Abstract
Chronic intermittent hypoxia (CIH) models repetitive bouts of arterial hypoxemia that occur in humans suffering from obstructive sleep apnea. CIH has been linked to persistent activation of arterial chemoreceptors and the renin-angiotensin system, which have been linked to chronic elevations of sympathetic nerve activity (SNA) and mean arterial pressure (MAP). Because Fos and FosB are transcription factors involved in activator protein (AP)-1 driven central nervous system neuronal adaptations, this study determined if CIH causes increased Fos or FosB staining in brain regions that regulate SNA and autonomic function. Male Sprague Dawley rats were instrumented with telemetry transmitters for continuous recording of MAP and heart rate (HR). Rats were exposed to continuous normoxia (CON) or to CIH for 8 h/day for 7 days. CIH increased MAP by 7–10 mmHg without persistently affecting HR. A separate group of rats was killed 1 day after 7 days of CIH for immunohistochemistry. CIH did not increase Fos staining in any brain region examined. Staining for FosB/ΔFosB was increased in the organum vasculosum of the lamina terminalis (CON: 9 ± 1; CIH: 34 ± 3 cells/section), subfornical organ (CON: 7 ± 2; CIH: 31 ± 3), median preoptic nucleus (CON 15 ± 1; CIH: 38 ± 3), nucleus of the solitary tract (CON: 9 ± 2; CIH: 28 ± 4), A5 (CON: 3 ± 1; CIH: 10 ± 1), and rostral ventrolateral medulla (CON: 5 ± 1; CIH: 17 ± 2). In the paraventricular nucleus, FosB/ΔFosB staining was located mainly in the dorsal and medial parvocellular subnuclei. CIH did not increase FosB/ΔFosB staining in caudal ventrolateral medulla or supraoptic nucleus. These data indicate that CIH induces an increase in FosB/ΔFosB in autonomic nuclei and suggest that AP-1 transcriptional regulation may contribute to stable adaptive changes that support chronically elevated SNA.
Keywords: sympathetic nerve activity, chronic intermittent hypoxia
patients with obstructive sleep apnea (OSA) demonstrate significantly elevated muscle sympathetic nerve activity (SNA) that has been linked to increased risk of cardiovascular complications, including hypertension (10, 60). A consistent observation is that the exaggerated SNA associated with OSA is maintained even during periods of the day when patients are awake and normoxic. Importantly, Continuous positive airway presssure (CPAP) therapy during sleep reduces the elevation of SNA and lowers mean arterial pressure (MAP) among CPAP-tolerant patients (10, 22). These findings suggest that increased sympathetic tone and arterial hypertension associated with OSA are likely due to repetitive bouts of arterial hypoxemia, sleep disruptions, or both (45).
In 1992, Fletcher and colleagues (15) introduced an animal model to study effects of arterial hypoxemia associated with OSA. They exposed conscious rats to bouts of hypoxia during the nocturnal period by repetitively lowering the level of ambient oxygen. This model of chronic intermittent hypoxia (CIH) (15) produces a rapid increase in MAP that is sustained throughout the diurnal cycle. Increased arterial pressure associated with CIH is dependent on SNA (2, 34), the renin-angiotensin system (13, 16), and peripheral arterial chemoreceptors (14, 34). CIH also alters central respiratory control (1, 41, 48), enhances SNA responses to acute hypoxia (17, 29), and increases stress-induced adrenocorticotropic hormone release (36). While the effects of CIH on arterial chemoreceptor in the carotid bodies have been characterized (10, 44), the central neural mechanisms responsible for persistent sympathoexcitation and elevation of blood pressure remain unclear.
Immunohistochemical staining for Fos, a member of the activator protein (AP)-1 transcription factor family, has been widely used as an indicator of acute activation in the central nervous system (CNS) (7, 24, 43). Previous studies have used Fos staining as a marker for acute neural activation mediated by chemoreceptors (4, 11, 21, 27, 54, 56) and exposure to CIH (18, 55). Along with other transcription factors such as hypoxia-inducible factor-1, NFAT, and NF-kB, Fos expression has been shown to increase in response to in vitro and in vivo exposure to intermittent hypoxia (44, 51). Recent studies indicate that inducible accumulation of FosB, and/or its more stable splice variant ΔFosB, is an indicator of chronic or intermittent activation of the CNS (24, 46). In the present study, we examined Fos and FosB/ΔFosB in CNS regions related to autonomic function in adult male rats exposed to a 7-day CIH protocol. This protocol has been previously reported to alter synaptic function and visceral afferent integration in the nucleus of the solitary tract (NTS) (9, 61) and to increase MAP after 1 day of intermittent hypoxia exposure (25).
METHODS
Animals.
Adult male Sprague-Dawley rats (250–350 g; Charles River) were individually housed and maintained on a 14:10-h light-dark cycle and provided with ad libitum access to food and water. Animal procedures were conducted according to National Institutes of Health guidelines, and were approved by the Institutional Animal Care and Use Committee at the University of North Texas Health Science Center and the University of Texas Health Science Center at San Antonio.
Telemetry monitoring of blood pressure and heart rate.
A Dataquest IV radiotelemetry system (Data Sciences, St. Paul, MN) was used to continuously record MAP and heart rate (HR). With the use of gas anesthesia (isoflurane 2%), rats were implanted with an abdominal aortic catheter attached to a TA11PA-C40 radiotelemetry transmitter. The transmitter was secured to the abdominal muscle and remained in the abdominal cavity for the duration of the experiment. Two weeks were allowed for recovery from surgery. Blood pressure measurements obtained during a 10-s sampling period (500 Hz) were averaged and recorded every 10 min. Signals from radiotransmitters were measured at atmospheric pressure preoperatively and postmortem to quantify and correct for any signal drift over the course of the protocol (25). In most cases, no postmortem offset adjustments were required.
CIH protocol.
After postsurgical recovery, rats individually housed in their home cages were relocated to custom-built Plexiglas chambers 1 wk before beginning the treatment period as previously described (25). Rats were allowed to acclimate to the chambers at normoxia (21% O2) for 4 days before recording baseline cardiovascular data for 3 days. Thereafter, rats were exposed to CIH for 7 days from 0800 to 1600 h. The O2 concentration in the chambers was regulated using custom-built user-controlled timers that separately switched the flow of room air and nitrogen into each chamber. Flow rates of room air and nitrogen to each chamber were controlled separately using individual flow meters (University of Texas Health Science Center at San Antonio Instrumentation Services). The CIH protocol was as follows: 1) O2 was reduced from 21 to 10% in 105 s, 2) O2 was held at 10% for 75 s, 3) O2 was returned to 21% in 105 s, and 4) O2 was held at 21% for 75 s. Accordingly, each complete cycle lasted 6 min, allowing for 80 cycles/day. Chambers were maintained at 21% throughout the remainder of the light phase (2 h) and the dark phase (14 h). Control (CON) animals were housed in identical cages in the same room and were exposed to the same ambient noise and lighting conditions as rats exposed to CIH.
Immunohistochemistry.
Separate groups of CON and CIH rats were used for immunohistochemistry experiments. The morning after the last CIH exposure, rats were anesthetized with thiobutabarbital (100 mg/kg ip Inactin; Sigma), and a 1- to 2-ml sample of whole blood was collected via cardiac puncture for measurement of plasma osmolality and hematocrit as previously described (30). Plasma osmolality was measured using a vapor pressure osmometer (Wescor, Logan, UT).
Rats were perfused transcardially with PBS followed by 4% paraformaldehyde. Brains were postfixed for 1–2 h followed by cryoprotection in 20% sucrose (PBS) at 4°C until the brains sunk. Three sets of coronal 40-μm sections were preserved in cryoprotectant and stored at −20°C to be processed at a later time.
Separate sets of serial sections from each rat were processed for either Fos (1:30,000 rabbit anti-c-Fos Ab5; Calbiochem, San Diego, CA) or FosB [1:1,000 rabbit anti-FosB (102); Santa Cruz Biotechnology, Santa Cruz, CA] immunohistochemistry as previously described (30). The anti-FosB antibody used in this study does not discriminate full-length FosB from the splice variant ΔFosB. Therefore, results will be referred to as FosB/ΔFosB staining (30). Both sets were processed separately with a biotinylated horse anti-rabbit IgG (1:200; Vector Laboratories, Burlingame, CA) and reacted with an avidin-peroxidase conjugate (Vectastain ABC Kit; Vector Laboratories) and PBS containing 0.04% 3,3′-diaminobenzidine hydrochloride and 0.04% nickel ammonium sulfate for 10–11 min. Brain stem sections were then processed for dopamine-β-hydroxylase (DBH) immunofluorescence using a commercially available mouse anti-DBH primary antibody (1:1,000; Millipore, Billerica, MA) and a CY3-labeled anti-mouse secondary antibody (1:250; Jackson ImmunoResearch, West Grove, PA).
Tissue sections were inspected using an Olympus microscope (BX41) equipped for epifluorescence and an Olympus DP70 digital camera with DP manager software (version 2.2.1). Images were uniformly adjusted for brightness and contrast. Regions were identified using the rat brain stereotaxic atlas of Paxinos and Watson (47). Fos or FosB/ΔFosB-positive cells in each region were counted using ImageJ. Four to six sections were analyzed from each rat for each region except for the organum vasculosum of the lamina terminalis (OVLT), median preoptic nucleus (MnPO), and subfornical organ (SFO) because of the limited rostral-caudal dimensions of these areas. The subregions of the paraventricular nucleus (PVN) were analyzed separately as previously described (57). Counts from each section were averaged for each brain region. The doromedial hypothalamus and the perifornical area were not included in the regions analyzed for technical reasons.
Within the brain stem, the portion of NTS analyzed was from 300 μm caudal to obex extending rostrally 300–400 μm beyond the area postrema (53). This area corresponds to −13.2 to −14.6 mm posterior to bregma according to the atlas of Paxinos and Watson (47). The anterior border of the rostral ventrolateral medulla (RVLM) was defined by the caudal pole of the facial nucleus, and the rostral hypoglossal nucleus was used for the posterior border (53), which corresponds to −11.6 to −13.7 posterior to bregma (47).
Images of FosB/ΔFosB and DBH double labeling were collected using an inverted confocal microscope (Olympus IX50) equipped with a disk spin unit (IX2-DSU; Olympus), a motorized stage, and a mercury lamp epifluorescence system. Images of FosB/ΔFosB staining were collected using bright field illumination. Images were inverted and adjusted to remove background artifacts. Inverted images were pseudocolored and merged with an image of DBH staining of the same field obtained using the same procedure. Because FosB/ΔFosB staining (green) is restricted to the nucleus and DBH staining (red) is cytoplasmic, colocalization does not often merge to a uniform yellow appearance. Therefore, cells with green nuclei and red cytoplasm were counted as double labeled. The distribution of FosB/ΔFosB, DBH, and double-labeled cells in the NTS and ventrolateral medulla was mapped using an Olympus BX51 microscope equipped for epifluorescence, a Hamamatsu ORCA-ER-150 CCD (Leeds Instruments, Dallas, TX), and a motorized stage (Microbrightfield, Williston, VT). Images were collected, and composites were created using Neurolucida software (version 8; Microbrightfield).
Data analysis and statistics.
Effects of CIH on MAP during the dark phase and during the 8-h period of CIH exposure were analyzed separately by two-way repeated-measures ANOVA. Student-Neumann-Keuls post hoc tests were used to identify differences between groups for each phase of each day. The significance level was P < 0.05.
Fos and FosB/ΔFosB counts were analyzed by one-way ANOVA with the Student-Newman-Keuls t-test for post hoc analysis. Significance was set at P < 0.05. All values are presented as means ± SE.
RESULTS
Effects of CIH on MAP, HR, and hematology.
CIH significantly increased MAP throughout the 7-day protocol. This effect was observed in both the light phase when the treatment was being applied and during the normoxic dark phase (Fig. 1). MAP returned to baseline 2 days after the end of the 7-day CIH treatment in both the light and dark phase. Animals in subsequent experiments were killed on the morning of the first day of recovery. During CIH, mean HR did not change significantly from baseline during the light or dark phases. CIH also significantly increased hematocrit above normoxic controls (CON: 45.6 ± 0.9%; CIH: 49.6 ± 0.3%; P < 0.05). However, osmolality was unaffected (CON: 294 ± 2 mosmol/l; CIH: 297 ± 1 mosmol/l; P > 0.05).
Fig. 1.
Effect of 7 days of chronic intermittent hypoxia (CIH) on mean arterial pressure (MAP) and heart rate (HR) during the light phase (left) and the normoxic dark phase (right). All rats were normoxic for 3 days of baseline (bs). Data shown for the light phase are mean pressures from 0800 to 1600 h during CIH (ih). Following CIH exposure, rats were allowed to recover for 3 days (p). Animals in subsequent experiments were killed on the morning following ih7. CON, control. *P < 0.05 vs. control; n = 6–8 rats.
Effects of CIH on Fos staining.
No increase in Fos expression was detected in structures of the lamina terminalis (SFO, OVLT, MnPO), PVN, or hindbrain nuclei following CIH (Fig. 2). Because brain tissue was obtained >16 h after the last episode of intermittent hypoxia, the lack of Fos staining is not surprising, since Fos expression generally subsides within several hours of withdrawal of a typical acute or chronic stimulus (42).
Fig. 2.
No. of Fos-positive cells following 7 days of CIH in the organum vasculosum of the lamina terminalis (OVLT), subfornical organ (SFO), median preoptic nucleus (MnPO), supraoptic nucleus (SON), and parvocellular paraventricular nucleus (PVN) (A) and in the area postrema (AP), nucleus of the solitary tract (NTS), caudal ventrolateral medulla (CVLM), and rostral ventrolateral medulla (RVLM) (B); n = 5/group.
Effects of CIH on forebrain FosB/ΔFosB staining.
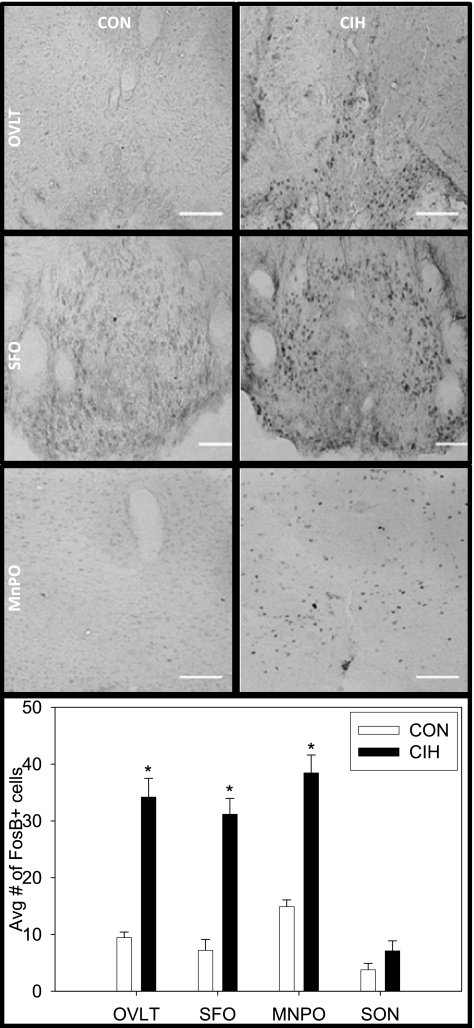
CIH significantly increased the numbers of FosB/ΔFosB-positive cells in the lamina terminalis but not in the supraoptic nucleus (Fig. 3). Within the lamina terminalis region, the OVLT, SFO, and MnPO all demonstrated increased FosB/ΔFosB staining after CIH. Both the dorsal and ventral MnPO showed significant FosB/ΔFosB staining in CIH rats. Representative images of staining in the OVLT, SFO, and ventral MnPO are also shown in Fig. 3.
Fig. 3.
Representative digital images of FosB/ΔFosB staining in CON rats (left) and rats exposed to CIH for 7 days (right). Depicted in order from top to bottom are: the OVLT, SFO, and the subcommissural MnPO. Each pair of CON and CIH images was adjusted for uniform brightness and contrast. Below the photomicrographs are the mean no. of FosB/ΔFosB-positive cells in the OVLT, SFO, MnPO, and SON. Scale bar = 50 μm; *P < 0.05 vs. CON; n = 6–8/group.
Within the PVN, CIH significantly increased FosB/ΔFosB staining in the dorsal and medial parvocellular subdivisions at all rostrocaudal levels examined (Fig. 4). Staining was also observed in the lateral parvocellular subregion of caudal PVN (Fig. 4). A small but significant increase in FosB/ΔFosB staining was detected in rostral lateral magnocellular PVN neurons but not in other caudal magnocellular subregions (see Fig. 4, levels 1 and 2).
Fig. 4.
Representative digital images of FosB/ΔFosB staining in PVN regions of CON rats (left) and 7-day CIH rats (middle). Three levels are depicted, and parvocellular and magnocellular subdivisions are diagrammed on the control column. Each pair of CON and CIH images was adjusted for uniform brightness and contrast. The column on the right indicates the mean no. of FosB/ΔFosB-positive cells counted in the indicated level of PVN. *P < 0.05 vs. CON; n = 6–8/group at each level. dp, Dorsal parvocellular; mp, medial parvocellular; pm, posterior magnocellular; lp, lateral parvocellular, Scale bar = 100 μm.
Effects of CIH on hindbrain FosB/ΔFosB staining.
CIH also significantly increased FosB/ΔFosB-positive cells in the NTS, RVLM, and A5 region but not the caudal ventrolateral medulla or area postrema (Fig. 5). Figure 6 shows FosB/ΔFosB staining in noradrenergic cells of the A5 regions. Figure 7 shows that staining in the NTS was located bilaterally in the caudal and subpostremal portions of the nucleus. A moderate but statistically significant increase in FosB/ΔFosB staining also was observed in DBH-positive neurons of the caudal NTS (CON: 2.3 ± 1.0 cells/section; CIH: 5.4 ± 1.0 cells/section; P < 0.02; n = 5/group). Although the FosB/ΔFosB-positive cells in the RVLM were intermingled with DBH-positive neurons, CIH was not associated with significant double labeling in this region (CON: 1.6 ± 1; CIH: 2.4 ± 0.5). Maps demonstrating the anatomical location of FosB/ΔFosB, DBH, and double labeling for the ventrolateral medulla from a representative control and CIH-treated rat are also shown in Fig. 7.
Fig. 5.
Mean no. of FosB/ΔFosB-positive cells in the AP, NTS, CVLM, RVLM, and in dopamine-β-hydroxylase-positive (DBH+) cells of the A5 noradrenergic region. *P < 0.05 vs. CON; n = 6–8.
Fig. 6.
Representative digital images of FosB/ΔFosB (top) and DBH (middle) immunostaining and colocalization (FosB is pseudo-colored green, bottom) in the A5 noradrenergic region of the brain stem from a CON rat (left) and a 7-day CIH rat (right). White arrows indicate cells with colocalized staining.
Fig. 7.
Neuroanatomical mapping of FosB/ΔFosB (filled triangles), DBH (open squares), and double-labeled (closed circles) cells to representative hindbrain sections of the caudal, subpostremal, and rostral NTS and ventrolateral medulla from a CON rat (A) and a 7-day CIH rat (B). Images are ordered caudal to rostral from left to right.
DISCUSSION
The main findings of this study are that CIH increased FosB/ΔFosB staining in several brain regions involved in endocrine and autonomic control. This coincided with the persistent increase in MAP produced by CIH in a separate group of rats. Hypoxic episodes did produce transient tachycardias that were quickly reversed during the intervening bouts of normoxia. Thus the average HR in the light phase was not elevated significantly compared with controls, and there was no persistent effect of CIH on dark-phase HR. On the other hand, the increases in MAP and FosB/ΔFosB staining are present for at least 16 h after the last episode of intermittent hypoxia. Fos staining was not increased significantly in these regions, as previously reported (36). In the present study, we used a 7-day CIH protocol that significantly increased dark-phase MAP after 1 day of intermittent hypoxia. Whereas other laboratories have shown similar increases in arterial pressure using longer protocols (12, 51), a 7-day protocol was used to facilitate identification of possible mechanisms that contribute to the initial increase in sympathetic outflow before overt changes in end-organ function. Fletcher et al. (13) has reported that there is no further increase in arterial pressure after 7 days up to 35 days of CIH. Whether the relatively short protocol duration contributed to the lack of Fos staining in the current study remains to be determined. Nevertheless, these findings are consistent with the reported time courses of Fos and FosB/ΔFosB expression (28, 42) and suggest that exposure to CIH leads to an accumulation of FosB/ΔFosB immunoreactive protein among the populations of neurons examined.
FosB/ΔFosB are members of the AP-1 family of transcription factors (24, 46). Along with Fos, FRA-1, and FRA-2, FosB proteins heterodimerize with Jun proteins to regulate the activity of genes containing the cognate AP-1 regulatory site TGS(C/G)TCA (24). These heterodimers have been shown to produce transactivation or suppression of AP-1-regulated genes. Jun proteins are reported to have high basal expression in several regions of the CNS (24, 46). Like Fos, FosB and its splice variants have low constitutive expression in the CNS and are induced following synaptic activation (7, 24, 46). FosB and its more stable splice variant ΔFosB have a longer time course and more stable expression relative to Fos and therefore can accumulate in association with intermittent or chronic stimulation (24, 46).
Changes in FosB expression related to CIH are also of interest because FosB/ΔFosB has been linked to neural plasticity (24, 46). Nestler and colleagues first identified the stability of ΔFosB (5) and demonstrated that fosB knockout mice fail to develop drug sensitization (26). Studies in the nucleus accumbens using virally mediated gene transfer have identified a number of downstream target genes that are regulated by ΔFosB, which include glutamate receptor (GluR) subunits and cell signaling proteins (39). Vialou et al. (59) have shown that fosB regulation of GluR2 in the nucleus accumbens is critical to the development of behavioral resilience in mice. These observations suggest that FosB/ΔFosB, through its stability and transcriptional regulatory activity, may mediate adaptive processes in the nervous system.
Different intermittent hypoxia paradigms have been shown to produce acute and chronic adaptations in central control of respiratory and autonomic function (19, 41, 52). Malik et al. (37) demonstrated that the respiratory adaptations produced by continuous hypobaric hypoxia are compromised in fosB knockout mice. Therefore, as in drug addiction and episodic seizure, FosB/ΔFosB may play a functional role in regulating changes in gene expression in neural networks involved in cardiorespiratory function. For example, FosB/ΔFosB may contribute to CIH-induced changes in neuronal function that produce neurogenic hypertension (2, 15), exaggerated stress responses (36), and hypersensitivity of autonomic pathways (1, 19, 61).
Areas demonstrating significant increases in FosB/ΔFosB staining included regions of NTS that receive primary afferents from arterial chemoreceptors and baroreceptors, as well as the parvocellular PVN and RVLM, which contain neurons that project to sympathetic preganglionic neurons in the spinal cord. Previous studies have shown that CIH is associated with changes in chemoreceptor and baroreceptor afferent activity that could contribute to chronic activation of NTS (33, 35, 52). It is also possible that increased FosB/ΔFosB staining in NTS is secondary to the increase in MAP associated with CIH. We also observed increased FosB staining in DBH-positive neurons in the NTS, suggesting that A2 neurons also are chronically/intermittently activated by CIH. Catecholaminergic neurons in the NTS have been shown to express Fos and to increase their firing rate in response to acute hypoxia (4, 20). Such neurons could contribute to effects of CIH on autonomic and endocrine function through their afferent projections to the hypothalamus (36).
Similarly, chronic or intermittent activation of the PVN and RVLM could contribute to increased sympathetic outflow associated with CIH as well as enhanced autonomic and neuroendocrine responses following CIH. The role of these regions in central autonomic function is well established (8, 20), and recent studies indicate that PVN projections to the RVLM contribute to increased arterial pressure and respiratory control in CIH-exposed rats (31, 32).
Whereas areas such as NTS and PVN have been shown to be acutely influenced by carotid body chemoreceptor activation (6, 8), increased FosB/ΔFosB staining was also observed in the lamina terminalis. A previous report on CNS effects of acute chemoreceptor activation or hypoxia did not report increased Fos staining in the lamina terminalis (8).
Studies have reported that the renin-angiotensin system contributes to increased arterial pressure associated with CIH in adult rats (13, 16). Furthermore, chronic losartan treatment has been shown to prevent increases in SNA and changes in carotid body function associated with CIH (38). Although additional research concerning the contribution of the renin-angiotensin system in hypertension associated with CIH is needed, it seems reasonable to speculate that increased FosB staining in the lamina terminalis could be due to activation of the renin-angiotensin system, which has been reported to acutely increase Fos in the lamina terminalis (40, 50). The lamina terminalis could contribute to increased sympathetic outflow during CIH through projections to the PVN. Electrophysiological evidence indicates that MnPO neurons that project to the PVN are activated by circulating ANG II (58). The ventral portion of the lamina terminalis is part of the anteroventral third ventricular region (AV3V), which has been shown to be involved in many different forms of experimental hypertension (3). More recent studies have focused on the role of specific AV3V nuclei in autonomic regulation and blood pressure control. For example, lesions of the MnPO or the SFO have been shown to attenuate angiotensin-dependent hypertension (23, 49). Thus the renin-angiotensin system could influence sympathetic outflow during CIH not only through its effects on the carotid body but also through the lamina terminalis.
Perspectives and Significance
Like dehydration and drug administration, CIH stimulates the accumulation of FosB/ΔFosB in CNS neurons comprising autonomic regions that participate in several models of hypertension and cardiovascular disease. However, the accumulation of FosB/ΔFosB was neither limited to regions receiving arterial chemosensory input nor to autonomic motor regions. This suggests that multiple CNS mechanisms may be involved in physiological responses to CIH. Given the role of FosB/ΔFosB in neural plasticity, its presence in these regions may also be an indication of altered translational activity that could affect cell signaling and synaptic activity.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant P01 HL-88052.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol 529: 215–219, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bao G, Metreveli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol 83: 95–101, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Brody MJ, Fink GD, Buggy J, Haywood JR, Gordon FJ, Johnson AK. The role of the anteroventral third ventricle (AV3V) region in experimental hypertension. Circ Res 43: I2–I13, 1978 [DOI] [PubMed] [Google Scholar]
- 4. Buller KM, Smith DW, Day TA. NTS catecholamine cell recruitment by hemorrhage and hypoxia. Neuroreport 10: 3853–3856, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. J Neurosci 17: 4933–4941, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cruz JC, Bonagamba LG, Machado BH, Biancardi VC, Stern JE. Intermittent activation of peripheral chemoreceptors in awake rats induces Fos expression in rostral ventrolateral medulla-projecting neurons in the paraventricular nucleus of the hypothalamus. Neuroscience 157: 463–472, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Curran T, Morgan JI. Fos: an immediate-early transcription factor in neurons. J Neurobiol 26: 403–412, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Dampney RA, Horiuchi J. Functional organisation of central cardiovascular pathways: studies using c-fos gene expression. Prog Neurobiol 71: 359–384, 2003 [DOI] [PubMed] [Google Scholar]
- 9. de Paula PM, Tolstykh G, Mifflin SW. Chronic intermittent hypoxia alters NMDA and AMPA-evoked currents in NTS neurons receiving carotid body chemoreceptor inputs. Am J Physiol Regul Integr Comp Physiol 292: R2259–R2265, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 90: 47–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol 348: 161–182, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Fletcher EC. Physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol 90: 1600–1605, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Fletcher EC, Bao G, Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension 34: 309–314, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Fletcher EC, Lesske J, Behm R, Miller CC, 3rd, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol 72: 1978–1984, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Fletcher EC, Lesske J, Qian W, Miller CCd, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension 19: 555–561, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Fletcher EC, Orolinova N, Bader M. Blood pressure response to chronic episodic hypoxia: the renin-angiotensin system. J Appl Physiol 92: 627–633, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Greenberg HE, Sica A, Batson D, Scharf SM. Chronic intermittent hypoxia increases sympathetic responsiveness to hypoxia and hypercapnia. J Appl Physiol 86: 298–305, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Greenberg HE, Sica AL, Scharf SM, Ruggiero DA. Expression of c-fos in the rat brainstem after chronic intermittent hypoxia. Brain Res 816: 638–645, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Griffioen KJ, Kamendi HW, Gorini CJ, Bouairi E, Mendelowitz D. network responses to acute intermittent hypoxia. J Neurophysiol 97: 2059–2066, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Hayward LF, Von Reitzenstein M. c-Fos expression in the midbrain periaqueductal gray after chemoreceptor and baroreceptor activation. Am J Physiol Heart Circ Physiol 283: H1975–H1984, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Hedner J, Darpo B, Ejnell H, Carlson J, Caidahl K. Reduction in sympathetic activity after long-term CPAP treatment in sleep apnoea: cardiovascular implications. Eur Respir J 8: 222–229, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Hendel MD, Collister JP. Contribution of the subfornical organ to angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 288: H680–H685, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Rev 28: 370–490, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Hinojosa-Laborde C, Mifflin SW. Sex differences in blood pressure response to intermittent hypoxia in rats. Hypertension 46: 1016–1021, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Hiroi N, Brown JR, Haile CN, Ye H, Greenberg ME, Nestler EJ. FosB mutant mice: loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine's psychomotor and rewarding effects. Proc Natl Acad Sci USA 94: 10397–10402, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirooka Y, Polson JW, Potts PD, Dampney RA. Hypoxia-induced Fos expression in neurons projecting to the pressor region in the rostral ventrolateral medulla. Neuroscience 80: 1209–1224, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Howe BM, Bruno SB, Higgs KA, Stigers RL, Cunningham JT. FosB expression in the central nervous system following isotonic volume expansion in unanesthetized rats. Exp Neurol 187: 190–198, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Huang J, Lusina S, Xie T, Ji E, Xiang S, Liu Y, Weiss JW. Sympathetic response to chemostimulation in conscious rats exposed to chronic intermittent hypoxia. Respir Physiol Neurobiol 166: 102–106, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Ji LL, Fleming T, Penny ML, Toney GM, Cunningham JT. Effects of water deprivation and rehydration on c-Fos and FosB staining in the rat supraoptic nucleus and lamina terminalis region. Am J Physiol Regul Integr Comp Physiol 288: R311–R321, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Kc P, Balan KV, Tjoe SS, Martin RJ, Lamanna JC, Haxhiu MA, Dick TE. Increased vasopressin transmission from the paraventricular nucleus to the rostral medulla augments cardiorespiratory outflow in chronic intermittent hypoxia-conditioned rats. J Physiol 588: 725–740, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kc P, Dick TE. Modulation of cardiorespiratory function mediated by the paraventricular nucleus. Respir Physiol Neurobiol 174: 55–64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lai CJ, Yang CC, Hsu YY, Lin YN, Kuo TB. Enhanced sympathetic outflow and decreased baroreflex sensitivity are associated with intermittent hypoxia-induced systemic hypertension in conscious rats. J Appl Physiol 100: 1974–1982, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia–influence of chemoreceptors and sympathetic nervous system. J Hypertens 15: 1593–1603, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Lin M, Liu R, Gozal D, Wead WB, Chapleau MW, Wurster R, Cheng ZJ. Chronic intermittent hypoxia impairs baroreflex control of heart rate but enhances heart rate responses to vagal efferent stimulation in anesthetized mice. Am J Physiol Heart Circ Physiol 293: H997–H1006, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Ma S, Mifflin SW, Cunningham JT, Morilak DA. Chronic intermittent hypoxia sensitizes acute hypothalamic-pituitary-adrenal stress reactivity and Fos induction in the rat locus coeruleus in response to subsequent immobilization stress. Neuroscience 154: 1639–1647, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Malik MT, Peng YJ, Kline DD, Adhikary G, Prabhakar NR. Impaired ventilatory acclimatization to hypoxia in mice lacking the immediate early gene fos B. Respir Physiol Neurobiol 145: 23–31, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Marcus NJ, Li YL, Bird CE, Schultz HD, Morgan BJ. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: role of the angiotensin II type 1 receptor. Respir Physiol Neurobiol 171: 36–45, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci 6: 1208–1215, 2003 [DOI] [PubMed] [Google Scholar]
- 40. McKinley MJ, Gerstberger R, Mathai ML, Oldfield BJ, Schmid H. The lamina terminalis and its role in fluid and electrolyte homeostasis. J Clin Neurosci 6: 289–301, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Intermittent hypoxia and respiratory plasticity. J Appl Physiol 90: 2466–2475, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Miyata S, Tsujioka H, Itoh M, Matsunaga W, Kuramoto H, Kiyohara T. Time course of Fos and Fras expression in the hypothalamic supraoptic neurons during chronic osmotic stimulation. Brain Res Mol Brain Res 90: 39–47, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Morgan JI, Curran T. Immediate-early genes: ten years on. Trends Neurosci 18: 66–67, 1995 [PubMed] [Google Scholar]
- 44. Nanduri J, Yuan G, Kumar GK, Semenza GL, Prabhakar NR. Transcriptional responses to intermittent hypoxia. Respir Physiol Neurobiol 164: 277–281, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation 100: 2332–2335, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci USA 98: 11042–11046, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1997 [Google Scholar]
- 48. Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA 100: 10073–10078, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ployngam T, Collister JP. An intact median preoptic nucleus is necessary for chronic angiotensin II-induced hypertension. Brain Res 1162: 69–75, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Potts PD, Hirooka Y, Dampney RA. Activation of brain neurons by circulating angiotensin II: direct effects and baroreceptor-mediated secondary effects. Neuroscience 90: 581–594, 1999 [DOI] [PubMed] [Google Scholar]
- 51. Prabhakar NR, Fields RD, Baker T, Fletcher EC. Intermittent hypoxia: cell to system. Am J Physiol Lung Cell Mol Physiol 281: L524–L528, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Prabhakar NR, Peng YJ, Jacono FJ, Kumar GK, Dick TE. Cardiovascular alterations by chronic intermittent hypoxia: importance of carotid body chemoreflexes. Clin Exp Pharmacol Physiol 32: 447–449, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Randolph RR, Li Q, Curtis KS, Sullivan MJ, Cunningham JT. Fos expression following isotonic volume expansion of the unanesthetized male rat. Am J Physiol Regul Integr Comp Physiol 274: R1345–R1352, 1998 [DOI] [PubMed] [Google Scholar]
- 54. Sato M, Severinghaus JW, Basbaum AI. Medullary CO2 chemoreceptor neuron identification by c-fos immunocytochemistry. J Appl Physiol 73: 96–100, 1992 [DOI] [PubMed] [Google Scholar]
- 55. Sica AL, Greenberg HE, Scharf SM, Ruggiero DA. Immediate-early gene expression in cerebral cortex following exposure to chronic-intermittent hypoxia. Brain Res 870: 204–210, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Smith DW, Buller KM, Day TA. Role of ventrolateral medulla catecholamine cells in hypothalamic neuroendocrine cell responses to systemic hypoxia. J Neurosci 15: 7979–7988, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stocker SD, Hunwick KJ, Toney GM. Hypothalamic paraventricular nucleus differentially supports lumbar and renal sympathetic outflow in water-deprived rats. J Physiol 563: 249–263, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stocker SD, Toney GM. Median preoptic neurons projecting to the hypothalamic paraventricular nucleus respond to osmotic, circulating ANG II, and baroreceptor input. J Physiol 568: 599–615, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, 3rd, Watts EL, Wallace DL, Iniguez SD, Ohnishi YH, Steiner MA, Warren BL, Krishnan V, Bolanos CA, Neve RL, Ghose S, Berton O, Tamminga CA, Nestler EJ. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci 13: 745–752, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wolk R, Kara T, Somers VK. Sleep-disordered breathing and cardiovascular disease. Circulation 108: 9–12, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Zhang W, Carreno FR, Cunningham JT, Mifflin SW. Chronic sustained and intermittent hypoxia reduce function of ATP-sensitive potassium channels in nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 295: R1555–R1562, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]