Abstract
Plasma membrane electron transport (PMET), a cytosolic/plasma membrane analog of mitochondrial electron transport, is a ubiquitous system of cytosolic and plasma membrane oxidoreductases that oxidizes cytosolic NADH and NADPH and passes electrons to extracellular targets. While PMET has been shown to play an important role in a variety of cell types, no studies exist to evaluate its function in insulin-secreting cells. Here we demonstrate the presence of robust PMET activity in primary islets and clonal β-cells, as assessed by the reduction of the plasma membrane-impermeable dyes WST-1 and ferricyanide. Because the degree of metabolic function of β-cells (reflected by the level of insulin output) increases in a glucose-dependent manner between 4 and 10 mM glucose, PMET was evaluated under these conditions. PMET activity was present at 4 mM glucose and was further stimulated at 10 mM glucose. PMET activity at 10 mM glucose was inhibited by the application of the flavoprotein inhibitor diphenylene iodonium and various antioxidants. Overexpression of cytosolic NAD(P)H-quinone oxidoreductase (NQO1) increased PMET activity in the presence of 10 mM glucose while inhibition of NQO1 by its inhibitor dicoumarol abolished this activity. Mitochondrial inhibitors rotenone, antimycin A, and potassium cyanide elevated PMET activity. Regardless of glucose levels, PMET activity was greatly enhanced by the application of aminooxyacetate, an inhibitor of the malate-aspartate shuttle. We propose a model for the role of PMET as a regulator of glycolytic flux and an important component of the metabolic machinery in β-cells.
Keywords: insulin secretion, pancreatic β-cells, NAD(P)H, cytosolic oxidoreductase, extracellular oxidants
the discovery of the plasma membrane electron transport system (PMET) was driven by the observation that membrane-impermeable oxidants are reduced by intact cells (reviewed in Ref. 10). PMET has since been identified as a ubiquitous critical player in a variety of cellular functions, including redox homeostasis (6, 11, 32, 33), cell proliferation and survival (8, 19, 47), and cellular defense (26).
Physically, PMET consists of a complex network of cytosolic and plasma membrane-associated oxidoreductases that transfer electrons from cytosolic-reducing equivalents, NADH and NADPH, via CoQ10 in the plasma membrane to extracellular electron acceptors (10). Several enzymes implicated in PMET activity include cytosolic NADH-cytochrome B5 oxidoreductase (43), cytosolic NAD(P)H-quinone oxidoreductase (NQO1) (7), formerly known as diaphorase, and the so far unnamed NQO1 analog with higher affinity for NADPH than NADH, which performs the same two electron redox reactions as NQO1 (24). The components of the PMET system vary by cell type; phagocytes, for instance, express specialized PMET enzymes, and NADPH oxidases, such as NOX2, and cells simultaneously express multiple enzymes capable of supporting PMET activity.
Operation of PMET results in the reoxidation of cytosolic reduced nicotinamides NADH and NADPH. NADH and NADPH are generated in the cytosol by processes that, dependent on the cell type, include glycolysis, the pentose phosphate pathway, and pyruvate cycling pathways. Besides ferric ion and ascorbate (10, 27), a primary extracellular electron acceptor is molecular oxygen (2, 8, 32, 39). Indeed, it is estimated that the 10–15% of cellular oxygen consumption that is potassium cyanide (KCN) resistant (and therefore independent of mitochondrial metabolism) is mediated by the reduction of molecular oxygen through PMET (28). PMET activity is routinely measured by the reduction of membrane-impermeable extracellular oxidants, such as ferricyanide (9), toluidine blue O polyacrylamide (1), or, most commonly used, the membrane-impermeable tetrazolium dye 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-1) (5). WST-1 is unlike the older generation of tetrazolium dyes, such as 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide, that carry a net positive charge that enables cellular uptake and subsequent reduction by mitochondrial enzymes such as succinate dehydrogenase (4). WST-1 instead carries a net negative charge that makes it impermeable to cell membranes. WST-1 is reduced by an intermediate electron acceptor, 1-methoxy-5-methyl-phenazinium methyl sulfate (mPMS), which also fails to penetrate the mitochondrial membrane (25, 30). In addition, the mitochondrial fuel succinate only marginally affects WST-1 reduction in the presence of mitochondrial fractions (14), indicating that mPMS acquires electrons from sources independent of complex II. This demonstrates that the WST-1/mPMS system acquires electrons at the cell surface or plasma membrane and reduces WST-1 via two single electron reduction events (14).
Both NADH and NADPH are generated as a consequence of glucose metabolism in β-cells. Pyruvate, the end product of glycolysis, enters mitochondrial metabolism via both decarboxylation and carboxylation pathways (reviewed in Ref. 23). Pyruvate carboxylation is linked to pyruvate cycling (13, 29, 37) and the resulting production of NADPH, which has been proposed to act as an important coupling mediator of insulin secretion (22). Glycolytically derived NADH must be reoxidized back to NAD+ to allow glycolysis to continue. This is accomplished in β-cells partly via mitochondrial malate/aspartate and glycerolphosphate shuttles (12), and via PMET, as we suggest here and by an earlier study which demonstrated that NADH could be reoxidized despite blockage of mitochondrial shuttles in pancreatic β-cells. The “presence of an unknown cytosolic factor re-oxidizing cytosolic NADH,” was suggested (12, 45) based on the observation that, despite blockage of mitochondrial shuttles, glycolytic flux remained unchanged (12).
Here we demonstrate for the first time the presence of a robust glucose-dependent PMET activity in pancreatic β-cells, mediated in part by the cytosolic oxidoreductase NQO1. This activity was found to be upregulated following treatment of cells with aminooxyacetate (AOA, a malate aspartate shuttle inhibitor) as well as the respiratory inhibitors cyanide, rotenone, and antimycin A. These data demonstrate that PMET activity is upregulated under conditions of mitochondrial inhibition and suggest that PMET is an important part of the metabolic network in β-cells.
MATERIALS AND METHODS
Materials.
Potassium ferricyanide (hexacyanoferrate) was from Fluka, WST-1 was from GenScript, collagenase was from Roche, and FCS was from Hyclone. All other chemicals were from Sigma Aldrich unless otherwise specified.
Cell and islet preparation and culture.
Clonal INS-1 832/13 cells, provided by Dr. Christopher Newgard (Duke University) were maintained and cultured as described previously (20). Male CD-1 mice (Charles River) were killed by halothane. All procedures were performed in accordance with the Institutional Guidelines for Animal Care in compliance with United States Public Health Service regulations and were approved by the Institutional Animal Care and Use Committee at the Marine Biological Laboratory. Pancreatic islets were isolated by collagenase (Roche, Indianapolis, IN) digestion as previously described (16). Islets were used after an overnight culture in RPMI supplemented with 10% FCS (Hyclone), penicillin/streptomycin, and 5 mM glucose.
PMET activity assay.
Following a 2-h preincubation in KRB buffer (in mM: 140 NaCl, 30 HEPES, pH 7.4, 4.6 KCl, 1 MgSO4, 0.15 Na2HPO4, 0.4 KH2PO4, 5 NaHCO3, and 2 mM CaCl2) supplemented with 2 or 4 mM glucose, INS-1 832/13 cells (96-well plates) or intact islets (20 islets/microtube) were exposed to the indicated concentrations of glucose or other metabolites and incubated in the presence of 250 μM WST-1 or 250 μM ferricyanide for 1 h. The presence of intermediate electron carriers 1-mPMS and CoQ1 was obligatory for WST-1 and ferricyanide reduction, respectively (unpublished observations). Reduction of WST-1 to WST-1-formazan and reduction of ferricyanide to ferrocyanide was monitored by the change in absorbance at 450 and 420 nm, with a reference absorbance at 650 or 500, respectively, using a Shimadzu UV mini-1240 spectrophotometer. In parallel, WST-1 or ferricyanide solution was applied to empty wells or microtubes, and the resulting control values were subtracted from the corresponding values obtained in the presence of INS-1 832/13 cells or islets. Reduction of WST-1 to formazan results in an increase in absorbance at 450 nm, whereas reduction of ferricyanide to ferrocyanide results in a decrease in absorbance at 420 nm. Calibration curves were constructed using known amounts of WST-1-formazan and ferricyanide. PMET activities were calculated as the amount of formazan formed per 105 cells per hour or the amount of ferricyanide consumed per 105 cells per hour.
Construction of adenoviruses.
Recombinant, replication-deficient type 5 adenoviruses expressing human NQO1 from Origene were custom-constructed by Vector BioLabs (Philadelphia, PA). The expression of NQO1 is under the control of a cytomegalovirus promoter that also directs expression of green fluorescent protein from the internal ribosome entry site. A control virus was constructed in parallel. Viral titers were determined by the plaque formation assay.
Western blot analysis.
Equal amounts of protein were resolved by gradient (4–20%) SDS-PAGE and electrotransferred to nitrocellulose membranes. Following overnight blocking with 5% nonfat dry milk, membranes were probed with NQO1 antibody (Santa Cruz, CA), and proteins were visualized by enhanced chemiluminescence (Thermo Scientific, Rockford, IL).
Determination of nucleotides.
Adenine nucleotides were determined using NAD+/NADH and ATP/ADP kits (Abcam, Cambridge, MA) according to manufacturer protocols.
Insulin secretion.
Cells were preincubated for 2 h in the presence of 2 mM (INS-1 832/13 cells) or 4 mM (mouse islets) glucose in KRB buffer. The amount of released insulin was determined after 60 min of static incubation in the presence of secretory fuels using an ELISA kit (Alpco Diagnostics, Salem, NH). Data were normalized for protein content determined by the Micro-BCA Protein Assay kit (Pierce, Rockford, IL).
Intracellular Ca2+.
Cells were loaded for 30 min at 37°C with 0.5 μmol/l fura 2-AM (Molecular Probes). After being loaded, cells were washed three times and incubated for 15 min to allow cleavage of intracellular fura 2-AM by cytosolic esterases. Following the trypsinization, cell suspension was added to the KRB buffer in cuvette, positioned inside a temperature-controlled chamber maintained at 37°C. The cuvette content was stirred to ensure proper mixing. Fluorescence was measured at 340/380-nm excitation and 510-nm emission on a fluorescence spectrophotometer (FluoroMax-3; Horiba Jobin Yvon), and Ca2+ was determined as described previously (17).
Glucose oxidation and utilization.
INS-1 832/13 cells were grown to 80% confluence in 8 T-150 flasks, trypsinized, and combined. An aliquot was then counted, and the remaining suspension was centrifuged (4°C, 500 rpm for 5 min). The cells were then resuspended in basal (2 mM glucose) KRB to a concentration of 1.0 × 107 cells/ml. One milliliter of cells was added to 25-ml Ehlenmeyer flasks containing 2 ml of basal KRB (contains 2 mM nonstimulatory glucose). Any remaining cells were analyzed for protein. After a 2-h preincubation period in a 37°C shaker water bath under a 5% CO2-95% O2 atmosphere, the flasks were sealed airtight, and glucose metabolic rates were then determined over the course of 2 h at 37°C by addition of glucose to a final concentration of either 2 or 10 mM, in the absence or presence of WST-1/1-mPMS (or ferricyanide/CoQ1) and tracer mixtures of radiolabeled glucose. Glucose utilization and oxidation were determined simultaneously from conversion of [5-3H]glucose (1 μCi/flask) to 3H2O and production of 14CO2 from [U-14C]glucose (0.2 μCi/flask). All metabolic conditions were performed in triplicate. Metabolism was stopped, and evolution of dissolved H2CO3 to the gas phase was measured by injection of 600 μl of 3 M perchloric acid to the cell suspension. 13CO2 was quantitatively trapped in 300 μl phenethylamine-methanol (1:1) within 2 h after acidification. The recovery of 14CO2 was 99.5 ± 2.5% in control experiments with [14C]bicarbonate under all substrate combinations. 3H2O specific activity was quantified in the water purified by vacuum distillation of an aliquot of the quenched media. Conversion of radiolabeled glucose to 3H2O and 14CO2 was determined by liquid scintillation counting of 3H and 14C disintegrations per minute in the quenched media compared with the purified 3H2O and entrapped 14CO2.
Statistical analysis.
Data are expressed as means ± SE. Significance was determined for multiple comparisons using one-way ANOVA; a P value of <0.05 was considered significant.
RESULTS
Characterization of PMET activity in β-cells.
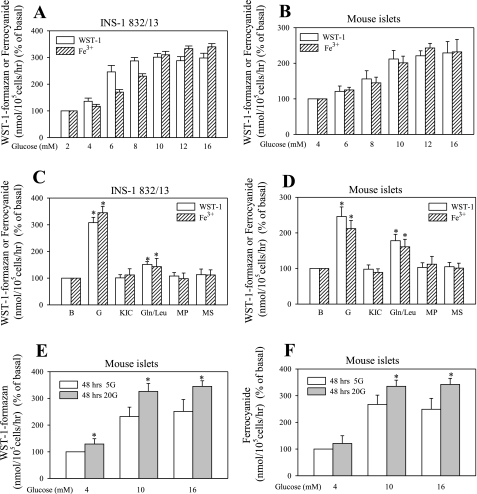
Although the ferricyanide- and WST-1-dependent reductive PMET systems have been described in various cell types (reviewed in Ref. 18), they have not been characterized in β-cells. To assess PMET activity in β-cells in relation to their functional status (insulin release), PMET activity was measured in cells exposed to a range of glucose concentrations. Basal (a level of glucose that corresponds to fasting glucose and that does not significantly stimulate insulin release) was 2 mM for INS-1 832/13 and 4 mM for islets. A lower basal glucose concentration (2 mM) was applied to INS-1 832/13 cells because these clonal cells are left shifted in their dose response to glucose, in contrast to native islet β-cells (20). Stimulatory (levels of glucose that correspond to postprandial levels and that stimulate insulin release) ranged from 4 to 16 mM. In addition, the effect of other fuels on PMET, applied at a concentration of 10 mM, was tested. WST-1 reduction readily took place in both preparations, and this activity was glucose dependent (Fig. 1). As in neurons (46), ferricyanide reduction was accomplished only in the presence of CoQ1 (data not shown), in contrast to HeLa cells, which can directly reduce ferricyanide (40).
Fig. 1.
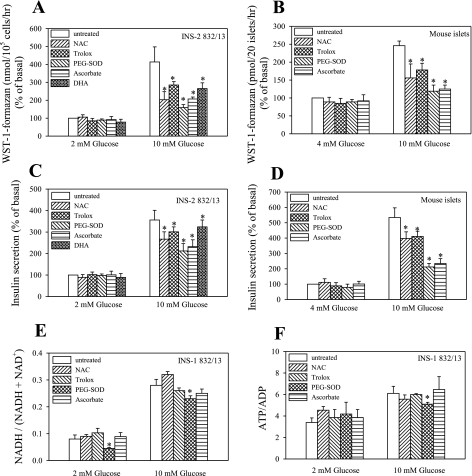
Effect of metabolic fuels on plasma membrane electron transport (PMET) activity. Following a 2-h preincubation period in the presence of 2 or 4 mM glucose, confluent INS-1 832/13 cells (96-well plates) or isolated mouse islets (20 islets/0.5-ml tube) were treated with glucose ranging from 2(or 4) to 16 mM (A and B, glucose dose response) or various metabolic fuels at 10 mM concentrations for 1 h in the presence of 2 (INS-1 832/13) or 4 (islets) mM glucose (C and D). E and F: effect of long-term glucose exposure of islets. The amount of WST-1-formazan (product of WST-1 reduction) and ferrocyanide (product of ferricyanide reduction) is expressed as %control, obtained by exposure of INS-1 832/13 cells to 2 mM glucose and islets to 4 mM glucose for 1 h. Basal PMET activity at 2 mM glucose for INS-1 832/13 cells was 588 ± 33 pmol formazan·10−5 cells·h−1 and 9.9 ± 2.5 pmol ferrocyanide·10−5 cells·h−1. Basal PMET activity at 4 mM glucose for islets was 18.2 ± 2.2 pmol formazan·20 islets−1·h−1 and 1.75 ± 0.2 pmol ferrocyanide·20 islets−1·h−1. Data are means ± SE from 2–4 independent experiments performed in triplicate measurements. *P < 0.05 compared with 2 mM glucose. G, glucose; KIC, ketoisocaproate; Gln/Leu, glutamine/leucine; MP, methyl-pyruvate; MS, methyl-succinate.
The level of PMET activity was found to be dependent on the glucose concentration (Fig. 1, A and B) and reached a maximum response between 8 and 12 mM glucose. Therefore, 10 mM glucose was chosen for further experiments, since the response was already saturated at this concentration. Other metabolic fuels did not show significant changes in PMET activity, with the exception of the amino acid combination of glutamine plus leucine, which caused about a 50% increase over the basal activity, measured under 2 mM (INS-1 832/13) or 4 mM (islets) glucose concentration (Fig. 1, C and D). To determine if PMET activity was affected by long-term exposure to a normoglycemic or a hyperglycemic environment, isolated mouse islets were cultured in media containing 5 mM glucose (mimics normoglycemic conditions) or 20 mM glucose (mimics hyperglycemic conditions) for 48–72 h. Culture of islets in a high glucose environment resulted in upregulation of PMET activity (Fig. 1, E and F).
Effect of mitochondrial inhibitors on PMET.
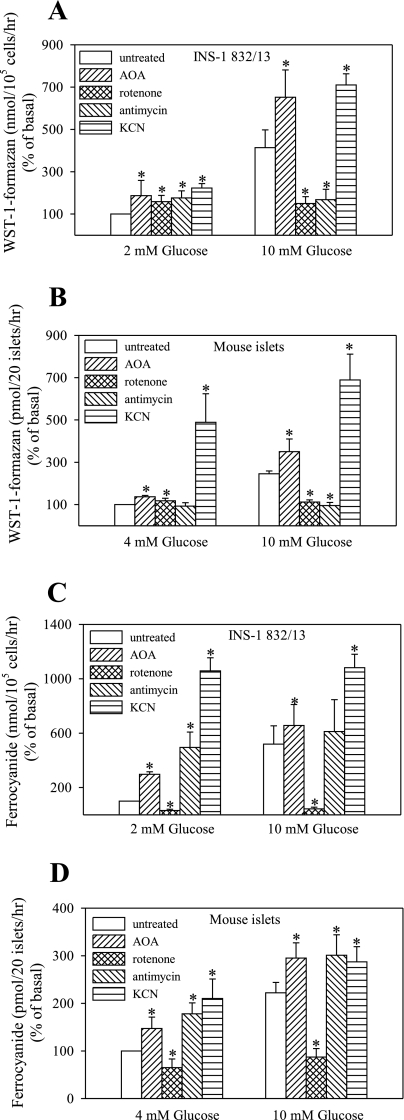
The effects of various inhibitors of mitochondrial function are summarized in Fig. 2. Reoxidation of glycolytically derived NADH in β-cells is thought to occur primarily via mitochondrial shuttles. To determine the role of the shuttle system in PMET, activity was measured in the presence of AOA, a malate aspartate shuttle inhibitor. AOA significantly elevated both WST-1 and ferricyanide reduction at both basal (2 or 4 mM) and stimulatory levels of glucose (10 mM). KCN, a cytochrome oxidase inhibitor, markedly enhanced reduction of both compounds under basal and stimulatory glucose concentrations, suggesting that PMET activity is enhanced in the face of mitochondrial failure. Although WST-1 and ferrricyanide reduction shared similar features in response to KCN and AOA, these systems differed markedly in their responses to rotenone and antimycin A. Rotenone and antimycin inhibited glucose-stimulated WST-1 reduction, whereas they increased basal WST-1 reduction. Glucose-stimulated as well as basal ferricyanide reduction was inhibited by rotenone, whereas it was increased by antimycin A. This suggests that there are differences between these two PMET systems in β-cells.
Fig. 2.
Effect of mitochondrial inhibitors on PMET activity. INS-1 832/13 cells (96-well plates) and mouse islets (20 islets/0.5-ml tube) were preincubated for 2 h in 2 or 4 mM glucose, respectively. PMET activity was measured by the formation of WST-1-formazan (A and B) or ferrocyanide (C and D). *P < 0.05 compared with the corresponding control value. Rotenone and antimycin were applied at 10 μM, aminooxyacetate (AOA) at 1 mM, and KCN at 0.5 mM final concentration. Data are means ± SE from 3–4 independent experiments performed in triplicate measurements.
Effect of NQO1 inhibition and overexpression on PMET and insulin secretion.
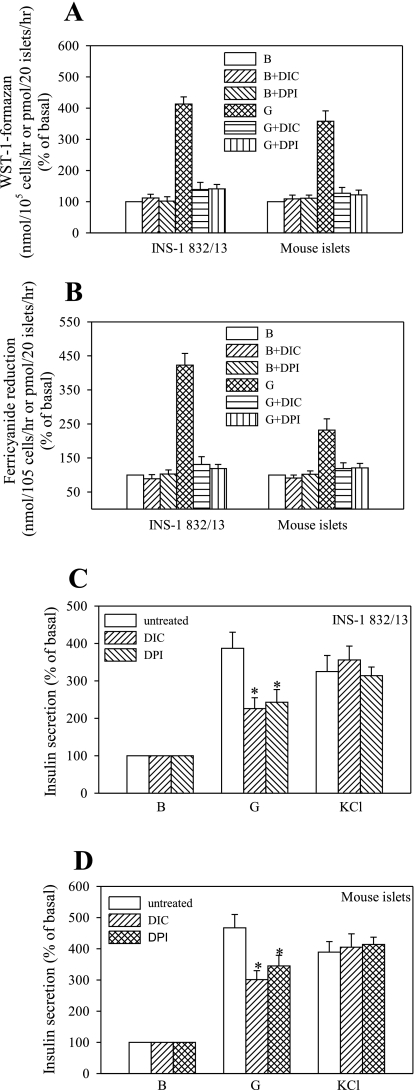
NQO1 is a cytosolic oxidoreductase that uses NADH or NADPH as a substrate and has been shown to facilitate WST-1 reduction via PMET-mediated redox cycling (41). Although not affecting basal activity significantly, dicoumarol (DIC, 2 μM), an inhibitor of NQO1, completely abolished glucose-stimulated PMET in both preparations. The same effect was observed after treatment of cells with the flavoprotein inhibitor diphenylene iodonium (DPI). The effect of DIC and DPI on WST-1 and ferricyanide reduction activities is summarized in Fig. 3. Both DPI and DIC significantly reduced the insulin secretory response to glucose but not to the depolarizing agent KCl, suggesting that these agents interfere with cell intermediary metabolism.
Fig. 3.
Effect of dicoumarol and diphenylene iodonium on PMET activity and insulin secretion. INS-1 832/13 cells and mouse islets were preincubated as described in Fig. 2 and were further incubated in the absence or presence of 2 μM dicoumarol or diphenylene iodonium. PMET activity was measured by formation of WST-1-formazan (A) or ferrocyanide (B). Insulin secretion was performed in parallel (C). Basal insulin secretion was 87.6 ± 5.6 ng insulin·mg protein−1·h−1 and 6.8 ± 0.6 ng insulin·20 islets−1·h−1. Data are means ± SE from 3 independent experiments performed in triplicate measurements. DIC, dicoumarol; DPI, diphenylene iodonium.
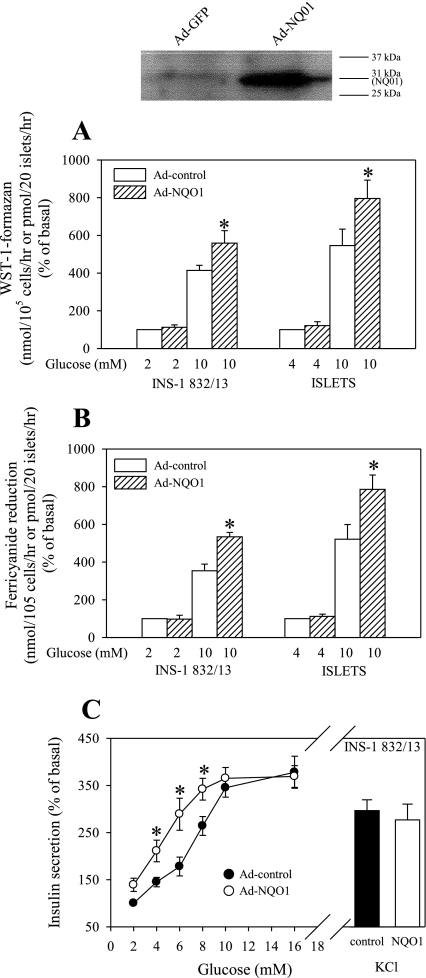
The opposite effect was observed following overexpression of NQO1 via adenovirus-mediated delivery, which significantly elevated NQO1 protein levels (Fig. 4) and NQO1 activity levels >10-fold (data not shown). NQO1 overexpression resulted in a substantial increase in both WST-1 and ferricyanide reduction under stimulatory glucose levels (Fig. 4, A and B). NQO1 overexpression affected insulin secretion by increasing sensitivity to glucose at lower stimulatory glucose levels (between 4 and 8 mM), whereas it did not significantly affect secretion in response to 16 mM glucose or the depolarizing agent KCl (Fig. 4C). The intracellular calcium rise in response to lower stimulatory glucose levels or maximal stimulatory glucose level was not changed in NQO1-overexpressing cells (data not shown).
Fig. 4.
Effect of NAD(P)H-quinone oxidoreductase (NQO1) overexpression on the PMET activity. Cells or freshly isolated islets were infected with type 5 adenoviruses expressing human NQO1 (Ad-NQO1) or control virus (Ad-control) at 5 × 105 plaque-forming units/ml. Following 48 h culture, PMET activity, determined as WST-1-formazan (A) or ferricyanide (B) formation, was measured as described in materials and methods. C: effect of NQO1 overexpression on insulin secretion. Data are means ± SE from 3 independent experiments performed in triplicate measurements. *P < 0.05 compared with the corresponding control value.
Effect of antioxidants on PMET and insulin secretion.
A key feature of the PMET system is the regulation of superoxide levels (34). It has been suggested by Tan and Berridge (40) that PMET-mediated reduction of cell-impermeable tetrazolium dyes is mediated by reactive oxygen intermediates (ROI). Thus, we tested the effect of various antioxidants on PMET activity. Several antioxidants were able to inhibit PMET activity, with the most potent effect due to superoxide dismutase (Fig. 5). Although these compounds had a generally marginal effect in the presence of basal, nonstimulatory glucose, they greatly reduced PMET activity at the stimulatory glucose concentration (Fig. 5, A and B). Antioxidants have been shown here (Fig. 5, C and D) and previously by others to inhibit glucose-stimulated insulin secretion (3, 35). To determine if these antioxidants affected the ATP-dependent K+ (KATP) channel-dependent pathway and early metabolic events, NADH-to-NAD+ and ATP-to-ADP ratios were determined in parallel (Fig. 5, E and F). However, antioxidants did not substantially alter these ratios, with the exception of PEG-SOD. This suggests that metabolic routes other than the KATP channel-dependent pathway are affected by antioxidants.
Fig. 5.
Effect of antioxidants on PMET activity and insulin secretion. Following the 2-h preincubation period, INS-1 832/13 cells (A) or islets (B) were exposed to N-acetylcysteine (NAC, 0.4 mM), Trolox (0.5 mM), polyethylene glycol superoxide dismutase (PEG-SOD) (30 U/ml), and ascorbate (25 μg/ml). Dehydroascorbae (DHA, 250 μM) was present during the preincubation period to elevate intracellular ascorbate levels. The amount of WST-1-formazan was determined as described in materials and methods. Insulin secretion (C and D) and nucleotide ratios (E and F) were performed in parallel. Data are means ± SE from 2–4 independent experiments performed in triplicate measurements. *P < 0.05 compared with the corresponding control value.
Effect of WST-1 and ferricyanide on β-cell intermediary metabolism.
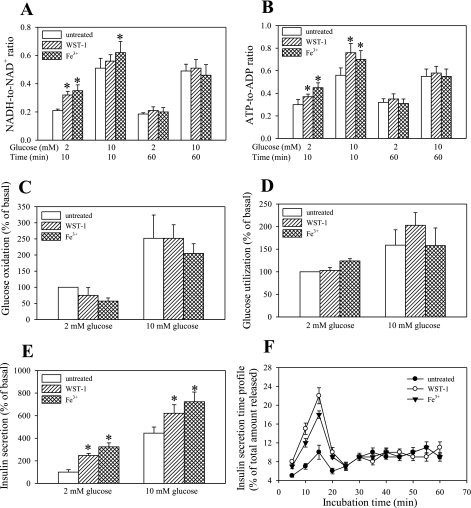
It has been reported that the addition of tetrazolium dyes has a stimulatory effect on cell metabolism (10). To evaluate the possible metabolic effect of WST1 and ferricyanide on β-cells, NADH and ATP levels in the presence of 2 and 10 mM glucose were determined after exposure of cells to these compounds. Both NADH-to-NAD+ and ATP-to-ADP ratios were transiently elevated under these conditions, but no sustained increase following 60 min of treatment was observed (Fig. 6, A and B). Glucose oxidation and glucose utilization (a measure of the glycolytic flux; Fig. 6, C and D) over a 60-min period were not significantly altered. The effect of WST-1 and ferricyanide on total insulin output over the 60 min (elevated; Fig. 6E) was on closer examination found to be located in the first phase of insulin secretion (Fig. 6F), suggesting that exposure of cells to WST-1 and ferricyanide causes secretion of insulin rather by altering plasma membrane excitability, then having a sustained effect on intermediary metabolism.
Fig. 6.
Effect of WST-1 and ferricyanide on nucleotide levels, glucose oxidation and utilization, and insulin secretion. INS-1 832/13 cells were exposed to WST-1 or ferricyanide and the NADH-to-NAD+ ratio (A), ATP-to-ADP ratio (B), glucose oxidation (C) and utilization (D), and insulin secretion (E and F) were determined as described in materials and methods. Data are means ± SE from 2–3 independent experiments performed in duplicate measurements. *P < 0.05 compared with the corresponding control value.
DISCUSSION
PMET is a system of membrane-bound and membrane-associated oxidoreductase enzymes, electron carriers within the plasma membrane, and extracellular electron acceptors, including ascorbate, ubiquinone, and molecular oxygen (reviewed in Ref. 10). The PMET pathway was first characterized in HeLa cells (38) and later found to be present in a variety of cancer cell lines, plant cells, and mammalian cells (10). In HeLa cells, PMET activity was described as a glycolysis-dependent pathway with activity proportional to the concentration of NADH (38). Furthermore, the PMET system is stimulated by cyanide, likely because of inhibition of NADH oxidation within the mitochondria, which ultimately results in elevated cytosolic NADH (31).
Role of PMET in glycolytic NADH reoxidation.
Reoxidation of cytosolic NADH back to NAD+ is crucial for the maintenance of glycolytic flux and is conventionally thought to occur via mitochondrial shuttles. However, the existence of an unidentified cytosolic factor in β-cells responsible for reoxidation of cytosolic NADH has been hypothesized based on the observation that, despite the blockage of both the malate aspartate shuttle and the glycerolphosphate shuttle, glycolytic flux remains unchanged (12). PMET serves as an excellent candidate for this “unknown cytosolic factor,” since reoxidization of glycolytically derived NADH is one of the functions of PMET operation (5). Our data support this notion by demonstrating that PMET activities are enhanced significantly in INS-1 832/13 cells and isolated islets following exposure to AOA, a malate-aspartate shuttle inhibitor (Fig. 2).
Cyanide and antimycin A both stimulated WST-1 and ferricyanide reduction under basal glucose. This is likely caused by the failure of mitochondrial oxidation of NADH, forcing NADH oxidation to occur solely through alternative pathways such as PMET (Fig. 7). Except for weak stimulation of basal WST-1 reduction, rotenone severely inhibited glucose-stimulated PMET activities. In mitochondria, rotenone inhibits complex I by preventing reduction of ubiquinone to ubiquinol. Because interconversion between ubiquionone and ubiquinol is also required for PMET operation, rotenone may therefore affect PMET at this step.
Fig. 7.
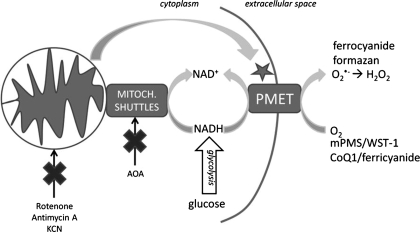
Model for the role of PMET in β-cells. PMET is measured by the transplasma membrane reduction of couplers [1-methoxy-5-methyl-phenazinium methyl sulfate (mPMS) or CoQ1], followed by the reduction of membrane-impermeable indicator dyes (WST-1 or ferricyanide). The activity of the PMET system is proportional to the intracellular concentration of NADH. Glucose undergoes glycolysis, increasing the cytosolic concentration of NADH, resulting in PMET stimulation. Application of mitochondrial inhibitors of complex I, III, and IV prevents mitochondrial oxidation of NADH, stimulating a rise in cytosolic NADH, which also results in enhanced PMET activity. Similarly, treatment with the malate/aspartate shuttle inhibitor AOA blocks mitochondrial-mediated reoxidation of NADH, leading to enhanced PMET activity.
Role of NQO1 in PMET and insulin secretion.
NQO1 is a cytosolic NADH-NADPH oxidoreductase that has recently been implicated in PMET-dependent redox cycling in epithelial cells (42). Interestingly, DIC, an inhibitor of NQO1, inhibits insulin secretion, suggesting the role of this enzyme for β-cell function, although a mechanism for that inhibition has not been described. Other flavin inhibitors such as DPI also inhibit insulin secretion (21). We have shown that DIC and DPI inhibit PMET activity and insulin secretion (Fig. 3). Overexpression of NQO1 potentiated PMET activity and insulin secretion in the presence of stimulatory glucose levels, suggesting that NQO1 is an integral component of the PMET, as well as insulin secretory machinery, in β-cells.
Role of PMET in ROI generation.
In β-cells, PMET activity was reduced significantly by the application of antioxidants, an effect also observed in HeLa cells (40). In contrast to findings in astrocytes, where pretreatment of cells with dehydroascorbate (DHA, oxidized form of ascorbate) was shown to increase intracellular ascorbate levels and increase ferricyanide reduction (27), DHA pretreatment of β-cells decreased both WST-1 (Fig. 5) and ferricyanide reduction (data not shown). It is possible that differences in the PMET and DHA/ascorbate transport network between astrocytes and β-cells are responsible for these differences. In parallel to their effect on PMET, antioxidant inhibition of GSIS has been reported, and ROI have been suggested as potential modulators of GSIS (35). Ascorbate, which was reported to recover insulin secretion in ascorbate-deprived pig scorbutic islets (44), was shown to severely inhibit insulin secretion from normal mouse islets (3), similar to our findings reported here.
While ROI are generally thought to be mitochondrial in origin, recent evidence points to cytosolically generated ROI and their role in GSIS (reviewed extensively in Ref. 15). Generation of ROI in the cytosol via PMET operation has been documented to take place in mitochondrial-deficient rho minus cells (28). Because the gradient of ROI has been suggested to be very steep in islets (36), cytosolically generated ROI via PMET will represent an advantage since their generation will occur conveniently in the proximity of the possible targets involved in insulin secretion.
In summary, we have demonstrated for the first time the existence of a robust PMET system in pancreatic β-cells, measured by the reduction of the cell-impermeable tetrazolium dye WST-1 and the iron compound ferricyanide. We have shown that PMET activity is dependent on glucose concentration and is elevated under conditions of mitochondrial inhibition. Furthermore, our data suggest that the cytosolic oxidoreductase NQO1 is a part of the β-cell PMET network. Studies are underway to further investigate the role of NQO1 on β-cell metabolism and insulin secretion using islets from NQO1 knockout animals. Future studies may investigate our hypothesis that PMET-mediated reoxidation of NADH is required for insulin secretion.
GRANTS
The work was supported by American Diabetes Association Grant 7-08-JF-18; nih Grants R56DK-088093 (E. Heart), P41-RR-001395 (P. J. S. Smith), DK-063984 (P. J. S. Smith), and DK-071071 (G. W. Cline); the Alexander Trust Fund (J. P. Gray); and the USCGA Center for Advanced Studies (J. P. Gray).
DISCLOSURES
Joshua Gray is a Professor at the U.S. Coast Guard Academy. The views presented here are his own and not necessarily those of the Academy or other branches of the U.S. government. The authors report no competing interests.
ACKNOWLEDGMENTS
We thank X. Zhao for excellent technical assistance and M. Meow for helpful comments and support in the preparation of this manuscript.
REFERENCES
- 1.Audi SH, Bongard RD, Okamoto Y, Merker MP, Roerig DL, Dawson CA. Pulmonary reduction of an intravascular redox polymer. Am J Physiol Lung Cell Mol Physiol 280: L1290–L1299, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Baker MA, Lawen A. Plasma membrane NADH-oxidoreductase system: a critical review of the structural and functional data. Antioxid Redox Signal 2: 197–212, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Bergsten P, Moura AS, Atwater I, Levine M. Ascorbic acid and insulin secretion in pancreatic islets. J Biol Chem 269: 1041–1045, 1994 [PubMed] [Google Scholar]
- 4.Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev 11: 127–152, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Berridge MV, Tan AS. Trans-plasma membrane electron transport: a cellular assay for NADH- and NADPH-oxidase based on the extracellular, superoxide-mediated reduction of sulfonated tetrazolium salt WST-1. Protoplasma 205: 74–82, 1998 [Google Scholar]
- 6.Berridge MV, Tan AS. Cell-surface NAD(P)H-oxidase: relationship to trans-plasma membrane NADH-oxidoreductase and a potential source of circulating NADH-oxidase. Antioxid Redox Signal 2: 277–288, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Bongard RD, Lindemer BJ, Krenz GS, Merker MP. Preferential utilization of NADPH as the endogenous electron donor for NAD(P)H:quinone oxidoreductase 1 (NQO1) in intact pulmonary arterial endothelial cells. Free Radic Biol Med 46: 25–32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brightman AO, Wang J, Miu RK, Sun IL, Barr R, Crane FL, Morre DJ. A growth factor- and hormone-stimulated NADH oxidase from rat liver plasma membrane. Biochim Biophys Acta 1105: 109–117, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Clark MG, Partick EJ, Patten GS, Crane FL, Low H, Grebing C. Evidence for the extracellular reduction of ferricyanide by rat liver. A trans-plasma membrane redox system. Biochem J 200: 565–572, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crane FL, Low H, Morre DJ. Historical perspective. In: Oxidoreduction at the Plasma Membrane: Relation to Growth and Transport, edited by Crane FL, Morre DJ, Low H. Boca Raton, FL: CRC, 1990, p. 1–27 [Google Scholar]
- 11.Crane FL, Sun IL, Clark MG, Grebing C, Low H. Transplasma-membrane redox systems in growth and development. Biochim Biophys Acta 811: 233–264, 1985 [DOI] [PubMed] [Google Scholar]
- 12.Eto K, Tsubamoto Y, Terauchi Y, Sugiyama T, Kishimoto T, Takahashi N, Yamauchi N, Kubota N, Murayama S, Aizawa T, Akanuma Y, Aizawa S, Kasai H, Yazaki Y, Kadowaki T. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science 283: 981–985, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Farfari S, Schultz V, Corkey BE, Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic b-cells. Possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes 49: 718–726, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Goodwin CJ, Holt SJ, Downes S, Marshall NJ. Microculture tetrazolium assays: a comparison between two new tetrazolium salts, XTT and MTS. J Immunol Methods 179: 95–103, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Gray JP, Heart E. Usurping the mitochondrial supremacy: extramitochondrial sources of reactive oxygen intermediates and their role in beta cells metabolism and insulin secretion. Toxicol Mech Methods 20: 167–174, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Heart E, Cline GW, Collis LP, Pongratz RL, Gray J, Smith PJ. Role for malic enzyme, pyruvate carboxylation and mitochondrial malate import in the glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 296: E1354–E1362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heart E, Yaney GC, Corkey RF, Schultz V, Luc E, Liu L, Deeney JT, Shirihai O, Tornheim K, Smith PJS, Corkey BE. Ca2+, NAD(P)H and membrane potential changes in pancreatic beta-cells by methyl succinate: comparison with glucose. Biochem J 403: 197–205, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herst PM, Berridge MV. Plasma membrane electron transport: a new target for cancer drug development. Curr Mol Med 6: 895–904, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Herst PM, Petersen T, Jerram P, Baty J, Berridge MV. The antiproliferative effects of phenoxodiol are associated with inhibition of plasma membrane electron transport in tumour cell lines and primary immune cells. Biochem Pharmacol 74: 1587–1595, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Hohmeier HE, Newgard CB. Cell lines derived from pancreatic islets. Mol Cell Endocrinol 228: 121–128, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Imoto H, Sasaki N, Iwase M, Nakamura U, Oku M, Sonoki K, Uchizono Y, Iida M. Impaired insulin secretion by diphenyleneiodium associated with perturbation of cytosolic Ca2+ dynamics in pancreatic beta-cells. Endocrinology 149: 5391–5400, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Ivarsson R, Quintens R, Dejonghr S, Tsukamoto K, Veld P, Renstrom E, Schuit FC. Redox control of exocytosis. Regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes 54: 2132–2142, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 295: E1287–E1297, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kishi T, Takahashi T, Usui A, Okamoto T. Ubiquinone redox cycle as a cellular antioxidant defense system. Biofactors 10: 131–138, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Kugler P. Quantitative dehydrogenase histochemistry with exogenous electron carriers (PMS, MPMS, MB). Histochemistry 75: 99–112, 1982 [DOI] [PubMed] [Google Scholar]
- 26.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Lane DJ, Robinson SR, Czerwinska H, Lawen A. A role for Na+/H+ exchangers and intracellular pH in regulating vitamin C-driven electron transport across the plasma membrane. Biochem J 428: 191–200, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Larm JA, Vaillant F, Linnane AW, Lawen A. Up-regulation of the plasma membrane oxidoreductase as a prerequisite for the viability of human Namalwa rho 0 cells. J Biol Chem 269: 30097–30100, 1994 [PubMed] [Google Scholar]
- 29.MacDonald MJ. Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. Further implication of cytosolic NADPH in insulin secretion. J Biol Chem 270: 20051–20058, 1995 [PubMed] [Google Scholar]
- 30.Marshall NJ, Goodwin CJ, Holt SJ. A critical assessment of the use of microculture tetrazolium assays to measure cell growth and function. Growth Regul 5: 69–84, 1995 [PubMed] [Google Scholar]
- 31.Merker MP, Audi SH, Bongard RD, Lindemer BJ, Krenz GS. Influence of pulmonary arterial endothelial cells on quinone redox status: effect of hyperoxia-induced NAD(P)H:quinone oxidoreductase 1. Am J Physiol Lung Cell Mol Physiol 290: L607–L619, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Morre DJ, Brightman AO. NADH oxidase of plasma membranes. J Bioenerg Biomembr 23: 469–489, 1991 [DOI] [PubMed] [Google Scholar]
- 33.Navas P, Alcain FJ, Buron I, Rodriquez-Aguilera JC, Villalba JM, Morre DM, Morre DJ. Growth factor-stimulated trans plasma membrane electron transport in HL-60 cells. FEBS Lett 299: 223–226, 1992 [DOI] [PubMed] [Google Scholar]
- 34.O'Donnell VB, Azzi A. High rates of extracellular superoxide generation by cultured human fibroblasts: involvement of a lipid-metabolizing enzyme. Biochem J 318: 805–812, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 56: 1783–1791, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Pi J, Zhang Q, Fu J, Woods CG, Hou Y, Corkey BE, Collins S, Andersen ME. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol 244: 77–83, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ronnebaum SM, Ilkayeva O, Burgess SC, Joseph JW, Lu D, Stevens RD, Becker TC, Sherry AD, Newgard CB, Jensen MV. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insuloin secretion. J Biol Chem 281: 30593–30602, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Sun IL, Crane FL, Grebing C, Low H. Properties of a transplasma membrane electron transport system in HeLa cells. J Bioenerg Biomembr 16: 583–595, 1984 [DOI] [PubMed] [Google Scholar]
- 39.Sun IL, Crane FL, Low H, Grebing C. Transplasma membrane redox stimulates HeLa cell growth. Biochem Biophys Res Commun 125: 649–654, 1984 [DOI] [PubMed] [Google Scholar]
- 40.Tan AS, Berridge MV. Distinct trans-plasma membrane redox pathways reduce cell-impermeable dyes in HeLa cells. Redox Rep 9: 302–306, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Tan AS, Berridge MV. Evidence for NAD(P)H:quinone oxidoreductase 1 (NQO1)-mediated quinone-dependent redox cycling via plasma membrane electron transport: a sensitive cellular assay for NQO1. Free Radic Biol Med 48: 421–429, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Tan AS, Berridge MV. Evidence for NAD(P)H:quinone oxidoreductase 1 (NQO1)-mediated quinone-dependent redox cycling via plasma membrane electron transport: a sensitive cellular assay for NQO1. Free Radic Biol Med 48: 421–429, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Villalba JM, Navarro F, Cordoba F, Serrano A, Arroyo A, Crane FL, Navas P. Coenzyme Q reductase from liver plasma membrane: purification and role in trans-plasma-membrane electron transport. Proc Natl Acad Sci USA 92: 4887–4891, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wells WW, Dou CZ, Dybas LN, Jung CH, Kalbach HL, Xu DP. Ascorbic acid is essential for the release of insulin from scorbutic guinea pig pancreatic islets. Proc Natl Acad Sci USA 92: 11869–11873, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westermark PO, Kotaleski JH, Bjorklund A, Grill V, Lansner A. A mathematical model of the mitochondrial NADH shuttles and anaplerosis in the pancreatic beta-cell. Am J Physiol Endocrinol Metab 292: E373–E393, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Wright MV, Kuhn TB. CNS neurons express two distinct plasma membrane electron transport systems implicated in neuronal viability. J Neurochem 83: 655–664, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Zurbriggen R, Dreyer JL. The plasma membrane NADH-diaphorase is active during selective phases of the cell cycle in mouse neuroblastoma cell line NB41A3. Its relation to cell growth and differentiation. Biochim Biophys Acta 1312: 215–222, 1996 [DOI] [PubMed] [Google Scholar]