Abstract
The mammalian target of rapamycin (mTOR) signaling exists in two complexes: mTORC1 and mTORC2. Neurotensin (NT), an intestinal hormone secreted by enteroendocrine (N) cells in the small bowel, has important physiological effects in the gastrointestinal tract. The human endocrine cell line BON abundantly expresses the NT gene and synthesizes and secretes NT in a manner analogous to that of N cells. Here, we demonstrate that the inhibition of mTORC1 by rapamycin (mTORC1 inhibitor), torin1 (both mTORC1 and mTORC2 inhibitor) or short hairpin RNA-mediated knockdown of mTOR, regulatory associated protein of mTOR (RAPTOR), and p70 S6 kinase (p70S6K) increased basal NT release via upregulating NT gene expression in BON cells. c-Jun activity was increased by rapamycin or torin1 or p70S6K knockdown. c-Jun overexpression dramatically increased NT promoter activity, which was blocked by PD98059, an mitogen-activated protein kinase kinase (MEK) inhibitor. Furthermore, overexpression of MEK1 or extracellular signal-regulated kinase 1 (ERK1) increased c-Jun expression and NT promoter activity. More importantly, PD98059 blocked rapamycin- or torin1-enhanced NT secretion. Consistently, rapamycin and torin1 also increased NT gene expression in Hep3B cells, a human hepatoma cell line that, similar to BON, expresses high levels of NT. Phosphorylation of c-Jun and ERK1/2 was also increased by rapamycin and torin1 in Hep3B cells. Finally, we showed activation of mTOR in BON cells treated with amino acids, high glucose, or serum and, concurrently, the attenuation of ERK1/2 and c-Jun phosphorylation and NT secretion. Together, mTORC1, as a nutrient sensor, negatively regulates NT secretion via the MEK/ERK/c-Jun signaling pathway. Our results identify a physiological link between mTORC1 and MEK/ERK signaling in controlling intestinal hormone gene expression and secretion.
Keywords: gastrointestinal, p70 S6 kinase, regulatory associated protein of mTOR
the mammalian target of rapamycin (mTOR) is a highly conserved serine-threonine kinase (67). mTOR consists of two distinct, independently regulated complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 consists of mTOR regulatory associated protein (RAPTOR), mLST8 (also known as GβL), and proline-rich AKT substrate (40 kDa) (PRAS40) (28). Ribosomal p70S6 kinase (p70S6K) and eukaryotic translation initiation factor-4E (eIF4E) binding protein 1 (4E-BP1) are two of the best characterized downstream effectors in the regulation of mRNA translation (47). mTORC2 consists of mTOR rapamycin-insensitive companion (RICTOR), mLST8, mSin1, and protein observed with RICTOR (PROTOR) (28). mTORC2 directly phosphorylates the serine-473 site of Akt (59). mTORC2 is also involved in actin cytoskeleton reorganization (58) and phosphorylates protein kinase Cα (PKCα) (29).
The phosphoinositide 3-kinases (PI3K)/Akt/mTOR and the Ras/Raf/mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) pathways are two important survival pathways in cells and are frequently dysregulated in cancer (14, 20). The cross-talk between the PI3K/Akt and Ras/Raf/MEK/ERK cascades has been implicated in certain human cancer cells (13, 48, 55, 57). The Ras/Raf/MEK/ERK and the PI3K/Akt pathways converge at the level of Raf-1 or B-Raf and Akt, as both Raf-1 and B-Raf contain Akt consensus sites (27, 71). Akt directly phosphorylates Raf-1 on Ser-259 and results in a decrease in Raf-1 activity (71). B-Raf activity can be negatively regulated by Akt through phosphorylation of B-Raf multiple residues (27). Recently, the negative feedback loop in which mTORC1 inhibition leads to Akt and ERK activation has been described (17, 26). The feedback inhibition of PI3K/Akt activity by mTOR has been demonstrated in human breast cancer cell lines and tumor tissues from patients treated with the rapamycin derivative everolimus (RAD001) (51). Treatment of human lung cancer cells with rapamycin increased Akt phosphorylation, which was suppressed by the PI3K inhibitor LY294002, suggesting the requirement of PI3K in this process (63). mTORC1 activates p70S6K, which inhibits IRS-1 through serine phosphorylation and subcellular redistribution and targets IRS-1 for proteasomal degradation, thereby downregulating Akt (33, 64). In pancreatic β-cells, the feedback inhibitory loop is IRS-2-dependent due to its abundant expression and functional importance (5, 40). mTOR inhibition induced ERK activation through p70S6K-PI3K-Ras signaling (9). The mTOR inhibition-induced feedback activation of ERK was observed in biopsies from patients treated with RAD001 or cancer cell lines and mouse models treated with rapamycin (26).
In general, mTOR-dependent pathways are essential for cell growth by regulating a number of cellular functions, including protein synthesis, ribosomal biogenesis, metabolism, cell cycle, and autophagy (3). In addition, mTORC1 controls trafficking of nutrient transporters (19), for example, glucose (7, 56) and amino acid transporters (18), suggesting involvement in the transport of vesicles containing hormone peptide. Increasing evidence shows an important role for TOR signaling and the control of vesicular trafficking in yeast (53). TOR is associated with intracellular vesicular structures and exists in the same cellular fractions as endosomes (11, 38), suggesting a role in endocytosis. The involvement of TOR in endocytosis was also demonstrated in Drosophila fat body cells (34). mTOR was demonstrated to be structurally related to the class III PI3K hVps34, which has well-characterized roles in endocytosis. Furthermore, mTOR is localized to the endoplasmic reticulum (ER) and Golgi, cellular components involved in the secretory pathway (16, 45), suggesting that mTOR has functions related to hormone peptide synthesis and maturation in ER and Golgi. More recently, Xu et al. (68, 69) showed the colocalization of phospho-mTOR (Ser2448) and ghrelin, a gastric hormone, in the mouse fundic mucosa; relative to normal fed mice, levels of both gastric preproghrelin and circulating ghrelin were increased in fasted mice in which mTOR signaling was inhibited. In contrast, ghrelin production was decreased in mice following intraperitoneal injection of rapamycin or in obese mice in which mTOR signaling was elevated.
Our laboratory is focused on better delineating the signaling mechanisms regulating intestinal hormone secretion. NT, a tridecapeptide, is produced and secreted by enteroendocrine (N) cells localized in the distal small bowel (21, 22). NT has numerous physiological functions in the gastrointestinal (GI) tract including effects on GI motility, facilitation of fatty acid translocation, stimulation of pancreatic secretion, and stimulation of intestinal growth (21, 22). Previously, using the novel BON endocrine cell line, which was established and characterized in our laboratory from a pancreatic carcinoid tumor (10, 23, 52), we have shown that the phorbol 12-myristate 13-acetate (PMA), a PKC activator, stimulated NT secretion through a mechanism involving PKC/protein kinase D (41, 42, 44). We also reported that forskolin (FSK), an agent that elevates intracellular cAMP level, stimulated NT secretion through signaling pathways mediated by the cAMP-dependent protein kinase (PKA) and the exchange protein directly activated by cAMP (Epac) (43). Given the importance of mTOR signaling on protein synthesis and cell metabolism, the purpose of the current study was to determine whether mTOR signaling affects NT peptide release.
MATERIALS AND METHODS
Materials.
Rapamycin, a selective mTORC1 inhibitor (6), and all the antibodies used in this study, except for the antibodies mentioned below, were from Cell Signaling Technology (Danvers, MA). Cell lysis buffer for Western blot was also from Cell Signaling. c-Jun, JunB, JunD, and c-Fos antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-c-Fos antibody was from Invitrogen. Torin1, a newly developed ATP-competitive inhibitor that suppresses both mTORC1 and mTORC2 (65), was provided by Drs. Gray and Sabatini (Harvard Medical School, Boston, MA). Plasmids containing short hairpin RNA (shRNA) targeting mTOR, RAPTOR, and mTOR rapamycin-insensitive companion (RICTOR) as well as the nontargeting control (NTC) shRNA were from Addgene (Cambridge, MA). The wild-type p70S6K, the active form of p70S6K-T389E, T7-ERK1, the constitutively active MEK1-D218/D222 (MEK1-DD), and pJC6-GL3 (c-Jun promoter containing −225 to +150 of the murine c-jun promoter) plasmids were also from Addgene. The human NT reporter plasmid containing the NT promoter (−373/+23) cloned upstream of the luciferase gene in SacI/SmaI-digested pXP1 was from Dr. Paul Dobner (University of Massachusetts, Worcester, MA). Construction of the 5′ deletions (−122 and −42) was performed using a PCR-based strategy and described previously (15). The overexpression plasmids of wild-type (WT) and dominant negative c-Jun (Tam67) in pcDNA3.1 vector were from Dr. Michael Birrer (National Cancer Institute, Rockville, MD). p70S6K shRNA lentiviral particles were from Sigma MISSION (St. Louis, MO). The NT enzyme immunoassay (EIA) kit was from Phoenix Pharmaceuticals (Belmont, CA). The NuPAGE BisTris gels for Western blot were from Invitrogen. The ECL detection system was from GE Healthcare (Piscataway, NJ). The RNeasy Kit was from Qiagen (Valencia, CA). The Dual Luciferase Reporter Assay System was from Promega (San Luis Obispo, CA). DMEM/F12 powder without amino acids was from US Biological (Swampscott, MA).
Cell culture.
The BON cell line was derived from a human pancreatic carcinoid tumor and characterized in our laboratory (23, 52). BON cells are maintained in a 1:1 mixture of Dulbecco's modified Eagle's medium (DMEM) and nutrient mixture, F12K, supplemented with 5% fetal bovine serum (growth medium) in 5% CO2 at 37°C. The BON cell line was last authenticated in October 2009 at the Johns Hopkins Genetic Resources Core Facility with short tandem repeat analysis using an Identifiler identification kit (Applied Biosystems). The Hep3B human hepatoma cells were purchased from ATCC (Manassas, VA) and cultured in minimum essential medium (Eagle) with 2 mM l-glutamine, 0.1 mM nonessential amino acids, and 1.0 mM sodium pyruvate, and 10% FBS. The 293FT packaging cells were purchased from Invitrogen and cultured in DMEM complemented with 10% FBS, 2 mM l-glutamine, 0.1 mM MEM nonessential amino acids (growth medium).
Generation of stable knockdown cell lines.
shRNA lentivirus was produced by the modified protocol from Addgene. Briefly, 293FT packaging cells cultured in 60-mm dishes were cotransfected with shRNA vector (1 μg) and two packaging plasmids 750 ng psPAX2 (Addgene no. 12260) and 250 ng pMD2G (Addgene no. 12259) by Lipofectamine 2000 and incubated in growth medium overnight. Subsequently, the cells were cultured in complete medium (growth medium plus 1 mM MEM sodium pyruvate) for 24 h; the supernatant containing the lentiviral particles was collected, filtered through a 0.45-μm SFCA sterile syringe filter, and used to infect target cells. BON cells in six-well plates (5 × 105 cells/well) were incubated with the viral supernatant in the presence of Polybrene (5 μg/ml) for 24 h; cells were then incubated with growth medium for an additional 24 h. The infected cells were subcultured in 100-mm dishes in fresh medium containing puromycin (2.5 μg/ml). Puromycin-resistant cell pools were collected, and effective knockdown was monitored by Western blot analysis.
Transfection, cell treatments, and luciferase assay.
BON cells were plated in 24-well plates (n = 3) (2.4 × 105 cells/well); the next day, cells were either transfected with NT promoter (−373/+23) alone or cotransfected with NT promoter plasmids (−373, −122, or −42) and expression plasmids (c-Jun, T7-ERK1, MEK1-DD, or p70S6K) with Lipofectamine 2000 in serum-free medium. The medium was changed with growth medium 3 h after transfection. For rapamycin or torin1 treatment, cells were incubated in growth medium containing rapamycin (20 nM) or torin1 (250 nM) at varying times or increasing doses for 24 h. For combination treatment with inhibitors, cells were pretreated with PD98059 for 1 h followed by combination of PD98059 and rapamycin or torin1 for 24 h. Firefly and Renilla luciferase activities were determined in cell lysates with the Dual Luciferase Reporter Assay System as previously described (8). The relative NT promoter luciferase activity was normalized by TK-Renilla activity.
NT EIA for NT secretion and intracellular NT content.
To measure basal NT secretion, cells were plated in 24-well plates at a density of 2.4 × 105 cells/well and replaced with fresh growth medium the next day. Cells were grown for 24 h, and medium was collected and stored in −80°C for NT secretion assays. NT secretion was measured by an NT EIA kit as described previously (41, 43). Intracellular NT content was measured from the parallel cell lysates (50 μg) using the NT EIA kit. Data obtained from NT EIA assays were normalized by protein concentration from parallel cell lysates.
Protein preparation and Western blot analysis.
Protein preparation and Western blotting were performed as described previously (42, 43). In brief, the cells were lysed with lysis buffer and equal amounts of protein were resolved on NuPAGE BisTris gels and electrophoretically transferred to polyvinylidene difluoride membranes; the membranes were incubated with primary antibodies overnight at 4°C followed by secondary antibodies conjugated with horseradish peroxidase. Membranes were developed using the ECL detection system.
RNA isolation, one-step real-time RT-PCR, and Northern blot analysis.
Cells were plated in 12-well plates (n = 3) and total RNA was isolated from cells using RNeasy Kit. Real-time RT-PCR was performed as described previously (41). The target gene NTS (ID: Hs00175048_m1) was analyzed (n = 3). Reactions for each sample were performed in duplicate. For Northern blot analysis, total RNA (10 μg) was run in 1.2% agarose-formaldehyde gels and transferred to supported nitrocellulose. Membranes were hybridized to a random-primed 32P-labeled human NT cDNA probe (kindly provided by Dr. Paul Dobner). After hybridization with the GAPDH probe, a control for equality of RNA loading, membranes were washed again and signals detected by autoradiography.
Statistical analysis.
Descriptive statistics including mean and standard deviation were calculated to summarize NT secretion, intracellular NT content, mRNA levels, luciferase activity, and cell proliferation for each cell culture condition. Bar graphs were generated to represent mean (± SD) fold changes of increase or decrease in treated groups relative to control. Within each experiment, comparisons across groups were accomplished using one- or two-way analysis of variance models, and pairwise comparisons were subsequently performed using contrast statements. Adjustment in P values due to several pairwise testing within each experiment was performed using the Holm's procedure. Trend tests for dose or time comparisons were likewise performed. Normality assumptions of the parametric tests for each outcome were assessed. Adjusted P values <0.05 were considered statistically significant.
RESULTS
Inhibition of mTORC1 increases NT secretion.
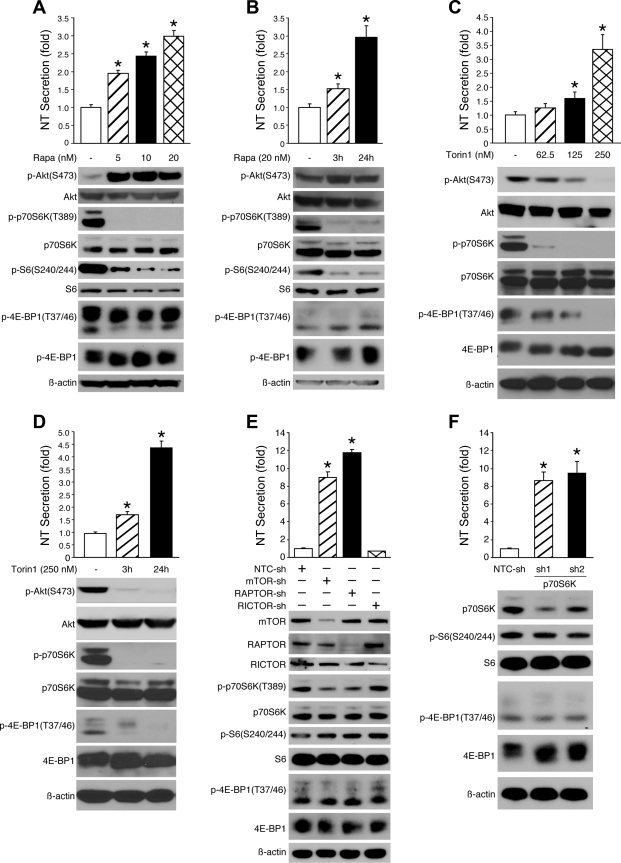
Previously, we have utilized the BON cell line as a novel endocrine cell model to study the signaling pathways involved in the regulation of agonist-stimulated (e.g., PMA and FSK) NT secretion (42, 43). In the present study, we sought to determine whether mTOR signaling mediates agonist-stimulated NT secretion. BON cells were pretreated with rapamycin for 30 min followed by stimulation with either PMA or FSK for another 30 min and then NT secretion was quantified. PMA treatment slightly increased, whereas FSK failed to affect, phosphorylation of p70S6K (T389) (data not shown). Moreover, rapamycin treatment failed to affect either PMA- or FSK-stimulated NT secretion (data not shown). We next determined whether mTOR signaling regulates constitutive (i.e., basal) NT secretion. BON cells were treated with rapamycin or torin1 for 24 h, without any stimulation, and NT release was quantitated. BON cells treated with rapamycin or torin1 were viable and demonstrated normal morphology at the dose and time course used in the study (data not shown). Surprisingly, rapamycin treatment, at all concentrations, significantly increased NT secretion (Fig. 1A, top); a 1.5-fold induction of NT secretion was noted at 3 h with an approximate 3-fold increase noted at 24 h after treatment (Fig. 1B, bottom). As shown in Fig. 1, A and B, bottom, induction of Akt (S473) phosphorylation was noted in cells treated with various doses of rapamycin (5–20 nM) for 24 h or 20 nM of rapamycin over a time course, which is consistent with other studies showing rapamycin-induced feedback activation of Akt (17); rapamycin treatment decreased p70S6K(T389) and S6(S240/244) phosphorylation, whereas phosphorylation of 4E-BP1(T37/46) was not altered, which is consistent with findings in other cell types (12). Similar to rapamycin, torin1 increased NT secretion in a dose-dependent fashion (Fig. 1C, top); NT secretion was noted to be increased at 3 and 24 h after torin1 treatment (Fig. 1D, top). As shown in Fig. 1, C and D, bottom, torin1 treatment decreased Akt (S473) phosphorylation; phosphorylation of p70S6K(T389) as well as 4E-BP1(T37/46) was also inhibited. Therefore, the results indicate that acute rapamycin treatment has no effect on agonist-stimulated NT secretion; however, prolonged inhibition of mTOR signaling increases basal NT release.
Fig. 1.
Inhibition of mammalian target of rapamycin (mTOR)C1 signaling increased neurotensin (NT) secretion. A and B: BON cells were treated with rapamycin (Rapa) at various doses for 24 h (A) or with 20 nM rapamycin over a time course (0, 3, and 24 h) (B); medium was collected for basal NT release measurements (top) (*P < 0.05 vs. vehicle). Cells were lysed for Western blot analysis (bottom). C and D: BON cells were treated with torin1 at different doses (C) or 250 nM torin1 over a time course (D); NT secretion was measured (top) (*P < 0.05 vs. vehicle). Cells were lysed and Western blot was performed (bottom). E and F: NT enzyme immunoassay (EIA) assays were performed to measure the basal NT secretion from stable cell lines with suppression of mTOR, regulatory associated protein of mTOR (RAPTOR), and mTOR rapamycin-insensitive companion (RICTOR) or p70S6 kinase (p70S6K) by short hairpin RNA (shRNA) (top) (*P < 0.05 vs. NTC-sh); Western blots show the suppression of mTOR, RAPTOR, RICTOR, and p70S6K expression and phosphorylation of the downstream effectors (bottom).
To further define the role of the mTOR complexes on NT secretion, we generated stable BON cell lines expressing shRNA targeting mTOR, RAPTOR, or RICTOR. NT secretion was significantly increased in cells expressing either mTOR or RAPTOR shRNA but not RICTOR shRNA (Fig. 1E, top), demonstrating that mTORC1, but not mTORC2, mediates the effect of mTOR inhibition-induced NT release, which is consistent with the effects of rapamycin. As shown in Fig. 1E, bottom, Western blot analysis confirmed the decreases of mTOR, RAPTOR, and RICTOR protein expression; inhibition of p70S6K(T389) phosphorylation was noted in cells expressing mTOR or RAPTOR shRNA; surprisingly, phosphorylation of S6 (S240/244) and 4E-BP1(T37/46) was not altered by knockdown of mTOR or RAPTOR. The finding that inhibition of mTORC1 increased NT secretion, which is inconsistent with the well-known mTORC1 function in the positive regulation of mRNA translation and protein synthesis, suggested that mTORC1-mediated translation and protein synthesis are not a predominant mechanism contributing to the increased NT secretion.
To confirm whether p70S6K is involved in NT secretion downstream of mTORC1, we next established p70S6K knockdown BON cell lines by stable expression of p70S6K shRNA; two stable cell lines with separate p70S6K shRNA sequences (sh1 and sh2) were selected. p70S6K knockdown significantly increased NT secretion as noted in Fig. 1F (top); knockdown of p70S6K is demonstrated in the two stable cell lines by Western blot (Fig. 1F, bottom). Again, neither S6 nor 4E-BP1 phosphorylation was significantly altered. These results further demonstrate that inhibition of mTORC1 increases NT secretion; however, mTORC1/p70S6K-mediated effects on translation and protein synthesis do not appear to be involved in this process, suggesting that the increase of NT secretion by mTORC1 inhibition is via a mechanism independent of mRNA translation.
mTORC1 suppression increases NT mRNA expression and intracellular NT content.
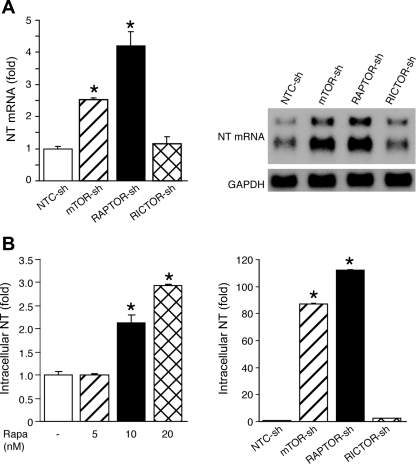
mTOR signaling has been implicated in the regulation of gene expression (either increase or decrease) (54, 67). Therefore, we hypothesized that NT secretion was mediated by mTORC1 at the transcriptional level. To test this hypothesis, we first analyzed NT mRNA levels. NT mRNA expression was enhanced by knockdown of mTOR or RAPTOR, but not RICTOR, as confirmed by real time PCR (Fig. 2A, left) and by Northern blot analysis (Fig. 2A, right). We next examined the intracellular NT content by EIA. Rapamycin treatment (Fig. 2B, left) and knockdown of mTOR or RAPTOR (Fig. 2B, right) increased intracellular NT content in BON cells. In addition, neither NT mRNA nor intracellular NT levels were altered in cells with either stable knockdown of Akt1 or Akt2 or overexpression of Akt1 (data not shown), suggesting that the feedback induction of Akt by mTORC1 inhibition is not involved in NT secretion. Collectively, inhibition of mTORC1 increases NT mRNA expression; therefore, the induction of NT release results from increased NT mRNA and intracellular NT protein content.
Fig. 2.
Inhibition of mTORC1 increased NT mRNA and intracellular NT content. A: total RNA was isolated from cells stably expressing NTC-sh, mTOR-sh, RAPTOR-sh, and RICTOR-sh. Real-time PCR was performed to quantitate NT mRNA expression (left) (*P < 0.05 vs. NTC-sh); Northern blot was performed to further confirm increased NT mRNA expression (right). B: BON cells were treated with various doses of Rapa (0, 5, 10, and 20 nM) for 24 h; cells were lysed and 50 μg protein was used for NT EIA to measure intracellular NT content (left) (*P < 0.05 vs. vehicle). Proteins were isolated from cells stably expressing NTC-sh, mTOR-sh, RAPTOR-sh, and RICTOR-sh. Proteins (50 μg) were used to measure intracellular NT content (right) (*P < 0.05 vs. NTC-sh).
mTORC1 inhibition induces activation of AP-1/c-jun and ERK1/2.
The activator protein 1 (AP-1) is a family of transcription factors that form homodimers or heterodimers composed of the Fos, Jun, and ATF proteins (2). The Jun proteins c-Jun, JunB, and JunD are core members of AP-1 proteins with c-Jun being the major component (36). We and others have reported that the proximal NT promoter region contains an AP-1/cAMP response element (CRE) binding site that binds Jun family (c-Jun and JunD) and ATF/CREB proteins and is critical for constitutive NT expression (24, 37). mTOR has been implicated in the regulation of Jun proteins (62). Therefore, we next screened the expression of Jun family proteins and c-Fos in BON cells with mTORC1 inhibition. As shown in Fig. 3A, p70S6K inhibition increased c-Jun (S63) phosphorylation as well as the total c-Jun expression; however, the alteration of JunB, JunD, and c-Fos expression was not noted. In addition, we also found that the phosphorylation of ERK1/2, a well-known upstream regulator of AP-1 proteins (35), was increased in p70S6K knockdown cells.
Fig. 3.
Inhibition of mTORC1 increased phosphorylation of c-Jun and ERK1/2 in BON cells. A: proteins were isolated from BON cells with stable p70S6K knockdown and Western blot performed. B and C: BON cells were treated with Rapa at different concentrations for 24 h or 20 nM for different time course (B) or various doses of torin1 for 24 h (C); Western blot was performed (left). The density was quantified from 3 experiments (right). D: BON cells were treated with torin1 at various doses for 24 h and Western blot performed. Density was quantified from 3 experiments.
We next examined the correlation of ERK and c-Jun phosphorylation in BON cells treated with rapamycin or torin1. As shown in Fig. 3B, left, rapamycin treatment induced both phosphorylated and total c-Jun expression in a time- and dose-dependent fashion; ERK1/2 phosphorylation was also increased by rapamycin treatment. Consistent with these results, torin1 treatment increased c-Jun phosphorylation and total protein expression as well as ERK1/2 phosphorylation in a dose-dependent manner (Fig. 3C). Consistent with our results, it has been reported that the activation of c-Jun, after phosphorylation by mitogen-activated protein kinase (MAPK), is accompanied by a reduction in c-Jun ubiquitination and consequent stabilization of the protein, causing an increase in total protein (49). The density of the phosphorylated and total c-Jun bands in Fig. 3, B–D, was quantified from three experiments (right). It was demonstrated that the mTOR-induced feedback activation of ERK is through p70S6K-PI3K-Ras pathways and appears to be independent of Akt (9); preincubation with wortmannin, an irreversible inhibitor of PI3K, for 24 h attenuated rapamycin-induced ERK activation. To test this in our system, we pretreated BON cells with wortmannin (100 nM) for 30 min followed by combination treatment with rapamycin and wortmannin for 24 h. We found that rapamycin-induced ERK phosphorylation was not altered in BON cells treated with wortmannin (data not shown), suggesting that rapamycin-induced ERK phosphorylation is independent of PI3K in BON cells. However, the mechanisms responsible for this induction, mediated by mTORC1 inhibition, require further investigation. Taken together, c-Jun and ERK1/2 activity was increased in BON cells in the presence of rapamycin or torin1. These results suggest that ERK/c-Jun signaling plays a role in the mTORC1-regulated NT gene expression in BON cells.
mTORC1 inhibition increases c-jun-mediated NT gene promoter activity.
We further determined whether c-Jun regulates NT promoter activity by luciferase assay. A human NT promoter construct (−373/+23), which contains the proximal AP-1/CRE site and more distal CRE and AP-1 sites (4), was used for these studies. Previously, we have shown that this region is sufficient to direct high-level, constitutive NT expression in BON cells (15). BON cells were cotransfected with the NT promoter and the c-Jun wild-type or the dominant negative mutant (Tam67). Compared with the control vector, wild-type c-Jun dramatically increased NT promoter activity; however, Tam67 cotransfection resulted in a slight, but not significant, decrease in NT promoter activity (Fig. 4A, left). We also cotransfected the NT promoter, with increasing concentrations of the wild-type c-Jun plasmid (0.1, 0.2, and 0.4 μg) and repeated the luciferase assays. Increased NT promoter activity was noted in a dose-dependent fashion (Fig. 4A, right).
Fig. 4.
mTORC1 mediated c-Jun-regulated NT promoter activity. A: BON cells were cotransfected with NT −373/+23 promoter, and the wild-type c-Jun or the dominant negative c-Jun (Tam67) and luciferase assays were performed (left) (*P < 0.05 vs. vector); BON cells were cotransfected with the NT −373/+23 promoter and various doses of c-Jun (0.1, 0.2 and 0.4 μg) and luciferase assays performed (right) (*P < 0.05 vs. vector). B: BON cells were cotransfected with c-Jun and the NT promoter (−42, −122, and −373) and the control vector pXP1; luciferase assays performed (*P < 0.05 vs. pXP1 alone; †P < 0.05 vs. −373 alone; ‡P < 0.05 vs. −122 alone). C: BON cells were cotransfected with the NT −373/+23 promoter and the wild-type p70S6K, and the active mutant p70S6K/T389E and luciferase assays were performed (left) (*P < 0.05 vs. vector); cotransfection of NT promoter-373 and c-Jun plasmids in BON cells with p70S6K knockdown and luciferase assay performed (right) (*P < 0.05 vs. NTC-sh). D: BON cells were transfected with pJC6-GL3 (c-Jun promoter) and treated with rapamycin for 24 h; luciferase assay was performed (*P < 0.05 vs. vector alone; †P < 0.05 vs. vector plus Rap). E: transfection of pJC6-GL3 in p70S6K knockdown cells and luciferase assay performed (left) (*P < 0.05 vs. NTC-sh); cotransfection of pJC6-GL3 with wild-type p70S6K or the control vector and luciferase assay performed (right) (*P < 0.05 vs. control vector).
To determine whether the proximal AP-1/CRE site or the more distal CRE or AP-1 site is involved in the effects of c-Jun in increasing NT promoter activity, deletion plasmids of NT-122, which only contains the proximal AP-1/CRE site, and NT-42, which contains the NT TATA box, were cotransfected with c-Jun. As shown in Fig. 4B, a similar high-level NT promoter induction with c-Jun cotransfection was noted for the −373 or −122 promoter fragments compared with the promoterless control vector pXP1; NT promoter induction was not noted using the −42 minimal promoter. This result indicates that c-Jun is acting through the proximal AP-1/CRE site to regulate NT expression. Conversely, overexpression of both the wild-type and active p70S6K-T389E decreased NT promoter activity (Fig. 4C, left). c-Jun-enhanced NT promoter activity was further increased in cells with p70S6K shRNA knockdown (Fig. 4C, right). These findings demonstrate that c-Jun plays an important positive role in the regulation of NT gene expression downstream of the mTORC1/p70S6K signaling pathway.
We next determined whether mTORC1 regulates c-Jun promoter activity. BON cells were transfected with the c-Jun promoter plasmid (pJC-GL3) and treated with rapamycin. c-Jun promoter activity was increased by rapamycin treatment (Fig. 4D). Furthermore, p70S6K knockdown increased c-Jun promoter activity (Fig. 4E, left); overexpression of p70S6K decreased c-Jun promoter activity (Fig. 4E, right). All together, these results demonstrate that inhibition of mTORC1 increased NT gene expression through upregulation of c-Jun protein activity.
MEK1/ERK1/2 mediates c-jun-regulated NT gene promoter activity downstream of mTORC1.
We next determined whether ERK1/2 is functionally involved in c-Jun-regulated NT gene expression. We found that both phosphorylated and total c-Jun expression was upregulated by ERK1 overexpression (Fig. 5A, left). Overexpresssion of MEK1-DD, a constitutively active form of MEK1, also increased both phosphorylated and total c-Jun protein levels (Fig. 5A, right). Furthermore, ERK1 overexpression increased NT secretion (Fig. 5B). PD98059 treatment significantly attenuated NT secretion resulting from rapamycin or torin1 treatment (Fig. 5C, top). Induction of c-Jun and ERK1/2 expression by rapamycin or torin1 was inhibited by PD98059 (Fig. 5C, bottom). Consistently, overexpression of various concentrations of ERK1 plasmid (0.1, 0.2, and 0.4 μg) increased NT promoter activity in a dose-dependent fashion (Fig. 5D). Importantly, the induction of NT promoter activity by c-Jun was blocked by PD98059, an MEK inhibitor (Fig. 5E). In contrast, NT promoter induction was not blocked by either JNK II (a JNK/MAPK inhibitor) or SB 203580 (a p38/MAPK inhibitor) (data not shown). These results further demonstrate that mTORC1/p70S6K regulates NT gene expression via the MEK/ERK/c-Jun signaling pathway in BON cells.
Fig. 5.
Mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK)-mediated c-Jun-regulated NT promoter activity. A: BON cells were transiently transfected with T7-ERK1 (left) or MEK1-DD (right) plasmids, and Western blot was performed. B: BON cells were transiently transfected with T7-ERK1 and NT EIA performed. C: cells were pretreated with PD98059 (10 μM) for 1 h followed by combination treatment with rapamycin or torin1 for 24 h; NT secretion was measured by NT EIA (*P < 0.05 vs. DMSO; †P < 0.05 vs. Rapa alone; ‡P < 0.05 vs. torin1 alone). D: BON cells were cotransfected with the NT −373/+23 promoter and increasing doses of T7-ERK1; luciferase assays were performed (*P < 0.05 vs. vector). E: BON cells were cotransfected with the NT −373/+23 promoter and c-Jun as well as the control vector; cells were treated with PD98059 (10 μM) for 24 h and luciferase assays performed (*P < 0.05 vs. vector; †P < 0.05 vs. c-Jun only).
mTORC1 signaling regulates NT gene expression in Hep3B human hepatoma cells.
Our previous studies showed that the NT gene is abundantly expressed in a human hepatoma cell line Hep3B. Hep3B cells express the NT gene at levels comparable to BON cells (15). To further establish the effects of mTORC1 signaling on NT gene expression, we treated Hep3B cells with rapamycin (20 nM) or torin1 (250 nM) for 24 h; real-time PCR was performed. Consistent with the findings in BON cells, both rapamycin and torin1 treatment increased NT mRNA expression approximately two- and threefold, respectively (Fig. 6A). Importantly, expression of both phosphorylated and total c-Jun was increased by rapamycin and torin1 treatment in a time-dependent fashion (Fig. 6B). Induction of c-Jun and ERK1/2 phosphorylation was also noted in Hep3B cells treated with rapamycin and torin1 at various concentrations (Fig. 6C). These results further demonstrate that mTORC1 inhibition upregulates NT gene expression and that the ERK/c-Jun signaling pathway is downstream of mTORC1.
Fig. 6.
Rapamycin and torin1 increased NT mRNA and intracellular content in Hep3B human hepatoma cells. A: Hep3B cells were plated in 24-well plates and treated with Rapa (20 nM) or torin1 (250 nM) for 24 h (n = 2); total RNA was extracted and real time PCR performed using triplicate samples (*P < 0.05 vs. vehicle). B: Hep3B cells were treated with Rapa (20 nM) or torin1 (250 nM) over a time course, and Western blot was performed. C: Hep3B cells were treated with various doses of Rapa (left) or torin1 (right) for 24 h, and Western blot was performed.
mTORC1 plays a negative role in nutrient-regulated NT secretion.
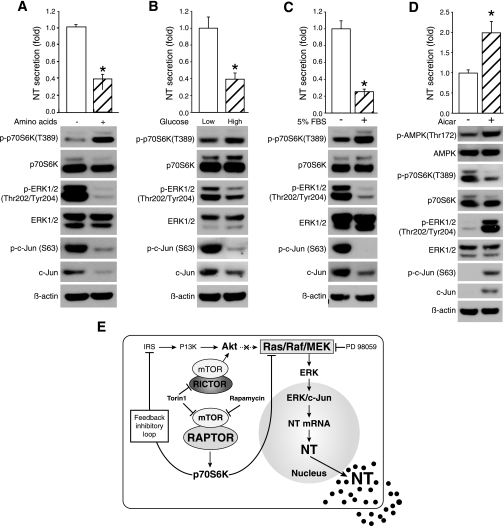
It is known that mTOR is activated by nutrients or serum (60). Furthermore, nutrients are well-known regulators of intestinal hormone secretion (31, 50). Consistently, NT secretion was decreased in BON cells after readdition of amino acids (normal DMEM/F12 medium without serum) compared with the group without amino acids (DMEM/F12 medium without amino acids) (Fig. 7A, top). Compared with low glucose, NT release was inhibited from cells by high glucose treatment (Fig. 7B, top). Phosphorylation of p70S6K (T389) was increased, whereas phosphorylation of ERK1/2 (The202/Tyr204) and c-Jun (S63) was decreased by both amino acids and high glucose (Fig. 7, A and B, bottom, respectively). Furthermore, NT secretion was decreased from BON cells in growth medium (5% FBS) compared with that in serum-free medium (Fig. 7C, top). Phosphorylation of p70S6K (T389) was increased by 5% FBS; conversely, phosphorylation of ERK1/2 (The202/Tyr204) and c-Jun (S63) was inhibited by 5% FBS (Fig. 7C, bottom).
Fig. 7.
Nutrients negatively regulate NT release. A: BON cells were incubated in medium without amino acids for 30 min and then treated with medium with or without amino acids for 1 h; medium was collected and NT EIA performed (top) (*P < 0.05 vs. amino acid free); cells were lysed for Western blot (bottom). B: BON cells were treated with low or high glucose in the absence of FBS for 24 h; medium was collected for NT EIA (top) (*P < 0.05 vs. low glucose), and cells were lysed for Western blot (bottom). C: BON cells were serum-starved overnight; cells were incubated with fresh medium with or without serum (5% FBS) for 1 h; medium was collected and NT EIA performed (top) (*P < 0.05 vs. serum free); cells were lysed, and Western blot was performed (bottom). D: BON cells were serum-starved overnight and treated with or without AICAR (1 mM) for 1 h in the absence of serum; medium was collected and cells lysed for NT EIA (*P < 0.05 vs. vehicle) and Western blot, respectively. E: proposed schematic of mTORC1/p70S6K/MEK/ERK/c-Jun signaling pathway in the control of NT gene expression and secretion in BON cells.
The AMP-activated protein kinase (AMPK) and mTOR are key regulators of cellular energy balance. AMPK responds to energy stress by suppressing cell growth and biosynthetic processes, in part through its inhibition of the mTORC1 pathway (32). Stimulation of AMPK kinase activity using 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), a pharmacological activator of AMPK, inhibits mTOR signaling (30). Consistently, AICAR treatment increased NT secretion (Fig. 7D, top) and concurrently the phosphorylation of AMPK (S179), ERK1/2 (The202/Tyr204), and c-Jun (S63) but decreased phosphorylation of p70S6K (T389) (Fig. 7D, bottom). Taken together, mTOR signaling plays a negative role in nutrient- or energy-regulated NT secretion through inhibiting ERK/c-Jun pathway.
DISCUSSION
The regulation of mTOR signaling by nutrients, which are mediators of intestinal peptide secretion, suggests a central role for mTOR and its component proteins in the control of basal and stimulated peptide release. Here, we examined the role of mTOR signaling in the regulation of NT peptide secretion and gene expression. We show that: 1) inhibition of mTORC1 signaling increased NT secretion in BON cells; 2) mTORC1 inhibition enhanced NT mRNA expression in both BON and Hep3B cells; and 3) mTORC1 regulates NT gene expression via MEK/ERK/c-Jun signaling.
mTORC1 is known to increase mRNA translation and protein synthesis via the two downstream substrates p70S6K and 4E-BP1 (46). mTOR is likely involved in the regulation of peptide release from certain endocrine cells by increasing protein synthesis (70). Support of this notion is provided by findings that leucine supplementation augments pancreatic β-cell function and insulin secretion in malnourished rats, which is associated with increased PI3K and mTOR protein contents, suggesting that activation of the PI3K/mTOR pathway may play a role in this process (25). In our previous studies, we found that acute treatment of PMA or FSK stimulated NT secretion via PKC- or cAMP-mediated signaling pathways from the BON endocrine cell line (41–44). However, we failed to detect any effects of rapamycin treatment (30 min) on the PMA- or FSK-stimulated NT secretion, suggesting that mTOR signaling is not involved in PKC- or cAMP-mediated NT release. Surprisingly, we found that inhibition of mTORC1/p70S6K significantly increased constitutive (unstimulated) NT secretion, demonstrating that mTOR signaling plays a negative role in the control of NT release. These results also demonstrate that different mechanisms are involved in the PMA- or FSK-stimulated and mTORC1 inhibition-induced NT secretion. Secretion stimulated by agonists is usually mediated by PKC or cAMP, which affects rapid Ca2+ flux or vesicle transport. The constitutive secretion is mediated by gene regulation. These results suggest that a unique mechanism is involved in the effects of mTOR/p70S6K. The finding that neither S6 nor 4E-BP1 was involved in this process further indicates that the role of mTOR/p70S6K in the regulation of NT secretion is translation independent.
Utilizing a combination of chemical inhibitors (rapamycin and torin1) or shRNA-mediated knockdown, we demonstrate that inhibition of mTORC1 promotes NT secretion by increasing NT mRNA expression. Similar to our findings, Xu et al. (68, 69) reported that intraperitoneal injection of rapamycin in C57BL/6J mice significantly increased gastric ghrelin mRNA expression, preproghrelin levels, and circulating ghrelin compared with control mice. Shimizu et al. (61) noted that rapamycin treatment increased neuropeptide Y gene expression in hypothalamic organotypic cultures. TOR signaling has been implicated in the regulation (either increase or decrease) of gene expression in yeast and mammalian cells. DNA microarray analysis revealed that rapamycin treatment increased the expression of ∼150 genes, whereas another 150 genes were decreased in yeast (66). Using transcriptional profiling, Peng et al. (54) showed that, in human BJAB B-lymphoma cells and murine CTLL-2 T lymphocytes, rapamycin treatment either increased or decreased the expression of numerous genes involved in nutrient and protein metabolism.
Multiple elements within the NT promoter contribute to basal, as well as stimulated NT gene expression, including a proximal AP-1/CRE-like motif, and a more distal AP-1 consensus binding site (24). In the present study, we demonstrate that overexpression of c-Jun acting through the proximal AP-1/CRE site dramatically increased NT promoter activity in BON cells; inhibition of mTORC1 increased NT gene expression by upregulating c-Jun expression. Osteopontin (OPN), a member of small integrin binding ligand N-linked glycoprotein family, regulates tumor progression through activation of various transcription factors and expression of their downstream effector gene(s) in breast cancer (1). Overexpression of mTOR inhibits OPN-induced AP-1-DNA binding and transcriptional activity; rapamycin treatment enhanced OPN-induced effects (1). Reversely, Staber et al. (62) showed that the inhibition of mTOR downregulates JunB protein levels by translational control in NPM-ALK-positive lymphoma cells. Together, these results demonstrate that inhibition of mTOR signaling can regulate transcription factor expression in either a positive or negative fashion.
The regulation of AP-1 proteins by the MEK/ERK pathway has been well characterized (35). In our present study, we found that overexpression of ERK1 and MEK1-DD increased both c-Jun phosphorylation and total protein expression, which is consistent with previous studies (49). Also, overexpression of ERK1 significantly increased NT promoter activity and NT secretion, demonstrating the importance of ERK signaling in the regulation of constitutive NT release. Importantly, c-Jun-mediated NT promoter induction and rapamycin- and torin1-increased NT secretion were blocked by PD98059 treatment. Therefore, these findings further demonstrate the interaction of mTOR/p70S6K and ERK/AP-1 signaling pathways in NT gene regulation. The peptidyl-prolyl cis-trans isomerase Pin1 interacts with p70S6K and enhances insulin-induced ERK1/2 phosphorylation and AP-1 activity, whereas the inhibition of p70S6K activity by rapamycin suppressed insulin-induced ERK1/2 phosphorylation and AP-1 activity in SK-HEP-1 cells, a human hepatocellular carcinoma cell line (39).
In summary, we found that inhibition of mTORC1 signaling by rapamycin, torin1, and shRNA-mediated knockdown enhances NT secretion by increasing NT gene expression in the endocrine cell line BON (Fig. 7E). We demonstrate that mTORC1 inhibition induced feedback activation of ERK/c-Jun that plays a positive role in the regulation of NT gene expression. Importantly, the physiological evidence showing that NT secretion was decreased by amino acids, high glucose, or serum and the simultaneous activation of mTOR signaling and inhibition of ERK/c-Jun further support the importance of the interaction of mTORC1 and MEK/ERK/c-Jun signaling pathways in the control of nutrient-regulated NT release.
GRANTS
This work was supported by National Institutes of Health Grants 2R37-AG10885, R01-DK48489, P01-DK35608, and K01-CA10209 and 5R01-CA133429-03 (to T. Gao).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Donna A. Gilbreath, Jennifer Rogers, and Nathan L. Vanderford for manuscript preparation and Yimei Han for assistance with statistical analyses.
REFERENCES
- 1. Ahmed M, Kundu GC. Osteopontin selectively regulates p70S6K/mTOR phosphorylation leading to NF-kappaB dependent AP-1-mediated ICAM-1 expression in breast cancer cells. Mol Cancer 9: 101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta 1072: 129–157, 1991 [DOI] [PubMed] [Google Scholar]
- 3. Anthony JC, Anthony TG, Kimball SR, Jefferson LS. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr 131: 856S–860S, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Bean AJ, Dagerlind A, Hokfelt T, Dobner PR. Cloning of human neurotensin/neuromedin N genomic sequences and expression in the ventral mesencephalon of schizophrenics and age/sex matched controls. Neuroscience 50: 259–268, 1992 [DOI] [PubMed] [Google Scholar]
- 5. Briaud I, Dickson LM, Lingohr MK, McCuaig JF, Lawrence JC, Rhodes CJ. Insulin receptor substrate-2 proteasomal degradation mediated by a mammalian target of rapamycin (mTOR)-induced negative feedback down-regulates protein kinase B-mediated signaling pathway in beta-cells. J Biol Chem 280: 2282–2293, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Brunn GJ, Hudson CC, Sekulic A, Williams JM, Hosoi H, Houghton PJ, Lawrence JC, Jr, Abraham RT. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 277: 99–101, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Buller CL, Loberg RD, Fan MH, Zhu Q, Park JL, Vesely E, Inoki K, Guan KL, Brosius FC., 3rd A GSK-3/TSC2/mTOR pathway regulates glucose uptake and GLUT1 glucose transporter expression. Am J Physiol Cell Physiol 295: C836–C843, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cai Q, Li J, Gao T, Xie J, Evers BM. Protein kinase Cdelta negatively regulates hedgehog signaling by inhibition of Gli1 activity. J Biol Chem 284: 2150–2158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest 118: 3065–3074, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carraway RE, Mitra SP, Evers BM, Townsend CM., Jr BON cells display the intestinal pattern of neurotensin/neuromedin N precursor processing. Regul Pept 53: 17–29, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Chen EJ, Kaiser CA. LST8 negatively regulates amino acid biosynthesis as a component of the TOR pathway. J Cell Biol 161: 333–347, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci USA 105: 17414–17419, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dai R, Chen R, Li H. Cross-talk between PI3K/Akt and MEK/ERK pathways mediates endoplasmic reticulum stress-induced cell cycle progression and cell death in human hepatocellular carcinoma cells. Int J Oncol 34: 1749–1757, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene 26: 3279–3290, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Dong Z, Wang X, Zhao Q, Townsend CM, Jr, Evers BM. DNA methylation contributes to expression of the human neurotensin/neuromedin N gene. Am J Physiol Gastrointest Liver Physiol 274: G535–G543, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Drenan RM, Liu X, Bertram PG, Zheng XF. FKBP12-rapamycin-associated protein or mammalian target of rapamycin (FRAP/mTOR) localization in the endoplasmic reticulum and the Golgi apparatus. J Biol Chem 279: 772–778, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Easton JB, Kurmasheva RT, Houghton PJ. IRS-1: auditing the effectiveness of mTOR inhibitors. Cancer Cell 9: 153–155, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Edinger AL, Linardic CM, Chiang GG, Thompson CB, Abraham RT. Differential effects of rapamycin on mammalian target of rapamycin signaling functions in mammalian cells. Cancer Res 63: 8451–8460, 2003 [PubMed] [Google Scholar]
- 19. Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell 13: 2276–2288, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 9: 550–562, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Evers BM. Endocrine gene neurotensin: molecular mechanisms and a model of intestinal differentiation. World J Surg 26: 799–805, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Evers BM. Neurotensin and growth of normal and neoplastic tissues. Peptides 27: 2424–2433, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Evers BM, Townsend CM, Jr, Upp JR, Allen E, Hurlbut SC, Kim SW, Rajaraman S, Singh P, Reubi JC, Thompson JC. Establishment and characterization of a human carcinoid in nude mice and effect of various agents on tumor growth. Gastroenterology 101: 303–311, 1991 [DOI] [PubMed] [Google Scholar]
- 24. Evers BM, Wang X, Zhou Z, Townsend CM, Jr, McNeil GP, Dobner PR. Characterization of promoter elements required for cell-specific expression of the neurotensin/neuromedin N gene in a human endocrine cell line. Mol Cell Biol 15: 3870–3881, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Filiputti E, Rafacho A, Araujo EP, Silveira LR, Trevisan A, Batista TM, Curi R, Velloso LA, Quesada I, Boschero AC, Carneiro EM. Augmentation of insulin secretion by leucine supplementation in malnourished rats: possible involvement of the phosphatidylinositol 3-phosphate kinase/mammalian target protein of rapamycin pathway. Metabolism 59: 635–644, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Grant S. Cotargeting survival signaling pathways in cancer. J Clin Invest 118: 3003–3006, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guan KL, Figueroa C, Brtva TR, Zhu T, Taylor J, Barber TD, Vojtek AB. Negative regulation of the serine/threonine kinase B-Raf by Akt. J Biol Chem 275: 27354–27359, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 12: 9–22, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell 11: 859–871, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Guo D, Hildebrandt IJ, Prins RM, Soto H, Mazzotta MM, Dang J, Czernin J, Shyy JY, Watson AD, Phelps M, Radu CG, Cloughesy TF, Mischel PS. The AMPK agonist AICAR inhibits the growth of EGFRvIII-expressing glioblastomas by inhibiting lipogenesis. Proc Natl Acad Sci USA 106: 12932–12937, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gutierrez-Aguilar R, Woods SC. Nutrition and L and K-enteroendocrine cells. Curr Opin Endocrinol Diabetes Obes 18: 35–41, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haruta T, Uno T, Kawahara J, Takano A, Egawa K, Sharma PM, Olefsky JM, Kobayashi M. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol 14: 783–794, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Hennig KM, Colombani J, Neufeld TP. TOR coordinates bulk and targeted endocytosis in the Drosophila melanogaster fat body to regulate cell growth. J Cell Biol 173: 963–974, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 270: 16483–16486, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol 9: 240–246, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Kislauskis E, Dobner PR. Mutually dependent response elements in the cis-regulatory region of the neurotensin/neuromedin N gene integrate environmental stimuli in PC12 cells. Neuron 4: 783–795, 1990 [DOI] [PubMed] [Google Scholar]
- 38. Kunz J, Schneider U, Howald I, Schmidt A, Hall MN. HEAT repeats mediate plasma membrane localization of Tor2p in yeast. J Biol Chem 275: 37011–37020, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Lee NY, Choi HK, Shim JH, Kang KW, Dong Z, Choi HS. The prolyl isomerase Pin1 interacts with a ribosomal protein S6 kinase to enhance insulin-induced AP-1 activity and cellular transformation. Carcinogenesis 30: 671–681, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Leibowitz G, Cerasi E, Ketzinel-Gilad M. The role of mTOR in the adaptation and failure of beta-cells in type 2 diabetes. Diabetes Obes Metab 10, Suppl 4: 157–169, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Li J, Chen LA, Townsend CM, Jr, Evers BM. PKD1, PKD2, and their substrate Kidins220 regulate neurotensin secretion in the BON human endocrine cell line. J Biol Chem 283: 2614–2621, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li J, Hellmich MR, Greeley GH, Jr, Townsend CM, Jr, Evers BM. Phorbol ester-mediated neurotensin secretion is dependent on the PKC-α and -δ isoforms. Am J Physiol Gastrointest Liver Physiol 283: G1197–G1206, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Li J, O'Connor KL, Cheng X, Mei FC, Uchida T, Townsend CM, Jr, Evers BM. Cyclic adenosine 5′-monophosphate-stimulated neurotensin secretion is mediated through Rap1 downstream of both Epac and protein kinase A signaling pathways. Mol Endocrinol 21: 159–171, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Li J, O'Connor KL, Greeley GH, Jr, Blackshear PJ, Townsend CM, Jr, Evers BM. Myristoylated alanine-rich C kinase substrate-mediated neurotensin release via protein kinase C-delta downstream of the Rho/ROK pathway. J Biol Chem 280: 8351–8357, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Liu X, Zheng XF. Endoplasmic reticulum and Golgi localization sequences for mammalian target of rapamycin. Mol Biol Cell 18: 1073–1082, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10: 307–318, 2009 [DOI] [PubMed] [Google Scholar]
- 47. Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene 25: 6416–6422, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. Regulation of Raf-Akt Cross-talk. J Biol Chem 277: 31099–31106, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Musti AM, Treier M, Bohmann D. Reduced ubiquitin-dependent degradation of c-Jun after phosphorylation by MAP kinases. Science 275: 400–402, 1997 [DOI] [PubMed] [Google Scholar]
- 50. Newsholme P, Gaudel C, McClenaghan NH. Nutrient regulation of insulin secretion and beta-cell functional integrity. Adv Exp Med Biol 654: 91–114, 2010 [DOI] [PubMed] [Google Scholar]
- 51. O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66: 1500–1508, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Parekh D, Ishizuka J, Townsend CM, Jr, Haber B, Beauchamp RD, Karp G, Kim SW, Rajaraman S, Greeley G, Jr, Thompson JC. Characterization of a human pancreatic carcinoid in vitro: morphology, amine and peptide storage, and secretion. Pancreas 9: 83–90, 1994 [DOI] [PubMed] [Google Scholar]
- 53. Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature 436: 78–86, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Peng T, Golub TR, Sabatini DM. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol 22: 5575–5584, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Reusch HP, Zimmermann S, Schaefer M, Paul M, Moelling K. Regulation of Raf by Akt controls growth and differentiation in vascular smooth muscle cells. J Biol Chem 276: 33630–33637, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Robinson KA, Buse MG. Mechanisms of high-glucose/insulin-mediated desensitization of acute insulin-stimulated glucose transport and Akt activation. Am J Physiol Endocrinol Metab 294: E870–E881, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science 286: 1738–1741, 1999 [DOI] [PubMed] [Google Scholar]
- 58. Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14: 1296–1302, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol 17: 596–603, 2005 [DOI] [PubMed] [Google Scholar]
- 60. Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40: 310–322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shimizu H, Arima H, Ozawa Y, Watanabe M, Banno R, Sugimura Y, Ozaki N, Nagasaki H, Oiso Y. Glucocorticoids increase NPY gene expression in the arcuate nucleus by inhibiting mTOR signaling in rat hypothalamic organotypic cultures. Peptides 31: 145–149, 2010 [DOI] [PubMed] [Google Scholar]
- 62. Staber PB, Vesely P, Haq N, Ott RG, Funato K, Bambach I, Fuchs C, Schauer S, Linkesch W, Hrzenjak A, Dirks WG, Sexl V, Bergler H, Kadin ME, Sternberg DW, Kenner L, Hoefler G. The oncoprotein NPM-ALK of anaplastic large-cell lymphoma induces JUNB transcription via ERK1/2 and JunB translation via mTOR signaling. Blood 110: 3374–3383, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res 65: 7052–7058, 2005 [DOI] [PubMed] [Google Scholar]
- 64. Takano A, Usui I, Haruta T, Kawahara J, Uno T, Iwata M, Kobayashi M. Mammalian target of rapamycin pathway regulates insulin signaling via subcellular redistribution of insulin receptor substrate 1 and integrates nutritional signals and metabolic signals of insulin. Mol Cell Biol 21: 5050–5062, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 284: 8023–8032, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tsang CK, Zheng XFTORin(g) the nucleus. Cell Cycle 6: 25–29, 2007 [DOI] [PubMed] [Google Scholar]
- 67. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 124: 471–484, 2006 [DOI] [PubMed] [Google Scholar]
- 68. Xu G, Li Y, An W, Li S, Guan Y, Wang N, Tang C, Wang X, Zhu Y, Li X, Mulholland MW, Zhang W. Gastric mammalian target of rapamycin signaling regulates ghrelin production and food intake. Endocrinology 150: 3637–3644, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xu G, Li Y, An W, Zhao J, Xiang X, Ding L, Li Z, Guan Y, Wang X, Tang C, Zhu Y, Wang N, Li X, Mulholland M, Zhang W. Regulation of gastric hormones by systemic rapamycin. Peptides 31: 2185–2192, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yang J, Chi Y, Burkhardt BR, Guan Y, Wolf BA. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr Rev 68: 270–279, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 286: 1741–1744, 1999 [DOI] [PubMed] [Google Scholar]