Abstract
The personality trait of extraversion has been linked to the network of brain systems controlling sensitivity to cues of reward and generating approach behavior in response, but little is known about whether extraverts’ neural circuits are especially sensitive to social stimuli, given their preference for social engagement. Utilizing event-related potential (ERP) methodology, this study demonstrates that variation on the extraversion dimension is associated with the extent to which social stimuli evoke enhanced allocation of attention. Specifically, higher scores on extraversion were found to be associated with higher amplitudes of the P300 component of the ERPs elicited by human faces. This finding suggests that social stimuli carry enhanced motivational significance for individuals characterized by high extraversion, and that individual differences in personality are related to meaningful individual differences in neural responses to social stimuli.
Keywords: Extraversion, Event-related potentials, P300
INTRODUCTION
Extraversion, a fundamental personality dimension, captures the social aspect of personality. Extraverts have a preference for seeking, engaging in, and enjoying social interactions, whereas introverts prefer to avoid social situations and tend to be reserved, withdrawn, or shy in social settings (Costa & McCrae, 1980; John, 1990). From the early personality and trait theorists (Allport, 1937; Eysenck, 1967) through contemporary social neuroscience (e.g., Canli, 2004; Depue, 2007; Wright et al., 2006), there continues to be a quest for physiological and neural substrates of personality traits, and extraversion in particular. Among the findings pertaining to the neurobiological correlates of extraversion (a comprehensive review of which is outside this paper’s scope) are positive correlations with neural activity in dopaminergically innervated, reward-sensitive regions, including the ventral striatum, amygdale, and medial prefrontal cortices (Cohen, Young, Baek, Kessler, & Ranganath, 2005; Depue & Collins, 1999; Johnson et al., 1999), although, as noted by Canli (2004), it is clear that personality factors such as extraversion are most likely widely distributed in the brain. Yet, notwithstanding the multitude of studies, the core question of whether extraverts’ neural circuits are more sensitive to social stimuli per se, befitting the very definition of extraversion, has yet to be addressed. Given that social engagement and preference for other people’s company is one of the fundamental features of extraversion (cf. Ashton, Lee, & Paunonen, 2002), it is essential to establish whether social stimuli, such as images of humans, are indeed assigned differential weights in the brains of extraverts relative to introverts.
Electrophysiological indices of brain activity, such as event-related potentials (ERPs), are well suited to address this question as they directly measure brain responses to discrete stimuli. Briefly, ERPs are derived from an electroencephalogram (EEG) by means of signal averaging, and are thought to arise from the synchronous activities of neuronal populations engaged in processing of information at hand. Among many identified ERP components, the P300 component is known as a marker of expectancy-related cognitive operations and as such might prove useful in investigating whether extraverts’ neural circuits are activated by social stimuli to a higher degree than those of introverts.
It has been well established that the amplitude of the P3001—a positive-going ERP component with a peak latency of approximately 300 to 500 ms (contingent on stimulus modality and task difficulty) following the onset of the eliciting event and maximum amplitudes measured at centro-parietal scalp sites—is proportional to the amount of attentional resources engaged in processing a given stimulus (Donchin & Coles, 1988; Johnson, 1988). The P300 is traditionally assessed using an “oddball” paradigm, in which one is presented with a sequence of events representing two distinct categories that vary along a given dimension, with one category occurring less frequently. A larger P300 is elicited by the events representing the low-probability (oddball) category (Donchin, 1981), even in the absence of instructions to categorize along a relevant dimension (Farwell & Donchin, 1991; Ito & Cacioppo, 2000).
Importantly, in addition to the objective frequency of the stimuli that the subject is facing, the P300 amplitude is further affected by the extent to which these stimuli have an intrinsic psychological relevance for the subject. For instance, Johnston and Wang (1991) showed that identical pictures elicited different P300 amplitudes in women at different phases of the menstrual cycle, such that pictures of babies and male models evoked larger P300s in women in the high-progesterone phase as compared to women in the low-progesterone phase. Recently, Fishman, Goldman, and Donchin (2008) have demonstrated P300 sensitivity to individual-specific experiences with (and beliefs about the outcomes of) alcohol use by employing experimental stimuli evoking a wide range of consequences of alcohol ingestion. Only those participants who reported frequent consumption of alcohol in large amounts and believed in “positive” effects of alcohol exhibited large P300 when presented with stimuli suggesting opposite (i.e., negative) effects of drinking. Further, Gray, Ambady, Lowenthal, & Deldin (2004) have shown that autobiographical, self-relevant information, such as one’s hometown or pet’s name, elicited increased P300 amplitudes, which were not significantly smaller than the P300 in response to the neutral/objective oddballs to which the subjects were explicitly instructed to direct their attention. Taken together, these findings (along with those by others; cf. Rosenfeld, Biroschak, & Furedy, 2005 on autobiographical items and P300) suggest that otherwise neutral or “objectively” chosen stimuli have a potential to become subjectively relevant—due to prior exposure, subjective preferences, or other individual history—and, as a result, take on additional psychological significance, which adds another source of variability to the P300 amplitude. This notion is encapsulated by a recent theory positing that P300 amplitude might reflect the extent to which processed information is motivationally significant or subjectively salient, through the activity of the locus coeruleus–norepinephrine (LC-NE) system, which may be measurable at the scalp as the P300 (Nieuwenhuis, Aston-Jones, & Cohen, 2005).
Within this framework, the present study utilized the P300 component of the ERPs to test the hypothesis that extraverts, who by definition enjoy and seek the company of others, would show increased P300 amplitudes when human faces serve as experimental stimuli, as compared to other, nonsocial stimuli. The key factor on which this prediction is based is the assumption that extraverts and introverts have differential motivational values that they assign to social stimuli, which, at the level of ERPs, should elicit differential P300 effects. The main question addressed by this study is whether the neural circuitry in individuals characterized by high sociability (i.e., extraverts) is more sensitive to processing information with social content, in comparison to introverts.
METHOD
Twenty-eight healthy young adults (15 females) between the ages of 18 and 40 (mean age = 21.5, SD = 4.58) participated in the study. Participants were recruited as part of an ongoing multicenter research program and screened to rule out history of central nervous system (CNS) disorder or injury, current or past psychiatric conditions, and current use of medications affecting CNS. The average number of years of formal education was 13.5 years (SD = 1.4); the sample’s ethnic composition was quite diverse, with 43% reporting their ethnicity as Caucasian, 35% as Asian-American, 11% as Hispanic, 7% as African- American, and 4% as Native American. Individual differences in extraversion were assessed using the 48-item Extraversion scale of the NEO Personality Inventory Revised (NEO PI-R; Costa & McCrae, 1992), from which Extraversion T-scores were calculated based on gender-specific normative data. The Extraversion scale was administered following the ERP task, to avoid any unintended priming that might occur with use of introspective questions about one’s personality.
A P300-eliciting “oddball” task was designed to assess whether, in individuals with high Extraversion scores, human faces evoke more attention allocation (i.e., elicit larger P300 in response to oddball targets) than nonsocial, but otherwise comparable, visual stimuli. As reviewed above, a standard oddball task requires that stimuli be clearly classifiable into two distinct categories (e.g., high- vs. low-tone pitches, or X vs. O letters), while one category is presented much more frequently (e.g., on 80% of the trials) than the other. Such an uneven probability setup robustly elicits large P300 amplitudes in response to the infrequent—oddball—stimuli, signifying enhanced resource allocation to an out-of-ordinary event. Utilizing this reliable experimental design, pictures of faces (males vs. females), as a social condition, and flowers (purple vs. yellow), as a nonsocial but equally complex visual control condition, were used as follows. Thirty color headshots of faces with neutral facial expression (NimStim Face Stimulus Set; Tottenham et al., 2009; 15 of each gender, matched for ethnicity) were used in Blocks 1 and 3, while 30 images of either purple or yellow flowers (15 of each) were used in Blocks 2 and 4. In each block, stimuli from two distinct categories (males and females in Blocks 1 and 3; purple and yellow flowers in Blocks 2 and 4) were presented semirandomly, with one of the categories appearing on 80% of the trials (e.g., male; purple flower) and the other, “target” event (e.g., female; yellow flower) appearing on 20% of the trials (targets were counterbalanced between the blocks). A semirandom presentation, with the same stimulus prevented from being presented on two consecutive trials, was chosen to avoid potential sequential effects (i.e., reduced P300 amplitude in response to targets appearing on successive trials; cf. Duncan-Johnson & Donchin, 1977; Johnson & Donchin, 1980) that might obscure differences between target and nontarget trials. Participants were instructed to respond (by a key press) each time they saw a specified target (i.e., oddball event). It was predicted that individuals with high Extraversion scores would exhibit larger P300 amplitudes in response to oddball events in the Face (social) in comparison to the Flower (non-social) blocks, despite equivalent probability (.20) of the oddball targets in the two conditions.
Overall, the task consisted of four blocks of 60 trials each (semirandomly drawn from the 30 available images), which, given a target probability of .20, yielded 24 oddball trials for each Faces and Flowers condition. Each trial consisted of a 500 ms presentation of a fixation cross, followed by an 800 ms stimulus presentation, to which ERPs were time-locked, with an interstimulus interval (ISI) of 1000 ms. EEG data were recorded using NetStation 4.0, an EEG recording system (Electrical Geodesics; EGI, Eugene, OR), with a 64-channel Geodesic Sensor Net with Ag/AgCl electrodes. Data were sampled at a rate of 250/s and filtered offline with a 0.1 to 40 Hz bandpass filter. The filtered data were segmented into epochs starting 100 ms before stimulus onset to 900 ms after stimulus onset, subjected to automated artifact detection (>70 µV in any one of the channels), corrected for vertical and horizontal eye movements (Gratton, Coles, & Donchin, 1983), re-referenced to a linked-mastoid reference, and baseline-corrected using the average of the 100 ms prestimulus epoch. Artifact-free trials were then averaged by experimental condition generating four separate average waveforms: oddball (target) vs. frequent stimuli, separately for Faces and Flowers conditions. The average number of artifact-free trials was 21.15 (SD = 3.07) for the Face targets and 20.07 (SD = 3.30) for the Flower targets.
For objective, data-driven, measurement of the P300 amplitude, its magnitude was determined by principal components analysis (PCA), a formal multivariate procedure which has a number of advantages over traditional peak measures (see Donchin & Heffley, 1978; Spencer, Dien, & Donchin, 2001). PCA decompositions were based on covariance association matrices and solutions were rotated using the Varimax procedure to maximize the amount of variance associated with the smallest number of variables; the number of components to be rotated was determined by the Scree test (Cattell, 1966). Correlational analysis with PCA-derived P300 amplitude as primary outcome variable was employed as the main inferential analytic method. Correlational analysis, rather than group variance analysis, was chosen given the continuous nature of the Extraversion construct.
RESULTS
The participants’ Total Extraversion T-scores ranged from 35 to 73, representing a wide spectrum of Extraversion: The NEO-PI-R manual interprets T scores of 56 to 65 as high and scores of 35 to 44 as low; while T > 65 and T < 35 are interpreted as very high and very low, respectively. The mean T-score for the sample was 56.2 (SD = 10.9). Extraversion scores were not significantly correlated with either age or years of formal education (both r values < .08, p values > .67). There was no significant correlation between Extraversion and accuracy as measured by error rates (r = −.08, p = .66), most likely due to the overall high accuracy of performance on this task (mean accuracy = .98, SD = .03).
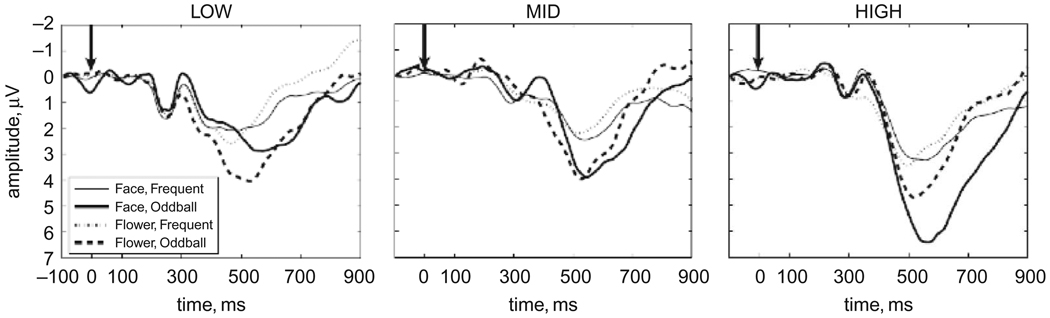
Figure 1 represents the ERP waveforms at the parietal Pz electrode (where P300 is typically at its maximum) for the Faces and Flowers conditions, averaged across individuals with low, mid-range and high Extraversion scores, based on the tertiary split of the sample (with cutoff points of 33% and 66% of the sample Total Extraversion T-scores distribution, resulting in semi-equal groups of n = 9, 10, and 9, respectively.2) The P300, a characteristic large positive deflection with a peak latency of about 500 ms following the stimulus onset, appears to vary systematically across these groups: While the P300 elicited by Flower oddballs appears to be unchanged between the groups, as was expected (since the intrinsic significance of flowers was not hypothesized to vary according to one’s extraversion level), the amplitude of the P300 elicited by Face oddballs appears to vary as a function of participant’s extraversion, such that the smallest positivity is observed in those with low Extraversion scores (i.e., introverts) and the largest positivity is seen in those with high Extraversion scores (i.e., extraverts). To quantify these observable differences, the P300 amplitude values were first derived by applying the spatio-temporal principal component analysis (PCA) to the data,3 as described in Methods. Based on the scalp distribution (i.e., highest loadings in the parietal electrodes) and the temporal variance accounted for (i.e., highest loadings in the 500 ms range, the time window corresponding to the peak positivity emerged in the averaged data; see Figure 1), a P300-like component was identified. Its PCA-derived factor scores for each experimental condition were used as primary dependent variables in all analyses.
Figure 1.
Event-related potentials at Pz (midline parietal electrode, where P300 is at its maximum) averaged for individuals with low, mid-range and high extraversion scores, based on the tertiary split of the sample. Black vertical arrows (corresponding to zero time) mark stimulus onset. Positive voltages are plotted as downward deflections.
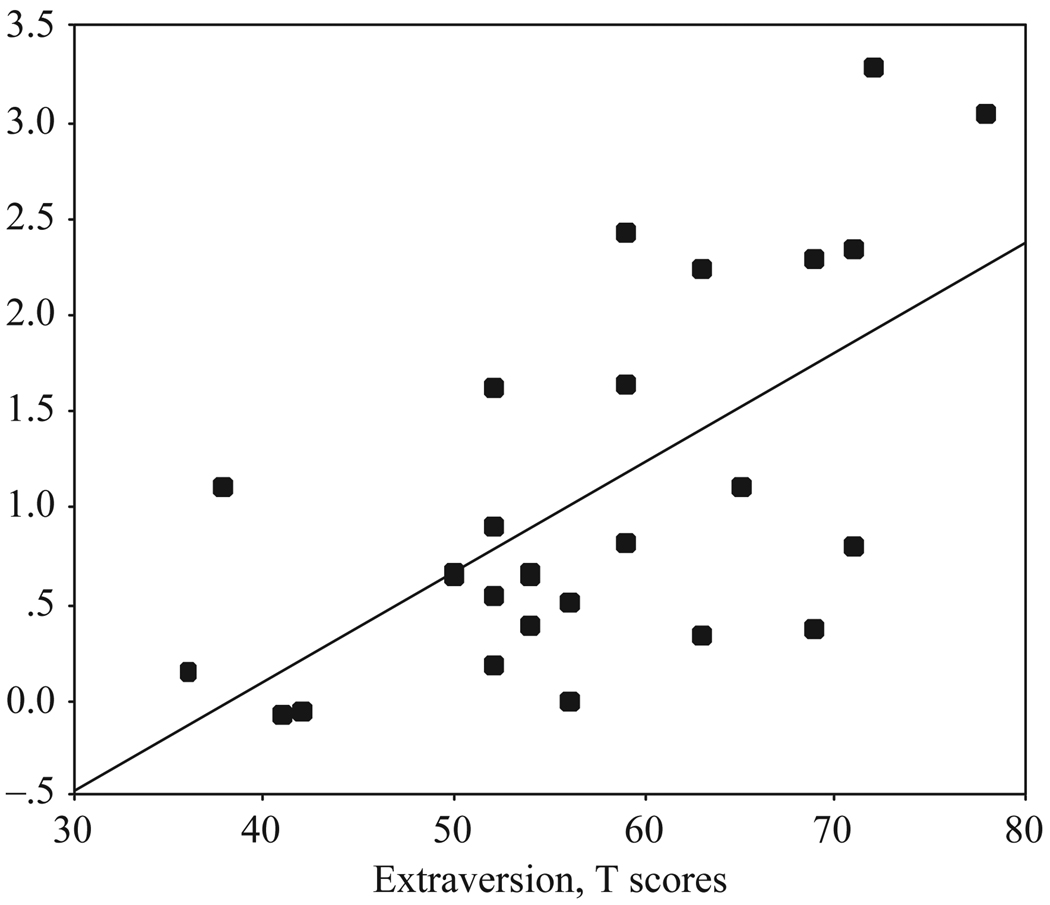
Using the PCA-derived magnitude of the P300 amplitude, a correlation analysis revealed that, as predicted, the P300 amplitude elicited by oddball Face stimuli correlated significantly with Extraversion scores (r = .54, p = .006), such that the higher an individual’s Extraversion score, the larger the P300 in response to Face oddballs (Figure 2). A bootstrapped correlation analysis using 10,000 samples computed a 95% confidence interval ranging from .27 to .75 (SE = 0.12), indicating that this effect was not driven by outlying values. Similar results were obtained when analyzing the so-called P300 effect determined as a difference wave between frequent and oddball Face trials (r = .50, p = .005), suggesting that the association between Face-elicited P300 and Extraversion is stable across different methods of calculating the P300. On the other hand, there was no significant (or sizeable) correlation between individuals’ Extraversion scores and P300 amplitude in response to non-social (i.e., flower) oddballs (r = .09, p = .32), indicating that the association between Extraversion and P300 was specific to social stimuli / faces. Finally, partial correlation analysis was used to rule out any potential confound of age on the Extraversion/Faces P300 effect. The magnitude of the partial correlation between Extraversion scores and Face-oddball P300 remained very similar to the zero-order correlation (r = .53, p = .009), indicating that controlling for age had little effect on the strength of the relationship between P300 amplitude and one’s Extraversion.
Figure 2.
Scatter plot of Extraversion scores and PCA-derived P300 amplitude (P300 factor scores). The value of the factor scores (Y-axis) is a unitless dimension.
DISCUSSION
The study’s main finding is that variation on the Extraversion dimension is strongly associated with the extent to which social stimuli evoke enhanced allocation of attention. The higher one’s score on Extraversion, the larger the index of attention allocation (P300 to oddball targets) to human faces. This finding suggests that faces have increased motivational significance for individuals characterized by high extraversion. Importantly, face (social) and flower (nonsocial) stimuli appeared with the same frequency in different blocks. The central difference between these two types of stimuli was the assumed absence of personal relevance of flowers – in contrast to faces – to participants across different levels of extraversion. In other words, these two stimulus categories were conceived to have differential motivational or rewarding value for those high on extraversion, and thereby were expected to elicit differential P300 amplitude in those individuals. This hypothesis was supported by the present data.
The finding that extraverts showed larger P300 amplitudes in response to oddball social stimuli (but not to oddball nonsocial stimuli) supports the idea that human faces are especially noteworthy for these individuals, in comparison to other visual stimuli with equivalent stimulus properties and frequency of occurrence. In contrast, smaller P300 amplitudes found in introverts in responses to faces suggest that human faces are not a particularly attention-grabbing category of visual information for these individuals. Overall, these results suggest that the sociability characterizing extraverts, including enjoyment of social activities and preference for social interactions over being alone, might be associated with enhanced processing of social stimuli, likely due to a heightened intrinsic psychological significance that such stimuli carry for extraverts. Importantly, this effect does not generalize to all categories of visual stimuli, as demonstrated by lack of such association between extraversion and P300 elicited by nonsocial visual stimuli (in this study, images of flowers).
In sum, the results support the notion of differential neurobiological processes associated with two distinct personality profiles characterized by social approach and social withdrawal. Although a causal relationship cannot be inferred from these results (i.e., it is unclear whether one’s extraversion/introversion might lead to specific alterations in neural circuitry via different lifetime experiences, including more or less social contact, or whether differential brain circuitry determines one’s extraversion), these findings suggest that individual differences in personality are related to meaningful individual differences in neural responses to social stimuli. Future research may utilize this methodology to further explore the impact of intrinsic biology vs. the cumulative effect of experience on personality development during earlier life stages.
Finally, given the recent evidence of the LC-NE system involvement in generation of the P300 (Nieuwenhuis et al., 2005; see also Polich, 2007), it is conceivable that this system might be implicated in the expression of the personality dimension descriptively captured as extraversion (and its main facet of social engagement).4 Although highly speculative, it may be worth considering the possibility that the P300 may serve as a probe of the processing pathways sustaining the extraverts’ bias toward seeking and enjoying social interactions. That is, within a few hundred milliseconds of being exposed to a social stimulus, the nervous system is already passing along a signal that is consistent with differential behavioral patterns encapsulated by the personality trait of extraversion: In extraverts this signal is biased towards allowing preferential access to the limited pool of attentional resources, while in introverts social stimuli are not granted such preferential status. Thus, given the currently discussed LC-NE hypothesis of the P300 etiology and the variability of the P300 elicited by social stimuli observed along the extraversion continuum in the present study, the LC-NE system might be another fundamental explanation for the difference in nervous system function between extraverts and introverts, perhaps originating with overall arousal, as has been suggested by early personality theorists (Eysenck, 1967; Eysenck & Eysenck, 1985).
Acknowledgments
This report is based on work supported by the National Institute of Child Health and Human Development (NICHD) grant P01 HD 33113 awarded to UB and the National Institute of Mental Health (NIMH) fellowship 5 T32 MH20002 awarded to IF.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Also sometimes referred to as the P3b component (cf. Polich, 2003, 2004).
The three groups did not differ on age, F (2, 27) = 1.02, p = .38; however, as expected based on the NEO-PI manual and norms, the Low Extraversion group included significantly more females than the Mid- and High Extraversion groups, F(2, 27) = 5.22, p = .01; pairwise comparison p values = .01. Both Mid- and High Extraversion groups were characterized by equal number of males and females (pairwise comparison p = .46). There was no difference between the groups with respect to the reaction time (RT) to either faces, F(2, 27) = 1.54, p = .23, or flowers, F(2, 27) = 2.01, p = .15.
To capture variance across electrode sites, a spatial PCA was conducted on a covariance matrix with the voltage readings at each of the 65 electrodes (64 plus reference) as variables, and time points across conditions and subjects as cases: 250 time points (1000 ms epochs, sampled every 4 ms) × 4 conditions × 28 participants. Using the Scree test, 8 spatial factors, accounting for 88.6% of the total variance, were extracted for Varimax rotation. Next, to achieve the analogous reduction in dimensionality in the temporal domain, a temporal PCA was conducted, with the data matrix consisting of spatial factor scores associated with the time points (250) as variables, and 8 spatial factors × 4 conditions × 28 participants as cases. The Scree test suggested retention of 8 temporal factors accounting for 93.1% of the variance, which were then rotated to simple structure using Varimax.
While we are aware of the dopaminergic hypothesis of extraversion first put forward by Depue (1995), evidence for this model has been inconsistent (cf. Wilt & Revelle, 2009).
REFERENCES
- Allport GW. Personality: A psychological interpretation. New York: Holt; 1937. [Google Scholar]
- Ashton MC, Lee K, Paunonen SV. What is the central feature of extraversion? Social attention versus reward sensitivity. Journal of Personality and Social Psychology. 2002;83:245–252. [PubMed] [Google Scholar]
- Canli T. Functional brain mapping of extraversion and neuroticism: Learning from individual differences in emotion processing. Journal of Personality. 2004;72(6):1105–1132. doi: 10.1111/j.1467-6494.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- Cattell RB. The scree test for the number of factors. Multivariate Behavioral Research. 1966;1(2):140–161. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Young J, Baek JM, Kessler C, Ranganath C. Individual differences in extraversion and dopamine genetics reflect reactivity of neural reward circuitry. Cognitive Brain Research. 2005;25(3):851–861. doi: 10.1016/j.cogbrainres.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Influence of extraversion and neuroticism on subjective well-being: Happy and unhappy people. Journal of Personality and Social Psychology. 1980;38:668–678. doi: 10.1037//0022-3514.38.4.668. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI): Professional Manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Depue RA. Neurobiological factors in personality and depression. European Journal of Personality. 1995;9(5):413–439. [Google Scholar]
- Depue RA. Psychobiology of personality. In: Caccioppo J, editor. Handbook of social and affective neurosciences. New York, NY: Cambridge University Press; 2007. [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;28(3):313–395. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Donchin E. Surprise! … Surprise? Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:357–374. [Google Scholar]
- Donchin E, Heffley E. Multivariate analysis of event-related potential data: A tutorial review. In: Otto D, editor. Multidisciplinary perspectives in event-related potential research. Washington, DC: US Government Printing Office; 1978. pp. 555–572. [Google Scholar]
- Duncan-Johnson CC, Donchin E. On quantifying surprise: The variation in event-related potentials with subjective probability. Psychophysiology. 1977;14:456–467. doi: 10.1111/j.1469-8986.1977.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. The biological basis of personality. Springfield, IL: Thomas; 1967. [Google Scholar]
- Eysenck HJ, Eysenck MW. Personality and individual differences: A natural science approach. New York, NY: Plenum; 1985. [Google Scholar]
- Farwell LA, Donchin E. The truth will out: Interrogative polygraphy (“lie detection”) with event-related brain potentials. Psychophysiology. 1991;28:531–547. doi: 10.1111/j.1469-8986.1991.tb01990.x. [DOI] [PubMed] [Google Scholar]
- Fishman I, Goldman M, Donchin E. The P300 as an electrophysiological probe of alcohol expectancy. Experimental and Clinical Psychopharmacology. 2008;16(4):341–356. doi: 10.1037/a0012873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gray HM, Ambady N, Lowenthal WT, Deldin P. P300 as an index of attention to self-relevant stimuli. Journal of Experimental Social Psychology. 2004;40:216–224. [Google Scholar]
- Ito TA, Cacioppo JT. Electrophysiological evidence of implicit and explicit categorization processes. Journal of Experimental Social Psychology. 2000;36:660–676. [Google Scholar]
- John OP. The “Big Five” factor taxonomy: Dimensions of personality in the natural language and in questionnaires. In: Pervin L, editor. Handbook of personality theory and research. New York, NY: Guilford; 1990. pp. 66–100. [Google Scholar]
- Johnson R., Jr . The amplitude of the P300 component of the event-related potential: Review and synthesis. In: Ackles PK, Jennings JR, Coles MGH, editors. Advances in psychophysiology. Vol. 3. Greenwich, CT: JAI Press; 1988. pp. 69–137. [Google Scholar]
- Johnson R, Jr, Donchin E. P300 and stimulus categorization: Two plus one is not so different from one plus one. Psychophysiology. 1980;17:167–178. doi: 10.1111/j.1469-8986.1980.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Johnson DL, Wiebe JS, Gold SM, Andreason NC, Hichwa RD, Watkins GL, et al. Biological bases of extraversion: A positron emission tomographical study. American Journal of Psychiatry. 1999;156:252–257. doi: 10.1176/ajp.156.2.252. [DOI] [PubMed] [Google Scholar]
- Johnston VS, Wang XT. The relationship between the menstrual phase and the P300 component of the ERP. Psychophysiology. 1991;28:400–409. doi: 10.1111/j.1469-8986.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychological Bulletin. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Polich J. Overview of P3a and P3b. In: Polich J, editor. Detection of change: Event-related potential and fMRI findings. Norwell, MA: Kluwer Academic Press; 2003. pp. 83–98. [Google Scholar]
- Polich J. Neuropsychology of P3a and P3b: A theoretical overview. In: Moore N, Arikan K, editors. Brainwaves and mind: Recent developments. Wheaton, IL: Kjellberg Press; 2004. pp. 15–29. [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld JP, Biroschak JR, Furedy JJ. P300-based detection of concealed autobiographical versus incidentally acquired information in target and non-target paradigms. International Journal of Psychophysiology. 2005;60(3):251–259. doi: 10.1016/j.ijpsycho.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology. 2001;38:343–358. [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilt J, Revelle W. Extraversion. In: Leary M, Hoyle R, editors. Handbook of individual differences in social behavior. New York: Guilford Press; 2009. pp. 27–45. [Google Scholar]
- Wright CI, Williams D, Feczko E, Barrett LF, Dickerson BC, Schwartz CE, et al. Neuroanatomical correlates of extraversion and neuroticism. Cerebral Cortex. 2006;16(12):1809–1819. doi: 10.1093/cercor/bhj118. [DOI] [PubMed] [Google Scholar]