Abstract
Infusion of a μ-opioid receptor (MOR) agonist into the nucleus accumbens (NAcc) drives voracious food intake, an effect hypothesized to occur through increased tastant palatability. While intake of many palatable foods is elevated by MOR stimulation, this manipulation has a preferential effect on fatty food ingestion. Consumption of high-fat foods is increased by NAcc MOR stimulation even in rats that prefer a carbohydrate-rich alternative under baseline conditions. This suggests that NAcc MOR stimulation may not simply potentiate palatability signals and raises the possibility that mechanisms mediating fat intake may be distinct from those underlying intake of other tastants. The present study was conducted to investigate the physiological mechanisms underlying the effects of NAcc MOR stimulation on fatty food intake. In experiment 1, we analyzed lick microstructure in rats ingesting Intralipid to identify the changes underlying feeding induced by infusion of a MOR-specific agonist into the NAcc. MOR stimulation in the NAcc core, but not shell, increased burst duration and first-minute licks, while simultaneously increasing the rate and duration of Intralipid ingestion. These results suggest that MOR activation in the core increases Intralipid palatability and attenuates inhibitory postingestive feedback. In experiment 2, we measured the effects of MOR stimulation in the NAcc core on consumption of nonnutritive olestra. A MOR-specific agonist dose dependently increased olestra intake, demonstrating that caloric signaling is not required for hyperphagia induced by NAcc MOR stimulation. Feeding induced by drug infusion in both experiments 1 and 2 was blocked by a MOR antagonist. In experiment 3, we determined whether MOR activation in the NAcc core could attenuate satiety-related signaling caused by infusion of the melanocortin agonist MTII into the third ventricle. Suppression of intake caused by MTII was reversed by MOR stimulation. Together, our results suggest that MOR stimulation in the NAcc core elevates fatty food intake through palatability mechanisms dependent on orosensory cues and suppression of satiety signals inhibiting food intake.
Keywords: nucleus accumbens, food reward
understanding the neural mechanisms that promote food intake exceeding metabolic need is a fundamentally important step in addressing the growing health problem of overweight and obesity. While food intake is determined by the convergence of many neural signaling pathways (31), central opioid signaling acting in motivation/reward circuits may be particularly important in driving excessive food consumption, as these brain circuits are involved in determining the reinforcing value of tastants (3, 24, 28).
Activation of μ-opioid receptors (MORs) in the nucleus accumbens (NAcc) constitutes a particularly potent stimulus for food intake. Even in satiated rats, infusion of the MOR-specific agonist [d-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) into the NAcc increases food intake (2, 29, 42, 44). This behavioral effect is hypothesized to occur through opioid-induced increases in tastant palatability. Several convergent lines of evidence support this hypothesis. First, NAcc MOR stimulation increases intake of palatable tastants such as high-fat chow, sucrose, and saccharin, but has little effect on substances with hedonically neutral tastes, such as water or standard rat chow (24, 46). This finding is consistent with a mechanism in which MOR activation potentiates palatability-related signals. Second, DAMGO infusion into the NAcc increases positive taste reactivity displays occurring in response to an intraorally infused sucrose solution (27, 28, 37). These orofacial reactions are correlated with the hedonic impact of tastants (19), suggesting MOR stimulation increases the palatability of the sucrose solution. Third, analysis of lick microstructure indicates that elevated sucrose consumption following infusion of DAMGO into the NAcc occurs through increases in the duration of licking bursts (41). Previous work has shown that burst duration covaries with tastant palatability, with more preferred tastants eliciting longer burst durations (14, 39). Together, these findings suggest increases in palatability underlie consumption driven by NAcc MOR stimulation.
While NAcc MOR stimulation can increase intake of many food items, it is particularly effective as well as selective in increasing the intake of fatty foods. In animals offered a choice of high-fat or high-carbohydrate foods, infusion of DAMGO into the NAcc selectively increases consumption of the high-fat diet (44). This selective effect is independent of baseline preference, as even carbohydrate-preferring rats more than triple their average consumption of the high-fat diet without significant changes in consumption of the high-carbohydrate diet. This finding has interesting implications for the mechanisms underlying opioid effects in the NAcc on food intake. Specifically, it challenges the idea that opioid-induced hyperphagia results solely through potentiation of palatability signals. If this were the case, NAcc DAMGO would be expected to increase consumption of preferred foods, independent of macronutrient content.
The independence of NAcc MOR-induced hyperphagia from baseline preference raises the possibility that the physiological mechanisms underlying potentiation of fatty food intake may differ from those supporting increased consumption of other palatable tastants. To investigate this possibility, we performed a series of experiments designed to elucidate the physiological mechanisms underlying the effects of NAcc MOR stimulation on consumption of fatty tastants. In experiment 1, we used lick microstructure analysis during consumption of a palatable fat emulsion to compare concentration-dependent changes in palatability to hyperphagia induced by NAcc DAMGO infusion. In experiment 2, we assessed the effects of NAcc MOR stimulation on consumption of noncaloric olestra. Finally, in experiment 3, we assessed interactions between NAcc DAMGO-induced hyperphagia and melanocortin-mediated satiety signals.
MATERIALS AND METHODS
Animals and Experimental Overview
Individually housed male Sprague-Dawley rats (n = 86; 350–550 g) maintained on a 12:12-h light-dark cycle (lights on at 7 AM) were used in all experiments. Rats were allowed ad libitum access to food (8640 Teklad 22/5 Rodent Diet; Harlan, Indianapolis, IN) and water throughout the experimental period. Rats were used in four experiments, studies of: 1) lick microstructure (experiment 1a, Intralipid concentration series, n = 16 rats; experiment 1b, NAcc DAMGO infusion, n = 19 for core infusions, n = 14 for shell infusions); 2) noncaloric olestra intake (n = 12); 3) interaction of DAMGO and the melanocortin agonist MTII signaling (n = 16); and 4) the effects of pretreatment with the MOR-specific antagonist d-Phe-Cys-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP) on DAMGO-induced hyperphagia (n = 9 rats for studies of olestra intake, n = 12 rats for studies of Intralipid intake). Rats used in experiment 2 were a subset of those used in experiment 3, but different groups were used for other experiments. All procedures were approved by the University of Utah Medical School Animal Care and Use Committee and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgery
With the exception of rats used in the Intralipid concentration series (experiment 1a), all rats received bilateral cannulae directed at the NAcc. Rats were implanted either with cannulae directed at the NAcc shell (experiment 1b), core [experiments 1b, 2, 3, and 4 (olestra intake)] or both (experiment 4 [Intralipid intake]). For cannula implantation, anesthesia was induced and maintained with isoflurane (induction, 5%; maintenance, 2% in O2). Twenty-four-gauge stainless steel cannulae (Small Parts, Miramar, FL) were targeted bilaterally to the NAcc core at AP, +1.4; ML, ±1.85; DV, −6.5 and the NAcc shell at AP, +1.4; ML, ±0.75; DV, −6.5. Rats used in experiment 3 were also implanted with cannulae directed at the third ventricle. Stereotaxic coordinates for the third ventricle were AP, −2.3; ML, 1.7; DV, −8.5 with cannulae implanted at a 10-degree angle (tilted toward the midline) to avoid the central sagittal sinus. For all implantation sites, cannula placements terminated 1 mm dorsal to the targeted injection site, and injectors extended 1 mm beyond the cannulae tips. All animals received lactated Ringer solution (3 ml sc), buprenorphine (0.1 mg/kg ip), and 1 wk of recovery postsurgery.
Experiment 1a
Lick microstructure during Intralipid concentration series.
In initial studies of lick microstructure, licking patterns were analyzed in rats given access to an ascending series of Intralipid (Baxter, Deerfield, IL) concentrations (1, 3, and 10%). Intralipid is a stable emulsion of soybean oil that rodents find highly palatable (32). This experiment was carried out to allow comparison of results obtained after NAcc DAMGO infusion with concentration-dependent changes in palatability. In this and other experiments, rats were handled daily for a minimum of 1 wk before beginning experiments. Thereafter, rats were habituated to daily 2-h sessions in operant chambers equipped with photobeam lickometers (MedAssociates, Georgia, VT). During these habituation sessions, rats were provided 2-h access to 1% (wt/vol) Intralipid. Daily presentations continued until mean consumption levels stabilized (<15% difference on consecutive days). Lick microstructure was then analyzed (detailed in Analysis of lick microstructure below) from data gathered on the last day of Intralipid exposure (second day of stable intake levels). This procedure was then repeated with 3% and 10% Intralipid solutions. Intralipid intake was quantified by weighing lick bottles before and after each session; licks were time-stamped and recorded digitally for off-line analysis of lick microstructure.
Experiment 1b
Lick microstructure during NAcc MOR stimulation of the core and shell.
In this experiment, lick microstructure of 5% Intralipid intake following NAcc MOR stimulation of the NAcc core or shell was analyzed. Rats were first habituated to daily 2-h sessions of 5% Intralipid access, using the procedure described in experiment 1a. Rats were then infused bilaterally with control saline and 10, 50, and 250 ng/μl of DAMGO (Sigma-Aldrich, St. Louis, MO) in volumes of 0.5 μl/NAcc in a Latin-square design. Each rat was infused once with each of the doses. In this and all subsequent infusion experiments, doses were randomized across days, and rats were infused every other day, allowing a rest day between infusions. Immediately following infusions, rats were placed in operant chambers for 2-h Intralipid access.
Analysis of lick microstructure.
A series of quantitative analyses were carried out to analyze licking behavior over short and longer behavior epochs. Temporal parameters used in this analysis were based on those used in previous studies (14, 39, 41). Briefly, bursts of near-continuous licking were defined by interlick intervals (ILIs) < 1 s in duration. That is, each burst started with the first lick after an ILI ≥ 1 s and ended with the last lick preceding the next ILI ≥ 1 s; licks occurring within each burst were thus separated by ILIs < 1 s long. Burst number (the total number of bursts occurring over the 2-h session) and burst duration were calculated for each experimental session. Burst analysis was of particular interest in our experiments as burst duration has been shown to covary with tastant palatability (1, 14, 39).
Longer epochs of consumption, meals, were defined by ILIs < 10 m. Meal duration was measured by the time elapsed from the first to the last lick of a meal. In addition, the latency to begin licking was defined as the time elapsed from the session start time until the first lick of a meal. Finally, first-minute licks were quantified to capture licking behavior early in the session, before inhibitory feedback from postingestive signaling could suppress consumption. Like burst duration, initial lick rate has been shown to covary with tastant palatability (11, 35).
Experiment 2
DAMGO effects on olestra intake.
In this experiment, we tested the effects of DAMGO infusion into the NAcc core on consumption of olestra (DairyLean J, generously donated by Procter and Gamble, Cincinnati, OH), a nonnutritive sucrose polyester. Rats were first habituated during 30-min sessions in which olestra was presented in a ceramic bowl in the home cage. After intake stabilized, drug testing began. Rats were infused bilaterally with concentrations of control saline and 0.05, 0.5, and 5 μg/μl of DAMGO (0.5 μl/NAcc). Our previous results (Katsuura Y and Taha SA, unpublished) suggest that high concentrations of DAMGO effectively stimulate intake of solid foods but not liquids. Because the olestra used in these experiments has a near-solid (gel-like) consistency at room temperature, the highest DAMGO concentration used in this experiment was higher than that used in experiment 1. This allowed characterization of DAMGO effects over a broader range of concentrations, extending to higher concentrations that we anticipated would continue to effectively elicit hyperphagia. Intake was measured by weighing olestra before and after the session. Ad libitum water was available during these sessions.
Experiment 3
Interaction of NAcc, DAMGO, and MTII signaling.
In this experiment, we tested the effects of coinfusion of DAMGO (control saline or 250 ng/μl, 0.5 μl/NAcc) into the NAcc core and the melanocortin agonist MTII (Phoenix Pharmaceuticals, Mountain View, CA; control saline or 0.8 μg in 1 μl) into the third ventricle on consumption of a high-fat chow (Bio-Serv, Frenchtown, NJ). In the high-fat chow formulation used, fat constituted 60% of total energy (protein, 15%; carbohydrate, 25%). The MTII dose used was based on previously published data indicating that this dose was sufficient to robustly inhibit consumption of a high-fat diet (9). Rats were habituated to a 1-h presentation of high-fat chow. After habituation, rats were infused with four drug combinations: saline (NAcc) + saline (third ventricle); DAMGO+saline; saline+MTII; and DAMGO+MTII. Sessions were videotaped for subsequent scoring of feeding behavior. Observers blind to drug condition scored each session, recording the time at which each feeding episode began and ended. Feeding duration after each drug combination was subsequently analyzed in 10-min bins. Ad libitum water was available during these sessions.
Experiment 4
Effects of the MOR-specific antagonist CTAP on DAMGO-induced hyperphagia.
In this experiment, we tested the effects of CTAP (2.2 μg/μl, 0.5 μl/NAcc) pretreatment on DAMGO-induced consumption. DAMGO doses of 125 ng/NAcc and 2.5 μg/NAcc were used in these experiments in studies of Intralipid and olestra consumption, respectively (corresponding to the highest doses used in experiments 1b and 2). Studies of CTAP effects on DAMGO-induced Intralipid intake were carried out in a group of 12 rats, each of which was implanted with bilateral cannulae directed at both the NAcc core and shell. After recovery from surgery, rats were infused with four drug combinations into each NAcc site: saline, DAMGO, CTAP, and CTAP+DAMGO. CTAP was infused 10 min prior to subsequent DAMGO infusion. A separate group of nine rats with bilateral cannulae placed in the NAcc core was used in studies of CTAP effects on DAMGO-induced olestra intake. Drug infusions of saline, DAMGO, CTAP, and CTAP+DAMGO into the NAcc core were tested in these animals.
Statistical Analysis
Data were analyzed using repeated-measures (RM) ANOVA or RM ANOVA on ranks for normal and nonnormal data distributions, respectively. Data from experiment 1a were analyzed using one-way RM ANOVA with Intralipid concentration as the independent variable and lick microstructure/consumption parameters (volume consumed, caloric intake, meal duration, first-minute licks, burst duration, and burst number) as dependent variables. Two-way RM ANOVA was performed with Intralipid concentration and time as independent variables and lick rate as the dependent variable. For data gathered in experiment 1b, statistical analyses of the same dependent variables were carried out with DAMGO concentration as the independent variable. One-way RM ANOVA was used for all tests except for burst duration, for which RM ANOVA on ranks was performed. Data from experiment 2 were analyzed using RM ANOVA on ranks, with DAMGO concentration as the independent variable and olestra intake as the dependent variable. One-way RM ANOVA was used to analyze data collected in experiment 3 to determine the effects of drug treatment (independent variable) on total consumption and latency to begin feeding (dependent variables). The time course of feeding was analyzed using two-way RM ANOVA with drug treatment and time as independent variables and time spent feeding as the dependent variable. One-way RM ANOVA was used to analyze data collected in experiment 4 to determine the effects of drug treatment (independent variable) on consumption (dependent variable). Except where noted, the Holm-Sidak method was used for post hoc testing.
Histology
Rats were deeply anesthetized with sodium pentobarbital (40 mg/kg Beuthenasia-D) and perfused first with physiological saline and then 4% formaldehyde. Brains were extracted, cryoprotected overnight in 30% sucrose, and cryostat sectioned. Sections were stained with methyl red to anatomically localize cannula placements. Cannulae directed at the NAcc shell were mislocalized in one rat. Data from this rat were excluded from further analysis.
RESULTS
Experiment 1a: Lick Microstructure During Consumption of Intralipid Concentration Series
In our initial experiment, we characterized lick microstructure as a function of concentration-dependent changes in palatability during consumption of Intralipid. Results from this experiment provided a context for understanding NAcc DAMGO-induced changes in licking behavior, studied in experiment 1b.
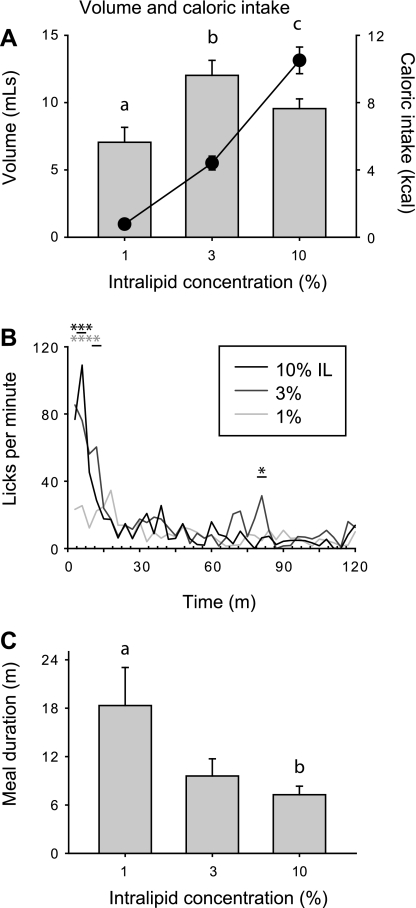
Mean intake of Intralipid solution varied as a function of concentration [Fig. 1A, bars; main effect of concentration, F(2, 30) = 11.0, P < 0.001], with significantly different mean intake for each Intralipid concentration (all P < 0.05). Mean consumption of Intralipid peaked at the 3% concentration, but relatively lower levels of 10% Intralipid intake likely resulted from increased satiety-related inhibitory feedback, rather than lower preference. Consistent with this interpretation, caloric intake increased monotonically as a function of Intralipid concentration [Fig. 1A, circles; main effect of concentration, F(2,30) = 120.5, P < 0.001; all P < 0.05]. Relative to 1% Intralipid, licking rates for the two higher concentrations were significantly elevated in the early portions of the meal, in approximately the first 9 min of the session [Fig. 1B; significant interaction of time and Intralipid concentration, F(78, 1170) = 2.8, P < 0.001]. Average meal durations were longest during consumption of 1% Intralipid (Fig. 1C), likely because rats continued to sample this solution intermittently over a protracted period without reaching substantial levels of satiety, as occurred for 3% and 10% Intralipid [main effect of concentration; F(2, 30) = 4.3, P < 0.05].
Fig. 1.
A: mean ± SE intake of 1, 3, and 10% Intralipid during 2-h sessions (bars, units on left y-axis) and mean caloric intake for each solution (circles, units on right y-axis). Different letters indicate statistically significant differences (for both volume and calories consumed). B: mean lick rate over the course of experimental sessions. Asterisks indicate significant differences between lick rates during 10% (black) and 3% (gray) Intralipid consumption vs. 1%. Underlined asterisks indicate significant differences relative to both of the other Intralipid concentrations. C: mean meal duration. Different letters indicate statistically significant differences.
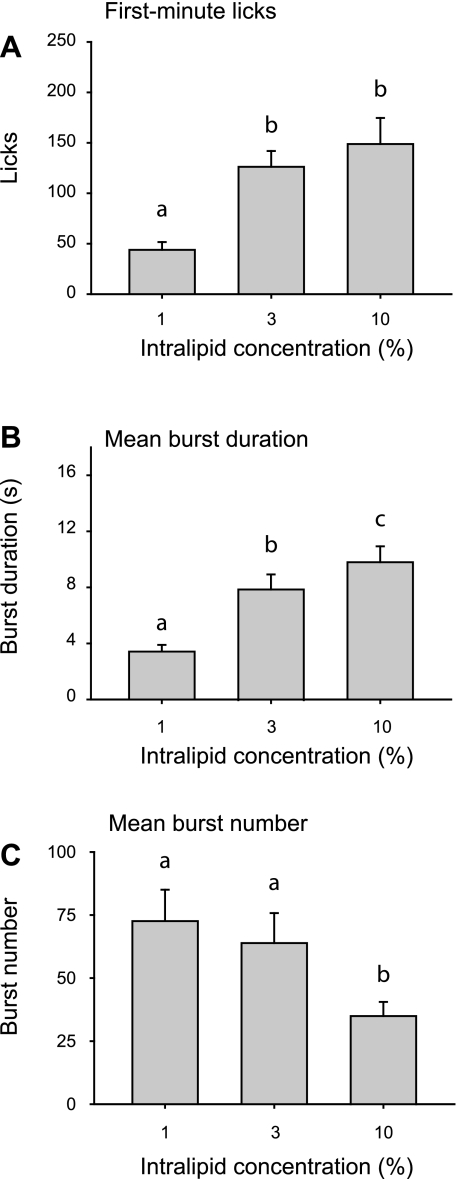
Licks confined to the first minute were directly correlated with Intralipid concentration [Fig. 2A; main effect of concentration; F(2, 30) = 10.1, P < 0.001]. Similarly, lick burst duration increased monotonically with concentration [Fig. 2B; main effect of concentration; F(2, 30) = 36.1, P < 0.001]. Both of these measures covary with tastant palatability (14, 39), suggesting that elevated caloric intake at higher Intralipid concentrations was driven by increased palatability. Changes in burst number did not contribute to increased intake of 3% and 10% Intralipid relative to the 1% solution. Indeed, there was a significant concentration-dependent decrease in the total number of bursts [Fig. 2C; main effect of burst duration; F(2, 30) = 10.1, P < 0.001], consistent with increased inhibitory feedback arising from the postingestive consequences of consuming solutions with higher caloric density (12, 15).
Fig. 2.
Mean first-minute licks (A), burst duration (B), and burst number (C) for Intralipid concentration series. Different letters indicate statistically significant differences.
Experiment 1b: Lick Microstructure of Intralipid Consumption Following DAMGO Infusion into the NAcc Core and Shell
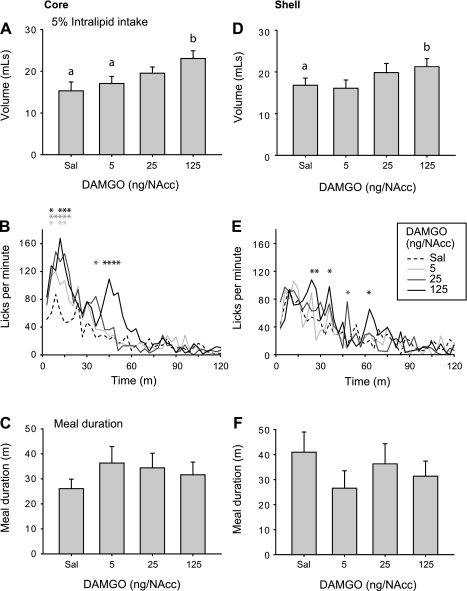
Infusion of DAMGO into the NAcc core dose dependently increased total Intralipid intake [Fig. 3A; main effect of DAMGO concentration, F(3, 54) = 9.0, P < 0.001]. Licking rates were elevated relative to control saline infusions for all three DAMGO concentrations [Fig. 3B; significant interaction of DAMGO concentration and time; F(117, 2106) = 1.9, P < 0.001] during roughly the first 15 min of the session (significant differences from saline infusion indicated by asterisks in Fig. 3B). Interestingly, infusion of the highest DAMGO concentration resulted in a protracted period of elevated lick rates, which differed significantly from lick rates following saline infusion both at early (first ∼15 min of session) and later time points in the session (beginning at ∼40 min). There were no significant changes in meal duration after infusion of any DAMGO concentration [Fig. 3C; F(3, 54) = 1.0, P = 0.39].
Fig. 3.
[d-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) effects on 5% Intralipid consumption after infusion into the nucleus accumbens (NAcc) core (A–C) or shell (D–F). A and D: 5% Intralipid consumption following NAcc DAMGO infusion. Different letters indicate statistically significant differences. Mean ± SE lick rate (B and E) and meal duration (C and F) following NAcc DAMGO infusion. Asterisks indicate significant differences relative to saline control (Sal).
Infusion of DAMGO into the NAcc shell resulted in a more modest increase in overall levels of Intralipid consumption [Fig. 3D; main effect of DAMGO concentration, F(3,39) = 4.2, P < 0.05]. Licking rates were intermittently elevated following DAMGO infusion relative to control saline [Fig. 3E; significant interaction of DAMGO concentration and time; F(117, 1521) = 1.3, P < 0.05]. Infusion of DAMGO into the NAcc shell did not alter meal duration [Fig. 3F; F(3,39) = 1.0, P = 0.4].
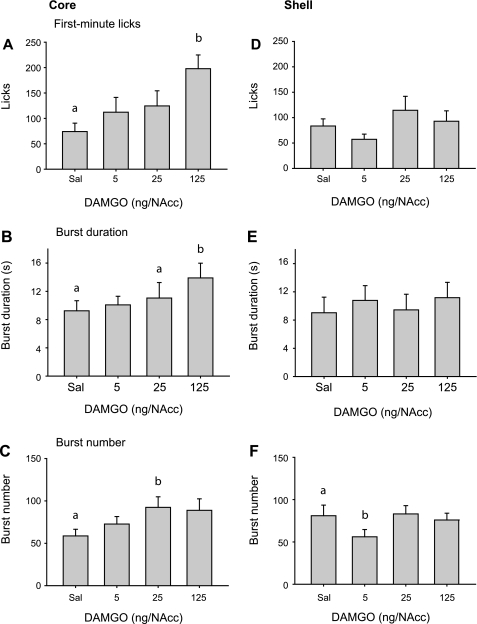
The effects of DAMGO infusion into the NAcc core on first-minute licks and burst duration (Fig. 4, A and B) paralleled the effects of increasing Intralipid concentration. Both measures increased dose dependently [main effect of DAMGO concentration; F(3, 54) = 3.9, P < 0.05 for first-minute licks; χ2(3) = 13.4, P < 0.01 for burst duration]. For both measures, post hoc testing indicated significant differences relative to saline infusion after the highest dose of DAMGO (P < 0.05, Holm-Sidak and Tukey tests, respectively). Burst number was significantly increased by DAMGO infusion into the NAcc core [main effect of concentration, F(3,54) = 3.4, P < 0.05], but differed significantly from saline infusion only for the 25 ng/NAcc dose of DAMGO (P < 0.05).
Fig. 4.
DAMGO effects on 5% Intralipid consumption after infusion into the NAcc core (A–C) or shell (D–F). Mean ± SE first-minute licks (A and D), burst duration (B and E), and burst number (C and F) following NAcc DAMGO infusion. Different letters indicate statistically significant differences.
Infusion of DAMGO into the NAcc shell had no significant effect on first-minute licks [Fig. 4D; F(3,39) = 1.5, P = 0.23] or burst duration [Fig. 4E; F(3,39) = 1.8, P = 0.17]. However, Intralipid consumption induced by the highest dose of DAMGO was significantly correlated with changes in burst duration (r = 0.57, P < 0.05; Pearson Product Moment Correlation; data not shown), demonstrating that intake driven by MOR stimulation was accompanied by longer burst durations. Burst number was significantly altered by DAMGO infusion into the NAcc shell [Fig. 4F; F(3,39) = 4.2, P < 0.05]. Post hoc testing indicated that burst number was significantly decreased following only the lowest dose of DAMGO relative to saline infusion (P < 0.05).
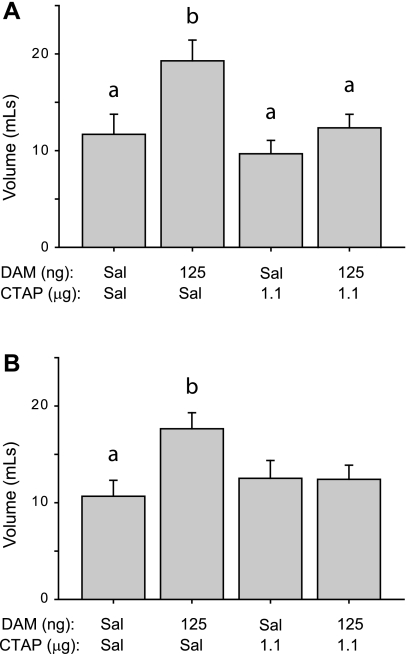
Pretreatment with the MOR-specific antagonist CTAP blocked Intralipid intake induced by DAMGO infusion into the core (Fig. 5A) or shell (Fig. 5B). For both infusion sites, there was a main effect of drug treatment [core, F(3, 43) = 5.5, P < 0.01; shell, F(3, 47) = 3.3, P < 0.05]. Post hoc testing indicated that Intralipid intake was significantly elevated after DAMGO infusion into the NAcc core relative to saline, CTAP, and DAMGO+CTAP infusions (P < 0.05). For NAcc shell infusions, post hoc testing indicated that DAMGO treatment significantly elevated consumption relative to saline treatment (P < 0.05).
Fig. 5.
Mean ± SE 5% Intralipid intake after DAMGO (DAM) and/or d-Phe-Cys-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP) infusion into the NAcc core (A) and the NAcc shell (B). Different letters indicate statistically significant differences.
Experiment 2: Effects of DAMGO Infusion into the NAcc Core on Consumption of Nonnutritive Olestra
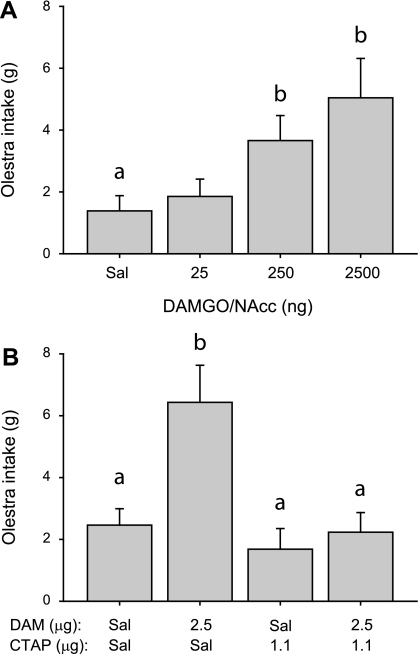
Infusion of DAMGO into the NAcc core more effectively increased Intralipid consumption than infusion into the shell (Figs. 3 and 4). We therefore targeted only core infusion sites in experiments 2 and 3. Infusion of DAMGO into the NAcc core dose dependently increased consumption of olestra during 30-min sessions [Fig. 6A; main effect of drug concentration, χ2(3) = 17.0, P < 0.001]. Intake after both the 250 and 2,500 ng/NAcc doses of DAMGO differed significantly from intake after saline infusion (post hoc Tukey test, P < 0.05).
Fig. 6.
A: mean ± SE olestra intake after DAMGO infusion into the NAcc core. B: mean olestra intake after DAMGO and/or CTAP infusion into the NAcc core. Different letters indicate statistically significant differences.
Pretreatment of the NAcc core with the MOR-specific antagonist CTAP blocked DAMGO-induced consumption of olestra [Fig. 6B; main effect of drug treatment, F(3,35) = 7.8, P < 0.001]. Post hoc testing indicated that consumption after DAMGO infusion was significantly higher than that following saline, CTAP, or DAMGO+CTAP infusion (P < 0.05).
Experiment 3: Interactions of MOR Stimulation in the NAcc Core with MTII Signaling
In experiment 1b, infusion of the highest dose of DAMGO (125 ng) into the NAcc core resulted in significantly elevated lick rates over a prolonged period (Fig. 3B), consistent with a DAMGO-induced suppression of satiety-related signals acting to curb intake (34, 36). To directly examine the ability of NAcc MOR stimulation to suppress or reverse satiety signals, we studied the effects of coinfusing DAMGO into the NAcc core and the melanocortin agonist MTII into the third ventricle.
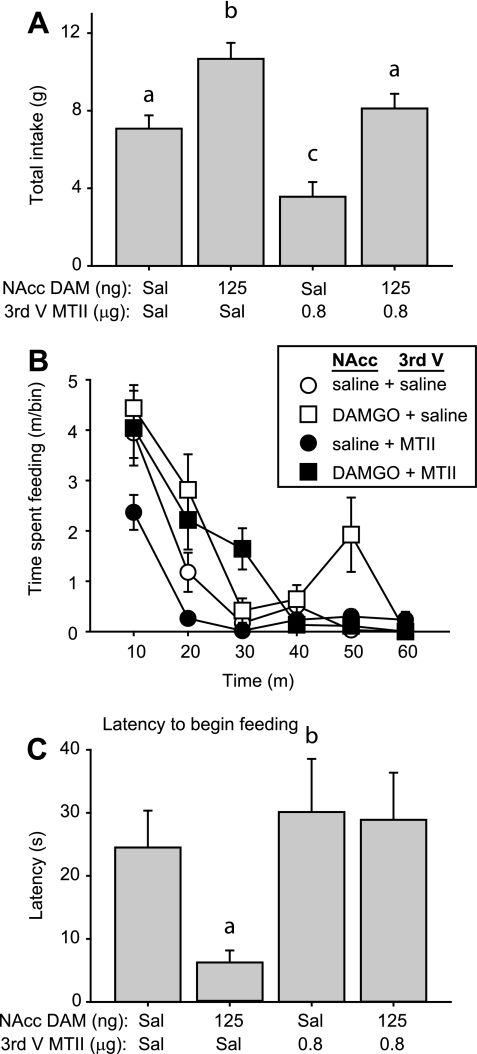
Drug treatment significantly altered consumption of high-fat chow consumed in a 1-h session [Fig. 7A; main effect of drug F(3, 45) = 14.9, P < 0.001]. DAMGO (NAcc) + saline (third ventricle) infusion significantly increased high-fat chow intake relative to that following control saline (NAcc and third ventricle) infusions (P < 0.05). Conversely, infusion of saline+MTII robustly decreased total intake relative to control infusions, causing a 50% decline in intake (mean ± SE of 7.1 ± 0.7 vs. 3.6 ± 0.8 g, P < 0.05). When DAMGO was coinfused with MTII, it reversed this suppression of food intake but did not cause hyperphagia; consumption was significantly higher than that following saline+MTII infusion, but still lower than that measured after DAMGO+saline infusions (both P < 0.05).
Fig. 7.
A: mean ± SE consumption of high-fat chow following drug infusion into the NAcc core (saline or 125 ng DAMGO/NAcc) and third ventricle (third V; saline or 0.8 μg MTII). B: time spent feeding during consecutive 10-min bins during 1-h test session. C: latency to begin feeding. Different letters indicate statistically significant differences.
Analysis of the time course of feeding revealed distinct temporal patterns of intake following each drug combination [Fig. 7B; significant interaction of drug × time, F(15, 225) = 3.4, P < 0.001]. Relative to saline-only infusions, DAMGO+saline infusion resulted in an elevation of consummatory behavior both early and late in the feeding session (significantly different from saline control at 20 and 50 min, P < 0.05), similar to DAMGO effects observed during Intralipid intake (Fig. 3B). MTII infusion into the third ventricle resulted in a rapid decline in ingestive behavior, significantly different from control values at the earliest time point measured (P < 0.05 relative to control at 10 m). Coinfusion of DAMGO reversed the MTII-mediated early suppression of intake, restoring the amount of time spent feeding to values that did not differ from those observed after DAMGO+saline (P > 0.05, NS, comparing feeding time after DAMGO+MTII to DAMGO+saline over 0–40 m). DAMGO+MTII infusion resulted in significantly more time spent feeding compared with intake after saline+MTII (significantly different from 10–30 m, P < 0.05) or control saline infusions (different at 30 m, P < 0.05). However, the late portion of DAMGO-induced food intake was suppressed by coinfusion of MTII (DAMGO+saline significantly different from DAMGO+MTII at 50 m, P < 0.05).
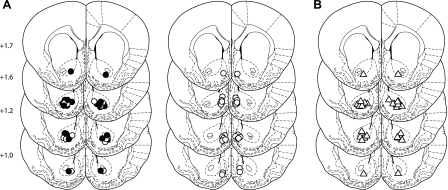
In some behavioral contexts, DAMGO infusion into the NAcc causes a transient suppression of feeding before subsequent hyperphagia (24, 41). However, we found that DAMGO+saline infusions resulted in a significant decrease in the latency to begin consumption of high-fat chow [Fig. 7C, main effect of drug χ2(3) = 15.7, P = 0.001]. MTII alone had no effect on the latency to begin feeding (P > 0.05 compared with control saline), but coinfusion of MTII with DAMGO reversed the rapid onset of feeding caused by DAMGO, restoring the latency to begin feeding to control levels (P > 0.05, compared with control saline). Infusion sites for all experiments were confined to targeted core and shell subregions of the NAcc (Fig. 8).
Fig. 8.
Infusion sites. A: circles indicate NAcc core (left) and shell (right) infusion sites for experiment 1b (lick microstructure). Black circles (NAcc core, left) indicate core infusion sites also used in experiment 2 (olestra intake). B: triangles indicate NAcc core infusion sites for experiment 3 (NAcc, DAMGO, and icv MTII coinfusion). Numbers (left) indicate anterioposterior position of coronal slices.
DISCUSSION
Our results demonstrate that Intralipid consumption induced by DAMGO infusion into the NAcc is associated with changes in lick microstructure suggestive of increased palatability, specifically, increased burst duration and first-minute licks. Both of these parameters, as well as the total volume of Intralipid consumed, were robustly increased following MOR stimulation in the NAcc core but not the shell. This finding is consistent with a previous mapping study demonstrating greater sensitivity of core vs. medial shell sites to DAMGO-induced hyperphagia (45). Notably, however, mapping studies utilizing morphine infusion to induce hyperphagia suggest that medial shell sites tend to be more effective than core sites in eliciting hyperphagia (2, 28). Pharmacological differences in the agonist as well as doses used may account for these divergent results.
Our finding of a primary role for the NAcc core in opioid-induced hyperphagia runs counter to the general proposal that shell and core regions show functional specialization for, respectively, reward-related processing and motor-associated function (16, 23, 43). Considerable evidence supports this hypothesis (8, 22, 33); however, our data suggest that opioid effects on food intake may not conform to this general pattern. Our results show that DAMGO infusion into the NAcc core potently increases palatability-associated lick microstructure parameters of burst duration and first-minute licks, while infusion into the medial shell has much more modest effects. Previous work has shown that positive taste reactivity displays are increased following DAMGO infusion into the NAcc shell (27); to our knowledge, however, similar experiments have not been carried out following infusion into the NAcc core, and so the relative sensitivity of these sites to the effects of MOR stimulation on hedonic taste reactivity is unknown. Interestingly, palatability-related electrophysiological responses are found in both the core and shell regions (30, 40), supporting potential roles for both areas in taste reward.
Because core infusions of DAMGO most effectively induced hyperphagia in lick microstructure experiments, we focused on this area in subsequent experiments. Taken together, results from these experiments support a two-part mechanism for hyperphagia elicited by stimulation of MORs in the NAcc core during consumption of a fatty tastant. Our analysis of lick microstructure showed that both burst duration and first-minute licks, each of which has previously been shown to covary with tastant palatability (14, 39), were dose dependently increased by infusion of DAMGO into the NAcc core (Fig. 4, A–B). These changes paralleled those observed after concentration-dependent increases in palatability (Fig. 2, A–B). In addition, NAcc MOR stimulation increased consumption of noncaloric olestra (Fig. 6). This latter finding suggests that orosensensory cues alone are a sufficient substrate for opioid-potentiated consumption, which clearly can occur in the absence of postingestive signaling derived from caloric content. Together these data provide evidence that increases in tastant palatability play a primary role in expression of NAcc DAMGO-induced hyperphagia for fatty foods.
Results from our lick microstructure experiments suggest that MOR stimulation in the NAcc core also attenuated satiety-related inhibitory feedback, resulting in extended periods of consumption. Inhibitory postingestive signaling decreases licking rates, meal duration, and burst number (13–15); decreases in the latter two measures were evident when comparing lick microstructure during 10% vs. 1% Intralipid intake (Figs. 1C and 2C). DAMGO infusion generally acted to counter these changes associated with satiety-related signaling. Infusion of a high dose of DAMGO (125 ng/NAcc) into the core resulted in sustained elevation of lick rates (Fig. 3B) and maintained meal duration and burst number at levels similar to those occurring after control saline infusion (Figs. 3C and 4C). In addition, infusion of NAcc DAMGO was able to reverse the suppressive effects of intracerebroventricular infusion of MTII on high-fat chow intake (Fig. 7A). These results show that suppression of satiety-related signaling contributes to the hyperphagia observed after NAcc DAMGO infusion.
Comparing NAcc DAMGO effects on intake of high-fat vs. high-carbohydrate foods.
The effects of NAcc DAMGO on lick microstructure during fatty tastant intake were qualitatively similar to those that occurred during sucrose consumption, which we described in a previous study (41). For both sucrose and Intralipid consumption, hyperphagia induced by DAMGO infusion into the NAcc was associated with increased burst duration, suggesting that palatability-related changes play a central role in increasing consumption after NAcc MOR stimulation, independent of the macronutrient content of the solution ingested.
Previous studies have shown that NAcc MOR stimulation preferentially increases high-fat chow intake, even in rats that show baseline preferences for a high-carbohydrate alternative (44). This suggests that mechanisms underlying hyperphagia mediated by NAcc MOR stimulation may not be mediated exclusively by changes to tastant palatability, provided that palatability itself is a principal driver of baseline food preference. In particular, this behavioral finding suggests that DAMGO is unlikely to act simply by potentiating palatability-related neural responses that have been identified in a subset of NAcc neurons (30, 40). Our results, however, suggest qualitatively similar changes relating chiefly to increases in palatability cause DAMGO-induced hyperphagia of both Intralipid and sucrose, albeit with some differences in the magnitude of the changes induced. A number of potential mechanisms could underlie the observed similarity in NAcc DAMGO effects on sucrose and Intralipid. It may be, for instance, that there is some segregation of palatability responses evoked by sweet and fatty tastants in the NAcc, and that these types of responses differ in their sensitivity to MOR stimulation. Electrophysiological studies of NAcc responses to fatty and sweet tastants will be important in addressing this possibility.
MOR stimulation in the NAcc core reverses melanocortin-driven satiety.
Results from lick microstructure studies suggested that, in addition to increasing palatability, DAMGO infusion into the NAcc core attenuated satiety-related inhibitory signaling. To determine whether NAcc DAMGO could attenuate pharmacologically induced satiety, we studied interactions of MOR stimulation of the NAcc core and intracerebroventricular administration of MTII, the melanocortin 3/4 agonist. It is well established that central melanocortin signaling plays a critical role in neural control of food intake (4, 9, 10, 17). Centrally administered melanocortin agonists robustly inhibit food intake, while the melanocortin inverse agonist agouti-related protein (AgRP) drives hyperphagia (18, 21, 25). Our focus in these experiments on the melanocortin pathway arose from previous observations that like NAcc MOR stimulation, AgRP-induced hyperphagia results in preferential intake of fatty foods, and moreover, that AgRP effects are dependent on opioid signaling (7, 20).
Coinfusion of DAMGO into the NAcc core with MTII into the third ventricle resulted in a reversal of the anorexigenic effects of MTII, which inhibited consumption of a high-fat chow when administered alone (Fig. 7A). DAMGO coinfusion did not increase consumption of chow beyond control levels, however; the combined effect of the two drugs was very close to the sum of the two drugs when administered alone. Temporal analysis of feeding showed that DAMGO was effective in reversing the early hypophagia (≤20 min) apparent when MTII was administered alone (Fig. 7B). However, the effects of MTII predominated later in the session (at 50 min), blocking DAMGO-induced consumption.
Our results differ from those recently reported by Zheng et al. (48). In that study, administration of as little as 0.02 nmol of MTII into the lateral ventricle resulted in robust inhibition of high-fat chow intake induced by unilateral infusion of DAMGO (250 ng) into the NAcc, while a higher dose of 0.5 nmol of MTII completely blocked DAMGO effects. In our experiment a dose of 0.8 μg MTII (0.8 nmol), while potently suppressing high-fat chow when infused alone (50% decrease in intake from control levels), had only partial effects on DAMGO-induced consumption, blocking the late but not early periods of hyperphagia.
A number of differences between experimental paradigms might contribute to these divergent outcomes, including different infusion sites for MTII (third ventricle in our study vs. lateral ventricle) and DAMGO (bilateral core vs. unilateral shell), as well as differences in the timing of drug infusion (concurrently vs. MTII 15 min before DAMGO). MOR stimulation in the core, rather than the shell, is more effective in stimulating high-fat chow intake (45), and this may have contributed to the absence of DAMGO-induced reversal of MTII effects in the Zheng et al. (48) study. A further difference in the behavioral paradigm used may be particularly relevant. Zheng et al. allowed rats an initial 30-min period of access to high-fat chow prior to drug infusion and subsequent measurement of further intake during a 2-h session. The substantially increased postingestive load occurring after consumption during this presatiation period may account both for the increased potency of MTII and the inability of NAcc DAMGO to reverse MTII effects in their study. Consistent with this interpretation, NAcc DAMGO was able to reverse the early but not late effects of MTII administration in our study.
It is interesting to consider the potential mechanisms through which NAcc DAMGO alters both palatability and satiety-related signaling. An obvious possibility is that initial changes in palatability are required for ensuing changes in satiety and that the DAMGO-induced increases in the hedonic impact of food items drive suppression of satiety signaling. However, an alternative possibility is that NAcc MOR stimulation elicits changes in palatability and satiety signaling in parallel, rather than through serial signaling. Consistent with this possibility, previous studies have shown that NAcc DAMGO effects on hyperphagia and taste reactivity can be dissociated anatomically (27, 38). MOR stimulation in a circumscribed portion of the NAcc shell increases both food intake and positive taste reactivity; however, stimulation in the surrounding volume of the NAcc increases food intake without concurrent increases in taste reactivity. Provided that changes in taste reactivity are a faithful index of palatability (5), this result suggests that NAcc MOR stimulation is capable of eliciting increased consumption independent of changes in palatability.
Perspectives and Significance
The growing prevalence of overweight and obesity poses a dangerous threat to health. Palatability-driven consumption of abundantly available, inexpensive, energy-dense foods is an important contributor to intake in excess of metabolic demand (26, 47). MOR stimulation in the NAcc may play an important role in this process, as it drives an intense hyperphagia and, moreover, selectively promotes consumption of high-fat foods, which are an important driver of weight gain in humans (6, 24, 44). Our results demonstrate that MOR stimulation in the NAcc core induces hyperphagia, driven by increased palatability of fatty tastants, independent of postingestive caloric signaling. Furthermore, we show that MOR activation in the NAcc core is sufficient to partially reverse satiety signaling induced by the melanocortin agonist MTII. Future work focused on mechanisms through which MOR stimulation suppresses satiety signaling will be important in understanding root causes of weight gain caused by palatability-driven intake.
GRANTS
This research was supported by National Institute for Mental Health Grant MH-082325, and funds from the Office of the Vice-President for Research at the University of Utah, the Brain & Behavior Research Foundation, and the March of Dimes.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Andrea Schwager and Vicki Skelton for critical comments on drafts of this manuscript.
REFERENCES
- 1. Baird JP, St John SJ, Nguyen EA. Temporal and qualitative dynamics of conditioned taste aversion processing: combined generalization testing and licking microstructure analysis. Behav Neurosci 119: 983–1003, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Bakshi VP, Kelley AE. Striatal regulation of morphine-induced hyperphagia: an anatomical mapping study. Psychopharmacology (Berl) 111: 207–214, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 191: 439–459, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Benoit SC, Schwartz MW, Lachey JL, Hagan MM, Rushing PA, Blake KA, Yagaloff KA, Kurylko G, Franco L, Danhoo W, Seeley RJ. A novel selective melanocortin-4 receptor agonist reduces food intake in rats and mice without producing aversive consequences. J Neurosci 20: 3442–3448, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev 24: 173–198, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Blundell JE, Lawton CL, Cotton JR, Macdiarmid JI. Control of human appetite: implications for the intake of dietary fat. Annu Rev Nutr 16: 285–319, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Brugman S, Clegg DJ, Woods SC, Seeley RJ. Combined blockade of both micro - and κ-opioid receptors prevents the acute orexigenic action of Agouti-related protein. Endocrinology 143: 4265–4270, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Carlezon WA, Jr, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci 16: 3112–3122, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clegg DJ, Benoit SC, Air EL, Jackman A, Tso P, D'Alessio D, Woods SC, Seeley RJ. Increased dietary fat attenuates the anorexic effects of intracerebroventricular injections of MTII. Endocrinology 144: 2941–2946, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411: 480–484, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Davis JD. The effectiveness of some sugars in stimulating licking behavior in the rat. Physiol Behav 11: 39–45, 1973 [DOI] [PubMed] [Google Scholar]
- 12. Davis JD. Some new developments in the understanding of oropharyngeal and postingestional controls of meal size. Nutrition 15: 32–39, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Davis JD, Campbell CS. Peripheral control of meal size in the rat. Effect of sham feeding on meal size and drinking rate. J Comp Physiol Psychol 83: 379–387, 1973 [DOI] [PubMed] [Google Scholar]
- 14. Davis JD, Perez MC. Food deprivation- and palatability-induced microstructural changes in ingestive behavior. Am J Physiol Regul Integr Comp Physiol 264: R97–R103, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Davis JD, Smith GP, Sayler JL. Closing the pylorus decreases the size of large meals in the rat. Physiol Behav 63: 191–196, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Deutch AY, Cameron DS. Pharmacological characterization of dopamine systems in the nucleus accumbens core and shell. Neuroscience 46: 49–56, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Ellacott KL, Cone RD. The central melanocortin system and the integration of short- and long-term regulators of energy homeostasis. Recent Prog Horm Res 59: 395–408, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385: 165–168, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 143: 263–279, 1978 [DOI] [PubMed] [Google Scholar]
- 20. Hagan MM, Rushing PA, Benoit SC, Woods SC, Seeley RJ. Opioid receptor involvement in the effect of AgRP-(83–132) on food intake and food selection. Am J Physiol Regul Integr Comp Physiol 280: R814–R821, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, Van Der Ploeg LH, Woods SC, Seeley RJ. Long-term orexigenic effects of AgRP-(83–132) involve mechanisms other than melanocortin receptor blockade. Am J Physiol Regul Integr Comp Physiol 279: R47–R52, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Kelley AE. Functional specificity of ventral striatal compartments in appetitive behaviors. Ann NY Acad Sci 877: 71–90, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev 27: 765–776, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav 76: 365–377, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Lu XY, Nicholson JR, Akil H, Watson SJ. Time course of short-term and long-term orexigenic effects of Agouti-related protein (86–132). Neuroreport 12: 1281–1284, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr 139: 629–632, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do μ-opioids cause increased hedonic impact of sweetness? J Neurosci 25: 11777–11786, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Res 863: 71–86, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Ragnauth A, Moroz M, Bodnar RJ. Multiple opioid receptors mediate feeding elicited by μ- and δ-opioid receptor subtype agonists in the nucleus accumbens shell in rats. Brain Res 876: 76–87, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron 45: 587–597, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Sclafani A. Fat and sugar flavor preference and acceptance in C57BL/6J and 129 mice: experience attenuates strain differences. Physiol Behav 90: 602–611, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci 23: 6295–6303, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith GP. The direct and indirect controls of meal size. Neurosci Biobehav Rev 20: 41–46, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Smith GP. John Davis and the meanings of licking. Appetite 36: 84–92, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Smith GP. (Editor). Satiation: From Gut to Brain. New York: Oxford University Press, 1998 [Google Scholar]
- 37. Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci 27: 1594–1605, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci 25: 8637–8649, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci 112: 678–694, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Taha SA, Fields HL. Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J Neurosci 25: 1193–1202, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taha SA, Katsuura Y, Noorvash D, Seroussi A, Fields HL. Convergent, not serial, striatal and pallidal circuits regulate opioid-induced food intake. Neuroscience 161: 718–733, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Will MJ, Pratt WE, Kelley AE. Pharmacological characterization of high-fat feeding induced by opioid stimulation of the ventral striatum. Physiol Behav 89: 226–234, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience 50: 751–767, 1992 [DOI] [PubMed] [Google Scholar]
- 44. Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by μ-opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther 285: 908–914, 1998 [PubMed] [Google Scholar]
- 45. Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal μ-opioid stimulation: microinjection mapping and fos expression. Neuroscience 99: 267–277, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a μ-opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 159: 415–423, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Zheng H, Berthoud HR. Neural systems controlling the drive to eat: mind versus metabolism. Physiology (Bethesda) 23: 75–83, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Zheng H, Townsend RL, Shin AC, Patterson LM, Phifer CB, Berthoud HR. High-fat intake induced by μ-opioid activation of the nucleus accumbens is inhibited by Y1R-blockade and MC3/4R-stimulation. Brain Res, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]