Abstract
Identification of factors that direct embryonic stem (ES) cell (ESC) differentiation into functional cardiomyocytes is essential for successful use of ESC-based therapy for cardiac repair. Neuregulin-1 (NRG1) and microRNA play important roles in the cardiac differentiation of ESCs. Understanding how NRG1 regulates microRNA will provide new mechanistic insights into the role of NRG1 on ESCs. It may also lead to the discovery of novel microRNAs that are important for ESC cardiac differentiation. The objective of this study was to assess the microRNA expression profile during NRG1-induced ESC cardiac differentiation. Murine ESCs were incubated with a recombinant NRG1β or an inhibitor of ErbB2 or ErbB4 during hanging drop-induced cardiac differentiation. The expression of cardiac-specific markers and microRNAs was analyzed by RT-PCR and microRNA array, respectively. We found that the expression of NRG1 and the ErbB receptors was increased during hanging drop-induced cardiac differentiation of ESCs. NRG1 stimulation during a specific developmental window enhanced, while inhibition of the ErbB2 or ErbB4 receptor inhibited, cardiac differentiation of ESCs. NRG1 increased the expression of mmu-miR-296–3p and mmu-miR-200c*, and decreased mmu-miR-465b-5p. Inhibition of mmu-miR-296–3p or mmu-miR-200c* decreased, while inhibition of mmu-miR-465–5p increased, the differentiation of ESCs into the cardiac lineage. This is the first report demonstrating that microRNAs are differentially regulated by NRG1-ErbB signaling during cardiac differentiation of ESCs. This study has also identified new microRNAs that are important for ESC cardiac differentiation.
Keywords: stem cells
there is an intensive effort to develop stem cell-based strategies for cardiac repair (1, 13, 16). Embryonic stem cells (ESCs) can develop into definitive cardiomyocytes and are therefore ideal for this purpose (17, 27, 41). We and others have shown that injection of ESCs into cardiac myocardium improves cardiac function in cardiac infarction mouse and rat models (26, 27, 37). However, ESCs are pluripotent and have the tendency to form teratomas (3, 16, 29). Methods for directing ESC differentiation into mature and functional cardiomyocytes are needed to achieve a safe and effective therapeutic outcome (1, 14, 22).1
Neuregulin-1 (NRG1) and its ErbB receptors are essential for the development of the heart (12, 23, 25). Deletion of the NRG1, ErbB2, or ErbB4 gene causes ventricular trabecular malformation and embryonic lethality (12, 23, 25). NRG1 is also capable of converting embryonic cardiomyocytes into cells of the cardiac conduction system (30, 31). The NRG1 gene is a member of the epidermal growth factor (EGF) gene family. NRG1 is synthesized and secreted by the endocardium and the endothelium of the cardiac microvasculature (9, 11). The receptors of NRG1 proteins are members of the EGF receptor family, which include ErbB1–4. NRG1 proteins bind directly to ErbB3 and ErbB4 and recruit ErbB2 as a coreceptor (42). In embryonic mouse hearts, ErbB2 and ErbB 4 receptors are expressed in the myocardium, while the ErbB3 receptor is expressed in mesenchymal cells of the endocardial cushion (12, 23, 25). NRG1 activates ErbB2 and ErbB4 receptors on the cardiomyocyte, thereby activating multiple downstream signaling pathways to regulate key functions of the cardiomyocyte (24). Studies have shown that NRG1 promotes the cardiac differentiation of ESCs (18, 35, 38).
MicroRNAs (miR) are a class of conserved noncoding small RNAs that regulate gene expression by targeting mRNA (4). It is now recognized that microRNAs are important for the regulation of cardiac development (7, 32, 39). Studies have shown that miR-1 is involved in cardiogenesis in Drosophila (20, 34) and mouse (45). Targeted deletion of miR-1-2 leads to cardiac ventricular septal defect formation during embryogenesis (44). Muscle-specific miR-1 or miR-133 overexpression promotes the mesodermal formation of ESCs (5, 15). These studies suggest that microRNAs are key regulators of ESC cardiac differentiation. Identification of novel microRNAs that are important for ESC cardiac differentiation as well as factors that regulate these microRNAs will have significant impact on the development of new strategies to effectively direct ESC differentiation into the cardiac lineage.
We hypothesize that NRG1 may induce cardiac differentiation of ESCs by modulating microRNA function. In this study, we identified microRNAs that are differentially regulated by NRG1-ErbB signaling and are important for ESC cardiac differentiation.
METHODS
ESC culture and differentiation.
Cells from the murine undifferentiated ES cell line, ES-D3 (American Type Culture Collection, Manassas, VA), were maintained on mitotic inactive mouse embryonic fibroblast feeder cells (Millipore, Billerica, MA) in ES-qualified DMEM. The medium contained 15% fetal bovine serum, 1% β-mercaptoethanol, 1% nucleosides, 1% penicillin-streptomycin, 1% nonessential amino acids, 1% l-glutamine, and 103 U/ml ESGRO (mouse leukemia inhibitory factor, mLIF; Millipore, Billerica, MA). The hanging drop-induced differentiation was initiated by culturing ESCs in hanging drops (500 cells/20 μl). The differentiation medium contained 10% fetal bovine serum, 1% β-mercaptoethanol, 1% nucleosides, 1% penicillin-streptomycin, 1% nonessential amino acids, and 1% l-glutamine in DMEM (40). Embryoid bodies (EBs) were formed 3 days after the initiation of the hanging drop culture. EBs were transferred into petri dishes containing differentiation medium for an additional 2 days. Cells were then moved into 0.1% gelatin-coated tissue plates containing differentiation medium for culture. Cells were harvested at different points for analyses.
NRG1 solvent (20 mM sodium acetate, 100 mM sodium sulfate, 1% mannitol, and 100 mM l-arginine, pH 6.5), recombinant human NRG1β [recombinant human glial growth factor 2 (rhGGF2), 100 ng/ml, a gift from Acorda Therapeutics], ErbB2 receptor inhibitor AG825 (1 μM, Calbiochem, San Diego, CA), or a ErbB1/ErbB2/ErbB4 receptor inhibitor (1 nM, catalog no. 324840, Calbiochem) was added in the culture medium at different time points.
RNA isolation and semiquantitative RT-PCR.
Total RNA was prepared from ESCs and ESC-derived cells using TRIzol reagent (Invitrogen, Carlsbad, CA). Reverse transcription (RT) was performed by using Superscript III reverse transcriptase (Invitrogen). Semiquantitative PCR was performed using gene-specific primers (Table 1).
Table 1.
Primers for semiquantitative RT-PCR
| Target Gene | Forward/Reverse (5′—3′) |
|---|---|
| OCT3/4 | GTTCTGCGGAGGGATGGCATACTGT/GTTCTCATTGTTGTCGGCTTCCTCC |
| Brachyury | GCTAACTAACGAGATGATTGTGACC/CTATGAACTGGGTCTCGGGAAAGCA |
| cTNT | CGTAGAAGAGGTTGGTCCTGATGAA/TGTACCCTCCAAAGTGCATCATGTT |
| cTNI | AAAAAGTCTAAGATCTCCGCCTCCA/GGTTTTCCTTCTCAATGTCCTCCTT |
| MLC2a | CAGGCACAACGTGGCTCTTCTAATG/GGGTGATGATGTAGCAGAGAGACTT |
| α-Sarcomeric actin | TGGAAGAAGAAATCGCCGCACTCGT/TCTTCTCTCTGTTAGCTTTGGGGTT |
| GATA4 | CCTGGAAGACACCCCAATCTCGATA/TTTATTCAGGTTCTTGGGCTTCCGT |
| NKX2.5 | TCCTGCATGCTGGCCGCCTTCA/CCTGCCGCTGTCGCTTGCACTTGTA |
| MEF2c | GAGGATAATGGATGAGCGTAACAGA/GTTATGGCTGGACACTGGGATGGTA |
| ErbB1 | GGAGGATGTAGTTGATGCTGATGAG/GGGCTGATTGTGATAGACAGGGTTC |
| ErbB2 | TTCTACCGTTCACTGCTGGAGGATG/CCAGGGGAGCAACGTAGCCATCAGT |
| ErbB3 | ACACAGCCTGCTTACTCCCGTCACC/CGCATTTCCTCATACCCTTGTTCAG |
| ErbB4 | TTGGTCCCCCAGGCTTTCAACATAC/ACACAAAAGGGTTCTCTTCCACAGG |
| GAPDH | CTTCCAGGAGCGAGACCCCACTAAC/CGGACACATTGGGGGTAGGAACACG |
cTNT, cardiac troponin T; cTNI, cardiac troponin I; MLC2a, myosin light chain 2a; MEF2c, myocyte enhancer factor 2c.
Real-time PCR.
Real-time PCR was performed to measure the expression of microRNAs. Total RNA (10 ng) was reverse transcribed in a 15-μl reaction buffer containing 50 units of Multiscribe reverse transcriptase and microRNA-specific RT primers (Taqman microRNA assay, Applied Biosystems, Foster City, CA). Real-time quantitative PCR was performed by Taqman microRNA assay using microRNA-specific PCR primers and Taqman MGB probes labeled with FAM fluorescence (Applied Biosystems). The amplification program was as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 10 s and 60°C for 1 min. SnoRNA202 was used as loading control. MicroRNA expression was analyzed by the ΔΔCT method. The primers and probes for microRNAs were as follows: mmu-miR-296–3p (part no. 4395212), mmu-miR-200c* (part no. 4395397), mmu-miR-465b-5p (part no. 4395615), and mouse snoRNA202 (part no. 4380914).
Real-time PCR was performed to assess the expression of NRG1 and cardiac-specific genes. cDNA (200 ng) was used for real-time PCR by Taqman gene assay (Applied Biosystems). The amplification program was described as above. The relative expression level was calculated using the ΔΔCT method. 18S rRNA was used as loading control. The primers and probes for NRG1α were forward primer, 5′-TCAAACCCCTCAAGATACT-3′; reverse primer, 5′-GTACATCTTGCTCCAGTGA-3′; and probe, 5′-FAM-TGCAAGTGCCAACCTGGA-3′ (FAM-labeled). The primers and probes for NRG1β were forward primer, 5′-GTCAAACCCCTCAAGATAC-3′; reverse primer, 5′-CGTAGTTTTGGCAACGATC-3′; and probe, 5′-TET-TGCAAGTGCCCAAATGAG-3′ (TET-labeled). The other primers and probes were cardiac troponin T (cTNT; Mm00441922_m1), NKX2.5 (Mm00657783_m1), α-fetoprotein (AFP; Mm00431715_m1), CD31 (Mm01246167_m1), Pax6 (Mm00443081_m1), brachyury (Mm01318252_m1), and 18S rRNA (part no. 4310893E).
Western blot analysis.
Cells were washed in phosphate-buffered-saline (pH 7.4) once and lysed in a lysis buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 10 mM sodium pyrophosphate, 20 mM β-glycerophosphate, 10 mM Na3VO4, 1 mM NaF, 1 mM PMSF, and protease inhibitor cocktail tablet (Roche Diagnostics, Indianapolis, IN). The cell lysates were centrifuged at 12,000 g for 15 min at 4°C and the supernatant was saved. Proteins were quantified using the Bradford assay (Bio-Rad, Hercules, CA). Equal amounts of protein were separated by SDS-PAGE and transferred to Whatman nitrocellulose membrane (pore size 0.2 μm, Fisher Scientific, Pittsburgh, PA). Membranes were probed with antibodies against mouse phosphorylated ErbB1 (Tyr1173), ErbB2 (Tyr877), ErbB3 (Tyr1289), ErbB4 (Tyr1284), ERK, Akt, and total ErbB1, ErbB2, ErbB3, ErbB4, ERK, Akt (Cell Signaling Technology, Danvers, MA), cTNT, NKX2.5, connexin 40 at 4°C overnight, followed by horseradish peroxidase-conjugated secondary antibody (Sigma-Aldrich, St. Louis, MO) for 1 h at room temperature. Blotted proteins were visualized using an enhanced chemiluminescence (ECL) system (GE Healthcare, Piscataway, NJ). GAPDH was used as loading control. Stripping and reprobing were performed as described by the manufacturer (Pierce, Rockford, IL).
Measurements of beating EBs.
The differentiation of mouse ESCs was performed as described above. For each experiment, 100 EBs were counted under microscopy at different time points, and the percentage of EBs that contained beating areas was calculated. In NRG1-treated ESCs, NRG1 was added at different time points and measurements of beating EBs were performed on day 9.
MicroRNA array.
Total RNA was prepared using TRIzol reagent (Invitrogen). The concentration and integrity of RNA were evaluated by Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA). MicroRNA array was performed using Taqman Rodent MicroRNA Array (version 2.0; Applied Biosystems). In brief, Taqman microRNA array included stem-loop RT and real-time PCR. Megaplex RT primer pools were used for RT which converted >500 of mouse microRNAs into cDNAs. Real-time PCR was performed using Taqman Rodent MicroRNA Array (version 2.0) following the manufacturer's instruction. The relative expression of microRNAs was calculated using the ΔΔCT method and normalized to the snoRNA expression. The fold changes of microRNAs in the cells from treated groups were calculated. Agglomerative hierarchical clustering was performed using Cluster program (8).
MicroRNA inhibition assay.
Inhibitors for mmu-miR-296–3p, mmu-miR-200c*, and mmu-miR-465b-5p, as well as negative control (Applied Biosystems), were transfected into undifferentiated ES-D3 cells using Lipofectamine 2000 reagents according to the manufacturer's instruction (Invitrogen). The next day, the differentiation of ES-D3 was induced by the hanging drop method. Cells were harvested on day 3 of hanging drop-induced differentiation. The expression of mmu-miR-296–3p, mmu-miR-200c*, and mmu-miR-465b-5p was analyzed by Taqman microRNA assay as described above.
Statistical analysis.
Data are presented as means ± SE and represent at least three independent experiments. Comparison of means was performed using Student's t-test. Differences were considered significant with P < 0.05.
RESULTS
Expression of NRG1 and the ErbB receptors during hanging drop-induced murine ESC differentiation.
The differentiation of murine ESC into cardiomyocytes was induced by the hanging drop method as described previously (40). First, we measured the expression of OCT3/4, an undifferentiated ESC marker (2), and brachyury, an early mesodermal marker (21). OCT3/4 was highly expressed in undifferentiated ESCs. It was decreased upon ESC differentiation. Brachyury was not detected in undifferentiated ESCs. It was increased at day 3 and day 5 of ESC differentiation and decreased after day 5 (Fig. 1A). Cardiac-specific structural proteins cTNT, cardiac troponin I (cTNI), myosin light chain 2a (MLC2a), and α-sarcomeric actin and transcriptional factors GATA4 and NKX2.5 were increased during the course of the cardiac differentiation of ESCs (Fig. 1, B and C). We detected EBs that contained beating areas on day 7 of the differentiation. The percentage of beating EBs reached 80% on day 9 (Fig. 1D).
Fig. 1.
Hanging drop-induced cardiac differentiation of murine embryonic stem cells (ESCs). ESCs were cultured as hanging drops from day 0–3 (D0–3). The formed embryoid bodies (EBs) were cultured in suspension for additional 2 days (day 3–5) and then cultured on gelatin-coated dishes. Cells were harvested at different time points. A: semiquantitative RT-PCR assessment of mRNA expression of OCT3/4 and Brachyury during ESC differentiation. GAPDH was used as loading control. B: semiquantitative RT-PCR assessment of mRNA expression of cardiac-specific genes during ESC differentiation. GAPDH was used as loading control. C: real-time PCR assessment of cardiac troponin T (cTNT) and NKX2.5 mRNA expression during ESC differentiation. 18S rRNA was used as loading control. The results were obtained from three independent experiments. D: percentage of EBs that contained beating areas. Murine ESC differentiation was performed as described above. The percentage of EBs with beating areas was determined at each indicated time point. In total, 100 EBs were counted in each experiment. Three independent experiments were performed.
Alternative splicing of EGF-like receptor binding domain of NRG1 produces α- and β-isoforms of NRG1 (9). We measured the mRNA expression of NRG1α and NRG1β during ESC differentiation. NRG1α expression was gradually increased during the course of differentiation. NRG1β expression was increased during day 5 to day 7 and again from day 10 to day 14 (Fig. 2A). The mRNA and total protein levels of the ErbB1, ErbB2, ErbB3, and ErbB4 receptors were increased during ESC cardiac differentiation (Fig. 2, B and C). The phosphorylation (activation) of ErbB2 was increased on day 5 and again on day 8 (Fig. 2D). The increase of NRG1β expression and ErbB2 activation was associated with the mesoderm formation of ESCs, as well as the increase of cardiac-specific genes and the emerging of beating areas in EBs. These results suggest that NRG1-ErbB signaling may be important for the cardiac differentiation of ESCs.
Fig. 2.
Expression of neuregulin-1 (NRG1) and ErbB receptors during hanging drop-induced murine ESC differentiation. A: real-time PCR assessment of mRNA expression of NRG1α and NRG1β during ESC differentiation. 18S rRNA was used as loading control. The results are from three independent experiments. B: mRNA expression of ErbB receptors during ESC differentiation. C: protein expression of ErbB receptors during ESC differentiation. D: phosphorylation levels of ErbB receptors during ESC differentiation. mRNA expression of ErbB1, ErbB2, ErbB3, and ErbB4 was measured by semiquantitative RT-PCR. The phosphorylation and total ErbB receptor levels were measured by Western blot analysis. GAPDH was used as loading control.
NRG1-ErbB signaling is pivotal for ESC differentiation into cardiomyocytes.
We investigated whether NRG1 treatment promoted the differentiation of ESCs into the cardiac lineage. We treated the cells with NRG1 during different stages of ESC differentiation (Fig. 3A) and assessed cardiac differentiation of ESCs on day 9. When cells were treated during day 5 to day 7, NRG1 increased the mRNA expression of cTNT, MLC2a, myocyte enhancer factor 2c (MEF2C), and GATA4 (Fig. 3B) and the protein level of NKX2.5, cTNT, and connexin 40 (Fig. 3C). Connexin 40 is a marker for the cardiac conduction system (30). In addition, NRG1 treatment during day 5 to day 7 significantly increased the number of beating EBs (Fig. 3D). On the other hand, the expression levels of endoderm marker AFP (6), vascular endothelial marker CD31, and ectoderm marker Pax6 (paired box gene 6) (43) were not changed by NRG1 stimulation (Fig. 3E).
Fig. 3.
NRG1 promoted hanging drop-induced cardiac differentiation of murine ESCs. A: schematic diagram of NRG1 stimulation protocol during hanging drop-induced murine ESC differentiation. Cells were incubated with solvent or NRG1 (100 ng/ml) during the different developmental windows shown. Cells were then washed and incubated without NRG1 for additional days. Cells were collected on day 9 for analyses. B: semiquantitative RT-PCR assessment of the mRNA level of cardiac-specific genes in NRG1-treated ESCs. mRNA expression of cTNT, myosin light chain 2a (MLC2a), myocyte enhancer factor 2c (MEF2c), and GATA4 was measured by semiquantitative RT-PCR. GAPDH was used as loading control. Densitometric quantification is shown at right. Ctrl, control. C: protein level of cardiac-specific genes in NRG1-treated ESCs. Protein expression of cTNT, NKX2.5, and connexin 40 was measured by Western blot analysis. GAPDH was used as loading control. Densitometric quantification is shown at right. D: percentage of beating EBs in NRG1-treated ESCs. Cells were treated with NRG1 during day 0–3, day 3–5, day 5–7, or day 7–9. The percentage of beating EBs was measured on day 9. E: mRNA expression of α-fetoprotein (AFP), CD31, and Pax6. Cells were treated with NRG1 during day 5–7. mRNA levels of AFP, CD31, and Pax6 were measured by real-time PCR on day 9. *P < 0.05 vs. control; **P < 0.01 vs. control.
NRG1 stimulation increased the phosphorylation of ErbB2, ErbB3, and ErbB4 in ESC-derived cells (Fig. 4, A and B). AG825, an ErbB2 inhibitor (10), abolished the effect of NRG1 on ErbB2 phosphorylation (Fig. 4A). The effects of NRG1 on ErbB2 and ErbB4 phosphorylation (activation) were also abolished by an ErbB1/ErbB2/ErbB4 inhibitor (Fig. 4B). When we treated cells with AG825 or this ErbB1/ErbB2/ErbB4 inhibitor during day 5 to day 7 of the ESC differentiation, the protein and mRNA expression levels of NKX2.5, as well as mRNA of cTNT, were significantly decreased (Fig. 4, C and D). These were associated with decreased phosphorylation of ERK1/2 and Akt (Fig. 4C), two major downstream signaling pathways of NRG1-ErbB signaling. ErbB2 and/or ErbB4 inhibitors also decreased the percentage of beating EBs at day 8 and day 9 of the ESC differentiation (Fig. 4E). ErbB1/ErbB2/ErbB4 inhibition induced a more robust decrease of cardiac markers and the percentage of beating EBs. These data demonstrated that NRG1-ErbB signaling is pivotal for the differentiation of ESCs into functional cardiomyocytes.
Fig. 4.
Inhibition of ErbB2 or ErbB4 receptors inhibited hanging drop-induced cardiac differentiation of murine ESCs. A: inhibition of ErbB2 abolished NRG1-induced ErbB2 activation in ESCs. Six days after the initiation of hanging drop-induced ESC differentiation, cells were treated with NRG1 (100 ng/ml) for 2 h. An ErbB2 inhibitor (AG825, 1 μM) was added 1 h before NRG1 treatment. Total and phosphorylated ErbB receptor levels were measured by Western blot analysis. Densitometric quantification is shown at right. B: ErbB1/ErbB2/ErbB4 inhibitor abolished NRG1-induced activations of ErbB2 and ErbB4. Cells were treated and total and phosphorylated ErbB receptor levels were measured as described in A. Densitometric quantification is shown at right. C: inhibition of the ErbB2 and/or ErbB4 receptor decreased the protein level of NKX2.5 and the phosphorylation of Akt and ERK1/2. Cells were incubated with AG825 (1 μM) or an ErbB1/ErbB2/ErbB4 inhibitor (1 nM) during day 5–7. Cells were then incubated without the inhibitor and collected on day 9. NKX2.5, pAkt, and pERK1/2 were measured by Western blot analysis. GAPDH was used as loading control. D: inhibition of the ErbB2 and/or ErbB4 receptor decreased the mRNA of NKX2.5 and cTNT. mRNA expression of NKX2.5 and cTNT was assessed by real-time PCR. The results are from three independent experiments. *P < 0.05 vs. control. E: inhibition of the ErbB2 and/or ErbB4 receptor decreased the percentage of EBs that contained beating areas. The percentage of EBs containing beating areas was measured at each indicated time point. In total, 100 EBs were counted in each experiment. The results are from three independent experiments. *P < 0.05 vs. control.
The expression of microRNAs was differentially regulated by NRG1 stimulation or ErbB receptor inhibition during hanging drop-induced ESC differentiation.
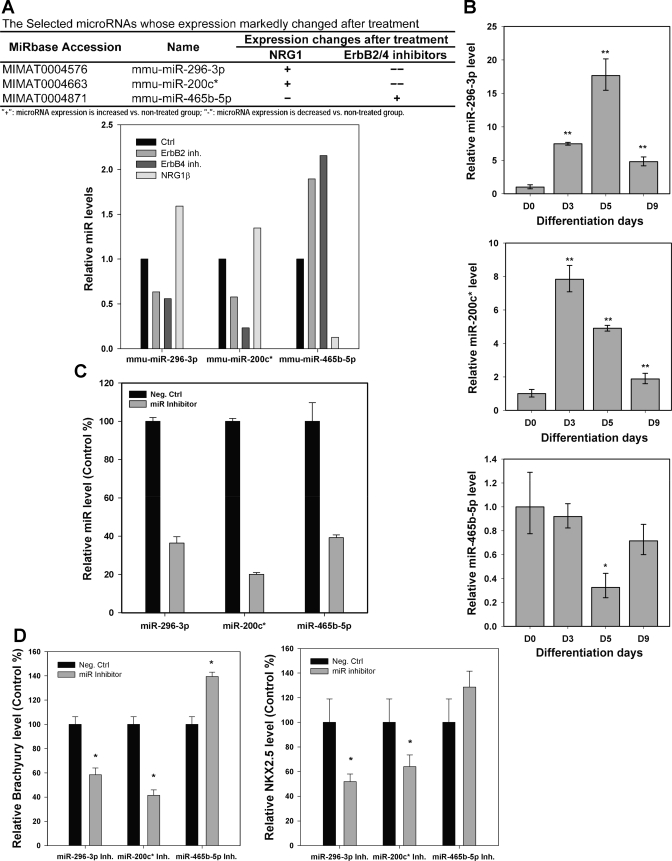
We performed microRNA profiling in NRG1 or ErbB inhibitor-treated ESCs during hanging drop-induced differentiation. In total, the expression of 592 microRNAs was analyzed. We identified microRNAs that were upregulated by NRG1 stimulation but downregulated by ErbB2 and/or ErbB4 inhibition. We also identified microRNAs that were inhibited by NRG1 but were increased by ErbB2 and/or ErbB4 inhibition (Supplemental data; Supplemental Material for this article is available online at the Journal website). By using quantitative real-time PCR, we confirmed that the expression of mmu-miR-296–3p was increased 60% by NRG1, while decreased 36% and 44% by AG825 and ErbB1/ErbB2/ErbB4 inhibitor, respectively. The expression of mmu-miR-200c* was increased 34% by NRG1, while decreased 42% and 77% by AG825 and ErbB1/ErbB2/ErbB4 inhibitor, respectively. The expression of mmu-miR465b-5p was decreased 87% by NRG1, while increased 89% and 110% by AG825 and ErbB1/ErbB2/ErbB4 inhibitor, respectively (Fig. 5A).
Fig. 5.
A: microRNA analysis of mmu-miR-296–3p, mmu-miR-200c*, and mmu-miR-465b-5p was differentially regulated by NRG1 and ErbB receptor inhibition. Cardiac differentiation of ESCs was performed by the hanging drop method. Cells were incubated with NRG1, ErbB2 inhibitor AG825 (1 μM), or ErbB1/ErbB2/ErbB4 inhibitor (1 nM) during day 5–7. Cells were then incubated without stimulation. RNA was collected on day 9. Accession number and name of the microRNAs are shown. B: mmu-miR-296–3p, mmu-miR-200c*, and mmu-miR-465b-5p were differentially expressed during hanging drop-induced mesoderm formation of ESCs. The expression of mmu-miR-296–3p and mmu-miR-200c* was increased during hanging drop-induced ESC differentiation. Expression of mmu-miR- 465b-5p was decreased during hanging drop-induced ESC differentiation. ESC differentiation was induced by the hanging drop method. RNA was collected on days 0, 3, 5, and 9. Expression of mmu-miR-296–3p, mmu-miR-200c*, and mmu-miR-465b-5p was assessed by real-time PCR. Data were normalized to snoRNA202 and are presented as fold expression relative to the mRNA level of undifferentiated ESCs (day 0). The results are from three independent experiments. *P < 0.05 vs. D0; **P < 0.01 vs. D0. C: expression of microRNAs was inhibited by anti-miR inhibitors. ES-D3 cells were transfected with anti-miR inhibitors or scrambled negative control and then the differentiation of ESCs was induced by the hanging drop method. On day 3 of ESC differentiation, RNA was collected and the expression of microRNAs was measured by real-time PCR. Data were normalized to snoRNA202 and are presented as a percentage of microRNA vs. control expression. D: expression of brachyury and NKX2.5 in differentiated ESCs that were transfected with anti-miR inhibitors. RNA collected in C was used for assessing the expression of brachyury and NKX2.5 by real-time PCR. Data were normalized to 18S rRNA and are presented as percent expression of individual microRNA vs. control transfection. *P < 0.05 vs. control.
Differential expression of mmu-miR-296–3p, mmu-miR-200c*, and mmu-miR-465b-5p during mesoderm formation.
We further measured the expression of mmu-miR-296–3p, mmu-miR-200c*, and mmu-miR-465b-5p in undifferentiated ESCs and in ESC-derived cells 3 and 5 days after the initiation of hanging drop, at which point mesoderm formation was detected (Fig. 1A). We found that mmu-miR-296–3p and mmu-miR-200c* expression levels were significantly increased during day 3 to day 5 of ESC differentiation (Fig. 5B). On the contrary, mmu-miR-465b-5p was significantly decreased at day 5 of ESC differentiation (Fig. 5B).
Inhibition of mmu-miR-296–3p or mmu-miR-200c* decreased, while inhibition of mmu-miR-465b-5p increased, cardiac differentiation of ESCs.
To test whether these microRNAs are important for the cardiac differentiation of ESCs, we used microRNA inhibitors to decrease the expression of these microRNAs in undifferentiated ESCs (Fig. 5C) and tested whether decreased expression of these microRNAs would affect the cardiac differentiation of ESCs. As shown in Fig. 5D, 3 days after the initiation of the hanging drop, Brachyury and NKX2.5 were significantly decreased in cells derived from ESCs with mmu-miR-296–3p and mmu-miR-200c* inhibition while increased in cells derived from ESCs with mmu-miR-465b-5p inhibition. These results suggest that mmu-miR-296–3p and mmu-miR-200c* enhance, while mmu-miR-465b-5p inhibits, the cardiac differentiation of ESCs.
DISCUSSION
This is the first report demonstrating that microRNAs are differentially regulated by NRG1-ErbB signaling during cardiac differentiation of ESCs. New microRNAs that are important for ESC cardiac differentiation have been identified.
Identification of proper conditions that effectively direct ESC differentiation into functional cardiomyocytes is crucial in ESC-based cell therapies for cardiac repair (14). This includes identification of factors that promote the cardiac differentiation of ESCs and determination of how and when to use these factors during the developmental stages of ESCs (14, 22). Factors, including growth factors, microRNAs, and chemicals that are important for cardiac differentiation of ESCs are being discovered (14, 33, 36, 39).
MicroRNAs have emerged as important modulators of ESC cardiac differentiation (7, 39). Despite intensive research, limited numbers of microRNAs that are important for ESC cardiac differentiation have been discovered (5, 15). Given the fact that NRG1-ErbB signaling promotes the cardiac differentiation of ESCs, we reasoned that by identifying microRNAs that are regulated by NRG1-ErbB signaling, we might discover new microRNA candidates that are important for ESC cardiac differentiation; we might also improve our understanding of how NRG1 enhances the cardiac differentiation of ESCs. We analyzed the microRNA expression profile in NRG1 or ErbB inhibitor-treated ESCs. We have discovered novel microRNAs that are differentially regulated by NRG1 or ErbB inhibition during hanging drop-induced ESC cardiac differentiation. We further show that these microRNAs are differentially expressed during the mesodermal formation of ESCs. In addition, inhibition of these microRNAs either inhibits or enhances cardiac differentiation of ESCs. These results suggest that these newly identified microRNAs are important for regulating the cardiac differentiation of ESCs.
This study has identified a specific developmental window during which time the effects of NRG1 for promoting the cardiac differentiation of ESCs are most evident. This is in contrast with previous studies in which NRG1 stimulation was conducted during an almost entire course of ESC differentiation (35). Studies have shown that identification of the developmental stage for specific stimulation is important in directing ESC cardiac differentiation. For example, stimulation of Wnt/β-catenin signaling enhances cardiac differentiation of ESCs during EB formation, whereas it suppresses cardiomyogenesis in the late stage of ESC differentiation (28). Smad2 activation improves endodermal and mesodermal induction in the early stage of ESC differentiation, but it inhibits cardiomyogenesis in the late stage (19). Our results have shown that NRG1 does not have negative regulatory effects on ESC cardiac differentiation; however, stimulation of NRG1-ErbB signaling at a specific developmental stage is crucial for effectively directing ESC differentiation into the cardiac lineage.
Previous studies have shown that NRG1 promotes embryonic cardiomyocyte differentiation into cells of the cardiac conduction system (31). In this study, we have further shown that NRG1 promotes the expression of specific markers of the cardiac conduction system during hanging drop-induced cardiac differentiation of ESCs. Furthermore, NRG1 increases the number of beating EBs. These results suggest that NRG1 has the capacity to direct ESC differentiation into mature and functional cardiomyocytes.
In summary, by assessing the microRNA expression profile during NRG1-stimulated ESC cardiac differentiation, we discovered novel microRNAs that are important for the cardiac differentiation of ESCs.
GRANTS
This research was funded by National Heart, Lung, and Blood Institute Grant HL-52864 (to J. P. Morgan), American Heart Association Scientist Development Grant 0635549T (to X. Yan), and American Heart Association Grant-In-Aid 10GRNT4710003 (to X. Yan).
DISCLOSURES
X. Yan receives research material [recombinant NRG1 (rhGGF2)] from Acorda Therapeutics Inc.; A. O. Caggiano is an employee of Acorda Therapeutics Inc.
Supplementary Material
ACKNOWLEDGMENTS
We thank Acorda Therapeutics Inc. for providing recombinant NRG1.
Footnotes
This article is the topic of an Editorial Focus by K. Lemmens and G. W. De Keulenaer (24a).
REFERENCES
- 1. Blin G, Neri T, Stefanovic S, Puceat M. Human embryonic and induced pluripotent stem cells in basic and clinical research in cardiology. Curr Stem Cell Res Ther 5: 215–226, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122: 947–956, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brignier AC, Gewirtz AM. Embryonic and adult stem cell therapy. J Allergy Clin Immunol 125: S336–S344, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Callis TE, Wang DZ. Taking microRNAs to heart. Trends Mol Med 14: 254–260, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet 38: 228–233, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cho CH, Parashurama N, Park EY, Suganuma K, Nahmias Y, Park J, Tilles AW, Berthiaume F, Yarmush ML. Homogeneous differentiation of hepatocyte-like cells from embryonic stem cells: applications for the treatment of liver failure. FASEB J 22: 898–909, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Cordes KR, Srivastava D. MicroRNA regulation of cardiovascular development. Circ Res 104: 724–732, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95: 14863–14868, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res 284: 14–30, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol 35: 1473–1479, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Fuller SJ, Sivarajah K, Sugden PH. ErbB receptors, their ligands, and the consequences of their activation and inhibition in the myocardium. J Mol Cell Cardiol 44: 831–854, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature 378: 390–394, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Gersh BJ, Simari RD, Behfar A, Terzic CM, Terzic A. Cardiac cell repair therapy: a clinical perspective. Mayo Clin Proc 84: 876–892, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horton RE, Millman JR, Colton CK, Auguste DT. Engineering microenvironments for embryonic stem cell differentiation to cardiomyocytes. Regen Med 4: 721–732, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Ivey KN, Muth A, Arnold J, King FW, Yeh RF, Fish JE, Hsiao EC, Schwartz RJ, Conklin BR, Bernstein HS, Srivastava D. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell 2: 219–229, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joggerst SJ, Hatzopoulos AK. Stem cell therapy for cardiac repair: benefits and barriers. Expert Rev Mol Med 11: e20, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest 108: 407–414, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim HS, Cho JW, Hidaka K, Morisaki T. Activation of MEK-ERK by heregulin-beta1 promotes the development of cardiomyocytes derived from ES cells. Biochem Biophys Res Commun 361: 732–738, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Kitamura R, Takahashi T, Nakajima N, Isodono K, Asada S, Ueno H, Ueyama T, Yoshikawa T, Matsubara H, Oh H. Stage-specific role of endogenous Smad2 activation in cardiomyogenesis of embryonic stem cells. Circ Res 101: 78–87, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Kwon C, Han Z, Olson EN, Srivastava D. MicroRNA1 influences cardiac differentiation in Drosophila and regulates Notch signaling. Proc Natl Acad Sci USA 102: 18986–18991, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lacaud G, Keller G, Kouskoff V. Tracking mesoderm formation and specification to the hemangioblast in vitro. Trends Cardiovasc Med 14: 314–317, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 25: 1015–1024, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 378: 394–398, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Lemmens K, Doggen K, De Keulenaer GW. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation 116: 954–960, 2007 [DOI] [PubMed] [Google Scholar]
- 24a. Lemmens K, De Keulenaer GW. Paving new paths for neuregulin-1-assisted cardiac regenerative medicine. Focus on “Improving murine embryonic stem cell differentiation into cardiomyocytes with neuregulin-1: differential expression of microRNA” (May 4, 2011). doi: 10.1152/ajpcell.00137.2011 [DOI] [PubMed]
- 25. Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature 378: 386–390, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Min JY, Yang Y, Converso KL, Liu L, Huang Q, Morgan JP, Xiao YF. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J Appl Physiol 92: 288–296, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, Passier R, Tertoolen L. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation 107: 2733–2740, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci USA 103: 19812–19817, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, Muskheli V, Pabon L, Reinecke H, Murry CE. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J 21: 1345–1357, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Patel R, Kos L. Endothelin-1 and neuregulin-1 convert embryonic cardiomyocytes into cells of the conduction system in the mouse. Dev Dyn 233: 20–28, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Rentschler S, Zander J, Meyers K, France D, Levine R, Porter G, Rivkees SA, Morley GE, Fishman GI. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc Natl Acad Sci USA 99: 10464–10469, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sartipy P, Olsson B, Hyllner J, Synnergren J. Regulation of ‘stemness’ and stem cell differentiation by microRNAs. IDrugs 12: 492–496, 2009 [PubMed] [Google Scholar]
- 33. Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci USA 97: 11307–11312, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sokol NS, Ambros V. Mesodermally expressed Drosophila microRNA-1 is regulated by Twist and is required in muscles during larval growth. Genes Dev 19: 2343–2354, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suk Kim H, Hidaka K, Morisaki T. Expression of ErbB receptors in ES cell-derived cardiomyocytes. Biochem Biophys Res Commun 309: 241–246, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Takahashi T, Lord B, Schulze PC, Fryer RM, Sarang SS, Gullans SR, Lee RT. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation 107: 1912–1916, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Wang JF, Yang Y, Wang G, Min J, Sullivan MF, Ping P, Xiao YF, Morgan JP. Embryonic stem cells attenuate viral myocarditis in murine model. Cell Transplant 11: 753–758, 2002 [PubMed] [Google Scholar]
- 38. Wang Z, Xu G, Wu Y, Guan Y, Cui L, Lei X, Zhang J, Mou L, Sun B, Dai Q. Neuregulin-1 enhances differentiation of cardiomyocytes from embryonic stem cells. Med Biol Eng Comput 47: 41–48, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Williams AH, Liu N, van Rooij E, Olson EN. MicroRNA control of muscle development and disease. Curr Opin Cell Biol 21: 461–469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wobus AM, Guan K, Yang HT, Boheler KR. Embryonic stem cells as a model to study cardiac, skeletal muscle, and vascular smooth muscle cell differentiation. Methods Mol Biol 185: 127–156, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res 91: 501–508, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Zhang X, Huang CT, Chen J, Pankratz MT, Xi J, Li J, Yang Y, Lavaute TM, Li XJ, Ayala M, Bondarenko GI, Du ZW, Jin Y, Golos TG, Zhang SC. Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell 7: 90–100, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1–2. Cell 129: 303–317, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature 436: 214–220, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.