Abstract
Renal tubular cell apoptosis is a significant component of obstruction-induced renal injury, and it results in a progressive loss in renal parenchymal mass during renal obstruction. Although IL-18 is an important mediator of inflammatory renal disease and renal fibrosis, its role in obstruction-induced renal tubular cell apoptosis remains unclear. To study this, male C57BL6 wild-type mice and C57BL6 mice transgenic for human IL-18-binding protein (IL-18BP Tg) were subjected to renal obstruction vs. sham operation. The kidneys were harvested after 1 or 2 wk and analyzed for IL-18 production, apoptosis, caspase activity, and Fas/Fas Ligand (FasL) expression. HK-2 cells were similarly analyzed for apoptosis and proapoptotic signaling following 3 days of direct exposure to IL-18 vs. control media. Renal obstruction induced a significant increase in IL-18 production, renal tubular cell apoptosis, caspase activation, and FasL expression. IL-18 neutralization, on the other hand, significantly reduced obstruction-induced apoptosis, caspase-8 and caspase-3 activity, and FasL expression. In vitro experiments similarly demonstrate that IL-18 stimulation induces apoptosis, FasL expression, and increases active caspase-8 and caspase-3 expression in a dose-dependent fashion. siRNA knockdown of FasL gene expression, however, significantly reduced IL-18-induced apoptosis. This study reveals that IL-18 is a significant mediator of obstruction-induced tubular cell apoptosis, and it demonstrates that IL-18 stimulates proapoptotic signaling through a FasL-dependent mechanism.
Keywords: apoptosis, renal dysfunction, cytokine, kidney
upper urinary tract obstruction is a common cause of renal dysfunction in both children and adults and is characterized by collecting system dilatation, significant tubulointerstitial fibrosis, and a progressive loss in renal parenchymal mass secondary to apoptotic tubular cell death (33). Renal tubular cells are extremely sensitive to obstruction-induced apoptosis with peak apoptotic cell death occurring within 1 to 2 wk of renal obstruction (3, 4). A number of inflammatory mediators and growth factors have been implicated in obstruction-induced renal apoptosis, most notably transforming growth factor (TGF)-β1 and TNF-α (8, 21, 24–26). Interleukin (IL)-18 is a proinflammatory cytokine implicated in the pathogenesis of many inflammatory renal diseases, including urinary tract infection, ischemia-reperfusion injury, autoimmune conditions, allograft rejection, and obstructive nephropathy (1, 5, 10, 18, 31). While IL-18's proinflammatory action has been attributed, in part, to the induction of downstream cytokine activity (27, 34, 35), we previously showed that IL-18 induces renal fibrosis and tubular cell epithelial-mesenchymal transition independent of TGF-β1 and TNF-α activity (1).
IL-18 is synthesized as a biologically inactive precursor (pro-IL-18) and is cleaved into an active molecule by the intracellular cysteine protease ICE or caspase-1 (6, 12). In humans, urinary IL-18 levels have been shown to be a sensitive and early marker of renal tubular damage from ischemic and posttransplantation ATN, with elevated urinary IL-18 levels predictive of acute tubular injury before creatinine levels and urine output become altered (30, 31). In animal models of renal ischemia-reperfusion injury, IL-18 has been shown to exacerbate acute tubular necrosis (7, 22, 23), and Liang et al. (19) demonstrated that direct IL-18 stimulation induces apoptosis in renal tubular cells (TECs) in vitro. IL-18 has been shown to induce apoptosis in a variety of cells through both TNF-α- and Fas/FasL-dependent mechanisms (11, 20, 28). In endothelial cells, IL-18 upregulates surface TNFR1 expression and thereby increases the susceptibility of endothelial cells to undergo TNF-induced, but not Fas-induced, apoptosis (20). On the other hand, IL-18 enhances FasL expression and induces apoptosis in NK cells and human myelomonocytic KG-1 cells through a Fas-dependent mechanism (15, 28). A Fas-dependent mechanism of IL-18-induced apoptosis has also been reported in hepatocytes (11).
Since IL-18 has recently been shown to have an important role in obstruction-induced renal fibrosis and TEC injury independent of TGF-β1 and TNF-α activity (1), we hypothesized that IL-18 is an important mediator of obstruction-induced renal tubular cell apoptosis through a Fas-dependent mechanism. To study this, we examined renal cortical IL-18 production, apoptotic cell death, active caspase expression, and Fas/FasL expression in male C57B16 wild-type (WT) mice and mice transgenic for IL-18BP using a well-established model of unilateral ureteral obstruction (UUO). C57BL6 mice transgenic for IL-18-binding protein (IL-18BP Tg) have been previously demonstrated to overexpress human IL-18-binding protein isoform a and reliably inhibit IL-18 activity (9). In addition, human proximal tubular cells (HK-2) were directly stimulated with IL-18 and the cells were subsequently examined for apoptotic cell death, FasL expression, and active caspase expression.
MATERIALS AND METHODS
Animals, experimental groups, and operative techniques.
The animal protocol was reviewed and accepted by the Animal Care and Research Committee of the Indiana University School of Medicine. Male C57BL6 mice transgenic for IL-18BP Tg were generously donated by Dr. Charles Dinarello from the University of Colorado Health Science Center (Denver, CO). These mice overexpress human IL-18-binding protein isoform a and reliably inhibit IL-18 activity, but do not have any notable phenotype (9). The genotype of the mice was confirmed with a PCR analysis of extracted DNA samples from tail snips (5′ primer, 5′-ACA CCT GTC TCG CAG ACC AC-3′ and 3′ primer, 5′-TCA GCT GCT CCA GCA CCA A-3′) as described by Fantuzzi et al. (9) and overexpression of serum levels of human IL-18BP was confirmed using an ELISA (Human IL-18BP DuoSet ELISA; R&D Systems) (1) before utilization of the animals.
Male WT or IL-18BP Tg mice weighing 25–30 g (6 animals per group) were anesthetized with isofluorane and subjected to either sham operation or left UUO. For obstructed animals, the left ureter was isolated and completely ligated with 5–0 silk suture. Sham-operated mice underwent an identical surgical procedure without ureteral ligation. One or two weeks postoperatively, mice were reanesthetized, the left kidneys were removed and snap-frozen in liquid nitrogen, and the animals were subsequently killed.
Tissue homogenization.
A portion of each renal cortex was homogenized after the samples had been diluted in 10 Vol of homogenate buffer per gram of tissue [10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EGTA, 1 mM DTT, and Complete Protease Inhibitor tabs (Boehringer Mannheim, Indianapolis, IN)] using a vertishear tissue homogenizer. Renal homogenates were then centrifuged at 3,000 g for 15 min at 4°C, and the supernatants were stored at −80°C until the ELISA assays or Western blots could be performed.
Cell culture and IL-18 stimulation.
The human proximal tubular cell line HK-2 was cultured in keratinocyte serum-free medium + 5 ng/ml epidermal growth factor and 40 mg/ml bovine extract + 100 U/ml penicillin and 100 U/ml of streptomycin. The cells were passaged weekly by trypsinization (0.05% Trypsin-EDTA) following formation of a confluent monolayer and placed in serum-free media 24 h before stimulation. Recombinant mature IL-18 (R&D Systems, Minneapolis, MN) was added to the cells at concentrations of 0.1 ng/ml, 1 ng/ml, 10 ng/ml, or 100 ng/ml, with untreated cells serving as controls. The cells were exposed to the single dose of IL-18 vs. untreated media for 3 days and both supernatants and cell lysates were harvested (4 plates per treatment group).
FasL siRNA transfection.
HK-2 cells (2.5 × 105 cells/well) were cultured in a six-well dish and placed in serum-free medium 18 h before transfection. Cells were than reversely transfected with 40 pmol siRNA (Silencer Select Negative Control #1 siRNA or Silencer Select Predesigned human Fas ligand siRNA, Ambion) using 6.5 μl of Lipofectamine 2000 reagent (Invitrogen). After 6 h, cells were treated with either vehicle or 100 ng/ml IL-18 (R&D Systems) for 3 days. Conditioned medium, total RNA, and protein were collected for future ELISA, RT-PCR, and Western blot analysis.
Apoptosis ELISA.
A portion of the renal cortex from each kidney was homogenized with a vertishear tissue homogenizer after 4 Vol of ice-cold 50 mM Tris·HCl (pH 7.4) was added. The samples were agitated on ice for 30 min. Four volumes of 0.1% Triton X-100 were added to each sample, and the samples were agitated on ice for 60 min. The samples were then centrifuged at 13,000 rpm for 20 min at 4°C and supernatants were collected. HK-2 cells were cultured and stimulated for 3 days with increasing concentrations of IL-18 as described above. The treated and untreated culture medium was collected and centrifuged at 13,000 rpm for 5 min at 4°C and supernatants were collected. A photometric enzyme immunoassay for the quantitative determination of cytoplasmic histone-associated DNA fragments representative of apoptosis (Cell Death Detection ELISA Plus, Roche Diagnostics; Mannheim, Germany) was performed using 20 μl of supernatant from each tissue or cell culture sample as described by the manufacturer. The samples were tested in duplicate, and the results were expressed as optical density per milligram of protein. The assay allows for the specific detection of mono- and oligonucleosomes in as few as 5 × 102 cell equivalents per well.
TdT-mediated dUTP nick end labeling assay.
Three transverse 6-μm renal tissue sections were prepared from each sample using a cryostat and fixed in 4% paraformaldehyde and 30% sucrose. Fluorometric DNA strand breaks representative of apoptosis were detected in each tissue section using terminal deoxynucleotidyl transferase incorporation of fluorescein-dUTP (ApopTag Red in situ Apoptosis Detection Kit, Temecula, CA). After cellular permeabilization with 20 μg/ml Proteinase K for 15 min, the tissue sections were exposed to terminal deoxynucleotidyl transferase fluorescein labeling for 1 h. The tissue sections were then washed in PBS and exposed to Anti-Digoxigenin conjugate for 30 min. The tissue was then washed again and counterstained (10 μg/ml bis-benzimide) for 10 min. The specimens were mounted in an antiquenching medium (ProLong Antifade Kit; Molecular Probes, Eugene, OR) and maintained at −4°C until the microscopic examination could be performed. The number of fluorescent nuclei was quantified per high-powered field in each treatment group and compared. Samples were analyzed in triplicate. The characteristic morphologic features of apoptosis (i.e., nuclear condensation) were also correlated with nuclear fluorescence.
ApopTag peroxidase in situ apoptosis detection.
Three transverse 6-μm paraffin-embedded renal tissue sections were obtained from each sample, deparaffinized, and pretreated with proteinase K (20 μg/ml) for 15 min. Following quenching of endogenous peroxidase activity in 3% hydrogen peroxide, samples were incubated with working-strength terminal deoxynucleotidyl transferase (TdT) enzyme for 1 h according to the manufacturer's instructions (kit S7100; Chemicon, Temecula, CA). Tissue samples were then incubated with anti-digoxignenin conjugate for 30 min, washed, and developed by applying peroxidase substrate (DAB, diaminobenzidine) to each specimen for 5 min. DNA strand breaks representative of apoptosis were detected in each tissue section using terminal deoxynucleotidyl transferase incorporation of digoxigenin-nucleotide. Slides were then counterstained with hematoxylin and eosin and the cell types undergoing apoptosis were determined.
Flow cytometry.
Apoptosis in HK-2 cells induced by IL-18 was quantitated by flow cytometry using an Annexin V-FITC Apoptosis Detection Kit I (BD Biosciences). HK-2 cells were exposed to IL-18 stimulation or control media as described above, then harvested, and washed twice with cold PBS. Cells (1 × 105) were resuspended in 100 μl of 1× binding buffer. Five microliters of annexin V-FITC and 5 μl of propidium iodide were then added to the cells, and the cells were incubated for 15 min at room temperature in darkness. An additional 400 μl of 1× binding buffer were then added to each sample. Apoptotic cell death was analyzed using FACScan and CellQuest software (BD Biosciences).
Western blot analysis.
Protein extracts from homogenized samples (30 μg/lane) or cell lysates (20 μg/lane) were subjected to SDS-PAGE electrophoresis on a Tris-glycine gel and transferred to a polyvinylidene fluoride membrane. Immunoblotting was performed by incubating each membrane in 5% dry milk for 1 h, followed by incubation with an anti-caspase-3 p17 antibody (1:500 overnight at 4°C, Cell Signaling) or an anti-caspase-8 p20 antibody [1:200 for 2 h at room temperature (RT); Santa Cruz Biotechnology, Santa Cruz, CA]. After being washed three times in TBST, each membrane was incubated for 1 h at RT with a peroxidase-conjugated secondary antibody (1:2,000 for caspase-3, 1:5,000 for caspase-8). Equivalent protein loading in each lane was confirmed by stripping and reblotting each membrane for GAPDH (1:20,000 for 30 min at RT, secondary 1:20,000 for 30 min at RT; Biodesign International, Saco, ME). The membranes were developed using enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ), and the density of each band was determined using NIH image analysis software and expressed as a percentage of GAPDH density.
Real-time PCR for Fas and FasL.
Total RNA was extracted from renal cortical tissue or cell lysates by homogenization in TRIzol (GIBCO BRL, Gaithersburg, MD) and then isolated by precipitation with chloroform and isopropanol. Total RNA (0.5 μg) was subjected to cDNA synthesis using iScript (Bio-Rad, Hercules, CA). cDNA from each sample was analyzed for Fas or Fas-L using a TaqMan gene expression assay (RT-PCR; Applied Biosystems, Foster City, CA). FAM Dye/MGB-labeled probes for GAPDH (4352932E Applied Biosystems) served as endogenous controls. Primers used were Fas (Mm00433237_m1; HS00163653_m1) and FasL (Mm00438864_m1; Hs00899442_m1).
Statistical analysis.
Data are presented as means ± SE. Differences at the 95% confidence level were considered significant. The experiment groups were compared using one-way ANOVA with post hoc Bonferroni-Dunn (JMP 5.0.1). For cell culture experiments using varying concentrations of IL-18, a multiple comparison analysis was performed in all pairs using Tukey-Kramer honestly significant difference. Data are presented as means ± SD.
RESULTS
Obstruction-induced apoptosis.
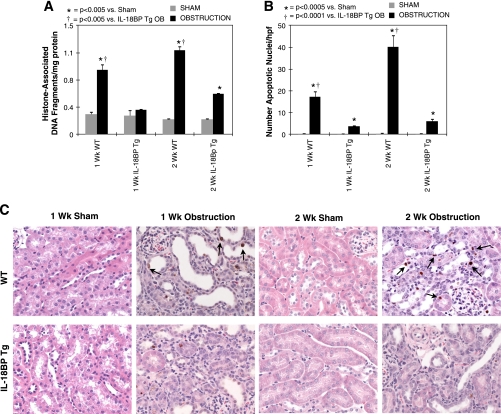
To assess the role of IL-18 in obstructed-induced renal cell apoptosis, samples from each treatment group were analyzed quantitatively for cytoplasmic histone-associated DNA fragments, a marker of apoptosis, and examined histologically for apoptotic nuclei with a TdT-mediated dUTP nick end labeling and ApopTag assay. Sham-treated samples demonstrated low levels of cytoplasmic histone-associated DNA fragments and minimal apoptotic staining in each treatment group. In contrast, WT animals exhibited a significant increase in cytoplasmic histone-associated DNA fragments (Fig. 1A) and apoptotic cell death (Fig. 1, B and C) in response to both 1 and 2 wk of renal obstruction, with the number of apoptotic nuclei most prominent at the 2-wk time point. Apoptotic nuclei localized to both tubular epithelial cells, and to a lesser extent, the interstitium on immunohistochemical staining (Fig. 1C). IL-18BP Tg animals, on the other hand, exhibited no increase in cytoplasmic histone-associated DNA fragments and a significant reduction in apoptotic cell death in response to both 1 and 2 wk of renal obstruction.
Fig. 1.
Renal cell apoptosis following unilateral ureteral obstruction (UUO). A: renal cortical histone-associated DNA fragment accumulation (ELISA) in wild-type (WT) and IL-18-binding protein transgenic (IL-18BP Tg) mice exposed to sham operation or 1 or 2 wk of UUO. B: graph depicting the number of apoptotic nuclei (TdT-mediated dUTP nick end labeling) per high-powered field (×400) in each treatment group. C: photographs (×400) depicting apoptotic nuclei (ApopTag) in renal cortical tissue sections counterstained with hematoxylin and eosin from WT and IL-18BP Tg mice exposed to sham operation or 1 or 2 wk of UUO. Apoptotic nuclei are stained brown and identified with black arrows.
IL-18-induced apoptosis in TEC.
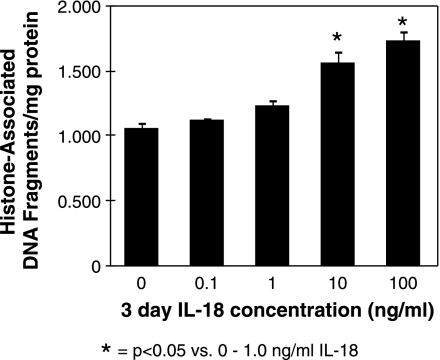
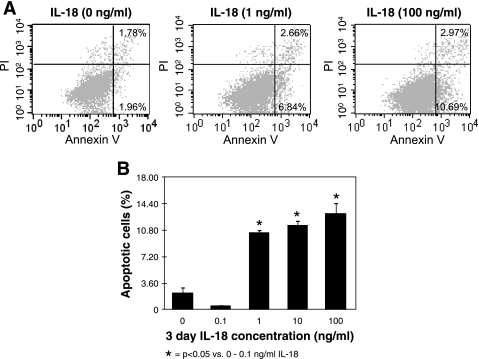
IL-18's ability to directly stimulate tubular epithelial cell apoptosis was subsequently evaluated by exposing HK-2 cells to various concentrations of IL-18 in vitro. A significant increase in cytoplasmic histone-associated DNA fragments (Fig. 2), and an increase in the percentage of apoptosis as determined by flow cytometry (Fig. 3, A and B), was detected in cells stimulated with IL-18 in a dose-dependent fashion. These findings, in conjunction with our observations in vivo, suggest that IL-18 is an important mediator of renal cell apoptosis.
Fig. 2.
Human proximal tubular cells (HK-2) histone-associated DNA fragment accumulation (ELISA) following IL-18 stimulation in vitro. Graphic representation of cell lysate histone-associated DNA fragment accumulation in response to 3 days of cell stimulation with varying concentrations of recombinant human IL-18 (0–100 ng/ml).
Fig. 3.
Flow cytometric analysis of HK-2 cells following IL-18 stimulation in vitro. A: flow cytometry for HK-2 cells stained with annexin V (apoptosis) and propidium iodide (PI; necrosis) following 3 days of stimulation with varying concentrations of recombinant human IL-18 (0–100 ng/ml). B: graphic representation of HK-2 cell percent apoptosis (flow cytometry) in response to IL-18 stimulation.
Caspase activation.
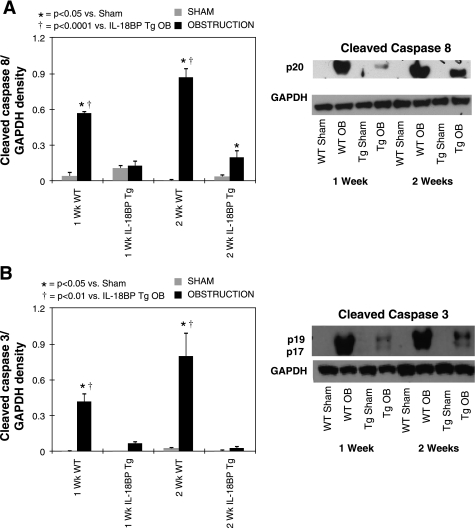
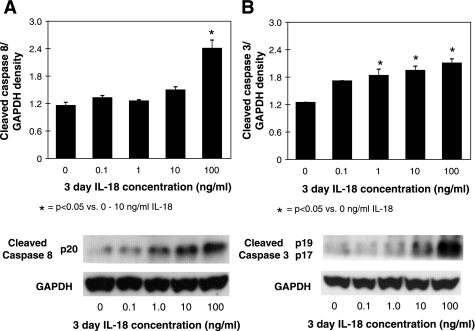
To elucidate the signaling pathway involved in IL-18-mediated renal cell apoptosis, active caspase-8 and -3 expression was evaluated in renal cortical tissue samples and in HK-2 cells in response to direct IL-18 stimulation in vitro. Active caspase-8 and -3 expression remained low in sham-treated animals, but increased significantly in response to 1 wk, and increased further in response to 2 wk of renal obstruction (Fig. 4, A and B). A marked reduction in active caspase-8 and -3 expression was detected in the IL-18BP Tg mice exposed to the same degree of obstruction. HK-2 cells subjected to graded IL-18 stimulation similarly demonstrated a significant increase in active caspase-8 and -3 expression in response to IL-18 in a dose-dependent fashion (Fig. 5). These data suggest that IL-18's regulatory role in renal cell apoptosis involves caspase signaling.
Fig. 4.
Renal cortical active caspase-3 and caspase-8 expression following UUO. A: gel photograph and densitometric analysis of active caspase-8 expression represented as a percentage of GAPDH in WT and IL-18BP Tg exposed to sham operation or 1 or 2 wk of UUO. B: gel photograph and densitometric analysis of active caspase-3 expression represented as a percentage of GAPDH in WT and IL-18BP Tg mice exposed to sham operation or 1 or 2 wk of UUO.
Fig. 5.
HK-2 active caspase-3 and caspase-8 expression following IL-18 stimulation in vitro. A: gel photograph and densitometric analysis of active caspase-8 expression represented as a percentage of GAPDH in HK-2 cells following 3 days of cell stimulation with varying concentrations of recombinant human IL-18 (0–100 ng/ml). B: gel photograph and densitometric analysis of active caspase-3 expression represented as a percentage of GAPDH in HK-2 following 3 days of cell stimulation with varying concentrations of recombinant human IL-18 (0–100 ng/ml).
Fas and FasL expression.
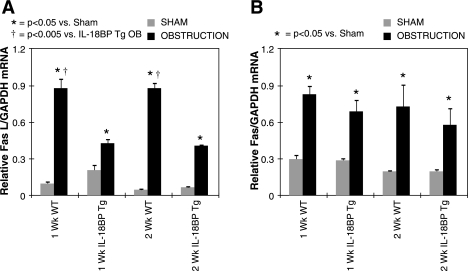
Since Fas and Fas ligand expression are increased during renal obstruction (4), and IL-18 stimulates FasL expression (11, 15) and Fas-FasL-dependent cytotoxity in a variety of cells (13), the impact of IL-18 activity on Fas/Fas ligand expression during renal obstruction and in HK-2 cells in vitro was evaluated. As expected, Fas and FasL expression increased significantly in WT mice in response to 1 and 2 wk of renal obstruction (Fig. 6). FasL expression, but not Fas expression, was significantly reduced; however, in IL-18BP Tg mice exposed to the same degree of obstruction. These results demonstrate that IL-18 induces apoptotic signaling in renal cells, in part, by stimulating FasL expression and suggest a Fas-dependent pathway for IL-18-induced renal cell apoptosis.
Fig. 6.
Quantitative renal cortical FasL and Fas mRNA expression following UUO. A: quantitative FasL mRNA expression represented as a percentage of GAPDH in WT and IL-18BP Tg animals exposed to sham operation or 1 or 2 wk of UUO. B: quantitative Fas mRNA expression represented as a percentage of GAPDH in WT and IL-18BP Tg animals exposed to sham operation or 1 or 2 wk of UUO.
HK-2 cell FasL expression and impact of FasL knockdown during IL-18 stimulation.
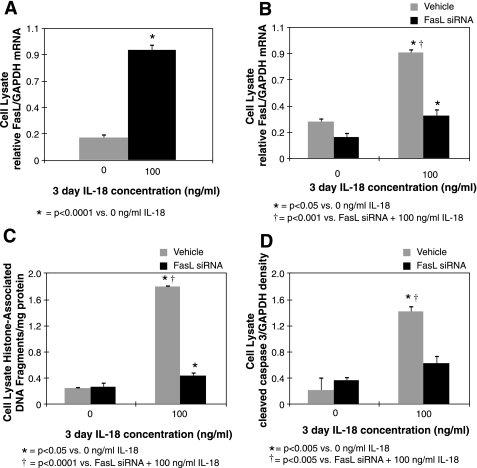
To further evaluate FasL as a mediator of IL-18-induced renal tubular cell apoptosis, FasL expression was evaluated in vitro in HK-2 cells exposed to IL-18. FasL expression was significantly higher in cells exposed to IL-18 stimulation (100 ng/ml for 3 days) compared with controls (Fig. 7A), and cells exposed to siRNA knockdown of FasL before IL-18 stimulation exhibited a significant reduction in FasL gene expression (Fig. 7B), apoptotic cell death (Fig. 7C), and active caspase 3 expression (Fig. 7D). These results provide evidence that IL-18 induces renal tubular cell apoptosis by stimulating FasL expression.
Fig. 7.
HK-2 FasL expression and apoptosis in response to knockdown of FasL gene expression. A: HK-2 quantitative FasL mRNA expression represented as a percentage of GAPDH following cell stimulation with recombinant human IL-18 (100 ng/ml for 3 days). B: HK-2 quantitative FasL mRNA expression represented as a percentage of GAPDH following cell stimulation with recombinant human IL-18 (100 ng/ml for 3 days) in the presence or absence of FasL siRNA (40 pmol). C: HK-2 lysate histone-associated DNA fragment accumulation following cell stimulation with recombinant human IL-18 (100 ng/ml for 3 days) in the presence or absence of FasL siRNA (40 pmol). D: densitometric analysis of active caspase-3 expression represented as a percentage of GAPDH in HK-2 lysates following cell stimulation with recombinant human IL-18 (100 ng/ml for 3 days) in the presence or absence of FasL siRNA (40 pmol).
DISCUSSION
The proinflammatory cytokine, IL-18, has been shown to induce apoptosis in HK-2 cells in vitro (19) and has been identified as an important mediator of obstruction-induced renal fibrosis and tubular cell injury independent of TGF-β1 and TNF-α activity (1). This is the first study to demonstrate that IL-18 has a significant role in proapoptotic signaling during renal obstruction, and it reveals that IL-18's proapoptotic activity is mediated, in part, through a Fas/FasL-dependent pathway. IL-18 induces the gene expression of several proapoptotic factors including TNF-α, IL-1, Fas ligand, and multiple chemokines (7), and it has been shown to stimulate apoptotic cell death in a variety of cells through both TNF-α- and Fas-dependent mechanisms (11, 20, 28). Since IL-18 has previously been shown to mediate renal tubular cell injury during obstruction independent of TNF-α and TGF-β1 activity (1), the role of IL-18 in obstruction-induced apoptosis and the impact of IL-18 on Fas/FasL-dependent apoptotic signaling was evaluated.
We previously showed that renal cortical IL-18 levels are three times higher in kidneys exposed to 1 or 2 wk of obstruction compared with sham-operated controls and that IL-18 neutralization ameliorates obstruction-induced renal fibrosis (1). Our current findings indicate that IL-18 neutralization also significantly reduces obstruction-induced renal cell apoptosis and cytoplasmic histone-associated DNA fragment levels at both 1 and 2 wk of obstruction. IL-18's direct cytotoxic effect on renal tubular cells was further evaluated in vitro, with results demonstrating a dose-dependent increase in cytoplasmic histone-associated DNA fragments and the percentage of apoptosis as determined by flow cytometry in HK-2 cells stimulated with IL-18. These results corroborate previous observations by Liang et al. (19) in HK-2 cells. While a general reduction in inflammatory signaling during IL-18 neutralization may lead to reduced apoptotic cell death in vivo, these results indicate that IL-18 has a direct proapoptotic effect in tubular epithelial cells.
IL-18's impact on proapoptotic signaling was subsequently evaluated by examining caspase-8 and caspase-3 activity during UUO and in TECs in vitro. Active caspase-8 and -3 expression was significantly increased at 1 wk and increased further after 2 wk of obstruction. IL-18 neutralization, however, dramatically reduced obstruction-induced active caspase-8 and -3 expression at both time points. This suggests that IL-18 activates an extrinsic proapoptotic signaling pathway during renal obstruction. Similarly, upon direct stimulation of HK-2 cells in vitro, IL-18 induced a dose-dependent increase in both active caspase-8 and active caspase-3 expression. Since our previous work elucidated IL-18's significant role in obstruction-induced renal fibrosis and TEC injury in vitro independent of any effect on TGF-β1 and TNF-α activity (1), we hypothesized that IL-18 stimulates extrinsic proapoptotic signaling through a Fas/FasL-dependent mechanism.
Upon FasL binding to the death receptor Fas, the adaptor protein FADD is recruited to the receptor, resulting in caspase-8 activation and subsequent apoptotic cell death (16, 32). It has previously been shown that the Fas/FasL system regulates ischemia-induced cardiac myocyte and renal cell apoptosis, as mice with disrupted Fas/FasL systems are protected from myocyte and tubular epithelial cell death (14, 17, 29). While IL-18's proapoptotic activity is mediated through a TNF-α-dependent mechanism in some cells (20), IL-18 has been shown to upregulate Fas/FasL expression and activate proapoptotic signaling through a Fas/FasL-dependent pathway in cardiac endothelial cells, NK cells, human myelomonocytic KG-1 cells, and hepatocytes (2, 11, 15, 28). Our results demonstrate that Fas and FasL expression are significantly increased in response to renal obstruction, and furthermore, that obstruction-induced FasL expression is significantly reduced in the presence of IL-18 neutralization. Our in vitro studies similarly reveal that FasL expression is significantly increased in TECs in response to IL-18 stimulation and demonstrate that cells exposed to siRNA knockdown of FasL before IL-18 stimulation exhibit a significant reduction in FasL gene expression, apoptotic cell death, and active caspase-3 expression. This suggests that IL-18 stimulates FasL expression in TECs in vitro and during obstruction and that IL-18-mediated renal cell apoptosis is dependent, in part, on the Fas/FasL signaling pathway.
Despite advancements in urologic surgery, upper urinary tract obstruction remains an important cause of renal failure. The development of renal injury during obstruction is primarily a consequence of increased proinflammatory signaling and the subsequent release and activation of multiple profibrotic and proapoptotic mediators. This is the first study to demonstrate that IL-18 stimulates renal tubular cell apoptosis and proapoptotic signaling through a Fas/FasL-dependent pathway during renal obstruction and in TECs in vitro. A greater understanding of IL-18's role in renal injury and the mechanisms involved in IL-18-induced apoptosis may provide new therapeutic strategies to protect against renal dysfunction in the future.
GRANTS
This research was supported by National Institutes of Health Grants DK065892 and DK08165 (to K. K. Meldrum).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Bani-Hani AH, Leslie JA, Asanuma H, Dinarello CA, Campbell MT, Meldrum DR, Zhang H, Hile K, Meldrum KK. IL-18 neutralization ameliorates obstruction-induced epithelial-mesenchymal transition and renal fibrosis. Kidney Int 76:500–511, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Chandrasekar B, Vemula K, Surabhi RM, Li-Weber M, Owen-Schaub LB, Jensen LE, Mummidi S. Activation of intrinsic and extrinsic proapoptotic signaling pathways in interleukin-18-mediated human cardiac endothelial cell death. J Biol Chem 279:20221–20233, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Chevalier RL, Smith CD, Wolstenholme J, Krajewski S, Reed JC. Chronic ureteral obstruction in the rat suppresses renal tubular Bcl-2 and stimulates apoptosis. Exp Nephrol 8:115–122, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Choi YJ, Baranowska-Daca E, Nguyen V, Koji T, Ballantyne CM, Sheikh-Hamad D, Suki WN, Truong LD. Mechanism of chronic obstructive uropathy: increased expression of apoptosis-promoting molecules. Kidney Int 58:1481–1491, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Daemen MA, van't Veer C, Wolfs TG, Buurman WA. Ischemia/reperfusion-induced IFN-gamma up-regulation: involvement of IL-12 and IL-18. J Immunol 162:5506–5510, 1999 [PubMed] [Google Scholar]
- 6. Dinarello CA. IL-18: a TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol 103:11–24, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Dinarello CA, Novick D, Puren AJ, Fantuzzi G, Shapiro L, Muhl H, Yoon DY, Reznikov LL, Kim SH, Rubinstein M. Overview of interleukin-18: more than an interferon-gamma inducing factor. J Leukoc Biol 63:658–664, 1998 [PubMed] [Google Scholar]
- 8. Docherty NG, O'Sullivan OE, Healy DA, Fitzpatrick JM, Watson RW. Evidence that inhibition of tubular cell apoptosis protects against renal damage and development of fibrosis following ureteric obstruction. Am J Physiol Renal Physiol 290:F4–F13, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Fantuzzi G, Banda NK, Guthridge C, Vondracek A, Kim SH, Siegmund B, Azam T, Sennello JA, Dinarello CA, Arend WP. Generation and characterization of mice transgenic for human IL-18-binding protein isoform a. J Leukoc Biol 74:889–896, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Faust J, Menke J, Kriegsmann J, Kelley VR, Mayet WJ, Galle PR, Schwarting A. Correlation of renal tubular epithelial cell-derived interleukin-18 upregulation with disease activity in MRL-Faslpr mice with autoimmune lupus nephritis. Arthritis Rheum 46:3083–3095, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Finotto S, Siebler J, Hausding M, Schipp M, Wirtz S, Klein S, Protschka M, Doganci A, Lehr HA, Trautwein C, Khosravi-Far R, Strand D, Lohse A, Galle PR, Blessing M, Neurath MF. Severe hepatic injury in interleukin 18 (IL-18) transgenic mice: a key role for IL-18 in regulating hepatocyte apoptosis in vivo. Gut 53:392–400, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 386:619–623, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Gracie JA. Interleukin-18 as a potential target in inflammatory arthritis. Clin Exp Immunol 136:402–404, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamar P, Song E, Kokeny G, Chen A, Ouyang N, Lieberman J. Small interfering RNA targeting Fas protects mice against renal ischemia-reperfusion injury. Proc Natl Acad Sci USA 101:14883–14888, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hashimoto W, Osaki T, Okamura H, Robbins PD, Kurimoto M, Nagata S, Lotze MT, Tahara H. Differential antitumor effects of administration of recombinant IL-18 or recombinant IL-12 are mediated primarily by Fas-Fas ligand- and perforin-induced tumor apoptosis, respectively. J Immunol 163:583–589, 1999 [PubMed] [Google Scholar]
- 16. Justo P, Sanz AB, Lorz C, Egido J, Ortiz A. Lethal activity of FADD death domain in renal tubular epithelial cells. Kidney Int 69:2205–2211, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Lee P, Sata M, Lefer DJ, Factor SM, Walsh K, Kitsis RN. Fas pathway is a critical mediator of cardiac myocyte death and MI during ischemia-reperfusion in vivo. Am J Physiol Heart Circ Physiol 284:H456–H463, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Leslie JA, Meldrum KK. The role of interleukin-18 in renal injury. J Surg Res 145:170–175, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Liang D, Liu HF, Yao CW, Liu HY, Huang-Fu CM, Chen XW, Du SH. Effects of interleukin 18 on injury and activation of human proximal tubular epithelial cells. Nephrology (Carlton) 12:53–61, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Marino E, Cardier JE. Differential effect of IL-18 on endothelial cell apoptosis mediated by TNF-alpha and Fas (CD95). Cytokine 22:142–148, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Meldrum KK, Meldrum DR, Hile KL, Yerkes EB, Ayala A, Cain MP, Rink RC, Casale AJ, Kaefer MA. p38 MAPK mediates renal tubular cell TNF-α production and TNF-α-dependent apoptosis during simulated ischemia. Am J Physiol Cell Physiol 281:C563–C570, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, Schrier RW, Edelstein CL. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest 107:1145–1152, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Melnikov VY, Faubel S, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL. Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest 110:1083–1091, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Misseri R, Meldrum DR, Dinarello CA, Dagher P, Hile KL, Rink RC, Meldrum KK. TNF-α mediates obstruction-induced renal tubular cell apoptosis and proapoptotic signaling. Am J Physiol Renal Physiol 288:F406–F411, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Misseri R, Rink RC, Meldrum DR, Meldrum KK. Inflammatory mediators and growth factors in obstructive renal injury. J Surg Res 119:149–159, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Miyajima A, Chen J, Lawrence C, Ledbetter S, Soslow RA, Stern J, Jha S, Pigato J, Lemer ML, Poppas DP, Vaughan ED, Felsen D. Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int 58:2301–2313, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Netea MG, Fantuzzi G, Kullberg BJ, Stuyt RJ, Pulido EJ, McIntyre RC, Jr, Joosten LA, Van der Meer JW, Dinarello CA. Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J Immunol 164:2644–2649, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Ohtsuki T, Micallef MJ, Kohno K, Tanimoto T, Ikeda M, Kurimoto M. Interleukin 18 enhances Fas ligand expression and induces apoptosis in Fas-expressing human myelomonocytic KG-1 cells. Anticancer Res 17:3253–3258, 1997 [PubMed] [Google Scholar]
- 29. Ortiz A, Justo P, Catalan MP, Sanz AB, Lorz C, Egido J. Apoptotic cell death in renal injury: the rationale for intervention. Curr Drug Targets Immune Endocr Metabol Disord 2:181–192, 2002 [PubMed] [Google Scholar]
- 30. Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol 16:3046–3052, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis 43:405–414, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Schneider P, Tschopp J. Apoptosis induced by death receptors. Pharm Acta Helv 74:281–286, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Truong LD, Sheikh-Hamad D, Chakraborty S, Suki WN. Cell apoptosis and proliferation in obstructive uropathy. Semin Nephrol 18:641–651, 1998 [PubMed] [Google Scholar]
- 34. Tsutsui H, Matsui K, Kawada N, Hyodo Y, Hayashi N, Okamura H, Higashino K, Nakanishi K. IL-18 accounts for both TNF-alpha- and Fas ligand-mediated hepatotoxic pathways in endotoxin-induced liver injury in mice. J Immunol 159:3961–3967, 1997 [PubMed] [Google Scholar]
- 35. Wyburn K, Wu H, Chen G, Yin J, Eris J, Chadban S. Interleukin-18 affects local cytokine expression but does not impact on the development of kidney allograft rejection. Am J Transplant 6:2612–2621, 2006 [DOI] [PubMed] [Google Scholar]