Abstract
Alzheimer's disease (AD) is a progressive neurodegenerative disease which begins with insidious deterioration of higher cognition and progresses to severe dementia. Clinical symptoms typically involve impairment of memory and at least one other cognitive domain. Owing to the exponential increase in the incidence of AD with age, the aging population across the world has seen a congruous increase in AD, emphasizing the importance of disease-altering therapy. Current therapeutics on the market, including cholinesterase inhibitors and N-methyl-D-aspartate receptor antagonists, provide symptomatic relief but do not alter progression of the disease. Therefore, progress in the areas of prevention and disease modification may be of critical interest. In this review, we summarize novel AD therapeutics that are currently being explored, and also mechanisms of action of specific drugs within the context of current knowledge of AD pathologic pathways.
Keywords: Alzheimer's disease, amyloid, antioxidants, cholinesterase inhibitors, luteinizing hormone, mitochondrial therapy, neurodegenerative drugs, NMDA antagonists, tau
Introduction
Alzheimer's disease (AD) is a neurodegenerative disease that initially presents clinically with an insidious impairment of cognitive function, and within 5–10 years results in debilitation, often encompassing severe memory loss, confusion, behavior and personality changes, speech dysfunction, an inability to live independently, and ultimately a near-vegetative state. Death from pneumonia is typical [Smith, 1998]. As the most common cause of dementia in the elderly, AD is an increasingly problematic medical, economic, and social threat to societies with growing elderly populations. Strongly correlated with age, the incidence of AD is 1% for those 60 years of age or less, and roughly doubles every 5 years thereafter [Ziegler-Graham et al. 2008]. Considering prevalence, 4 million people are currently afflicted with AD in the United States and the elderly population is growing precipitously [Qiu et al. 2007]. Current estimates for the year 2050 place the number of people in the United States suffering from AD at approximately 16 million [Brookmeyer et al. 2007].
Although presentation of clinical symptoms may differ significantly between individuals, accurate diagnosis is made in 80–90% of cases and is based on clinical assessment paired with continuously improving radiologic techniques. Microscopically, AD is characterized by several hallmark pathological lesions, namely neuritic plaques, neurofibrillary tangles (NFTs), neuropil threads, and diffuse plaques [Kaminsky et al. 2010]. NFTs are filamentous aggregates of hyperphosphorylated tau protein which encircle or displace the neuronal nucleus; the intracellular, insoluble lesions are highly resistant to cellular clearance in vivo. Neuritic plaques, conversely, are found extracellularly and are composed primarily of aggregated amyloid-β (Aβ) protein. Although plaques and tangles have long been seen as the characteristic pathological structures of AD, they are no longer considered the primary progenitors of the disease pathway, but instead are viewed as downstream sequelae of prior cellular insults [Zhu et al. 2007; Smith et al. 2000]. Alternatively, oxidative stress and dysfunction of mitochondrial dynamics are now considered two primary role-players in the early pathological cascade [Smith et al. 2010; Wang et al. 2009; Petersen et al. 2007; Zhu et al. 2001]. If early oxidative stress and mitochondrial dysfunction are allowed to proceed unabated, secretion and aggregation of Aβ and NFTs, as well as microglial and astrocytic activation, neuroinflammation, and cell cycle aberration soon follow [Bonda et al. 2010a, 2009; Mancuso et al. 2008; Zhu et al. 2004]. As such, additional components of neuritic plaques include proinflammatory cytokines, apolipoproteins, α1-antichymotrypsin, and various constituents of the complement cascade. While the precise role of senile plaques and NFTs in the pathologic progression of AD is contentious and the subject of ongoing research [Castellani et al. 2009, 2008], pathological changes ultimately result in neuronal death first evident in the entorhinal cortex, then the hippocampus and isocortex, and finally the neocortex [Whitehouse, 2006; Burns et al. 1997].
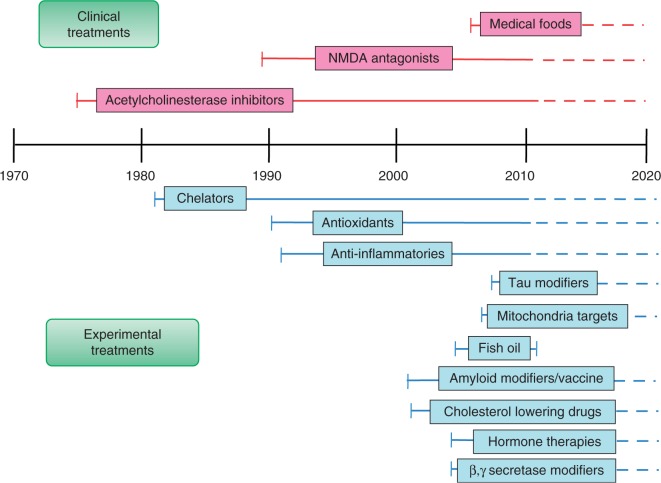
Owing to the loss of cortical neurons and cholinergic function in AD, initial therapeutic research targeted cholinergic deficiency [Farlow and Evans, 1998]. Consequently, cholinesterase inhibitors (ChEIs) were the first pharmaceutical agents approved in the treatment of AD [Moreira et al. 2006]. This strategy, although valuable in mild-to-moderate cases, only provides symptomatic relief and does not alter disease progression [Giacobini, 2002, 2001, 2000]. In addition to ChEIs, the N-methyl-D-aspartate (NMDA) receptor antagonist, memantine, is the only additional drug currently approved by the US Food and Drug Administration (FDA) for treatment in AD and is indicated in moderate-to-severe cases [Kornhuber et al. 1989]. Overactivation of NMDA receptors by excess synaptic glutamate is thought to lead to neuronal death through excitotoxicity. Similar to ChEIs, memantine shows some symptomatic benefit [van Dyck et al. 2007; McShane et al. 2006]. Given the paucity and ineffectiveness of symptomatic treatments, novel methods of AD treatment are increasingly being pursued and span from modifying disease-specific protein targets to additions to the diet including fish oil or pharmacologically created medical food supplements (Figure 1).
Figure 1.
Timeline of the emergence of various experimental and clinical therapies for Alzheimer's disease.
In addition to symptomatic treatments, novel drug development includes an emphasis on prevention of primary cellular insults which initiate AD, and intervention measures which modulate the neurotoxic pathways once in motion. As such, this paper will review therapeutic interventions under current investigation divided among three categories: preventative, disease-modifying, and symptomatic treatment.
Since the AD pathological pathway is multifactorial involving a host of genetic, environmental, and behavioral components, potential therapeutic targets are numerous [Shah et al. 2008], leaving some room for cautious optimism (Figure 1).
Preventative approaches
Given the lack of current pharmacological options that target the primary disease process, and the probable advanced nature of the disease when clinically symptomatic [Su et al. 2010; Castellani et al. 2001], a preventative approach appears to have some merit. Estimates in fact suggest that if therapeutic interventions delay disease onset by a even 1 year, approximately 9.2 million fewer cases of AD would be observed in 2050 than expected [Brookmeyer et al. 2007]. Despite logistical difficulties in research, several large clinical studies focusing on preventative strategies such as antioxidant therapy, protection of mitochondrial dynamics, use of cholesterol-lowering drugs, and anti-inflammatory treatment have noteworthy findings.
Antioxidant therapy
Recent evidence suggests that oxidative stress plays an early and active role in the AD pathological cascade [Zhu et al. 2007]. Reactive oxygen species (ROS), such as the highly reactive molecules superoxide (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (OH•−) are naturally created in the mitochondria as the cell generates adenosine triphosphate (ATP) through cellular respiration [Petersen et al. 2007]. Although the cell has defensive machinery to reduce ROS such as the enzymes superoxide dismutase and catalase, some ROS inevitably leak from mitochondria and damage literally every cellular component including phospholipids, proteins, and notably, mitochondrial DNA (mtDNA) [Ansari and Scheff, 2010]. As ROS damage shows an increased incidence in AD and occurs temporally early in the initiation of the disease [Zhu et al. 2004, 2001], antioxidant therapy may present a valuable preventative strategy [Aliev et al. 2008; Liu et al. 2007].
Readily available through dietary supplementation, naturally occurring antioxidants, such as several vitamin moieties, present an ideal preventative measure. Evidence of protection from AD by supplements such as antioxidant vitamins (E, C, and carotenoids) and B vitamins (B6, B12, and folate) is inconsistent [Middleton and Yaffe, 2009; Gillette Guyonnet et al. 2007; Perrig et al. 1997]. However, several large longitudinal studies found folate supplementation is associated with a reduced risk of AD [Corrada et al. 2005], combination therapy with vitamins E and C is associated with reduced incidence and prevalence of AD [Zandi et al. 2004], and treatment with vitamin E may slow the progression of AD [Sano et al. 1997]. Additional studies that control carefully for confounding factors are certainly warranted in the case of antioxidant vitamin protection.
In addition to naturally occurring antioxidant vitamins, several other drugs are under investigation for the prevention of ROS-based damage. As mitochondria are the principal producers of ROS [Lee et al. 2004] and mitochondrial components undergo the initial oxidative insults temporally [Zhu et al. 2006, 2004; Aliev et al. 2003; Ogawa et al. 2002], mitochondrial antioxidant pharmaceuticals present a promising target for therapeutic development. One such therapy in development involves treatment with Coenzyme Q10 (CoQ10), the protein responsible for carrying high- energy electrons from complex I and complex II in the electron transport chain (ETC) through subsequent oxidation and reduction. Studies show potential neuroprotective effects of CoQ10 including the suppression of toxic free-radical production, reduction of ROS injury, and stabilization of mitochondrial function [Lee et al. 2009; Wadsworth et al. 2008; Beal, 2004] Despite positive study results, CoQ10 treatment presents important obstacles. First, proper functioning of CoQ10 within the cellular respiration pathway requires an intact and fully functional ETC. Oxidatively damaged mitochondria, observed early in AD, often do not contain intact ETCs as many enzymes involved with oxidative phosphorylation become irreversibly damaged; thus, CoQ10 administration to cells with mitochondria exhibiting early stages of dysfunction is ineffective [Lass et al. 1999]. In addition, oral administration of CoQ10 did not significantly increase the protein levels of CoQ10 in the brain [Kwong et al. 2002]. This finding suggests that the current formulation of CoQ10 lacks the ability to penetrate the blood-brain barrier (BBB). Owing to the drawbacks of CoQ10 therapy, other moieties, which possess greater BBB permeability and do not require an intact ETC for proper functioning, are under investigation.
One such formulation is a triphenylphosphonium-linked ubiquinone derivative known as MitoQ [Murphy, 2001]. Like CoQ10, MitoQ is shown to inhibit ROS production, protect mitochondrial oxidative phosphorylation, preserve mitochondrial structural integrity, and ultimately prevent cell death [Murphy and Smith, 2007]. In addition, MitoQ addresses the shortcomings of CoQ10; MitoQ is shown to effectively operate in the absence of an intact ETC [Lu et al. 2008] and concentrate several hundred-fold in mitochondria [Smith et al. 2004]. At this time, MitoQ remains on the horizon for AD treatment as the therapy was found to be effective in phase II clinical trials for liver damage associated with HCV infection.
Additional antioxidants under investigation for future treatment of AD include acetyl-L-carnitine (ALCAR) and R-α-lipoic acid (LA). Differing combinations of these two antioxidants are shown to reduce cellular insults by ROS, and in particular, prevent mitochondrial abnormalities in mouse models of AD [Siedlak et al. 2009], and prevent cognitive decline and/or restore cognitive functioning in aged rats and dogs [Long et al. 2009; Milgram et al. 2007; Ames and Liu, 2004; Liu et al. 2002a, 2002b, 2002c] Notably, mitochondrial structural integrity was preserved and the number of mitochondria increased in the hippocampal region by ALCAR/LA administration [Aliev et al. 2009]. While much investigation remains to be completed regarding the role antioxidants may or may not play in AD treatment, the above-mentioned therapies under current investigation present exciting possibilities.
Anti-inflammatory therapy
Although the precise role of inflammatory processes in AD pathogenesis remains elusive, several in vitro and in vivo animal and human studies implicate neuroinflammation in disease progression [Moore and O'Banion, 2002; Kalaria, 1999]. In particular, activated microglia, the resident guardian macrophages of the central nervous system (CNS), and reactive astrocytes, the most abundant CNS cells responsible for a multitude of critical functions including buffering of extracellular environment, preservation of the BBB, and generation of energy substrates, are involved directly in neuroinflammation. In the presence of an inflammatory stimulus, reactive astrocytes and activated microglia express and/or secrete increased concentrations of cytokines, chemokines, ROS, growth factors, major histocompatibility complex type II, and complement proteins: all pro-inflammatory mediators. In relation to AD, human postmortem studies and transgenic (Tg) animal models of AD show neuritic plaque development associated with glial activation [Apelt and Schliebs, 2001; Wegiel et al. 2001], suggest microglia assist in conversion of Aβ to its fibrillar form, induce diffuse plaques to aggregate into neuritic plaques [Sasaki et al. 1997; Cotman et al. 1996; Griffin et al. 1995; Mackenzie et al. 1995], and induce glutamate release contributing to excitotoxicity [Piani et al. 1992]. Owing to an increasing body of research suggesting a progenitive role of neuroinflammation in AD, several anti-inflammatory therapies have been studied.
Notably, several large longitudinal studies show use of anti-inflammatory drugs are associated with a lower incidence of AD [Cornelius et al. 2004; Lindsay et al. 2002; in t’ Veld et al. 2001]. In particular, findings show that nonaspirin nonsteroidal anti-inflammatory drugs (NSAIDs) protect against the development of AD [Etminan et al. 2003; McGeer et al. 1996], increased duration of NSAID use is associated with a lower relative risk for AD [Stewart et al. 1997], and naproxen in particular has a positive preventative effect [Cole and Frautschy, 2010]. Importantly, although anti-inflammatory administration shows preventative potential, several studies show treatment of patients with clinically diagnosed mild-to-moderate AD has no effect [Reines et al. 2004; Aisen et al. 2003, 2000; Van Gool et al. 2001]. This lends further support that once early pathological pathways, including neuroinflammation, initiate, preventative strategies quickly become futile.
Disease-modifying approaches
While preventing the onset of AD entirely is an ideal aim, the multitude of factors influencing the onset and progression of AD as well as poorly understood nature of the primary pathogenic processes and outright misconceptions inherent in leading hypotheses, make prevention unlikely; therefore, therapies that blunt the secondary effects of established disease are in high demand for both current and future AD patients.
Stabilization of mitochondrial dynamics
One such critical secondary cellular insult is disruption of mitochondrial function. Mitochondria are highly dynamic organelles which constantly fuse and divide within cells in response to energy demands [Chan, 2006]. Given that 95% of the cell's energy is provided by the citric acid (TCA) cycle and oxidative phosphorylation of mitochondria, and neurons have extremely high metabolic demands, proper functioning of mitochondria is absolutely critical to neuronal viability. As mentioned prior, the integrity of mitochondrial structure and utility are significantly compromised in AD [Wang et al. 2008a, 2008b; Hirai et al. 2001]; mitochondria, therefore, present a valuable potential target for the modulation of AD.
In addition to the prior-mentioned mitochondrial antioxidants, dimebon is believed to target mitochondrial dysfunction in AD. Specifically, evidence indicates dimebon binds to and blocks the mitochondrial permeability pore (MPP) [Bachurin et al. 2003]. The MPP is a multiprotein complex involving both the inner and outer mitochondrial membranes which serves to regulate the transfer of ions and proteins in and out of mitochondria with particular focus on maintaining intracellular Ca2+ homeostasis. Early in the pathological pathway of AD, oxidative stress and other neurotoxic insults can irreversibly open the MPP eliciting significant cellular stress [Parks et al. 2001]. Through closure of faulty MPPs, dimebon may serve as a potent neuroprotector. Importantly, although dimebon showed unprecedented success in an initial relatively small phase II trial in patients with mild-to-moderate AD [Doody et al. 2008], a recently completed double-blind large-scale phase III clinical trial indicated a discouraging similarity in cognitive outcomes between dimebon and control groups. While the fate of dimebon is yet to be determined, the strategy of attenuating mitochondrial dysfunction in AD will certainly continue to be the subject of much future inquiry.
Cessation of luteinizing hormone expression
Dyshomeostasis of hormones, luteinizing hormone (LH) in particular, appears to play a significant role in AD. Substantiating this assertion, the incidence of AD in women who experience severe hypothalamic-pituitary-gonadal hormone changes with menopause, particularly an elevation of circulating LH, is approximately two times higher than men [Jorm and Jolley, 1998]. Evaluation of patients with Down's syndrome (DS) lend further evidence supporting the role of LH in AD. Men with DS are twice as likely as women with DS to develop AD, showing the reverse trend of the normal population [Bonda et al. 2010b]. Demonstrating the same phenomenon as postmenopausal women, men with DS have elevated levels of circulating LH suggesting a direct link between risk for AD and LH level [Webber et al. 2007a; Casadesus et al. 2006]. In fact, elevated LH is the only hormonal similarity discovered to date which connects the predisposition to AD observed in normal women and men with DS. Many recent investigations have, therefore, been launched into the role of LH in AD and the evaluation of LH-based treatments.
In short, patients afflicted with AD show serum concentrations of LH approximately twice as high as age-matched controls [Short et al. 2001; Bowen et al. 2000]. Elevated LH is observed in the AD vulnerable hippocampus [Bowen et al. 2002], and LH appears to encourage Aβ deposition into senile plaques [Webber et al. 2007b; Bowen et al. 2004]. A breakthrough on the treatment front, the novel therapeutic leuprolide acetate is shown to reduce LH levels by down-regulating the expression of gonadotropin releasing hormone (GnRH) receptor levels in the anterior pituitary [Marlatt et al. 2005]. This appears to have a significant positive effect in AD as leuprolide acetate reduces Aβ deposition in rats [Okada et al. 1996], and modulates cognition in amyloid-β protein precursor (AβPP) overexpressing Tg mice and humans [Casadesus et al. 2006] (see ClinicalTrials.gov, NCT00076440). In addition, high doses of leuprolide acetate attenuated cognitive decline (ADCS-CGIC, ADAS-Cog) and stabilized activities of daily living (ADCS-ADL) in phase II clinical trials of patients with mild-to-moderate AD (see http://www.secinfo.com/d14D5a.z6483.htm, pp. 56–64). Although much study is still required, LH-ablating therapies are quite promising as a novel treatment avenue.
Tau-focused therapies
As tau hyperphosphorylation and aggregation into pathological NFTs is a significant aspect in the development of AD, tau-focused therapeutic advancement is of much current interest. Notably, the abundance of NFTs in AD vulnerable brain regions is correlated with cognitive decline [Castellani et al. 2006; Nunomura et al. 2006], and NFT accumulation is therefore used to definitively diagnose AD postmortem [Perry et al. 1985]. Consequently, targeting tau to inhibit NFT build up or encourage NFT degradation may prove to be an important therapeutic strategy.
Inhibition of tau hyperphosphorylation.
Evidence suggests the hyperphosphorylation of tau precedes NFT formation. Therefore, the strategy of inhibiting tau phosphorylation is under investigation as a potential AD modulating therapy. In particular, tau phosphorylation at threonine 231, and serines 235 and 262 appears to be responsible for the fibrillization and aggregation that produces NFTs. Glycogen synthase kinase 3 (GSK3) is believed to be the primary kinase which induces the hyperphosphorylation of tau [Shiurba et al. 1996] as it is shown to colocalize to NFTs in AD vulnerable neurons [Imahori and Uchida, 1997], and Tg mice engineered to overexpress GSK3 show increased tau hyperphosphorylation and AD symptomology [Hernandez et al. 2002; Lucas et al. 2001]. Hence, several therapeutic agents aimed at inhibiting GSK3 are in early stages of development and show promising initial results. In this regard, administration of the GSK3 inhibitor, LiCl, to tau-overexpressing Tg mice prevented the development of NFTs [Engel et al. 2006], reduced tau phosphorylation [Noble et al. 2005], and reduced the concentration of insoluble tau [Perez et al. 2003]. In addition, other GSK3 inhibitors, SB216763 and CHIR-98014 [Selenica et al. 2007], as well as a nonspecific kinase inhibitor, SRN-003-556 [Hampel et al. 2009], are under early investigation for efficacy in AD treatment.
In addition to preventing tau phosphorylation by direct inhibition of tau kinases, another strategy under inquiry is the inhibition of the β-N-acetylglucosamine (O-GlcNAc) cleaving enzyme O-GlcNAcase. Tau is shown to be postranslationally glycosylated with O-GlcNAc at the same threonine and serine residues that become pathologically phosphorylated. Consequently, O-GlcNAc glycosylation acts as a competitive inhibitor to tau hyperphosphorylation and subsequent aggregation into NFTs [Liu et al. 2004; Lefebvre et al. 2003], so preventing the cleavage of O-GlcNAc may therefore be a valuable strategy. In this regard, thiamet-G, a potent O-GlcNAcase inhibitor, induced a reduction of tau phosphorylation at threonine 231, and serines 396 and 404 in rats [Liu et al. 2004]. Although the molecules under current investigation which seek to inhibit tau hyperphosphorylation are in early stages and many obstacles still must be addressed, tau phosphorylation as a target in AD treatment remains valid nonetheless.
Inhibition of tau oligomerization and fibrillization.
Following tau hyperphosphorylation, monomeric tau begins to aggregate into oligomers which proceed to further accumulate into fibrils. Therefore, in lieu of hyperphosphorylation inhibition, another potential strategy seeks to prevent formation of toxic oligomers and fibrils [Brunden et al. 2010, 2009]. Closest to the therapeutic market in this category, methylene blue, a histologic dye, recently completed a phase II clinical trial with positive results and will begin phase III trials soon [Neugroschl and Sano, 2009]. Several additional tau aggregation inhibitors are under preclinical research as well and show promise for the future [Crowe et al. 2009; Larbig et al. 2007; Pickhardt et al. 2005].
Degradation of hyperphosphorylated tau.
In addition to the prevention of hyperphosphorylation and aggregation of tau, an additional strategy aimed at ameliorating presumed toxic deposition of phosphorylated tau is to increase its intracellular degradation through the ubiquitin proteosome system [Brunden et al. 2009]. Evidence suggests this may be accomplished through the inhibition of heat shock protein 90 (HSP90). HSP90 serves to refold denatured proteins and its inhibition is believed to attenuate the preservation of phosphorylated tau, therefore enhancing its degradation [Dickey et al. 2007; Zhang and Burrows, 2004]. Notably, the HSP90 inhibitor, EC102, reduced the amount of hyperphosphorylated tau in the brains of Tg mice engineered to overexpress tau [Luo et al. 2007] and efficiently inhibited HSP90 in human cortical AD homogenates at 1000-fold lower concentrations than control homogenates [Dickey et al. 2007] indicating a clinically safe dosing range is available. It is important to note that the ubiquitin proteosome degradation system involves the precise threading of a denatured peptide through the small opening of the cylindrical proteosome. Therefore, once hyperphosphorylated tau becomes fibrillized, it no longer is an available target for this system being too wide to enter the proteosome. Nevertheless, tau degradation remains an exciting and promising strategy in the modification and attenuation of destructive AD pathology.
Amyloid-focused therapy
Along with hyperphosphorylated tau tangles, Aβ plaques compose the second hallmark pathological marker of AD. Notably, increasing evidence indicates that Aβ may actually be a protective measure used by the cell to attenuate damage by oxidative stress [Zhu et al. 2007, 2001], as Aβ has antioxidant properties [Hayashi et al. 2007; Nakamura et al. 2007; Kontush, 2001; Kontush et al. 2001; Rottkamp et al. 2001; Cuajungco et al. 2000], and the appearance of mitochondrial abnormalities and ROS precedes that of Aβ deposition [Petersen et al. 2007]. However, the possibility that oversecretion of Aβ (perhaps caused by oxidative stress), and eventual Aβ aggregation into senile plaques, is a toxic event that is implicit in the literature even though it is somewhat dated in recent studies focusing on soluble oligomeric species. Hence, current amyloid-focused treatment strategies in development aim to prevent the accumulation and aggregation of insoluble Aβ and/or clear Aβ plaques postformation. Still, soluble Aβ peptides may similarly be protective in vivo as an ameliorative response to free-radical toxicity [Nunomura et al. 2010; Masters et al. 1985].
Inhibition of Aβ accumulation and aggregation.
One therapy method currently under investigation seeks to prevent the toxicity associated with Aβ by inhibiting its accumulation a priori. In this vein, the modulation of enzymes responsible for the cleavage of AβPP to potentially detrimental Aβ peptides is one strategy of interest. The aspartyl proteases β- and γ-secretase work in sequence to cleave AβPP to Aβ; consequently, drug treatment aims to inhibit the enzymes and thereby limit concentrations of extracellular Aβ [Lundkvist and Naslund, 2007; Marlatt et al. 2005]. Numerous pharmacological agents, including NCT00594568, NCT00762411, GS1-136, and MK0752 [Neugroschl and Sano, 2009], are under current study; most notably, the β-secretase inhibitor, posiphen, is in phase I clinical trials [Sabbagh, 2009], and the g-secretase inhibitor, LY450139, is in phase III clinical trials [Lundkvist and Naslund, 2007; Barten et al. 2005; Wong et al. 2004]. Results of these trials are much anticipated and will further elucidate the future role of β- and γ-secretase inhibitors in AD treatment. In addition to prevention of Aβ accumulation, the inhibition of Aβ aggregation is of much current interest. In this regard, nanoparticle metal chelators present an exciting future avenue to accomplish anti-aggregation of Aβ. Redox metals iron and copper are shown to be elevated in AD brain [Smith et al. 2010b; Honda et al. 2004] and appear to induce Aβ oxidation and self-assembly [Exley, 2006; Jobling et al. 2001; Atwood et al. 2000; Bush et al. 1994].
Consequently, metal chelators attempt to obstruct the interaction of Aβ and redox metals to prevent aggregation in AD vulnerable neurons. Metal chelators such as ethylenediaminetetracetic acid (EDTA), desferrioxamine, and iodochlorhydroxyquin (clioquinol) [Ritchie et al. 2003; Regland et al. 2001; Crapper McLachlan et al. 1991] can prevent the detrimental interaction between redox metals and Aβ [Liu et al. 2006; Opazo et al. 2002; Schubert and Chevion, 1995]. Unfortunately however, they cannot cross the BBB. To address this, current studies suggest utilizing nanoparticle conjugation to efficiently deliver metal chelators across the BBB [Liu et al. 2009]. Specifically, the Nano-N2PY conjugate has been shown to thwart Aβ-induced toxicity in neuronal cell lines without impacting cell proliferation and growth [Liu et al. 2009]. Nanoparticle-conjugated metal chelators remain in early-stage preparation as the pharmacological agents have yet to be administered in an animal model, but present a promising future treatment nonetheless.
Further along in drug development, the neurohormone melatonin also shows promise as an inhibitor of Aβ aggregation. In detail, long-term administration of melatonin to Aβ-overexpressing Tg mice significantly attenuated senile plaque deposition in the entorhinal cortex and hippocampus [Olcese et al. 2009]. This effect was most likely due to melatonin's ability to prevent fibrillization of Aβ [Poeggeler et al. 2001; Pappolla et al. 1998; Fraser et al. 1991], although melatonin also had the beneficial effects of reducing oxidative stress and pro-inflammatory cytokines [Olcese et al. 2009]. The clinical safety of melatonin administration in humans is confirmed, so all that remains is the completion of a large-scale phase III clinical trial of melatonin in AD patients which will most likely take place in the near future.
Finally, in addition to nanoparticle-conjugated metal chelators and melatonin, the cyclohexanehexol stereoisomer, scyllo-inositol (ELND005), is under current study for Aβ aggregation prevention [Sabbagh, 2009]. Specifically, the molecule showed a reduction of insoluble Aβ and reversed cognitive decline in Aβ-overexpressing Tg mice [Rogers et al. 1993]. With phase I trials complete, ELND005 will produce much anticipated results from a phase II clinical trial in mid-2010 [Scharf et al. 1999].
Degradation of Aβ.
Once Aβ begins to accumulate and aggregate, a third treatment strategy may prove to be efficacious: degradation of insoluble extracellular Aβ. Immunotherapy directed against Aβ oligomers is highly touted as the solution to Aβ deposition despite a high risk of adverse effects and questionable patient outcomes [Smith et al. 2002; Perry et al. 2000]. Active immunotherapy involves introducing an Aβ antigen into the host system to induce the production of antibodies which bind Aβ and target the insoluble peptide oligomers for degradation and clearance. The initial trial of active immunotherapy with compound AN1792, an aggregated amyloid peptide, was prematurely terminated due to the development of meningoencephalitis in a significant percentage of enrolled patients (6%). This phenomenon was attributed to aberrant T-cell activation [Marlatt et al. 2005]. Hence, several new Aβ antigens are in development which produce lowered T-cell activation, including ACC-001 and CAD106, both of which are in current phase II trials.
As an alternative to active immunization, passive immunotherapy involves the injection of a preformulated anti-Aβ antibody, thus necessitating much less involvement of the patients own immune system. In this category, the humanized anti-Aβ monoclonal antibody, bapineuzumab (AAB-001), is in phase III clinical trials (see ClinicalTrials.gov, NCT00667810) and unfortunately shows a paucity of positive results. Specifically, patients treated with bapineuzumab show no temporal improvements in cognition (ADAS-cog), excluding post hoc analyses (see ClinicalTrials.gov, NCT00676143, NCT00575055, and NCT00574132). In addition to bapineuzumab, several other passive immunotherapeutics are in various phases of clinical development including phase II immunoglobulin and PF-04360365, and phase III solanezumad (LY2062430) [Relkin et al. 2009] (see ClinicalTrials.gov, NCT00329082, NCT00749216, NCT00905372, NCT00904683, and NCT00722046). As noted previously, evidence suggests Aβ is actually an antioxidant elaborated by neurons as a neuroprotective agent against primary cellular insults involved in AD pathogenesis; therefore, as we have long contended, amyloid degradation strategies will likely continue to be disappointing because of a fundamentally flawed paradigm [Smith et al. 2002; Perry et al. 2000].
Symptomatic approaches
Since preventative and disease-modifying treatments are presently unproven, additional symptomatic treatments in addition to the ChEIs mentioned are worth considering as an attempt to decrease the burden on those already afflicted with AD and improve functional outcome with symptomatic AD. Such approaches are scarce, although one potential avenue is antidepressant therapy.
Approximately 35% of AD patients present with the crippling symptom of depression at some point in the disease course [Arbus et al. 2010]. Depression is believed to appear in AD due to an insufficiency of the neurotransmitters serotonin, norepinephrine, and dopamine resulting from cortical atrophy. Consequently, the use of selective serotonin reuptake inhibitors (SSRIs) in AD has been widely studied. Although there are mixed results with particular drugs within the class [Rosenberg et al. 2010], as a whole, SSRIs seem to improve symptoms of depression in AD patients and significantly enhance quality of life [Siddique et al. 2009]. In addition, in patients treated with ChEIs, the addition of SSRIs to the treatment regimen may actually protect against cognitive decline [Tan and Kutlu, 1991]. Although antidepressants do not appear to target the disease process of AD [Hampel et al. 2009], their use is still extremely valuable in symptomatic relief. Moreover, antidepressant therapy is, to date, the only therapeutic intervention that has shown significant improvement in subjects with mild cognitive impairment [Doody et al. 2009].
Conclusions
Although AD pharmaceuticals on the market today, including ChEIs and NMDA antagonists, fail to alter disease progression, the development of new agents utilizing novel treatment strategies is certain. Numerous preventative treatments, specifically those aiming to avert oxidative stress (i.e. antioxidants) and neuroinflammation, are in the clinical testing phases and offer some hope for upstream therapy that may blunt the neurodegenerative process. Similarly, pharmaceuticals that stabilize mitochondrial function, ablate LH production, and obstruct the detrimental effects of hyperphosphorylated tau and Aβ, may play a role either alone or, more likely, in combination with other approaches. Since, to date, the neurodegenerative process has been unaffected by the spectrum of approaches, a broadening of that spectrum, rather than a narrowing of the focus on failed constructs, is the logical approach.
Footnotes
This work was supported by the National Institutes of Health (grant number R01 AG028679 to MAS and R01 AG031852 to XWZ) and the Alzheimer's Association.
Mark A. Smith is, or has in the past been, a paid consultant for, owns equity or stock options in, and/or receives grant funding from Anavex, Canopus BioPharma, Medivation, Neurotez, Neuropharm, Panacea Pharmaceuticals, and Voyager Pharmaceuticals. Dr Perry is a paid consultant for Neurotez and Takeda Pharmaceuticals.
References
- Aisen P.S., Davis K.L., Berg J.D., Schafer K., Campbell K., Thomas R.G., et al. (2000) A randomized controlled trial of prednisone in Alzheimer's disease. Alzheimer's Disease Cooperative Study. Neurology 54: 588–593 [DOI] [PubMed] [Google Scholar]
- Aisen P.S., Schafer K.A., Grundman M., Pfeiffer E., Sano M., Davis K.L., et al. (2003) Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: A randomized controlled trial. JAMA 289: 2819–2826 [DOI] [PubMed] [Google Scholar]
- Aliev G., Liu J., Shenk J.C., Fischbach K., Pacheco G.J., Chen S.G., et al. (2009) Neuronal mitochondrial amelioration by feeding acetyl-L-carnitine and lipoic acid to aged rats. J Cell Mol Med 13: 320–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliev G., Obrenovich M.E., Reddy V.P., Shenk J.C., Moreira P.I., Nunomura A., et al. (2008) Antioxidant therapy in Alzheimer's disease: Theory and practice. Mini Rev Med Chem 8: 1395–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliev G., Smith M.A., Obrenovich M.E., de la Torre J.C., Perry G. (2003) Role of vascular hypoperfusion-induced oxidative stress and mitochondria failure in the pathogenesis of Azheimer disease. Neurotox Res 5: 491–504 [DOI] [PubMed] [Google Scholar]
- Ames B.N., Liu J. (2004) Delaying the mitochondrial decay of aging with acetylcarnitine. Ann N Y Acad Sci 1033: 108–116 [DOI] [PubMed] [Google Scholar]
- Ansari M.A., Scheff S.W. (2010) Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol 69: 155–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelt J., Schliebs R. (2001) Beta-amyloid-induced glial expression of both pro- and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res 894: 21–30 [DOI] [PubMed] [Google Scholar]
- Arbus C., Gardette V., Bui E., Cantet C., Andrieu S., Nourhashemi F., et al. (2010) Antidepressant use in Alzheimer's disease patients: Results of the REAL.FR cohort. Int Psychogeriatr 22: 120–128 [DOI] [PubMed] [Google Scholar]
- Atwood C.S., Scarpa R.C., Huang X., Moir R.D., Jones W.D., Fairlie D.P., et al. (2000) Characterization of copper interactions with alzheimer amyloid beta peptides: Identification of an attomolar-affinity copper binding site on amyloid beta1-42. J Neurochem 75: 1219–1233 [DOI] [PubMed] [Google Scholar]
- Bachurin S.O., Shevtsova E.P., Kireeva E.G., Oxenkrug G.F., Sablin S.O. (2003) Mitochondria as a target for neurotoxins and neuroprotective agents. Ann N Y Acad Sci 993: 334–344, discussion 345–339. [DOI] [PubMed] [Google Scholar]
- Barten D.M., Guss V.L., Corsa J.A., Loo A., Hansel S.B., Zheng M., et al. (2005) Dynamics of {beta}-amyloid reductions in brain, cerebrospinal fluid, and plasma of {beta}-amyloid precursor protein transgenic mice treated with a {gamma}-secretase inhibitor. J Pharmacol Exp Ther 312: 635–643 [DOI] [PubMed] [Google Scholar]
- Beal M.F. (2004) Mitochondrial dysfunction and oxidative damage in Alzheimer's and Parkinson's diseases and coenzyme Q10 as a potential treatment. J Bioenerg Biomembr 36: 381–386 [DOI] [PubMed] [Google Scholar]
- Bonda D.J., Bajic V.P., Spremo-Potparevic B., Casadesus G., Zhu X., Smith M.A., et al. (2010a) Cell Cycle Aberrations and Neurodegeneration: A Review. Neuropathol Appl Neurobiol 36: 157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonda D.J., Castellani R.J., Lee H.G., Tabaton M., Casadesus G., Perry G., et al. (2010b) Down syndrome and Alzheimer disease: A “multi-hit” hypothesis based on multiple contributing factors resulting in pleotrophic consequences, In: Urbano K.V. (ed.). Advances in Genetics Research, Vol. 2, Nova Science Publishers: Hauppauge, NY, pp. 87–97 [Google Scholar]
- Bonda D.J., Evans T.A., Santocanale C., Llosa J.C., Vina J., Bajic V.P., et al. (2009) Evidence for the progression through S-phase in the ectopic cell cycle re-entry of neurons in Alzheimer disease. Aging (Albany NY) 1: 382–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen R.L., Isley J.P., Atkinson R.L. (2000) An association of elevated serum gonadotropin concentrations and Alzheimer disease? J Neuroendocrinol 12: 351–354 [DOI] [PubMed] [Google Scholar]
- Bowen R.L., Smith M.A., Harris P.L., Kubat Z., Martins R.N., Castellani R.J., et al. (2002) Elevated luteinizing hormone expression colocalizes with neurons vulnerable to Alzheimer's disease pathology. J Neurosci Res 70: 514–518 [DOI] [PubMed] [Google Scholar]
- Bowen R.L., Verdile G., Liu T., Parlow A.F., Perry G., Smith M.A., et al. (2004) Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-beta precursor protein and amyloid-beta deposition. J Biol Chem 279: 20539–20545 [DOI] [PubMed] [Google Scholar]
- Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi H.M. (2007) Forecasting the global burden of Alzheimer's disease. Alzheimers Dement 3: 186–191 [DOI] [PubMed] [Google Scholar]
- Brunden K.R., Ballatore C., Crowe A., Smith A.B., III, Lee V.M., Trojanowski J.Q. (2010) Taudirected drug discovery for Alzheimer's disease and related tauopathies: A focus on tau assembly inhibitors. Exp Neurol 223: 304–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunden K.R., Trojanowski J.Q., Lee V.M. (2009) Advances in tau-focused drug discovery for Alzheimer's disease and related tauopathies. Nat Rev Drug Discov 8: 783–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns A., Whitehouse P., Arendt T., Forsti H. (1997) Alzheimer's disease in senile dementia: Loss of neurones in the basal forebrain. Whitehouse, P., Price, D., Struble, R., Clarke, A., Coyle, J. and Delong, M. Science (1982), 215, 1237–1239. Int J Geriatr Psychiatry 12: 7–10 [DOI] [PubMed] [Google Scholar]
- Bush A.I., Pettingell W.H., Multhaup G., Paradis M., Vonsattel J.P., Gusella J.F., et al. (1994) Rapid induction of Alzheimer A beta amyloid formation by zinc. Science 265: 1464–1467 [DOI] [PubMed] [Google Scholar]
- Casadesus G., Webber K.M., Atwood C.S., Pappolla M.A., Perry G., Bowen R.L., et al. (2006) Luteinizing hormone modulates cognition and amyloid-beta deposition in Alzheimer APP transgenic mice. Biochim Biophys Acta 1762: 447–452 [DOI] [PubMed] [Google Scholar]
- Castellani R.J., Harris P.L., Sayre L.M., Fujii J., Taniguchi N., Vitek M.P., et al. (2001) Active glycation in neurofibrillary pathology of Alzheimer disease: N(epsilon)-(carboxymethyl) lysine and hexitol-lysine. Free Radic Biol Med 31: 175–180 [DOI] [PubMed] [Google Scholar]
- Castellani R.J., Lee H.G., Siedlak S.L., Nunomura A., Hayashi T., Nakamura M., et al. (2009) Reexamining Alzheimer's disease: Evidence for a protective role for amyloid-beta protein precursor and amyloid-beta. J Alzheimers Dis 18: 447–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani R.J., Lee H.G., Zhu X., Nunomura A., Perry G., Smith M.A. (2006) Neuropathology of Alzheimer disease: Pathognomonic but not pathogenic. Acta Neuropathol (Berl) 111: 503–509 [DOI] [PubMed] [Google Scholar]
- Castellani R.J., Nunomura A., Lee H.G., Perry G., Smith M.A. (2008) Phosphorylated tau: Toxic, protective, or none of the above. J Alzheimers Dis 14: 377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D.C. (2006) Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 22: 79–99 [DOI] [PubMed] [Google Scholar]
- Cole G.M., Frautschy S.A. (2010) Mechanisms of action of non-steroidal anti-inflammatory drugs for the prevention of Alzheimer's disease. CNS Neurol Disord Drug Targets 9: 140–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius C., Fastbom J., Winblad B., Viitanen M. (2004) Aspirin, NSAIDs, risk of dementia, and influence of the apolipoprotein E epsilon 4 allele in an elderly population. Neuroepidemiology 23: 135–143 [DOI] [PubMed] [Google Scholar]
- Corrada M.M., Kawas C.H., Hallfrisch J., Muller D., Brookmeyer R. (2005) Reduced risk of Alzheimer's disease with high folate intake: The Baltimore Longitudinal Study of Aging. Alzheimers Dement 1: 11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C.W., Tenner A.J., Cummings B.J. (1996) beta-Amyloid converts an acute phase injury response to chronic injury responses. Neurobiol Aging 17: 723–731 [DOI] [PubMed] [Google Scholar]
- McLachlan D.R. Crapper, Dalton A.J., Kruck T.P., Bell M.Y., Smith W.L., Kalow W., et al. (1991) Intramuscular desferrioxamine in patients with Alzheimer's disease. Lancet 337: 1304–1308 [DOI] [PubMed] [Google Scholar]
- Crowe A., Huang W., Ballatore C., Johnson R.L., Hogan A.M., Huang R., et al. (2009) Identification of aminothienopyridazine inhibitors of tau assembly by quantitative high-throughput screening. Biochemistry 48: 7732–7745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuajungco M.P., Goldstein L.E., Nunomura A., Smith M.A., Lim J.T., Atwood C.S., et al. (2000) Evidence that the beta-amyloid plaques of Alzheimer's disease represent the redox-silencing and entombment of abeta by zinc. J Biol Chem 275: 19439–19442 [DOI] [PubMed] [Google Scholar]
- Dickey C.A., Kamal A., Lundgren K., Klosak N., Bailey R.M., Dunmore J., et al. (2007) The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest 117: 648–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody R.S., Ferris S.H., Salloway S., Sun Y., Goldman R., Watkins W.E., et al. (2009) Donepezil treatment of patients with MCI: A 48-week randomized, placebo-controlled trial. Neurology 72: 1555–1561 [DOI] [PubMed] [Google Scholar]
- Doody R.S., Gavrilova S.I., Sano M., Thomas R.G., Aisen P.S., Bachurin S.O., et al. (2008) Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer's disease: A randomised, double-blind, placebo-controlled study. Lancet 372: 207–215 [DOI] [PubMed] [Google Scholar]
- Engel T., Goni-Oliver P., Lucas J.J., Avila J., Hernandez F. (2006) Chronic lithium administration to FTDP-17 tau and GSK-3beta overexpressing mice prevents tau hyperphosphorylation and neurofibrillary tangle formation, but pre-formed neurofibrillary tangles do not revert. J Neurochem 99: 1445–1455 [DOI] [PubMed] [Google Scholar]
- Etminan M., Gill S., Samii A. (2003) Effect of non-steroidal anti-inflammatory drugs on risk of Alzheimer's disease: Systematic review and meta-analysis of observational studies. BMJ 327: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley C. (2006) Aluminium and iron, but neither copper nor zinc, are key to the precipitation of betasheets of Abeta_{42} in senile plaque cores in Alzheimer's disease. J Alzheimers Dis 10: 173–177 [DOI] [PubMed] [Google Scholar]
- Farlow M.R., Evans R.M. (1998) Pharmacologic treatment of cognition in Alzheimer's dementia. Neurology 51(Suppl 1): S36-S44, discussion S65-S37. [DOI] [PubMed] [Google Scholar]
- Fraser P.E., Nguyen J.T., Surewicz W.K., Kirschner D.A. (1991) pH-dependent structural transitions of Alzheimer amyloid peptides. Biophys J 60: 1190–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobini E. (2000) Cholinesterase inhibitors stabilize Alzheimer's disease. Ann N Y Acad Sci 920: 321–327 [DOI] [PubMed] [Google Scholar]
- Giacobini E. (2001) Do cholinesterase inhibitors have disease-modifying effects in Alzheimer's disease? CNS Drugs 15: 85–91 [DOI] [PubMed] [Google Scholar]
- Giacobini E. (2002) Long-term stabilizing effect of cholinesterase inhibitors in the therapy of Alzheimer' disease. J Neural Transm Suppl 62: 181–187 [DOI] [PubMed] [Google Scholar]
- Guyonnet S. Gillette, Van Kan G. Abellan, Andrieu S., Gateau P. Barberger, Berr C., Bonnefoy M., et al. (2007) IANA task force on nutrition and cognitive decline with aging. J Nutr Health Aging 11: 132–152 [PubMed] [Google Scholar]
- Griffin W.S., Sheng J.G., Roberts G.W., Mrak R.E. (1995) Interleukin-1 expression in different plaque types in Alzheimer's disease: Significance in plaque evolution. J Neuropathol Exp Neurol 54: 276–281 [DOI] [PubMed] [Google Scholar]
- Hampel H., Ewers M., Burger K., Annas P., Mortberg A., Bogstedt A., et al. (2009) Lithium trial in Alzheimer's disease: A randomized, single-blind, placebo-controlled, multicenter 10-week study. J Clin Psychiatry 70: 922–931 [PubMed] [Google Scholar]
- Hayashi T., Shishido N., Nakayama K., Nunomura A., Smith M.A., Perry G., et al. (2007) Lipid peroxidation and 4-hydroxy-2-nonenal formation by copper ion bound to amyloid-beta peptide. Free Radic Biol Med 43: 1552–1559 [DOI] [PubMed] [Google Scholar]
- Hernandez F., Borrell J., Guaza C., Avila J., Lucas J.J. (2002) Spatial learning deficit in transgenic mice that conditionally over-express GSK-3beta in the brain but do not form tau filaments. J Neurochem 83: 1529–1533 [DOI] [PubMed] [Google Scholar]
- Hirai K., Aliev G., Nunomura A., Fujioka H., Russell R.L., Atwood C.S., et al. (2001) Mitochondrial abnormalities in Alzheimer's disease. J Neurosci 21: 3017–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Casadesus G., Petersen R.B., Perry G., Smith M.A. (2004) Oxidative stress and redoxactive iron in Alzheimer's disease. Ann N Y Acad Sci 1012: 179–182 [DOI] [PubMed] [Google Scholar]
- Imahori K., Uchida T. (1997) Physiology and pathology of tau protein kinases in relation to Alzheimer's disease. J Biochem 121: 179–188 [PubMed] [Google Scholar]
- Veld B.A. T', Ruitenberg A., Hofman A., Launer L.J., van Duijn C.M., Stijnen T., et al. (2001) Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N Engl J Med 345: 1515–1521 [DOI] [PubMed] [Google Scholar]
- Jobling M.F., Huang X., Stewart L.R., Barnham K.J., Curtain C., Volitakis I., et al. (2001) Copper and zinc binding modulates the aggregation and neurotoxic properties of the prion peptide PrP106-126. Biochemistry 40: 8073–8084 [DOI] [PubMed] [Google Scholar]
- Jorm A.F., Jolley D. (1998) The incidence of dementia: A meta-analysis. Neurology 51: 728–733 [DOI] [PubMed] [Google Scholar]
- Kalaria R.N. (1999) Microglia and Alzheimer's disease. Curr Opin Hematol 6: 15–24 [DOI] [PubMed] [Google Scholar]
- Kaminsky Y.G., Marlatt M.W., Smith M.A., Kosenko E.A. (2010) Subcellular and metabolic examination of amyloid-beta peptides in Alzheimer disease pathogenesis: Evidence for Abeta(25-35). Exp Neurol 221: 26–37 [DOI] [PubMed] [Google Scholar]
- Kontush A. (2001) Alzheimer's amyloid-beta as a preventive antioxidant for brain lipoproteins. Cell Mol Neurobiol 21: 299–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontush A., Berndt C., Weber W., Akopyan V., Arlt S., Schippling S., et al. (2001) Amyloid-beta is an antioxidant for lipoproteins in cerebrospinal fluid and plasma. Free Radic Biol Med 30: 119–128 [DOI] [PubMed] [Google Scholar]
- Kornhuber J., Bormann J., Retz W., Hubers M., Riederer P. (1989) Memantine displaces [3H]MK-801 at therapeutic concentrations in postmortem human frontal cortex. Eur J Pharmacol 166: 589–590 [DOI] [PubMed] [Google Scholar]
- Kwong L.K., Kamzalov S., Rebrin I., Bayne A.C., Jana C.K., Morris P., et al. (2002) Effects of coenzyme Q(10) administration on its tissue concentrations, mitochondrial oxidant generation, and oxidative stress in the rat. Free Radic Biol Med 33: 627–638 [DOI] [PubMed] [Google Scholar]
- Larbig G., Pickhardt M., Lloyd D.G., Schmidt B., Mandelkow E. (2007) Screening for inhibitors of tau protein aggregation into Alzheimer paired helical filaments: A ligand based approach results in successful scaffold hopping. Curr Alzheimer Res 4: 315–323 [DOI] [PubMed] [Google Scholar]
- Lass A., Forster M.J., Sohal R.S. (1999) Effects of coenzyme Q10 and alpha-tocopherol administration on their tissue levels in the mouse: Elevation of mitochondrial alpha-tocopherol by coenzyme Q10. Free Radic Biol Med 26: 1375–1382 [DOI] [PubMed] [Google Scholar]
- Lee J., Boo J.H., Ryu H. (2009) The failure of mitochondria leads to neurodegeneration: Do mitochondria need a jump start? Adv Drug Deliv Rev 61: 1316–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.J., Jeong S.Y., Karbowski M., Smith C.L., Youle R.J. (2004) Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 15: 5001–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre T., Ferreira S., Dupont-Wallois L., Bussiere T., Dupire M.J., Delacourte A., et al. (2003) Evidence of a balance between phosphorylation and O-GlcNAc glycosylation of Tau proteins-a role in nuclear localization. Biochim Biophys Acta 1619: 167–176 [DOI] [PubMed] [Google Scholar]
- Lindsay J., Laurin D., Verreault R., Hebert R., Helliwell B., Hill G.B., et al. (2002) Risk factors for Alzheimer's disease: A prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol 156: 445–453 [DOI] [PubMed] [Google Scholar]
- Liu F., Iqbal K., Grundke-Iqbal I., Hart G.W., Gong C.X. (2004) O-GlcNAcylation regulates phosphorylation of tau: A mechanism involved in Alzheimer's disease. Proc Natl Acad Sci U S A 101: 10804–10809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Men P., Harris P.L., Rolston R.K., Perry G., Smith M.A. (2006) Nanoparticle iron chelators: A new therapeutic approach in Alzheimer disease and other neurologic disorders associated with trace metal imbalance. Neurosci Lett 406: 189–193 [DOI] [PubMed] [Google Scholar]
- Liu G., Men P., Kudo W., Perry G., Smith M.A. (2009) Nanoparticle-chelator conjugates as inhibitors of amyloid-beta aggregation and neurotoxicity: A novel therapeutic approach for Alzheimer disease. Neurosci Lett 455: 187–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Atamna H., Kuratsune H., Ames B.N. (2002a) Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann N Y Acad Sci 959: 133–166 [DOI] [PubMed] [Google Scholar]
- Liu J., Head E., Gharib A.M., Yuan W., Ingersoll R.T., Hagen T.M., et al. (2002b) Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: Partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci U S A 99: 2356–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Killilea D.W., Ames B.N. (2002c) Age-associated mitochondrial oxidative decay: Improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats acetyl-L- carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci U S A 99: 1876–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Xie F., Rolston R., Moreira P.I., Nunomura A., Zhu X., et al. (2007) Prevention and treatment of Alzheimer disease and aging: Antioxidants. Mini Rev Med Chem 7: 171–180 [DOI] [PubMed] [Google Scholar]
- Long J., Gao F., Tong L., Cotman C.W., Ames B.N., Liu J. (2009) Mitochondrial decay in the brains of old rats: Ameliorating effect of alpha-lipoic acid and acetyl-L-carnitine. Neurochem Res 34: 755–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C., Zhang D., Whiteman M., Armstrong J.S. (2008) Is antioxidant potential of the mitochondrial targeted ubiquinone derivative MitoQ conserved in cells lacking mtDNA? Antioxid Redox Signal 10: 651–660 [DOI] [PubMed] [Google Scholar]
- Lucas J.J., Hernandez F., Gomez-Ramos P., Moran M.A., Hen R., Avila J. (2001) Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J 20: 27–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist J., Naslund J. (2007) Gamma-secretase: A complex target for Alzheimer's disease. Curr Opin Pharmacol 7: 112–118 [DOI] [PubMed] [Google Scholar]
- Luo W., Dou F., Rodina A., Chip S., Kim J., Zhao Q., et al. (2007) Roles of heat-shock protein 90 in maintaining and facilitating the neurodegenerative phenotype in tauopathies. Proc Natl Acad Sci U S A 104: 9511–9516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie I.R., Hao C., Munoz D.G. (1995) Role of microglia in senile plaque formation. Neurobiol Aging 16: 797–804 [DOI] [PubMed] [Google Scholar]
- Mancuso M., Orsucci D., Siciliano G., Murri L. (2008) Mitochondria, mitochondrial DNA and Alzheimer's disease What comes first?. Curr Alzheimer Res 5: 457–468 [DOI] [PubMed] [Google Scholar]
- Marlatt M.W., Webber K.M., Moreira P.I., Lee H.G., Casadesus G., Honda K., et al. (2005) Therapeutic opportunities in Alzheimer disease: One for all or all for one? Curr Med Chem 12: 1137–1147 [DOI] [PubMed] [Google Scholar]
- Masters C.L., Simms G., Weinman N.A., Multhaup G., McDonald B.L., Beyreuther K. (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A 82: 4245–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer P.L., Schulzer M., McGeer E.G. (1996) Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: A review of 17 epidemiologic studies. Neurology 47: 425–432 [DOI] [PubMed] [Google Scholar]
- McShane R., Sastre A. Areosa, Minakaran N. (2006) Memantine for dementia. Cochrane Database Syst Rev 2: CD003154. [DOI] [PubMed] [Google Scholar]
- Middleton L.E., Yaffe K. (2009) Promising strategies for the prevention of dementia. Arch Neurol 66: 1210–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgram N.W., Araujo J.A., Hagen T.M., Treadwell B.V., Ames B.N. (2007) Acetyl-L-carnitine and alpha-lipoic acid supplementation of aged beagle dogs improves learning in two landmark discrimination tests. FASEB J 21: 3756–3762 [DOI] [PubMed] [Google Scholar]
- Moore A.H., O'Banion M.K. (2002) Neuroinflammation and anti-inflammatory therapy for Alzheimer's disease. Adv Drug Deliv Rev 54: 1627–1656 [DOI] [PubMed] [Google Scholar]
- Moreira P.I., Zhu X., Nunomura A., Smith M.A., Perry G. (2006) Therapeutic options in Alzheimer's disease. Expert Rev Neurother 6: 897–910 [DOI] [PubMed] [Google Scholar]
- Murphy M.P. (2001) Development of lipophilic cations as therapies for disorders due to mitochondrial dysfunction. Expert Opin Biol Ther 1: 753–764 [DOI] [PubMed] [Google Scholar]
- Murphy M.P., Smith R.A. (2007) Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol 47: 629–656 [DOI] [PubMed] [Google Scholar]
- Nakamura M., Shishido N., Nunomura A., Smith M.A., Perry G., Hayashi Y., et al. (2007) Three histidine residues of amyloid-beta peptide control the redox activity of copper and iron. Biochemistry 46: 12737–12743 [DOI] [PubMed] [Google Scholar]
- Neugroschl J., Sano M. (2009) An update on treatment and prevention strategies for Alzheimer's disease. Curr Neurol Neurosci Rep 9: 368–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble W., Planel E., Zehr C., Olm V., Meyerson J., Suleman F., et al. (2005) Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci USA 102: 6990–6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A., Castellani R.J., Lee H.G., Moreira P.I., Zhu X., Perry G., et al. (2006) Neuropathology in Alzheimer's disease: Awaking from a hundred-year-old dream. Sci Aging Knowledge Environ 8: pe10. [DOI] [PubMed] [Google Scholar]
- Nunomura A., Tamaoki T., Tanaka K., Motohashi N., Nakamura M., Hayashi T., et al. (2010) Intraneuronal amyloid beta accumulation and oxidative damage to nucleic acids in Alzheimer disease. Neurobiol Dis 37: 731–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa O., Zhu X., Perry G., Smith M.A. (2002) Mitochondrial abnormalities and oxidative imbalance in neurodegenerative disease. Sci Aging Knowledge Environ 41: pe16. [DOI] [PubMed] [Google Scholar]
- Okada H., Doken Y., Ogawa Y. (1996) Persistent suppression of the pituitary-gonadal system in female rats by three-month depot injectable microspheres of leuprorelin acetate. J Pharm Sci 85: 1044–1048 [DOI] [PubMed] [Google Scholar]
- Olcese J.M., Cao C., Mori T., Mamcarz M.B., Maxwell A., Runfeldt M.J., et al. (2009) Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. J Pineal Res 47: 82–96 [DOI] [PubMed] [Google Scholar]
- Opazo C., Huang X., Cherny R.A., Moir R.D., Roher A.E., White A.R., et al. (2002) Metalloenzymelike activity of Alzheimer's disease beta-amyloid. Cu-dependent catalytic conversion of dopamine, cholesterol, and biological reducing agents to neurotoxic H(2)O(2). J Biol Chem 277: 40302–40308 [DOI] [PubMed] [Google Scholar]
- Pappolla M., Bozner P., Soto C., Shao H., Robakis N.K., Zagorski M., et al. (1998) Inhibition of Alzheimer beta-fibrillogenesis by melatonin. J Biol Chem 273: 7185–7188 [DOI] [PubMed] [Google Scholar]
- Parks J.K., Smith T.S., Trimmer P.A., Bennett J.P., Jr, Parker W.D., Jr (2001) Neurotoxic Abeta peptides increase oxidative stress in vivo through NMDA-receptor and nitric-oxide-synthase mechanisms, and inhibit complex IV activity and induce a mitochondrial permeability transition in vitro. J Neurochem 76: 1050–1056 [DOI] [PubMed] [Google Scholar]
- Perez M., Hernandez F., Lim F., Diaz-Nido J., Avila J. (2003) Chronic lithium treatment decreases mutant tau protein aggregation in a transgenic mouse model. J Alzheimers Dis 5: 301–308 [DOI] [PubMed] [Google Scholar]
- Perrig W.J., Perrig P., Stahelin H.B. (1997) The relation between antioxidants and memory performance in the old and very old. J Am Geriatr Soc 45: 718–724 [DOI] [PubMed] [Google Scholar]
- Perry G., Nunomura A., Raina A.K., Smith M.A. (2000) Amyloid-beta junkies. Lancet 355: 757. [DOI] [PubMed] [Google Scholar]
- Perry G., Rizzuto N., Autilio-Gambetti L., Gambetti P. (1985) Paired helical filaments from Alzheimer disease patients contain cytoskeletal components. Proc Natl Acad Sci U S A 82: 3916–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R.B., Nunomura A., Lee H.G., Casadesus G., Perry G., Smith M.A., et al. (2007) Signal transduction cascades associated with oxidative stress in Alzheimer's disease. J Alzheimers Dis 11: 143–152 [DOI] [PubMed] [Google Scholar]
- Piani D., Spranger M., Frei K., Schaffner A., Fontana A. (1992) Macrophage-induced cytotoxicity of N-methyl-D-aspartate receptor positive neurons involves excitatory amino acids rather than reactive oxygen intermediates and cytokines. Eur J Immunol 22: 2429–2436 [DOI] [PubMed] [Google Scholar]
- Pickhardt M., Gazova Z., von Bergen M., Khlistunova I., Wang Y., Hascher A., et al. (2005) Anthraquinones inhibit tau aggregation and dissolve Alzheimer's paired helical filaments in vitro and in cells. J Biol Chem 280: 3628–3635 [DOI] [PubMed] [Google Scholar]
- Poeggeler B., Miravalle L., Zagorski M.G., Wisniewski T., Chyan Y.J., Zhang Y., et al. (2001) Melatonin reverses the profibrillogenic activity of apolipoprotein E4 on the Alzheimer amyloid Abeta peptide. Biochemistry 40: 14995–15001 [DOI] [PubMed] [Google Scholar]
- Qiu C., De Ronchi D., Fratiglioni L. (2007) The epidemiology of the dementias: An update. Curr Opin Psychiatry 20: 380–385 [DOI] [PubMed] [Google Scholar]
- Regland B., Lehmann W., Abedini I., Blennow K., Jonsson M., Karlsson I., et al. (2001) Treatment of Alzheimer's disease with clioquinol. Dement Geriatr Cogn Disord 12: 408–414 [DOI] [PubMed] [Google Scholar]
- Reines S.A., Block G.A., Morris J.C., Liu G., Nessly M.L., Lines C.R., et al. (2004) Rofecoxib: No effect on Alzheimer's disease in a 1-year, randomized, blinded, controlled study. Neurology 62: 66–71 [DOI] [PubMed] [Google Scholar]
- Relkin N.R., Szabo P., Adamiak B., Burgut T., Monthe C., Lent R.W., et al. (2009) 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging 30: 1728–1736 [DOI] [PubMed] [Google Scholar]
- Ritchie C.W., Bush A.I., Mackinnon A., Macfarlane S., Mastwyk M., MacGregor L., et al. (2003) Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: A pilot phase 2 clinical trial. Arch Neurol 60: 1685–1691 [DOI] [PubMed] [Google Scholar]
- Rogers J., Kirby L.C., Hempelman S.R., Berry D.L., McGeer P.L., Kaszniak A.W., et al. (1993) Clinical trial of indomethacin in Alzheimer's disease. Neurology 43: 1609–1611 [DOI] [PubMed] [Google Scholar]
- Rosenberg P.B., Drye L.T., Martin B.K., Frangakis C., Mintzer J.E., Weintraub D., et al. (2010) Sertraline for the treatment of depression in Alzheimer disease. Am J Geriatr Psychiatry 18: 136–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottkamp C.A., Raina A.K., Zhu X., Gaier E., Bush A.I., Atwood C.S., et al. (2001) Redox-active iron mediates amyloid-beta toxicity. Free Radic Biol Med 30: 447–450 [DOI] [PubMed] [Google Scholar]
- Sabbagh M.N. (2009) Drug development for Alzheimer's disease: Where are we now and where are we headed? Am J Geriatr Pharmacother 7: 167–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M., Ernesto C., Thomas R.G., Klauber M.R., Schafer K., Grundman M., et al. (1997) A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N Engl J Med 336: 1216–1222 [DOI] [PubMed] [Google Scholar]
- Sasaki A., Yamaguchi H., Ogawa A., Sugihara S., Nakazato Y. (1997) Microglial activation in early stages of amyloid beta protein deposition. Acta Neuropathol 94: 316–322 [DOI] [PubMed] [Google Scholar]
- Scharf S., Mander A., Ugoni A., Vajda F., Christophidis N. (1999) A double-blind, placebocontrolled trial of diclofenac/misoprostol in Alzheimer's disease. Neurology 53(1): 197–201 [DOI] [PubMed] [Google Scholar]
- Schubert D., Chevion M. (1995) The role of iron in beta amyloid toxicity. Biochem Biophys Res Commun 216: 702–707 [DOI] [PubMed] [Google Scholar]
- Selenica M.L., Jensen H.S., Larsen A.K., Pedersen M.L., Helboe L., Leist M., et al. (2007) Efficacy of small-molecule glycogen synthase kinase-3 inhibitors in the postnatal rat model of tau hyperphosphorylation. Br J Pharmacol 152: 959–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R.S., Lee H.G., Xiongwei Z., Perry G., Smith M.A., Castellani R.J. (2008) Current approaches in the treatment of Alzheimer's disease. Biomed Pharmacother 62: 199–207 [DOI] [PubMed] [Google Scholar]
- Shiurba R.A., Ishiguro K., Takahashi M., Sato K., Spooner E.T., Mercken M., et al. (1996) Immunocytochemistry of tau phosphoserine 413 and tau protein kinase I in Alzheimer pathology. Brain Res 737: 119–132 [DOI] [PubMed] [Google Scholar]
- Short R.A., Bowen R.L., O'Brien P.C., Graff-Radford N.R. (2001) Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin Proc 76: 906–909 [DOI] [PubMed] [Google Scholar]
- Siddique H., Hynan L.S., Weiner M.F. (2009) Effect of a serotonin reuptake inhibitor on irritability, apathy, and psychotic symptoms in patients with Alzheimer's disease. J Clin Psychiatry 70: 915–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedlak S.L., Casadesus G., Webber K.M., Pappolla M.A., Atwood C.S., Smith M.A., et al. (2009) Chronic antioxidant therapy reduces oxidative stress in a mouse model of Alzheimer's disease. Free Radic Res 43: 156–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.A. (1998) Alzheimer disease. Int Rev Neurobiol 42: 1–54 [DOI] [PubMed] [Google Scholar]
- Smith M.A., Atwood C.S., Joseph J.A., Perry G. (2002) Predicting the failure of amyloid-beta vaccine. Lancet 359: 1864–1865 [DOI] [PubMed] [Google Scholar]
- Smith M.A., Nunomura A., Zhu X., Takeda A., Perry G. (2000) Metabolic, metallic, and mitotic sources of oxidative stress in Alzheimer disease. Antioxid Redox Signal 2: 413–420 [DOI] [PubMed] [Google Scholar]
- Smith M.A., Zhu X., Tabaton M., Liu G., McKeel D.W., Jr, Cohen M.L., et al. (2010a) Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J Alzheimers Dis 19: 363–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.A., Zhu X., Tabaton M., Liu G., McKeel D.W., Jr, Cohen M.L., et al. (2010b) Increased iron and free radical generation in preclinical alzheimer disease and mild cognitive impairment. J Alzheimers Dis 19: 363–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.A., Kelso G.F., James A.M., Murphy M.P. (2004) Targeting coenzyme Q derivatives to mitochondria. Methods Enzymol 382: 45–67 [DOI] [PubMed] [Google Scholar]
- Stewart W.F., Kawas C., Corrada M., Metter E.J. (1997) Risk of Alzheimer's disease and duration of NSAID use. Neurology 48: 626–632 [DOI] [PubMed] [Google Scholar]
- Su B., Wang X., Zheng L., Perry G., Smith M.A., Zhu X. (2010) Abnormal mitochondrial dynamics and neurodegenerative diseases. Biochim Biophys Acta 1802: 135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan U., Kutlu N. (1991) The distribution of paw preference in right-, left-, and mixed pawed male and female cats: The role of a female right-shift factor in handedness. Int J Neurosci 59: 219–229 [DOI] [PubMed] [Google Scholar]
- van Dyck C.H., Tariot P.N., Meyers B., Malca Resnick E. (2007) A 24-week randomized, controlled trial of memantine in patients with moderate-to-severe Alzheimer disease. Alzheimer Dis Assoc Disord 21: 136–143 [DOI] [PubMed] [Google Scholar]
- Van Gool W.A., Weinstein H.C., Scheltens P., Walstra G.J. (2001) Effect of hydroxychloroquine on progression of dementia in early Alzheimer's disease: An 18-month randomised, double-blind, placebo-controlled study. Lancet 358: 455–460 [DOI] [PubMed] [Google Scholar]
- Wadsworth T.L., Bishop J.A., Pappu A.S., Woltjer R.L., Quinn J.F. (2008) Evaluation of coenzyme Q as an antioxidant strategy for Alzheimer's disease. J Alzheimers Dis 14: 225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Su B., Fujioka H., Zhu X. (2008a) Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer's disease patients. Am J Pathol 173: 470–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Su B., Siedlak S.L., Moreira P.I., Fujioka H., Wang Y., et al. (2008b) Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A 105: 19318–19323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Su B., Zheng L., Perry G., Smith M.A., Zhu X. (2009) The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer's disease. J Neurochem 109(Suppl. 1): 153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber K.M., Casadesus G., Bowen R.L., Perry G., Smith M.A. (2007a) Evidence for the role of luteinizing hormone in Alzheimer disease. Endocr Metab Immune Disord Drug Targets 7: 300–303 [DOI] [PubMed] [Google Scholar]
- Webber K.M., Perry G., Smith M.A., Casadesus G. (2007b) The contribution of luteinizing hormone to Alzheimer disease pathogenesis. Clin Med Res 5: 177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J., Wang K.C., Imaki H., Rubenstein R., Wronska A., Osuchowski M., et al. (2001) The role of microglial cells and astrocytes in fibrillar plaque evolution in transgenic APP(SW) mice. Neurobiol Aging 22: 49–61 [DOI] [PubMed] [Google Scholar]
- Whitehouse P.J. (2006) Quality of life: The bridge from the cholinergic basal forebrain to cognitive science and bioethics. J Alzheimers Dis 9(3 Suppl): 447–453 [DOI] [PubMed] [Google Scholar]
- Wong G.T., Manfra D., Poulet F.M., Zhang Q., Josien H., Bara T., et al. (2004) Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem 279: 12876–12882 [DOI] [PubMed] [Google Scholar]
- Zandi P.P., Anthony J.C., Khachaturian A.S., Stone S.V., Gustafson D., Tschanz J.T., et al. (2004) Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: The Cache County Study. Arch Neurol 61: 82–88 [DOI] [PubMed] [Google Scholar]
- Zhang H., Burrows F. (2004) Targeting multiple signal transduction pathways through inhibition of Hsp90. J Mol Med 82: 488–499 [DOI] [PubMed] [Google Scholar]
- Zhu X., Castellani R.J., Takeda A., Nunomura A., Atwood C.S., Perry G., et al. (2001) Differential activation of neuronal ERK, JNK/SAPK and p38 in Alzheimer disease: The ‘two hit’ hypothesis. Mech Ageing Dev 123: 39–46 [DOI] [PubMed] [Google Scholar]
- Zhu X., Lee H.G., Perry G., Smith M.A. (2007) Alzheimer disease, the two-hit hypothesis: An update. Biochim Biophys Acta 1772: 494–502 [DOI] [PubMed] [Google Scholar]
- Zhu X., Perry G., Moreira P.I., Aliev G., Cash A.D., Hirai K., et al. (2006) Mitochondrial abnormalities and oxidative imbalance in Alzheimer disease. J Alzheimers Dis 9: 147–153 [DOI] [PubMed] [Google Scholar]
- Zhu X., Raina A.K., Perry G., Smith M.A. (2004) Alzheimer's disease: The two-hit hypothesis. Lancet Neurol 3: 219–226 [DOI] [PubMed] [Google Scholar]
- Ziegler-Graham K., Brookmeyer R., Johnson E., Arrighi H.M. (2008) Worldwide variation in the doubling time of Alzheimer's disease incidence rates. Alzheimers Dement 4: 316–323 [DOI] [PubMed] [Google Scholar]