Abstract
To test the hypothesis that Na+/H+ exchanger (NHE) regulatory factor 2 (NHERF2) is necessary for multiple aspects of acute regulation of NHE3 in intact mouse small intestine, distal ileal NHE3 activity was determined using two-photon microscopy/SNARF-4F in a NHERF2-null mouse model. The NHERF2-null mouse ileum had shorter villi, deeper crypts, and decreased epithelial cell number. Basal rates of NHE3 activity were reduced in NHERF2-null mice, which was associated with a reduced percentage of NHE3 in the apical domain and an increase in intracellular NHE3 amount but no change in total level of NHE3 protein. cAMP, cGMP, and elevated Ca2+ due to apical exposure to UTP all inhibited NHE3 activity in wild-type mouse ileum but not in NHERF2-null mice, while inhibition by hyperosmolarity occurred normally. The cAMP-increased phosphorylation of NHE3 at aa 552; levels of PKAIIα and cGMP-dependent protein kinase II (cGKII); and elevation of Ca2+ were similar in wild-type and NHERF2-null mouse ileum. Luminal lysophosphatidic acid (LPA) stimulated NHE3 in wild-type but not in NHERF2-null ileum. In conclusion, 1) there are subtle structural abnormalities in the small intestine of NHERF2-null mouse which include fewer villus epithelial cells; 2) the decreased basal NHE3 activity and reduced brush border NHE3 amount in NHERF2-null mice show that NHERF2 is necessary for normal basal trafficking or retention of NHE3 in the apical domain; 3) hyperosmolar inhibition of NHE3 occurs similarly in wild-type and NHERF2-null ileum, demonstrating that some inhibitory mechanisms of NHE3 are not NHERF2 dependent; 4) cAMP inhibition of NHE3 is NHERF2 dependent at a step downstream of cAMP/PKAII phosphorylation of NHE3 at aa 552; 5) cGMP- and UTP-induced inhibition of NHE3 are NHERF2 dependent at steps beyond cGKII and the UTP-induced increase of intracellular Ca2+; and 6) LPA stimulation of NHE3 is also NHERF2 dependent.
Keywords: Ca2+; adenosine 3′,5′-cyclic monophosphate; guanosine 3′,5′-cyclic monophosphate; trafficking; Na+/H+ exchanger regulation; Na+/H+ exchanger regulatory factor; lysophosphatidic acid
the brush border sodium/hydrogen exchanger, NHE3, belongs to the SLC9A gene family of proteins and is predominantly expressed in the apical surface of small and large intestine and proximal tubules of the kidney where it accounts for the majority of intestinal and renal Na+ absorption (39). NHE3 activity is highly regulated. Much of this regulation involves large signaling complexes which NHE3 forms with multiple binding partners, some of which bind to the intracellular COOH-terminal ∼377 aa of NHE3 (12, 14, 15).
The Na+/H+ exchanger regulatory factor (NHERF) family of multi-PDZ domain containing proteins (NHERFs 1–4) are scaffolds that bind to the middle of the intracellular COOH terminus of NHE3 and form some of the signaling complexes that regulate NHE3 (12–14, 36, 38). The NHERF proteins are present in the apical domain of intestinal and renal proximal tubule Na-absorptive cells and bind multiple proteins in addition to NHE3. These scaffold proteins contain two to four related PDZ domains, and NHERF1 and NHERF2 in addition contain a COOH-terminal ezrin/radixin/moesin binding domain that anchors brush border proteins to the actin cytoskeleton in the intestinal microvilli (4, 5).
The reasons for having four closely related scaffolding proteins in a similar domain in a single cell have been partially resolved with the recognition that the NHERFs are present in somewhat different although closely opposed areas in the apical domain of Na+ absorptive cells. NHERF1 and 3 are present at the outer microvillus and NHERF 2 and 4 at the inner microvillus with NHERF4 also cystoplasmic (12, 33, 36, 37). In addition, the NHERFs bind somewhat different proteins, although they also bind many of the same proteins. In addition, phosphorylation appears to play a role in regulation of NHERF1 but not NHERF2. NHERF1 is phosphorylated as part of signaling in which it participates, which leads at least to decreased binding to NHERF1 substrates when S77 is phosphorylated (33). NHERF2 has not yet been shown to be phosphorylated.
The role of the NHERFs in NHE3 regulation is only partially defined. Transfection of specific NHERFs into cell lines lacking that NHERF, knocking down NHERFs by short hairpin RNA in cells that endogenously express that NHERF, or study of knockout mouse models of the NHERF alone or when several members of this gene family are simultaneously knocked out have been used to identify the role of each NHERF in NHE3 regulation. In cell models, NHERF2 has been involved in a majority of NHE3 regulatory signaling pathways, including inhibition by elevated cAMP (35), cGMP (6), and Ca2+ (17, 21) and stimulation by lysophosphatidic acid (LPA; 9, 20) and d-glucose (23). Of note, however, was that NHERF2 was not present in all cell lines studied and overexpression of NHERF2 was often used to evaluate its regulatory function in epithelial cells. The role of NHERF2 in NHE3 regulation has also been evaluated in mice on a FVB/N background (8, 24). Basal NHE3 activity was not different from that of wild type (WT) in NHERF2-null jejunum, ileum, distal ileum, and colon (8). In addition, cAMP inhibited NHE3 similarly in WT and NHERF2-null jejunum, ileum, and colon, but, surprisingly, there was no forskolin effect on either WT or NHERF2 distal ileum (8). Elevated Ca2+ inhibition of NHE3 was NHERF2 dependent in jejunum and colon, and heat-stable Escherichia coli enterotoxin (STa) inhibited NHE3 in jejunum only in the presence of NHERF2 (8). Given the apparent importance of NHERF2 in some examples of both NHE3 stimulation and inhibition both in cell models and in a mouse model on one specific background (FVB/N), we undertook a comprehensive evaluation of NHE3 regulation in C57BL/6 mice in a specific intestinal segment (distal ileum), with effects of NHERF2 expression determined on basal NHE3 activity and on a spectrum of acute regulation, including inhibition of NHE3 by cAMP, cGMP, elevated Ca2+ and hyperosmolarity, and stimulation by LPA, the goal being to allow separation of general dependence of NHE3 regulation on NHERF2 vs. effects that are cell, background, or intestinal segment specific.
EXPERIMENTAL PROCEDURES
Chemicals.
SNARF-4F-AM and Fluo-3-AM were from Invitrogen; nigericin, probenecid, EIPA, 8-bromoadenosine-3′,5′- cyclic monophosphate ( 8-Br-cAMP ), forskolin, heat-stable E. coli enterotoxin (STa), UTP, and LPA were from Sigma; and HOE-694 was a kind gift provided by Juergen Punter (Sanofi/Hoechst, Germany).
Antibodies.
Rabbit polyclonal antibodies to NHE3 (Ab1381) and NHERF2 (Ab2570) were previously characterized (16, 34). Anti-actin and anti-5-bromo-1-(2-deoxy-β-d-ribofuranosyl) uracil (5-BrdU) antibodies were from Sigma. Anti-PKA antibodies were from Santa Cruz. Anti-cGMP-dependent protein kinase II (cGKII) polyclonal antibodies and anti-NHE3 P552 monoclonal antibodies were as described previously (6, 18, 19).
Animals.
NHERF2 knockout (KO) mice in a FVB/N genetic background were from the de Jonge laboratory, as reported (3). These mice were bred into a C57BL/6 background (Charles River Laboratories, Wilmington, MA), and NHERF2−/− mice were studied at least in the F12 generation.
Male NHERF2−/− and WT C57BL/6 mice were studied between 12–14 wk of age. The mice were maintained under standard light and climate conditions in the animal facility of the Johns Hopkins University School of Medicine with ad libitum access to water and chow. Experiments with animals were carried out using protocols approved by the Animal Care and Use Committee of The Johns Hopkins University.
Metabolic studies.
Five experiments were performed with three WT and three NHERF2 KO mice used for each experiment. Animals were individually housed in metabolic cages for 4 days after a 48-h period of acclimatization. The amounts of food and water taken by each mouse were measured daily as was the urine and stool volume and weight. To determine the percentage of water in stool, fresh feces were collected from individual mice, weighed at once, and heated at 80°C overnight before reweighing.
Isolation of ileum for Na+/H+ exchange activity assays.
Mice were euthanized by cervical dislocation. The abdomen was immediately opened by midline incision, and distal ileum (∼3 cm in length ending 1 cm proximal to the ileo-cecal junction) was excised and placed immediately in cold Na+ buffer (in mM: 138 NaCl, 5 KCl, 2 CaCl2, 1 MgSO4, 1 NaH2PO4, 25 glucose, 1 probenecid, and 20 HEPES, pH 7.4) and opened along the mesenteric border. Six- to eight-millimeter pieces were mounted with Krazy Glue (Elmer's Productions) onto a glass coverslip with the mucosal surface facing up. All preparations were performed on ice. We showed previously that this glue used for mounting had no autofluorescent signal and did not affect tissue viability (26).
Measurement of NHE3 activity by two-photon microscopy/SNARF-4F-AM loading, imaging, and analysis.
The protocol for imaging intracellular pH of mouse ileum using two-photon microscopy was developed and described by us in detail previously (26). By using a ×40/1.00 numerical aperture water immersion objective (Nikon), the images of the ileal villus epithelial cells loaded with the pH-sensitive dye, SNARF-4F-AM in Na+ buffer, were visualized using a two-photon laser-scanning microscope (MRC-1024MP, Bio-Rad, Hercules, CA) powered by a wide-band, infrared (780 mm) combined photo-diode pump laser and mode-locked titanium sapphire laser (Tsunami Ti Sa laser, Spectra-Physics, Mountain View, CA). The 8-bit images were recorded and stored after which fluorescence intensity was calculated off-line using MetaMorph software (version 5.0; Molecular Devices) as described previously (26). Ileum (muscle layers intact) was loaded with 20 μM SNARF-4F in Na+ buffer at 37°C for 35 min with 95% O2-5% CO2 gassing and then placed in a perfusion chamber (RC-21BDW, Warner Instruments) on a heated platform (PH Series, Warner Instruments) on the microscope stage (25°C) and perfused, using a peristaltic pump (Ismatec Reglo), over the tissue at 1 ml/min with Na+ buffer for 15 min at room temperature. Tissue was then acidified using a prepulse with 60 mM NH4Cl for 20 min, and then perfused with N-methyl-d-glucamine (NMDA) buffer (same as Na+ buffer with NMDA replacing Na+) for 10–15 min. To eliminate the contribution of NHE1 and NHE2 activity, 50 μM HOE-694 was added to the NMDA buffer. To monitor pH recovery due to Na+/H+ exchange activity, the NMDA buffer was switched back to the Na+ buffer which also contained HOE-694. No pH recovery occurred in the presence of 100 μM EIPA. Regulation of NHE3 was carried out with antagonists and agonists present in all solutions, such that tissues were exposed for 75–80 min to 100 μM 8-Br-cAMP, 10 nM STa, 300 μM UTP, and 50 μM LPA. In contrast, when the effect of hyperosmolarity on NHE3 activity was determined, 50 mM mannitol was added only to the final Na+ buffer. Probenecid (1 mM) was present in all perfusates to prevent SNARF-4F leakage (10).
Images for each optical section (0–50 μm from villus tip at 5-μm steps) were taken at 580 and 640 nm and stored, as described previously (26). These conditions allowed quantifiable signals to be studied up to depths of 40–50 μm from the villus tip with study of cells generally performed ∼10–20 μm from the villus tip. Calibration of 640/580 ratio was performed using the K+/nigericin method (22) for external pH values: 6.1–6.3; 6.7–6.8; 7.3–7.5. Analyses of collected images were performed as described previously (26). Since the intracellular buffering power was not different in distal ileum from WT and NHERF2-null mice (see below), the Na+/H+ exchange activity of NHE3 is reported only as the initial rate in change in intracellular pH (pHi). This was calculated as the initial steep change in pHin over time (usually ∼1–2 min after Na addition), using linear curve fit analysis (Origin 4.0) and presented as ΔpH/min.
Determination of the intrinsic intracellular buffering power.
The samples of tissue (distal ileum with muscle layers intact) were prepared and loaded with SNARF-4F and examined by using two-photon microscopy as for study of NHE3 activity. Determination of intracellular buffering power (βi) was performed as previously described by Boyarsky et al. (2) by using decreasing NH4Cl concentrations (60–1 mM in 10 mM steps with NMDA replaced by NH4Cl in NMDA buffer) and measuring the resultant changes in pHi. βi was calculated over a wide range of pHin (pH 6.0–8.0) with analysis done combining experimental points over every 0.4 pH units. The generated buffering curves showed no difference in intrinsic buffering power in distal ileum between WT and NHERF2−/− mice (Supplemental Fig. S1).
Measurement of intracellular Ca2+ in ileal tissue with Fluo-3-AM employing two-photon microscopy.
Ileal tissue isolation and preparation for Ca2+ measurements were performed as for examination of NHE3 activity. Fluo-3-AM (10 μM) was loaded into tissue in Na+ buffer at 37°C for 30 min in the presence of 95% O2-5% CO2. Maximal excitation for Fluo-3 was obtained at 780 nm with emission at 530 nm, and the two-photon approach was identical to what we used for evaluation of pHi. Images of tissue loaded with dye were taken before and after exposure of tissue to UTP (300 μM), collected, and stored for off-line analysis. Images were analyzed using MetaMorph software. Changes in intracellular Ca2+ are presented as the relative fluorescence of Fluo-3 (F/F0), where F0 is the initial fluorescence and F is the final fluorescence (28).
Immunofluorescence staining of ileum for NHE3 and NHERF2.
Ileal tissue samples were fixed in 3.5% paraformaldehyde in PBS at 4°C and paraffin embedded. Histological sections (4 μm thick) were mounted onto Superfrost microscope slides (Fisher Scientific, Arlington, VA) and heat fixed. Slides were microwaved for antigen recovery in 10 mM sodium citrate buffer, pH 6 (Sigma Chemical, St. Louis, MO) at power level setting 9 (model NN-C980B Conventional Microwave Oven, Panasonic, Secaucus, NJ) for 2–5 min. After cooling for 30 min, sections were washed in PBS and preblocked with 5% normal goat serum (NGS) in PBS for 30 min at room temperature. Sections were incubated for 1 h at room temperature and then overnight at 4°C with polyclonal anti-NHERF2 or -NHE3 antibodies, diluted 1:500 in 5% NGS-PBS. Ileal sections were then washed twice in PBS for 10 min and incubated for 1 h at room temperature with anti-rabbit Alexa-Fluor secondary antibodies for each diluted 1:100. Sections were washed twice with PBS and autofluorescence was quenched with 1% Sudan Black (Sigma) in 70% methanol for 10 min at room temperature. Sections were counterstained with Hoechst 33342 (Invitrogen) and mounted with Gel Mount (Sigma). Ileal sections were imaged using a Zeiss LSM 510/META confocal fluorescence microscope (×63 objective, oil immersion).
Isolation of ileal total membrane and cytosol from WT and NHERF2−/− mice.
All procedures were done on ice or at 4°C. Ileum was rinsed with ice-cold 0.9% saline and opened along the mesenteric border, and ileal villus cells were scraped and placed into homogenization buffer A [in mM: 60 mannitol, 2.4 mM Tris (pH 7.1), 1 EGTA, 1 β-glycerol-PO4, 2 Na3VO4, and 1 phenylalanine]. Protease inhibitor cocktail (1:100; Sigma) and phosphorhamidon (1:1,000) were added to buffer. Cells were then homogenized at 4°C with a polytron (10 times for 10 s at speed 5 with a 20-s interval between each burst) followed by homogenization of samples in a glass-teflon homogenizer. The homogenates were centrifuged at 2,000 g for 10 min at 4°C to remove cell debris and nuclei and designated as total lysates. Lysates were then centrifuged at 150,000 g for 60 min, and total membrane (TM) pellets were collected. The resulting TMs were resuspended in buffer B (in mM: 300 mannitol, 20 HEPES, pH 7.4, 5 Mg-gluconate, 1 Na3VO4, 1 β-glycerol-PO4, 1 phenylalanine, and protease inhibitors added as in buffer A). The protein concentrations of the TM samples were measured with bicinchoninic acid (Sigma) and subsequently analyzed by SDS-PAGE and Western blotting using primary antibodies for NHE3, PKAIIα, cGKII, NHERF2 and actin and with fluorescently labeled secondary goat anti-mouse or anti-rabbit IRDye TM 700 or TM 800 antibodies (Rockland). The fluorescence intensity of detected protein bands was visualized by the Odyssey system (LI-COR).
Tissue isolation for morphometric/histologic studies of small intestine and colon.
Age-matched WT and NHERF2 KO mice were euthanized by cervical dislocation. The whole intestine and colon were removed and washed twice with cold PBS (pH 7.4). The length of small intestine and colon was measured. For histological studies of ileal tissue, pieces of ileum as defined above were cut open and fixed in 10% neutral-buffered formalin overnight at 4°C. Fixed tissue was embedded vertically in paraffin, and 4-μm sections were prepared, deparaffinized in xylene, then rehydrated through a series of graded ethanol exposures, and then stained with hematoxylin and eosin (H&E) or with periodic acid-Schiff (goblet cells). Length of villi and depth of crypts were computed from H&E digital images using MetaMorph software (version 5.0; Molecular Devices). Numbers of epithelial, goblet, and Paneth cells were counted manually using H&E and periodic acid-Schiff digital images, respectively, with MetaMorph software. For morphometry, at least 10–15 fields of villi and crypts from six mice of each genotype were examined.
Cell proliferation assay.
Eight-week-old WT and NHERF2 KO mice were injected intraperitoneally with 5-BrdU (100 mg/kg; Sigma) and euthanized 2 h later. Tissue preparation for BrdU staining was performed as described above, with modifications listed. Sections were fixed in 4% paraformaldehyde for 4 h at room temperature. Fixed tissue was then embedded vertically in paraffin and sectioned (4 μm). After deparaffinization and rehydration in ethanol, tissues were washed in cold PBS and incubated with H2O2 (3%) and NaN3 (1 mM) in PBS for 20 min at room temperature to block endogenous peroxidase activity. DNA was denatured by incubating tissues with HCl (1 N) for 15 min at 37°C. To decrease antigen masking by DNA-binding proteins or histones, tissues were digested with trypsin (1 mg/ml Sigma) for 30 min at room temperature. After being washed in cold PBS, tissues were exposed to Blocking Reagent (Roche) for 1 h at room temperature. Anti-BrdU monoclonal antibody (Sigma) was used at 1:100 dilution and incubated with tissues overnight at 4°C, then washed three times for 5 min in Tris-buffered saline (in mM: 25 Tris, pH 7.5, and 150 NaCl) and incubated with secondary fluorescent (Alexa-Fluor 488, 1:100) antibodies for 1 h, washed again, and analyzed by confocal microscopy. Fluorescent image acquisition of tissue was performed using a Zeiss 510 LSM/META confocal imaging system. Eight-bit images were collected and stored. BrdU-labeled cells in crypts were manually counted in a blinded fashion and data are presented from three WT and three NHERF2-null mice studied in each of two identical experiments, with three to four crypts per field studied from six fields for each mouse.
Phosphorylation of NHE3 assay in ileal tissues treated with forskolin.
Ileum harvested as above was preincubated for 15 min at 37°C in Ringer-HCO3−/25 mM glucose, gassed at 95% O2-5% CO2, and then exposed at 37°C to 10 μM forskolin or equal volume of DMSO (as control) for 15 min. Specimens studied in parallel and handled in an identical manner were processed to isolate total lysate. SDS-PAGE and Western blot analysis with anti-phospho-552-NHE3 antibodies were performed, as described previously (19).
Statistics.
Values are presented as means ± SE. Statistical significance was determined using Student's unpaired and paired t-tests and one-way ANOVA. P < 0.05 was considered significant.
RESULTS
Characteristics of NHERF2−/− mice in C57BL/6 background.
NHERF2-null male mice bred into a C57BL/6 background appeared grossly like WT mice and had similar weights (Table 1). Metabolic cage experiments (Table 1) demonstrated that NHERF2−/− and WT mice consumed similar amounts of food and water. There were no differences in the urine volume and stool number. The percentage of water in stool of NHERF2−/− mice was ∼8% higher than in WT, but this difference was not statistically significant (P = 0.08).
Table 1.
Results of metabolic cage analysis
| WT (n = 14) | NHERF2 KO (n = 13) | P Value | |
|---|---|---|---|
| Body wt, g | 21.8 ± 0.7 | 21.6 ± 0.6 | NS |
| Food intake, g/day | 5.5 ± 0.7 | 4.8 ± 0.6 | NS |
| Water intake, ml/day | 5.2 ± 1.4 | 5.0 ± 0.8 | NS |
| Urine, ml/day | 1.1 ± 0.3 | 1.0 ± 0.4 | NS |
| Feces, mg/day | 1.1 ± 0.3 | 1.4 ± 0.3 | NS |
| % H2O in feces | 59.4 ± 1.9 | 63.9 ± 1.3 | NS |
Values are means ± SE.
WT, wild type; NHERF2, Na+/H+ exchanger regulatory factor 2; KO, knockout; NS, not significant.
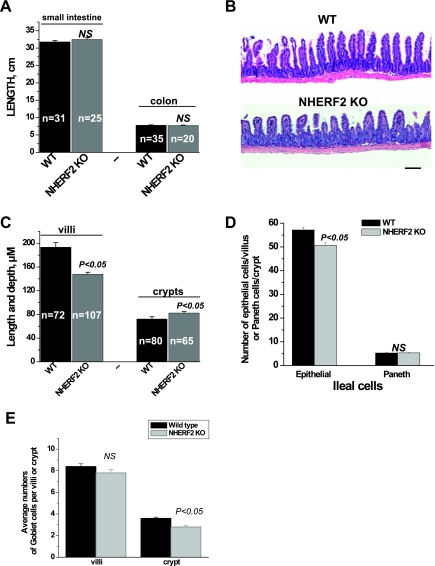
Macroscopic examination of small intestine and colon also demonstrated no gross abnormalities. As shown by Fig. 1A, the lengths of the small intestine (32.5 ± 0.4 vs. 31.8 ± 0.4 cm) and colon (7.8 ± 0.1 vs. 7.8 ± 0.1 cm) of NHERF2−/− were not altered compared with WT mice.
Fig. 1.
Characteristics of Na+/H+ exchanger (NHE) regulatory factor 2-null (NHERF2−/−) mice bred in C57BL/6 background. A: measurement of length of total small intestine and colon shows no differences in 8- to 10-wk-old male wild-type (WT) vs. NHERF2−/− mice. NS, not significant; KO, knockout; n, no. of animals studied. B and C: ileal villi were shorter in NHERF2−/− mice. Hematoxylin and eosin staining of ileal tissue (∼3 cm pieces of ileum obtained ending 1 cm proximal to the cecum) showed that NHERF2−/− mice had shorter villi but deeper crypts compared with WT mice. Villi of knockout mice are wider than WT due to expansion of lamina propria. Scale bar in B, 100 μm. D: NHERF2-null ileum had a lower number of columnar epithelial cells along the villus/crypt axis but had no change in the number of Paneth cells. E: goblet cells in NHERF2−/− ileal villi were similar in number to WT ileal tissue, but goblet cells in the NHERF2−/− crypts were reduced compared with WT.
Changes in ileum in NHERF2−/− mice.
Since the goal of our study was to determine the role of NHERF2 in regulation of NHE3 activity in distal ileum, we restricted the histological studies to this part of the small intestine, the distal 3 cm ending ∼1 cm proximal to the cecum. Histological analysis of ileum of NHERF2−/− mice showed some mild abnormalities compared with WT ileum. As shown by Fig. 1, B and C, in NHERF2−/− mice the villus-to-crypt ratio was decreased (2.1 ± 0.1) compared with the ratio in WT (3.2 ± 0.2, P < 0.05). The changes in villus-to-crypt ratio might be explained by changes in size of villus and/or crypt. Morphometric analysis of length of villi and depth of crypts (Fig. 1C) showed that the villi in ileum of NHERF2−/− are shorter compared with WT ileum (147.8 ± 3.4 μm, vs. 193.4 ± 7.8 μm, P < 0.05) while the depth of crypts in NHERF2−/− ileum was increased in comparison with WT (82.4 ± 2.6 vs. 72.3 ± 3.7 μm, P < 0.05). Figure 1B also shows that the thickness of villi from NHERF2−/− ileum was increased compared with WT. Analysis of histological sections of ileal tissue from six different mice showed that this increase in villus thickness occurred because of expansion of the lamina propria. We determined whether the reduction of villus length was due to changes in the numbers and/or size of the columnar epithelial cells. The number of epithelial cells (Fig. 1D) per ileal villus from NHERF2−/− mice was decreased in comparison with WT (50.7 ± 1.0 and 57.2 ± 1.2, respectively, P < 0.05). There were no gross differences in size of single columnar epithelial cells. The effect of absence of NHERF2 protein on the number of other cell types in NHERF2−/− ileal tissue was determined, including Paneth and goblet cells. The number of Paneth cells per crypt (Fig. 1D) was unchanged in ileal tissue of NHERF2−/− mice compared with WT (5.3 ± 0.1 vs. 5.3 ± 0.1). While the number of goblet cells in NHERF2-null villi was not changed, the number in the crypts was decreased compared with WT (Fig. 1E).
Epithelial proliferation is not altered in NHERF2−/− mice.
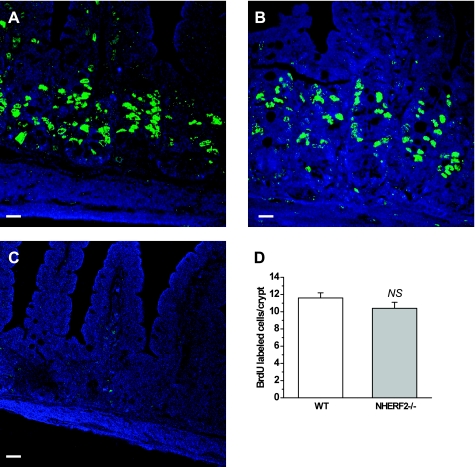
BrdU incorporation was used to assess epithelial proliferation. As in WT ileum, BrdU-positive cells in NHERF2−/− ileum were restricted to the crypts and transit-amplifying cell population. The number of BrdU-positive cells was not significantly altered in crypts of NHERF2−/− mice compared with WT ileal crypts (Fig. 2).
Fig. 2.
Cell proliferation in ileum from NHERF2−/− mice is not different from WT. A–C: 5-bromo-1-(2-deoxy-β-d-ribofuranosyl) uracil (BrdU) incorporation (green) was quantitated after 2 h of labeling in crypt cells of WT (A), NHERF2−/− ileum (B), and ileal tissue from NHERF2−/− injected with PBS as control (C). D: morphometry of BrdU-incorporated cells 2 h after injection in WT and NHERF2−/− ileal tissue revealed no difference in number of cells stained with anti-BrdU antibodies. Scale bars in A–C, 20 μm.
NHERF2 is required for maintaining normal basal rate of NHE3 activity in mouse ileum.
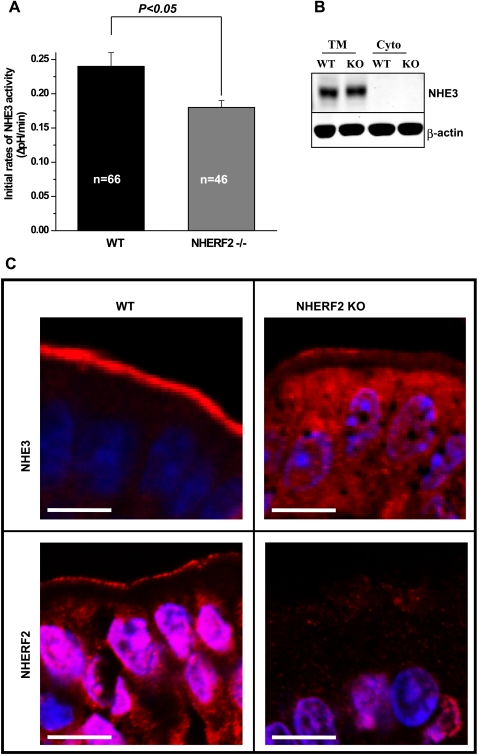
The initial rate of NHE3 activity after intracellular acidification in distal ileum from NHERF2−/− mouse was determined. To assess NHE3 activity in intact tissue, changes in intracellular pH were measured using the pH-sensitive dye SNARF-4F-AM and two-photon microscopy under basal conditions after NH4+ prepulse. The epithelial cells studied were ∼10–20 μm from the villus tip to avoid including dying cells at the villus tip. Ileal tissue from WT and NHERF2−/− mice demonstrated the same resting pH before NH4+ prepulse [7.52 ± 0.08 (n = 23) and 7.48 ± 0.08 (n = 20) for WT and NHERF2 KO, respectively]. After NH4+ prepulse, pHi values reached were not significantly different in distal ileum of WT (pH 6.33 ± 0.06, n = 33) and NHERF2−/− (pH 6.23 ± 0.04, n = 39) mice. Basal NHE3 activity (Fig. 3A) in ileum from NHERF2−/− mice was lower than in WT (ΔpH/min, 0.24 ± 0.02 vs. 0.18 ± 0.01, P < 0.05). This result indicates that NHERF2 is required for setting/maintaining basal NHE3 activity.
Fig. 3.
Ileal brush border (BB) NHE3 activity is NHERF2 dependent. A: the initial rate of NHE3 activity was decreased in NHERF2−/− ileal epithelial cells compared with WT ileum. The tissue was loaded with 20 μM SNARF-4F, and EIPA-sensitive basal NHE3 activity in ileum was determined by two-photon microscopy as the initial rate of Na+-induced alkalinization after an acute acid load. Fifty micromolar HOE-694 was present to eliminate the contribution of NHE1 and NHE2 activities to initial rates. Results are means ± SE. n, no. of mice studied. B: the total amount of NHE3 in NHERF2−/− ileal mucosa [total membrane (TM)] was similar to that of WT mice. Equal amounts of protein of total membranes and cytosolic fractions (Cyto) were separated on 10% SDS-PAGE and immunoblotted for NHE3 (1:1,000 dilution of primary antibody) and actin (1:3,000). A representative experiment that was repeated 3 times is shown. C: distribution of NHE3 (red) in ileum of NHERF2−/− mice compared with WT ileal tissue. Intact ileum was obtained from WT and NHERF2−/− mice, fixed, and paraffin embedded. Histological sections were stained with polyclonal antibodies to NHE3 (top left for WT and top right for NHERF2−/− ileum) and NHERF2 (bottom left for WT and bottom right for NHERF2−/− ileum), then visualized by fluorescent secondary antibodies and confocal microscopy. For WT ileum, NHE3 and NHERF2 were predominantly localized to the BB of epithelial cells. Some intracellular staining for NHERF2 was present. For NHERF2−/− ileum, the distribution of NHE3 was changed. There was a smaller NHE3 pool in the BB compared with WT. More intracellular NHE3 staining was present. NHERF2 expression was present in the apical domain of WT ileum but, as expected, was completely lost in the apical domain of epithelial cells from NHERF2−/− mice. Scale bar in C, 10 μm.
The expression level of NHE3 remains unchanged, but cellular distribution is altered in NHERF2−/− ileum.
To understand the molecular mechanism of decreased basal NHE3 activity, we performed Western blotting of total membrane fractions isolated from ileum of NHERF2−/− and WT mice. As shown in Fig. 3B, the total amount of membrane associated NHE3 was not different in NHERF2−/− and WT mice. However, immunohistochemical analysis revealed differences in the distribution of NHE3 in WT and NHERF2−/− ileum. In WT mice (Fig. 3C, top left), NHE3 (red fluorescence) was localized predominantly to the brush border of the ileum, whereas in NHERF2-null ileum the amount of brush border NHE3 was decreased while intracellular NHE3 was increased (Fig. 3C, top right). Figure 3C, bottom left, shows the presence of NHERF2 protein (red fluorescence) in WT ileum where it is mostly in the apical domain. In Fig. 3C, bottom right, the absence of immunofluorescence in NHERF2−/− ileum is shown. The difference in distribution of NHE3 with lower amount of protein in the brush border of NHERF2−/− ileum is the likely explanation for the reduced NHE3 activity in NHERF2−/− mice. The decrease in the number of villus epithelial cells is likely to contribute to a further decrease in total NHE3 activity. Of note, the ratiometric fluorescence technique used here to measure NHE3 activity measures rates for a similar surface area in a few epithelial cells repeated in at least three to four individual villi and is not usually affected by changes in total villus surface area or number of cells.
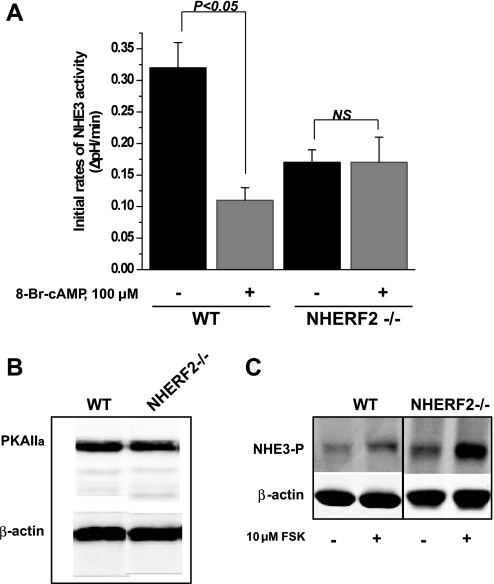
Inhibition of NHE3 activity by cAMP is abolished in ileum of NHERF2−/− mice.
In the PS120 fibroblast cell line stably expressing exogenous NHE3 and NHERF1 or NHERF2, the presence of either NHERF1 or NHERF2 is necessary and sufficient for cAMP-mediated inhibition of NHE3 activity (35). Our previous study of NHERF1−/− ileal tissue demonstrated that NHERF1 is not required for cAMP-induced inhibition of ileal NHE3 activity (26). Therefore, we examined the role of NHERF2 in cAMP-mediated inhibition of ileal NHE3 activity. Figure 4A shows that 100 μM 8-Br-cAMP decreased NHE3 activity in ileum from WT mice but had no effect in ileum from NHERF2-null mice. This result indicates that the effect of cAMP is dependent on NHERF2 in mouse ileal epithelial cells, and that NHERF1 is not able to reconstitute the inhibitor effect of cAMP in distal ileum. Next, the mechanism of cAMP inhibition of ileal NHE3 activity was studied.
Fig. 4.
cAMP-dependent inhibition of NHE3 activity requires NHERF2 in ileal villus Na+-absorptive cells. A: basal NHE3 activity and 8-bromoadenosine-3′,5′- cyclic monophosphate ( 8-Br-cAMP) effect (100 μM) are shown as means ± SE in WT (n = 20) and NHERF2−/− (n = 10) ileum; n, no. of mice studied. P values represent comparison of effect of 8-Br-cAMP in WT and NHERF2−/− conditions. Note reduced basal NHERF activity in NHERF2-null mice compared with WT. B: Western blots of PKAIIα show no change in the expression level in WT vs. NHERF2−/− total ileal lysates. Control of protein loading was with anti-β-actin. Representative experiment that was repeated 3 times is shown. C: NHE3 in ileal tissue from NHERF2−/− mice is phosphorylated at amino acid residue 552 after cAMP exposure similarly to WT ileal tissue. Ileal tissue from WT and NHERF2−/− mice was excised and incubated with 10 μM forskolin (FSK) and then most villus cells obtained by scrapping the mucosa and total lysates prepared. Representative Western blot shows separation of equal amount of proteins on 10% SDS-PAGE, and immunostaining with antibodies that recognize phosphorylated NHE3 at aa residue 552 increased with cAMP in both WT and NHERF2-null. Loading control was β-actin, which was used for normalization. A representative experiment that was repeated 3 times is shown.
Expression of PKAIIα and level of cAMP-mediated phosphorylation of NHE3 at amino acid residue 552 are normal in NHERF2−/− mice.
Previously, we showed that in mouse ileum, cAMP-mediated inhibition of NHE3 activity occurs entirely through a PKA-dependent pathway with no contribution of exchange protein directly activated by cAMP (EPAC) (26). NHERF2 knockout in ileum reduced cAMP-mediated NHE3 inhibition. Consequently, we determined the level of PKAIIα expression in NHERF2−/− ileum. As shown by Fig. 4B, the expression of PKAIIα in total ileal membranes from WT and NHERF2-null mice was similar. It is known that PKAIIα phosphorylates NHE3 at two amino acid residues, 552 and 605, which are needed for cAMP to inhibit NHE3 (18). Western blot analysis of total lysate in Fig. 4C shows that increased phosphorylation of NHE3 at amino acid residue 552 occurs similarly in ileum from WT and NHERF2-null mice treated with forskolin (10 μM) for 15 min. The phosphorylation of NHE3 at amino acid 605 could not be reproducibly detected in mouse ileum by immunoblotting. Since cAMP-mediated phosphorylation of NHE3 is normal at least at the 552 amino acid residue in NHERF2-null ileum, we conclude that NHERF2 might be involved in a step in cAMP inhibition of NHE3 activity downstream of NHE3 phosphorylation by protein kinase A.
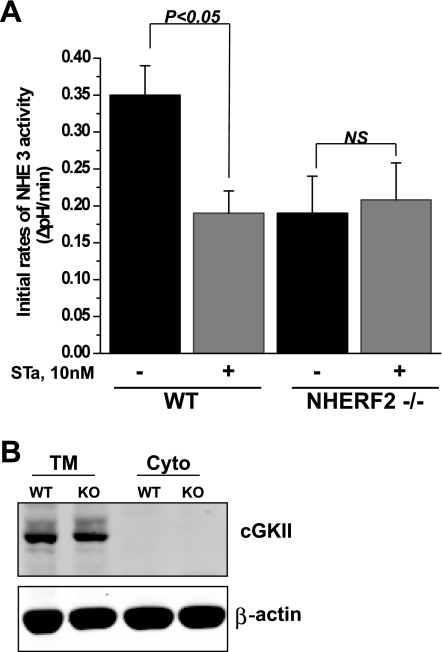
Inhibition of NHE3 activity by cGMP/cGKII is abolished in ileum of NHERF2−/− mice.
NHERF2 is required for cGMP regulation of NHE3 activity in cell models of NHE3 regulation. NHERF2 reconstitutes inhibition of NHE3 activity by cGMP in PS120 cells, opossum kidney (OK) polarized renal proximal tubule cells, and polarized intestinal Caco-2 cells when the cells transiently express cGKII but not in the absence of this kinase (6, 30). To study the role of NHERF2 in regulation of NHE3 activity by cGMP in intact distal ileum, 10 nM heat-stable toxin (STa) from E. coli was used. This toxin binds to the same apical membrane receptor as endogenous guanylin and activates guanyl cyclase C to elevate intracellular cGMP (29). In Fig. 5A is shown that apically placed heat-stable toxin reduced the NHE3 activity by 46% in ileum from WT mice, while in NHERF2-null ileum the effect of toxin was abolished. The evaluation of cGKII expression by immunoblotting total membranes from WT and NHERF2−/− ileum indicates that the level of kinase was not different in WT and NHERF2-null mice (Fig. 5B). These results demonstrate that NHERF2 is necessary for cGMP/cGKII-mediated inhibition of NHE3 activity by a process that is not associated with abnormal levels of cGKII, similar to the situation with cAMP-mediated inhibition.
Fig. 5.
NHERF2 is necessary for NHE3 inhibition by heat-stable Escherichia coli enterotoxin (cGMP) in ileum. A: basal NHE3 activity and STa effect (10 nM) are shown as means ± SE in WT (n = 10) and NHERF2−/− (n = 10) ileum. P values represent comparison of effect of STa in WT and NHERF2−/− compared with untreated control conditions. B: representative Western blotting of cGMP-dependent protein kinase II (cGKII) shows no changes in the expression level or distribution of protein in WT and NHERF2−/− total membrane and cytosolic fractions. β-Actin was used as a protein loading control. A representative experiment that was repeated 3 times with similar results is shown.
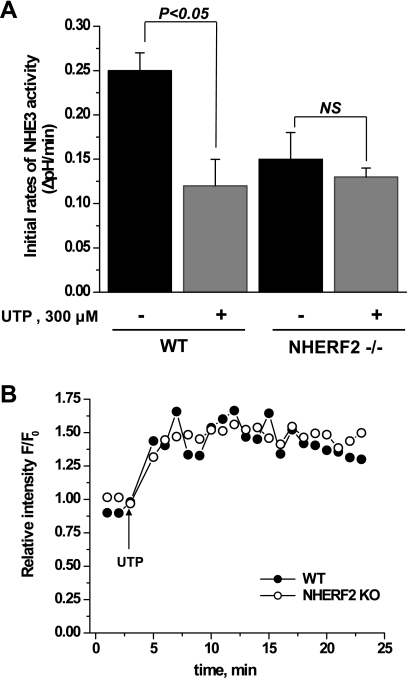
NHERF2 is necessary for Ca2+-mediated inhibition of NHE3 activity.
In the PS120 cell and Caco-2 models, NHERF2 has been shown to be necessary for Ca2+ inhibition of NHE3 activity (17, 21, 30). To determine the role of NHERF2 in NHE3 regulation in intact ileal tissue, we treated ileum with UTP (300 μM) applied apically. In WT ileum (Fig. 6A), UTP inhibited NHE3 activity but had no effect on NHE3 activity in NHERF2−/− ileum. UTP increases intracellular Ca2+ in airway epithelial cells (25, 31). However, the mechanism of the UTP effect on small intestinal epithelial cells is unknown. Therefore, the UTP effect on intracellular Ca2+ was evaluated in ileum. For this purpose, ileal tissue was loaded with the Ca-sensitive dye, Fluo-3-AM (10 μM). UTP elevated the ileal Ca2+ concentration [increased the relative fluorescence (F/F0 ratio) of the Fluo-3 dye], which was monitored with a two-photon microscope. Figure 6B shows that UTP caused a similar rapid and prolonged (20 min) increase in Ca2+ in both WT and NHERF2−/− ileum. Thus, the lack of UTP inhibition of NHE3 in NHERF2 knockout mice confirms in intact ileum that NHERF2 is necessary for elevated Ca2+-mediated inhibition of NHE3 activity.
Fig. 6.
Ca2+-dependent inhibition of NHE3 activity requires NHERF2 in ileum. A: ileum was loaded with 20 μM SNARF-4F in Na+-containing buffer in the presence of vehicle or 300 μM UTP for 30 min at 37°C. UTP was present in all perfusion buffers. Initial NHE3 activity rates and UTP effect on NHE3 activity were determined. Data shown are means ± SE of 5 experiments for WT mice and for NHERF2−/− mice. P values are comparison of UTP effect with basal NHE3 activity in both WT and NHERF2−/− (unpaired t-test). B: UTP elevated Ca2+ similarly in WT and NHERF2−/− ileum. Time course is shown of UTP-induced Ca2+ response in ileal tissue from WT and NHERF2−/− mice. Intact ileal tissue was loaded with 10 μM Fluo-3 for 30 min at 37°C and mounted into a flow chamber, and images were stored and analyzed as described in experimental procedures. UTP caused a rapid increase in fluorescence ratio F/F0, demonstrating rapid increase in intracellular Ca2+, which remained elevated for at least 20 min in both WT and NHERF2−/− ileum.
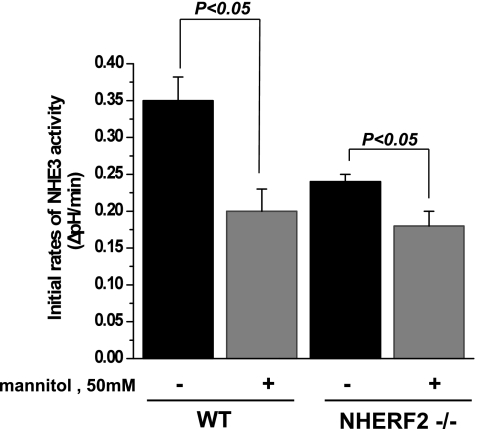
Hyperosmolarity inhibits NHE3 activity similarly in both WT and NHERF2−/− ileal tissue.
The inhibitory effects of cAMP, cGMP, and UTP on NHE3 activity were all abolished in ileal tissue from NHERF2−/− mice. The question thus arises whether NHERF2 is necessary for all inhibitory regulation of NHE3 activity. To address this question, we examined the effect of hyperosmolarity on NHE3 activity in NHERF2−/− ileum. It is known that hyperosmolarity inhibits NHE3 activity acutely and that this process does not involve NHE3 phosphorylation or trafficking of NHE3 in cell models (27). To increase osmolarity in Na+ buffer, we used mannitol (50 mM). As shown by Fig. 7, addition of 50 mM mannitol to Na+ buffer decreased NHE3 activity in WT ileum by 37 ± 9% (n = 5 for control and treated ileum, P < 0.05). Hyperosmolarity similarly decreased NHE3 activity in NHERF2−/− ileum by 23 ± 9% (n = 5 for control and treated mice, P < 0.05). The percentage of NHE3 inhibition with mannitol was not significantly different in WT and NHERF2-null ileum. These results show that NHERF2-null ileum can respond similarly to WT for at least one form of acute inhibition.
Fig. 7.
Hyperosmolar inhibition of ileal NHE3 activity occurs similarly in WT and NHERF2−/− ileum. Fifty millimolar mannitol was added only to the final Na buffer in studies measuring ileal NHE3 activity (see experimental procedures). Basal NHE3 activity and effect of mannitol are shown as means ± SE in WT (n = 10) and NHERF2−/− (n = 10) ileum. Hyperosmolarity significantly inhibited NHE3 activity in both WT and NHERF2−/− ileum. P values represent comparison of effect of hyperosmolarity in WT and NHERF2 ileum by one-way ANOVA.
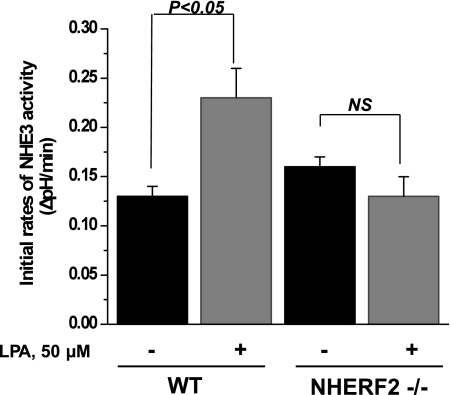
LPA treatment increase in NHE3 activity in distal mouse ileum is NHERF2 dependent.
As shown above, intracellular elevation of cAMP, cGMP, and calcium inhibits NHE3 activity in mouse distal ileum, which in all cases is NHERF2 dependent. Earlier, we showed that LPA increases NHE3 activity in polarized renal proximal tubule cells (OK cell line) and this activation is NHERF2 dependent (7, 20). More recently, LPA was shown to stimulate NHE3 activity in Caco-2 cells and to increase net fluid absorption in mouse intestine in a NHERF2-dependent manner (24). To date, LPA has not been shown to affect NHE3 activity in distal mouse ileum. In Fig. 8 is shown that luminal exposure of distal ileum to 50 μM LPA for 30 min (20 min in NH4+ buffer and 10 min in NMDA buffer) increases NHE3 activity in distal ileum from WT mice from 0.13 ± 0.01 ΔpH/min in control to 0.23 ± 0.03 ΔpH/min in LPA-treated ileum (n = 4 for control and treated tissues, P < 0.05). There was no LPA effect on NHE3 activity in distal ileum from NHERF2−/− mice. The NHE3 activity in NHERF2−/− ileum under control and LPA-treated conditions was 0.16 ± 0.01 and 0.13 ± 0.02 ΔpH/min, respectively (n = 4 for each group; P = not significant).
Fig. 8.
Lysophosphatidic acid (LPA)-dependent stimulation of NHE3 activity is NHERF2 dependent in ileum. Ileum was loaded with 20 μM SNARF-4F in Na+-containing buffer for 30 min at 37°C. Vehicle (0.1% fat-free BSA in PBS) or 50 μM LPA was added to NH4+-containing buffer for 20 min and to NMDA-containing buffer for 10 min. Initial NHE3 activity rates and LPA effect on NHE3 activity were determined. Data shown are means ± SE of 4 experiments for WT mice and for NHERF2−/− mice. P values are comparison of LPA effect with basal NHE3 activity in WT and NHERF2−/− (unpaired t-test).
DISCUSSION
These studies examined the effect of knocking out NHERF2 in basal and acutely regulated NHE3 activity in distal ileum of C57BL/6 mice. The results support conclusions gained from our previous studies, that members of the NHERF multi-PDZ domain family are necessary for regulation of many aspects of NHE3 activity in an intestinal segment and agonist-specific manner. The current studies were performed in distal ileum (defined as the distal ∼third of the ileum). We studied NHE3 regulation in this segment because both human and mouse distal ileum have some specificity for vitamin B12 and Na-dependent bile salt absorption, demonstrating that it functions as a unique intestinal transporting region (1, 11).
The current studies demonstrated that in distal ileum, NHERF2 is necessary for 1) setting basal NHE3 activity, since knocking out NHERF2 lowers basal NHE3 activity; 2) inhibition of NHE3 by elevation of cAMP, cGMP, and Ca2+; and 3) stimulation of NHE3 by LPA. In contrast, NHERF2 is not necessary for hyperosmolar inhibition of NHE3 activity.
We also began mechanistic studies to understand how NHERF2 is involved in setting NHE3 activity. Some ileal structural changes occurred in the NHERF2 knockout ileum. These included decreased villus length, increased crypt depth, and a decrease in total number of villus epithelial cells. The two-photon microscopy/SNARF-4F fluorescence technique used to measure NHE3 activity in villus epithelial cells uses a ratiometric approach, and thus the decrease in villus length or number of epithelial cells, or even changes in surface area, did not contribute to the results. These findings do indicate, however, that the effect of NHERF2 knockout on intestinal NHE3 activity was even larger than indicated in these studies due to magnification of effects of reduced NHE3 activity by reduced absorptive surface area/less number of villus Na-absorptive cells on rates of Na absorption. What accounts for these ileal structural changes is unknown but is not explained by changes in proliferation in the NHERF2 knockout mice compared with WT.
The decreased basal rate of NHE3 activity in ileal villus epithelial cells in NHERF2-null mice appears to be due to less brush border NHE3, with normal total membrane NHE3 amount, and more intracellular NHE3 (Fig. 3). The explanation for these findings is likely due either to failure of NHE3 to traffic to the apical domain or failure to fix the NHE3 that does traffic to the apical membrane domain. Of note, these results differed from NHERF2 knockout mice on a FVB/N background, in which basal intestinal (jejunal, ileal, distal ileal, and colon) NHE3 activity was not changed compared with WT. This correlated with similar total expression of NHE3 in jejunum and colon of WT vs. NHERF2-null mice (8). In contrast, in Caco-2 cells in which NHERF2 was knocked down, basal NHE3 activity and surface NHE3 were both increased, although there was no change in total NHE3 expression (30). The latter finding was interpreted to indicate that NHERF2 played a role in maintaining a storage pool of NHE3 which was not accessible to apical labeling with biotin and which moved more apically when NHERF2 was reduced (23). Thus, there do not appear to be consistent differences in basal NHE3 activity comparing WT and models with reduced or absent NHERF2 expression across cells or even intestinal segments of mice from different backgrounds. While the studies in Caco-2 cells and mouse distal ileum suggest a role for NHERF2 in setting the basal location of NHE3 at the base of microvilli or in the intervillus cleft or terminal web, based on the method used to localize NHE3, additional mechanistic studies of this role for NHERF2 are needed.
Further studies were performed of acute regulation of NHE3. Since most of the acute regulatory processes that affect NHE3 in distal ileum are NHERF2 dependent, it was necessary to ask whether all aspects of regulation of NHE3 were NHERF2 dependent. That the previously described (27) hyperosmolar percent inhibition of ileal NHE3 activity was quantitatively similar in NHERF2-null mice to the effect in WT mice served as a positive control for NHERF2 dependence of regulation of NHE3 activity and showed that failure of cAMP, cGMP, and elevated Ca2+ to inhibit NHE3 activity in NHERF2-null ileum was not a general phenomenon.
Both elevated cGMP/cGKII and Ca2+ inhibition of NHE3 were NHERF2 dependent, with NHERF1 and NHERF3, which are present in mouse small intestinal brush border membrane vesicles in these mice in increased amounts (6, 38), unable to make up for the loss of NHERF2. These data support results obtained in the past by studying effects of cGKII-specific agonists (8-cpt-cGMP) in the presence of cGKII and of elevated Ca2+ in inhibiting NHE3 in fibroblasts, polarized OK cells, and Caco-2 cells (6, 30), as well as in mouse jejunum on a FVB/N background. For these effects, NHERF2 takes part in forming signaling complexes that regulate NHE3. NHERF2 binds to the NHE3 COOH terminus but at the same time binds to specific signaling molecules, which is one way for conferring specificity to its regulatory role. For instance, for cGMP inhibition of NHE3 studied in PS120 fibroblasts and OK cells, NHERF2 bound to cGKII which also bound to the plasma membrane directly, which caused us to suggest that NHERF2 acted as a G kinase-anchoring protein (6). Similarly for elevated Ca2+ inhibition of NHE3, again in PS120 cells and rabbit ileum, NHERF2 bound α-actinin-4 with elevated Ca2+ with the α-actinin-4 EF hands domain presumably magnifying the elevation of Ca2+ near the intracellular COOH terminus of NHE3 (17). The similarity of these aspects of acute NHE3 regulation in NHERF2 knockout ileum and some cell lines lacking endogenous NHERF2 suggests that these models can be used to further probe the mechanisms of these aspects of NHE3 regulation. Please note, that this is the initial demonstration that apically administered UTP elevated intracellular Ca2+ in mouse small intestine and inhibited NHE3 activity in WT mice.
cAMP inhibition of NHE3 was NHERF2 dependent in mouse ileum. This is the aspect of NHE3 inhibition in which tissue specificity of a NHERF2-dependent effect has been most clearly demonstrated. In PS120 cells and Caco-2 cells, either NHERF1 or NHERF2 is sufficient to allow cAMP inhibition of NHE3. This is not true in ileum, in which NHERF2 is necessary and cAMP inhibition does not occur in the absence of NHERF2 as demonstrated here. In this case, neither NHERF1 nor NHERF3, both of which are present in the mouse villus Na-absorptive cells, was able to reconstitute cAMP inhibition of NHE3. Please note that in mouse proximal tubule, cAMP inhibition of NHE3 is NHERF1 dependent and thus no clear pattern of cAMP-dependent inhibition of NHE3 activity has emerged in which the effect is dependent on either NHERF1 or NHERF2. What accounts for this tissue and cell specificity of NHE3 inhibition by cAMP is not known. What is known from our studies, however, is that the NHERF2 dependence probably occurs downstream of PKA-dependent phosphorylation of mouse ileal brush border NHE3, since PKAII levels are normal and cAMP phosphorylates ileal NHE3 normally, at least at aa 552, in NHERF2-null mice.
This is the initial demonstration that some acute stimulation of NHE3 in intact small intestine (by LPA) is NHERF2 dependent. LPA is an inflammatory mediator which is increased in the reparative stages of inflammation. We previously reported that LPA acutely stimulates NHE3 activity in polarized OK cells (20), and this recently was shown to be dependent on the LPA5 apical membrane receptor (24). In a cell model, 1) the LPA stimulation of NHE3 was associated with elevation of intracellular Ca2+, the magnitude of which was NHERF2 dependent and related to PLCβ3 binding to NHERF2 (9); and 2) the magnitude of the NHE3 stimulation correlated with the extent of elevation of intracellular Ca2+ (9). Thus the LPA stimulation of NHE3 is another of several examples of conditions in which an elevation of intracellular Ca2+ is associated with stimulation of NHE3 activity rather than with the more common inhibition of NHE3 activity, as occurs with carbachol and serotonin (36–38).
Thus having defined specific NHERF2-dependent aspects of mouse distal ileal tissue structure, basal transport, cAMP, cGMP, and Ca2+-dependent acute inhibition and LPA acute stimulation, the challenge remains to understand mechanistically in more detail what accounts for the role NHERF2 plays in this regulation. The clues identified are that some aspects of the effects of NHERF1 and/or NHERF2 in NHE3 regulation exhibit organ and tissue specificity (basal and cAMP), while other aspects of NHE3 regulation always appear to be NHERF2 dependent (cGMP/cGKII, elevated Ca2+, LPA), while still other regulatory aspects of NHE3 are not at all NHERF2 dependent (hyperosmotic inhibition). The current studies indicate that further mechanistic studies of how NHERF2 contributes to NHE3 regulation can be performed in the simplest polarized Na-absorptive epithelial cell modes, such as Caco-2 cells, with concentration on the roles of NHERF2 that are seen uniformly across cell lines, tissues, and different mouse backgrounds.
GRANTS
This work was supported in part by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Grants RO1DK26523, RO1DK61765, PO1DK072084, R24DK64388 (The Hopkins Basic Research Digestive Diseases Development Core Center), and T32DK2007632 and by the Hopkins Center for Epithelial Biology.
DISCLOSURES
M. Donowitz is a part owner of Tranzmembrane, Inc.
REFERENCES
- 1. Bijvelds MJ, Jorna H, Verkade HJ, Bot AG, Hofmann F, Agellon LB, Sinaasappel M, de Jonge HR. Activation of CFTR by ASBT-mediated bile salt absorption. Am J Physiol Gastrointest Liver Physiol 289: G870–G879, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Boyarsky G, Ganz MB, Sterzel RB, Boron WF. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3−. Am J Physiol Cell Physiol 255: C844–C856, 1988 [DOI] [PubMed] [Google Scholar]
- 3. Broere N, Hillesheim J, Tuo B, Jorna H, Houtsmuller AB, Shenolikar S, Weinman EJ, Donowitz M, Seidler U, de Jonge HR, Hogema BM. Cystic fibrosis transmembrane conductance regulator activation is reduced in the small intestine of Na+/H+ exchanger 3 regulatory factor 1 (NHERF-1)- but not NHERF-2-deficient mice. J Biol Chem 282: 37575–37584, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Cha B, Donowitz M. The epithelial brush border Na+/H+ exchanger NHE3 associates with the actin cytoskeleton by binding to ezrin directly and via PDZ domain-containing Na+/H+ exchanger regulatory factor (NHERF) proteins. Clin Exp Pharmacol Physiol 35: 863–871, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Cha B, Kenworthy A, Murtazina R, Donowitz M. The lateral mobility of NHE3 on the apical membrane of renal epithelial OK cells is limited by the PDZ domain proteins NHERF1/2, but is dependent on an intact actin cytoskeleton as determined by FRAP. J Cell Sci 117: 3353–3365, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Cha B, Kim JH, Hut H, Hogema BM, Nadarja J, Zizak M, Cavet M, Lee-Kwon W, Lohmann SM, Smolenski A, Tse CM, Yun C, de Jonge HR, Donowitz M. cGMP inhibition of Na+/H+ antiporter 3 (NHE3) requires PDZ domain adapter NHERF2, a broad specificity protein kinase G-anchoring protein. J Biol Chem 280: 16642–16650, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Cha B, Zhu XC, Chen W, Jones M, Ryoo S, Zachos NC, Chen TE, Lin R, Sarker R, Kenworthy AK, Tse M, Kovbasnjuk O, Donowitz M. NHE3 mobility in brush borders increases upon NHERF2-dependent stimulation by lysophosphatidic acid. J Cell Sci 123: 2434–2443, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen M, Sultan A, Cinar A, Yeruva S, Riederer B, Singh AK, Li J, Bonhagen J, Chen G, Yun C, Donowitz M, Hogema B, de Jonge H, Seidler U. Loss of PDZ-adaptor protein NHERF2 affects membrane localization and cGMP- and [Ca2+]- but not cAMP-dependent regulation of Na+/H+ exchanger 3 in murine intestine. J Physiol 588: 5049–5063, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choi JW, Lee-Kwon W, Jeon ES, Kang YJ, Kawano K, Kim HS, Suh PG, Donowitz M, Kim JH. Lysophosphatidic acid induces exocytic trafficking of Na(+)/H(+) exchanger 3 by E3KARP-dependent activation of phospholipase C. Biochim Biophys Acta 1683: 59–68, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Chu S, Montrose MH. Non-ionic diffusion and carrier-mediated transport drive extracellullar pH regulation of mouse colonic crypts. J Physiol 494: 783–793, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coffey JW, Hansen HJ, Miller ON. Studies on the interaction of vitamin B12: intrinsic factor and receptors. 3. Chemical and biological properties of vitamin B12 combining substances from the stomach, serum, and ascites fluid of the mouse. Arch Biochem Biophys 110: 117–123, 1965 [DOI] [PubMed] [Google Scholar]
- 12. Donowitz M, Cha B, Zachos NC, Brett CL, Sharma A, Tse CM, Li X. NHERF family and NHE3 regulation. J Physiol 567: 3–11, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donowitz M, Li X. Regulatory binding partners and complexes of NHE3. Physiol Rev 87: 825–872, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Donowitz M, Mohan S, Zhu CX, Chen TE, Lin R, Cha B, Zachos NC, Murtazina R, Sarker R, Li X. NHE3 regulatory complexes. J Exp Biol 212: 1638–1646, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donowitz M, Singh S, Salahuddin FF, Hogema BM, Chen Y, Gucek M, Cole RN, Ham A, Zachos NC, Kovbasnjuk O, Lapierre LA, Broere N, Goldenring J, deJonge H, Li X. Proteome of murine jejunal brush border membrane vesicles. J Proteome Res 6: 4068–4079, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Hoogerwerf WA, Tsao SC, Devuyst O, Levine SA, Yun CH, Yip JW, Cohen ME, Wilson PD, Lazenby AJ, Tse CM, Donowitz M. NHE2 and NHE3 are human and rabbit intestinal brush-border proteins. Am J Physiol Gastrointest Liver Physiol 270: G29–G41, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Kim JH, Lee-Kwon W, Park JB, Ryu SH, Yun CH, Donowitz M. Ca(2+)-dependent inhibition of Na+/H+ exchanger 3 (NHE3) requires an NHE3-E3KARP-alpha-actinin-4 complex for oligomerization and endocytosis. J Biol Chem 277: 23714–23724, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Kocinsky HS, Dynia DW, Wang T, Aronson PS. NHE3 phosphorylation at serines 552 and 605 does not directly affect NHE3 activity. Am J Physiol Renal Physiol 293: F212–F218, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Kocinsky HS, Girardi AC, Biemesderfer D, Nguyen T, Mentone S, Orlowski J, Aronson PS. Use of phospho-specific antibodies to determine the phosphorylation of endogenous Na+/H+ exchanger NHE3 at PKA consensus sites. Am J Physiol Renal Physiol 289: F249–F258, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Lee-Kwon W, Kawano K, Choi JW, Kim JH, Donowitz M. Lysophosphatidic acid stimulates brush border Na+/H+ exchanger 3 (NHE3) activity by increasing its exocytosis by an NHE3 kinase A regulatory protein-dependent mechanism. J Biol Chem 278: 16494–16501, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Lee-Kwon W, Kim JH, Choi JW, Kawano K, Cha B, Dartt DA, Zoukhri D, Donowitz M. Ca2+-dependent inhibition of NHE3 requires PKCα which binds to E3KARP to decrease surface NHE3 containing plasma membrane complexes. Am J Physiol Cell Physiol 285: C1527–C1536, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Levine SA, Montrose MH, Tse CM, Donowitz M. Kinetics and regulation of three cloned mammalian Na+/H+ exchangers stably expressed in a fibroblast cell line. J Biol Chem 268: 25527–25535, 1993 [PubMed] [Google Scholar]
- 23. Lin R, Murtazina R, Cha B, Chakraborty M, Sarker R, Chen TE, Lin Z, Hogema BM, de Jonge HR, Seidler U, Turner JR, Li X, Kovbasnjuk O, Donowitz M. d-Glucose acts via sodium/glucose cotransporter 1 to increase NHE3 in mouse jejunal brush border by a Na+/H+ exchange regulatory factor 2-dependent process. Gastroenterology 140: 560–571, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin S, Yeruva S, He P, Singh AK, Zhang H, Chen M, Lamprecht G, de Jonge HR, Tse M, Donowitz M, Hogema BM, Chun J, Seidler U, Yun CC. Lysophosphatidic acid stimulates the intestinal brush border Na(+)/H(+) exchanger 3 and fluid absorption via LPA(5) and NHERF2. Gastroenterology 138: 649–658, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Michoud MC, Tolloczko B, Martin JG. Effects of purine nucleotides and nucleoside on cytosolic calcium levels in rat tracheal smooth muscle cells. Am J Respir Cell Mol Biol 16: 199–205, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Murtazina R, Kovbasnjuk O, Zachos NC, Li X, Chen Y, Hubbard A, Hogema BM, Steplock D, Seidler U, Hoque KM, Tse CM, De Jonge HR, Weinman EJ, Donowitz M. Tissue-specific regulation of sodium/proton exchanger isoform 3 activity in Na(+)/H(+) exchanger regulatory factor 1 (NHERF1) null mice. cAMP inhibition is differentially dependent on NHERF1 and exchange protein directly activated by cAMP in ileum versus proximal tubule. J Biol Chem 282: 25141–25151, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Nath SK, Hang CY, Levine SA, Yun CH, Montrose MH, Donowitz M, Tse CM. Hyperosmolarity inhibits the Na+/H+ exchanger isoforms NHE2 and NHE3: an effect opposite to that on NHE1. Am J Physiol Gastrointest Liver Physiol 270: G431–G441, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Ohata H, Yamada H, Niioka T, Yamamoto M, Momose K. Optical bioimaging: from living tissue to a single molecule: calcium imaging in blood vessel in situ employing two-photon excitation fluorescence microscopy. J Pharm Sci 93: 242–247, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Qian X, Prabhakar S, Nandi A, Visweswariah SS, Goy MF. Expression of GC-C, a receptor-guanylate cyclase, and its endogenous ligands uroguanylin and guanylin along the rostrocaudal axis of the intestine. Endocrinology 141: 3210–3224, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Sarker R, Valkhoff VE, Zachos NC, Lin R, Cha B, Chen TE, Guggino S, Zizak M, Dejonge H, Hogema B, Donowitz M. NHERF1 and NHERF2 are necessary for multiple but usually separate aspects of basal and acute regulation of NHE3 activity. Am J Physiol Cell Physiol 300: C771–C782, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Uzlaner N, Priel Z. Interplay between the NO pathway and elevated [Ca2+]i enhances ciliary activity in rabbit trachea. J Physiol 516: 179–190, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Voltz JW, Brush M, Sikes S, Steplock D, Weinman EJ, Shenolikar S. Phosphorylation of PDZ1 domain attenuates NHERF-1 binding to cellular targets. J Biol Chem 282: 33879–33887, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Wade JB, Welling PA, Donowitz M, Shenolikar S, Weinman EJ. Differential renal distribution of NHERF isoforms and their colocalization with NHE3, ezrin, and ROMK. Am J Physiol Cell Physiol 280: C192–C198, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Yun CH, Lamprecht G, Forster DV, Sidor A. NHE3 kinase A regulatory protein E3KARP binds the epithelial brush border Na+/H+ exchanger NHE3 and the cytoskeletal protein ezrin. J Biol Chem 273: 25856–25863, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Yun CH, Oh S, Zizak M, Steplock D, Tsao S, Tse CM, Weinman EJ, Donowitz M. cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci USA 94: 3010–3015, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zachos NC, Hodson C, Kovbasnjuk O, Li X, Thelin WR, Cha B, Milgram S, Donowitz M. Elevated intracellular calcium stimulates NHE3 activity by an IKEPP (NHERF4) dependent mechanism. Cell Physiol Biochem 22: 693–704, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zachos NC, Kovbasnjuk O, Donowitz M. Regulation of intestinal electroneutral sodium absorption and the brush border Na+/H+ exchanger by intracellular calcium. Ann NY Acad Sci 1165: 240–248, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zachos NC, Li X, Kovbasnjuk O, Hogema B, Sarker R, Lee LJ, Li M, de Jonge H, Donowitz M. NHERF3 (PDZK1) contributes to basal and calcium inhibition of NHE3 activity in Caco-2BBe cells. J Biol Chem 284: 23708–23718, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443, 2005 [DOI] [PubMed] [Google Scholar]