Abstract
Ingestion of high-fat, high-calorie diets is associated with hyperphagia, increased body fat, and obesity. The mechanisms responsible are currently unclear; however, altered leptin signaling may be an important factor. Vagal afferent neurons (VAN) integrate signals from the gut in response to ingestion of nutrients and express leptin receptors. Therefore, we tested the hypothesis that leptin resistance occurs in VAN in response to a high-fat diet. Sprague-Dawley rats, which exhibit a bimodal distribution of body weight gain, were used after ingestion of a high-fat diet for 8 wk. Body weight, food intake, and plasma leptin levels were measured. Leptin signaling was determined by immunohistochemical localization of phosphorylated STAT3 (pSTAT3) in cultured VAN and by quantifaction of pSTAT3 protein levels by Western blot analysis in nodose ganglia and arcuate nucleus in vivo. To determine the mechanism of leptin resistance in nodose ganglia, cultured VAN were stimulated with leptin alone or with lipopolysaccharide (LPS) and SOCS-3 expression measured. SOCS-3 protein levels in VAN were measured by Western blot following leptin administration in vivo. Leptin resulted in appearance of pSTAT3 in VAN of low-fat-fed rats and rats resistant to diet-induced obesity but not diet-induced obese (DIO) rats. However, leptin signaling was normal in arcuate neurons. SOCS-3 expression was increased in VAN of DIO rats. In cultured VAN, LPS increased SOCS-3 expression and inhibited leptin-induced pSTAT3 in vivo. We conclude that VAN of diet-induced obese rats become leptin resistant; LPS and SOCS-3 may play a role in the development of leptin resistance.
Keywords: lipopolysaccharide, high-fat diet, suppressor of cytokine signaling-3
the gut plays a crucial role in sensing luminal contents that is important for the efficient digestion and absorption of ingested nutrients but also in integration of other physiological processes that regulate metabolism and energy expenditure, such as pancreatic β-cell function, hepatic function, and also food intake (34). In response to the presence or absence of food, enteroendocrine cells of the gut release anorexic or orexigenic hormones, respectively. The first site of integration of these signals occurs at the level of vagal afferent neurons (VAN), which project to and stimulate second-order neurons in the dorsal vagal complex of the hindbrain (27). VAN express receptors for many of the anorexigenic and orexigenic hormones released from the gut wall and mediate changes in gastrointestinal function and food intake in response to luminal nutrients (18).
Leptin is a gut and adipose tissue-derived hormone that regulates a range of biological functions and processes, including energy intake and expenditure, body fat, neuroendocrine systems, autonomic function, and insulin and glucose balance (12). Leptin receptors, LepRb, are located on neurons throughout the central nervous system as well as on VAN and other peripheral tissue. Leptin activation of its receptor leads to phosphorylation of the transcription factor signal transducer and activator of transcription 3 (STAT3) (1, 5), and this has been used as an important indicator of leptin signaling in vivo (29).
Genetic mutations in leptin, its receptor, or signaling pathways activated by leptin lead to the development of obesity. In obese humans and in rodent models of high-fat (HF), high-energy, diet-induced obesity, plasma leptin levels are elevated as a result of an increased adiposity, and this is associated with a blunted response to exogenous leptin. Leptin resistance has been widely reported in neurons of the arcuate nucleus of obese animals and is associated with a lack of STAT3 phosphorylation (pSTAT3) in response to exogenous leptin (3, 4, 28). Both suppressor of cytokine signaling-3 (SOCS-3) and protein tyrosine phosphatase 1B (PTP1B) have been shown to play a role in the development of leptin resistance. Neuronal deletion of SOCS-3 in mice leads to enhanced leptin-induced phosphorylation of STAT3 in the hypothalamus, greater body weight loss, and suppression of food intake and protects against diet-induced obesity (23). PTP1B deficiency in mice results in hypersensitivity to leptin and reduced weight and adiposity and increased activity and energy expenditure (2). These observations suggest that these inhibitory signaling molecules play a crucial role in mediating changes in leptin sensitivity.
It has been demonstrated recently that the absence of LepR signaling in other neuronal populations, such as the ventromedial nucleus (17), the ventral tegmental area (22), and the caudal nucleus tractus solitarius and area postrema (21), is also important in the regulation of energy homeostasis. Given that VAN play a major role in the regulation of meal size and duration and express leptin receptors, we hypothesized that leptin resistance could also occur in VAN in diet-induced obesity. The specific aims of the current study were to determine 1) whether leptin resistance occurs in VAN from obese rats by measuring phosphorylation of STAT3 both in vivo following exogenous leptin administration and in vitro by exposure of VAN in short-term culture to leptin and 2) the possible mechanism of leptin resistance in VAN, focusing specifically on the microbial breakdown product lipopolysaccharide (LPS), found to be upregulated in obese animals (11, 13) and to increase SOCS-3 expression (33).
MATERIALS AND METHODS
Rats.
Sprague-Dawley rats (initial weight 180 g, n = 36; Harlan, San Diego, CA) were kept on regular laboratory rodent chow (Purina 5008) or HF diet (45% kcal/g fat; Research Diets D12451) for 8 wk, during which time body weight and food intake were measured every 3 days. Blood samples were collected at week 8. After week 8, on respective diets tissue was collected for immunohistochemistry or for protein measurements, and epididymal, mesenteric, and retroperitoneal fat pads from the left side of the animals were collected and weighed. A further group of rats (n = 12) were kept on chow and used to collect nodose ganglia for culture of VAN. The animals and procedures used were in accordance with the guidelines of the University of California Davis Institutional Animal Care and Use Committees, which also approved the procedures.
Peptides and drugs.
Leptin (rat) and LPS (Escherichia coli 0111:B4 purified by ethanol extraction) were obtained from Sigma (St. Louis, MO).
Cell culture.
Desheathed nodose ganglia were dissected under aseptic conditions and digested for 120 min at 37°C in 3 ml of Ca2+ and Mg2+ free HBSS containing 6 mg of collagenase type Ia (Roche Diagnostics, Indianapolis, IN), as described previously (16). Cells were maintained in culture for 72 h, at which time they were transferred to serum-free medium for 1 h before treatment with leptin (10 mg/ml) either alone or in combination with LPS (10–50 ng/ml) for 2 h or CCK (10 nM).
Immunohistochemistry.
Cryostat sections of fixed nodose ganglia (5 μm) were mounted on polysine-coated slides (Polysine; MenzelGlaser, Braunschweig, Germany) and processed for immunohistochemistry with an antibody raised against pSTAT3 phosphorylated at tyrosine 705 (D3A7; Cell Signaling Technology, Beverly, MA). Cultured neurons were fixed in 4% paraformaldehyde in PBS (30 min RT) and processed for immunohistochemistry with antibodies raised against TLR4 (Imgenex, San Diego, CA), pSTAT3 (Cell Signaling Technology), and SOCS-3 (Cell Signaling Technology). Secondary antibodies were used as appropriate and included donkey anti-rabbit immunoglobulin conjugated with Alexa Fluor 488 and donkey anti-goat immunoglobulin conjugated with Alexa Fluor 546 (Molecular Probes, Eugene, OR). Specificity of immunostaining was determined by omitting the primary antibody and by preincubation with an excess of appropriate peptide where available. Samples were mounted in Vectashield with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Peterborough, UK) for nuclear localization. Images were collected using an Olympus spinning disc confocal microscope (BX61 System; Olympus, Melville, NY). The number of immunoreactive cells in longitudinal sections of nodose ganglia were counted passing through the whole length of the nodose ganglion (6).
Western blotting.
As described previously (13), briefly, 10 μg of protein was used, and unless otherwise stated in the figure legends, samples were loaded into precast 10% BisTris gel, and the gel was run for 50 min at 200 V (Invitrogen Power Case 500). The proteins were transferred for 1 h. Primary antibodies were left to incubate overnight [total STAT3 Ab, STAT3 phosphorylated at tyrosine 705 (D3A7) Ab, SOCS-3, Erk p42/44, Akt, and pSTAT5; Cell Signaling Technology]. GAPDH was used as a loading control (14C10, rabbit mAb; Cell Signaling Technology). Total STAT3 was not used as a loading control for pSTAT3 to prevent stripping of Western blots, which we found resulted in loss of signal. The film was analyzed by Imagequant version 5.1 software (Amersham Biosciences, Amersham, UK).
Measurement of circulating leptin levels.
Blood samples were collected from the tail of fasted rats following 6 wk on respective diets. Blood was centrifuged (1,000 g for 10 min), and the plasma was removed and stored at −80°C. Leptin was measured by ELISA according to the manufacturer's protocol (Alpco Diagnostics, Salem, NH).
Statistics.
Statistical analysis was performed using Prism software (Prism 5.0; GraphPad Software, La Jolla, CA). Two-way ANOVA was used to analyze energy intake data (time and diet were used as the variables). One-way ANOVA was performed to analyze Western blot and immunohistochemistry quantification, with diet used as a variable, and differences among groups were analyzed using multiple comparison procedures (Bonferroni method). Differences were considered significant if P < 0.05. Data are means ± SE. Different letters in Figs. 1–5 denote significant differences between groups.
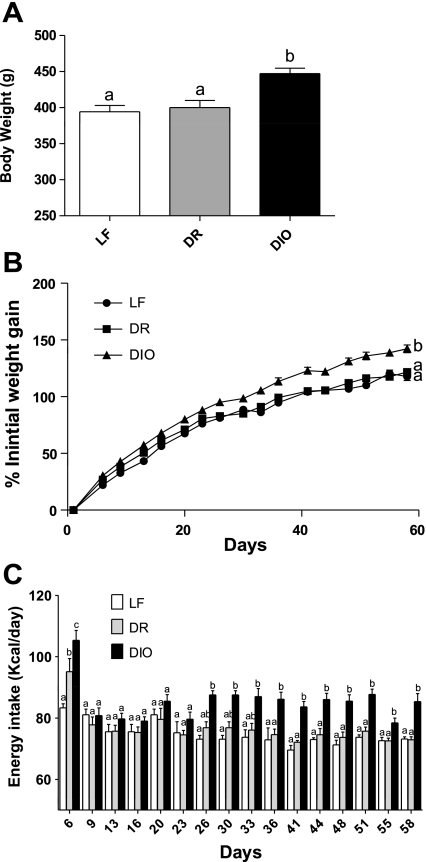
Fig. 1.
Effect of a high-fat (HF) diet on body weight and food intake A: body weight of rats ingesting either a HF (45% kcal) or low-fat (LF) diet for 8 wk. There was a significant increase in body weight in diet-induced obese (DIO) rats compared with diet-induced obesity-resistant (DR) and LF animals (DIO vs. LF, P < 0.01, DIO vs. DR, P < 0.01). B: the increase in body weight of DIO, DR, and LF rats expressed as %initial body weight. DIO rats became significantly heavier than LF-fed animals after 2 wk of HF diet (P < 0.05) and after 4 wk of a HF diet compared with the DR rats (P < 0.001). C: food intake over the 8 wk on the diets. In the 1st week, all rats on the HF diet had a significantly higher energy intake than LF-fed rats, but this initial hyperphagia lasted for only 1 wk, after which there was no significant difference in caloric intake between the groups until week 4. At week 4, the DIO group had a significantly higher energy intake than LF rats (P < 0.01). By week 5 of a HF diet, the DIO had a higher caloric intake than the DR rats (P < 0.05); n = 12 rats/group. Data are expressed as means ± SE.
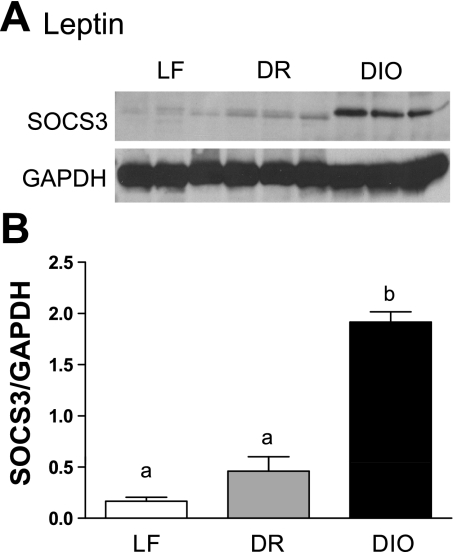
Fig. 5.
Effect of HF diet on suppressor of cytokine signaling-3 (SOCS-3) expression in nodose ganglia. A: Western blot of SOCS-3 expression in nodose ganglia of LF, DR, and DIO rats after 8 wk on respective diets following leptin injection (80 μg/kg ip). SOCS-3 is low in nodose ganglia from LF-fed or DR rats; in contrast, SOCS-3 is markedly increased in nodose ganglia of DIO rats. B: quantification of Western blot demonstrating that SOCS-3 is upregulated in DIO rats compared with LF and DR rats (P < 0.001); n = 3.
RESULTS
Effect of a HF diet on body weight and food intake.
As reported previously, two different phenotypes emerged within the HF group; animals with the highest body weight at 8 wk were assigned to the diet-induced obese (DIO) group, and the other rats were designated diet-induced obese resistant (DR). After 8 wk, DIO rats had a significantly higher body weight compared with low-fat-fed (LF) control (P < 0.01; Fig. 1A) or DR rats (P < 0.01), but there was no significant difference between DR and LF controls (P > 0.05). DIO rats had a significant increase in body weight gain compared with the LF controls after 2 wk on a HF diet (42.7 ± 1.4 vs. 32.7 ± 0.9%, P < 0.05) and after 4 wk on a HF diet compared with the DR rats (95.1 ± 2.2 vs. 82.8 ± 1.1%, P < 0.001; Fig. 1B).
In the first week on a HF diet, both DIO and DR groups had a higher caloric intake than LF (P < 0.001 and P < 0.05, respectively). Following acclimation to the HF diet in the first week, DR rats had equal calorific intake to the LF controls. The DIO animals had a consistently higher caloric intake than LF and DR rats, but this became significant only after 4 wk on a HF diet compared with the LF rats (P < 0.01; Fig. 1C) and after 5 wk of HF feeding compared with the DR rats (P < 0.05).
Effect of a HF diet on adiposity and circulating leptin.
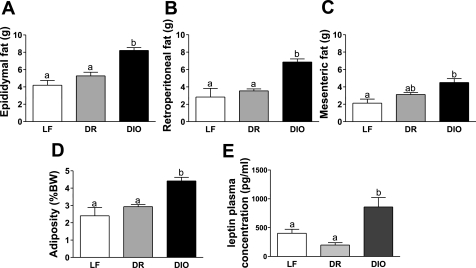
Epidydimal and retroperitoneal fatpad weights were elevated in DIO rats compared with LF and DR rats (DIO vs. LF, P < 0.001; DIO vs. DR, P < 0.001; Fig. 2, A and B). There was a significant increase in mesenteric fat pad weight in DIO rats compared with LF rats but not DR rats (DIO vs. LF, P < 0.01; DIO vs. DR, P > 0.05; Fig. 2C). The adiposity index of DIO rats was higher than LF and DR rats despite DIO rats weighing more than LF and DR rats (DIO vs. LF, P < 0.001; DIO vs. DR, P < 0.01; Fig. 2D). Fasting plasma leptin levels were measured in rats fed LF or HF diets for 8 wk. Circulating leptin levels were elevated in DIO rats compared with DR or LF rats after 8 wk on the respective diets (DIO vs. LF, P < 0.05; DIO vs. DR, P < 0.01; Fig. 2E).
Fig. 2.
Effect of 8-wk consumption of a HF diet on adiposity and circulating leptin levels. A–C: weight of epididymal (A), retroperitoneal (B), and mesenteric fat pads (C) collected from left side of LF, DR, and DIO rats after 8 wk on respective diets. D: combined weight of collected adipose tissue as percentage of body weight (%BW). E: circulating leptin levels in fasted LF, DR, and DIO rats after 8 wk on respective diets. a,bSignificant differences between groups with different letters.
Effect of HF diet on leptin signaling in VAN.
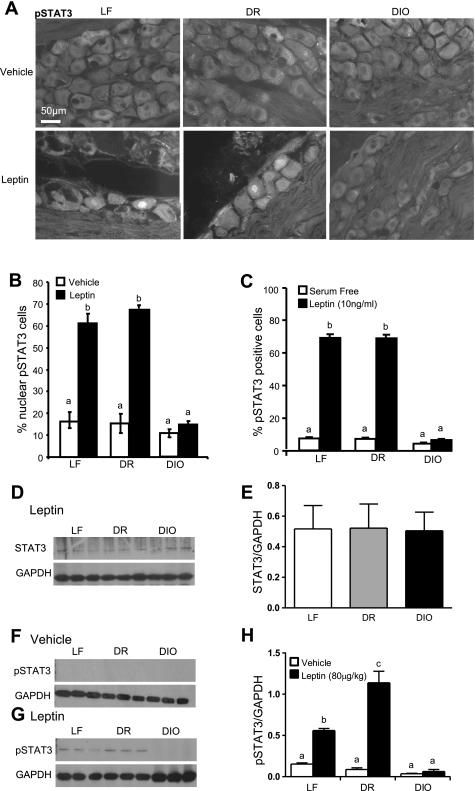
Leptin signaling was measured in nodose ganglia of rats fed LF or HF diets for 8 wk using immunolocalization of pSTAT3. In saline-treated LF, DR, and DIO rats, basal nuclear pSTAT3 immunoreactivity was largely cytoplasmic or absent; there was no significant difference in the number of neurons with nuclear immunoreactivity for pSTAT3 between the three groups (P > 0.05; Fig. 3, A and B). Administration of leptin (80 μg/kg ip, 1 h) significantly increased nuclear localization of pSTAT3 in VAN of LF and DR rats compared with saline-treated rats (P < 0.01; Fig. 3, A and B). However, leptin had no effect on pSTAT3 nuclear translocation in VAN from DIO rats fed a HF diet for 8 wk (P > 0.05). Similarly, exposure of cultured VAN from LF or DR rats to leptin (10 ng/ml, 2 h) significantly increased nuclear pSTAT3 but had no effect in VAN taken from DIO rats fed a HF diet for 8 wk (Fig. 3C).
Fig. 3.
Effect of 8-wk consumption of a HF diet on phosphorylated STAT (pSTAT3) immunoreactivity in nodose ganglia. A: photomicrographs to show immunoreactivity for pSTAT3 in images of nodose ganglia from LF, DR, and DIO rats after 8 wk on respective diets following treatment with either vehicle (400 μl ip saline) or leptin (80 μg/kg ip). In vehicle-treated rats, there was some immunoreactivity of pSTAT3 in the cytoplasm; leptin treatment increased nuclear localization of pSTAT3 in nodose neurons from LF and DR but not DIO rats. B: quantification of pSTAT3 immunoreactivity from nodose ganglia expressed as a percentage of the total neuron population; n = 4 rats/group, total no. of neurons >500. C: leptin also induced an increase in nuclear localization of pSTAT3 immunoreactivity in cultured vagal afferent neurons; data expressed as a percentage of the total cultured neuron population; n = 6/group; total no. of neurons >500. D: total STAT3 protein measured by Western blot in nodose ganglia of LF, DR, and DIO rats after 8 wk on respective diets in response to leptin. E: quantification of Western blots. There was no difference in total STAT3 expression in the nodose ganglia of LF, DR, or DIO. F and G: levels of pSTAT3 protein measured by Western blot in nodose ganglia of LF, DR, and DIO rats after 8 wk on respective diets following treatment with either saline (400 μl ip) or leptin injection (80 μg/kg ip). Leptin increased pSTAT3 in nodose ganglia of LF and DR rats, but no pSTAT3 was detectable in the nodose ganglion of DIO rats. G: loading of protein from the nodose ganglia of DIO rats was doubled compared with DR and LF to demonstrate complete absence of pSTAT3. H: quantification of Western blots. The nodose ganglia of LF (P < 0.01) and DR (P < 0.001) rats showed an increase in pSTAT3 expression in response to administration of leptin compared with saline, but there was no change in pSTAT3 expression in the nodose ganglia of DIO; n = 6. Data are expressed as means ± SE. a–cSignificant differences between groups with different letters.
Western blot was used to determine protein expression of pSTAT3 in protein extracts of the nodose ganglia of rats fed LF or HF diets for 8 wk. Total STAT3 expression was found to remain unchanged in LF, DR, or DIO rats (Fig. 3D). There was little pSTAT3 in nodose tissue from saline-treated LF, DR, or DIO rats (Fig. 3F). Administration of leptin (80 μg/kg ip, 1 h) significantly increased pSTAT3 levels in LF (P < 0.01) and DR rats (P < 0.001) but not DIO rats despite the amount of protein being loaded twice (P > 0.05; Fig. 2, G and H).
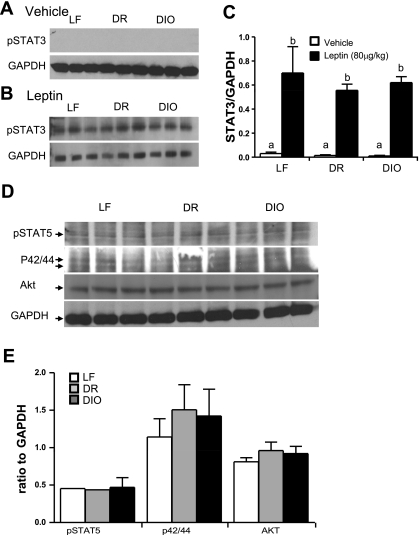
To determine whether leptin resistance was present in the arcuate nucleus of the same rats fed LF or HF diet for 8 wk, protein expression of pSTAT3 was measured by Western blot. There was little pSTAT3 in saline-treated LF, DR, and DIO rats (Fig. 4A). Administration of leptin (80 μg/kg ip) significantly increased pSTAT3 levels in all three groups (P < 0.01; Fig. 4, A–C).
Fig. 4.
Effect of consumption of a HF diet on leptin signaling in the arcuate nucleus. A and B: Western blot of pSTAT3 expression in the arcuate nucleus of LF, DR, and DIO rats after 8 wk on respective diets following administration of either saline (400 μl ip) or leptin (80 μg/kg ip). Administration of leptin increased pSTAT3 in nodose ganglia from LF and DR rats but not DIO rats. C: quantification of Western blots demonstrating that leptin increased STAT3 phosphorylation compared with saline in LF (P < 0.01), DR (P < 0.05), and DIO (P < 0.01) rats; n = 3 rats/group. D: phosphorylated STAT5, p42/44, and Akt in the arcuate nucleus of LF, DR, and DIO rats after 8 wk on respective diets following leptin injection (80 μg/ml ip). E: quantification of Western blot demonstrating that leptin signaling is not disturbed in LF, DR, and DIO rats fed respective diets for 8 wk; n = 3. a,bSignificant differences with different letters.
In the arcuate nucleus, leptin has also been shown to phosphorylate STAT5, ERK p42/44, and phosphatidylinositol 3-kinase (PI3K) (30). Following administration of leptin (80 μg/kg ip), DIO rats expressed similar levels of pSTAT5 compared with LF and DR rats (P > 0.05; Fig. 4, E and F). Leptin signaling via p42/44 was unaffected in DIO rats compared with LF or DR rats (P > 0.05; Fig. 4, E and F). Akt was also used as a marker of leptin-induced PI3K signaling; Akt levels were similar in the arcuate nucleus of DIO rats compared with LF and DR rats following leptin administration (P > 0.05; Fig. 4, E and F).
Effect of HF diet on SOCS-3 expression in VAN.
It has been shown previously that, in arcuate neurons, leptin resistance is mediated at least in part by an increase in expression of SOCS-3. Therefore, protein expression of SOCS-3 was measured by Western blot of nodose ganglia from rats fed LF or HF diets for 8 wk following administration of leptin (80 μg/kg ip). In the nodose ganglia of rats fed a low-fat diet or in DR rats there was little SOCS-3 protein; however, there was a significant increase in SOCS-3 protein in nodose ganglia from DIO rats (P < 0.001; Fig. 5, A and B).
Effect of LPS on VAN.
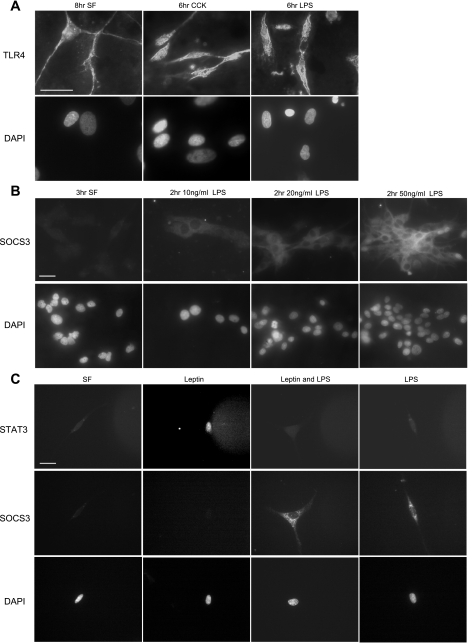
Immunohistochemistry was used to detect the presence of Toll-like receptor-4 (TLR4) on cultured VAN. TLR4 was localized to the plasma membrane and to the cytoplasm of VAN. There was no change in TLR4 abundance or cellular localization in VAN stimulated with CCK for 6 h (10 nM) or stimulated with LPS for 6 h (50 ng/ml) (Fig. 6A).
Fig. 6.
Exposure of vagal afferent neurons to LPS increases expression of SOCS-3 and inhibits leptin-induced phosphorylation of STAT3. A: photomicrographs of nodose neurons in culture immunostained for Toll-like receptor-4 (TLR-4). TLR-4 immunoreactivity was localized to both the plasma membrane and the membrane of neurons; exposure to either CCK or LPS had no observable effect on either levels of expression or cellular localization. B: photomicrographs of SOCS-3 (top) and nuclear staining (bottom) from cultured vagal afferent neurons in response to increasing doses of LPS (0–50 ng/ml). SOCS-3 is dose-dependently upregulated in response to LPS; n = 6. C: photomicrographs of phosphorylated STAT3 (top) and nuclear staining (bottom) from cultured vagal afferent neurons under serum-free conditions in response to leptin alone, LPS alone, or leptin and LPS together. LPS alone had no effect on pSTAT3 but increased expression of SOCS-3. LPS inhibited leptin-induced STAT3 phosphorylation; n = 8. DAPI, 4′,6-diamidino-2-phenylindole.
Treatment of VAN with LPS (0–50 ng/ml, 2 h) induced SOCS-3 expression in a dose-dependent manner (Fig. 6B). Leptin alone (10 ng/ml, 2 h) increased pSTAT3. LPS alone (20 ng/ml, 2.5 h) had no effect on STAT3 phosphorylation. However, LPS (20 ng/ml) 30 min prior to stimulating VAN with leptin (10 ng/ml, 2 h) inhibited leptin-induced STAT3 phosphorylation (Fig. 6C).
DISCUSSION
In this study, we demonstrate that leptin resistance develops in VAN of DIO rats fed a HF diet for 8 wk but not in DR or LF control rats and that this occurs in the absence of measurable leptin resistance in the arcuate nucleus of the hypothalamus. It has been shown previously that, in arcuate neurons of the hypothalamus, leptin resistance is induced by elevated levels of SOCS-3. Here we demonstrate SOCS-3 levels are elevated in nodose ganglia from DIO rats. Moreover, induction of SOCS-3 by the bacterial breakdown product LPS in cultured VAN results in an inability of leptin to phosphorylate STAT3. Taken together, the data suggest that LPS may be a triggering factor in the onset of leptin resistance in VAN of DIO rats.
Sprague-Dawley rats ingesting a HF diet were used as a model of diet-induced obesity. As described previously (13, 24, 25), these rats carry either a polygenic susceptibility (DIO) or resistance (DR) to obesity. After 8 wk on a HF diet, DR rats maintained a body weight similar to control rats fed a LF diet, whereas DIO rats had significantly greater body weight. This is a useful model to discriminate between the effects of feeding a HF diet from the obese phenotype.
After 8 wk on a low-fat diet, leptin signaling in VAN of control rats was intact. However, DIO rats had reduced pSTAT3 protein expression and reduced nuclear localization of pSTAT3 compared with DR or LF control rats, strongly indicating the onset of leptin resistance in VAN in these rats. It is currently unclear how early DIO rats develop leptin resistance in VAN. However, physiological processes rarely occur abruptly and are usually incremental in nature, so the fact that leptin signaling via STAT3 is completely abolished in VAN of DIO rats fed a HF diet for 8 wk suggests that leptin resistance in these neurons may develop sooner.
Interestingly, in the arcuate nucleus of the same rats, we found no evidence of altered leptin signaling at 8 wk. Previous work has reported leptin resistance in the arcuate nucleus of Sprague-Dawley rats fed a HF diet for 12 wk (36). This suggests that leptin resistance in VAN may occur prior to leptin resistance in VAN. Similar data has also been reported in DIO mice (19, 26), in which LF and DIO mice responded normally to intraperitoneal injections of leptin within the first 8 wk on low-fat or HF diets. It was reported that, after 15–20 wk on a HF diet, the DIO mice developed leptin resistance in the arcuate nucleus.
A previous study in our laboratory found no significant difference in the gene expression of the leptin receptor (Ob1R) between LF, DR, or DIO rats (32). Therefore, decreased Ob1R levels are unlikely to explain the reduction in leptin signaling in the VAN of DIO rats. In the arcuate nucleus, SOCS-3 has been demonstrated to inhibit leptin signaling by preventing STAT3 phosphorylation and is thought to play an important role in the development of leptin resistance. Here we find that the nodose ganglia of DIO rats have elevated SOCS-3 levels after 8 wk on a HF diet, and this is associated with a reduction in leptin-induced STAT3 phosphorylation. Furthermore, in cultured VAN we demonstrate that SOCS-3 is present in neurons lacking nuclear pSTAT3 following leptin injection. Therefore, we suggest that SOCS-3 is a putative mechanism for the onset of leptin resistance in DIO rats. In addition to SOCS-3, other negative regulators of leptin signaling have been shown to be involved in the induction of leptin signaling in the central nervous system, such as PTP1B (2, 20) and endoplasmic reticulum (ER) stress (31). Further work is required to determine whether PTP1B or proteins involved in ER stress are expressed in VAN and whether these mechanisms are involved in the development of leptin resistance in these neurons.
We report that at 8 wk DIO rats have increased adiposity and a subsequent increase in plasma leptin levels. Leptin has previously been found to rapidly induce SOCS-3 mRNA in the hypothalamus, resulting in leptin resistance (4). Therefore, it is possible that elevated levels of circulating leptin are responsible for the leptin resistance in VAN.
Consumption of a HF diet has also been found to increase plasma LPS levels (11, 13). Chronic low-dose LPS infusion in mice fed laboratory chow resulted in increased body weight and inflammation (11). These data suggest that LPS could be an important triggering factor linking inflammation to HF diet-induced obesity. Recent work from our laboratory has shown that both DIO and DR rats fed a HF diet developed an “obese microbiota” (13). However, we reported that in the same strain of rats fed the same HF diet for the same amount of time used in this study, DIO rats had elevated LPS levels compared with DR and LF control rats as a result of impaired tight junction function and intestinal permeability (13). Vagal afferent nerve terminals innervating the gut are in close proximity to the LPS release site and could be a mechanism by which LPS signaling occurs. To test this hypothesis, we used cultured VAN to determine whether these neurons express the Toll-like receptor required for LPS signaling and whether LPS signaling could be detected.
Cultured VAN have previously been characterized and demonstrated to maintain their phenotype in culture (6, 14–16). We further validate this technique in this study by demonstrating that cultured VAN of obese animals also conserve their phenotype in culture. We found that cultured VAN from DR and LF animals responded to leptin, whereas DIO animals failed to induce STAT3 phosphorylation in response to leptin similarly to that seen in nodose ganglia from in vivo rats. Using this method, we found that cultured VAN expressed TLR4, indicating that LPS could activate these neurons.
A number of receptors have been found to be differentially expressed in VAN depending on the feeding state of the animal (6–10); however, TLR4 expression was unchanged in cultured VAN under conditions that mimic feeding and fasting in vivo (6, 14). LPS has been found to induce SOCS-3 expression in macrophage and microglia (33), leading us to hypothesize that LPS induce SOCS-3 expression in VAN, thereby resulting in leptin resistance in these neurons. We found that LPS dose-dependently upregulated SOCS-3 in cultured VAN and crucially demonstrated that LPS could inhibit leptin-induced signaling via pSTAT3 in these neurons. From these data, we conclude that changes in gut microbiota and changes in gut permeability could be signaled directly to the brain via the vagus nerve and that chronic exposure to low-level LPS could lead to the development of leptin resistance in VAN.
It is unclear whether LPS could induce leptin resistance in the arcuate nucleus. There is evidence that proinflammatory signals in the hypothalamus result in impaired leptin signaling (35) and that NF-κB signaling, an important downstream effector of LPS, induces SOCS-3 expression in the arcuate nucleus (37). Our data shed no light on the matter and suggest only that LPS levels are insufficient following 8 wk ingestion of a HF diet in Sprague-Dawley rats to effect leptin signaling in the arcuate nucleus.
In conclusion, we demonstrate for the first time that leptin resistance develops in VAN. We noted that this occurred in the absence of measurable leptin resistance in the arcuate nucleus in the same animals at the same time. We provide evidence that VAN of DIO rats have elevated SOCS-3 expression compared with LF or DR rats and that this could account for the development of leptin resistance in these rats. We found elevated circulating levels of leptin and LPS and suggest these as putative mechanisms for the onset of leptin resistance in VAN.
GRANTS
This work funded by National Institutes of Health Grant DDK-41004 (H. E. Raybould).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421: 856–859, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med 12: 917–924, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bjørbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem 274: 30059–30065, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Bjørbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell 1: 619–625, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG, Jr, Rossetti L. Critical role of STAT3 in leptin's metabolic actions. Cell Metab 4: 49–60, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burdyga G, de Lartigue G, Raybould HE, Morris R, Dimaline R, Varro A, Thompson DG, Dockray GJ. Cholecystokinin regulates expression of Y2 receptors in vagal afferent neurons serving the stomach. J Neurosci 28: 11583–11592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burdyga G, Lal S, Spiller D, Jiang W, Thompson D, Attwood S, Saeed S, Grundy D, Varro A, Dimaline R, Dockray GJ. Localization of orexin-1 receptors to vagal afferent neurons in the rat and humans. Gastroenterology 124: 129–139, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci 24: 2708–2715, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burdyga G, Spiller D, Morris R, Lal S, Thompson DG, Saeed S, Dimaline R, Varro A, Dockray GJ. Expression of the leptin receptor in rat and human nodose ganglion neurones. Neuroscience 109: 339–347, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol 290: G1289–G1297, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Confavreux CB, Levine RL, Karsenty G. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol 310: 21–29, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol 299: G440–G448, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Lartigue G, Dimaline R, Varro A, Dockray GJ. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J Neurosci 27: 2876–2882, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Lartigue G, Dimaline R, Varro A, Raybould H, De la Serre CB, Dockray GJ. Cocaine- and amphetamine-regulated transcript mediates the actions of cholecystokinin on rat vagal afferent neurons. Gastroenterology 138: 1479–1490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Lartigue G, Lur G, Dimaline R, Varro A, Raybould H, Dockray GJ. EGR1 Is a target for cooperative interactions between cholecystokinin and leptin, and inhibition by ghrelin, in vagal afferent neurons. Endocrinology 151: 3589–3599, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49: 191–203, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Dockray GJ, Burdyga G. Plasticity in vagal afferent neurons during feeding and fasting: mechanisms and significance. Acta Physiol (Oxf) 201: 313–321, 2011 [DOI] [PubMed] [Google Scholar]
- 19. El-Haschimi K, Pierroz DD, Hileman SM, Bjørbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105: 1827–1832, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283: 1544–1548, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, Di Leone RJ, Bence KK, Grill HJ. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab 11: 77–83, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, Di Leone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51: 801–810, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjørbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med 10: 734–738, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 273: R725–R730, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Levin BE, Sullivan AC. Glucose-induced sympathetic activation in obesity-prone and resistant rats. Int J Obes 13: 235–246, 1989 [PubMed] [Google Scholar]
- 26. Lin X, Chavez MR, Bruch RC, Kilroy GE, Simmons LA, Lin L, Braymer HD, Bray GA, York DA. The effects of a high fat diet on leptin mRNA, serum leptin and the response to leptin are not altered in a rat strain susceptible to high fat diet-induced obesity. J Nutr 128: 1606–1613, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Moran TH, Ladenheim EE, Schwartz GJ. Within-meal gut feedback signaling. Int J Obes Relat Metab Disord 25, Suppl 5: S39–S41, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 10: 739–743, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145: 4880–4889, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Myers MG, Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 21: 643–651, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 9: 35–51, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Paulino G, Barbier de la Serre C, Knotts TA, Oort PJ, Newman JW, Adams SH, Raybould HE. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am J Physiol Endocrinol Metab 296: E898–E903, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qin H, Roberts KL, Niyongere SA, Cong Y, Elson CO, Benveniste EN. Molecular mechanism of lipopolysaccharide-induced SOCS-3 gene expression in macrophages and microglia. J Immunol 179: 5966–5976, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Raybould HE. Nutrient sensing in the gastrointestinal tract: possible role for nutrient transporters. J Physiol Biochem 64: 349–356, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Velloso LA, Araujo EP, de Souza CT. Diet-induced inflammation of the hypothalamus in obesity. Neuroimmunomodulation 15: 189–193, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Wilsey J, Zolotukhin S, Prima V, Scarpace PJ. Central leptin gene therapy fails to overcome leptin resistance associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 285: R1011–R1020, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135: 61–73, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]