Abstract
β-Adrenergic receptor (β-AR) activation elevates cAMP levels in fat cells and triggers both metabolic and transcriptional responses; however, the potential interactions between these pathways are poorly understood. This study investigated whether lipolysis affects β-AR-mediated gene expression in adipocytes. Acute β3-adrenergic receptor (β3-AR) stimulation with CL 316,243 (CL) increased expression of PKA-targeted genes PCG-1α, UCP1, and NOR-1 in mouse white fat. Limiting lipolysis via inhibition of hormone-sensitive lipase (HSL), a direct target of PKA, sharply potentiated CL induction of PCG-1α, UCP1, and NOR-1. CL also induced greater expression of PKA-targeted genes in white fat of HSL-null mice compared with wild-type littermates, further indicating that HSL activity limits PKA-mediated gene expression. Inhibiting HSL in 3T3-L1 adipocytes also potentiated the induction of PGC-1α, UCP1, and NOR-1 by β-AR activation, as did siRNA knockdown of adipose triglyceride lipase, the rate-limiting enzyme for lipolysis. Conversely, treatments that promote intracellular fatty acid accumulation suppressed induction of PGC-1α and UCP1 through β-AR stimulation. Analysis of β-adrenergic signaling indicated that excessive intracellular fatty acid production inhibits adenylyl cyclase activity and thereby reduces PKA signaling to the nucleus. Lastly, partially limiting lipolysis by inhibition of HSL increased the induction of oxidative gene expression and mitochondrial electron transport chain activity in white adipose tissue and facilitated fat loss in mice treated for 5 days with CL. Overall, our results demonstrate that fatty acids limit the upregulation of β-AR-responsive genes in white adipocytes and suggest that limiting lipolysis may be a novel means of enhancing β-AR signaling.
Keywords: protein kinase A; adenylyl cyclase; adipose tissue; adenosine 3′,5′-cyclic monophosphate; hormone-sensitive lipase; adipose triglyceride lipase
chronic activation of β-adrenergic receptors (β-AR) in rodents induces a thermogenic program in white adipose tissue involving both metabolic and transcriptional responses in fat cells (20). These responses are mediated via activation of adenylyl cyclase (AC), which increases cyclic AMP (cAMP) levels and activates protein kinase A (PKA). The most salient metabolic effect of β-AR activation in adipocytes is the stimulation of lipolysis. Release of free fatty acids is accomplished by PKA-mediated phosphorylation, which indirectly activates adipose triglyceride lipase (ATGL) and directly activates hormone-sensitive lipase (HSL) (14). Concurrently, β-AR/PKA promotes the transcription of genes involved in lipid oxidation, including peroxisome proliferator-activated receptor (PPAR)γ coactivator 1α (PGC-1α) (43), uncoupling protein 1 (UCP1) (1), and neuron-derived orphan receptor-1 (NOR-1) (42). PGC-1α is a transcriptional coactivator of nuclear receptors such as the PPARs and functions as a master regulator of mitochondrial biogenesis and lipid oxidation (49). PGC-1α is important in eliciting the effects of β-AR since mice deficient in PGC-1α are cold sensitive (30, 32) and have increased body fat (32). UCP1, an equally important target of β-AR activation, is a mitochondrial protein that uncouples oxidative phosphorylation, thereby allowing for high substrate oxidation and low ATP synthesis (39). NOR-1 is a PKA-responsive orphan nuclear receptor that increases UCP1 expression (27). Moreover, fatty acids mobilized by β-AR stimulation can also trigger uncoupling activity of UCP1 (39). Thus, lipolysis, gene transcription, and oxidative metabolism are thought to be functionally linked in adipocytes. However, the role that lipolysis plays in regulating the transcription of β-AR-targeted genes is unknown.
Long-term pharmacological activation of β3-adrenergic receptors (β3-ARs) in rodents reduces body fat and improves insulin sensitivity and plasma lipid profile (11, 15), and these beneficial effects are thought to occur in part by increased lipid oxidation in adipose tissue. At the cellular level, chronic stimulation of β3-ARs increases expression of genes involved in mitochondrial function and fatty acid oxidation (12), producing a phenotype similar to brown fat (21). Whereas β3-AR activation is known to increase gene expression by PKA phosphorylation of specific transcription factors (44), the role that lipolysis plays is not known. Lipolysis generates fatty acids that are ligands for the family of nuclear receptor PPARs (1, 46) and may be important for β3-AR-mediated “browning” of white adipose tissue (WAT) (31). On the other hand, excessive fatty acids can produce inflammation in white adipose tissue, which decreases expression of adipocyte-specific genes (12) and promotes insulin resistance (16). Furthermore, exogenous fatty acids are known to inhibit cAMP production in adipocytes (10); however, it is not known whether endogenously mobilized fatty acids affect PKA-mediated gene expression.
Here we report that pharmacological suppression of fatty acid mobilization during β3-AR activation potentiates the induction of PGC-1α, UCP1, and NOR-1 in WAT of mice. HSL-knockout (KO) mice, which are resistant to diet-induced obesity, exhibited increased induction of β3-AR/PKA target genes. In 3T3-L1 adipocytes, inhibition of HSL or knockdown of ATGL augmented β-AR-coupled expression of PGC-1α, UCP1, and NOR-1. Promoting intracellular accumulation of fatty acids in 3T3-L1 adipocytes suppressed the expression of PGC-1α and UCP1. Moreover, elevation of intracellular fatty acids reduced the accumulation of cAMP, and this effect was localized to adenylyl cyclase. Finally, inhibition of HSL over 5 days enhanced CL 316,243 (CL)-induced markers of fat oxidation and mitochondrial electron transport activity in WAT and augmented body fat loss. These data suggest that mobilized fatty acids limit the upregulation of PKA targeted genes and that limiting lipolysis might be a means of facilitating the induction of an oxidative phenotype in WAT.
METHODS
Animal studies.
Three animal experiments were performed to examine the role of HSL in the acute or chronic effects of the β3-AR agonist (CL) treatment. Male C57BL/6J (BL6; Jackson Laboratories) mice (n = 7–8) were used at an age of between 8 and 10 wk. The first experiment examined the effects of HSL inhibition on β3-AR induction of gene expression. Mice were pretreated with the selective HSL inhibitor BAY 59-9435 (BAY; 30 mg/kg) (6) or 0.5% methylcellulose (MC) by gavage for 1 h before intraperitoneal injection of CL (10 nmol) or sterile water. Epididymal WAT (EWAT) was collected 3 h after CL or H2O treatment in RNAlater at −80°C. For measurement of plasma fatty acids and glycerol, mice were fed ad libitum, and blood was collected via retroorbital bleed 45 min after CL treatment. Serum fatty acids were quantified using the NEFA C kit (Wako) and glycerol using free glycerol reagent (Sigma). A second experiment examined β3-AR induction of gene expression in mice deficient for HSL and wild-type littermates. HSL-KO mice on a BL6 background were obtained from Dr. Frederic Kraemer (Stanford University) and bred and genotyped, as described previously (36). Male or female animals were treated with CL or water for 6 h, and tissue was collected as stated above. Finally, a third experiment examined the subacute effects of pharmacological HSL inhibition. Male BL6 mice were treated daily for 5 days with BAY (30 mg/kg) or MC via gavage, followed by an injection of CL (10 nmol) or saline 1 h later. Body composition was determined by NMR (EchoMRI) prior to drug treatment (day 0) and after 5 days. Glucose was measured using a One Touch glucometer (Lifescan). Tissues for the subacute experiment were processed as described above. In situ electron transport chain activity was examined in EWAT minces by measuring the reduction of 2,3,5-triphenyltetrazolium chloride (TTC; Sigma), as described previously (31). All animal studies were approved by the Institutional Animal Care and Use Committee at Wayne State University.
Cell culture studies.
3T3-L1 cells obtained from American Type Culture Collection (CL-173) were differentiated into adipocytes as described previously (34) and used 9–10 days postdifferentiation. Cells were placed overnight in Dulbecco's modified Eagle's medium (DMEM) without serum, and medium was changed to DMEM without phenol red containing 0.1% bovine serum albumin (BSA) or the indicated amount of BSA. Cells were treated with the HSL inhibitor BAY (5 μM) or DMSO for 30 min followed by isoproterenol (10 μM), forskolin (4 μM; Sigma), or the cAMP analog 8-bromo-cAMP [8-Br-cAMP (1 mM); Sigma] for 3 h. Long-chain acyl-CoA synthetase (ACSL) activity was inhibited with triacsin C (5 μM; Sigma) for 30 min followed by isoproterenol treatment (10 μM, 3 h). For measurement of cAMP, 3T3-L1 adipocytes were pretreated with BAY or triacsin C, treated as above, but stimulated for 10 min with isoproterenol. Cells were lysed in 0.1 M HCl and 0.2% Triton-X-100, and cAMP levels were quantified on neutralized supernatants by ELISA (Biomedical Technologies) as suggested by the manufacturer. Intracellular fatty acids were quantified as described previously (35).
Knockdown of ATGL was performed using small interfering RNA (siRNA), as described previously (24). Briefly, adipocytes were trypsinized and replated (1.8 × 105 cells/well) in collagen-coated 24-well plates containing 100 nM siRNA against ATGL (siATGL; Dharmacon M-040220-01) or nontargeting siRNA (siCON; Dharmacon D-001210-01). At 72 h posttransfection, medium was changed and cells were stimulated with 10 μM isoproterenol for 3 h/group in triplicate. Proteins were extracted in RIPA lysis buffer (25 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 0.5% Na deoxycholate, 1% NP-40, 0.5% sodium dodecyl sulfate, and 1 mM EDTA) containing protease inhibitors (Roche) from triplicate wells for each siRNA. Western blot was performed using antibodies against ATGL (13), HSL (provided by Dr. A. Chaudhry) (34), and GAPDH (Millipore) as described (36). Glycerol and fatty acids were quantified as stated above for animal studies.
cAMP response element (CRE) reporter assays were performed using a cAMP-responsive reporter plasmid (pADneo2 C6-BGL) provided by Dr. Adolf Himmler (19). Five days after induction of differentiation, 3T3-L1 adipocytes were trypsinized and replated (3.6 × 105 cells/well) in collagen-coated 12-well plates containing medium and 2 μg of pADneo2 C6-BGL, 0.08 μg of β-galactosidase (β-gal), and 4 μl of Lipofectamine 2000 (Invitrogen). On the following day, transfection medium was changed to DMEM containing 0.1% BSA, and cells were treated in duplicate with BAY (5 μM), triacsin C (5 μM), or DMSO for 30 min, followed by treatment with forskolin (10 μM) for 6 h. Cells were harvested in 150 μl of Cell Culture Lysis Reagent (Promega), and 20 μl was assayed in duplicate. Luciferase activity was measured in luciferase assay buffer (15 mM KH2PO4, 15 mM MgSO4, 4 mM EDTA, 2 mM ATP, 1 mM dithiothreitol) with 15 μg of d-luciferin (Gold Biotechnology) using a MicroLumatPlus LB96V luminometer (Berthold Technologies). β-Gal activity was measured in 100 mM sodium phosphate buffer, pH 7.3, 1 mM MgCl2, and 50 mM β-mercaptoethanol with o-nitrophenyl-β-d-galactopyranoside at 420 nm (Versamax; Molecular Devices). Luciferase activity was normalized to β-gal, and four separate experiments were analyzed by two-way ANOVA. Results are reported as fold of forskolin.
Gene expression analysis.
Adipose tissue RNA was extracted in Trizol (Invitrogen) and the aqueous phase purified on an RNAeasy (Qiagen) column. RNA from 3T3-L1 adipocytes was isolated using a Nucleospin RNA II kit (Machery-Nagel). Total RNA was reverse transcribed using Superscript III (Invitrogen) with oligo(dT) primers (Fermentas). Thirty to fifty nanograms of cDNA was analyzed in a 20-μl quantitative PCR reaction (ABsolute Blue QPCR SYBR; ThermoScientific) with 80 nM of primers. Expression data were normalized to the housekeeping gene peptidylprolyl cis-trans isomerase A (PPIA) using the ΔΔCT method (2−ΔΔCT) (33). NOR-1 cDNA was amplified using forward primer 5′-GCTTAAAGGACCAGAGG-3′ and reverse primer 5′-TGTCAAGGAAGAGCTTGTCG-3′, elongation of very long-chain fatty acids-like 3 (Elovl3) using forward primer 5′-GAGAAAGGATGCCACACAAC-3′ and reverse primer 5′-GAGGCTCCATCTTTCTTTCC-3, UCP1 using forward primer 5′-TGGCCTCTCCAGTGGATGTG-3′ and reverse primer 5′-CGTGGTCTCCCAGCATAGAAG-3, PGC-1α using forward primer 5′-ACACCTGTGACGCTTTCGCTG-3′ and reverse primer 5′-CATTTGAAGGGGTCGCCCTTG-3, CCAAT/enhancer-binding protein-β (C/EBPβ) using forward primer 5′-GCAAGAGCCGCGACAAG -3′ and reverse primer 5′-GGCTCGGGCAGCTGCTT-3, and ATGL using forward primer 5′-TGGGTCCTGCCCCACTAAGA -3′ and reverse primer 5′-TGTAGTGGCTGGTGAAAGGT-3. Primers for long-chain acyl-CoA dehydrogenase (Lcad), cytochrome c oxidase subunit VIIIb (Cox8b), PPARα, and PPARγ have been described previously (31).
Statistical analysis.
Data are reported as means ± SE. Data were analyzed by one- or two-way ANOVA using GraphPad Prism 5 (GraphPad Software). Post hoc comparisons were performed using Bonferroni posttest.
RESULTS
Inhibition of HSL potentiates β3-AR gene expression in WAT.
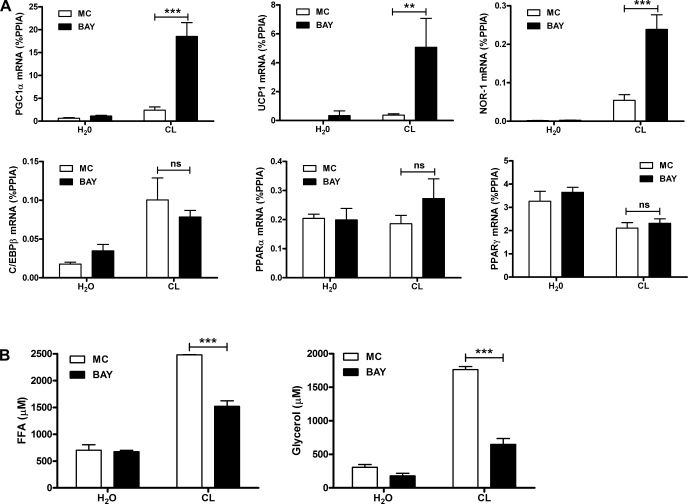
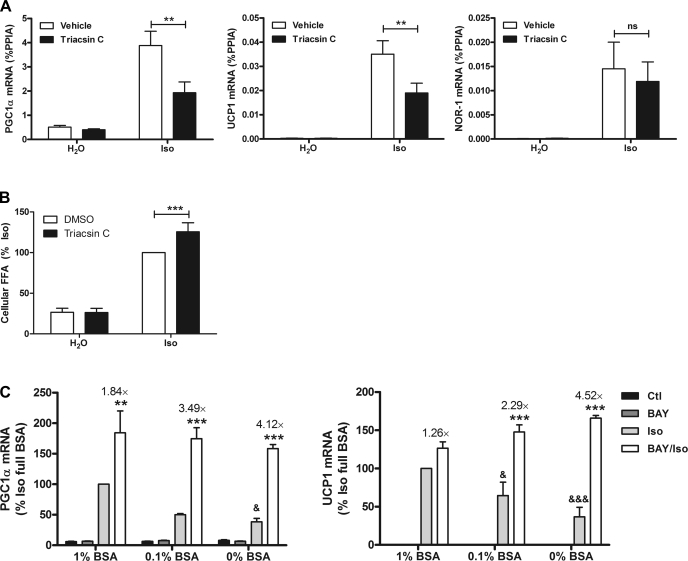
Adipose tissue lipolysis is mediated by the concerted action of ATGL and HSL. We initially focused our attention on HSL using pharmacological inhibition. HSL is a major adipocyte lipase acting on triacylglycerol (TAG) and diacylglycerol (DAG) as substrates responsible for liberating up to two or three free fatty acids in response to PKA activation (26). BAY is a highly selective inhibitor of HSL, does not inhibit the other adipocyte TAG lipase ATGL, and has no effect in HSL-null mice (36). We first examined regulation of PGC-1α and UCP1 mRNA, which are known to mediate metabolic effects of β-AR activation in white fat. We also measured the expression of NOR-1, a gene that is highly induced in response to β-adrenergic stimulation and is thought to play a role in UCP1 induction (27). Treatment with CL for 3 h elevated levels of PKA targets PGC-1α, UCP1, and NOR-1 in epididymal WAT, as expected. Inhibition of HSL with BAY potentiated the expression of PGC-1α, UCP1, and NOR-1 greater than fivefold but did not modify basal expression (Fig. 1A). We also evaluated whether BAY affected expression of key adipocyte transcription factors. CL elevated expression of C/EBPβ, but BAY did not modify the effect. CL reduced the expression of nuclear receptor PPARγ and had no effect on PPARα expression. BAY did not modify basal expression of PPARγ or PPARα or the suppression of PPARγ by CL. As expected, BAY reduced the levels of serum fatty acids after β3-AR activation and suppressed glycerol levels but did not have an effect on basal levels (Fig. 1B). These results suggest that HSL activity limits the expression of PKA-targeted genes in WAT.
Fig. 1.
Reducing lipolysis by inhibition of hormone-sensitive lipase (HSL) potentiates PKA-targeted genes during β3-adrenergic receptor (β3-AR) activation in white adipose tissue (WAT) of mice. A: mice were pretreated with BAY 59-9435 (BAY) or methylcellulose (MC) for 1 h, followed by CL 316,243 (CL) treatment (10 nmol) or vehicle (H2O) for 3 h, and epididymal WAT (EWAT) was collected and analyzed for mRNA expression by quantitative PCR (qPCR). Data are from 8 mice/group, and the effect of BAY is indicated. B: serum fatty acid and glycerol levels in mice (n = 2–3) pretreated with BAY or vehicle (MC) after 45 min of CL (10 nmol). The effect of BAY is indicated. ***P < 0.001; **P < 0.01. ns, Nonsignificant. PGC-1α, peroxisome proliferator-activated receptor (PPAR)γ coactivator-1α; UCP1, uncoupling protein 1; %PPIA, percentage of peptidylprolyl cis-trans isomerase A; NOR-1, neuron-derived orphan receptor-1; C/EBPβ, CCAAT/enhancer-binding protein-β; FFA, free fatty acid.
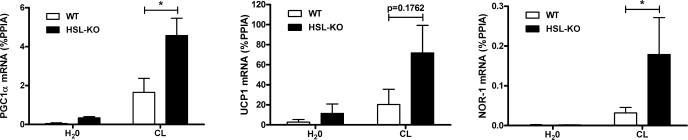
The role of HSL was further evaluated by measuring the expression of PKA-targeted genes in EWAT of HSL-KO mice. CL upregulated the expression of PGC-1α and NOR-1 in wild-type mice, and this induction was significantly greater in HSL-KO mice (Fig. 2). The effect of CL on UCP1 expression was variable and did not reach statistical significance (P = 0.09).
Fig. 2.
HSL knockout (HSL-KO) mice have increased expression of β3-AR-targeted genes in EWAT. mRNA levels were measured by qPCR and normalized to %PPIA from 3–7 mice/group. The difference between wild-type (WT) and HSL-KO is indicated (*P < 0.05).
Limiting lipolysis potentiates β-AR induction of PKA-targeted genes in 3T3-L1 adipocytes.
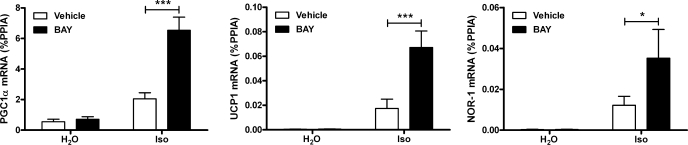
The preceding in vivo experiments suggested that fatty acids produced by HSL reduce β3-AR signaling and the expression of PKA-targeted genes in WAT of mice. Adipose tissue consists of numerous cells, including adipocytes, preadipocytes, vascular cells, and macrophages. 3T3-L1 adipocytes were used as an in vitro cell culture model to test whether lipolysis can directly modify gene expression in adipocytes. Treatment with isoproterenol, a general β-AR agonist, elevated the mRNA levels of PGC-1α, UCP1, and NOR-1 (Fig. 3). Pretreatment with the HSL inhibitor BAY significantly potentiated β-AR stimulation of PGC-1α, UCP1, and NOR-1.
Fig. 3.
Inhibition of HSL in 3T3-L1 adipocytes potentiates β-AR induction of PKA-targeted genes. 3T3-L1 adipocytes were treated with BAY or vehicle (DMSO), followed by 10 μM isoproterenol (Iso) or water (H2O) for 3 h. Gene expression was measured by qPCR and normalized to %PPIA. Data are from 5 separate experiments, and the effect of BAY is indicated (***P < 0.001; *P < 0.05).
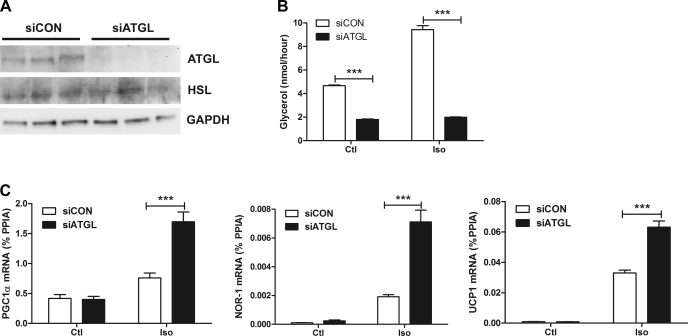
HSL acts on TAG and DAG to generate free fatty acids (26); however, HSL is rate limiting for degradation of DAG, raising the possibility that accumulation of DAG might promote PKA-mediated gene expression rather than fatty acids inhibiting it. To discriminate between these two possibilities, we performed siRNA knockdown of ATGL, which is the rate-limiting enzyme for PKA-mediated DAG production in adipocytes. siRNA knockdown of ATGL reduced ATGL protein to almost undetectable levels by Western blot (Fig. 4A) and abolished isoproterenol-induced lipolysis (Fig. 4B). Protein levels of HSL were unaffected, indicating that there was no effect of siRNA treatment on differentiation (Fig. 4A). Knockdown of ATGL significantly potentiated isoproterenol induction of PGC-1α, UCP1, and NOR-1 (Fig. 4C). These results strongly indicate that fatty acids limit β-AR-mediated expression of PKA target genes in adipocytes.
Fig. 4.
Knockdown of adipose triglyceride lipase (ATGL) potentiates β-AR induction of PKA-targeted genes. A: Western blot for ATGL, HSL, and GAPDH from 3 separate wells per small interfering (si)RNA. GAPDH serves as a loading control. B: glycerol levels from basal and stimulated (10 μM) control siRNA (siCON) and siATGL adipocytes. The effect of ATGL knockdown (siATGL) is indicated. C: siCON- and siATGL-treated adipocytes were stimulated for 3 h with 10 μM Iso or H2O. Gene expression was measured by qPCR and normalized to %PPIA. The effect of ATGL knockdown (siATGL) is indicated. Data are from an experiment performed in triplicate and are representative of 2 independent experiments. ***P < 0.001. Ctl, control.
Intracellular fatty acids reduce β-AR-coupled gene expression in 3T3-L1 adipocytes.
We next examined whether increasing intracellular fatty acids by blocking reesterification with triacsin C would suppress PKA-mediated gene expression. Triacsin C is an inhibitor of long-chain ACSLs, which control the conversion of fatty acids to acyl-CoA derivatives (7). This activation of fatty acids is necessary for downstream processes, including the reesterification of fatty acids into TAG, which occurs during adrenergic activation (29). Treatment with triacsin C did not modify the basal levels of PGC-1α, UCP1, or NOR-1. In contrast, triacsin C reduced β-AR induction of PGC-1α and UCP1 (Fig. 5A). The induction of NOR-1 was highly variable in this experiment, and the effect of ACSL inhibition did not reach statistical significance. Importantly, triacsin C elevated intracellular fatty acid levels during stimulation, but not under basal conditions (Fig. 5B). These results suggest that it is the intracellular accumulation of fatty acids that inhibit induction of PGC-1α and UCP1 and not the formation of fatty acyl-CoA. Indeed, it seems likely that esterification and/or oxidation of acyl-CoAs positively regulate β-AR-mediated induction of PKA target genes.
Fig. 5.
Intracellular fatty acids suppress the expression of β-AR-targeted genes. A: 3T3-L1 adipocytes were treated with 5 μM triacsin C or vehicle (DMSO), followed by 10 μM Iso or H2O. Gene expression was measured by qPCR from 7 separate experiments and normalized to %PPIA. The effect of triacsin C is indicated. B: 3T3-L1 adipocytes were treated as in A, but Iso treatment was for 1 h. Intracellular fatty acids were quantified, and statistical analysis was performed by repeated-measures ANOVA to determine the effect of triacsin C. C: 3T3-L1 adipocytes were incubated in indicated concentrations of BSA and treated with BAY (5 μM) or DMSO followed by Iso (10 μM) for 3 h. mRNA levels were quantified by qPCR, normalized to %PPIA, and expressed as %Iso in the presence of 1% BSA. One-way ANOVA was performed to compare Iso with BAY/Iso at each BSA concentration and to test the effect of Iso at different concentrations of BSA. The fold difference between Iso and BAY/Iso is indicated above the bars. Data are from 3 separate experiments performed in duplicate. **P < 0.01; ***P < 0.001; &P < 0.05; &&&P < 0.001.
An independent means of elevating intracellular fatty acids is to suppress efflux by limiting albumin availability (35). Systematically lowering BSA concentration in the culture medium suppressed the induction of PGC-1α and UCP1 gene expression (Fig. 5C). BAY increased the expression of PGC-1α and UCP1 at all concentrations of extracellular albumin. Importantly, the ability of BAY to potentiate gene expression was greatest when the concentration of BSA was lowest.
Fatty acids blunt β-AR induction of cAMP in 3T3-L1 adipocytes by inhibiting AC and reducing PKA signaling to the nucleus.
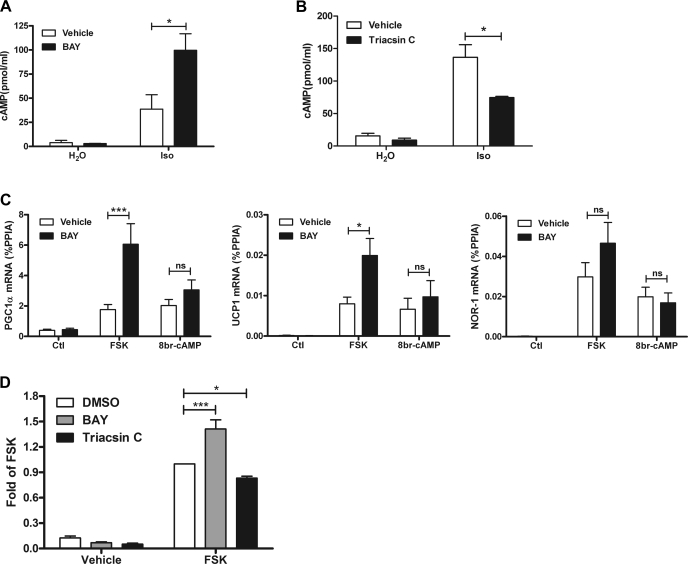
PGC-1α, UCP1, and NOR-1 are known to be regulated by PKA in white adipocytes, so we next examined whether fatty acid mobilization limits the production of cAMP. Treatment with isoproterenol elevated intracellular cAMP, and these levels were further increased by HSL inhibition (Fig. 6A). Conversely, triacsin C, which blunted β-AR stimulation of PGC-1α and UCP1, decreased β-AR-mediated production of cAMP (Fig. 6B). Neither BAY nor triacsin C modified basal cAMP levels.
Fig. 6.
Fatty acids suppress cAMP/PKA signaling at the level of adenylyl cyclase. A: 3T3-L1 adipocytes were treated with BAY followed by Iso for 10 min. cAMP levels were measured from 3 separate experiments, each performed in duplicate. The effect of BAY is indicated. B: 3T3-L1 adipocytes were treated with triacsin C followed by Iso for 10 min. cAMP levels were measured in 2 separate experiments, each performed in triplicate. The effect of triacsin C is indicated. C: 3T3-L1 adipocytes were treated with vehicle (DMSO) or BAY (5 μM) followed by forskolin (FSK; 4 μM) or 8-Br-cAMP (1 mM). mRNA levels were measured by qPCR and normalized to %PPIA. The BAY effect is indicated. D: 3T3-L1 adipocytes, transfected with a cAMP response element reporter, were treated with vehicle (DMSO), BAY, or triacsin C followed by FSK for 6 h. Reporter activity is expressed as fold of FSK, and statistical analysis was performed on the normalized data (relative light units/β-galactosidase OD) by repeated-measures ANOVA to determine the effect of BAY or triacsin C. ***P < 0.001; *P < 0.05.
The above data strongly indicate that intracellular fatty acids limit the generation of cAMP during β-AR stimulation, and this may explain the effect of HSL inhibition on gene expression. To localize the site within the β-AR/PKA cascade where fatty acids were inhibiting, we compared the effects of BAY on gene expression induced by forskolin, a direct activator of AC, with 8-Br-cAMP, which bypasses AC and directly activates PKA. We chose submaximal doses of forskolin and 8-Br-cAMP that were equieffective to 10 μM isoproterenol. Both forskolin and 8-Br-cAMP equally increased the expression of PGC-1α, UCP1, and NOR-1 (Fig. 6C). Inhibition of HSL enhanced forskolin-mediated PGC-1α and UCP1 expression, indicating that fatty acids inhibit post-β-AR receptor. Conversely, BAY did not affect gene expression induced by 8-Br-cAMP (Fig. 6C), indicating that the inhibitory effects of fatty acids on gene expression occur upstream of PKA. The expression of NOR-1 was variable, and the effect of BAY on forskolin-dependent NOR-1 expression did not reach statistical significance. Overall, these results indicate that intracellular fatty acids inhibit β-AR signaling at the level of AC.
PGC-1α, UCP1, and NOR-1 genes are controlled by various genetic elements (5, 28, 45) besides the CREs that confer PKA responsiveness (44). We tested whether fatty acids can specifically modulate CRE-mediated transcription in adipocytes using a CRE luciferase reporter (19). Treatment of 3T3-L1 adipocytes with forskolin greatly elevated levels of the CRE reporter, and this effect was enhanced by BAY and reduced by triacsin C (Fig. 6D). Neither BAY nor triacsin C modified basal levels of the CRE reporter. These results indicate that fatty acids reduce cAMP levels and decrease CRE-regulated transcription in adipocytes.
Limiting lipolysis enhances markers of white adipocyte browning and mitochondrial function in EWAT and promotes CL-induced body fat loss.
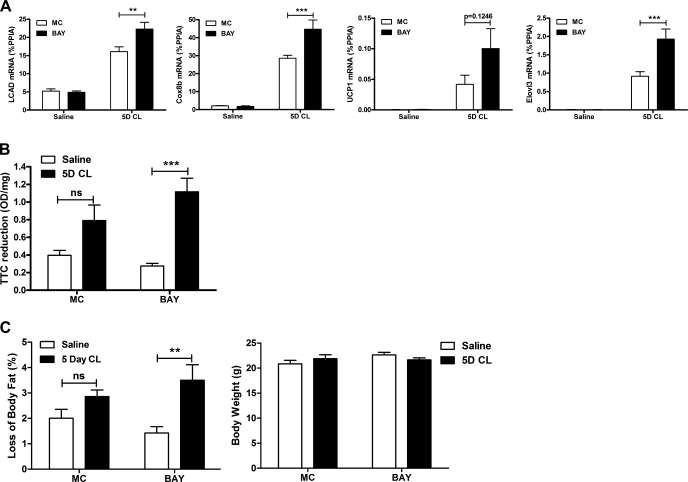
The above in vivo and in vitro data indicate that lipolysis restrains cAMP production and thereby limits induction of β-AR/PKA-targeted genes Thus, we hypothesized that limiting lipolysis could promote the expression of markers of white fat “browning” and contribute to body fat loss during CL treatment. We choose a period of 5 days of CL treatment, during which the induction of oxidative genes and body fat loss are maximal (36). Short-term CL treatment elevated the expression of very long-chain acyl-CoA dehydrogenase (Lcad), a marker of fat oxidation, the expression of Cox8b, a mitochondrial enzyme, and brown adipocyte markers UCP1 and Elovl3. BAY significantly enhanced the mRNA levels of Lcad, Cox8b, and Elovl3, although the levels of UCP1 did not reach statistical significance (Fig. 7A). CL tended to increase mitochondrial electron transport chain activity, as measured by the reduction of TTC; however, this effect was significant only within the BAY group (Fig. 7B). Similarly, short-term CL treatment significantly reduced body fat only in mice treated with both CL and BAY (Fig. 7C). No changes were observed in total body weight (Fig. 7C). Five-day CL treatment lowered serum fatty acid levels, and BAY did not enhance this effect (not shown). No differences were observed in plasma glucose (not shown).
Fig. 7.
Partial inhibition of lipolysis enhances markers of white fat browning and mitochondrial activity and promotes fat loss in mice. Mice (n = 8/group) were treated with BAY (30 mg/kg) or MC followed by CL (5D CL; 10 nmol) or saline repeatedly for 5 days. A: mRNA levels in EWAT were measured by qPCR and normalized to %PPIA. The effect of BAY on 5-day CL is shown. B: 2,3,5-triphenyltetrazolium chloride (TTC) reduction as a measurement of mitochondrial electron transport chain activity. The effect of 5D CL treatment is shown. C: body fat composition was measured in mice by MRI and expressed as loss of body fat. The effect of 5D CL is indicated. ***P < 0.001; **P < 0.01.
DISCUSSION
This study explored the relationship between lipolysis, gene transcription, and oxidative metabolism in white adipocytes. We found that pharmacological or genetic inhibition of HSL greatly potentiated PKA-mediated gene transcription in vivo and in vitro. Additionally, siRNA knockdown of ATGL, which blocks production of fatty acids and DAG, confirmed the role of fatty acids in inhibiting PKA-mediated gene transcription. Complimentary experiments that increased intracellular fatty acids further demonstrated the suppressive effects of fatty acids on β-AR-mediated gene expression.
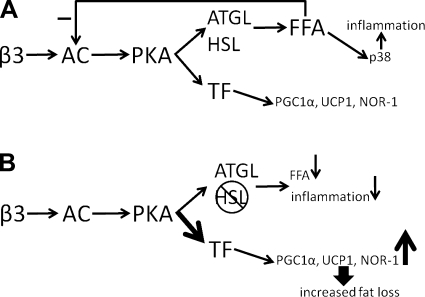
The current results demonstrate that acute β-AR activation mobilizes fatty acids, which negatively feed back on AC to limit the induction of PKA target genes (Fig. 8A). Fatty acids were previously shown to suppress cAMP production (10, 22), but the impact of this potential feedback on PKA-mediated gene expression was not known. Our data indicate that fatty acids suppress cAMP production at levels of β-adrenergic receptor occupancy that are submaximal with respect to the induction of PKA-targeted genes. Thus, inhibiting lipase activity relieves negative feedback on AC, allowing for increased levels of cAMP and greater expression of PKA-targeted genes (Fig. 8B). Chronic β3-AR agonist treatment in rodents is thought to promote body fat loss and systemic insulin sensitivity by increasing fatty acid mobilization and oxidation in situ. Lipase inhibition effectively dampens the relationship between cAMP production and lipolysis without affecting the relationship between cAMP and oxidative gene expression and thus provides a better balance between fatty acid mobilization and oxidation. This explains, perhaps counterintuitively, how inhibition of HSL promotes greater weight loss and oxidative gene expression during the early phase of CL treatment. Our data agree with other work demonstrating that enhanced cAMP signaling in fat cells promotes lipid oxidation and fat loss (48). It is important to note that, with HSL inhibition, ATGL is still capable of promoting the mobilization of fatty acids for release and oxidation.
Fig. 8.
Limiting lipolysis promotes β3-AR signaling. A: acute stimulation of the β3-AR activates adenylyl cyclase (AC) and PKA and subsequently ATGL and HSL and transcription factors (TF) that promote the transcription of genes such as PCG-1α, UCP1, and NOR-1. The mobilization of fatty acids causes inflammation and feedback to reduce AC activity. B: during inhibition of HSL in vivo, fatty acid mobilization is reduced, inflammation is suppressed, and the negative feedback on AC is relieved, thereby increasing expression of PKA-targeted genes and promoting fat loss.
Mice deficient for HSL are leaner and resistant to diet-induced obesity (18, 40, 51), suggesting that reduced fatty acid feedback might increase β-AR signaling and a lean phenotype. This is further supported by our data, in which we found that β3-AR induction of PGC-1α, UCP1, and NOR-1 was greater in HSL-KO mice. Recently, it was proposed that low retinoic acid levels in white fat of HSL-KO mice promote a lean phenotype by inducing a browning of white adipose tissue (50). The results from knockdown of ATGL and manipulation of fatty acid metabolism (triacsin C) and efflux (BSA removal) argue against a role for retinoids in our study since these methods modify fatty acid levels independently of HSL inhibition. Additionally, inhibition of HSL is known to promote accumulation of DAG (17), which could impair insulin sensitivity in muscle (52). Although we have not measured insulin sensitivity, HSL-KO mice have normal insulin-stimulated glucose uptake in WAT under normal diet conditions, with increased insulin sensitivity in fat tissue, muscle, and heart under high-fat diet (41).
We previously showed that β3-AR stimulation induces the expression of inflammatory genes in WAT (12). Subsequent work demonstrated that inflammatory cytokine expression is regulated by HSL-dependent fatty acid production and the activation of p38 MAPK (35, 36). Inhibition of p38 suppresses the expression of CCL2, IL-6, and PAI-1, but the expression of PGC-1α, UCP1, and NOR-1 is unaffected (results not shown), suggesting that p38 activation or inflammatory cytokines do not limit β3-AR induction of PKA-targeted genes. Thus, limiting lipolysis during β3-AR activation would have the independent benefits of suppressing inflammation and augmenting the expression of genes regulated by PKA (Fig. 8B). Recently, it was proposed that macrophages recruited to adipose tissue during activation of lipolysis function to limit subsequent fatty acid mobilization (25). Our results suggest that the feedback inhibition of AC is the initial response by which fat cells restrain lipolysis.
How fatty acids inhibit AC activity is currently not known. Saturated and unsaturated fatty acids equally limit isoproterenol-stimulated lipolysis in human adipocytes (4), suggesting a general role of long-chain fatty acids in inhibition of AC. Oleic acid inhibits AC activity in fat cell membranes under conditions that G protein-coupled receptor ligands lactate and β-hydroxybutyrate do not, indicating different mechanisms of action (9). Furthermore, fatty acids may modulate AC directly, since arachidonic acid inhibited AC activity independent of GTP-binding proteins (38). AC isoforms are differentially regulated by nucleoside inhibitors and forskolin (23), suggesting the possibility that fatty acids also differentially regulate AC isoforms. Fatty acid inhibition of AC is likely not specific to WAT since palmitic acid inhibits lipolysis in isolated brown adipocytes (3), whereas TAG hydrolysis activity inhibits AC activity in mouse cerebellum (37).
Metabolic remodelling of WAT by β3-AR is a dynamic process, and it is likely that limiting lipolysis might facilitate the early but not the late phases of remodeling (12, 31). In the early phase of β3-AR activation, adipose tissue has low capability for lipid oxidation, and the mobilization of fatty acids triggers inflammation and suppresses cAMP production. Limiting fatty acid production during acute β3-AR activation reduces inflammation (35) and facilitates the transition to an oxidative phenotype. In the late phase, the enhanced oxidative capacity of WAT provides a sink for mobilized fatty acids. We have shown previously that the deleterious effects of mobilized fatty acids are mitigated once oxidative capacity has expanded (31). Thus, allowing full activation of lipolysis may promote fat loss after full induction of oxidative gene expression has been achieved.
The main function of fat cells is to store and mobilize fatty acids in response to energetic demand. Fatty acids are toxic when their production exceeds efflux or oxidation, and feedback inhibition is one means of balancing fatty acid production with subsequent metabolism. In this regard, treatments that reduce systemic free fatty acids by promoting local oxidation (β3-AR agonists and CNTF) (8) or storage (47) have potent antidiabetic and anti-inflammatory effects (2). We suggest that this physiological feedback loop can be taken advantage of pharmacologically to augment PKA signaling and promote therapeutic remodeling of WAT.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-062292 to J. G. Granneman. E. P. Mottillo is supported by a Doctoral Research Award from the Canadian Institutes of Health Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Robert Mackenzie, Dr. Todd Leff, and Dr. Vickie Kimler for critically reading the manuscript. We thank members of the Granneman Laboratory for discussions and Zheng Zhu and Amanda Baker for technical support.
REFERENCES
- 1. Ahmed W, Ziouzenkova O, Brown J, Devchand P, Francis S, Kadakia M, Kanda T, Orasanu G, Sharlach M, Zandbergen F, Plutzky J. PPARs and their metabolic modulation: new mechanisms for transcriptional regulation? J Intern Med 262: 184–198, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 454: 470–477, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Bukowiecki LJ, Follea N, Lupien J, Paradis A. Metabolic relationships between lipolysis and respiration in rat brown adipocytes. The role of long chain fatty acids as regulators of mitochondrial respiration and feedback inhibitors of lipolysis. J Biol Chem 256: 12840–12848, 1981 [PubMed] [Google Scholar]
- 4. Burns TW, Langley PE, Terry BE, Robinson GA. The role of free fatty acids in the regulation of lipolysis by human adipose tissue cells. Metabolism 27: 1755–1762, 1978 [DOI] [PubMed] [Google Scholar]
- 5. Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BM, Collins S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol 24: 3057–3067, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Claus TH, Lowe DB, Liang Y, Salhanick AI, Lubeski CK, Yang L, Lemoine L, Zhu J, Clairmont KB. Specific inhibition of hormone-sensitive lipase improves lipid profile while reducing plasma glucose. J Pharmacol Exp Ther 315: 1396–1402, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Coleman RA, Lewin TM, Van Horn CG, Gonzalez-Baró MR. Do long-chain acyl-CoA synthetases regulate fatty acid entry into synthetic versus degradative pathways? J Nutr 132: 2123–2126, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Crowe S, Turpin SM, Ke F, Kemp BE, Watt MJ. Metabolic remodeling in adipocytes promotes ciliary neurotrophic factor-mediated fat loss in obesity. Endocrinology 149: 2546–2556, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Fain JN, Shephard RE. Inhibition of adenosine 3′:k′-monophosphate accumulation white fat acids, lactate, and beta-hydroxybutyrate. J Lipid Res 17: 377–385, 1976 [PubMed] [Google Scholar]
- 10. Fain JN, Shepherd RE. Free fatty acids as feedback regulators of adenylate cyclase and cyclic 3′:5′-AMP accumulation in rat fat cells. J Biol Chem 250: 6586–6592, 1975 [PubMed] [Google Scholar]
- 11. Ghorbani M, Claus TH, Himms-Hagen J. Hypertrophy of brown adipocytes in brown and white adipose tissues and reversal of diet-induced obesity in rats treated with a beta3-adrenoceptor agonist. Biochem Pharmacol 54: 121–131, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Granneman JG, Li P, Zhu Z, Lu Y. Metabolic and cellular plasticity in white adipose tissue I: effects of β3-adrenergic receptor activation. Am J Physiol Endocrinol Metab 289: E608–E616, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Granneman JG, Moore HP, Mottillo EP, Zhu Z, Zhou L. Interactions of perilipin-5 (Plin5) with adipose triglyceride lipase. J Biol Chem 286: 5126–5135, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Granneman JG, Moore HP. Location, location: protein trafficking and lipolysis in adipocytes. Trends Endocrinol Metab 19: 3–9, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Grujic D, Susulic VS, Harper ME, Himms-Hagen J, Cunningham BA, Corkey BE, Lowell BB. Beta3-adrenergic receptors on white and brown adipocytes mediate beta3-selective agonist-induced effects on energy expenditure, insulin secretion, and food intake. A study using transgenic and gene knockout mice. J Biol Chem 272: 17686–17693, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9: 367–377, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haemmerle G, Zimmermann R, Hayn M, Theussl C, Waeg G, Wagner E, Sattler W, Magin TM, Wagner EF, Zechner R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem 277: 4806–4815, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Harada K, Shen WJ, Patel S, Natu V, Wang J, Osuga J, Ishibashi S, Kraemer FB. Resistance to high-fat diet-induced obesity and altered expression of adipose-specific genes in HSL-deficient mice. Am J Physiol Endocrinol Metab 285: E1182–E1195, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Himmler A, Stratowa C, Czernilofsky AP. Functional testing of human dopamine D1 and D5 receptors expressed in stable cAMP-responsive luciferase reporter cell lines. J Recept Res 13: 79–94, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Himms-Hagen J, Cui J, Danforth E, Jr, Taatjes DJ, Lang SS, Waters BL, Claus TH. Effect of CL-316,243, a thermogenic β3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol Regul Integr Comp Physiol 266: R1371–R1382, 1994 [DOI] [PubMed] [Google Scholar]
- 21. Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 279: C670–C681, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Ho R, Russell TR, Asakawa T, Sutherland EW. Cellular levels of feedback regulator of adenylate cyclase and the effect of epinephrine and insulin. Proc Natl Acad Sci USA 72: 4739–4743, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hurley JH. Structure, mechanism, and regulation of mammalian adenylyl cyclase. J Biol Chem 274: 7599–7602, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Kilroy G, Burk DH, Floyd ZE. High efficiency lipid-based siRNA transfection of adipocytes in suspension. PLoS One 4: e6940, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest 120: 3466–3479, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res 43: 1585–1594, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Kumar N, Liu D, Wang H, Robidoux J, Collins S. Orphan nuclear receptor NOR-1 enhances 3′,5′-cyclic adenosine 5′-monophosphate-dependent uncoupling protein-1 gene transcription. Mol Endocrinol 22: 1057–1064, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar N, Wang H, Liu D, Collins S. Liver X receptor is a regulator of orphan nuclear receptor NOR-1 gene transcription in adipocytes. Int J Obes 33: 519–524, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Leibel RL, Hirsch J. A radioisotopic technique for analysis of free fatty acid reesterification in human adipose tissue. Am J Physiol Endocrinol Metab 248: E140–E147, 1985 [DOI] [PubMed] [Google Scholar]
- 30. Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3: e101, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li P, Zhu Z, Lu Y, Granneman JG. Metabolic and cellular plasticity in white adipose tissue II: role of peroxisome proliferator-activated receptor-α. Am J Physiol Endocrinol Metab 289: E617–E626, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jäger S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 119: 121–135, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Moore HP, Silver RB, Mottillo EP, Bernlohr DA, Granneman JG. Perilipin targets a novel pool of lipid droplets for lipolytic attack by hormone-sensitive lipase. J Biol Chem 280: 43109–43120, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Mottillo EP, Shen XJ, Granneman JG. beta3-adrenergic receptor induction of adipocyte inflammation requires lipolytic activation of stress kinases p38 and JNK. Biochim Biophys Acta 1801: 1048–1055, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mottillo EP, Shen XJ, Granneman JG. Role of hormone-sensitive lipase in β-adrenergic remodeling of white adipose tissue. Am J Physiol Endocrinol Metab 293: E1188–E1197, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Nakamura J, Okamura N, Usuki S. Inhibition of adenylylcyclase activity in mouse cerebellum membranes upon hydrolysis of triacylglycerols by triacylglycerol lipase, but not phospholipids by phospholipase A(2). Arch Biochem Biophys 393: 123–131, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Nakamura J, Okamura N, Usuki S, Bannai S. Inhibition of adenylyl cyclase activity in brain membrane fractions by arachidonic acid and related unsaturated fatty acids. Arch Biochem Biophys 389: 68–76, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta 1504: 82–106, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Osuga J, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A, Shionoiri F, Yahagi N, Kraemer FB, Tsutsumi O, Yamada N. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci USA 97: 787–792, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park SY, Kim HJ, Wang S, Higashimori T, Dong J, Kim YJ, Cline G, Li H, Prentki M, Shulman GI, Mitchell GA, Kim JK. Hormone-sensitive lipase knockout mice have increased hepatic insulin sensitivity and are protected from short-term diet-induced insulin resistance in skeletal muscle and heart. Am J Physiol Endocrinol Metab 289: E30–E39, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Pearen MA, Muscat GEO. Minireview: nuclear hormone receptor 4A signaling: implications for metabolic disease. Mol Endocrinol 24: 1891–1903, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92: 829–839, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Reusch JE, Colton LA, Klemm DJ. CREB activation induces adipogenesis in 3T3-L1 cells. Mol Cell Biol 20: 1008–1020, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rim JS, Kozak LP. Regulatory motifs for CREB-binding protein and Nfe2l2 transcription factors in the upstream enhancer of the mitochondrial uncoupling protein 1 gene. J Biol Chem 277: 34589–34600, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Ruby MA, Goldenson B, Orasanu G, Johnston TP, Plutzky J, Krauss RM. VLDL hydrolysis by LPL activates PPAR-alpha through generation of unbound fatty acids. J Lipid Res 51: 2275–2281, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Seufert J, Lubben G, Dietrich K, Bates PC. A comparison of the effects of thiazolidinediones and metformin on metabolic control in patients with type 2 diabetes mellitus. Clin Ther 26: 805–818, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Song Y, Altarejos J, Goodarzi MO, Inoue H, Guo X, Berdeaux R, Kim JH, Goode J, Igata M, Paz JC, Hogan MF, Singh PK, Goebel N, Vera L, Miller N, Cui J, Jones MR, CHARGE Consortium. GIANT Consortium. Chen YD, Taylor KD, Hsueh WA, Rotter JI, Montminy M. CRTC3 links catecholamine signalling to energy balance. Nature 468: 933–939, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spiegelman BM. Transcriptional control of mitochondrial energy metabolism through the PGC1 coactivators. Novartis Found Symp 287: 60–63; discussion 63–69, 2007 [PubMed] [Google Scholar]
- 50. Ström K, Gundersen TE, Hansson O, Lucas S, Fernandez C, Blomhoff R, Holm C. Hormone-sensitive lipase (HSL) is also a retinyl ester hydrolase: evidence from mice lacking HSL. FASEB J 23: 2307–2316, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Wang SP, Laurin N, Himms-Hagen J, Rudnicki MA, Levy E, Robert MF, Pan L, Oligny L, Mitchell GA. The adipose tissue phenotype of hormone-sensitive lipase deficiency in mice. Obes Res 9: 119–128, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277: 50230–50236, 2002 [DOI] [PubMed] [Google Scholar]