Abstract
Although thyroid hormone (TH) is known to exert important effects on the skeleton, the nuclear factors constituting the TH receptor coactivator complex and the molecular pathways by which TH mediates its effects on target gene expression in osteoblasts remain poorly understood. A recent study demonstrated that the actions of TH on myoblast differentiation are dependent on diabetes- and obesity-related protein (DOR). However, the role of DOR in osteoblast differentiation is unknown. We found DOR expression increased during in vitro differentiation of bone marrow stromal cells into osteoblasts and also in MC3T3-E1 cells treated with TH. However, DOR expression decreased during cellular proliferation. To determine whether DOR acts as a modulator of TH action during osteoblast differentiation, we examined whether overexpression or knockdown of DOR in MC3T3-E1 cells affects the ability of TH to induce osteoblast differentiation by evaluating alkaline phosphatase (ALP) activity. ALP activity was markedly increased in DOR-overexpressing cells treated with TH. In contrast, loss of DOR dramatically reduced TH stimulation of ALP activity in MC3T3-E1 cells and primary calvaria osteoblasts transduced with lentiviral DOR shRNA. Consistent with reduced ALP activity, mRNA levels of osteocalcin, ALP, and Runx2 were decreased significantly in DOR shRNA cells. In addition, a common single nucleotide polymorphism (SNP), DOR1 found on the promoter of human DOR gene, was associated with circulating osteocalcin levels in nondiabetic subjects. Based on these data, we conclude that DOR plays an important role in TH-mediated osteoblast differentiation, and a DOR SNP associates with plasma osteocalcin in men.
Keywords: thyroid hormone, bone, alkaline phosphatase, osteocalcin, single nucleotide polymorphism
osteoporosis poses a major public health threat, with 6 million fractures occurring annually in the US (8). Two defining features of osteoporosis include low bone mineral density and microarchitectural deterioration. Individuals with osteoporosis exhibit increased bone fragility, and therefore, they are at an increased risk for osteoporotic fracture. Therefore, studies that focus on the mechanisms regulating bone accretion and peak bone mass during active growth periods are of considerable importance in the prevention and treatment of osteoporosis. Several studies have implicated a role for thyroid hormone (TH) in skeletal development and maturation. For example, TH deficiency results in delayed bone age, disruption of epiphyseal growth plate formation, and short stature (4, 16). TH excess causes advanced bone age and premature fusion of growth plates and cranial sutures (35). Although there is considerable evidence regarding the importance of TH in skeletal development, the molecular mechanisms of TH action in bone are poorly understood.
It is known that TH regulates gene expression by interacting with a family of nuclear receptors known as thyroid hormone receptors (TR), which bind to TH response elements (TRE) as either a homodimer (TR/TR) or a heterodimer (TR/RXR) in the promoters of 3,3′,5-triiodo-l-thyronine (T3) target genes to facilitate activation of gene transcription (3). In the absence of TH ligand, corepressors such as nuclear receptor corepressor protein/silencing mediator of retinoid and thyroid hormone receptors are recruited to TR, and basal transcription is repressed (2, 35). However, in the presence of TH, ligand-bound TR undergoes a conformational change, thereby releasing the corepressors and recruiting coactivators such as CBP/p300, pCAF, and SRC-1 to the transcriptional complex to facilitate gene transcription (21, 47). Although TH action is mediated in part by nuclear receptors, chromatin remodeling, and known corepressors and coactivators, it remains unknown whether additional nuclear cofactors are recruited to the TR coactivator complex during osteoblast differentiation and bone formation. Therefore, the identification of potential signaling molecules involved in regulating TH action is essential for elucidating molecular pathways.
Recently, Baumgartner et al. (5) found that diabetes- and obesity-related protein (DOR), also known as tumor/transformation-related protein p53-inducible protein 2, is a key determinant of myoblast differentiation and modulates TH function in muscle cells. On the basis of the important role of DOR in mediating TH action and differentiation in myoblasts, we hypothesized that DOR is differentially expressed during osteoblast differentiation and modulates TH action in osteoblasts. To address this hypothesis, we evaluated the expression of DOR in response to known inducers of osteoblast differentiation and also examined whether DOR gain or loss of function alters TH action on osteoblast differentiation by evaluating the effect on alkaline phosphatase (ALP) activity. The gene expression of osteoblast-specific markers was also assessed in DOR-deficient cells stimulated by TH. This study provides experimental evidence that DOR acts as a stimulatory mediator of TH effects in osteoblasts and may be involved in regulating osteoblast differentiation. In addition, we provide preliminary evidence that a common DOR polymorphism associates with plasma osteocalcin levels.
MATERIALS AND METHODS
Reagents.
T3, l-thyroxine (T4), cycloheximide, ascorbic acid (AA), and β-glycerophosphate were purchased from Sigma-Aldrich (St. Louis, MO). Fibroblast growth factor (FGF) was obtained from R & D systems (Minneapolis, MN), and calf serum (CS) was from Atlanta Biologicals (Lawrenceville, GA). Lentiviral expression vectors of pCMVDR8.7, pMD2G, PLMVTHMScr (scramble control), PLMVTHMSi6 (DOR shRNA), and rabbit polyclonal antibody against DOR were produced as described (5). Antibodies to β-actin, β-tubulin, and histone H3 were purchased from Sigma-Aldrich.
Cell culture.
Bone marrow stromal cells (BMSCs) were isolated from the femur and tibia of mice and plated at 20 × 106 cells/90-mm petri dish. To induce differentiation into osteoblasts, BMSCs were treated with AA as described (14). Primary osteoblasts were isolated from the calvaria of 4-day-old C57BL/6J mice, as reported previously (30). All mice were housed at the Jerry L. Pettis Memorial Veterans Affairs (VA) Medical Center Veterinary Medical Unit (Loma Linda, CA) under standard approved laboratory conditions. Animal use protocols were approved by the Institutional Animal Care and Use Committee of the Jerry L. Pettis Memorial VA Medical Center. MC3T3-E1 mouse preosteoblasts from American Type Culture Collection (Manassas, VA) were grown in standard α-MEM growth medium containing 10% CS, penicillin (100 U/ml), and streptomycin (100 μg/ml). Twenty-four hours prior to factor treatment, cells were incubated in the presence of serum free medium consisting of α-MEM + 1% penicillin and streptomycin (PS). Cell culture treatments with appropriate factors (T3, T4, cycloheximide, FGF, and CS) were made in α-MEM + 0.1% bovine serum albumin + 1% PS. For differentiation experiments, the medium was also supplemented with AA and β-glycerophosphate.
RNA isolation and gene expression analysis.
RNA was extracted using Trizol and chloroform, and isolation was completed using the RNeasy mini kit (Qiagen, Valencia, CA). Reverse transcription was accomplished using Superscript (Invitrogen, Carlsbad, CA), and the cDNA was used for real-time RT-PCR. Real-time RT-PCR was performed to assess the gene expression of osteocalcin, ALP, and Runx2, as described previously (13). Gene-specific primers were used to amplify DOR (forward: 5′-CCAGCCTTTTCTTCAACACC-3′; reverse: 5′ GCCCCTCTGCAGTAAAACAG-3′). The housekeeping gene peptidylprolyl isomerase A was used as an internal control in the PCR reaction, and the fold change compared with control was calculated according to the formula 2−ΔΔCT.
Cloning of DOR.
Full-length coding sequence of DOR was amplified by PCR with the high-fidelity enzyme Pfx50 (Invitrogen), using Mus Musculus bone cDNA as a template, as described previously (26). The primer sequences are DOR forward (5′-GGGTCGACGCCACCATGCATCATCACCATCACCATTTCCAGCGCTTCACCAGCCTTTTC-3′) and DOR reverse (5′-CGCGGATCCTCAGTAGTTGAACTGGCGCTGGCACGG-3′). The PCR product was purified with GeneClean Spin kit (Qbiogene, Carlsbad, CA) and digested with BamHI and SalI (New England Biolabs, Ipswich, MA). The digested product was purified and then cloned into corresponding restriction sites of pY expression vector. DOR coding sequence was confirmed by DNA sequencing at Arizona State University (Tempe, AZ).
Murine leukemia virus-based viral vector (MLV-based vector) and lentivirus-based vector (HIV-based vectors) production and transduction.
To generate Murine leukemia virus-based viral vector (MLV-based vectors), in a 10-cm plate, 293T cells were transduced with a mixture of 20 μg of MLV-based expression vector [β-galactosidase (β-Gal) or DOR], 10 μg of MLV-GP expression vector, and 1 μg of VSV-G expression vector by CaPO4 precipitation. The conditioned medium containing the viral vectors was collected 48 h after the transduction (38). To generate lentiviral vectors, 293T cells were transduced with a mixture of 10 μg of lentiviral expression vector (scramble control or DOR shRNA), 7 μg of HIV-GP expression vector, pCMVD8.7, and 1 μg of VSV-G expression vector (5). MC3T3-E1 cells or primary murine calvaria osteoblasts were transduced as described previously (26, 38).
ALP activity assay.
ALP assay was performed to determine osteoblast differentiation. Cells were seeded at 8,000 cells/well in a 96-well plate containing α-MEM + 10% CS + 1% PS. The following day, the medium was changed to serum-free α-MEM for 24 h. The cells were incubated with T3 (0.1, 1, 3, and 10 ng/ml) or T4 (10, 100, and 1,000 ng/ml) for 72 h. All treatments contained AA (100 μg/ml) and β-glycerophosphate (10 mM). Cells were then rinsed with PBS and lysed with 0.1% Triton X-100 in 250 mM NaHCO3. Protein concentration was determined by BCA method (Thermo Fisher Scientific, Rockford, IL), and ALP activity was assayed as described previously (15).
Western blot.
Cytoplasmic and nuclear extracts of MC3T3-E1 cells were prepared as described previously (46). Equivalent amounts of protein were resolved on an SDS polyacrylamide gel (12%) and transferred to a PVDF membrane (Millipore, Billerica, MA). Membranes were blocked with 10% nonfat dry milk in Tris-buffered saline with 0.1% Tween 20 overnight with rotation at 4°C. The following day, the membranes were probed with specific antibody to DOR (1:1,000), β-actin (1:10,000), β-tubulin (1:10,000), or anti-histone H3 (1:10,000) for 1 h at room temperature. The membranes were washed and incubated with appropriate horseradish peroxidase-conjugated antibody (1:10,000; Sigma-Aldrich). Detection was performed with Super Signal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific).
Subjects.
For the analysis of polymorphisms, 306 Spanish nondiabetic men from the general population were recruited at Hospital Trueta (Girona, Spain) and subjected to phenotyping. All subjects had fasting plasma glucose <7.0 mM and 2-h postload plasma glucose <11.1 mM in a 75-g oral glucose tolerance test. Inclusion criteria were 1) BMI <40 kg/m2, 2) absence of systemic disease, and 3) absence of infection within the previous month. None of the control subjects were under medication or had evidence of metabolic disease other than obesity. All studies were approved by the ethics committees of the participating centers. All subjects provided written, informed consent prior to participation.
Genotyping.
DOR1 (rs2378256) was genotyped in the samples by restriction digestion with Dde1 (Invitrogen) following PCR amplification with 5′-AGGAGCCGGTAGGAGGGAGTGGAG-3′ (forward) and 5′-CGCCGGCGGAGACAGACAAAG-3′ (reverse). All analyses were performed by ANOVA using appropriately transformed variables within SPSS for Windows version 11.5.
Statistical analysis.
Data are expressed as means ± SE and were analyzed using Student's t-test or ANOVA (1-way or 2-way; Statistica 6, Tulsa, OK) as appropriate. Post hoc analysis was performed using Duncan's test. Values were considered statistically significant when P < 0.05.
RESULTS
DOR expression increases during osteoblast differentiation.
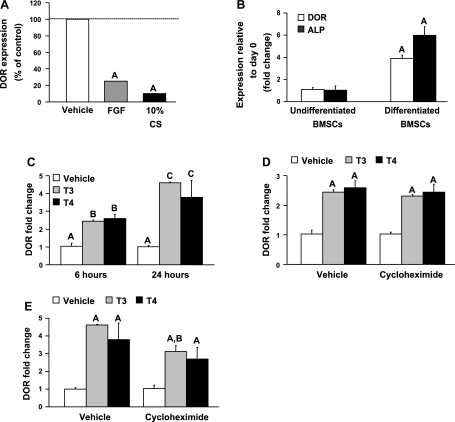
Treatments with FGF and CS that are known to promote proliferation and inhibit differentiation caused a drastic 75 and 90% reduction, respectively, in DOR expression in MC3T3-E1 osteoblasts (Fig. 1A). To determine whether DOR expression is regulated during osteoblast differentiation, as in the case of myoblast differentiation, we examined the effects of agents known to modulate osteoblast differentiation on DOR expression. DOR expression increased 3.8-fold during in vitro differentiation of BMSCs into osteoblasts (Fig. 1B). Differentiation was confirmed by increased ALP gene expression.
Fig. 1.
Diabetes- and obesity-related protein (DOR) expression during osteoblast proliferation and differentiation. A: DOR expression decreases during osteoblast proliferation. MC3T3-E1 cells were treated with or without fibroblast growth factor (FGF; 1 ng/ml) or 10% calf serum (CS) for 24 h. DOR expression was determined by real-time RT-PCR analysis. DOR expression in vehicle-treated control (ΔCT = 9.74 ± 0.07). Values are presented as %vehicle-treated control ± SE (n = 3). AP < 0.01 vs. vehicle. B: DOR expression increases during bone marrow stromal cell (BMSC) differentiation, as measured by real time RT-PCR. BMSCs were cultured with α-MEM containing 10% CS in the presence or absence of ascorbic acid (100 μg/ml) and β-glycerophosphate (10 mM) for 24 days to induce osteoblast differentiation. Alkaline phosphatase (ALP) gene expression was also assessed to confirm BMSC differentiation. DOR expression in undifferentiated BMSCs (ΔCT = 11.24 ± 0.27). Values are presented as fold change relative to day 0 ± SE (n = 6). AP < 0.001 vs. undifferentiated BMSCs. C: thyroid hormone (TH) stimulates DOR gene expression. MC3T3-E1 cells were incubated in the presence or absence of 3,3′,5-triiodothyronine (T3; 10 ng/ml) or l-thyroxine (T4; 100 ng/ml) for 6 or 24 h. DOR expression was determined by real-time RT-PCR analysis. Values are presented as fold change from corresponding vehicle control ± SE (n = 3–5). Data with different letters indicate significant difference at P < 0.05. Treatment groups with same letters are not statistically different. D and E: TH induction of DOR is acute and is minimally affected by de novo protein synthesis. MC3T3-E1 cells were pretreated with cycloheximide (1 μM) for 1 h prior to the addition of T3 (10 ng/ml) or T4 (100 ng/ml) for 6 (D) or 24 h (E). DOR expression was determined by real-time RT-PCR analysis. DOR expression was 60% lower in cycloheximide vehicle group compared with vehicle alone at 24 h. ΔCT = 9.39 ± 0.11, DOR expression in vehicle-treated control. Values are presented as fold change from corresponding vehicle control ± SE (n = 3–5). AP < 0.05 vs. vehicle control; BP < 0.05 vs. T3 alone.
To examine whether TH regulates DOR expression and whether the effect is acute, DOR mRNA expression levels were measured at 6 and 24 h. Treatment with T3 or T4 increased DOR expression at 6 (2.4- and 2.6-fold) and 24 h (4.6- and 3.8-fold), respectively (Fig. 1C). To examine whether TH induction occurs in the absence of de novo protein synthesis, MC3T3-E1 cells were treated with T3 or T4 in the presence of cycloheximide (1 μM inhibits >80% protein synthesis) for 6 or 24 h. Pretreatment with cycloheximide did not affect TH induction of DOR expression at 6 h (Fig. 1D). DOR expression was also induced at 24 h by T3 and T4 in the presence of cycloheximide; however, it was slightly reduced, suggesting a minor effect on protein synthesis (Fig. 1E).
DOR modulates TH-induced osteoblast differentiation.
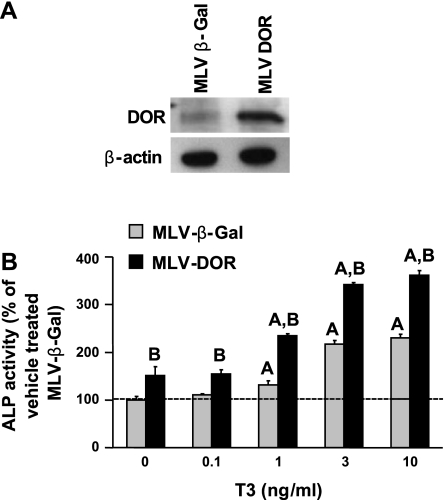
To determine the consequence of increased DOR expression during osteoblast differentiation in modulating TH biological effects, we overexpressed DOR or β-Gal in MC3T3-E1 cells and examined TH effects on ALP activity. Figure 2A shows increased DOR expression as determined by Western blot in MC3T3-E1 cells transduced with a retroviral vector expressing DOR compared with cells expressing β-Gal. As expected, TH increased ALP activity compared with vehicle control in both β-Gal- and DOR-overexpressing cells. However, DOR-overexpressing cells exhibited two- to fivefold higher ALP activity than β-Gal in response to T3 (Fig. 2B).
Fig. 2.
DOR overexpression exacerbates T3-induced increase in ALP activity. A: expression of DOR in MC3T3-E1 cells transduced with murine leukemia virus-β-galactosidase (MLV-β-Gal) or MLV-DOR as determined by Western blot using whole cell lysates. B: MLV-β-Gal- and MLV-DOR-overexpressing MC3T3-E1 cells were treated with T3 (0.1, 1, 3, and 10 ng/ml), and ALP activity was determined 72 h after treatment. Values are presented as %vehicle-treated MLV-β-Gal ± SE (n = 5–8). Basal ALP activity values of MLV-β-Gal (1.48 mU/mg protein ± 0.11) and DOR-overexpressing cells (2.52 mU/mg protein ± 0.18). Data were analyzed by 2-way ANOVA, and a significant interaction was observed between gene and treatment. AP < 0.05 vs. vehicle-treated control; BP < 0.05 vs. MLV-β-Gal at corresponding dose.
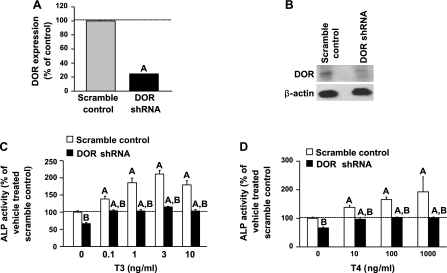
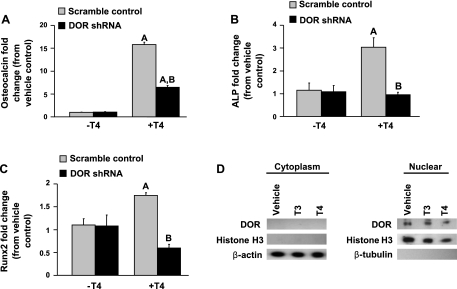
We next evaluated the consequence of DOR inhibition on TH-induced osteoblast differentiation by utilizing lentiviral particles expressing short hairpin RNA (shRNA) against DOR. DOR expression decreased by 75% at the mRNA level and 70% at the protein level (Fig. 3, A and B) in cells expressing DOR shRNA compared with scramble control. Basal ALP activity was reduced by 34% in DOR shRNA cells (Fig. 3, C and D). T3 treatment increased ALP activity in scramble control MC3T3-E1 cells twofold. However, ALP activity was markedly reduced by 96% at 10 ng/ml T3 in DOR shRNA cells compared with control shRNA cells (Fig. 3C). Because we found that T3, the biologically active form of TH, increased ALP activity, we next examined the effect of prohormone T4 in DOR shRNA cells. T4 treatment increased ALP activity in MC3T3-E1 cells in a dose-dependent manner, but at a much higher dose compared with T3. However, DOR shRNA cells exhibited 95% less ALP activity than scramble control at 100 ng/ml T4 (Fig. 3D).
Fig. 3.
Inhibition of DOR reduces TH stimulation of ALP activity in MC3T3-E1 cells. A: expression of DOR in MC3T3-E1 cells transduced with scramble control short-hairpin RNA (shRNA) or DOR shRNA as determined by real-time RT-PCR analysis. Values are presented as means ± SE (n = 4). AP < 0.01 vs. scramble control. B: DOR protein level as determined by Western blot using whole cell lysates. Scramble control and DOR shRNA MC3T3-E1 cells were treated with T3 (0.1, 1, 3, and 10 ng/ml; C) or T4 (10, 100, and 1,000 ng/ml; D), and ALP activity was determined 72 h after treatment. Values are presented as %vehicle-treated scramble control ± SE (n = 6–8). Basal ALP activity values of scramble control (0.35 mU/mg protein ± 0.01) and DOR shRNA (0.23 mU/mg protein ± 0.01). Data were analyzed by 2-way ANOVA, and a significant interaction was observed between gene and treatment. AP < 0.05 vs. vehicle-treated control; BP < 0.05 vs. scramble control at corresponding dose.
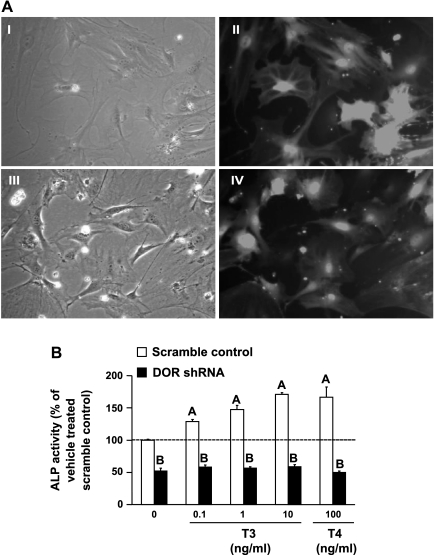
To confirm our results in MC3T3-E1 cells, ALP activity was also evaluated in primary mouse calvaria osteoblasts transduced with DOR shRNA. The transduction efficiency was determined by examining green fluorescent protein (GFP) fluorescence, since scramble and DOR lentiviral constructs also express GFP reporter gene. As shown in Fig. 4A, the transduction efficiency was >90% in primary calvaria osteoblasts transduced with scramble control shRNA and DOR shRNA. These results were also confirmed by sorting GFP-positive cells using FACS analysis (data not shown). Basal ALP activity was dramatically suppressed by 50% in DOR shRNA cells. As expected, ALP activity increased in a dose-dependent manner in scramble control shRNA osteoblasts, with a maximal increase of 71% at 10 ng/ml T3. In contrast, treatment with T3 or T4 failed to elicit a substantial increase in ALP activity in DOR shRNA cells (Fig. 4B).
Fig. 4.
TH-induced increase in ALP activity is severely reduced in primary calvaria osteoblasts transduced with DOR shRNA. A: transduction efficiency of primary calvaria osteoblasts transduced with scramble control or DOR shRNA. Pictures are representative of scramble control-bright field (I), scramble control-GFP fluorescence (II), DOR shRNA-bright field (III), and DOR shRNA-GFP fluorescence (IV). Images were taken at ×40 magnification with an Olympus IX70. B: scramble control and DOR shRNA primary calvaria osteoblasts were treated with T3 (0.1, 1, and 10 ng/ml) or T4 (100 ng/ml), and ALP activity was determined 72 h after treatment. Values are presented as %vehicle-treated scramble control ± SE (n = 6). Basal ALP activity values of scramble control (43.1 mU/mg protein ± 0.67) and DOR shRNA (21.2 mU/mg protein ± 1.5). Data were analyzed by 2-way ANOVA, and a significant interaction was observed between gene and treatment. AP < 0.05 vs. vehicle treated control; BP < 0.001 vs. scramble control at corresponding dose.
Inhibition of DOR impairs TH-induced expression of osteogenic markers.
On the basis of our finding that DOR shRNA cells exhibited reduced ALP activity, we next examined the gene expression of osteoblast-specific differentiation markers stimulated by T4 in scramble control and DOR shRNA MC3T3-E1 cells. Expression of osteocalcin, an osteoblast differentiation marker protein, increased 16-fold. However, osteocalcin expression decreased by 60% in DOR shRNA cells compared with scramble control shRNA (Fig. 5A). Expression of ALP, another osteoblast differentiation marker protein, increased threefold in scramble control, but T4 failed to stimulate ALP gene expression in DOR shRNA cells (Fig. 5B). We also evaluated the effect on Runx2, a major transcription factor involved in osteoblast differentiation. T4 treatment increased Runx2 gene expression nearly twofold in scramble control but failed to stimulate Runx2 gene expression in DOR shRNA cells (Fig. 5C). Basal gene expression was similar between untreated scramble control and untreated DOR shRNA cells. On the basis of our findings we would expect DOR to be a nuclear factor. Cell fractionation of the cytoplasm and nucleus from MC3T3-E1 cells revealed that DOR protein expression was present only in the nuclear extract in both vehicle- and TH-treated cultures (Fig. 5D). Furthermore, acute treatment with T3 or T4 did not alter nuclear localization of DOR, a finding consistent with an earlier report (5).
Fig. 5.
DOR deficiency decreases the gene expression of osteoblast-specific markers. Scramble control and DOR shRNA MC3T3-E1 cells were incubated in the presence or absence of T4 (100 ng/ml) for 24 (osteocalcin) or 72 h (ALP and Runx2). All treatments contained ascorbic acid (100 μg/ml) and β-glycerophosphate (10 mM). Expression of osteocalcin (A), ALP (B), and Runx2 (C) was determined by real-time RT-PCR. Values are presented as fold change ± SE (n = 4). AP < 0.05 vs. vehicle control; BP < 0.05 vs. scramble control. D: DOR is localized to the nucleus. DOR expression was evaluated by Western blot in MC3T3-E1 cells incubated in the presence or absence of T3 (10 ng/ml) or T4 (100 ng/ml) for 1 h. Cell lysates from cytosolic and nuclear fractions were analyzed, and histone H3, β-actin, and β-tubulin served as internal controls. Data are representative of 4 independent experiments (n = 4).
A single nucleotide polymorphism in DOR promoter associates with circulating osteocalcin in healthy subjects.
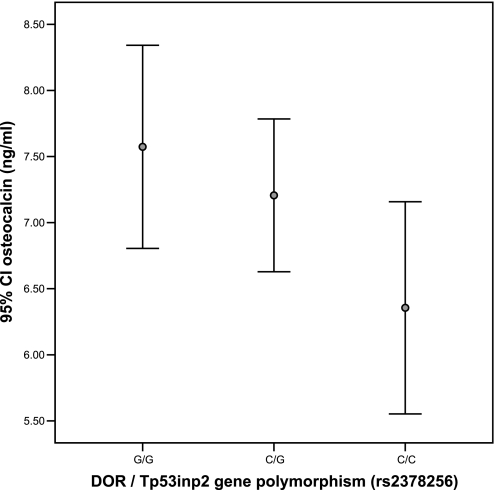
The human DOR gene (current HUGO ID: c20orf110, LocusLink ID 58476) contains 5 exons, although alternative splicing leads to at least five transcript variants. In the longest transcript (Ensembl ID OTTHUMT00020002515), the protein coding region starts at exon 3. DOR maps to chromosome 20q11.22. Resequencing of exonic sequence in 20 nondiabetic Spanish subjects, together with inspection of CELERA and public databases, failed to identify any common variants within the DOR coding sequence but did reveal a common variant (DOR1, rs2378256) located 23 bp upstream of the transcription start site (predicted, and as confirmed by 5′-RACE) (data not shown). Based on these data, we looked for possible genotype-phenotype correlations in a cohort of healthy individuals. Of the 306 nondiabetic, middle-aged Spanish men, those with the CC genotype (n = 55) showed lower osteocalcin levels in plasma compared with G carriers (Fig. 6). CC and G carrier subjects did not show any differences in age, BMI, or fasting glycemia (Table 1).
Fig. 6.
Ninety-five percent confidence interval (95% CI) of circulating osteocalcin levels according to DOR/Tp53inp2 gene polymorphism. Osteocalcin levels in G/G vs. C/C carriers were significantly different.
Table 1.
Clinical characteristics of subjects
| G/(−) Carriers (n = 251) | C/C Subjects (n = 55) | P Value | |
|---|---|---|---|
| Age, yr | 49.1 ± 12.4 | 50.6 ± 14.6 | 0.4 |
| BMI, kg/m2 | 31.2 ± 8.1 | 30.6 ± 7.7 | 0.6 |
| Fasting glucose, mg/dl | 100.9 ± 22.9 | 102.7 ± 20.4 | 0.6 |
| Osteocalcin, ng/ml | 7.31 ± 3.7 | 6.13 ± 2.8 | 0.01 |
Values are means ± SE. BMI, body mass index.
DISCUSSION
In this study, we provide the first experimental evidence that DOR is involved in mediating the effects of TH on osteoblast differentiation. Specifically, DOR overexpression potentiated the effect of TH on ALP activity. In contrast, inhibition of DOR attenuated the stimulatory effect of TH on ALP activity and also reduced TH-induced gene expression of osteoblast-specific markers. DOR expression was upregulated during osteoblast differentiation, and DOR was differentially regulated by TH. We also demonstrate that DOR was localized to the nucleus. In addition, a single nucleotide polymorphism (SNP), DOR1, found on the promoter of the human DOR gene was associated with plasma osteocalcin levels. Therefore, our data support an important role for DOR in mediating TH-induced osteoblast differentiation and associating with plasma osteocalcin levels in men.
To determine a role for DOR in osteoblasts, we first evaluated expression levels of DOR in response to agents that regulate proliferation and/or differentiation of osteoblasts. We found that DOR expression increased robustly during in vitro differentiation of bone marrow stromal cells into osteoblasts and also in the presence of T3 and T4, known inducers of osteoblast differentiation. This finding is consistent with reports by Baumgartner et al. (5), who described increased DOR expression during myoblast differentiation, and by Malik et al. (28), who found that T3 treatment of rat hepatocytes increased DOR expression. In contrast, DOR expression was significantly downregulated in the presence of FGF and CS, agents that stimulate proliferation. During the switch to cellular proliferation, the expression and/or activity of signaling molecules involved in cellular differentiation decreases. Whereas proliferation-specific genes such as c-fos/c-jun and histone are upregulated during proliferation, the expression of these genes is downregulated during the transition from cell cycle growth to differentiation. Others have demonstrated the inverse relationship that exists between the expression of proliferation- and differentiation-specific genes (22, 27, 41). T3 inhibits cell proliferation by downregulating histone and cell cycle-specific gene expression, but it increases the expression of ALP and osteocalcin markers of differentiated osteoblasts (12, 43, 45). Therefore, DOR downregulation observed during cell proliferation is consistent with a role of this protein in osteoblast differentiation.
In regard to the potential mechanisms regulating DOR expression during differentiation in osteoblasts, it is possible that the increased expression of DOR may be mediated by exogenously added ascorbic acid and/or local growth factors produced by differentiating osteoblasts. It is well established that bone morphogenetic protein-2 (BMP-2) and IGF-I increase during osteoblast differentiation. Therefore, it is feasible that the endogenous production of these factors may act as local growth factors in mediating DOR expression. In this regard, we found that ascorbic acid and/or BMP-2 increased the expression of DOR in C3H10T1/2 cells (data not shown). TH effects on DOR expression may be direct or indirect via growth factors. Using UMR-106 rat osteoblastic cells and fetal rat limb bone cultures, Lakatos et al. (24) observed that T3 and T4 increased the secretion of IGF-I into the conditioned medium. T3 and T4 treatment increases IGF-I expression, and IGF-I is important for mediating the anabolic effects of TH (18, 44). TH also influences the IGF/IGF-binding protein system in vivo in hyperthyroid patients (25).
It is also possible that T3 and T4 directly regulate DOR expression. DOR expression was acutely regulated by T3 and T4 at 6 h, suggesting that it may be an early-response gene. Moreover, we found that TH effect on DOR is independent of new protein synthesis. These data are consistent with a recent study by Malik et al. (28), who observed early gene expression of DOR in hepatocytes stimulated with T3. Sequence analyses of DOR promoter revealed four putative sites containing TRE consensus sequence (AGGTCA) located within the promoter region 5 kb upstream of the transcription start site. Further studies are needed to determine whether TH regulates transcription of DOR gene via its TRE.
If DOR is involved in mediating TH biological effects, then modulating DOR expression should influence TH effects on osteoblast differentiation. It is well established that TH plays a role in stimulating osteoblast differentiation. Accordingly, in our study ALP activity increased significantly following treatment with T3 and T4. These findings are consistent with previous studies in MC3T3-E1, ROS 17/2.8, and primary calvarial osteoblasts that have demonstrated stimulation of ALP activity in the presence of T3 and T4 (1, 20, 39, 45). The overexpression of DOR enhanced the effects of TH on ALP activity. In contrast, ALP activity was decreased substantially in DOR shRNA MC3T3E-1 cells and primary osteoblasts stimulated with TH. Therefore, these data demonstrate that DOR plays an important role in mediating TH signaling in osteoblasts during differentiation, and these findings are consistent with the role of DOR in myoblasts (5). However, ALP activity was not completely abolished in DOR-deficient cells. This observation raises the possibility that DOR may not be the sole mediator of TH signaling in osteoblasts and suggests that other factors are involved. Recently, several studies have described nongenomic effects of T3 and T4 (1, 7, 9, 19, 40). Therefore, additional mechanisms regulating TH action besides the genomic pathway may potentially be involved. However, in our experiments, it seems unlikely that DOR is a cytoplasmic factor that nongenomically modulates TH signaling since DOR was not found in the cytoplasm of MC3T3-E1 cells. Instead, our data demonstrated that DOR was detected predominantly in the nuclear fraction, suggesting that it may act via a genomic mechanism. Further studies are needed to examine this possibility.
It is well known that the biological actions of TH are mediated via TR, namely TRα and TRβ. Although both TR isoforms are expressed in bone, the major isoform in bone is TRα (3). The receptor binds as either a homodimer (TR/TR) or heterodimer (TR/RXR) at the TRE in the promoter of TH target genes to modulate gene transcription. However, it remains to be determined whether DOR interacts with the homodimer or heterodimer in osteoblasts.
We found that DOR loss of function in MC3T3-E1 cells and primary calvaria osteoblasts decreased basal ALP activity in the absence of exogenously added TH. Osteoblasts produce a number of growth factors, including BMP-2 and IGF-I during differentiation. It remains to be determined whether the effect is due to DOR mediating the effects of other factors besides TH. This observation was evident in primary osteoblasts derived from calvaria of 4-day-old C57BL/6J mice and in the preosteoblast cell line MC3T3-E1. The loss of DOR alone decreased myoblast gene expression in the absence of T3 (5), whereas we did not observe an effect on osteoblast gene expression in the present study. Potential explanations for these differences can be due to the cell type, anatomic origin, passage number, and differentiation status (11, 17).
Consistent with our findings, other studies have shown that T4 regulates gene expression of ALP and osteocalcin in osteoblasts (23, 42). Moreover, the suppression of DOR impaired T4-induced expression of osteoblast-specific markers. These findings suggest that loss of DOR reduces the osteogenic effect of TH in bone. These data are in agreement with a previous report showing that loss of DOR decreased TH-induced myoblast gene expression (5). Asai et al. (1) found that Runx2 expression was unchanged after stimulation with T3. In contrast, we observed a modest increase in Runx2 expression following treatment with T4. Potential explanations for these differences could be due to the length of treatment with TH (36 vs. 72 h) and/or the form of TH used in the experiments (T3 vs. T4). In our study, treatment with T3 and T4 induced DOR gene expression and increased ALP activity in both MC3T3-E1 cells and primary calvarial osteoblasts. T4 was used at a much higher dose since T3 is the biologically active form of TH and because TR has a higher affinity for T3 ligand (36). Consistent with a previous report (44), we did not observe a significant difference in the responsiveness of the cells between T4 (prohormone) and T3 (active form).
Presently, this is the first published study to demonstrate that a DOR SNP in humans associates with plasma levels of the bone formation marker osteocalcin. In agreement with this finding in humans, our in vitro studies demonstrated that inhibition of DOR decreased gene expression of osteocalcin and other bone formation markers. It is possible that DOR gene expression plays a significant role in modulating the expression of bone formation markers. This finding in humans further strengthens our contention that DOR is an important mediator of osteoblast functions and differentiation. Analysis of putative transcription factor binding sites using Transcription Element Search Software (40a) revealed a potential binding site for RXR and T3Rα in the region containing the DOR1 SNP. The binding site for RXR and T3Rα was conserved across the promoters of human, mouse, and rat. Further studies are needed to determine the functional significance of the polymorphism in mediating changes in DOR transcription and expression levels.
Besides its role in modulating TH action and cellular differentiation, other biological functions of DOR have been described in mammalian and drosophila systems. Bennetts et al. (6) demonstrated that DOR is involved in mouse embryonic development. It is expressed in the developing heart and nervous system as well as during limb and craniofacial development. DOR also plays a role in mammalian cell autophagy by recruiting specific proteins required for autophagosome development (29, 33, 34). Alterations in the processing or expression of DOR may also lead to tumorigenesis. Alternative splicing of DOR by heterogenous ribonucleoprotein A2 promotes ovarian cancer cell migration and invasion (31). A 700-kb deletion of chromosome 20 encompassing DOR was detected in hairy cell leukemia patients (32). In Drosophila melanogaster, DOR acts as a coactivator for ecdysone receptor (10). Collectively, these findings provide evidence that DOR has broad effects beyond modulation of TH action and is significant in various biological processes.
In conclusion, we have demonstrated that DOR is differentially regulated by TH and acts as a stimulatory mediator of TH effects in osteoblasts. DOR may also play a role in regulating osteoblast differentiation; however, other factors besides DOR may also be important in mediating TH effects on osteoblast differentiation. Further studies identifying such molecules will advance our understanding of the molecular mechanisms responsible for TH signaling in osteoblasts.
GRANTS
This work was supported by National Institutes of Health Grants R01-A-R48139, 1F31-AR-056204, and 5R25-GM-060507 and by Centro de Investigación Biomédica en Red de diabetes y Enfermedades Metabólicas Asociadas (Instituto de Salud Carlos III, Spain).
DISCLOSURES
No conflict of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Joe Rung-Aroon, Catrina Alarcon, Biblia Kim, and Vrajeta Joshi for their expert technical assistance. All in vitro work was performed with the facilities provided by the Department of Veterans Affairs in Loma Linda, CA. SNP study in human subjects was performed at Hospital Trueta and Universitat de Barcelona in Spain.
REFERENCES
- 1. Asai S, Cao X, Yamauchi M, Funahashi K, Ishiguro N, Kambe F. Thyroid hormone non-genomically suppresses Src thereby stimulating osteocalcin expression in primary mouse calvarial osteoblasts. Biochem Biophys Res Commun 387: 92–96, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Bassett J, Harvey C, Williams G. Mechanisms of thyroid hormone receptor-specific nuclear and extra nuclear actions. Mol Cell Endocrinol 213: 1–11, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bassett J, Williams G. Critical role of the hypothalamic-pituitary-thyroid axis in bone. Bone 43: 418–426, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bassett J, Williams G. The molecular actions of thyroid hormone in bone. Trends Endocrinol Metab 14: 356–364, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Baumgartner B, Orpinell M, Duran J, Ribas V, Burghardt H, Bach D, Villar A, Paz J, González M, Camps M, Oriola J, Rivera F, Palacín M, Zorzano A. Identification of a novel modulator of thyroid hormone receptor-mediated action. PLoS One 2: e1183, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bennetts J, Rendtorff N, Simpson F, Tranebjaerg L, Wicking C. The coding region of TP53INP2, a gene expressed in the developing nervous system, is not altered in a family with autosomal recessive non-progressive infantile ataxia on chromosome 20q11–q13. Dev Dyn 236: 843–852, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Bergh J, Lin H, Lansing L, Mohamed S, Davis F, Mousa S, Davis P. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 146: 2864–2871, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Burge R, Dawson-Hughes B, Solomon D, Wong J, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22: 465–475, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Davis P, Davis F, Cody V. Membrane receptors mediating thyroid hormone action. Trends Endocrinol Metab 16: 429–435, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Francis VA, Zorzano A, Teleman AA. dDOR is an EcR coactivator that forms a feed-forward loop connecting insulin and ecdysone signaling. Curr Biol 20: 1799–1808, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Fratzl-Zelman N, Glantschnig H, Rumpler M, Nader A, Ellinger A, Varga F. The expression of matrix metalloproteinase-13 and osteocalcin in mouse osteoblasts is related to osteoblastic differentiation and is modulated by 1,25-dihydroxyvitamin D3 and thyroid hormones. Cell Biol Int 27: 459–468, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Gouveia C, Schultz J, Bianco A, Brent G. Thyroid hormone stimulation of osteocalcin gene expression in ROS 17/2.8 cells is mediated by transcriptional and post-transcriptional mechanisms. J Endocrinol 170: 667–675, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Govoni K, Amaar Y, Kramer A, Winter E, Baylink D, Mohan S. Regulation of insulin-like growth factor binding protein-5, four and a half lim-2, and a disintegrin and metalloprotease-9 expression in osteoblasts. Growth Horm IGF Res 16: 49–56, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Govoni K, Lee S, Chadwick R, Yu H, Kasukawa Y, Baylink D, Mohan S. Whole genome microarray analysis of growth hormone-induced gene expression in bone: T-box3, a novel transcription factor, regulates osteoblast proliferation. Am J Physiol Endocrinol Metab 291: E128–E136, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Govoni K, Linares G, Chen S, Pourteymoor S, Mohan S. T-box 3 negatively regulates osteoblast differentiation by inhibiting expression of osterix and runx2. J Cell Biochem 106: 482–490, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harvey C, O'Shea P, Scott A, Robson H, Siebler T, Shalet S, Samarut J, Chassande O, Williams G. Molecular mechanisms of thyroid hormone effects on bone growth and function. Mol Genet Metab 75: 17–30, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Harvey C, Stevens D, Williams A, Jackson D, O'Shea P, Williams G. Analysis of thyroid hormone responsive gene expression in osteoblastic cells. Mol Cell Endocrinol 213: 87–97, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Huang B, Golden L, Tarjan G, Madison L, Stern P. Insulin-like growth factor I production is essential for anabolic effects of thyroid hormone in osteoblasts. J Bone Miner Res 15: 188–197, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Ishisaki A, Tokuda H, Yoshida M, Hirade K, Kunieda K, Hatakeyama D, Shibata T, Kozawa O. Activation of p38 mitogen-activated protein kinase mediates thyroid hormone-stimulated osteocalcin synthesis in osteoblasts. Mol Cell Endocrinol 214: 189–195, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Kasono K, Sato K, Han D, Fujii Y, Tsushima T, Shizume K. Stimulation of alkaline phosphatase activity by thyroid hormone in mouse osteoblast-like cells (MC3T3-E1): a possible mechanism of hyperalkaline phosphatasia in hyperthyroidism. Bone Miner 4: 355–363, 1988 [PubMed] [Google Scholar]
- 21. Kim S, Ho S, Hong S, Kim K, So E, Christoffolete M, Harney J. A novel mechanism of thyroid hormone-dependent negative regulation by thyroid hormone receptor, nuclear receptor corepressor (NCoR), and GAGA-binding factor on the rat cD44 promoter. J Biol Chem 280: 14545–14555, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Kockx M, McCabe L, Stein J, Lian J, Stein G. Influence of DNA replication inhibition on expression of cell growth and tissue-specific genes in osteoblasts and osteosarcoma cells. J Cell Biochem 54: 47–55, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Kung A, Ng F. A rat model of thyroid hormone-induced bone loss: effect of antiresorptive agents on regional bone density and osteocalcin gene expression. Thyroid 4: 93–98, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Lakatos P, Caplice M, Khanna V, Stern P. Thyroid hormones increase insulin-like growth factor I content in the medium of rat bone tissue. J Bone Miner Res 8: 1475–1481, 1993 [DOI] [PubMed] [Google Scholar]
- 25. Lakatos P, Foldes J, Nagy Z, Takacs I, Speer G, Horvath C, Mohan S, Baylink D, Stern P. Serum insulin-like growth factor-I, insulin-like growth factor binding proteins, and bone mineral content in hyperthyroidism. Thyroid 10: 417–423, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Linares G, Xing W, Govoni K, Chen S, Mohan S. Glutaredoxin 5 regulates osteoblast apoptosis by protecting against oxidative stress. Bone 44: 795–804, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Machwate M, Jullienne A, Moukhtar M, Marie P. Temporal variation of c-Fos proto-oncogene expression during osteoblast differentiation and osteogenesis in developing rat bone. J Cell Biochem 57: 62–70, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Malik IA, Baumgartner BG, Naz N, Sheikh N, Moriconi F, Ramadori G. Changes in gene expression of DOR and other thyroid hormone receptors in rat liver during acute-phase response. Cell Tissue Res 342: 261–272, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mauvezin C, Orpinell M, Francis V, Mansilla F, Duran J, Ribas V, Palacín M, Boya P, Teleman A, Zorzano A. The nuclear cofactor DOR regulates autophagy in mammalian and Drosophila cells. EMBO Rep 11: 37–44, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miyakoshi N, Richman C, Kasukawa Y, Linkhart T, Baylink D, Mohan S. Evidence that IGF-binding protein-5 functions as a growth factor. J Clin Invest 107: 73–81, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moran-Jones K, Grindlay J, Jones M, Smith R, Norman J. hnRNP A2 regulates alternative mRNA splicing of TP53INP2 to control invasive cell migration. Cancer Res 69: 9219–9227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nordgren A, Corcoran M, Sääf A, Bremer A, Kluin-Nelemans H, Schoumans J, Grandér D. Characterisation of hairy cell leukaemia by tiling resolution array-based comparative Genome hybridisation: a series of 13 cases and review of the literature. Eur J Haematol 84: 17–25, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Nowak J, Archange C, Tardivel-Lacombe J, Pontarotti P, Pébusque M, Vaccaro M, Velasco G, Dagorn J, Iovanna J. The TP53INP2 protein is required for autophagy in mammalian cells. Mol Biol Cell 20: 870–881, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nowak J, Iovanna J. TP53INP2 is the new guest at the table of self-eating. Autophagy 5: 383–384, 2009 [DOI] [PubMed] [Google Scholar]
- 35. O'Shea P, Guigon C, Williams G, Cheng S. Regulation of fibroblast growth factor receptor-1 (FGFR1) by thyroid hormone: identification of a thyroid hormone response element in the murine Fgfr1 promoter. Endocrinology 148: 5966–5976, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Oetting A, Yen PM. New insights into thyroid hormone action. Best Pract Res Clin Endocrinol Metab 21: 193–208, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Peng H, Chen S, Wergedal J, Polo J, Yee J, Lau K, Baylink D. Development of an MFG-based retroviral vector system for secretion of high levels of functionally active human BMP4. Mol Ther 4: 95–104, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Sato K, Han D, Fujii Y, Tsushima T, Shizume K. Thyroid hormone stimulates alkaline phosphatase activity in cultured rat osteoblastic cells (ROS 17/2.8) through 3,5,3′-triiodo-l-thyronine nuclear receptors. Endocrinology 120: 1873–1881, 1987 [DOI] [PubMed] [Google Scholar]
- 40. Scarlett A, Parsons M, Hanson P, Sidhu K, Milligan T, Burrin J. Thyroid hormone stimulation of extracellular signal-regulated kinase and cell proliferation in human osteoblast-like cells is initiated at integrin alphaVbeta3. J Endocrinol 196: 509–517, 2008 [DOI] [PubMed] [Google Scholar]
- 40a. Schug J, Overton GC. TESS: Transcription Element Search Software on the WWW. Computational Biology and Informatics Laboratory, School of Medicine, Univ. of Pennsylvania. http://www.cbil.upenn.edu/tess [26 February 1998].
- 41. Shalhoub V, Gerstenfeld L, Collart D, Lian J, Stein G. Downregulation of cell growth and cell cycle regulated genes during chick osteoblast differentiation with the reciprocal expression of histone gene variants. Biochemistry 28: 5318–5322, 1989 [DOI] [PubMed] [Google Scholar]
- 42. Suwanwalaikorn S, Van Auken M, Kang M, Alex S, Braverman L, Baran D. Site selectivity of osteoblast gene expression response to thyroid hormone localized by in situ hybridization. Am J Physiol Endocrinol Metab 272: E212–E217, 1997 [DOI] [PubMed] [Google Scholar]
- 43. van den Ent F, van Wijnen A, Last T, Bortell R, Stein J, Lian J, Stein G. Concerted control of multiple histone promoter factors during cell density inhibition of proliferation in osteosarcoma cells: reciprocal regulation of cell cycle-controlled and bone-related genes. Cancer Res 53: 2399–2409, 1993 [PubMed] [Google Scholar]
- 44. Varga F, Rumpler M, Klaushofer K. Thyroid hormones increase insulin-like growth factor mRNA levels in the clonal osteoblastic cell line MC3T3-E1. FEBS Lett 345: 67–70, 1994 [DOI] [PubMed] [Google Scholar]
- 45. Varga F, Rumpler M, Luegmayr E, Fratzl-Zelman N, Glantschnig H, Klaushofer K. Triiodothyronine, a regulator of osteoblastic differentiation: depression of histone H4, attenuation of c-fos/c-jun, and induction of osteocalcin expression. Calcif Tissue Int 61: 404–411, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Xing W, Archer TK. Upstream stimulatory factors mediate estrogen receptor activation of the cathepsin D promoter. Mol Endocrinol 12: 1310–1321, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Zhang J, Lazar M. The mechanism of action of thyroid hormones. Annu Rev Physiol 62: 439–466, 2000 [DOI] [PubMed] [Google Scholar]