although not the rate of oxidative ATP synthesis) is a function of the rate of glucose uptake. If true, the lower rates of glucose uptake present in insulin-insensitive subjects (even during a period of insulin administration) could account for the observed reductions of unidirectional Pi→ATP rates in these subjects (31, 38, 41).
Conclusions
We suggest that conventional MT-based measurements of Pi→ATP rate in skeletal and cardiac muscle, liver, and perhaps in other tissues as well (with the apparent exception of the brain) do not yield quantitative estimates of the rate of oxidative ATP synthesis unless the GAPDH/PGK contribution to the Pi→ATP rate is absent and/or the presence of significant mitochondrial activity in the ATP→Pi direction can be ruled out. This complication should be considered when interpreting MT Pi→ATP rate data obtained in any tissue. Our analysis based on considerations of the biochemical origins of measured Pi→ATP rates and basic enzyme kinetics is supported by non-MT-based studies of mitochondrial function in normal and insulin-insensitive skeletal muscle. We conclude that MT-based measurements of total skeletal muscle Pi→ATP rates recorded in insulin-insensitive and normal subjects are not indicative of the true net rate of oxidative (i.e., mitochondrial) ATP synthesis. Hence, these data cannot be used to infer a functionally significant limitation of mitochondrial capacity for oxidative ATP synthesis in insulin-insensitive resting skeletal muscle. The mechanism of the reduced Pi→ATP rates observed in insulin-insensitive resting skeletal muscle remains to be elucidated but may well be a consequence of the effects of decreased glucose uptake on the kinetics of the GAPDH/GPK enzyme couple.
GRANTS
This work was supported by National Centers for Research Resources (NCRR), National Institutes of Health Grant P41RR08079.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Footnotes
This article is the topic of an Editorial Focus by Balaban and Koretsky (1a).
In a chemical reaction A↔B, there are two unidirectional rates corresponding to the rates for reaction A→→→B and B→→→A. The net rate of formation of compound B in this chemical reaction is the difference between the unidirectional rate of A→→→B conversion minus the unidirectional rate of B→→→A conversion. At equilibrium, these two unidirectional rates are equal and the net rate of A or B formation is zero (thus the concentrations of A and B do not change even though they are being converted back and forth by the chemical reaction). Far out of equilibrium, one of these unidirectional rates is much larger than the other and the reaction proceeds in the direction of establishing chemical equilibrium.
REFERENCES
- 1a. Balaban RS, Koretsky AP. Interpretation of 31P NMR saturation transfer experiments: what you can't see might confuse you. Focus on “Standard magnetic resonance-based measurements of the Pi→ATP rate do not index the rate of oxidative phosphorylation in cardiac and skeletal muscles.” Am J Physiol Cell Physiol (April 13, 2011). doi:10.1152/ajpcell.00100.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1. Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, Shulman GI. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 56: 1376–1381, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Befroy DE, Petersen KF, Dufour S, Mason GF, Rothman DL, Shulman GI. Increased substrate oxidation and mitochondrial uncoupling in skeletal muscle of endurance-trained individuals. Proc Natl Acad Sci USA 105: 16701–16706, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50: 790–796, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brindle KM, Blackledge MJ, Challiss RA, Radda GK. 31P NMR magnetization-transfer measurements of ATP turnover during steady-state isometric muscle contraction in the rat hind limb in vivo. Biochemistry 28: 4887–4893, 1989 [DOI] [PubMed] [Google Scholar]
- 5. Brindle KM, Radda GK. 31P-NMR saturation transfer measurements of exchange between Pi and ATP in the reactions catalysed by glyceraldehyde-3-phosphate dehydrogenase and phosphoglycerate kinase in vitro. Biochim Biophys Acta 928: 45–55, 1987 [DOI] [PubMed] [Google Scholar]
- 6. Campbell-Burk SL, Jones KA, Shulman RG. 31P NMR saturation-transfer measurements in Saccharomyces cerevisiae: characterization of phosphate exchange reactions by iodoacetate and antimycin A inhibition. Biochemistry 26: 7483–7492, 1987 [DOI] [PubMed] [Google Scholar]
- 7. Chance B, Leigh JS, Jr, Kent J, McCully K, Nioka S, Clark BJ, Maris JM, Graham T. Multiple controls of oxidative metabolism in living tissues as studied by phosphorus magnetic resonance. Proc Natl Acad Sci USA 83: 9458–9462, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Feyter HM, Lenaers E, Houten SM, Schrauwen P, Hesselink MK, Wanders RJ, Nicolay K, Prompers JJ. Increased intramyocellular lipid content but normal skeletal muscle mitochondrial oxidative capacity throughout the pathogenesis of type 2 diabetes. FASEB J 22: 3947–3955, 2008 [DOI] [PubMed] [Google Scholar]
- 9. De Feyter HM, van den Broek NM, Praet SF, Nicolay K, van Loon LJ, Prompers JJ. Early or advanced stage type 2 diabetes is not accompanied by in vivo skeletal muscle mitochondrial dysfunction. Eur J Endocrinol 158: 643–653, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Decking UK, Skwirba S, Zimmermann MF, Preckel B, Thamer V, Deussen A, Schrader J. Spatial heterogeneity of energy turnover in the heart. Pflügers Arch 441: 663–673, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Du F, Zhu XH, Qiao H, Zhang X, Chen W. Efficient in vivo 31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. Magn Reson Med 57: 103–114, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Erecinska M, Wilson DF, Nishiki K. Homeostatic regulation of cellular energy metabolism: experimental characterization in vivo and fit to a model. Am J Physiol Cell Physiol 234: C82–C89, 1978 [DOI] [PubMed] [Google Scholar]
- 13. From AH, Zimmer SD, Michurski SP, Mohanakrishnan P, Ulstad VK, Thoma WJ, Ugurbil K. Regulation of the oxidative phosphorylation rate in the intact cell. Biochemistry 29: 3731–3743, 1990 [DOI] [PubMed] [Google Scholar]
- 14. Holloszy JO. Skeletal muscle “mitochondrial deficiency” does not mediate insulin resistance. Am J Clin Nutr 89: 463S–466S, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Hood DA, Gorski J, Terjung RL. Oxygen cost of twitch and tetanic isometric contractions of rat skeletal muscle. Am J Physiol Endocrinol Metab 250: E449–E456, 1986 [DOI] [PubMed] [Google Scholar]
- 16. Jucker BM, Dufour S, Ren J, Cao X, Previs SF, Underhill B, Cadman KS, Shulman GI. Assessment of mitochondrial energy coupling in vivo by 13C/31P NMR. Proc Natl Acad Sci USA 97: 6880–6884, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jucker BM, Ren J, Dufour S, Cao X, Previs SF, Cadman KS, Shulman GI. 13C/31P NMR assessment of mitochondrial energy coupling in skeletal muscle of awake fed and fasted rats. Relationship with uncoupling protein 3 expression. J Biol Chem 275: 39279–39286, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Kalliokoski KK, Knuuti J, Nuutila P. Relationship between muscle blood flow and oxygen uptake during exercise in endurance-trained and untrained men. J Appl Physiol 98: 380–383, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Kemp GA. The interpretation of abnormal 31P magnetic resonance saturation transfer measurements of Pi/ATP exchange in insulin-resistant skeletal muscle. Am J Physiol Endocrinol Metab 294: E640–E642, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Kingsley-Hickman P, Sako EY, Andreone PA, Cyr JA, Michurski S, Foker JE, From AH, Petein M, Ugurbil K. 31P NMR measurement of ATP synthesis rate in perfused intact rat hearts. FEBS Lett 198: 159–163, 1986 [DOI] [PubMed] [Google Scholar]
- 21. Kingsley-Hickman PB, Sako EY, Mohanakrishnan P, Robitaille PM, From AH, Foker JE, Ugurbil K. 31P NMR studies of ATP synthesis and hydrolysis kinetics in the intact myocardium. Biochemistry 26: 7501–7510, 1987 [DOI] [PubMed] [Google Scholar]
- 22. Kingsley-Hickman PB, Sako EY, Ugurbil K, From AH, Foker JE. 31P NMR measurement of mitochondrial uncoupling in isolated rat hearts. J Biol Chem 265: 1545–1550, 1990 [PubMed] [Google Scholar]
- 23. Kobayashi K, Neely JR. Control of maximum rates of glycolysis in rat cardiac muscle. Circ Res 44: 166–175, 1979 [DOI] [PubMed] [Google Scholar]
- 24. LaNoue KF, Jeffries FM, Radda GK. Kinetic control of mitochondrial ATP synthesis. Biochemistry 25: 7667–7675, 1986 [DOI] [PubMed] [Google Scholar]
- 25. Lebon V, Dufour S, Petersen KF, Ren J, Jucker BM, Slezak LA, Cline GW, Rothman DL, Shulman GI. Effect of triiodothyronine on mitochondrial energy coupling in human skeletal muscle. J Clin Invest 108: 733–737, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lei H, Ugurbil K, Chen W. Measurement of unidirectional Pi to ATP flux in human visual cortex at 7 T by using in vivo 31P magnetic resonance spectroscopy. Proc Natl Acad Sci USA 100: 14409–14414, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mogensen M, Sahlin K, Fernstrom M, Glintborg D, Vind BF, Beck-Nielsen H, Hojlund K. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 56: 1592–1599, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 115: 3587–3593, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nair KS, Bigelow ML, Asmann YW, Chow LS, Coenen-Schimke JM, Klaus KA, Guo ZK, Sreekumar R, Irving BA. Asian Indians have enhanced skeletal muscle mitochondrial capacity to produce ATP in association with severe insulin resistance. Diabetes 57: 1166–1175, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pellerin L, Magistretti PJ. Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. Neuroscientist 10: 53–62, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Petersen KF, Dufour S, Shulman GI. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med 2: e233, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Phillips D, Aponte AM, French SA, Chess DJ, Balaban RS. Succinyl-CoA synthetase is a phosphate target for the activation of mitochondrial metabolism. Biochemistry 48: 7140–7149, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Portman MA. Measurement of unidirectional P(i)–>ATP flux in lamb myocardium in vivo. Biochim Biophys Acta 1185: 221–227, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J, Wagner PD. Evidence of O2 supply-dependent V̇o2max in the exercise-trained human quadriceps. J Appl Physiol 86: 1048–1053, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Richardson RS, Saltin B. Human muscle blood flow and metabolism studied in the isolated quadriceps muscles. Med Sci Sports Exerc 30: 28–33, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Robitaille PM, Merkle H, Sako E, Lang G, Clack RM, Bianco R, From AH, Foker J, Ugurbil K. Measurement of ATP synthesis rates by 31P-NMR spectroscopy in the intact myocardium in vivo. Magn Reson Med 15: 8–24, 1990 [DOI] [PubMed] [Google Scholar]
- 37. Sako EY, Kingsley-Hickman PB, From AH, Foker JE, Ugurbil K. ATP synthesis kinetics and mitochondrial function in the postischemic myocardium as studied by 31P NMR. J Biol Chem 263: 10600–10607, 1988 [PubMed] [Google Scholar]
- 38. Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 87: 507–520, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schmid AI, Chmelik M, Szendroedi J, Krssak M, Brehm A, Moser E, Roden M. Quantitative ATP synthesis in human liver measured by localized 31P spectroscopy using the magnetization transfer experiment. NMR Biomed 21: 437–443, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Shen W, Tian R, Saupe KW, Spindler M, Ingwall JS. Endogenous nitric oxide enhances coupling between O2 consumption and ATP synthesis in guinea pig hearts. Am J Physiol Heart Circ Physiol 281: H838–H846, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Szendroedi J, Schmid AI, Chmelik M, Toth C, Brehm A, Krssak M, Nowotny P, Wolzt M, Waldhausl W, Roden M. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med 4: e154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thoma WJ, Ugurbil K. Saturation-transfer studies of ATP-Pi exchange in isolated perfused rat liver. Biochim Biophys Acta 893: 225–231, 1987 [DOI] [PubMed] [Google Scholar]
- 43. Ugurbil K, Kingsley-Hickman PB, Sako EY, Zimmer S, Mohanakrishnan P, Robitaille PM, Thoma WJ, Johnson A, Foker JE, From AH. 31P NMR studies of the kinetics and regulation of oxidative phosphorylation in the intact myocardium. Ann NY Acad Sci 508: 265–286, 1987 [DOI] [PubMed] [Google Scholar]
- 44. Xavier AR, Roselino JE, Resano NM, Garofalo MA, Migliorini RH, Kettelhut Ido C. Glyconeogenic pathway in isolated skeletal muscles of rats. Can J Physiol Pharmacol 80: 164–169, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Yan J, Young ME, Cui L, Lopaschuk GD, Liao R, Tian R. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation 119: 2818–2828, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yerby B, Deacon R, Beaulieu V, Liang J, Gao J, Laurent D. Insulin-stimulated mitochondrial adenosine triphosphate synthesis is blunted in skeletal muscles of high-fat-fed rats. Metabolism 57: 1584–1590, 2008 [DOI] [PubMed] [Google Scholar]