Abstract
Huntington's disease (HD), a neurodegenerative disorder caused by mutant huntingtin, is characterized by a catabolic phenotype. To determine the mechanisms underlying muscle wasting, we examined key signal transduction pathways governing muscle protein metabolism, apoptosis, and autophagy in R6/2 mice, a well-characterized transgenic model of HD. R6/2 mice exhibited increased adiposity, elevated energy expenditure, and decreased body weight and lean mass without altered food intake. Severe skeletal muscle wasting accounted for a majority of the weight loss. Protein synthesis was unexpectedly increased 19% in gastrocnemius muscle, which was associated with overactivation of basal and refeeding-stimulated mammalian target of rapamycin (mTOR) signaling, elevated Akt expression and Ser473 phosphorylation, and decreased AMPK Thr172 phosphorylation. Moreover, mRNA abundance of atrogenes muscle ring finger-1 and atrophy F-box, was markedly attenuated during fasting and refeeding, and the urinary excretion of 3-methylhistidine was decreased, arguing against a role for the ubiquitin proteasome-mediated proteolysis in the atrophy. In contrast, mRNA expression of several caspase genes and genes involved in the extrinsic or intrinsic apoptotic pathway, caspase-3/7, -8, and -9 activity, protein abundance of caspase-3 and -9, Fas, and Fadd, and cytochrome c release were elevated. Protein expressions of LC3B-I and -II, beclin-I, and atg5 and -7 in muscle were upregulated. Thus, mutant huntingtin in skeletal muscle results in increased protein synthesis and mTOR signaling, which is countered by activation of the apoptotic and autophagic pathways, contributing to an overall catabolic phenotype and the severe muscle wasting.
Keywords: mammalian target of rapamycin, protein synthesis, proteolysis, autophagy, apoptosis
huntington's disease (HD) is a progressive, neurodegenerative movement disorder caused by expanded polyglutamine repeats of the mutated huntingtin (mHtt) protein. Ubiquitous mHtt expression causes lesions not only in specific brain areas but also in peripheral tissues, including skeletal and cardiac muscle, pancreatic β-cells, adipcytes, and blood cells (23, 37, 40, 42, 45). In both peripheral tissues and neurons, mHtt causes accumulation of intracellular protein aggregates, impairment of energy metabolism, transcriptional deregulation, and programmed cell death (10, 48). HD patients lose weight, despite normal or even elevated food intake, and weight loss in HD patients and animal models is associated with increased energy expenditure and global muscle wasting, independent of locomotor activity (21, 59). The atrophic muscle phenotype is progressive in R6/2 mice (45). Metabolic profiling studies have reported decreased plasma branched-chain amino acids (BCAAs, including leucine, isoleucine, and valine) and insulin-like growth factor I (IGF-I) and increased plasma glucocorticoid levels in either HD patients or transgenic mice (6, 21, 30, 41). Collectively, these findings suggest that HD exhibits a catabolic phenotype. Moreover, metabolic alterations occur in the early stage of HD, preceding symptom onset, suggesting that the procatabolic state of HD may directly contribute to the disease progression (58).
Although the catabolic phenotype of HD is apparent, the underlying mechanisms for the erosion of lean body mass are not yet fully understood. Muscle atrophy is a comorbidity observed in catabolic diseases and conditions such as cancer, acquired immune deficiency syndrome, congestive heart failure, chronic obstructive pulmonary disease, renal failure, severe burns, sepsis, denervation, and disuse (20). Patients who have cachexia in these disease settings have a poor prognosis. Skeletal muscle is predominantly composed of structural proteins, i.e., myofibrils, and the major mechanism to maintain muscle mass is through the regulation of protein synthesis and degradation. Theoretically, a change in the normal balance between protein synthesis and protein degradation, i.e., a diminished protein synthesis and/or enhanced proteolysis, can lead to loss of muscle mass. The mammalian target of rapamycin (mTOR) signaling pathway, which is activated by nutrients, especially leucine, and hormones/growth factors such as insulin and IGF-I, regulates global protein synthesis (50). The protein degradation pathway responsible for much of the muscle atrophy is the ATP-dependent ubiquitin proteasome system (UPS) (7, 28, 60). Myofibrillar proteins are degraded primarily by the UPS (3, 12, 19, 54). However, this traditional view of the regulation of skeletal muscle mass has been recently challenged; i.e., muscle mass homeostasis likely proceeds via coordinated regulation of myocyte growth, differentiation, degeneration, and regeneration by a complex web of intricate signaling networks (46). Although the cause-effect relationship has not yet been established, selective activation of apoptotic pathways and a progressive impairment of regenerative mechanisms to replace damaged muscle are common characteristics of muscle atrophy seen in aging and a variety of catabolic diseases (2, 33, 46, 63). Additionally, the lysosomal proteolytic system (i.e., macroautophagy) may also be stimulated under conditions leading to muscle atrophy (5). Thus, the exact cellular and molecular mechanisms underlying muscle atrophy may differ among diseases and conditions.
The objective of this study was to determine the cellular signaling mechanism(s) responsible for skeletal muscle atrophy in R6/2 mice. We measured body composition and oxygen consumption (V̇o2) at different ages during the disease progression and examined signaling pathways controlling protein synthesis, UPS, apoptosis, and autophagy. Contrary to expectations, mTOR signaling activation and protein synthesis rate were significantly increased in skeletal muscle of R6/2 mice, consistent with the observed increases in Akt and decreased AMPK phosphorylation. Furthermore, muscle mRNA expression of key UPS genes was downregulated, and the urinary concentration of 3-methylhistidine (3-MH), a surrogate marker of UPS-mediated proteolysis, was decreased in these mice. In contrast, apoptotic signaling events from the intrinsic mitochondrial and the extrinsic death receptor-associated pathways as well as signaling components of autophagy were markedly elevated in muscle of these animals. Therefore, our data suggest that elevated apoptotic and autophagic signaling contribute to the severe muscle wasting seen in HD.
MATERIALS AND METHODS
Animals and treatments.
All animal experiments were approved by the IACUC at The Pennsylvania State University College of Medicine. Tg(HDexon1)62Gpb(ovary transplanted) female and B6CBAF1/J male mice ordered from Jackson Laboratories were bred to generate R6/2 transgenic mice and wild-type (WT) littermates for experiments. The R6/2 mouse is a severe and acute model of HD with motor and cognitive abnormality exhibited before 6 wk of age (8, 36). All mice were on the C57BL6/CBA mixed background. Mice at 3–4 wk of age were weaned and genotyped using a standard PCR protocol described on the Jackson's Laboratories website. The CAG repeat length in tail DNA samples was analyzed using the services of Laragen (Los Angeles, CA), and R6/2 mice with an average CAG size of 123 (108–131) were used for studies. All mice were maintained in an enriched environment according to the guidelines of Psychogenics Best Practices Protocols and housed in groups of up to five per cage (mixed genotypes, single sex) in a temperature- (∼21°C) and humidity-controlled environment with a 12:12-h light-dark cycle. Mice were provided free access to extended water spouts and diet pellets made from powdered wet normal chow (2018; Harlan Laboratories, Madison, WI). For signaling experiments (mRNA and protein expression), both male and female mice were used at the age of 11–12 wk when muscle atrophy was severe. Mice were anesthetized (Nembutal, 70 mg/kg) under conditions of randomly fed, fasted for 6 h, or refed for 3 h after a 21-h fast starting at 8 AM. Gastrocnemius and quadriceps muscles were collected and immediately freeze-clamped in liquid nitrogen and stored at −80°C. Mice were then euthanized by heart excision.
Energy expenditure, locomotor activity, and body composition.
V̇o2, respiratory quotient (RQ), and locomotor activity were measured using indirect calorimetry (Oxymax; Columbus Instruments, Columbus, OH) and infrared technology as previously described (51). Male R6/2 and WT mice at 4, 8, and 12 wk of age were placed in an indirect calorimeter to measure V̇o2, RQ, and locomotor activity for 24 h, and values during light and dark phases were calculated. Longitudinal changes in body composition were tracked noninvasively in conscious animals by use of a 1H-NMR analyzer (Bruker LF90 proton-NMR Minispec; Bruker Optics, Woodlands, TX) for rapid measurement of total body fluid and lean and adipose tissue mass according to the manufacturer's manual. Body weight-normalized daily food intake was measured in individually housed male mice at 7–8 wk of age for a period of 1 wk.
Protein synthesis and degradation.
Rates of protein synthesis in 11- to 12-wk-old randomly fed male mice were measured using the flooding-dose method to assess the incorporation of radioactive phenylalanine into protein, as previously described (51). As an index of protein degradation, 24-h urine samples were collected from 8- to 10-wk-old male mice by means of a mouse metabolic cage (Nalgene Labware, Rochester, NY), and 0.5 ml of urine was prepurified in a column filled with ion exchange resin Dowex 50Wx8–400 mesh (62). Acid and neutral amino acids were eliminated with 0.2 M pyridine, and 3-MH was eluted with 1.5 M pyridine and then measured using an amino acid analyzer (Biochrom, Cambridge, UK), which used a cation exchange column with post-column ninhydrin. Urinary creatinine was measured using a colorimetric detection kit (Enzo Life Sciences, Plymouth Meeting, PA), and the molar 3-MH/creatinine ratio was calculated.
Cytosolic and mitochondrial fractions of skeletal muscle.
Isolation of cellular fractions from skeletal muscle was performed as described (68). Approximately 100 mg of the fresh left quadriceps was first minced in 1:5 (wt/vol) ice-cold lysis buffer consisting of (in mM) 20 HEPES (pH 7.5), 250 sucrose, 140 KCl, 2 MgCl, 2.1 EDTA, 1 EGTA, 1 ABSF, 1 DTT, 2.5 ATP, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, and 0.01% protease inhibitor cocktail and then homogenized on ice in a polytron homogenizer with low speed and centrifuged for 10 min at 1,000 g at 4°C to pellet cellular debris and nuclei. The supernatant was collected and further centrifuged for 15 min at 14,000 g at 4°C. The resulting supernatant, representing the mitochondria-free cytosolic fraction, was collected and stored at −80°C. The mitochondrial pellet was twice resuspended in 1.5 ml of ice-cold lysis buffer and centrifuged for 15 min at 14,000 g at 4°C. The supernatant was decanted, and the mitochondrial pellet was resuspended in 50 μl of lysis buffer and stored at −80°C for Western analysis using an antibody for cytochrome c (BioLegend, San Diego, CA).
Caspase activity assay.
Randomly fed male and female R6/2 and WT mice at 12 wk of age were euthanized, and powdered gastrocnemius muscle was homogenized using a polytron at a low speed for 2 × 15 s in 650 μl of ice-cold lysis buffer (20 mM HEPES, pH 7.5, 250 mM sucrose,140 mM KCl, and 2 mM MgCl). After being centrifuged at 10,000 g in 4°C for 10 min, the supernatant was used for determining caspase-3, -8, and -9 activity using Caspase-Glo 3/7, 8, and 9 assay systems (Promega, Madison, WI), respectively. Fold changes of caspase activity between the two groups were normalized to measured protein concentrations.
mRNA expression using qRT-PCR array and ribonuclease protection assay.
Total RNA was isolated from gastrocnemius using combined reagents of TRIzol (Invitrogen, Carlsbad, CA) and an RNeasy kit (Qiagen, Valencia, CA) as previously described (35). The quality of RNA samples was verified by measuring the A260-to-A280 ratio (>1.8) and by electrophoresis on a BioAnalyzer using an RNA 6000 Nano LabChip (Agilent, Santa Clara CA). A PCR array kit focused on mouse apoptosis pathway (SAbiosciences, Frederick, MD) was used to measure mRNA expression of genes involved in apoptosis. Synthesis of first-strand cDNA, performance of real-time RT-PCR, and data analysis were carried out following the manufacturer's manual. Total RNA samples from two mice were pooled for each array in each 96-well plate, and four separate arrays were performed for each genotype. A web-based PCR array data analysis program provided by the company was used to calculate fold changes and P values using Student's t-test. For ribonuclease protection assay, riboprobes were synthesized from a multiprobe mouse cytokine template set (rCK-1; Pharmingen, San Diego, CA) using an in vitro transcription kit (Pharmingen). Primer sequences for muscle ring finger-1 (MuRF1) and muscle atrophy F-box (MAFbx) have been reported (32). The labeled riboprobe was hybridized with RNA overnight using a ribonuclease protection assay. Protected RNAs were separated using a 5% acrylamide gel, and dried gels were exposed to a PhosphorImager screen (Molecular Dynamics, Sunnyvale, CA). The resulting data were quantified using ImageQuant and normalized to L32.
Western blot analysis.
Aliquots of frozen powdered tissues were homogenized on ice in 3–7 volumes of a phospho-preserving, ice-cold homogenization buffer consisting of (in mmol/l) 20 HEPES (pH 7.4), 2 EGTA, 50 sodium fluoride, 100 KCl, 0.2 EDTA, 50 β-glycerophosphate, 1 DTT, 0.1 phenylmethanesulfonylfluoride, 1 benzamidine, and 0.5 sodium vanadate. Protein concentration was determined after centrifugation, and equal amounts of protein were resolved by electrophoresis and then transferred to PVDF membranes. The membranes were then immunoreacted with antibodies against S6K1, 4E-BP1, (Bethyl Laboratories, Montgomery, TX), procaspase-9 and -3, mTOR, pS2448-mTOR, pT389-S6K1, AMPK, pT172-AMPK, Akt, pS473-Akt, LC3B, beclin-I, atg5, atg7, GAPDH, and α-tubulin, (Cell Signaling, Danvers, MA), FAS and FADD (Santa Cruz Biotechnology, Santa Cruz, CA). To check for protein expression of regular internal house genes and to equalize protein loading, GAPDH, α-tubulin, or Ponceau S staining were used.
Electron microscopy and TUNEL assay.
The gastrocnemius was collected from two 12-wk-old male R6/2 and two WT mice, and tissue fixation and procession for electron microscopy were performed as previously described (52). The sections were examined in a JEM-1400 transmission electron microscopy (JEOL, Tokyo, Japan). The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed on sections (n = 5/mouse group of 7–8 wk of age) of routine paraformaldehyde-fixed, paraffin-embedded gastrocnemius by using the basic protocol in the DeadEnd TUNEL kit (Promega) with biotin-UTP followed by streptavidin-HRP (Jackson Immunoresearch, West Grove, PA), and counterstained with hematoxylin.
Statistical analysis.
A two-tailed nonpaired t-test was used to assess the difference between two groups. A one-way ANOVA or repeated-measures ANOVA with a Newman-Keuls posttest was used to analyze data obtained from more than two groups or multiple time points. Values are means ± SE. P < 0.05 was considered significantly different.
RESULTS
age-dependent alterations in body weight, fat and lean mass, organ weight, and energy expenditure in r6/2 mice.
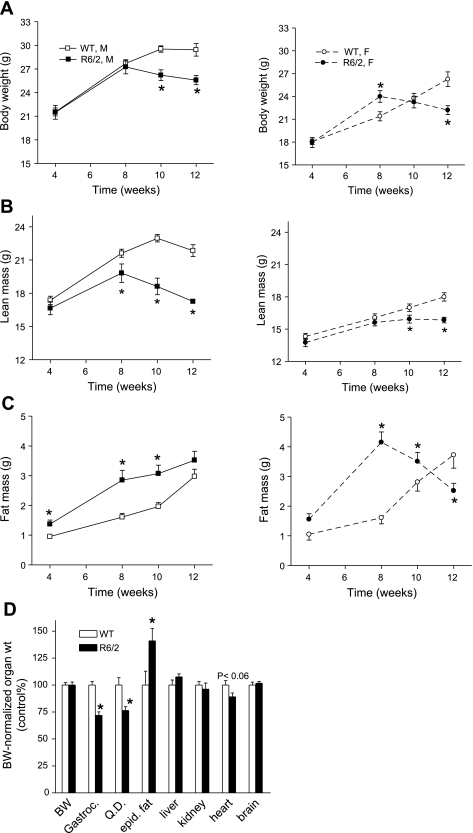
An NMR minspec analyzer was used to assess body composition in both male and female mice at 4, 8, 10, and 12 wk of age. The growth curves of WT and R6/2 mice diverged at week 8, when the motor dysfunction phenotype appeared (Fig. 1A). In WT mice, body weight gradually increased over the 12 wk of age in females (P < 0.05 between any time points) and plateaued at week 10 in males. In both male and female R6/2 mice, body weight was greater at week 8 than at week 4 (P < 0.05) but did not differ between weeks 8, 10, and 12. Compared with WT mice, body weights at week 12 were 13 and 15% lower in male and female R6/2 mice, respectively. In parallel with body weight loss, absolute lean mass (Fig. 1B) and its percentage of the total body weight [Supplemental Tables S1 and S2 (supplementary materials are found in the online version of this paper at the Journal website)] were lower in R6/2 than in WT mice, with a greater difference at an older age (18 and 12% lowered lean mass at week 12 in males and females, respectively). In contrast, fat mass and its percentage (Supplemental Tables S1 and S2) were higher in R6/2 mice (Fig. 1C). Compared with WT mice, there was a 40% increase in fat mass in male R6/2 mice even at week 4 and a 163% increase in female R6/2 mice at week 8. However, the fat mass at week 12 in female R6/2 mice was diminished 40% of its peak value at week 8 and was lower than in WT mice. These results indicate that both male and female R6/2 mice gained a significant amount of fat mass at an early age and then started to lose body weight and lean mass. Female R6/2 mice also lost fat mass as the disease progressed.
Fig. 1.
Age-dependent alterations of body weight (BW; A), lean mass (B), fat mass (C), and BW-normalized organ weights (D) measured in 12-wk-old mice. Gastroc., gastrocnemius; Q.D., quadriceps femoris; epid., epididymal. *P < 0.05, R6/2 vs. wild-type (WT) mice at each time point for each sex (male, left; female, right) in A–C; n = 8–26. *P < 0.05, R6/2 vs. WT, n = 10/group in D.
We also measured organ weights of male mice euthanized at week 12 (Fig. 1D). The body weight-normalized masses of gastrocnemius and quadriceps muscles were 28 and 24% lower, respectively, whereas epididymal fat weight was 41% higher in R6/2 mice compared with WT mice. The absolute weights of gastrocnemius and quadriceps muscles were 38 and 33% lowered, respectively, in R6/2 mice. There was a tendency for decreased heart weight in R6/2 mice; however, when normalized to the prevailing body weight, the weights of liver, kidney, and brain did not differ between the two groups. These data indicate that R6/2 mice exhibited severe muscle wasting, which contributed to, but was disproportionately larger than, the loss of body weight.
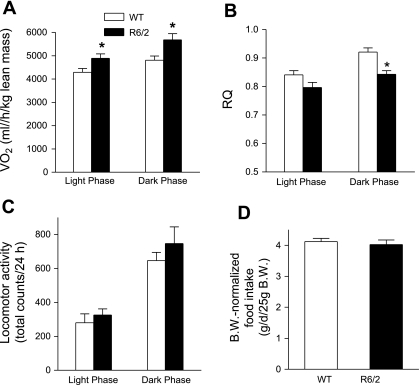
Using indirect calorimetry, we measured energy expenditure in male mice at 4, 8, and 12 wk of age. V̇o2 expressed relative to body weight did not differ between R6/2 and WT mice (data not shown). Since fat tissue has a lower metabolic rate and thereby makes a smaller contribution to whole-body V̇o2 compared with the other tissues, we normalized V̇o2 to lean body mass. Even at 4 wk of age, dark-phase V̇o2 was ∼9% higher in R6/2 than in WT mice (supplemental Fig. S1A). At week 8, light- and dark-phase V̇o2 per lean mass weight was 14 and 18% higher in R6/2 mice (Fig. 2A). At week 12, light- and dark-phase V̇o2 per lean mass weight was 28 and 17% higher compared with WT controls (Supplemental Fig. S1A).
Fig. 2.
O2 consumption (V̇o2; A), respiratory quotient (RQ; B), and locomotor activity (C) measured in 8-wk-old mice and food intake (D) measured in 7- to 8-wk-old mice. *P < 0.05, R6/2 vs. WT mice, n = 8/group in A–C; n = 10/group in D.
We then quantified locomotor activity and found no differences between the two groups of mice at weeks 8 and 12, except that the activity was slightly increased during the light phase in R6/2 mice at week 12 (Fig. 2C and Supplemental Fig. S1C). Thus, increased locomotor activity does not appear to contribute significantly to the elevated energy expenditure in these mice. Food intake did not differ between R6/2 and WT mice (Fig. 2D). Thus, a negative energy balance from elevated energy expenditure may lead to body weight loss. Dark-phase RQ in 4- and 8-wk-old R6/2 mice and light-phase RQ in 12-wk-old R6/2 mice were significantly lower than those in WT littermates (Fig. 2B and Supplemental Fig. S1B), suggesting that, compared with WT controls, R6/2 mice oxidized less carbohydrate as a fuel despite greater body fat accumulation in these animals.
Elevated protein synthesis and mTOR signaling in skeletal muscle of R6/2 mice.
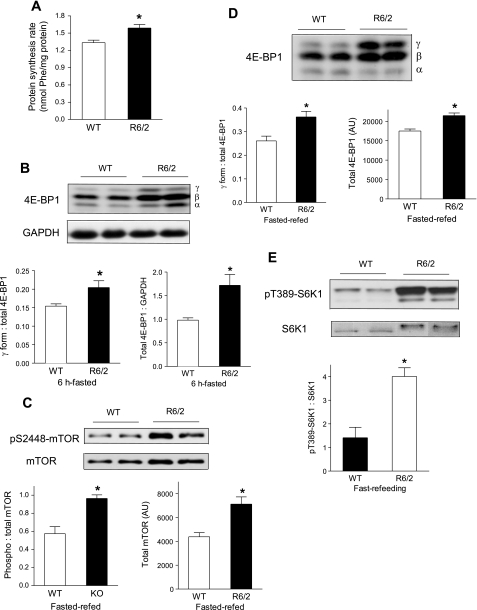
To explore the underlying mechanisms of severe muscle wasting in R6/2 mice, we assessed protein synthesis directly and proteolysis indirectly in these animals. We measured the incorporation of radiolabeled phenylalanine into muscle protein to determine the protein-synthetic rate. Unexpectedly, the in vivo-determined rate of protein synthesis in randomly fed mice was 19% higher in gastrocnemius of 12-wk-old R6/2 mice than in WT controls (Fig. 3A). In contrast, protein synthesis was unaltered in liver, brain, and kidney in R6/2 mice and decreased in liver and epididymal fat (Supplemental Fig. S2).
Fig. 3.
Elevated protein synthesis and mammalian target of rapamycin (mTOR) signaling in skeletal muscle of R6/2 mice. A: elevated protein synthesis rate in gastrocnemius of 12-wk-old male mice. *P < 0.05, R6/2 vs. WT mice, n = 10/group. B: Western blot analysis of eIF4E-binding protein-1 (4E-BP1) in gastrocnemius of 12-wk-old male mice fasted for 6 h, and ratio of hyperphosphorylated γ-isoform to total 4E-BP1 and total 4E-BP1 level normalized by GAPDH are shown. Western blot analysis of mTOR phosphorylation and total mTOR (C), 4E-BP1 (D), and T389 S6K1 phosphorylation (E) in 12-wk-old male mice after 21-h fasting then 3-h refeeding. *P < 0.05, n = 8 for WT and 5 for R6/2.
Protein synthesis is regulated in part by the mTOR signaling pathway, which is activated by growth factors such as insulin and IGF-I as well as nutrients such as amino acids, especially leucine, the concentrations of which rise during feeding. We first examined mTOR signaling after a 6-h fast. The ratio of the hyperphosphorylated γ-isoform of 4E-BP1 to total 4E-BP1 was higher in muscle of R6/2 mice than in WT mice (Fig. 3B). Total 4E-BP1 protein level, which was normalized to GAPDH, was also increased 74% in R6/2 mice compared with WT mice. These data suggest an elevated basal level of mTOR signaling in skeletal muscle of R6/2 transgenic mice at 12 wk of age.
Protein synthesis and mTOR signaling are robustly stimulated during the transition from fasting to refeeding. To avoid the variations of mTOR signaling in randomly fed mice, we next measured mTOR signaling in response to refeeding after a period of food deprivation. Twelve-week-old male mice were fasted for 21 h and then refed for 3 h, and muscle was collected for Western analysis. Both total mTOR protein level and the ratio of phosphorylated to total mTOR were ∼65% higher in R6/2 than in WT mice (Fig. 3C). The ratios of γ-isoform to total 4E-BP1 as well as to total 4E-BP1 protein level were ∼30% higher in R6/2 than in WT mice after refeeding (Fig. 3D). Although total S6K1 protein level did not differ (data not shown), the ratio of phosphorylated to total S6K1 was 2.8-fold higher in R6/2 than in WT mice (Fig. 3E). While basal mTOR signaling after a 21-h fast was slightly higher in R6/2 mice (data not shown), these data clearly demonstrated that, in response to refeeding, mTOR signaling activation was significantly enhanced in R6/2 mice. Furthermore, we measured mTOR signaling in response to insulin and leucine treatment in mice after an overnight fast and found that mTOR activation with both treatments was greater in R6/2 mice (data not shown). Collectively, these data indicate that overactivation of mTOR signaling is likely responsible for the increased protein synthesis detected in skeletal muscle of R6/2 mice.
Alterations in Akt and AMPK signaling in skeletal muscle of R6/2 mice.
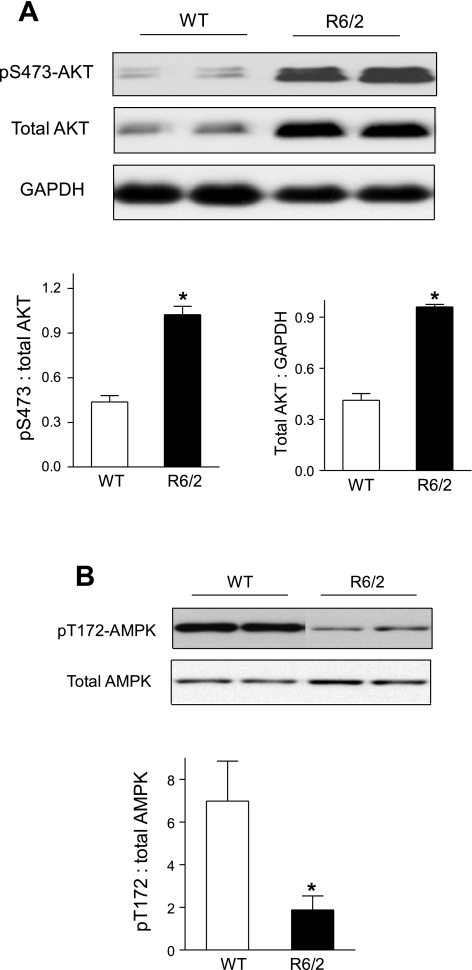
mTOR activation is potently stimulated by the insulin/IGF receptor-PI3K-Akt signaling cascade but inhibited by AMPK through the TSC1/2-Rheb-mTORC-1 interactions (24, 50). We therefore examined Akt protein expression and Ser473 phosphorylation in gastrocnemius of randomly fed R6/2 mice (Fig. 4A). Akt protein expression and Ser473 phosphorylation were both twofold higher in R6/2 than in WT mice, suggesting increased Akt activity in muscle of R6/2 mice. We also examined the expression and phosphorylation of AMPK, a key cellular energy sensor, in quadriceps muscle of randomly fed R6/2 mice (Fig. 4B). While total AMPK protein level was unaltered, Thr172 phosphorylation of AMPK was decreased by 67% in muscle of R6/2 mice compared with WT mice, suggesting a marked decrease of AMPK activity. Thus, the elevated Akt activity but decreased AMPK activity is consistent with elevated mTOR signaling in muscle of R6/2 mice.
Fig. 4.
Altered Akt and AMPK activation in skeletal muscle of R6/2 mice. Western blot analysis of Akt and pS473-Akt (A) and AMPK and pT172-AMPK (B) in quadriceps of randomly fed mice, *P < 0.05, n = 9 for WT and 7 for R6/2 mice.
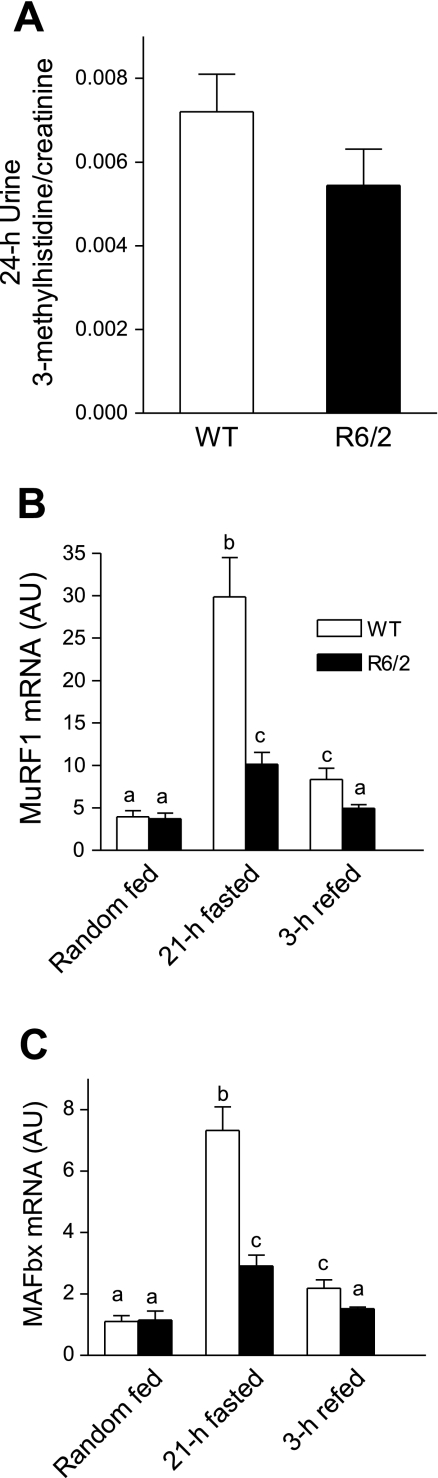
Diminished urinary 3-MH excretion and attenuated mRNA expression of atrogenes in skeletal muscle of R6/2 mice.
Skeletal muscle consists of major myofibrillar proteins such as α-actin and myosin, which contain a unique amino acid, 3-MH. When proteolysis of these proteins is elevated, the release of 3-MH into the circulation and its excretion into urine is increased. The urinary excretion of 3-MH is therefore used as a surrogate marker of muscle proteolysis. Creatinine is a breakdown product of creatine phosphate in muscle and is usually produced at a constant rate in a muscle mass-dependent manner. It is excreted into urine with little reabsorption by the kidney. Thus, the molar ratio of 3-MH to creatinine is a recognized index of muscle proteolysis (72). We found that the 24-h amounts of 3-MH and creatinine were 41 and 20% lower in R6/2 than in WT mice, respectively (Supplemental Fig. S3). The decreased creatinine level is consistent with decreased muscle mass in these mice. Although the 3-MH/creatinine ratio was not statistically different between the two groups of mice (P < 0.18; Fig. 5A), there was a trend for a decrease in R6/2 mice. Protein profiling of whole tissue lysis using silver staining of SDS-PAGE revealed no large differences in major protein bands (data not shown), suggesting that muscle atrophy in R6/2 mice did not result from specific degradation of major muscle structural proteins but resulted instead from approximately proportional losses of all cellular proteins.
Fig. 5.
Altered urinary molar 3-methyhistidine/creatinine ratio (A) and muscle mRNA expression of atrogenes muscle ring finger-1 (MuRF1; B) and muscle atrophy F-box (MAFbx; C) in gastrocnemius of randomly fed, 21-h-fasted and 3-h-refed R6/2 mice. In A, n = 10 for WT and 9 for R6/2 mice. In B and C, P < 0.05 between groups with different letters; for WT, n = 4, 4, and 5, and for R6/2 mice, n = 5, 6, and 6 in fed, fasted and refed states, respectively.
The UPS is thought to be a major pathway for proteolysis in skeletal muscle under catabolic conditions, and atrogenes such as MuRF1 and MAFbx are expressed specifically in striated muscle and are central players in the UPS-regulated turnover of sarcomeric proteins (7, 28, 60). MuRF1 and MAFbx are upregulated at the transcriptional level in various human and rodent models of muscle atrophy including fasting (66, 67, 69). As shown in Fig. 5C, MuRF1 mRNA did not differ between the two genotypes in the randomly fed state and was increased about fivefold during a 21-h fast from the random-fed baseline in WT mice; however, this increase was markedly attenuated in R6/2 mice. MuRF1 mRNA expression was markedly downregulated by 3-h refeeding in both genotypes, and its expression was 50% lower in R6/2 than in WT mice during refeeding. The change in MAFbx mRNA was comparable to that in MuRF1 mRNA (Fig. 5C).
Elevated apoptotic signaling in skeletal muscle of R6/2 mice.
Elevated apoptotic signaling has been observed in several catabolic states of skeletal muscle such as aging, sepsis, cancer cachexia, burn injury, and denervation (2, 17, 53). We therefore investigated whether apoptotic signaling is altered in R6/2 mice. Using an apoptotic pathway-specific qRT-PCR array, we measured mRNA expression of apoptotic genes in the gastrocnemius (Table 1). Compared with WT controls, mRNA levels were increased about twofold for caspase-1, -3, and -6 and 4.5-fold for caspase-9 (due to the variation and small sample size, some changes did not reach statistical significance, but a tendency was noted). The mRNA level of Cradd, which is associated with caspase-2, was about twofold higher. The mRNA levels for the death receptor Fas (CD95/Apo1), its ligand FasL, and death domain and binding cofactor FADD were increased 1.6- to 3.8-fold. The GAPGH mRNA abundance was significantly decreased in muscle of R6/2 mice, consistent with a microarray study (55) and an ∼10% decrease in GAPDH protein expression (Figs. 3B and 4A and quantitative data not shown). These data suggest that gene expression of apoptotic signaling components was coordinately upregulated in skeletal muscle.
Table 1.
Altered expression of genes involved in apoptosis detected using real-time qRT-PCR array
| Gene Name | Fold Difference | P Value |
|---|---|---|
| Gapdh | 0.37 | 0.013 |
| Bag1 | 1.42 | 0.033 |
| Casp1 | 1.85 | 0.049 |
| Casp6 | 1.87 | 0.088 |
| Cradd | 1.92 | 0.016 |
| Birc3 | 2.13 | 0.052 |
| Casp3 | 2.17 | 0.028 |
| Trp63 | 2.61 | 0.034 |
| Fas | 1.62 | 0.047 |
| Fasl | 3.05 | 0.058 |
| Fadd | 3.75 | 0.081 |
| Casp9 | 4.47 | 0.060 |
For both wild-type and R6/2 mice, n = 4.
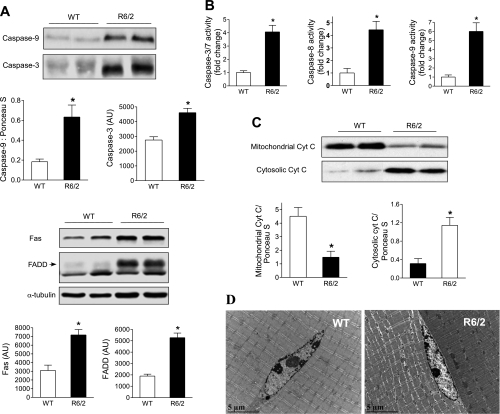
When measured using Western analysis, protein expression of procaspase-9 and -3 in R6/2 mice was increased and 3.5- and 1.7-fold, respectively, compared with WT mice (Fig. 6A, top). Protein expressions of Fas and FADD were increased 2.3- and 2.8-fold, respectively, in R6/2 mice (Fig. 6A, bottom). Finally, caspase activity was directly determined, and we found that the activity of caspase-3/7, -8, and -9 was increased four- to sixfold (Fig. 6B). However, despite a marked increase in the activity of several caspases, the activated forms of caspase-3 and -9 using the procaspase antibodies could not be detected in muscle samples of R6/2 mice. Nonetheless, the levels of mRNA, protein, and activity of several caspases were consistently elevated in R6/2 mice.
Fig. 6.
Elevated apoptosis signaling and mitochondrial cytochrome c (Cyt C) release in skeletal muscle of R6/2 mice. A: Western blot analysis of procaspase-9 and -3 in gastrocnemius and quantitative protein level normalized by Ponceau S staining for caspase-9 or unnormalized arbitrary units (AU) for caspase-3 (top). *P < 0.05 for caspase-9, n = 9 for WT and 7 for R6/2, and for caspase-3, n = 8/group. Western blot analysis of Fas and FADD in gastrocnemius and representative α-tubulin as a loading control are shown (bottom). *P < 0.05, n = 8/group. B: elevated activity of caspase-3, -8, and -9 were found in gastrocnemius of R/2 mice. *P < 0.05, n = 11 for each group. C: increased Cyt C release from mitochondria in quadriceps of R6/2 mice. Mitochondrial and cytosolic fractions of fresh quadriceps were isolated, and Western analysis was performed on each fraction; quantitative protein levels normalized by Ponceau S staining as a load control are shown. *P < 0.05, n = 9 for WT and 7 for R6/2 mice. D: representative electron microscopy revealed a degenerative myocyte with a relatively normal neighboring cell in R6/2 mice (right) and healthy myocytes in WT mice (left).
We next examined the possible involvement of mitochondrial dysfunction in mediating the increased apoptotic signaling in R6/2 mice. Cytochrome c (Cyt C) is a small heme protein found loosely associated with the inner membrane of the mitochondria, and it is an essential component of the electron transport chain involved in oxidative phosphorylation and ATP synthesis in mitochondria. Cyt C is released from mitochondria in response to proapoptotic stimuli and activates caspase-9 and -3, the executive caspase initiating cell killing (34). We isolated the cytosolic and mitochondrial fractions from fresh quadriceps muscle and performed Western blot of Cyt C in both fractions (Fig. 6C). Cytosolic Cyt C protein level was 3.6-fold higher in R6/2 than in WT mice, whereas the amount of mitochondrial Cyt C was decreased 67% in R6/2 mice, suggesting an elevated Cyt C release from mitochondria to cytoplasm in skeletal muscle of R6/2 mice.
Finally, we examined apoptosis in situ using the TUNEL assay. As shown in Supplemental Fig. S4, A and B, the number of TUNEL-positive nuclei was increased, as demonstrated in representative sections of skeletal muscle of 8-wk-old R6/2 mice. We also assessed muscle morphology using electron microscopy (EM). EM showed multifocal degeneration of sarcomeres within myofibers with hydropic degeneration of the sarcoplasm and probable dilatation of sarcoplasmic reticulum and mild Z-line streaming in the gastrocnemius of 12-wk-old R6/2 mice (Fig. 6D and Supplemental Fig. S4C), suggesting a possible myocyte death, and this abnormity was not observed in WT and 8-wk-old R6/2 mouse muscle. Muscle degeneration detected in muscle of 12-wk R6/2 mice is consistent with an age-dependent decrease in the muscle fiber diameter previously reported in R6/2 mice (45).
Elevated autophagy signaling in skeletal muscle of R6/2 mice.
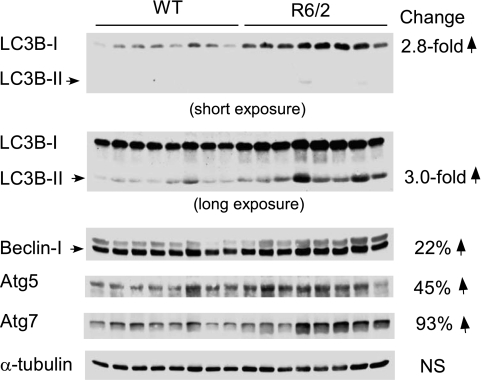
To investigate whether autophagy is involved in muscle wasting in HD, we analyzed protein expression of key players involved in autophagosome formation (Fig. 7). The microtubule-associated protein light chain LC3 is essential for the expansion of autophagosomes. The cytosolic form LC3B-I and the lipidated membrane bound form L3CB-II were increased 2.8- and 3-fold, respectively, in gastrocnemius of R6/2 mice compared with WT controls. However, the LC3B-I/LC3B-II ratio did not differ between the two groups. Beclin-1, which is associated with multimeric complex of macroautophagy regulatory proteins, was 22% higher in R6/2 mice. Also, the atg7 protein, which initiates the conjugation of atg12 to atg5 and LC3 to phosphatidylethanolamine, was 93% higher. Likewise, atg5, a protein essential for autophagosome precursor expansion and completion through a ubiquitin-like conjugation system was increased 45% in R6/2 mice. Thus, these data clearly demonstrate that signaling molecules of autophagy were elevated in skeletal muscle of R6/2 mice.
Fig. 7.
Elevated autophagy signaling protein expression in skeletal muscle of R6/2 mice. Western blot analysis of LC3B-I and II, Beclin-I, and atg5 and -7 in gastrocnemius and the change (R6/2 vs. WT mice) expressed as a fold change or percentage are shown. NS, not significantly different. ↑, P < 0.05, n = 8/group. α-tubulin as a loading control was also shown.
DISCUSSION
In the present study, we confirm and provide further evidence that R6/2 mice exhibit a profound peripheral phenotype that is characterized by catabolic symptoms of body weight loss, increased adiposity, severe muscle wasting, and elevated energy expenditure. The alterations of these parameters appeared to be age dependent. The accumulation of fat mass was detected as early as 4 wk of age in male mice, likely contributing to an increase in total body weight in 8-wk-old female R6/2 mice compared with WT littermates. The increased adiposity in R6/2 mice likely results from defective lipolysis (18). Starting at 8 wk of age, these animals displayed rapid body weight loss, decreased lean mass, and neurological abnormalities such as trembling and motor imbalance and died between 12 and 15 wk (8, 36). At 12 wk, body weight-normalized skeletal muscle mass (gastrocnemius and quadriceps) was 25% lower in R6/2 mice along with increased body fat and unaltered weights of other organs (Fig. 1D), suggesting that muscle wasting is a major cause of body weight loss. Another reason for the weight loss is the negative energy balance due to elevated whole body energy expenditure, which is often observed in a variety of catabolic conditions.
While the R6/2 mouse is an acute model of HD with rapid occurrence of typical clinical symptoms, their severe muscle atrophy prompted us to investigate the underlying molecular mechanisms by which skeletal muscle mass was dramatically reduced in such a short period of time. Muscle mass is maintained through a complex signaling network regulating muscle protein metabolism as well as myocyte degeneration and regeneration (46, 63). Most catabolic muscle wasting disorders exhibit a profound decrease in muscle protein synthesis. However, in the skeletal muscle of R6/2 mice there was an increase in protein synthesis and mTOR signaling, suggesting that the mechanisms must be different. We observed elevated mTOR signaling not only at the basal level but also in response to refeeding (Fig. 3) and leucine or insulin administration (data not shown) in the HD transgenic mice. Interestingly, we found that Akt protein expression and Akt phosphorylation in the basal condition (Fig. 4A) and when stimulated with insulin (data not shown) were markedly enhanced in muscle of R6/2 mice. Additionally, we found that basal AMPK phosphorylation in muscle was decreased, suggesting diminished AMPK activity. Both findings provide a molecular basis for the elevated mTOR signaling observed in muscle of these animals. Increased protein synthesis most likely results in elevated energy expenditure in R6/2 mice, as we recently demonstrated markedly increased energy expenditure associated with the activation of a futile protein turnover cycle in mice with disrupted first step of BCAA metabolism (51).
The decreased urinary 3-MH excretion and the tendency for a lower 3-MH/creatinine ratio, indexes of myofibrillar proteolysis, were unexpected. It has been shown that a nonlysosomal Ca2+-independent process is responsible for a rapid loss of cellular protein and dramatically elevated 3-MH in a denervated muscle model (19). It has been also reported that glucocorticoids activate the ATP-dependent and UPS-mediated proteolysis in skeletal muscle during fasting and that MuRF-1 and MuRF-2 together target a specific set of myofibrillar proteins, and MHC is a specific MuRF1 substrate (12, 66, 67). Additionally, it was shown that recombinant caspase-3 cleaved actomyosin, producing a characteristic, ∼14-kDa actin fragment (15). Thus, it appears that the UPS, not apoptotic activation, promotes selective myofibrillar proteolysis, which is associated with elevated 3-MH release. Our observation of lower MuRF1 and MAFbx gene expression during fasting and refeeding as well as lower urinary 3-MH in R6/2 mice agrees with relatively unaltered levels of MHC measured using immunoblotting and other major muscle proteins detected by silver staining (data not shown). Collectively, these data suggest that activation of the UPS is unlikely responsible for muscle atrophy of R6/2 mice.
Autophagy promotes bulk intracellular degradation of proteins and organelles and is stimulated in skeletal muscle under numerous pathological conditions (5). In a fly model of the neurodegenerative disease spinobulbar muscular atrophy, a compensatory autophagy was induced in response to impaired UPS function in a microtubule-associated histone deacetylase (HDAC6)-dependent manner (43). We demonstrated that protein expression levels of several key players in autophagy, such as LC3B-I and II, beclin, atg5, and agt 7, were all elevated in skeletal muscle of R6/2 mice (Fig. 6), suggesting a possible altered autophagic flux in HD mice. Heng et al. (22) recently showed early and sustained increased LC3-I, LC3-II, and p62 in brain of the HD knock-in HdhQ200 mouse model. It was also reported that, although there is an increased formation of autophagic vacuoles in HD cells, cargo is inadequately loaded into autophagosomes (39). How is autophagy signaling stimulated in muscle of R6/2 mice? Our finding of Akt and mTOR overactivation in muscle of R6/2 mice appears to contradict increased autophagy, because Akt inhibits autophagy by blocking FOXO3 (38). However, activation of IRS-2 by the insulin/IGF-I signaling cascade was reported to induce a macroautophagy-mediated clearance of the accumulated mHtt protein independently of Akt/mTOR activation (70). Thus, it remains to be determined whether autophagy is stimulated by this mechanism in muscle of R6/2 mice. Alternatively, endoplasmic reticulum (ER) stress due to a drastic defect in ER-associated degradation caused by misfolded proteins could initiate macroautophagy, as previously postulated (14, 16). A growing body of work in HD models suggests altered autophagy in response to mutant polyQ proteins and supports the view that increased autophagy is an important cytoprotective response to expanded repeat polyQ proteins in neurons (26, 39, 43, 44, 47). However, the role of autophagy in wasting skeletal muscle of HD is unclear, i.e., whether autophagy prompts cell survival, or autophagic cell death contributes to muscle atrophy as shown in some forms of neurodegenerative diseases and myopathy, and further studies are warranted (4, 57).
We detected significant increases in several markers of apoptosis in skeletal muscle of R6/2 mice. Increased caspase mRNA, protein and activity, and protein levels were consistently observed; mRNA and/or protein expression of Fas, FasL, and FADD, and other related genes was also increased, suggesting a possible activation of the death receptor extrinsic apoptotic pathway. Moreover, Cyt C release from mitochondria to cytosol was dramatically elevated. These in vivo data are in accord with the observed mitochondrial depolarization, Cyt C release, increased caspase-3, -8, and -9 activity, and defective cell differentiation in cultures of primary muscle cells from HD subjects (11). Elevated apoptotic signals were found in other HD cell types, such as increased Bax expression in B and T lymphocytes and monocytes from HD patients (1) as well as in the cortex of R6/1 HD mice (56). Increased Cyt C release from mitochondria and caspase-9 and -3 activity were reported in striatal neurons from HD patients and R6/2 mice of severe neuropathological grades (31). Stress-induced apoptotic cell death was found to be associated with caspase-3 activation and mitochondrial depolarization in lymphoblasts derived from HD patients (49). Furthermore, transgenic HD pigs, unlike mice expressing the same transgene, display typical apoptotic neurons with DNA fragmentation in their brains with activated caspase-3, although it is unknown whether this more severe apoptosis causes the death of these pigs at a very early age (71). Importantly, it was shown that neuro2a cells that express truncated NH2-terminal huntingtin (tNhtt) exhibited accumulated polyubiquitin-conjugated tNhtt and decreased proteasomal activity as well as increased Cyt C release and activated caspase-9 and -3-like proteases (29). Thus, our data and others' consistently demonstrate activated apoptotic signaling events in cell and animal models and patients of HD. Although our EM results showed relatively normal nuclear DNA at the light microscope level, TUNEL-positive skeletal fiber nuclei were prevalent in muscle of R6/2 mice.
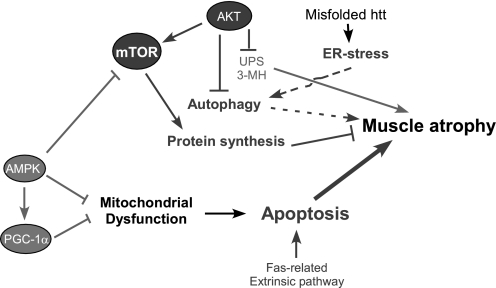
In summary, we have found that R6/2 mice exhibit muscle atrophy and apoptotic activation in the face of elevated protein synthesis as well as increased energy expenditure and adiposity. Mitochondrial dysfunction resulting from AMPK deactivation and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) downregulation might be central to these metabolic perturbations. Our data suggest complex interplays among the four signaling pathways of UPS, autophagy, apoptosis, and protein synthesis in skeletal muscle of R6/2 mice (Fig. 8). Mutant mHtt may induce ER stress as a primary trigger of autophagy activation to clear protein aggregates (61). Decreased energy production due to mitochondrial dysfunction in the face of elevated energy expenditure, which is associated with increased protein synthesis, could create an energy deficit, eventually leading to apoptotic muscle atrophy in HD. It has been shown that impaired mitochondrial biogenesis resulting from transcriptional repression of PGC-1α by mHtt in neurons, fat cells, and muscle plays an important role in HD pathogenesis (9, 13, 65). We now provide additional evidence of the deactivation of AMPK in muscle of R6/2 mice, consistent with unresponsive AMPK activation to chronic energy deprivation induced by a catabolic stressor, β-guanidinopropionic acid, in NLS-N171–82Q HD mice (9). AMPK is essential for mitochondrial biogenesis and regulation of PGC-1α, and PGC-1α overexpression was shown to improve sarcopenia (25, 27, 64, 73). Therefore, it is imperative to determine the mechanisms underlying AMPK deactivation and PGC-1α downregulation and whether reactivation of AMPK and PGC-1α can treat muscle atrophy of HD in animal models by improving mitochondrial function and deactivating apoptotic signaling in R6/2 mice.
Fig. 8.
Model of mechanisms underlying muscle atrophy in R6/2 mice. 3-MH, 3-methyhistidine; ER, endoplasmic reticulum; htt, huntingtin; UPS, ubiquitin proteasome system; PGC-1α; PPARγ coactivator-1α. Dark and light gray denote decrease and increase, respectively, in activity, gene expression, or exerting strength. Solid line, findings established in this study or in the literature; dotted line, not-yet-established but probable links. Arrow and ⊥, stimulation and inhibition, respectively.
GRANTS
This work was supported by grants from the National Institutes of Health (DK-062880 to C. J. Lynch, GM-38032 to C. H. Lang) and CHDI Management, Inc. (Early Discovery Initiative to P. She).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Anne Pruznak and Abid Kazi for technical assistance.
REFERENCES
- 1. Almeida S, Sarmento-Ribeiro AB, Januario C, Rego AC, Oliveira CR. Evidence of apoptosis and mitochondrial abnormalities in peripheral blood cells of Huntington's disease patients. Biochem Biophys Res Commun 374: 599–603, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Argiles JM, Lopez-Soriano FJ, Busquets S. Apoptosis signalling is essential and precedes protein degradation in wasting skeletal muscle during catabolic conditions. Int J Biochem Cell Biol 40: 1674–1678, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Attaix D, Aurousseau E, Combaret L, Kee A, Larbaud D, Ralliere C, Souweine B, Taillandier D, Tilignac T. Ubiquitin-proteasome-dependent proteolysis in skeletal muscle. Reprod Nutr Dev 38: 153–165, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Bahr BA, Bendiske J. The neuropathogenic contributions of lysosomal dysfunction. J Neurochem 83: 481–489, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Bechet D, Tassa A, Taillandier D, Combaret L, Attaix D. Lysosomal proteolysis in skeletal muscle. Int J Biochem Cell Biol 37: 2098–2114, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Bjorkqvist M, Petersen A, Bacos K, Isaacs J, Norlen P, Gil J, Popovic N, Sundler F, Bates GP, Tabrizi SJ, Brundin P, Mulder H. Progressive alterations in the hypothalamic-pituitary-adrenal axis in the R6/2 transgenic mouse model of Huntington's disease. Hum Mol Genet 15: 1713–1721, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J Neurosci 19: 3248–3257, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaturvedi RK, Adhihetty P, Shukla S, Hennessy T, Calingasan N, Yang L, Starkov A, Kiaei M, Cannella M, Sassone J, Ciammola A, Squitieri F, Beal MF. Impaired PGC-1alpha function in muscle in Huntington's disease. Hum Mol Genet 18: 3048–3065, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaturvedi RK, Calingasan NY, Yang L, Hennessey T, Johri A, Beal MF. Impairment of PGC-1alpha expression, neuropathology and hepatic steatosis in a transgenic mouse model of Huntington's disease following chronic energy deprivation. Hum Mol Genet 19: 3190–3205, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ciammola A, Sassone J, Alberti L, Meola G, Mancinelli E, Russo MA, Squitieri F, Silani V. Increased apoptosis, Huntingtin inclusions and altered differentiation in muscle cell cultures from Huntington's disease subjects. Cell Death Differ 13: 2068–2078, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E, Glass DJ. The E3 ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab 6: 376–385, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell 127: 59–69, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Ding WX, Yin XM. Sorting, recognition and activation of the misfolded protein degradation pathways through macroautophagy and the proteasome. Autophagy 4: 141–150, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, Price SR, Mitch WE. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest 113: 115–123, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duennwald ML, Lindquist S. Impaired ERAD and ER stress are early and specific events in polyglutamine. Genes Dev 22: 3308–3319, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dupont-Versteegden EE. Apoptosis in skeletal muscle and its relevance to atrophy. World J Gastroenterol 12: 7463–7466, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fain JN, Del Mar NA, Meade CA, Reiner A, Goldowitz D. Abnormalities in the functioning of adipocytes from R6/2 mice that are transgenic for the Huntington's disease mutation. Hum Mol Genet 10: 145–152, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Furuno K, Goodman MN, Goldberg AL. Role of different proteolytic systems in the degradation of muscle proteins during denervation atrophy. J Biol Chem 265: 8550–8557, 1990 [PubMed] [Google Scholar]
- 20. Glass D, Roubenoff R. Recent advances in the biology and therapy of muscle wasting. Ann NY Acad Sci 1211: 25–36, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Goodman AO, Murgatroyd PR, Medina-Gomez G, Wood NI, Finer N, Vidal-Puig AJ, Morton AJ, Barker RA. The metabolic profile of early Huntington's disease- a combined human and transgenic mouse study. Exp Neurol 210: 691–698, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Heng MY, Duong DK, Albin RL, Tallaksen-Greene SJ, Hunter JM, Lesort MJ, Osmand A, Paulson HL, Detloff PJ. Early autophagic response in a novel knock-in model of Huntington disease. Hum Mol Genet 19: 3702–3720, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hunt MJ, Morton AJ. Atypical diabetes associated with inclusion formation in the R6/2 mouse model of Huntington's disease is not improved by treatment with hypoglycaemic agents. Exp Brain Res Experimentelle Hirnforschung 166: 220–229, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Irrcher I, Ljubicic V, Kirwan AF, Hood DA. AMP-activated protein kinase-regulated activation of the PGC-1alpha promoter in skeletal muscle cells. PLoS One 3: e3614, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iwata A, Christianson JC, Bucci M, Ellerby LM, Nukina N, Forno LS, Kopito RR. Increased susceptibility of cytoplasmic over nuclear polyglutamine aggregates to autophagic degradation. Proc Natl Acad Sci USA 102: 13135–13140, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 104: 12017–12022, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jagoe RT, Goldberg AL. What do we really know about the ubiquitin-proteasome pathway in muscle atrophy? Curr Opin Clin Nutr Metab Care 4: 183–190, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Jana NR, Zemskov EA, Wang G, Nukina N. Altered proteasomal function due to the expression of polyglutamine-expanded truncated N-terminal huntingtin induces apoptosis by caspase activation through mitochondrial cytochrome c release. Hum Mol Genet 10: 1049–1059, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Jenkins BG, Andreassen OA, Dedeoglu A, Leavitt B, Hayden M, Borchelt D, Ross CA, Ferrante RJ, Beal MF. Effects of CAG repeat length, HTT protein length and protein context on cerebral metabolism measured using magnetic resonance spectroscopy in transgenic mouse models of Huntington's disease. J Neurochem 95: 553–562, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Kiechle T, Dedeoglu A, Kubilus J, Kowall NW, Beal MF, Friedlander RM, Hersch SM, Ferrante RJ. Cytochrome C and caspase-9 expression in Huntington's disease. Neuromol Med 1: 183–195, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Krawiec BJ, Nystrom GJ, Frost RA, Jefferson LS, Lang CH. AMP-activated protein kinase agonists increase mRNA content of the muscle-specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells. Am J Physiol Endocrinol Metab 292: E1555–E1567, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Lee CE, McArdle A, Griffiths RD. The role of hormones, cytokines and heat shock proteins during age-related muscle loss. Clin Nutr 26: 524–534, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Liang Y, She P, Wang X, Demarest K. The messenger RNA profiles in liver, hypothalamus, white adipose tissue, and skeletal muscle of female Zucker diabetic fatty rats after topiramate treatment. Metabolism 55: 1411–1419, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Lione LA, Carter RJ, Hunt MJ, Bates GP, Morton AJ, Dunnett SB. Selective discrimination learning impairments in mice expressing the human Huntington's disease mutation. J Neurosci 19: 10428–10437, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luthi-Carter R, Hanson SA, Strand AD, Bergstrom DA, Chun W, Peters NL, Woods AM, Chan EY, Kooperberg C, Krainc D, Young AB, Tapscott SJ, Olson JM. Dysregulation of gene expression in the R6/2 model of polyglutamine disease: parallel changes in muscle and brain. Hum Mol Genet 11: 1911–1926, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6: 458–471, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Martinez-Vicente M, Talloczy Z, Wong E, Tang G, Koga H, Kaushik S, de Vries R, Arias E, Harris S, Sulzer D, Cuervo AM. Cargo recognition failure is responsible for inefficient autophagy in Huntington's disease. Nat Neurosci 13: 567–576, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mihm MJ, Amann DM, Schanbacher BL, Altschuld RA, Bauer JA, Hoyt KR. Cardiac dysfunction in the R6/2 mouse model of Huntington's disease. Neurobiol Dis 25: 297–308, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mochel F, Charles P, Seguin F, Barritault J, Coussieu C, Perin L, Le Bouc Y, Gervais C, Carcelain G, Vassault A, Feingold J, Rabier D, Durr A. Early energy deficit in Huntington disease: identification of a plasma biomarker traceable during disease progression. PLoS ONE 2: e647, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Orth M, Cooper JM, Bates GP, Schapira AH. Inclusion formation in Huntington's disease R6/2 mouse muscle cultures. J Neurochem 87: 1–6, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB, Schwartz SL, DiProspero NA, Knight MA, Schuldiner O, Padmanabhan R, Hild M, Berry DL, Garza D, Hubbert CC, Yao TP, Baehrecke EH, Taylor JP. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 447: 859–863, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Qin ZH, Wang Y, Kegel KB, Kazantsev A, Apostol BL, Thompson LM, Yoder J, Aronin N, DiFiglia M. Autophagy regulates the processing of amino terminal huntingtin fragments. Hum Mol Genet 12: 3231–3244, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Ribchester RR, Thomson D, Wood NI, Hinks T, Gillingwater TH, Wishart TM, Court FA, Morton AJ. Progressive abnormalities in skeletal muscle and neuromuscular junctions of transgenic mice expressing the Huntington's disease mutation. Eur J Neurosci 20: 3092–3114, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Saini A, Al-Shanti N, Stewart CE. Waste management—cytokines, growth factors and cachexia. Cytokine Growth Factor Rev 17: 475–486, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Sarkar S, Rubinsztein DC. Huntington's disease: degradation of mutant huntingtin by autophagy. FEBS J 275: 4263–4270, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Sassone J, Colciago C, Cislaghi G, Silani V, Ciammola A. Huntington's disease: the current state of research with peripheral tissues. Exp Neurol 219: 385–397, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Sawa A, Wiegand GW, Cooper J, Margolis RL, Sharp AH, Lawler JF, Jr, Greenamyre JT, Snyder SH, Ross CA. Increased apoptosis of Huntington disease lymphoblasts associated with repeat length-dependent mitochondrial depolarization. Nat Med 5: 1194–1198, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell 103: 253–262, 2000 [DOI] [PubMed] [Google Scholar]
- 51. She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab 6: 181–194, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. She P, Zhou Y, Zhang Z, Griffin K, Gowda K, Lynch CJ. Disruption of BCAA metabolism in mice impairs exercise metabolism and endurance. J Appl Physiol 108: 941–949, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Siu PM, Alway SE. Response and adaptation of skeletal muscle to denervation stress: the role of apoptosis in muscle loss. Front Biosci 14: 432–452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Solomon V, Goldberg AL. Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem 271: 26690–26697, 1996 [DOI] [PubMed] [Google Scholar]
- 55. Strand AD, Aragaki AK, Shaw D, Bird T, Holton J, Turner C, Tapscott SJ, Tabrizi SJ, Schapira AH, Kooperberg C, Olson JM. Gene expression in Huntington's disease skeletal muscle: a potential biomarker. Hum Mol Genet 14: 1863–1876, 2005 [DOI] [PubMed] [Google Scholar]
- 56. Teles AV, Rosenstock TR, Okuno CS, Lopes GS, Bertoncini CR, Smaili SS. Increase in bax expression and apoptosis are associated in Huntington's disease progression. Neurosci Lett 438: 59–63, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ 12, Suppl 2: 1528–1534, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Underwood BR, Broadhurst D, Dunn WB, Ellis DI, Michell AW, Vacher C, Mosedale DE, Kell DB, Barker RA, Grainger DJ, Rubinsztein DC. Huntington disease patients and transgenic mice have similar pro-catabolic serum metabolite profiles. Brain 129: 877–886, 2006 [DOI] [PubMed] [Google Scholar]
- 59. van der Burg JM, Bacos K, Wood NI, Lindqvist A, Wierup N, Woodman B, Wamsteeker JI, Smith R, Deierborg T, Kuhar MJ, Bates GP, Mulder H, Erlanson-Albertsson C, Morton AJ, Brundin P, Petersen A, Bjorkqvist M. Increased metabolism in the R6/2 mouse model of Huntington's disease. Neurobiol Dis 29: 41–51, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Ventadour S, Attaix D. Mechanisms of skeletal muscle atrophy. Curr Opin Rheumatol 18: 631–635, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Vidal R, Caballero B, Couve A, Hetz C. Converging pathways in the occurrence of endoplasmic reticulum (ER) stress in Huntington's disease (Review). Curr Mol Med 11: 1–12, 2011 [DOI] [PubMed] [Google Scholar]
- 62. Vielma H, Mendez J, Druckenmiller M, Lukaski H. A practical and reliable method for determination of urinary 3-methylhistidine. J Biochem Biophys Methods 5: 75–82, 1981 [DOI] [PubMed] [Google Scholar]
- 63. Vinciguerra M, Musaro A, Rosenthal N. Regulation of muscle atrophy in aging and disease. Adv Exp Med Biol 694: 211–233, 2010 [DOI] [PubMed] [Google Scholar]
- 64. Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci USA 106: 20405–20410, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65. Weydt P, Pineda VV, Torrence AE, Libby RT, Satterfield TF, Lazarowski ER, Gilbert ML, Morton GJ, Bammler TK, Strand AD, Cui L, Beyer RP, Easley CN, Smith AC, Krainc D, Luquet S, Sweet IR, Schwartz MW, La Spada AR. Thermoregulatory and metabolic defects in Huntington's disease transgenic mice implicate PGC-1alpha in Huntington's disease neurodegeneration. Cell Metab 4: 349–362, 2006 [DOI] [PubMed] [Google Scholar]
- 66. Wing SS, Goldberg AL. Glucocorticoids activate the ATP-ubiquitin-dependent proteolytic system in skeletal muscle during fasting. Am J Physiol Endocrinol Metab 264: E668–E676, 1993 [DOI] [PubMed] [Google Scholar]
- 67. Witt SH, Granzier H, Witt CC, Labeit S. MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: towards understanding MURF-dependent muscle ubiquitination. J Mol Biol 350: 713–722, 2005 [DOI] [PubMed] [Google Scholar]
- 68. Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp Gerontol 45: 138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wray CJ, Mammen JM, Hershko DD, Hasselgren PO. Sepsis upregulates the gene expression of multiple ubiquitin ligases in skeletal muscle. Int J Biochem Cell Biol 35: 698–705, 2003 [DOI] [PubMed] [Google Scholar]
- 70. Yamamoto A, Cremona ML, Rothman JE. Autophagy-mediated clearance of huntingtin aggregates triggered by the insulin-signaling pathway. J Cell Biol 172: 719–731, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yang D, Wang CE, Zhao B, Li W, Ouyang Z, Liu Z, Yang H, Fan P, O'Neill A, Gu W, Yi H, Li S, Lai L, Li XJ. Expression of Huntington's disease protein results in apoptotic neurons in the brains of cloned transgenic pigs. Hum Mol Genet 19: 3983–3994, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Young VR, Munro HN. Ntau-methylhistidine (3-methylhistidine) and muscle protein turnover: an overview. Fed Proc 37: 2291–2300, 1978 [PubMed] [Google Scholar]
- 73. Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA 99: 15983–15987, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.