Abstract
Sex differences in sympathetic neural control during static exercise in humans are few and the findings are inconsistent. We hypothesized women would have an attenuated vasomotor sympathetic response to static exercise, which would be further reduced during the high sex hormone [midluteal (ML)] vs. the low hormone phase [early follicular (EF)]. We measured heart rate (HR), blood pressure (BP), and muscle sympathetic nerve activity (MSNA) in 11 women and 10 men during a cold pressor test (CPT) and static handgrip to fatigue with 2 min of postexercise circulatory arrest (PECA). HR increased during handgrip, reached its peak at fatigue, and was comparable between sexes. BP increased during handgrip and PECA where men had larger increases from baseline. Mean ± SD MSNA burst frequency (BF) during handgrip and PECA was lower in women (EF, P < 0.05), as was ΔMSNA-BF smaller (main effect, both P < 0.01). ΔTotal activity was higher in men at fatigue (EF: 632 ± 418 vs. ML: 598 ± 342 vs. men: 1,025 ± 416 a.u./min, P < 0.001 for EF and ML vs. men) and during PECA (EF: 354 ± 321 vs. ML: 341 ± 199 vs. men: 599 ± 327 a.u./min, P < 0.05 for EF and ML vs. men). During CPT, HR and MSNA responses were similar between sexes and hormone phases, confirming that central integration and the sympathetic efferent pathway was comparable between the sexes and across hormone phases. Women demonstrated a blunted metaboreflex, unaffected by sex hormones, which may be due to differences in muscle mass or fiber type and, therefore, metabolic stimulation of group IV afferents.
Keywords: cold pressor test, muscle sympathetic nerve activity, metaboreflex
premenopausal women typically have lower resting blood pressure (BP) (9, 14, 17) and lower incidence of cardiovascular disease (24) than similarly aged men, but the underlying mechanisms are not completely understood. The hormone profile of premenopausal women suggests that estrogen and/or progesterone provide a degree of cardioprotection (27, 39). BP reactivity has been described as one predictor of the future development of hypertension (1, 8). Based on this, we would expect to see sex differences in BP responses to tests such as static handgrip exercise, which is one way to assess BP control through a pressor response.
Static exercise invokes increases in heart rate (HR), BP, and muscle sympathetic nerve activity (MSNA) through two neural pathways: central command and the exercise pressor reflex (4, 12, 15). The exercise pressor reflex is a feedback system arising from mechano- (group III) and metabosensitive (group IV) afferent nerve endings within the skeletal muscle (4, 15). This feedback loop increases BP through increases in MSNA, which is one determinant of vasoconstriction in nonexercising muscles.
Previous studies regarding the effects of sex on sympathetic neural control during static exercise in humans are few and the findings are inconsistent. For example, it was found that women and men responded with comparable increases in MSNA during 1 min of static handgrip exercise, when changes were examined as a percent increase from baseline (18). In contrast, Ettinger et al. (6) reported that cardiovascular and vasomotor sympathetic responses to static handgrip were attenuated in women. Additionally, the same group reported that increases in MSNA during static handgrip varied with the phase of the menstrual cycle (7). MSNA, but not cardiovascular responses, were attenuated during the late follicular phase (10 to 12 days after the onset of menstruation, high estrogen/low progesterone) compared with the early follicular phase (EF; 1 to 4 days after the onset of menstruation, low estrogen/low progesterone) of the menstrual cycle (7). These studies, however, are difficult to interpret and do not allow for a direct comparison as they have not controlled for menstrual cycle status (18), use of hormonal birth control (18), use of hormone replacement therapy (6), have not performed static handgrip to fatigue to reach a common metabolic endpoint (6, 7), or have not provided a male cohort for direct sex comparisons (7).
Thus, based on the current literature, it is not clear whether true sex differences exist and how both estrogen and progesterone influence static handgrip outcomes. Our study differs from those conducted previously because we have 1) controlled for hormone status by excluding individuals taking oral contraceptives and hormone replacement therapies; 2) tested young premenopausal women during the low and high hormone phases of the menstrual cycle; 3) included a cohort of men for sex comparisons; and 4) had subjects perform static handgrip until fatigue so that all subjects reached a common metabolic end point (30). The purpose of this study was to test the hypothesis that premenopausal women would demonstrate an attenuated cardiovascular and vasomotor sympathetic response during static handgrip to fatigue and postexercise circulatory arrest (PECA) compared with men. We also hypothesized that the influence of the high sex hormone phase [midluteal (ML)] of the menstrual cycle in women would result in further blunting of these responses compared with the low hormone phase (EF).
MATERIALS AND METHODS
Subjects
Twenty-one (11 women, 10 men) healthy volunteers were studied. Descriptive characteristics of the subjects are outlined in Table 1. Exclusionary criteria included: significant medical history, smoking, recreational drug use, hormonal contraceptive use within the previous 6 months, and current pregnancy. Additionally, subjects that were endurance trained were excluded from participation. All female participants were normally menstruating (i.e., ∼28-day cycle). All subjects gave written informed consent to participate in the study, which was approved by the Institutional Review Boards at the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas.
Table 1.
Subject characteristics
| Women |
|||
|---|---|---|---|
| Variables | EF | ML | Men |
| Age, yr | 33 ± 10 | 33 ± 10 | 32 ± 10 |
| Height, cm | 165.9 ± 5.9* | 165.5 ± 6.1* | 176.0 ± 6.0 |
| Weight, kg | 63.7 ± 7.5* | 63.8 ± 7.7* | 79.1 ± 12.0 |
| Body surface area, m2 | 1.7 ± 0.1* | 1.7 ± 0.1* | 2.0 ± 0.2 |
| Body mass index, kg/m2 | 23.1 ± 2.1 | 23.3 ± 2.1 | 25.3 ± 3.1 |
Values are means ± SD; n = 11 women, 10 men.
EF, early follicular phase; ML, midluteal phase.
Different from men, P < 0.01.
Measurements
HR and BP.
HR was determined from lead II of the electrocardiogram (ECG). BP was assessed using two methods. Beat-by-beat arterial pressure [systolic BP (SBP); diastolic BP (DBP)] was estimated noninvasively by using finger photoplethysmography (model 1, Nexfin HD monitor; BMEYE, Amsterdam, The Netherlands). This method was used to examine BP changes during static handgrip to fatigue and the cold pressor test (CPT). BP was also obtained via electrosphygmomanometry (model 4240; SunTech Medical Instruments, Raleigh, NC) with a microphone placed over the brachial artery to detect Korotkoff sounds. This method was used to obtain baseline BP values when the subject was initially instrumented in our laboratory to ensure a resting steady state had been achieved before data collection began. The BP values we report were derived from the beat-by-beat determinations.
MSNA.
MSNA signals were obtained using the microneurographic technique (35). Briefly, a recording electrode was placed in the peroneal nerve at the popliteal fossa and a reference electrode was placed subcutaneously 2–3 cm from the recording electrode. The nerve signals were amplified (gain 70,000–160,000), band-pass filtered (700–2,000 Hz), full-wave rectified, and integrated with a resistance-capacitance circuit (time constant 0.1 s). Criteria for adequate MSNA recording included: 1) pulse synchrony, 2) facilitation during the hypotensive phase of the Valsalva maneuver and suppression during the hypertensive overshoot after release, 3) increases in response to breath holding, and 4) insensitivity to emotional stimuli (35).
Protocol
The first visit (screening) consisted of a 12-lead resting ECG and supine BP measurements. The second visit was comprised of the static handgrip and CPTs. Female subjects completed the second visit twice since they were tested once during the EF phase (days 1–4 when both estrogen and progesterone are low) of their menstrual cycles and once during the ML phase (days 19–22 when both sex hormones are high), with the order counterbalanced. The cycle phase was determined by the onset of menstruation and by detection of the luteinizing hormone surge by an ovulation prediction kit (OvuQuick; Quidel, San Diego, CA). Hormone concentrations were verified on each study day. For the 2 days prior to testing, all subjects were on an isocaloric constant diet consisting of 200 meq sodium, 100 meq potassium, and 1,000 mg calcium, while water intake was ad libitum. Female subjects submitted a urine sample for pregnancy testing prior to any of the experimental procedures.
The experiment was performed ≥ 2 h after a light meal and ≥ 48 h after the last caffeinated or alcoholic beverage was consumed. The laboratory was environmentally controlled with an ambient temperature of ∼25°C. Each subject was studied in the supine position. Prior to microneurography, subjects performed three brief (∼3 s) maximal contractions with his/her dominant hand to determine his/her maximal voluntary contraction (MVC) by using a handgrip dynamometer. Baseline data collection began at least 10 min after an acceptable nerve recording was obtained.
CPT.
We used the CPT as a secondary probe of sympathetic neural control between the sexes, as others have used it to assess the central integration of vasomotor processes and their efferent pathways (26, 31, 37). CPT-induced increases in BP and HR involve the activation of the rostral ventrolateral medulla and the nucleus ambiguous (26), two brain structures responsible for pressor and tachycardic responses.
Following 1 min of baseline, the dominant hand was immersed up to the wrist in an ice water bath (4°C) for 2 min, which was followed by 3 min of recovery. Subjects were instructed to avoid breath holding (confirmed by nasal cannula) and to stay as relaxed as possible.
Static handgrip to fatigue.
After at least a 5-min recovery phase from the CPT, static handgrip was performed at 40% of MVC until fatigue. This level of force was chosen since it has been previously shown that handgrip sustained at 40% and 60% of MVC elicited comparable increases in BP and MSNA at fatigue (30). Since individuals can sustain 40% of MVC for a longer period of time, this allowed for a better assessment of the time course, as well as for comparisons between studies (i.e., studies that stopped handgrip at 2 min). Once the exerted force declined to < 80% of the desired force for ≥ 2 s, a 2-min PECA (with an upper arm cuff inflated to 250 mmHg) phase began. During static handgrip exercise and PECA, subjects were instructed to avoid breath holding (confirmed by nasal cannula).
Data Analysis
Data were sampled at 625 Hz with a commercial data acquisition system (Biopac Systems, Santa Barbara, CA) and analyzed using LabView Software (National Instruments, Austin, TX). Beat-by-beat HR was calculated from the R-R interval of the ECG. Beat-by-beat SBP and DBP were estimated from the arterial waveforms.
MSNA bursts were identified by a computer program using a 3:1 signal-to-noise ratio threshold within a 0.5-s search window and an expected burst reflex latency of 1.3 s from the preceding R-wave (5). All bursts were confirmed by trained personnel. Burst areas of the integrated neurogram and BP were measured simultaneously on a beat-to-beat basis. Burst frequency (BF) was defined as the number of bursts per min, and burst incidence (BI) was used to normalize BF per 100 heart beats. Total activity was defined as the burst area of the rectified and integrated neurogram. We used a modified method developed by Sugiyama et al. (34) and Halliwill (13) where we assigned the largest burst amplitude during baseline a value of 100. Therefore, all other bursts within a testing session were normalized against this value. For comparisons between groups (men vs. women) and across sessions (EF vs. ML) we report the change in total activity from baseline (Δtotal activity).
Baseline MSNA, HR, and BP for both the static handgrip exercise and CPT were averaged for 1 min. The total handgrip time was divided evenly into five stages, and the data were presented as 20, 40, 60, 80, and 100% (at fatigue) (30). Stages during handgrip were divided accordingly because subjects performed handgrip until fatigue and this served as a way to normalize for intersubject variability. PECA 1, PECA 2, and recovery (REC 1, REC 2, REC 3) data were averaged each minute. Stages for the CPT were averaged every 30 s.
Statistical Analysis
Data are expressed as mean ± SD. A two-way repeated-measures ANOVA [group (men, EF, ML) × stage] was used to examine cardiovascular and MSNA responses to the static handgrip exercise and CPT between the sexes and menstrual phases. A separate two-way repeated-measures ANOVA [group (men, EF, ML) × stage] was used to assess differences between the sexes in terms of their response to these perturbations by examining the change (Δ) from baseline for each variable. Tukey's post hoc analysis was used when significance was found. All statistical analyses were performed using SigmaStat 3.11 (Systat Software, San Jose, CA). A P value of <0.05 was considered statistically significant.
RESULTS
Hormone Analyses
Estrogen was lower during the EF phase compared with the ML phase (32.4 ± 8.9 vs. 91.8 ± 46.7 pmol/l; P < 0.01). Progesterone was also lower during the EF phase (0.9 ± 0.5 vs. 11.1 ± 5.7 nmol/l; P < 0.01).
Static Handgrip to Fatigue
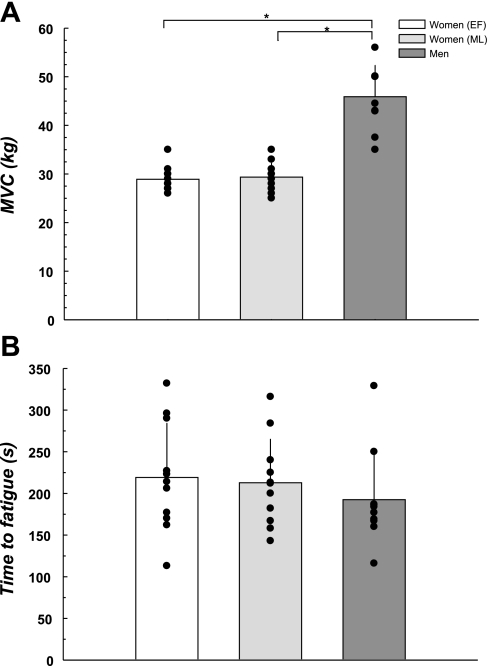
Figure 1 illustrates that the men had higher MVC compared with the women during both phases of the menstrual cycle (both P < 0.05) and that menstrual cycle phase did not influence MVC in the women (P = 0.43). The time to fatigue (Fig. 1) was comparable between the sexes and menstrual phases (P = 0.61).
Fig. 1.
Maximal voluntary contraction (MVC) and time to fatigue. A: MVC was greater in men compared with the women during both the low and high hormone phases of the menstrual cycle. B: time to fatigue during the static handgrip exercise was neither different between the groups nor affected by menstrual cycle phase. EF, early follicular; ML, midluteal. *Difference from the men, P < 0.05.
HR
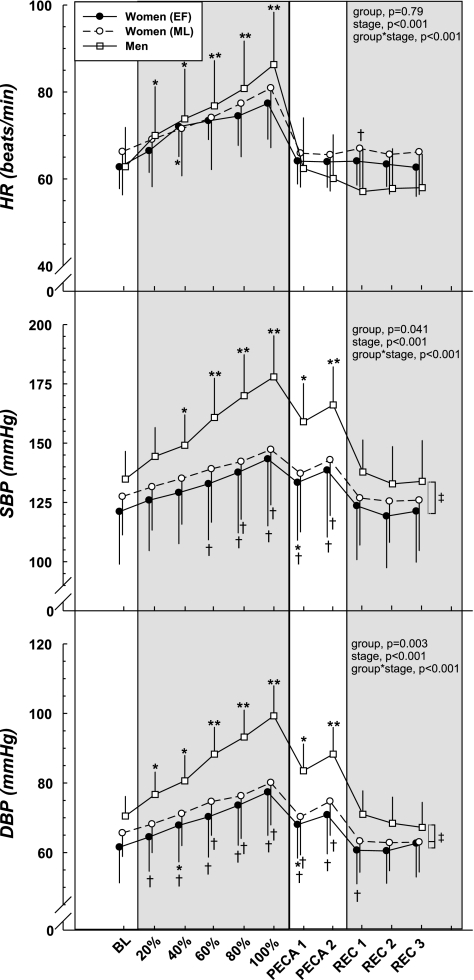
The cardiovascular variables in response to static handgrip exercise are illustrated in Table 2 and Fig. 2. HR increased progressively during handgrip and reached its peak at fatigue in all the subjects (P < 0.001). The absolute HR at fatigue was not different between the sexes; however, the increase in HR from baseline to fatigue (Table 2) was greater in men than in women (both phases P < 0.01). There was no difference in HR response from baseline to fatigue between the two phases of the menstrual cycle (P = 0.47). Finally, HR returned to baseline values during PECA in both women and men, with no difference between phases of the menstrual cycle.
Table 2.
Cardiovascular and vasomotor sympathetic outflow changes during static handgrip exercise and cold pressor test (CPT)
| Static Handgrip |
|||
|---|---|---|---|
| Variables | 100%, at fatigue | PECA 2 | CPT Peak |
| ΔHR, beats/min | |||
| Women, EF | 15 ± 7* | 1 ± 5 | 12 ± 7 |
| Women, ML | 12 ± 9* | −1 ± 6 | 13 ± 7 |
| Men | 24 ± 6 | −3 ± 6 | 11 ± 7 |
| ΔSBP, mmHg | |||
| Women, EF | 22 ± 13* | 17 ± 12* | 16 ± 12* |
| Women, ML | 16 ± 12* | 15 ± 13* | 18 ± 16* |
| Men | 43 ± 13 | 31 ± 10 | 31 ± 10 |
| ΔDBP, mmHg | |||
| Women, EF | 16 ± 6* | 9 ± 5* | 8 ± 7* |
| Women, ML | 12 ± 7* | 9 ± 7* | 9 ± 8* |
| Men | 29 ± 6 | 18 ± 6 | 20 ± 6 |
| ΔMSNA-BF, bursts/min | |||
| Women, EF | 18 ± 7* | 11 ± 8* | 18 ± 14 |
| Women, ML | 20 ± 12* | 10 ± 7* | 16 ± 11 |
| Men | 30 ± 7 | 18 ± 9 | 26 ± 10 |
| ΔMSNA-BI, bursts/100 heart beats | |||
| Women, EF | 20 ± 10 | 17 ± 14* | 24 ± 17 |
| Women, ML | 24 ± 16 | 16 ± 13* | 21 ± 16* |
| Men | 29 ± 7 | 31 ± 14 | 40 ± 18 |
| ΔTotal activity, a.u./min | |||
| Women, EF | 632 ± 418* | 354 ± 321* | 675 ± 785 |
| Women, ML | 598 ± 342* | 341 ± 199* | 504 ± 528 |
| Men | 1025 ± 416 | 599 ± 327 | 798 ± 575 |
All values are the differences from baseline, means ± SD.
PECA, postexercise circulatory arrest; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MSNA, muscle sympathetic nerve activity; BF, burst frequency; BI, burst incidence; a.u., arbitrary units.
Difference from men within stage, P < 0.05. Peak for CPT was defined as: HR, 0.5 min; SBP and DBP, 1.5 min; MSNA, 1.5 min.
Fig. 2.
Cardiovascular responses during static handgrip exercise and postexercise circulatory arrest (PECA). Static handgrip elicited increases in heart rate (HR), systolic blood pressure (SBP), and diastolic BP (DBP) where men demonstrated higher BP than the women. Similarly, PECA induced higher BP compared with baseline (BL) in both sexes with the women showing an attenuated response compared with the men. Low vs. high hormone status did not affect the responses to either static handgrip or PECA in women. REC, recovery. *Group difference compared with BL, P < 0.05. **Difference from BL (all groups), P < 0.05. †Sex difference within stage, P < 0.05. ‡Group difference (main effect), P < 0.05.
BP
The BP responses to static handgrip and circulatory arrest are shown in Table 2 and Fig. 2. SBP and DBP in all groups was higher at fatigue (all P < 0.001) and during PECA (all P < 0.001) compared with baseline. The men had higher SBP and DBP compared with women at fatigue and PECA (all P < 0.01), as well as demonstrated a greater ΔSBP and ΔDBP from baseline to fatigue and during PECA (all P < 0.01). Low vs. high sex hormone status did not influence the absolute BP or the magnitude of the change in BP in women.
MSNA
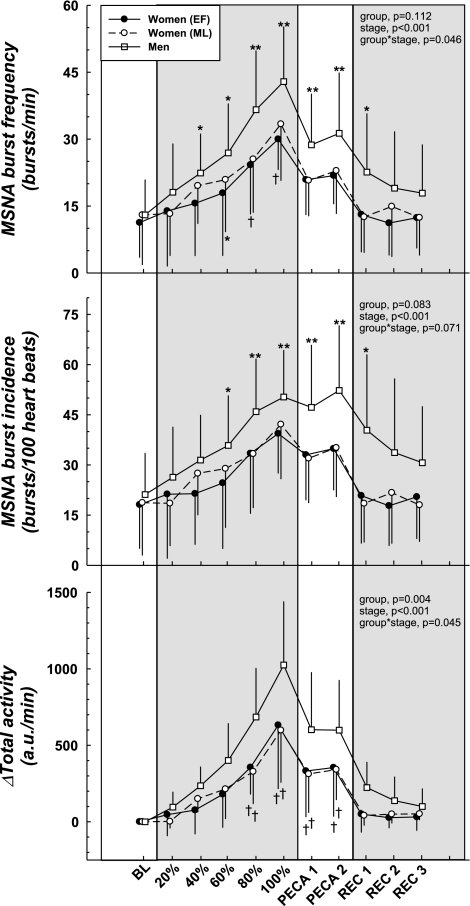
MSNA (Table 2 and Fig. 3) progressively increased during static handgrip in both sexes, reached the peak at fatigue, and remained elevated during PECA compared with baseline (all P < 0.05). The absolute MSNA-BF during handgrip was lower in women during the low hormone phase (EF, P = 0.01), as was the ΔMSNA-BF smaller (main effect, both P < 0.01) compared with the men. ΔMSNA-BI was attenuated in women compared with the men during both the low and high hormone phases (main effect, both P < 0.05). The women had a smaller Δtotal activity (for both EF and ML) compared with the men (all P < 0.05). The low vs. high sex hormone phase did not influence any of the MSNA responses to static handgrip or PECA.
Fig. 3.
Muscle sympathetic nerve activity (MSNA) responses during static handgrip exercise and PECA. Static handgrip elicited increases in MSNA-BF and MSNA-BI where men demonstrated higher values than the women. ΔTotal activity was also greater in the men compared with the women. Similarly, PECA induced higher MSNA compared with BL in both sexes with the women showing an attenuated response compared with the men. Low vs. high hormone phase of the menstrual cycle did not affect the responses to either handgrip or PECA in women. a.u., Arbitrary units. BF, burst frequency; BI, burst incidence. *Group difference compared with BL, P < 0.05. **Difference from BL (all groups), P < 0.05. †Sex difference within stage, P < 0.05. ‡Group difference (main effect), P < 0.05.
Vascular Transduction
During static handgrip (at fatigue and during PECA 2) the change in MSNA and DBP from baseline was lower in the women. However, there was no difference in the vascular transduction (ΔDBP/Δtotal activity) between the sexes in either condition (EF: 0.03 ± 0.02 units vs. ML: 0.04 ± 0.05 vs. men: 0.03 ± 0.01 at fatigue, P = 0.66; EF: 0.04 ± 0.04 vs. ML: 0.05 ± 0.07 vs. men: 0.04 ± 0.02 units during PECA2, P = 0.69).
CPT
HR.
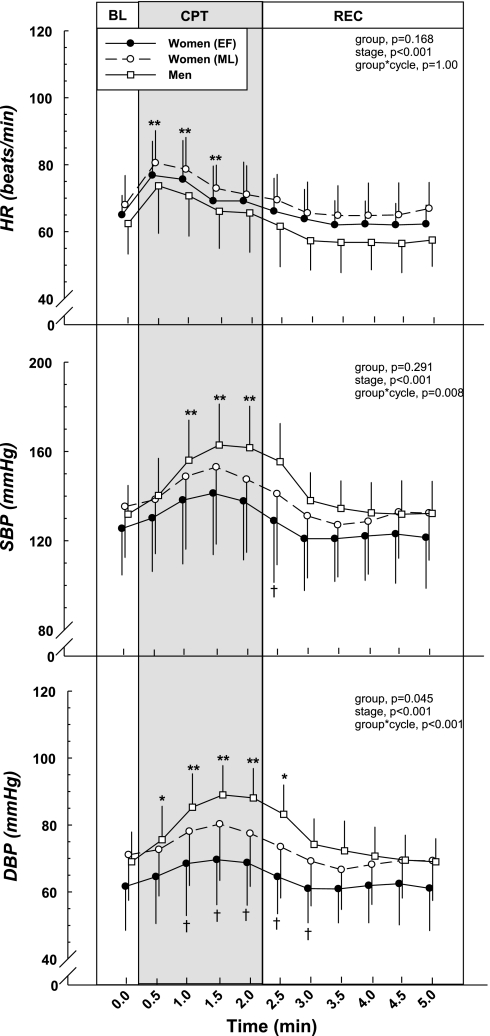
Figure 4 shows the cardiovascular responses to the CPT. HR increased immediately and was significantly higher compared with baseline during the CPT (P < 0.001). The change from baseline (ΔHR, Table 2) was similar between men and women, as well as between phases of the menstrual cycle.
Fig. 4.
Cardiovascular responses during the cold pressor test (CPT). The cold pressor stimulus elicited increases in HR, SBP, and DBP. HR responses were comparable between the sexes and between the low vs. high hormone phases of menstrual cycle. However, men demonstrated greater changes from BL in SBP and greater DBP compared with the women. *Group difference compared with BL, P < 0.05. **Difference from BL (all groups), P < 0.05. †Sex difference within stage (absolute difference), P < 0.05.
BP.
SBP and DBP (Table 2 and Fig. 4) also increased during the CPT in both groups (all P < 0.05). The magnitude of the change compared with baseline (ΔSBP and ΔDBP, Table 2) was significantly higher in the men during the peak response (at 1.5 min) compared with the women during both phases of the menstrual cycle (all P < 0.05). The absolute DBP was higher in men compared with women in the EF during the CPT (all P < 0.05). Although it appears that absolute SBP and DBP was higher during the high hormone phase compared with the low hormone phase of the menstrual cycle in the women, there was no statistical difference. The women demonstrated similar increases (Δ) in SBP and DBP during the both low and high hormone phases of the menstrual cycle.
MSNA.
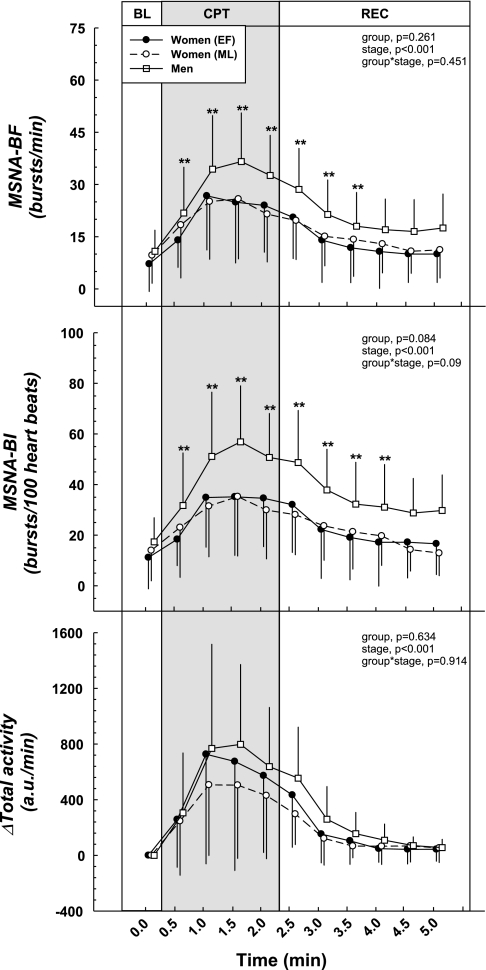
MSNA is illustrated in Fig. 5. MSNA-BF was significantly higher during the CPT compared with baseline (P < 0.001). MSNA-BI showed a similar trend (P < 0.001). ΔMSNA-BF was similar between the sexes; however, the men had a significantly greater ΔMSNA-BI compared with women in the ML phase because women had higher HRs (Table 2, P < 0.05). ΔTotal activity was not different between the sexes. Low vs. high sex hormone status did not affect any of the MSNA variables in the women.
Fig. 5.
MSNA responses during the CPT. The cold pressor stimulus elicited increases in MSNA-BF and MSNA-BI. No cycle phase × stage interaction was present, indicating MSNA responses were comparable between the sexes and between the low vs. high hormone phases of the menstrual cycle. ΔMSNA-BI was significantly different between the men and women during the ML phase, which was likely the result of a higher HR in the women. ΔTotal activity was similar between the sexes and menstrual phases. **Difference from BL (all groups), P < 0.05.
DISCUSSION
The present study is the first to comprehensively exam responses to static handgrip exercise and a cold pressor stimulus in a group of men and women, while controlling for low vs. high hormone phases in the women. The principal findings from the present study are that 1) women demonstrate attenuated BP and MSNA responses during static handgrip to fatigue and during PECA compared with men; 2) women and men demonstrate comparable increases in MSNA (ΔMSNA-BF and Δtotal activity) in response to a cold pressor stimulus; and 3) low vs. high sex hormone exposure does not influence the cardiovascular or vasomotor sympathetic responses to static handgrip exercise or to the CPT in women. Our results support the hypothesis that women would demonstrate a blunted sympathetic response during static handgrip to fatigue compared with the men; however, our results do not support the hypothesis that the high sex hormone phase results in further blunting of sympathetic responses to either static handgrip exercise or the CPT in the women.
We found that women demonstrated a similar contraction-induced peak HR during static handgrip to fatigue. Since this occurred with a concomitantly lower BP and vasomotor sympathetic response that persisted during PECA, when the contribution of central command and the stimulation of group III afferents was removed, it indicates that the disparate responses between the sexes are not likely due to the contribution of central command or the mechanoreflex. Moreover, it has been previously shown that central command contributes little to the rise in MSNA during static handgrip exercise (38). Since women and men had similar vasomotor sympathetic responses during the CPT, a nonexercise pressor stimulus, central processing and the efferent arm of the sympathetic nervous system are assumed to be comparably intact between the two groups. That is, the women are capable of achieving similar levels of MSNA as the men but the static handgrip to fatigue and PECA do not elicit the same response. Thus, the blunted response demonstrated by the women is likely to be explained by differences in the metaboreflex arising from the exercising muscle. The following section relates our principal findings in the context of the contributions of the mechanoreflex and the metaboreflex during static handgrip exercise and the PECA phase.
Contributions of the Mechano- and Metaboreflex
The separate contributions of the mechanoreceptor and the metaboreceptor during static exercise are difficult to assess. Mechanical deformation stimulates group III afferents and metabolites stimulate group IV afferents (20), both of which occur during static handgrip. The size of the active muscle mass has been thought to be a potential contributor to the magnitude of the increase in MSNA (29). For example, Seals (29) demonstrated that isometric handgrip evoked a larger increase in MSNA compared with isometric abduction of the first dorsal interosseus of the hand in the same subject. It is difficult, however, to extrapolate the findings of Seals (29) to those of intersubject differences. That is, do the men have higher MSNA during static exercise simply because a larger muscle mass is engaged? Ettinger et al. (6) addressed this issue by matching the sexes for anthropometric variables, as well as MVC (adductor pollicus) and time to fatigue, showing that women still had an attenuated MSNA response. Taken together, these studies suggest that activation of a larger muscle mass is associated with greater intrasubject increases in MSNA but does not necessarily explain intersubject differences. When size discrepancies are accounted for, there still remains a sex difference in the neural response to static exercise, which suggests the metaboreflex may be a larger contributor to the increase in MSNA and BP than the mechanoreflex.
Our findings are consistent with others who showed 1) baseline MSNA was comparable between the sexes (3, 6, 10, 11); 2) the low and high hormone phases of the menstrual cycle did not influence baseline MSNA (2, 10, 22, 23); and 3) women had a smaller change in MSNA from baseline during circulatory arrest after exercise (6). We interpret these findings as women demonstrating a reduced metaboreceptor response, which is independent of exposure to low or high sex hormone concentrations. Indeed, others have previously found, using [31P]-nuclear magnetic resonance spectroscopy, that women had lower concentrations of H+ and H2PO4− in the muscle (6); H+ and H2PO4− have been positively associated with increases in MSNA (33, 36).
Cellular metabolite concentration should be related to the net effect of production and clearance, which is influenced by muscle fiber type. Saito (28) showed that static exercise performed in muscle groups of differing fiber type composition (very low type II → high type II) elicited dissimilar increases in MSNA. For example, forearm (high type II) static exercise elicited the largest increase in MSNA compared with the soleus (very low type II) (28), suggesting that type II fibers are associated with greater production and/or a decrease in clearance of metabolic byproducts compared with type I fibers. Indeed, animal studies have shown that lactate uptake is higher in type I fibers compared with type II fibers (19). These findings imply that fiber type distribution may vary between the sexes. In fact, human studies of biopsied vastus lateralis muscles have confirmed this hypothesis (32). Simoneau and Bouchard (32) studied biopsies of 270 healthy sedentary individuals (126 women, 144 men), reporting that while large interindividual differences exist, on the average women had a significantly higher percentage of type I fiber distribution. Taken together, these studies indicate that women have a higher proportion of type I fibers in the vastus lateralis, which may also be true of other muscle beds. The smaller proportion of type II fibers may lead to lower production of metabolites and the greater proportion of type I fibers may increase their capacity to buffer the metabolites, which may result in a smaller MSNA response in women during static exercise.
Influence of Sex Hormones on Exercise Pressor Reflex
Ettinger et al. (7) demonstrated that the estrogen fluctuations throughout the menstrual cycle did not influence baseline (resting) MSNA but did influence the response to static handgrip exercise. They showed that the increase in MSNA was attenuated during the high estrogen phase with unopposed progesterone (7). We were unable to replicate their finding; however, our study was consistent with another group that examined MSNA during ischemic, rhythmic handgrip exercise during the EF and ML phases of the menstrual cycle (25). Thus, one possible explanation for the disparate findings between our study and Ettinger et al. (7) could be related to the contribution of progesterone. Ettinger et al. (7) compared days 1–4 (EF) to days 10–12 (preovulatory) when estrogen, but not progesterone, was different. We compared days 1–4 (EF) to days 19–22 (ML) or during the low hormone phase and the high hormone phase for both estrogen and progesterone. The role of progesterone in cardiovascular control is not well defined and no consensus exists. However, the interaction of estrogen and progesterone is important as premenopausal women are exposed to both during the course of the menstrual cycle, where neither acts in true isolation. Thus, based on the differences between these studies (7, 25) and ours, it appears that the presence of progesterone may alter clearance of metabolic byproducts. We also cannot leave out the possibility that differences in experimental design (handgrip to fatigue vs. terminating handgrip at 2 min) and/or data analysis played a role in these observed differences.
Despite the large fluctuations in both estrogen and progesterone throughout the menstrual cycle, the response of the women remained similar between the low hormone and high hormone phases of the cycle in our study. Thus, findings from our study indicate that the acute alterations in sex hormones in women do not alter the cardiovascular and vasomotor sympathetic activity responses during static handgrip exercise. This does not exclude the long-term effects of cyclical exposure to estrogen and/or progesterone, which may contribute to the overall sex differences in the exercise pressor reflex.
Sex Differences in BP Responsiveness During the CPT
It is worth noting that the women demonstrated reduced DBP responses to the CPT compared with the men, despite similarities in MSNA. We, however, did not find sex differences in vascular transduction or differences between the low and high hormone phases of the menstrual cycle, which is consistent with others that have shown no difference between the EF and ML phases (25). Certainly there are other factors to consider that influence vascular tone and BP responsiveness. For example, it has been reported that women have greater β2-adrenergic sensitivity (21). Additionally, DBP does not differentiate the degree of vasoconstriction in beds like the splanchnic and renal circulations but only provides an overall assessment of total peripheral resistance. Therefore, it is possible that MSNA alone (i.e., the skeletal muscle bed) does not reflect the activity of other circulations.
Limitations
There are at least four limitations in our study. First, we did not assess the separate roles of estrogen and progesterone by measuring responses in the late-follicular phase (high estrogen/low progesterone). Second, we cannot ignore that men produced a higher absolute force than women during static handgrip exercise. However, Seals (30) posited that at fatigue sympathetic outflow is independent of the level of force (>20% of MVC); therefore, having the subjects perform handgrip to fatigue served as a way of normalizing potential differences between the sexes. Third, we did not measure metabolic byproducts during handgrip and PECA in our subjects. This, however, does not change the overall finding that a sex difference exists in cardiovascular and neural responses during PECA, demonstrating that women have a blunted metaboreflex response. Fourth, we did not assess the interaction of the metaboreflex and the arterial baroreflex in the present study. Some studies (5, 16) have suggested that during PECA there is an increase in baroreflex sensitivity. We cannot reject the possibility that baroreflex sensitivity was altered differently between the women and men during PECA in the present study. However, Cui et al. (5) reported that the metaboreflex is more powerful in increasing MSNA than any baroreflex-mediated suppression of MSNA. During pharmacologic manipulation (bolus injection of sodium nitroprusside followed by phenylephrine) of BP, and, therefore, MSNA during PECA, Cui et al. (5) found that MSNA was still significantly higher than baseline during the phenylephrine portion of the maneuver. Yet, in the nonexercise control trial of their study, MSNA was suppressed, though not statistically significant, compared with baseline (5). Thus, it is not likely that the contribution of the baroreflex changes our findings. We acknowledge, however, that whether baroreflex sensitivity was differently altered during PECA between the two groups remains a separate and testable hypothesis, which was not the primary focus of this paper.
Perspectives and Significance
BP reactivity is one predictor for the future development of hypertension in young healthy individuals. Exposure to both estrogen and progesterone seem to confer a degree of cardioprotection in young premenopausal women. Indeed, premenopausal women demonstrate lower resting BP and lower incidence of hypertension. Based on these findings, we surmise that this cardioprotection will be lost during menopause and that BP reactivity will be comparable or even greater in elderly women when the incidence of hypertension and cardiovascular disease is higher than in men (39).
GRANTS
This research was supported by the National Institutes of Health K23 Grant HL-075283, the National Space Biomedical Research Institute Grant CA00701, and the Clinical and Translational Research Center (formerly, the General Clinical Research Center) Grant RR-00633.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGEMENTS
We are grateful to the individuals that participated in this study. We also thank Robin P. Shook, Jeffrey L. Hastings, M. Dean Palmer, Colin L. Conner, and Diane Bedenkop for valuable laboratory assistance.
REFERENCES
- 1. Armario P, del Rey RH, Martin-Baranera M, Almendros MC, Ceresuela LM, Pardell H. Blood pressure reactivity to mental stress task as a determinant of sustained hypertension after 5 years of follow-up. J Hum Hypertens 17: 181–186, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Carter JR, Lawrence JE, Klein JC. Menstrual cycle alters sympathetic neural responses to orthostatic stress in young, eumenorrheic women. Am J Physiol Endocrinol Metab 297: E85–E91, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carter JR, Ray CA. Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. Am J Physiol Heart Circ Physiol 296: H847–H853, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cui J, Wilson TE, Shibasaki M, Hodges NA, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during posthandgrip muscle ischemia in humans. J Appl Physiol 91: 1679–1686, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Ettinger SM, Silber DH, Collins BG, Gray KS, Sutliff G, Whisler SK, McClain JM, Smith MB, Yang QX, Sinoway LI. Influences of gender on sympathetic nerve responses to static exercise. J Appl Physiol 80: 245–251, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Ettinger SM, Silber DH, Gray KS, Smith MB, Yang QX, Kunselman AR, Sinoway LI. Effects of the ovarian cycle on sympathetic neural outflow during static exercise. J Appl Physiol 85: 2075–2081, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Falkner B, Kushner H, Onesti G, Angelakos ET. Cardiovascular characteristics in adolescents who develop essential hypertension. Hypertension 3: 521–527, 1981 [DOI] [PubMed] [Google Scholar]
- 9. Frey MA, Hoffler GW. Association of sex and age with responses to lower-body negative pressure. J Appl Physiol 65: 1752–1756, 1988 [DOI] [PubMed] [Google Scholar]
- 10. Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol 587: 2019–2031, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol 289: R109–R116, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226: 173–190, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Halliwill JR. Segregated signal averaging of sympathetic baroreflex responses in humans. J Appl Physiol 88: 767–773, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension 53: 571–576, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herr MD, Imadojemu V, Kunselman AR, Sinoway LI. Characteristics of the muscle mechanoreflex during quadriceps contractions in humans. J Appl Physiol 86: 767–772, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Ichinose M, Saito M, Wada H, Kitano A, Kondo N, Nishiyasu T. Modulation of arterial baroreflex control of muscle sympathetic nerve activity by muscle metaboreflex in humans. Am J Physiol Heart Circ Physiol 286: H701–H707, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Jarvis SS, Florian JP, Curren MJ, Pawelczyk JA. Sex differences in vasoconstrictor reserve during 70 deg head-up tilt. Exp Physiol 95: 184–193, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Jones PP, Spraul M, Matt KS, Seals DR, Skinner JS, Ravussin E. Gender does not influence sympathetic neural reactivity to stress in healthy humans. Am J Physiol Heart Circ Physiol 270: H350–H357, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Juel C, Honig A, Pilegaard H. Muscle lactate transport studied in sarcolemmal giant vesicles: dependence on fibre type and age. Acta Physiol Scand 143: 361–365, 1991 [DOI] [PubMed] [Google Scholar]
- 20. Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res 12: 429–439, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36: 1233–1238, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Lawrence JE, Klein JC, Carter JR. Menstrual cycle elicits divergent forearm vascular responses to vestibular activation in humans. Auton Neurosci 154: 89–93, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lawrence JE, Ray CA, Carter JR. Vestibulosympathetic reflex during the early follicular and midluteal phases of the menstrual cycle. Am J Physiol Endocrinol Metab 294: E1046–E1050, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J 111: 383–390, 1986 [DOI] [PubMed] [Google Scholar]
- 25. Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Nakamura T, Kawabe K, Sapru HN. Cold pressor test in the rat: medullary and spinal pathways and neurotransmitters. Am J Physiol Heart Circ Physiol 295: H1780–H1787, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 286: R233–R249, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Saito M. Differences in muscle sympathetic nerve response to isometric exercise in different muscle groups. Eur J Appl Physiol Occup Physiol 70: 26–35, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Seals DR. Influence of active muscle size on sympathetic nerve discharge during isometric contractions in humans. J Appl Physiol 75: 1426–1431, 1993 [DOI] [PubMed] [Google Scholar]
- 30. Seals DR. Influence of force on muscle and skin sympathetic nerve activity during sustained isometric contractions in humans. J Physiol 462: 147–159, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seals DR. Sympathetic activation during the cold pressor test: influence of stimulus area. Clin Physiol 10: 123–129, 1990 [DOI] [PubMed] [Google Scholar]
- 32. Simoneau JA, Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol Endocrinol Metab 257: E567–E572, 1989 [DOI] [PubMed] [Google Scholar]
- 33. Sinoway LI, Smith MB, Enders B, Leuenberger U, Dzwonczyk T, Gray K, Whisler S, Moore RL. Role of diprotonated phosphate in evoking muscle reflex responses in cats and humans. Am J Physiol Heart Circ Physiol 267: H770–H778, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Sugiyama Y, Matsukawa T, Suzuki H, Iwase S, Shamsuzzaman AS, Mano T. A new method of quantifying human muscle sympathetic nerve activity for frequency domain analysis. Electroencephalogr Clin Neurophysiol 101: 121–128, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979 [DOI] [PubMed] [Google Scholar]
- 36. Victor RG, Bertocci LA, Pryor SL, Nunnally RL. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest 82: 1301–1305, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Victor RG, Leimbach WN, Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension 9: 429–436, 1987 [DOI] [PubMed] [Google Scholar]
- 38. Victor RG, Pryor SL, Secher NH, Mitchell JH. Effects of partial neuromuscular blockade on sympathetic nerve responses to static exercise in humans. Circ Res 65: 468–476, 1989 [DOI] [PubMed] [Google Scholar]
- 39. Villablanca AC, Jayachandran M, Banka C. Atherosclerosis and sex hormones: current concepts. Clin Sci (Lond) 119: 493–513, 2010 [DOI] [PubMed] [Google Scholar]