Abstract
Although it is known that urocortin 1 (UCN) acts on both corticotropin-releasing factor receptors (CRF1 and CRF2), the mechanisms underlying UCN-induced anorexia remain unclear. In contrast, ghrelin, the endogenous ligand for the growth hormone secretagogue receptor, stimulates food intake. In the present study, we examined the effects of CRF1 and CRF2 receptor antagonists (CRF1a and CRF2a) on ghrelin secretion and synthesis, c-fos mRNA expression in the caudal brain stem, and food intake following intracerebroventricular administration of UCN. Eight-week-old, male Sprague-Dawley rats were used after 24-h food deprivation. Acylated and des-acylated ghrelin levels were measured by enzyme-linked immunosorbent assay. The mRNA expressions of preproghrelin and c-fos were measured by real-time RT-PCR. The present study provided the following important insights into the mechanisms underlying the anorectic effects of UCN: 1) UCN increased acylated and des-acylated ghrelin levels in the gastric body and decreased their levels in the plasma; 2) UCN decreased preproghrelin mRNA levels in the gastric body; 3) UCN-induced reduction of plasma ghrelin and food intake were restored by CRF2a but not CRF1a; 4) UCN-induced increase of c-fos mRNA levels in the caudal brain stem containing the nucleus of the solitary tract (NTS) was inhibited by CRF2a; and 5) UCN-induced reduction of food intake was restored by exogenous ghrelin and rikkunshito, an endogenous ghrelin secretion regulator. Thus, UCN increases neuronal activation in the caudal brain stem containing NTS via CRF2 receptors, which may be related to UCN-induced inhibition of both ghrelin secretion and food intake.
Keywords: corticotropin-releasing factor receptors, dorsal vagal complex, rikkunshito, digestive system hormone, anorexia
stressful life events have been associated with the onset or symptom exacerbation of several functional gastrointestinal disorders including functional dyspepsia and irritable bowel syndrome (24). Corticotropin-releasing factor (CRF) is a key mediator in the central nervous system and is secreted as an adaptive response to stress. In rodents, central CRF administration induces stress-like behaviors, including increased depression, decreased rearing activity, and suppression of food intake (5, 50). Urocortin 1 (UCN), a member of the mammalian CRF family, bears 45% sequence identity to CRF and acts as an endogenous ligand for CRF receptors (49). Although CRF and UCN mediate their actions through the activation of CRF1 and CRF2 receptors, CRF and UCN display different binding affinities for these receptors. CRF preferentially binds to CRF1 receptors, whereas UCN shows high affinity for both receptors; we therefore believe that UCN is useful for examining the actions of both CRF1 and CRF2 receptors. Central UCN administration suppresses feeding in rats and mice, and the suppression was shown to be at least partly CRF2 mediated in studies that used selective antagonists (7) or antisense oligonucleotides (38). In addition, the initial effect of food intake inhibition after central UCN injection is absent in mice lacking CRF1 receptors (4). Whereas CRF1 receptors mediate immediate mobilization of avoidance, defensive responses to threats, and acute interruption of feeding and digestion, CRF2 receptors mediate long-term responses by postponing appetite and digestion and modulating anxiety-like behaviors (57). Furthermore, CRF1 and CRF2 receptor pathways play important roles in stress-related alterations of feeding behavior and gut motor functions (41).
Ghrelin is a digestive system hormone, originally identified in the stomach as the endogenous ligand for the growth hormone secretagogue receptor GHS-R1a (15). In addition to the promotion of growth hormone secretion, ghrelin displays a wide spectrum of biological functions, including appetite stimulation, gastrointestinal motility, gastric acid secretion, glucose metabolism, cardiovascular action, and immune system modulation (48). The afferent vagus nerve is the major pathway conveying peripheral ghrelin signals to the brain, and the orexigenic effects of peripheral ghrelin are mainly mediated via the arcuate and paraventricular nuclei of the hypothalamus, a region involved in appetite regulation (9, 34). Ghrelin is the only known circulating orexigen, and most circulating ghrelin is produced in the stomach; reduction in the ghrelin concentration was observed after gastrectomy (1). Circulating ghrelin levels are regulated by acute and chronic energy statuses; i.e., its circulating levels increase during fasting and decrease with refeeding (11). In addition, the circulating levels are responsive to chronic stress (21, 28). Although several neurotransmitters and neuropeptides are reported to be involved in gastric ghrelin secretion (13, 27), their mechanisms remain unclear. The brain-gut axis plays an important role in gastrointestinal motor functions and appetite regulation, and the autonomic nervous system (ANS) forms a major link between the brain and stomach (16). ANS and stress hormones that act on ANS may both be involved in the regulation of gastric ghrelin secretion (13, 55).
It has been reported that UCN acutely increased whole body oxygen consumption and body temperature via central activation of sympathetic outflow (10). In addition, central UCN administration increases Fos immunoreactivity in the brain sites known to be involved in the control of appetite and energy homeostasis, e.g., the ventromedial hypothalamic nucleus (VMH), arcuate nucleus (ARC), central nucleus of the amygdala (CeA), paraventricular nucleus (PVN), and nucleus of the solitary tract (NTS) (3, 8, 37). The dorsal vagal complex (DVC), including NTS, is apparently crucial in the regulation of upper gastrointestinal functions; it is the brain stem integrative center that mediates the satiety reflex and relays autonomic neural responses to stress (6).
In the present study, we examined the effect of intracerebroventricular (icv) administration of UCN on ghrelin synthesis and secretion and the involvement of CRF1 and CRF2 receptors in this process. Gastric body and plasma ghrelin concentrations were measured as indicators of ghrelin secretion, and preproghrelin mRNA levels in the gastric body and hypothalamus were measured as indicators of ghrelin synthesis. In addition, using c-fos mRNA expression as a marker, involvement of CRF1 and CRF2 receptors in neuronal activation in the caudal brainstem containing DVC was measured after icv injection of UCN.
MATERIALS AND METHODS
Experimental animals.
Seven-week-old, male Sprague-Dawley rats weighing 210–230 g were purchased from Japan SLC, (Shizuoka, Japan). Rats were anesthetized by intraperitoneal (ip) injection of pentobarbital sodium (50 mg/kg) and placed on a stereotaxic frame. A stainless steel guide cannula (AG-8; Eicom, Kyoto, Japan) was implanted into the right lateral ventricle (coordinates: 0.8 mm posterior and 1.4 mm right lateral from the bregma, and 3.4 mm ventral from the skull surface) using a rat brain atlas (31). The rats were allowed to recover for more than 5 days before the experiments. All animals were housed in stainless steel cages maintained at a room temperature of 23 ± 2°C, relative humidity of 55 ± 10%, and a 12:12-h light-dark cycle (lights on from 0700 to 1900 daily). Rats were handled daily for 5 min by the investigators to minimize stress during experiments. Rats were manually restrained, and intravenous injections in rats were given by skilled investigators. Access to standard laboratory food was stopped 24 h before all experiments. Excluding food intake experiments, all experiments were performed between 0800 and 1300 to avoid confounding variables from the effect of diurnal rhythm. The food intake experiments were performed between 0800 and 1600.
All experimental procedures were performed according to the Guidelines for the Care and Use of Laboratory Animals and were approved by the Laboratory Animal Committee of Tsumura & Co. The animal protocol numbers applicable to the present study are 09–163, 10–023, 10–149, and 10–150.
Drugs and reagents.
Rat UCN and acylated ghrelin were purchased from Peptide Institute (Osaka, Japan); NBI27914 hydrochloride (NBI27914), a CRF1 receptor antagonist, was purchased from Wako Pure Chemical Industries (Osaka, Japan), and astressin2B trifluoroacetate salt (astressin2B), a CRF2 receptor antagonist, was purchased from Sigma-Aldrich Chemical (St. Louis, MO); the GHS-R antagonist (d-Lys3)-GHRP-6, was purchased from Bachem (Torrance, CA). Rikkunshito was used in the form of a powdered extract obtained by spray-drying a hot water extract of the following eight crude drugs: Atractylodis lanceae rhizoma (4.0 g), Ginseng radix (4.0 g), Pinelliae tuber (4.0 g), Hoelen (4.0g), Zizyphi fructus (2.0 g), Aurantii nobilis pericarpium (2.0 g), Glycyrrhizae radix (1.0 g), and Zingiberis rhizoma (0.5 g). Other reagents used for analysis were of the highest purity that were commercially available.
Effects of UCN on food intake, gastric emptying, and gastric acid secretion during CRF1 or CRF2 receptor blockade.
UCN and astressin2B were dissolved in phosphate-buffered saline (PBS, pH 6.9), and a solution of NBI27914 in 100% ethanol (final concentration of ethanol 0.1%) was suspended in PBS (pH 6.9). We had previously confirmed that PBS containing 0.1% ethanol did not affect feeding. PBS served as the vehicle control. Rats were administered intracerebroventricularly with PBS, CRF receptor antagonists, UCN, or UCN/CRF receptor antagonists. The doses of UCN used in the present study were selected on the basis of a study that characterized the anorectic effects of UCN (Fig. 1A). The doses of CRF antagonists used in the present study were selected on the basis of those reported by other researchers (2, 33) and preliminary studies that characterized the effects of CRF antagonists on UCN-induced anorexia. UCN (300 pmol/5 μl) and CRF receptor antagonist (12 nmol/5 μl NBI27914 or 2 nmol/5 μl astressin2B) were mixed. The total volume administered intracerebroventricularly was 10 μl per animal. After icv injections, a preweighed amount of chow was distributed to each cage. Food intake was measured at 1, 2, 4, and 6 h after icv injections.
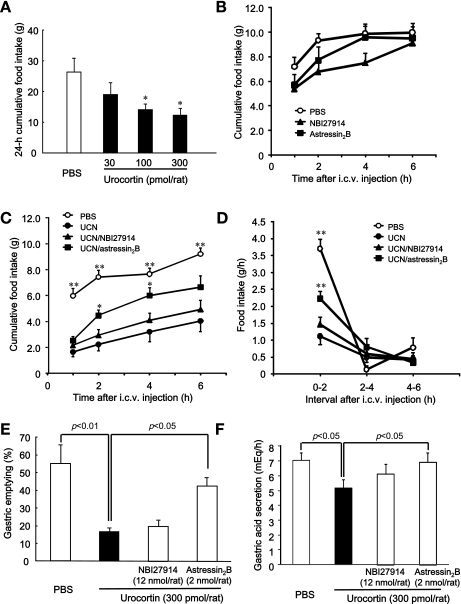
Fig. 1.
Effects of corticotropin-releasing factor (CRF) receptor antagonists on food intake, gastric emptying, and gastric acid secretion in rats after intracerebroventricular (icv) injection of urocortin 1 (UCN). A: effect of icv injection of UCN (30–300 pmol/rat) on food intake in 24-h-fasted rats. Columns express means ± SE (n=5–7). Significance was determined by a post hoc Dunnett's t-test following a one-way analysis of variance (ANOVA; *P < 0.05 vs. PBS-injected group). B: effect of icv injection of NBI27914 (12 nmol/rat) and astressin2B (2 nmol/rat) on cumulative food intake in 24-h-fasted rats. Points express means ± SE (n=5–6). C and D: effects of CRF receptor antagonists (NBI27914: 12 nmol/rat, astressin2B: 2 nmol/rat) on cumulative food intake (C) and food intake per hour (D) after icv injection of UCN (300 pmol/rat). Points express means ± SE (n=8). UCN (300 pmol/5 μl) and CRF receptor antagonists [CRF1 receptor antagonist: NBI27914 (12 nmol/5 μl) or CRF2 receptor antagonist: astressin2B (2 nmol/5 μl)] were combined. Total volume of injected icv was 10 μl per animal. Factorial two-way ANOVA revealed no significant effects of treatment [F(3,112)=54.823, P < 0.001] and time [F(3,112)=22.252, P < 0.001]. There was no significant effect of treatment × time [F(9,112)=0.565, P=0.823]. Significance with post hoc analyses (Dunnett's t-test) following ANOVA: *P < 0.05 or **P < 0.01 vs. UCN-injected group. E: effects of CRF receptor antagonists on gastric emptying in rats after icv injection of UCN. Columns express means ± SE (n=6). F: effects of CRF receptor antagonists on gastric acid secretion in rats after icv injection of UCN. Columns express means ± SE (n=28). Effect of UCN was evaluated by Student t-tests; effects of CRF receptor antagonists were evaluated by post hoc Dunnett's t-test after one-way ANOVA.
In another set of experiments, evaluating gastric emptying, fluorescein-labeled dextran (FD70; 12.5 mg/0.5 ml per rat; Invitrogen, Carlsbad, CA) was administered orally as a nonabsorbable marker to rats 30 min after icv injections. Rats were euthanized 30 min later, and their stomachs were excised immediately. The gastric contents labeled with FD70 were collected in 5 ml of PBS. The supernatant was passed through 0.45-μm filters (Millipore, Bedford, MA), and the absorbance was measured at wavelengths of 490 and 520 nm using the UV-1200 spectrophotometer (Shimadzu, Kyoto, Japan). Gastric emptying was calculated as gastric emptying (%)=100 − (A/B) × 100, where A represents the amount of FD70 remaining in the stomach and B the total amount administered.
Measurement of gastric acid secretion was performed according to a procedure described previously (54). The gastric juice was harvested 4 h after icv injection, and gastric acid output was measured by titration against 0.01 M NaOH.
Determination of ghrelin levels.
Rats were divided into four groups (n=8) and euthanized at 0, 1, 2, and 4 h after icv injection of UCN (300 pmol/10 μl); blood and gastric samples were then collected. In another set of experiments, rats were divided into four groups (n=8) and euthanized 2 h after icv injection of PBS, UCN, UCN + NBI 27914, or UCN + astressin2B; blood samples were then collected.
Blood (∼4 ml) from decapitated rats was collected in polypropylene tubes containing 5.0 mg EDTA and 2,000 kIU aprotinin and centrifuged at 10,000 g at 4°C for 3 min. The supernatant was acidified with 1 mol/l HCl (1/10 vol) and stored at −80°C until the ghrelin assay.
The gastric body was removed from decapitated rats and rinsed with saline. The rinsed tissue sample was boiled in water for 7 min and acidified with 1 mol/l HCl after cooling. Samples were then homogenized and centrifuged at 10,000 g at 4°C for 3 min. The supernatant was stored at −80°C until ghrelin assay.
The ghrelin level was determined using Active Ghrelin or Des-acyl Ghrelin Enzyme-Linked Immunoassay Kits (Mitsubishi Chemical Medicine, Tokyo, Japan). The lowest detectable levels of acylated ghrelin and des-acylated ghrelin were 2.7 and 12.3 fmol/ml, respectively. The intra-assay coefficients of variation for acylated ghrelin and des-acylated ghrelin were 0.8–4.8% and 2.2–5.5%, respectively, and the interassay coefficients of variation for acylated ghrelin and des-acylated ghrelin were 2.8–6.4% and 1.9–9.0%, respectively.
Real-time RT-PCR mRNA expression assay.
Rats were divided into four groups (n=8) and euthanized at 0, 1, 2, and 4 h after icv injection of UCN (300 pmol/10 μl), and the hypothalamus and gastric body were removed. In another set of experiments, rats were divided into four groups (n=15–17) and euthanized at 1, 2, and 4 h after icv injection of PBS, UCN, UCN + NBI 27914, or UCN + astressin2B. Gastric body and caudal brain stem samples (taken from the coronary side and including a 1-mm section of DVC) were collected.
Total RNA was extracted from the isolated gastric body using the RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Quantitative gene expression analysis was performed by real-time RT-PCR according to a procedure described previously (26). TaqMan Gene Expression Master Mix (Applied Biosystems) and Assays-on-Demand Gene Expression probes (GHRL: Rn00572319_m1; FOS: Rn02396759_m1; S18rRNA: Hs99999901_s1; RPS29: Rn00820645_g1; Applied Biosystems) were used in subsequent PCR reactions according the manufacturer's instructions. All real-time RT-PCR reactions were performed in triplicate.
In situ hybridization.
Rats were divided into four groups (n=2), anesthetized with pentobarbital sodium (50 mg/kg ip) at 0, 1, 2, and 4 h after icv injection of UCN (300 pmol/10 μl), and then transcardially perfused with saline, followed by tissue fixative (Genostaff, Tokyo, Japan). In another set of experiments, rats were divided into two groups (n=4), anesthetized with pentobarbital sodium (50 mg/kg ip) 1 h after icv injection of PBS or UCN (300 pmol/10 μl), and then transcardially perfused with saline followed by tissue fixative (Genostaff). Following perfusion, the rat brains were dissected. Fixed whole brains were cut along the coronary plane, embedded in paraffin, and sectioned at 6 μm. Paraffin-embedded tissue blocks and sections of rat brain for in situ hybridization were prepared according to procedures described previously (26). The cDNA template used for Fos was a 458-base pair fragment corresponding to bases 884–1341 of Fos cDNA (GenBank accession no. NM_022197.2). Sense and antisense cRNA probes for Fos mRNA were synthesized using the DIG RNA Labeling Kit (Roche, Basel, Switzerland) according to the manufacturer's protocol. c-Fos mRNA-positive cells were counted in four brain sections, and the average value was used.
Effects of acylated ghrelin and rikkunshito on food intake in UCN-injected rats.
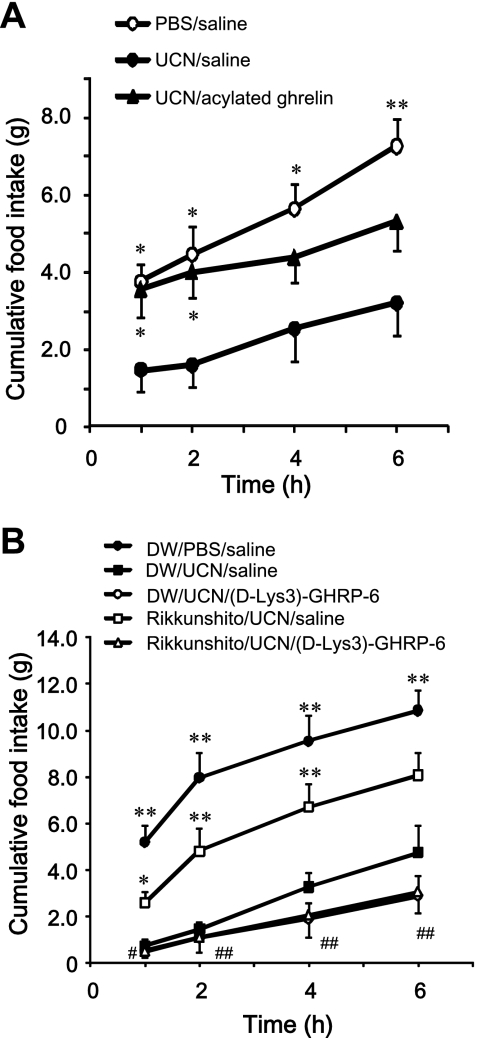
We first investigated the effect of a bolus injection of acylated ghrelin on food intake in rats administered UCN (300 pmol/10 μl). Acylated ghrelin (10 nmol/kg) was intravenously administered into the tail vein of rats 1 min after icv injection of UCN (300 pmol/10 μl). Preweighed chow was supplied to each cage following administration of acylated ghrelin. Cumulative food intake was calculated for 1, 2, and 4 h, immediately after acylated ghrelin administration. In another set of experiments, we investigated the effect of rikkunshito on the cumulative food intake at 1, 2, 4, and 6 h after icv injection of UCN (300 pmol/10 μl). Rikkunshito, a traditional Japanese medicine, increases plasma acylated ghrelin levels in healthy human volunteers, normal mice, and cisplatin-treated rats (23, 43). Therefore, we used rikkunshito as an endogenous acylated ghrelin secretion regulator in this experiment. Distilled water (10 ml/kg) or rikkunshito (1,000 mg/kg) was orally administered to rats. After 2 h, saline or (d-Lys3)-GHRP-6 (4 μmol/kg) was injected intravenously into the tail vein of rats 1 min after icv injection of PBS or UCN (300 pmol/10 μl). Cumulative food intake was calculated for 1, 2, 4, and 6 h, beginning immediately after icv injection.
Statistical analysis.
All values are represented as means ± SE. Statistical significance was evaluated by a one-way analysis of variance (ANOVA) followed by Dunnett's t-test or Student's t- test. Food intake data in this study were analyzed by two-way ANOVA. Sources of significant differences contributing to significant main effects after ANOVA were identified with a post hoc test (Dunnett's or Tukey-Kramer). P < 0.05 was considered statistically significant.
RESULTS
Effects of UCN on food intake, gastric emptying, and gastric acid secretion during CRF1 or CRF2 receptor blockade.
The icv injection of UCN at doses of 100 and 300 pmol per rat significantly decreased the 24-h cumulative food intake in rats deprived of food for 24 h compared with PBS-injected rats (P < 0.05) (Fig. 1A). In accordance with this result, the 300 pmol dose of UCN was used in subsequent experiments. The icv injection of NBI27914 at a dose of 12 nmol per rat or astressin2B at a dose of 2 nmol per rat did not affect cumulative food intake in rats without UCN injection (Fig. 1B). The icv injection of NBI27914 (12 nmol per rat) failed to alter the UCN-induced decrease in cumulative food intake, whereas icv injection of astressin2B (2 nmol per rat) significantly increased the cumulative food intake in UCN-treated rats (Fig. 1C). As shown in Fig. 1D, food intake per hour decreased between 0 and 2 h but not between 2 and 6 h after icv injection of UCN. The decrease in food intake between 0 and 2 h was blocked by astressin2B.
Gastric emptying and acid secretion in rats decreased significantly (P < 0.01, P < 0.05, respectively) after icv injection of UCN vs. those after icv injection of PBS. The UCN-induced decrease in gastric emptying and acid secretion was restored by astressin2B but not by NBI27914 (Fig. 1, E and F).
Acylated and des-acylated ghrelin levels in gastric body and plasma after icv injection of UCN.
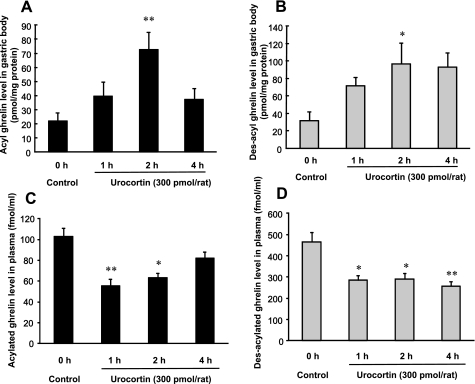
To investigate the effect of UCN on the secretion of acylated ghrelin, and des-acylated ghrelin, gastric body and plasma ghrelin levels were measured after icv injection of UCN. The levels of acylated and des-acylated ghrelin in the gastric body were significantly increased within 2 h in response to UCN administration (P < 0.05). Although increased acylated ghrelin levels in the gastric body decreased to basal levels at the end of 4 h, those of des-acylated ghrelin did not (Fig. 2, A and B). In contrast, after icv injection of UCN, the levels of acylated and des-acylated ghrelin in plasma were significantly decreased at 1 and 2 h and at 1, 2, and 4 h, respectively (Fig. 2, C and D). The time-dependent changes in both acylated and des-acylated ghrelin levels after icv injection of UCN revealed an inverse correlation between gastric and plasma ghrelin levels.
Fig. 2.
Acylated and des-acylated ghrelin levels in the gastric body (A and B) and plasma (C and D) after icv injection of UCN. Columns express means ± SE (n=6–8). Significance with post hoc Dunnett's t-test following one-way ANOVA: *P < 0.05 and **P < 0.01 vs. pre-UCN injection (control).
Preproghrelin mRNA expression in gastric body and hypothalamus after icv injection of UCN.
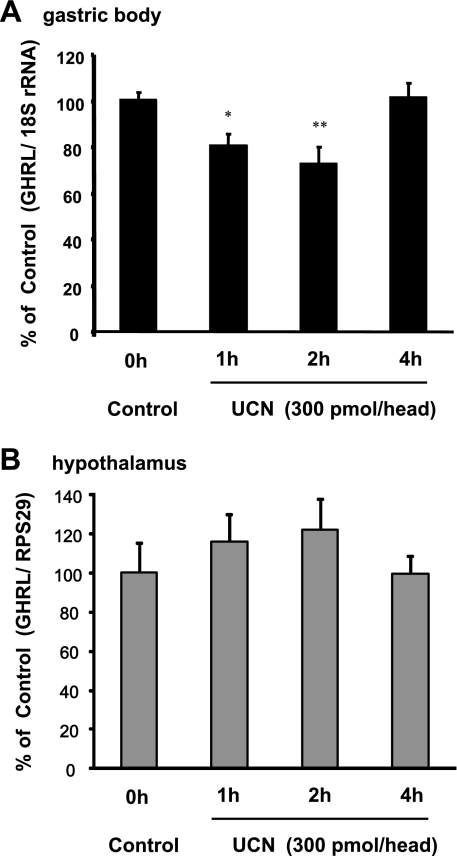
To investigate the effect of UCN on ghrelin synthesis, preproghrelin mRNA expression in the gastric body and hypothalamus was measured after icv injection of UCN. Gastric body preproghrelin mRNA levels significantly decreased 1 and 2 h after icv injection of UCN (Fig. 3A) but recovered to basal levels by the end of 4 h. In contrast, preproghrelin mRNA levels in the hypothalamus remained unchanged (Fig. 3B).
Fig. 3.
Preproghrelin mRNA expressions in the gastric body (A) and hypothalamus (B) after icv injection of UCN. Columns express means ± SE (n=8). Significance with post hoc Dunnett's t-test following one-way ANOVA: *P < 0.05 and **P < 0.01 vs. pre-UCN injection (control). Quantitative gene expression analysis was performed by real-time RT-PCR using ribosomal protein S29 or 18S ribosomal RNA as the reference gene.
Effects of CRF receptor antagonists on plasma ghrelin and gastric preproghrelin mRNA expression levels after icv injection of UCN.
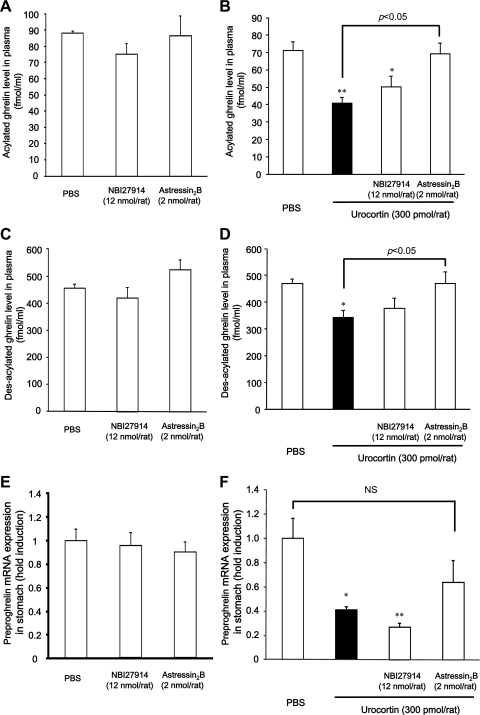
Plasma acylated and des-acylated ghrelin levels in rats after icv injection of UCN (300 pmol per rat) were significantly decreased compared with those in PBS-injected animals (P < 0.01, P < 0.05; Fig. 4, B and D). Plasma acylated and des-acylated ghrelin levels decreased after icv injection of UCN were restored by astressin2B but not by NBI27914 (Fig. 4, B and D). The level of gastric preproghrelin expression in rats following icv injection of UCN (300 pmol per rat) was significantly lower (P < 0.05) than that in PBS-injected rats (Fig. 4F). There was a tendency for astressin2B to restore the decreased level of gastric preproghrelin expression; however, there was no significant difference between the UCN-injected and UCN + astressin2B groups (Fig. 4F).
Fig. 4.
Effects of CRF antagonists on levels of plasma acylated ghrelin (A and B), plasma des-acylated ghrelin (C and D), and gastric preproghrelin mRNA expression (E and F) after icv injection of UCN. Rats were euthanized 2 h after icv injection, and blood samples were collected. A, C, and E: columns express means ± SE (n=5–6) B and D: columns express means ± SE (n=8) F: columns express means ± SE (n=15–17). Significance with post hoc Dunnett's t-test following one-way ANOVA: *P < 0.05 and **P < 0.01 vs. PBS-injected group.
The icv injection of NBI27914 or astressin2B did not affect plasma ghrelin and gastric preproghrelin mRNA levels in rats without UCN injection (Fig. 4, A, C, and E).
Effects of acylated ghrelin and rikkunshito on food intake in UCN-administered rats.
A bolus injection of acylated ghrelin induced a significant increase in cumulative food intake at 1 and 2 h after icv injection of UCN compared with that after icv injection of saline (Fig. 5A). Rikkunshito, an endogenous ghrelin secretion regulator, effectively recovered the decrease in cumulative food intake induced by UCN (Fig. 5B). Although the GHS-R1 antagonist (d-Lys3)-GHRP-6 did not affect the cumulative food intake induced by UCN, the effect of rikkunshito was blocked by (d-Lys3)-GHRP-6 (Fig. 5B).
Fig. 5.
Effects of acylated ghrelin (A) and rikkunshito (B) on food intake in UCN-injected rats. A: saline or acylated ghrelin (10 nmol/kg iv) was administrated into the tail vein of rats 1 min after icv injection of PBS or UCN (300 pmol/10 μl). Points express means ± SE (n=6). Factorial two-way ANOVA revealed significant effects of treatment [F(2,60)=20.909, P < 0.001] and time [F(3,60)=6.692, P < 0.001]. There was no significant effect of treatment × time [F(6,60)=0.432, P=0.855]. Significance with post hoc analyses (Dunnett's t-test) following ANOVA is indicated (*P < 0.05 or **P < 0.01 vs. UCN/saline group). B: distilled water (10 ml/kg) or rikkunshito (1,000 mg/kg) was administered orally in rats. After 2 h, saline or (d-Lys3)-GHRP-6 (4 μmol/kg iv) was administrated into the tail vein of rats 1 min after icv injection of PBS or UCN (300 pmol/10 μl). Points express means ± SE (n=7–8). Factorial two-way ANOVA revealed significant effects of treatment [F(4,124)=60.422, P < 0.001] and time [F(3,124)=26.750, P < 0.001]. There was no significant effect of treatment × time [F(12,124)=0.897, P=0.552]. Significance with post hoc analyses (Tukey-Kramer) following ANOVA: *P < 0.05 or **P < 0.01 vs. DW/UCN/saline group or #P < 0.05 or ##P < 0.01 vs. rikkunshito/UCN/saline group.
c-fos mRNA expression in hypothalamus and caudal brainstem after icv injection of UCN.
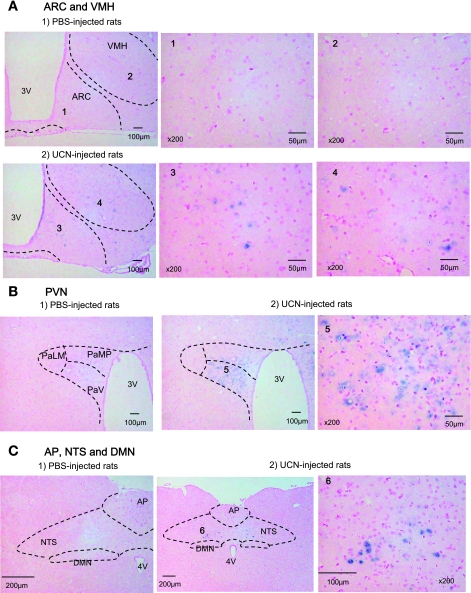
By use of c-fos mRNA expression as a marker, neural activation in the hypothalamus and caudal brainstem following icv injection of UCN was examined. c-fos mRNA was detected by in situ hybridization. The c-fos mRNA expression in the hypothalamus and caudal brainstem reached a peak at 1 h and increased until 4 h after UCN injection (data not shown). In the hypothalamus, ARC and VHM showed an increased number of c-fos mRNA-positive cells after UCN injection (Fig. 6A and Table 1). The increase in c-fos mRNA expression by UCN was significant in PVN (Fig. 6B and Table 1). In the caudal brain stem, c-fos mRNA expression was hardly observed in the dorsal motor nucleus of the vagus (DMV) after icv injection of UCN (Fig. 6C). In contrast, an increase in c-fos mRNA was observed in NTS after icv injection of UCN (Fig. 6C and Table 1). The increase in c-fos mRNA expression by UCN was slight in CeA (P=0.069) of the forebrain and in the rostral ventrolateral medulla (P=0.063) of the hindbrain (Table 1).
Fig. 6.
c-fos mRNA expression in the hypothalamus and dorsal vagal complex (DVC) after icv injection of UCN by in situ hybridization. c-fos mRNA expressions were measured at 1 h after icv injection of PBS or UCN (300 pmol/rat). ARC, arcuate nucleus; VMH, ventromedial hypothalamic nucleus; PVN, paraventricular nucleus; PaLM, paraventric hy, lat magnocell; PaMP, paraventric hy, med parvicell; PaV, paraventricular hy nu, ventral; 3V, 3rd ventricle; NTS, nucleus of the solitary tract; DMN, dorsal vagal nucleus; AP, area postrema; 4V, 4th ventricle.
Table 1.
Effects of icv injection of UCN on c-fos mRNA expression in several brain regions
| Mean Number of c-fos mRNA-Expressing Cells |
|||
|---|---|---|---|
| Brain region | PBS | UCN | P Value |
| ARC | 3 ± 2 | 75 ± 9 | <0.001 |
| VMH | 7 ± 1 | 182 ± 36 | <0.01 |
| PVN | 29 ± 9 | 927 ± 70 | <0.001 |
| CeA | 5 ± 2 | 47 ± 19 | 0.069 |
| NTS | 4 ± 1 | 87 ± 16 | <0.01 |
| RVLM | 5 ± 2 | 47 ± 18 | 0.063 |
Values are means ± SE; n=4. c-fos mRNA expressions were measured at 1 h after icv injection of PBS or urocortin 1 (UCN; 300 pmol/rat). ARC, arcuate nucleus; VMH, ventromedial hypothalamic nucleus; PVN, paraventricular nucleus; CeA, central nucleus of the amygdala; NTS, nucleus of the solitary tract; RVLM, rostral ventrolateral medulla. Effect of UCN on c-fos mRNA expression was compared to that of PBS using Student's t-test.
Effects of CRF antagonists on c-fos mRNA expression in caudal brain stem containing DVC after icv injection of UCN.
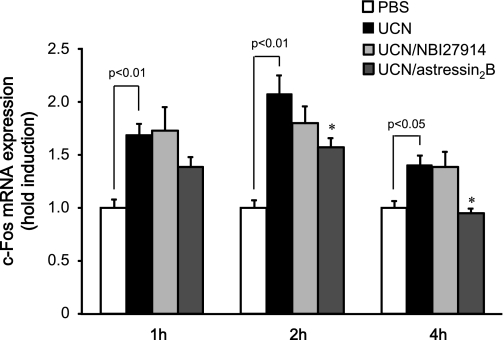
The involvement of both CRF1 and CRF2 receptors in neural activation in the caudal brainstem containing DVC following icv injection of UCN was examined. c-fos mRNA was detected by real-time RT-PCR. Measurement of c-fos mRNA expression levels in the caudal brain stem containing DVC by real-time RT-PCR demonstrated a significant difference between the PBS- and UCN-injected groups (Fig. 7). The increases of c-fos mRNA expression at 2 and 4 h after UCN injection were reduced significantly by astressin2B but not by NBI27914 (P < 0.05).
Fig. 7.
Effects of CRF antagonists (NBI27914: 12 nmol/rat, astressin2B: 2 nmol/rat) on c-fos mRNA expression in brainstems containing DVC after icv injection of UCN by real-time RT-PCR. c-fos mRNA was measured at 1, 2, and 4 h after icv injections. Columns express means ± SE (n=11–17). Effect of UCN was evaluated by Student's t-test. Significance with post hoc Dunnett's t-test following one-way ANOVA was *P < 0.05 vs. UCN-injected group.
DISCUSSION
The present study provided the following important insights into the mechanisms underlying the anorectic effects of UCN: 1) UCN increased acylated and des-acylated ghrelin levels in the gastric body and decreased their levels in the plasma; 2) UCN decreased preproghrelin mRNA levels in the gastric body; 3) UCN-induced reduction of plasma ghrelin and food intake were restored by CRF2 receptor antagonist but not CRF1 receptor antagonist; 4) UCN-induced increase of c-fos mRNA levels in the caudal brain stem containing the NTS was inhibited by CRF2 receptor antagonist; and 5) UCN-induced reduction of food intake was restored by exogenous ghrelin and rikkunshito, an endogenous ghrelin secretion regulator.
Ghrelin consists of two forms: active (acylated ghrelin) and inactive (des-acylated ghrelin) (48). Most plasma ghrelin is produced in the stomach, as demonstrated by the reduction in ghrelin concentration after gastrectomy (1). In the present study, icv injection of UCN induced a decrease in plasma acylated and des-acylated ghrelin levels and an increase in gastric acylated and des-acylated ghrelin levels. In addition, time-dependent changes in both acylated and des-acylated ghrelin levels after icv injection of UCN revealed an inverse correlation between gastric and plasma ghrelin levels. These results suggest that icv injection of UCN has an inhibitory effect on ghrelin secretion from the stomach into the circulation. Interestingly, although both the decreased level of plasma acylated ghrelin and the elevated level of gastric body acylated ghrelin were restored to basal levels 4 h after icv injection of UCN, the levels of des-acylated ghrelin were not restored. Ghrelin-producing cells exist as two types in the rat gastrointestinal tract (26), closed- and opened-type cells. Acylated ghrelin exists in closed-type cells, while des-acylated ghrelin exists in both closed- and opened-type cells (25). The differential localization of acylated and des-acylated ghrelin in the rat stomach accounts for their different responses to intragastric pH (25). We speculate that the difference in time-dependent changes in both acylated and des-acylated ghrelin levels after icv injection of UCN may result from changes in ghrelin O-acyltransferase activity, which converts des-acylated ghrelin to acylated ghrelin, and/or the differential secretory regulation of acylated and des-acylated ghrelin.
Although ghrelin is predominantly produced in the stomach, small amounts are also produced in the hypothalamus. It has been reported that the mechanisms of ghrelin synthesis in appetite regulation may differ between these two locations because preproghrelin mRNA expression in the stomach increases, whereas that in the hypothalamus decreases, after fasting (36). Therefore, we examined preproghrelin mRNA expression in these two locations after icv injection of UCN. UCN administration reduced the level of preproghrelin mRNA in the gastric body but not in the hypothalamus. Because inhibition of ghrelin secretion by UCN leads to accumulation of ghrelin in the stomach, the decrease of preproghrelin mRNA expression in the stomach may be transiently induced by a feedback mechanism in response to this accumulation.
CRF and UCN mediate their effects via two receptor subtypes, CRF1 and CRF2 (57). Because UCN binds and activates both CRF receptors with similarly high nanomolar potency (19, 57), UCN is useful for examining the actions of these receptors. To clarify the relative contributions of CRF receptor subtypes to the decrease in circulating ghrelin levels induced by UCN, we examined the effects of a selective CRF1 receptor antagonist (NBI27914) and a CRF2 receptor antagonist (astressin2B) on plasma ghrelin levels following icv injection of UCN. Administration of the CRF2 receptor antagonist restored the decrease in circulating ghrelin levels induced by UCN, whereas that of the CRF1 receptor antagonist did not. These results suggest that inhibition of ghrelin secretion from the stomach into circulation by icv injection of UCN may be induced via a central CRF2 receptor.
CRF receptors are expressed in specific regions of the brain; however, they are also detectable in peripheral sites, including the gastrointestinal tract and heart (40). Peripheral administration of UCN or CRF induces inhibition of gastric emptying and a reduction in food intake (22, 45). The effects induced by peripheral administration of UCN or CRF are inhibited by peripheral but not by central administration of CRF receptor antagonists (18, 53). These results indicate that the signaling systems involved in central and peripheral CRF receptors are independent. Furthermore, UCN and CRF are known to produce prolonged hypotension when administered systemically and transient hypertension when administered centrally (39, 55). To rule out the possibility that the decrease of ghrelin secretion induced by central administration of UCN (300 pmol/10 μl per rat) was produced by indirect effects due to leakage of the peptide into the peripheral bloodstream, we measured arterial blood pressure in freely moving rats after central administration of UCN. Peripheral (intravenous) UCN had reduced the mean arterial blood pressure (MAP) at 10 min after administration (mean ± SD: −5.5 ± 2.12 mmHg), while central, i.e., icv, administration of the same amount mildly increased MAP, 30 min after administration (mean ± SD: +4.67 ± 2.65 mmHg). These observations support the hypothesis that inhibition of ghrelin secretion from the stomach into circulation by icv injection of UCN may be induced via a central CRF2 receptor.
In the present study, decreased food intake, delayed gastric emptying, and decreased gastric acid secretion induced by UCN were recovered by icv injection of a CRF2 receptor antagonist. Because ghrelin stimulates food intake, gastric emptying, and gastric acid secretion (48), the decrease of ghrelin secretion after icv injection of UCN may be involved in the onset of decreased food intake, delayed gastric emptying, and decreased gastric acid secretion. To determine whether supplemental acylated ghrelin or endogenous acylated ghrelin secretion regulators reverse the decrease of food intake produced by UCN, we examined the effects of acylated ghrelin and rikkunshito on food intake in UCN-injected rats. Because it has recently been reported that rikkunshito, a traditional Japanese medicine, stimulates the secretion of endogenous acylated ghrelin in rats, mice, and humans (23, 44), we used rikkunshito as an endogenous acylated ghrelin secretion regulator. The present study demonstrated that supplementation with acylated ghrelin and rikkunshito reversed the decrease of food intake produced by UCN. These results further suggest that the decrease of food intake after icv injection of UCN may be partly due to the decrease of circulating ghrelin levels. In addition, although cumulative food intake for 6 h was decreased by UCN, food intake per hour was decreased between 0 and 2 h but not between 2 and 6 h, after icv injection of UCN. Plasma acylated ghrelin levels decreased at 1 and 2 h after icv injection of UCN, and the decrease was restored by 4 h. The correlation between the plasma acylated ghrelin level and food intake suggests that the change of the plasma acylated ghrelin level may contribute to feeding behavior in the early phase after UCN injection. In contrast, central UCN administration resulted in suppression of food intake that persisted throughout the 12-h observation period (12, 39). In the present study, we observed that cumulative food intake decreased during the 4- to 24-h period after icv injection of UCN (data not shown). Therefore, the decrease of food intake in the late phase after icv injection of UCN might have been due to factors other than ghrelin.
The expressions of Fos protein and c-fos mRNA are useful as markers of neuronal activation. Central administration of UCN increases Fos immunoreactivity in the forebrain and hindbrain structures associated with food intake, e.g., LSN, VMH, ARC, CeA, PVN, and NTS (3, 8, 37, 48). In the present study, the expression of c-fos mRNA was also increased after icv injection of UCN in ARC, VMH, PVN, and NTS. These cerebral areas are known to express high levels of CRF2 receptors (20). In addition, it has been reported that feeding is inhibited when UCN is injected into not only LSN (51), PVN (52), and VMH (29) of the forebrain but also DVC of the hindbrain (12). The contribution of the caudal brain stem has been demonstrated by Daniels et al. (8) using chronically maintained decerebrate and neurologically intact control rats given fourth-ventricle injections of UCN. Therefore, not only the forebrain but also the caudal brain stem may have primary sites of UCN action.
The DVC is crucial to the regulation of upper gastrointestinal functions and is the brain stem integrative center mediating the satiety reflex and relaying autonomic neural responses to stress (6). Although the mechanism of ghrelin secretion has not been elucidated, it has been reported that the ANS may be related to the regulation of gastric ghrelin secretion. Therefore, we examined the ability of UCN to activate nerve cells in DVC by assaying c-fos mRNA expression and found that an increase in c-fos mRNA was hardly observed in DMV, although it was observed in NTS after icv injection of UCN. Although the c-fos mRNA expression increased until 4 h after icv injection of UCN in the present study, the half-life of c-fos mRNA is generally about 10–15 min (56). However, c-fos mRNA expression at sustained levels for several hours has been reported after CRF administration (14), endotoxin administration (32), or prolonged immobilization stress (47). Therefore, the duration of c-fos mRNA expression may be maintained under some experimental conditions. In addition, the increases in c-fos mRNA expression 2 and 4 h after icv injection of UCN were reduced significantly by CRF2 but not CRF1 receptor antagonist in the caudal brain stem containing NTS. The increase in c-fos mRNA expression 1 h after icv injection of UCN was not reduced significantly by a CRF2 receptor antagonist, which may be due to an increase in c-fos mRNA expression induced by artifactual effects such as the stimulation resulting from vehicle injection. These results suggest that neuronal activation by icv injection of UCN in the caudal brain stem containing NTS may be mediated mainly via CRF2 receptors. NTS is an important site in not only mediating the vagovagal reflex but also innervating the region of the ventrolateral medulla (VLM) (30, 42). Neurons in the VLM are generally regarded as premotor sympathoexcitatory neurons that send efferents via the intermediolateral nucleus of the spinal cord to the celiac ganglia, which send sympathetic postsynaptic fibers to the stomach (17, 42, 46). In the present study, we observed an increase of c-fos mRNA expression in the VLM after icv injection of UCN. Therefore, we speculate that the inhibition of ghrelin secretion by UCN may be induced partly by the activation of sympathetic nerves.
In conclusion, UCN increased neuronal activation in the caudal brain stem containing NST via CRF2 receptors, which may be related to the inhibition of ghrelin secretion and food intake induced by UCN. This may explain the mechanism underlying the anorexia induced by physiological or psychological stress.
DISCLOSURES
K. Yakabi has received grant support from Tsumura & Co. M. Noguchi, Y. Harada, C. Sadakane, and T. Hattori are employed by Tsumura & Co. S. Ohno, S. Ro, T. Onouchi, M. Ochiai, H. Takabayashi, and K Takayama have no conflicts of interest to declare.
REFERENCES
- 1. Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 86: 4753–4758, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Bakshi VP, Newman SM, Smith-Roe S, Jochman KA, Kalin NH. Stimulation of lateral septum CRF2 receptors promotes anorexia and stress-like behaviors: functional homology to CRF1 receptors in basolateral amygdala. J Neurosci 27: 10568–10577, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benoit SC, Thiele TE, Heinrichs SC, Rushing PA, Blake KA, Steeley RJ. Comparison of central administration of corticotrophin-releasing hormone and urocortin on food intake, conditioned taste aversion, and c-Fos expression. Peptides 21: 345–351, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Bradbury MJ, McBurnie MI, Denton DA, Lee KF, Vale WW. Modulation of urocortin-induced hypophagia and weight loss by cortictropin-releasing factor receptor 1 deficiency in mice. Endocrinology 141: 2715–2724, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Britton DR, Koob GF, Rivier J, Vale W. Intraventricular corticotropin-releasing factor enhances behavioral effects of novelty. Life Sci 31: 363–367, 1982 [DOI] [PubMed] [Google Scholar]
- 6. Chigr F, Rachidi F, Segura S, Mahaut S, Tardivel C, Jean A, Najimi M, Moyse E. Neurogenesis inhibition in the dorsal vagal complex by chronic immobilization stress in the adult rat. Neuroscience 158: 524–536, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Cullen MJ, Ling N, Fosterr AC, Pelleymounter MA. Urocortin, corticotrophin releasing factor-2 receptors and energy balance. Endocrinology 142: 992–999, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Daniels D, Markison S, Grill HJ, Kaplan JM. Central structures necessary and sufficient for ingestive and glycemic responses to Urocortin I administration. J Neurosci 24: 50: 11457–11462, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123: 1120–1128, 2002 [DOI] [PubMed] [Google Scholar]
- 10. De Fanti BA, Martínez JA. Central urocortin activation of sympathetic-regulated energy metabolism in Wister rats. Brain Res 930: 37–41, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Drazen DL, Vahl TP, D'Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology 147: 23–30, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Grill HJ, Markison S, Ginsberg A, Kaplan JM. Long-term effects on feeding and body weight after stimulation of forebrain or hindbrain CRH receptors with urocortin. Brain Res 867: 19–28, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Hosoda H, Kangawa K. The autonomic nervous system regulates gastric ghrelin secretion in rats. Regul Pept 146: 12–18, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Imaki T, Shibasaki T, Hotta M, Demura H. Intracerebroventricular administration of corticotropin-releasing factor induces c-fos mRNA expression in brain regions related to stress responses: comparison with pattern of c-fos mRNA induction after stress. Brain Res 616: 114–125, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656–660, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Konturek SJ, Konturek JW, Pawlik T, Brzozowski T. Brain-gut axis and its role in the control of food intake. J Physiol Pharmacol 55: 137–154, 2004 [PubMed] [Google Scholar]
- 17. Laskey W, Polosa C. Characteristics of the sympathetic preganglionic neuron and its synaptic input. Prog Neurobiol 31: 47–84, 1988 [DOI] [PubMed] [Google Scholar]
- 18. Lenz HJ, Raedler A, Greten H, Vale WW, Rivier JE. Stress-induced gastrointestinal secretory and motor responses in rats are mediated by endogenous corticotropin-releasing factor. Gastroenterology 95: 1510–1517, 1988 [DOI] [PubMed] [Google Scholar]
- 19. Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA 98: 7570–7575, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J Neurosci 22: 991–1001, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci 11: 752–753, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martinez V, Wang L, Rivier JE, Vale W, Taché Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther 301: 611–617, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Matsumura T, Arai M, Yonemitsu Y, Maruoka D, Tanaka T, Suzuki T, Yoshikawa M, Imazeki F, Yokosuka O. The traditional Japanese medicine Rikkunshito increases the plasma level of ghrelin in humans and mice. J Gastroenterol 45: 300–307, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut 47: 861–869, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mizutani M, Atsuchi K, Asakawa A, Matsuda N, Fujimura M, Inui A, Kato I, Fujimiya M. Localization of acyl ghrelin- and des-acyl ghrelin-immunoreactive cells in the rat stomach and their responses to intragastric pH. Am J Physiol Gastrointest Liver Physiol 297: G974–G980, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Noguchi M, Yuzurihara M, Kase Y, Yasui T, Irahara M. Involvement of cytokine-induced neutrophil chemoattractant in hypothalamic thermoregulation of luteinizing hormone-releasing hormone. Endocrinology 149: 2899–2906, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Nonogaki K. Ghrelin and feedback systems. Vitam Horm 77: 149–170, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Ochi M, Tominaga K, Tanaka F, Tanigawa T, Shiba M, Watanabe T, Fujiwara Y, Oshitani N, Higuchi K, Arakawa T. Effect of chronic stress on gastric emptying and plasma ghrelin levels in rats. Life Sci 82: 862–868, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Ohata H, Shibasaki T. Effects of urocortin 2 and 3 on motor activity and food intake in rats. Peptides 25: 1703–1709, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Ondicova K, Mravec B. Multilevel interactions between the sympathetic and parasympathetic nervous systems: a minireview. Endocr Regul 44: 69–75, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 1982, p. 12 [Google Scholar]
- 32. Rivest S, Laflamme N. Neuronal activity and neuropeptide gene transcription in the brains of immune-challenged rats. J Neuroendocrinol 7: 501–525, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, DiGruccio M, Vaughan J, Reubi JC, Waser B, Koerber SC, Martinez V, Wang L, Taché Y, Vale W. Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med Chem 45: 4737–4747, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Ruter J, Kobelt P, Tebbe JJ, Avsar Y, Veh R, Wang L, Klapp BF, Wiedenmann B, Taché Y, Monnikes H. Intraperitoneal injection of ghrelin induces Fos expression in the paraventricular nucleus of the hypothalamus in rats. Brain Res 991: 26–33, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Sakata I, Nakamura K, Yamazaki M, Matsubara M, Hayashi Y, Kangawa K, Sakai T. Ghrelin-producing cells exist as two types of cells, closed- and opened-type cells, in the rat gastrointestinal tract. Peptides 23: 531–536, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Sato T, Fukue Y, Teranishi H, Yoshida Y, Kojima M. Molecular forms of hypothalamic ghrelin and its regulation by fasting and 2-deoxy-d-glucose administration. Endocrinology 146: 2510–2516, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Sinnayah P, Blair-West JR, McBurnie MI, Mckinley MJ, Oldfield BJ, Rivier J, Vale WW, Walker LL, Weisinger RS, Denton DA. The effect of urocortin on ingestive behaviours and brain Fos immunoreactivity in mice. Eur J Neurosci 18: 37–41, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Smagin GN, Howell LA, Ryan DH, De Souza EB, Harris RB. The role of CRF2 receptors in corticotrophin-releasing factor- and urocortin-induced anorexia. Neuroreport 9: 1601–1606, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Spina M, Merlo-Pich E, Chan RK, Basso AM, Rivier J, Vale W, Koob GF. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science 273: 1561–1564, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Stengel A, Taché Y. Neuroendocrine control of the gut during stress: corticotropin-releasing factor signaling pathways in the spotlight. Annu Rev Physiol 71: 219–239, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taché Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest 117: 33–40, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tada H, Fujita M, Harris M, Tatewaki M, Nakagawa K, Yamamura T, Pappas TN, Takahashi T. Neural mechanism of acupuncture-induced gastric relaxations in rats. Dig Dis Sci 48: 59–68, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Takeda H, Sadakane C, Hattori T, Katsurada T, Ohkawara T, Nagai K, Asaka M. Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology 134: 2004–2013, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Takeda H, Sadakane C, Hattori T, Katsurada T, Ohkawara T, Nagai K, Asaka M. Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology 134: 2004–2013, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Tanaka C, Asakawa A, Ushikai M, Sakoguchi T, Amitani H, Terashi M, Cheng K, Chaolu H, Nakamura N, Inui A. Comparison of the anorexigenic activity of CRF family peptides. Biochem Biophys Res Commun 390: 887–891, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Toth ZE, Gallatz K, Fodor M, Palkovits M. Decussations of the descending paraventricular pathways to the brainstem and spinal cord autonomic centers. J Comp Neurol 414: 255–266, 1999 [PubMed] [Google Scholar]
- 47. Trneĉková L, Rotllant D, Klenerová V, Hynie S, Armario A. Dynamics of immediate early gene and neuropeptide gene response to prolonged immobilization stress: evidence against a critical role of the termination of exposure to the stressor. J Neurochem 100: 905–914, 2007 [DOI] [PubMed] [Google Scholar]
- 48. van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev 25: 426–457, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature 378: 287–292, 1995 [DOI] [PubMed] [Google Scholar]
- 50. Vergoni AV, Bertolini A, Wikberg JE, Schioth HB. Corticotropin-releasing factor (CRF) induced anorexia is not influenced by a melanocortin 4 receptor blockage. Peptides 20: 509–513, 1999 [DOI] [PubMed] [Google Scholar]
- 51. Wang C, Kotz CM. Urocortin in the lateral septal area modulates feeding induced by orexin A in the lateral hypothalamus. Am J Physiol Regul Integr Comp Physiol 283: R358–R367, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Wang C, Mullet MA, Glass MJ, Billington CJ, Levine AS, Kotz CM. Feeding inhibition by urocortin in the rat hypothalamic paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol 280: R473–R480, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Wang L, Martinez V, Rivier JE, Taché Y. Peripheral urocortin inhibits gastric emptying and food intake in mice: differential role of CRF receptor 2. Am J Physiol Regul Integr Comp Physiol 281: R1401–R1410, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Yakabi K, Mimura H, Iwabuchi H, Ro S, Nakamura T. Interleukin-8 enhances tetragastrin-stimulated acid secretion in vivo. Dig Dis Sci 43: 2317–2321, 1998 [DOI] [PubMed] [Google Scholar]
- 55. Yang LZ, Tovote P, Rayner M, Kockskamper J, Pieske B, Spiess J. Corticotropin-releasing factor receptors and urocortins, links between the brain and the heart. Eur J Pharmacol 632: 1–6, 2010 [DOI] [PubMed] [Google Scholar]
- 56. Zangenehpour S, Chaudhuri A. Differential induction and decay curves of c-fos and zif268 revealed through dual activity maps. Brain Res Mol Brain Res 109: 221–225, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Zorrilla EP, Tache Y, Koob GF. Nibbling at CRF receptor control of feeding and gastrocolonic motility. Trends Pharmacol Sci 24: 421–427, 2003 [DOI] [PubMed] [Google Scholar]