Summary
Purpose
The γ-aminobutyric acid (GABA) degradation blocker γ-vinyl-GABA (VGB) is used clinically to treat seizures in both adult and immature individuals. The mechanism by which VGB controls developmental seizures is not fully understood. Specifically, whether the anticonvulsant properties of VGB arise only from its elevation of brain GABA levels and the resulting activation of GABA receptors, or also from associated mechanisms, remains unresolved. Corticotropin-releasing hormone (CRH), a neuropeptide present in many brain regions involved in developmental seizures, is a known convulsant in the immature brain and has been implicated in some developmental seizures. In certain brain regions, it has been suggested that CRH synthesis and release may be regulated by GABA. Therefore we tested the hypothesis that VGB decreases CRH gene expression in the immature rat brain, consistent with the notion that VGB may decrease seizures also by reducing the levels of the convulsant molecule, CRH.
Methods
VGB was administered to immature, 9-day-old rats in clinically relevant doses, whereas littermate controls received vehicle.
Results
In situ hybridization histochemistry demonstrated a downregulation of CRH mRNA levels in the hypothalamic paraventricular nucleus but not in other limbic regions of VGB-treated pups compared with controls. In addition, VGB-treated pups had increased CRH peptide levels in the anterior hypothalamus, as shown by radioimmunoassay.
Conclusions
These findings are consistent with a reduction of both CRH gene expression and secretion in the hypothalamus, but do not support an indirect anticonvulsant mechanism of VGB via downregulation of CRH levels in limbic structures. However, the data support a region-specific regulation of CRH gene expression by GABA.
Keywords: GABA, Seizures, Developmental, Infantile spasms, Vigabatrin
γ-Aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the central nervous system. The actions of GABA on the GABAA receptor suppress the initiation and propagation of seizures (1). Therefore drugs activating GABAA receptors or elevating GABA levels in the synaptic cleft by blocking the breakdown of this neurotransmitter or its reuptake have anticonvulsant properties (2). For example, the GABA transaminase blocker, γ-vinyl-GABA (VGB; vigabatrin), has been efficacious for seizures in adults as well as in infants and children (3,4). VGB also has been particularly useful for seizures that are age specific to the developmental period, such as infantile spasms (5,6). Indeed, the response to VGB treatment, which results in a dose-dependent elevation of brain GABA (7), is often significantly superior to the response to agents that activate GABA receptors directly (5). Therefore it is unclear whether the mechanism by which VGB controls developmental seizures involves solely GABA elevation and the consequent augmented inhibition in neuronal circuits, or other mechanisms related to enhanced brain GABA levels such as age-specific inhibition of production or secretion of excitatory neurotransmitters or neuromodulators.
A candidate proconvulsant mechanism in the developing brain is enhancement of glutamate-mediated excitation by the peptide corticotropin-releasing hormone (CRH). CRH has been shown essentially to “amplify” the excitatory input of glutamate to the hippocampal circuit, leading to epileptiform output (8). In addition, picomolar amounts of CRH have been shown to induce prolonged limbic seizures involving amygdala and hippocampus (9,10). These proconvulsant effects of CRH are largely age specific to the developmental period (11).
In the developing and mature brain, CRH functions as a neuromodulator in limbic circuits involved in mediating the response to stress, as well as in learning and memory functions (12,14). The peptide is highly expressed in brain regions associated with developmental seizures, such as hippocampus and amygdala (15,16). In addition, in vitro and recent in vivo studies suggested that CRH synthesis and secretion may be influenced by neurotransmitters such as GABA (17,18). This study used an immature animal model to determine whether the anticonvulsant effects of VGB may be mediated also by reduced expression of the convulsant molecule CRH.
MATERIALS AND METHODS
Animals and tissue preparation
All experiments were conducted in accordance with NIH guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care Committee. Timed-pregnancy Sprague–Dawley-derived rats (Zivic-Miller, Zelienople, PA, U.S.A.) were maintained in an NIH-approved animal facility on a 12-h light/dark cycle. In all, 36 animals were used in the following experiments. Controls included in each experiment were littermates of experimental pups. All pups were subjected to an intraperitoneal injection of either VGB (500 mg/kg) or an identical volume of vehicle, between 8 and 10 a.m. on postnatal day 9, to prevent potential diurnal variability in CRH–messenger RNA (mRNA) levels (19). Pups were then observed for an hour on a euthermic pad, and then returned to their home cages. Rats were permitted to survive for 24 h, a time point when VGB-induced brain GABA levels are maximal (20).
For immunocytochemistry, rats were perfused with 4% buffered paraformaldehyde, as described in detail elsewhere (15,21). For in situ hybridization histochemistry and for radioimmunoassay analysis of CRH, rats were decapitated within minutes of removal from home cages to minimize stress-related alteration of CRH expression (22,23). Brains were rapidly removed and subjected to one of two procedures. For sectioning and in situ hybridization, brains were frozen on powdered dry ice; for regional quantitation of CRH, brains were dissected on ice as described (23). Trunk blood was collected for evaluation of adrenocorticotropic hormone (ACTH) and corticosterone.
Immunocytochemistry
Matched sections from control and VGB-treated rats, spanning the rostrocaudal extent of the cerebrum, were immunostained for GAD-67 on slide or free floating, by using a modification of the standard Vector ABC protocol, as previously described (15). Sections from control and experimental brains were processed under identical conditions (on the same slide or in the same well).
In brief, after several washes with phosphate-buffered saline (PBS), sections were treated with 0.05% hydrogen peroxide for quenching endogenous peroxidase activity, and then preincubated in PBS containing 5% normal goat serum and 1.5% bovine serum albumin for blocking non-specific reactivity (PBS+). Sections were then incubated in a solution of polyclonal GAD-67 antibody, made in rabbit (Chemicon Inc., Temecula, CA, U.S.A.; final dilution, 1:5,000) in PBS+ containing 0.03% Triton-X-100, overnight at room temperature. After several rinses with PBS, sections were transferred to a solution containing 1% biotinylated goat anti-rabbit immunoglobulin G (IgG) with Triton-X-100 for 1 h at room temperature, followed by a 1-h incubation in 1% ABC solution (Vectastain; Vector Laboratories, Burlingame, CA, U.S.A.). The immunoreaction product was visualized by using 0.005% hydrogen peroxide and 0.05% 3,3 diaminobenzidine (DAB) with 0.5% NiCl enhancement.
In situ hybridization histochemistry (ISH)
ISH for detection of CRH–mRNA was performed as described previously (22-24). In brief, 20-μm coronal sections were collected on gelatin-coated slides and stored at −80°C. For ISH, tissue sections were thawed, air-dried, fixed in 4% buffered paraformaldehyde, dehydrated, and rehydrated through graded ethanols (22). Sections were exposed to 0.25% acetic anhydride in 0.1 M triethanolamine and dehydrated. Prehybridization (1 h) and hybridization (20 h) steps were performed at 40°C in a humidified chamber (22,24), by using a deoxyoligonucleotide probe complementary to the coding region of CRH–mRNA and 3′-end-labeled with 35S-dATP, at 3–4 × 105 cpm/section (24). Sections were then washed and apposed to film (Hyperfilm β-Max; Amersham, IL, U.S.A.) for 5–7 days. Selected sections also were dipped in emulsion (NTB-2; Eastman Kodak, Rochester, NY, U.S.A.) and exposed for 3–4 weeks.
Acquisition and quantitative analysis of CRH-mRNA ISH signal
Semiquantitative analysis of CRH–mRNA was performed after in situ hybridization (22,23). Digitized images of each brain were acquired by using a Studiostar scanner (AGFA, resolution 1,200 × 1,200 dots per inch) and analyzed by using the ImageTool software program (University of Texas Health Science Center, San Antonio, TX, U.S.A.; version 1.25). Densities were calibrated by using 14C standards and are expressed in μCi/g after correcting for background by subtracting the density of the hybridization signal over the corpus callosum. Anatomically matched sections from the hypothalamic paraventricular nucleus (PVN; six sections with highest signal per brain), central nucleus of amygdala (ACE), cingulate cortex, and hippocampus (CA 1, CA3a, CA3b) were analyzed from at least three brains per group. The significance of observed quantitative differences among experimental groups was evaluated by using the unpaired Student’s t test with Welch’s correction for unequal variance as needed.
Analysis of CRH peptide
Immediately after decapitation, brains were microdissected to isolate the medial basal hypothalamus (containing the median eminence and arcuate nucleus) and the anterior hypothalamus (containing paraventricular, suprachiasmatic, and supraoptic nuclei, as well as the anterior hypothalamic and medial preoptic areas). Tissue blocks were placed individually in microfuge tubes and frozen in powdered dry ice. CRH peptide was purified by a modification of a previously described procedure (25). In brief, tissue was thawed in extraction buffer [0.25N HCI, 0.25N acetic acid, 4.5 μg/ml pepstatin A (Sigma, St. Louis, MO, U.S.A.) and 0.5% β-mercaptoethanol] at 48°C for 3 min followed by homogenization (10 strokes with micro tissue grinder; Kontes, Vineland, NJ, U.S.A.) on ice. The homogenate was sonicated (15 s at 75% power of 0.8 duty cycle of Braun sonicator) a total of 4 times on ice. The preparation was centrifuged at 15,600 g for 30 min at 4°C. The supernatant was transferred to a new tube, the centrifugation was repeated, and the supernatant was desiccated to near dryness in a speed-vac. The preparation was resuspended in 400 μl of assay buffer (26) containing 1 μg/ml of phenol red and the pH was adjusted to 7.2–7.6 using NaOH.
Radioimmunoassay (RIA) for CRH peptide was performed as previously described (26) with slight modifications. Assay was performed in polypropylene RIA tubes (Fisher) with rabbit anti-rat CRH antibody (kindly provided by W. Vale, final dilution of 1:700,000) and 125I-Tyr-rat/human CRH (New England Nuclear; 30,000 cpm/tube). Calibrations to CRH standards were performed by using 1:l serial dilutions of CRH (Bachem, Torrance, CA, U.S.A.) ranging from 2,000 to 0.25 pg. The final precipitation was achieved by using Pansorbin (Calbiochem, La Jolla, CA, U.S.A.; final dilution, I:360).
Plasma hormone measurement
Plasma concentrations of ACTH and of corticosterone were determined by RIA by using commercial kits (Incstar, Stillwater, MN, and ICN, Irvine, CA, U.S.A., for ACTH and corticosterone, respectively).
RESULTS
Behavioral effects of VGB
Administration of VGB (500 mg/kg) did not lead to acute behavioral alterations compared with vehicle-injected controls. However, within 3–4 h of VGB treatment, pups became drowsy. VGB-treated rats were still asleep at 24 h after receiving VGB, but maintained normothermia and were observed to be groomed by the mother. However, they did not suckle, and lost an average of 2–3 g of their body weight during the 24 h after VGB administration.
Levels of the GABA-synthesizing enzyme, GAD-67
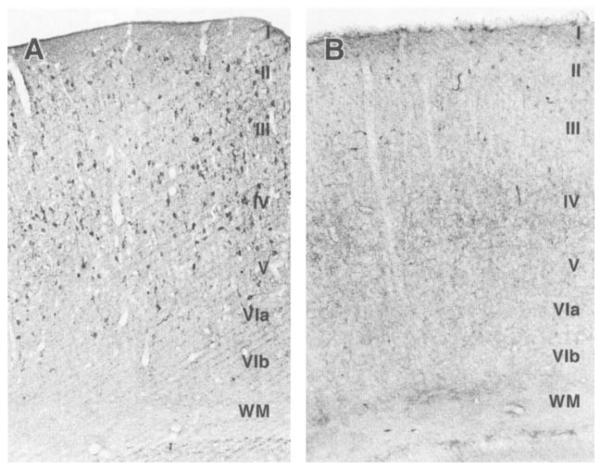
Because regional quantitation of GABA levels in the same brains analyzed for CRH expression and release is problematic, analysis of the abundance of the GABA-synthesizing enzyme was used as an indirect measure of GABA levels (27). GABA, via end-product inhibition. is known to down-regulate the expression of glutamic acid decarboxylase (GAD), the GABA-synthetic enzyme (27). Therefore immunocytochemistry for the GAD isoform, GAD-67, was used to study changes in the GABA system in the cerebral cortex after VGB treatment. GAD-67 immunoreacrivity was reduced in VGB-treated rats as compared with controls (Fig. 1). This included disappearance of GAD-67 immunoreactive neurons in the neocortex, leaving only a reduced number of labeled cells in the superficial layers. Furthermore, GAD-67 immunolabeling in the neuropil of the cortex, cingulate, piriform, and entorhinal cortical regions was lighter in the VGB-treated than control brains (data not shown).
FIG. 1.
VGB-induced reduction of GABA-synthesizing enzyme (GAD-67) abundance, consistent with elevation of GABA levels. Low-power view of immunoreactivity for glutamicacid-decarboxylase isoform GAD-67 in infant rat neocortex, showing the effect of VGB on GAD expression. A, B: From homologous portions of the parietal cortex of a 10-day-old control rat and its VGB-treated littermate, respectively. GAD-67 immunoreactive neurons are seen in all six cortical layers–indicated by roman numerals–of the control section (A). Abundance of GAD-67–immunolabeled cell bodies is substantially reduced after VGB treatment (B). WM, white matter. (Original magnification, ×100).
CRH–mRNA levels after VGB treatment
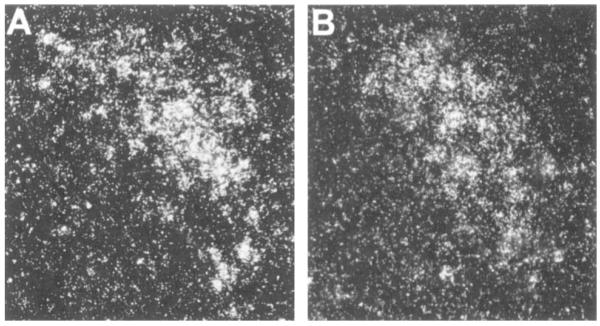
Quantitative analysis performed after in situ hybridization showed that CRH–mRNA levels in the hypothalamic PVN of VGB-treated pups were significantly lower than those of controls. To validate this analysis, two evaluation techniques were used: with the average value of all sections for each brain as an independent variable (leading to n = three to four per group), CRH–mRNA levels in VGB-treated rats was 0.1017 μCi/g, whereas that of controls was 0.2104 μCi/g (p < 0.05). When analyzing values directly from each section. (leading to n = 18–24 per group, see Fig. 2), CRH–mRNA in VGB-treated rats was 0.1017 μCi/g, whereas that of controls was 0.2079 μCi/g (p < 0.001; Fig. 2). Dark-field photomicrographs of PVN of control rats and those injected with VGB are shown in Fig. 3, demonstrating substantial reduction of CRH–mRNA expression in matched sections of the parvocellular PVN after VGB treatment. CRH–mRNA levels in the central nucleus of the amygdala, hippocampus, and cingulate cortex of VGB-treated rats were not significantly different from those in controls (Fig. 5).
FIG. 2.
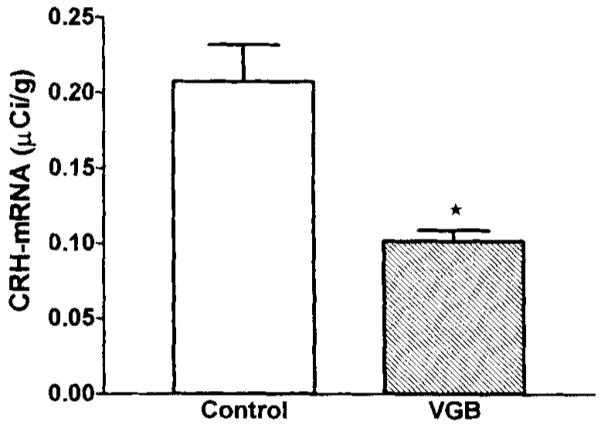
CRH–mRNA levels in the hypothalamic paraventricular nucleus (PVN) are decreased after VGB treatment. Quantitative analysis of autoradiograms of coronal sections (six per brain) at the level of the hypothalamus subjected to in situ hybridization for CRH–mRNA. Signal over the paraventricular nucleus (PVN) was quantified by using densitometric analysis. Values are expressed as mean ± SEM of 18–24 values per group. Significance of difference from vehicle-treated controls was determined by using the unpaired t test with Welch’s correction: *p < 0.001.
FIG. 3.
CRH–mRNA hybridization signal in the PVN is reduced after VGB treatment. Dark-field photomicrographs of the hypothalamic paraventricular region of control (A) and VGB-treated rats (B). Animals were killed 24 h after injections of saline (A) or VGB (B). Coronal sections at the level of the PVN were subjected to in situ hybridization by using an oligodeoxynucleotide probe directed at CRH–mRNA. The sections were dipped in emulsion and developed together (see text for details). A significant reduction in CRH–mRNA signal intensity is evident after VGB treatment. (Original magnification, ×200)
FIG. 5.
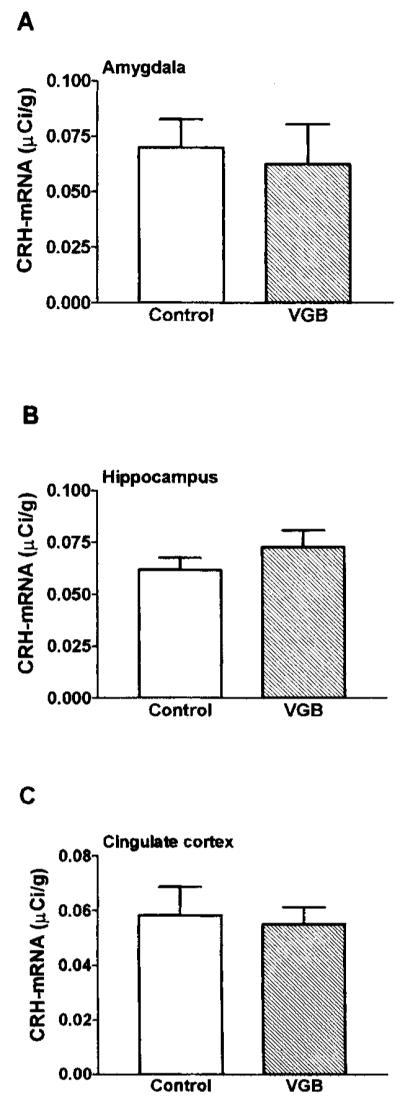
CRH–mRNA levels in central amygdala, hippocampus, and cingulate cortex are unchanged after VGB treatment. Quantitative analysis of autoradiograms of sections subjected to in situ hybridization for CRH–mRNA in amygdala, hippocampus, and cingulate cortex. Values are expressed as the mean ± SEM of at least four values per region per group. CRH–mRNA levels in these regions were not significantly different from those of vehicle-treated controls (p > 0.1).
CRH peptide levels after VGB treatment
RIA was used to measure the amount of the peptide, CRH, in the anterior hypothalamus (containing the paraventricular, suprachiasmatic, and supraoptic nuclei) and in the medial–basal hypothalamus (containing the median eminence and the arcuate nucleus). CRH peptide levels in the anterior hypothalamus of VGB-treated pups were significantly higher than the controls (p < 0.05; Fig. 4). In contrast, CRH peptide levels in the medial-basal hypothalamus were not affected by VGB treatment (not shown).
FIG. 4.
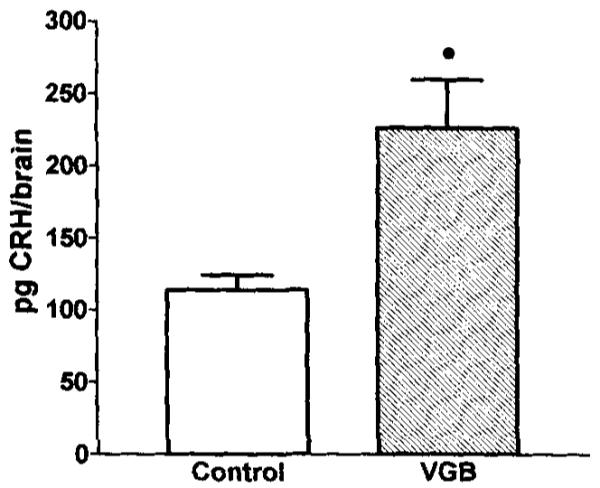
CRH peptide levels in anterior hypothalamus are increased by VGB treatment. Quantitative analysis of CRH peptide in the microdissected paraventricular and adjacent hypothalamic nulcei was achieved by using RIA. Rats were killed 24 h after saline or VGB treatment. Values are expressed as the mean CRH peptide content per rat, as obtained from five rats per group. Error bars represent the standard error of the mean. *Significantly different from saline controls (p < 0.05).
Plasma hormone levels after VGB treatment
Plasma ACTH and corticosterone levels were measured by RIA. Pups treated with VGB had significantly higher plasma corticosterone levels as compared with saline-treated controls (p < 0.05; Fig. 6A). The apparent increase of plasma ACTH levels of VGB-treated pups was not statistically significant (p < 0.1; Fig. 6B).
FIG. 6.
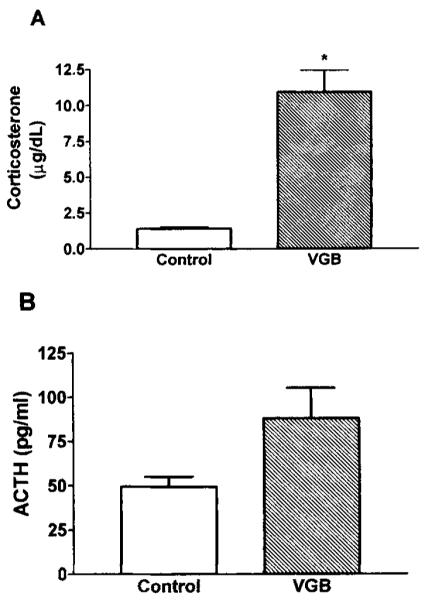
Plasma corticosterone but not ACTH levels are elevated after VGB treatment. Twenty-four hours after treatment of saline or VGB, corticosterone (A), and adrenocorticotropic hormone (ACTH, B) in trunk blood were measured by RIA. Bars represent the mean of three rats per group with error bars indicating SEM. In A, *p < 0.05, as determined by unpaired Student’s t test with Welch’s correction.
DISCUSSION
This study was designed to test the influence of GABA on CRH gene expression in specific brain regions of the developing rat brain. The major findings of the study are (a) VGB treatment under the experimental conditions of this study downregulated GAD-67 expression, consistent with elevated GABA levels; (b) VGB treatment resulted in lower levels of CRH–mRNA in the hypothalamic PVN but not in the limbic regions analyzed; (c) levels of the peptide CRH were higher in anterior hypothalamus of VGB-treated rats compared with controls; and (d) VGB treatment led to increased plasma corticosterone without significant alteration of ACTH levels.
VGB blocks GABA degradation by inhibiting both neuronal and glial GABA transaminase (28). With in vivo magnetic resonance spectroscopy, VGB administration has been shown to increase brain GABA levels in the human (29). For example, a single oral dose of 50 mg/kg resulted in a 40% increase in GABA over baseline within 2 h, and a 60% increase by 24 h (7,20). Thus in the adult, both a dose effect and a time effect of VGB on brain GABA have been found, with presumed maximal inhibition of GABA transaminase with ~100 mg/kg of VGB, and peak GABA accumulation at 24 h after administration (7,20). In the developing human, the recommended VGB doses for long-term treatment of seizures is 100–150 mg/kg/day (5), and in a series of three children, increased brain GABA have been reported (30). The VGB dose used in this study for immature rats (500 mg/kg) is comparable to clinically useful doses when calculated per body surface area, and the effect of this dose was assessed at the 24-h point, predicted to coincide with maximal brain GABA levels.
Alterations in GABA levels after VGB treatment were examined indirectly, by using immunocytochemistry detection of the GABA-synthesizing enzyme, GAD-67. This permitted estimates of both GABA abundance and CRH–mRNA levels on adjacent sections from the same experimental or control brain. Decreased brain GAD levels have been documented after VGB-induced brain GABA levels (27). Thus the reduction of GAD immunoreactivity found in our study, is consistent with increased brain GABA due to VGB-induced blocking of GABA transaminase (7,27).
Modulation of GABA is anticipated to influence CRH gene expression and secretion in key limbic regions of the developing brain. In hippocampus, both in Ammon’s horn and the dentate gyrus, a robust population of CRH-expressing neurons has been shown to consist of GAB-Aergic interneurons (15,31). Colocalization of CRH with GAD-65 and GAD-67 isoforms has been documented in virtually all of these neurons (15). Colocalization of CRH and GABA also has been demonstrated in a minority of CRH-expressing neurons of the hypothalamic PVN (32). However, most CRH-expressing neurons in the PVN contain neither the GABA-synthesizing enzyme GAD nor GABA (32,33). In addition, evidence for inhibitory input to CRH-expressing neurons via GABAergic innervation has been documented (32,34). To test whether the increased GABA associated with VGB treatment influenced CRH gene expression, in situ hybhdization was performed to measure steady-state levels of CRH–mRNA in limbic and hypothalamic regions. In addition, the relatively high levels of CRH peptide present in hypothalamus permitted quantitative analysis of CRH peptide with RIA.
VGB-treated rats were found to have a reduced level of CRH–mRNA in the PVN compared with the controls. This is consistent with a reduction of CRH gene expression by enhanced GABAergic receptor activation. Downregulation of CRH-mRNA levels was confined to the hypothalamus; there were no alterations of CRH gene expression in other brain regions (cingulate cortex, hippocampus, and amygdala) in response to VGB treatment. Because colocalization of CRH and GABA is found in hippocampus and cortex, but not hypothalamus, this finding suggests that the differential regiona1 regu1ation of CRH by GABAergic mechanisms is not mediated by autoinhibition of double-transmitter CRH–GABA neuronS by GABA.
Whereas VGB administration resulted in decreased CRH–mRNA levels in PVN, increased peptide levels were detected in tissue blocks consisting mainly of the PVN (with additional hypothalamic regions virtually devoid of CRH). Increased CRH peptide levels might be due to increased synthesis. However, this is unlikely in the face of reduced CRH-message levels. Alternatively, increased CRH peptide denotes inhibited secretion along axons leading to the median eminence and into the bloodstream (32). This possibility is supported by several in vitro studies showing that CRH secretion from hypothalamic slices is inhibited by GABA (17,35).
Aside from administration of VGB, other experimental procedures and manipulations used in this study are stressful and may therefore influence CRH synthesis and levels in the hypothalamus of the immature rat (22,23). For example, separation from the mother is a stressful circumstance, as is an intraperitoneal injection (22,36,37). However, the experimental design accounted for these variables by using litter-matched controls that were separated from the mother concurrent with the experimental group, received vehicle injection, and were killed with the VGB group. In addition, to eliminate potential diurnal variability in the levels of CRH–mRNA (19), all injections took place between 8 and 10 a.m. and timed killing was precisely at 24 h. Therefore it is unlikely that stress resulting directly from the experimental manipulations accounted for the differences in CRH–mRNA or CRH peptide levels between VGB-treated and control groups.
However, as mentioned earlier, VGB led to drowsiness followed by prolonged sleep. This resulted in lack of suckling and of food intake in the experimental group. Whether food deprivation for 24 h influences CRH–mRNA levels in the PVN has not been fully settled. Smith et al. (38) reported that maternal separation for 24 h—which is associated with food deprivation—decreased CRH–mRNA levels in the PVN. However, work from our laboratory (36), confirmed by other investigators (39), indicated that 24 h of food deprivation did not alter levels of CRH–mRNA in the PVN. Therefore we conclude that the decreased or absent suckling in VGB-treated rats is unlikely to account fully for the reduction in CRH–mRNA expression found in this study. In addition, food deprivation associated with separation from the mother for 24 h has been shown to elevate plasma corticosterone levels (36,37). Reinstitution of food intake via gavage restored plasma corticosterone to baseline levels (37). Plasma ACTH levels, which directly depend on CRH secretion, are not increased under the circumstances of maternal separation and food deprivation (37,40). Thus it is generally considered that the elevation of plasma corticosterone levels associated with lack of food intake (observed also in this study) is mediated via an effect on adrenal sensitivity to ACTH rather than modulation of CRH secretion.
In summary, VGB administration to immature rats depressed CRH gene expression in the hypothalamus but not in the hippocampus, amygdala, or cortex. Therefore it is concluded that an anticonvulsant effect of VGB via decreased CRH-mediated excitation is unlikely. However, a region-specific effect of VGB on the CRH neuron is demonstrated, which suggests alterations in the ability of VGB-treated humans to mount a hormonal response to stressful stimuli.
REFERENCES
- 1.Dichter MA. Basic mechanisms of epilepsy: targets for therapeutic intervention. Epilepsia. 1997;38(suppl 9):S2–6. doi: 10.1111/j.1528-1157.1997.tb05200.x. [DOI] [PubMed] [Google Scholar]
- 2.Meldrum BS. Identification and preclinical testing of novel anti-epileptic compounds. Epilepsia. 1997;38(suppl 9):S7–15. doi: 10.1111/j.1528-1157.1997.tb05204.x. [DOI] [PubMed] [Google Scholar]
- 3.Mumford JP, Dam M. Meta-analysis of European placebo controlled studies of vigabatrin in drug resistant epilepsy. Br J Clin Pharmacol. 1989;27(suppl 1):101S–7S. doi: 10.1111/j.1365-2125.1989.tb03469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lortie A, Chiron C, Dumas C, Mumford JP, Dulac O. Optimizing the indication of vigabatrin in children with refractory epilepsy. J Child Neurol. 1997;12:253–9. doi: 10.1177/088307389701200407. [DOI] [PubMed] [Google Scholar]
- 5.Aicardi J, Mumford JP, Dumas C, Wood S, Sabril IS Investigator and Peer Review Groups Vigabatrin as initial therapy for infantile spasms: a European retrospective survey. Epilepsia. 1996;37:638–42. doi: 10.1111/j.1528-1157.1996.tb00627.x. [DOI] [PubMed] [Google Scholar]
- 6.Chiron C, Dulac O, Luna D, et al. Vigabatrin in infantile spasms. Lancet. 1990;335:363–4. doi: 10.1016/0140-6736(90)90660-w. Letter. [DOI] [PubMed] [Google Scholar]
- 7.Petroff OA, Rothman DL, Behar KL, Mattson RH. Human brain GABA levels rise after initiation of vigabatrin therapy but fail to rise further with increasing dose. Neurology. 1996;46:1459–63. doi: 10.1212/wnl.46.5.1459. [DOI] [PubMed] [Google Scholar]
- 8.Hollrigel GS, Chen K, Baram TZ, Soltesz I. The pro-convulsant actions of corticotropin-releasing hormone in the hippocampus of infant rats. Neuroscience. 1998;84:71–9. doi: 10.1016/s0306-4522(97)00499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baram TZ, Hirsch E, Snead OC, Schultz L. Corticotropin-releasing hormone-induced seizures in infant rats originate in the amygdala. Ann Neurol. 1992;31:488–94. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21:471–6. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baram TZ, Schultz L. Corticotropin-releasing hormone is a rapid and potent convulsant in the infant rat. Dev Brain Res. 1991;61:97–101. doi: 10.1016/0165-3806(91)90118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 13.Heinrichs SC, Menzaghi F, Merlo Pich E, Britton KT, Koob GF. The role of CRF in behavioral aspects of stress. Ann N Y Acad Sci. 1995;771:92–104. doi: 10.1111/j.1749-6632.1995.tb44673.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang HL, Wayner MJ, Chai CY, Lee EHY. Corticotrophin-releasing factor produces a long-lasting enhancement of synaptic efficacy in the hippocampus. Eur J Neurosci. 1998;10:3428–37. doi: 10.1046/j.1460-9568.1998.00352.x. [DOI] [PubMed] [Google Scholar]
- 15.Yan XX, Toth Z, Schultz L, Ribak CE, Baram TZ. Corticotropin-releasing hormone (CRH)-containing neurons in the immature rat hippocampal formation: light and electron microscopic features and colocalization with glutamate decarboxylase and parvalbumin. Hippocampus. 1998;8:231–43. doi: 10.1002/(SICI)1098-1063(1998)8:3<231::AID-HIPO6>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray TS, Bingaman EW. The amygdala: corticotropin-releasing factor, steroids, and stress. Crit Rev Neurobiol. 1996;10:155–68. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- 17.Calogero AE, Gallucci WT, Chrousos GP, Gold PW. Interaction between GABAergic neurotransmission and rat hypothalamic corticotropin-releasing hormone secretion in vitro. Brain Res. 1988;463:28–36. doi: 10.1016/0006-8993(88)90523-9. [DOI] [PubMed] [Google Scholar]
- 18.Patchev VK, Shoaib M, Holsboer F, Almeida OF. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience. 1994;62:265–71. doi: 10.1016/0306-4522(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 19.Watts AG, Swanson LW. Diurnal variations in the content of preprocorticotropin-releasing hormone messenger ribonucleic acids in the hypothalamic paraventricular nucleus of rats of both sexes as measured by in situ hybridization. Endocrinology. 1989;125:1734–8. doi: 10.1210/endo-125-3-1734. [DOI] [PubMed] [Google Scholar]
- 20.Petroff OA, Rothman DL, Behar KL, Collins TL, Mattson RH. Human brain GABA levels rise rapidly after initiation of vigabatrin therapy. Neuralogy. 1996;47:1567–71. doi: 10.1212/wnl.47.6.1567. [DOI] [PubMed] [Google Scholar]
- 21.Toth Z, Yan XX, Heftoglu S, Ribak CE, Baram TZ. Seizure-induced neuronal injury: vulnerability to febrile seizures in an immature rat model. J Neurosci. 1998;18:4285–94. doi: 10.1523/JNEUROSCI.18-11-04285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi SJ, Baram TZ. Corticotropin-releasing hormone mediates the response to cold stress in the neonatal rat without compensatory enhancement of the peptide’s gene expression. Endocrinology. 1994;135:2364–8. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatalski CG, Guirguis C, Baram TZ. Corticotropin releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated acute stress in the immature rat. J Neuroendocrinol. 1998;10:663–9. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baram TZ, Lerner SP. Ontogeny of corticotropin releasing hormone gene expression in rat hypothalamus—comparison with somatostatin. Int J Dev Neurosci. 1991;9:473–8. doi: 10.1016/0736-5748(91)90033-i. [DOI] [PubMed] [Google Scholar]
- 25.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 26.Vale W, Vaughan J, Yamamoto G, et al. Assay of corticotropin releasing factor. Methods Enzymol. 1983;103:565–77. doi: 10.1016/s0076-6879(83)03040-2. [DOI] [PubMed] [Google Scholar]
- 27.Leach JP, Sills GJ, Butler E, Forrest G, Thompson GG, Brodie MJ. Neurochemical actions of vigabatrin and tiagabine alone and in combination in mouse cortex. Gen Pharmacol. 1997;28:715–9. doi: 10.1016/s0306-3623(96)00357-6. [DOI] [PubMed] [Google Scholar]
- 28.Jnng MJ, Palfreyman MG. Vigabatrin: mechanisms of action. In: Levy RH, Mattson RH, Meldrum BS, editors. Antiepileptic drugs. Raven Press; New York: 1995. pp. 903–13. [Google Scholar]
- 29.Trabesinger AH, Mueller S, Weber OM, et al. Effects of vigabatrin on brain GABA levels in patients with epilepsy monitored by magnetic resonance spectroscopy. Epilepsia. 1997;38:104. Abstract. [Google Scholar]
- 30.Hyder F, Rothman DL, Shevell M, Novotny EJ. Cerebral GABA in pediatric epilepsy. Epilepsia. 1997;38:127–8. Abstract. [Google Scholar]
- 31.Smith MA, Weiss SR, Berry RL, et al. Amygdala-kindled seizures increase the expression of corticotropin-releasing factor (CRF) and CRF-binding protein in GABAergic interneurons of the dentate hilus. Brain Res. 1997;745:248–56. doi: 10.1016/s0006-8993(96)01157-2. [DOI] [PubMed] [Google Scholar]
- 32.Meister B, Hokfelt T, Geffard M, Oertel W. Glutamic acid decarboxylase- and gamma-aminobutyric acid-like immunoreactivities in corticotropin-releasing factor-containing parvocellular neurons of the hypothalamic paraventricular nucleus. Neuroendocrinology. 1988;48:516–26. doi: 10.1159/000125058. [DOI] [PubMed] [Google Scholar]
- 33.Kovacs KJ, Bali B, Sawchenko PE. Activation of inhibitory circuitries during stress. Soc Neurosci Abs. 1998;24:544. Abstract. [Google Scholar]
- 34.Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol. 1996;10:371–94. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- 35.Plotsky PM, Otto S, Sutton S. Neurotransmitter modulation of corticotropin releasing factor secretion into the hypophysial-portal circulation. Life Sci. 1987;41:1311–7. doi: 10.1016/0024-3205(87)90211-6. [DOI] [PubMed] [Google Scholar]
- 36.Avishai-Eliner S, Yi SJ, Newth CJ, Baram TZ. Effects of maternal and sibling deprivation on basal and stress induced hypothalamic-pituitary-adrenal components in the infant rat. Neurosci Lett. 1995;192:49–52. doi: 10.1016/0304-3940(95)11606-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suchecki D, Rosenfeld P, Levine S. Maternal regulation of the hypothalamic-pituitary-adrenal axis in the infant rat: the roles of feeding and stroking. Dev Brain Res. 1993;75:185–92. doi: 10.1016/0165-3806(93)90022-3. [DOI] [PubMed] [Google Scholar]
- 38.Smith MA, Kim S-Y, Van Oers H, Levine S. Maternal deprivation and stress induce immediate early genes in the infant rat brain. Endocrinology. 1997;138:4622–8. doi: 10.1210/endo.138.11.5529. [DOI] [PubMed] [Google Scholar]
- 39.van Oers HJJ, De Kloet ER, Levine S. Persistent, but paradoxical, effects on HPA regulation of infants maternally deprived at different ages. Stress. 1997;2:249–61. doi: 10.3109/10253899709013745. [DOI] [PubMed] [Google Scholar]
- 40.Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski CG, Baram TZ. Differential regulation of the expression of corticotropin-releasing factor receptor type 2ÿ (CRF2) in hypothalamus and amygdala of the immature rat by sensory input and food intake. J Neurosci. 1999;19:3982–91. doi: 10.1523/JNEUROSCI.19-10-03982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]