Abstract
Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid metabolite formed by phosphorylation of sphingosine. S1P has been indicated to play a significant role in the cardiovascular system. It has been shown that the enzymes for S1P metabolism are expressed in the kidneys. The present study characterized the expression of S1P receptors in the kidneys and determined the role of S1P in the control of renal hemodynamics and sodium excretion. Real-time RT-PCR analyses showed that S1P receptors S1P1, S1P2, and S1P3 were most abundantly expressed in the renal medulla. Immunohistochemistry revealed that all three types of S1P receptors were mainly located in collecting ducts. Intramedullary infusion of FTY720, an S1P agonist, produced a dramatic increase in sodium excretion by twofold and a small but significant increase in medullary blood flow (16%). Administration of W146, an S1P1 antagonist, into the renal medulla blocked the effect of FTY720 and decreased the sodium excretion by 37% when infused alone. The antagonists of S1P2 and S1P3 had no effect. FTY720 produced additive natriuretic effects in combination with different sodium transporter inhibitors except amiloride, an epithelial sodium channel blocker. In the presence of nitric oxide synthase inhibitor l-NAME, FTY720 still increased sodium excretion. These data suggest that S1P produces natriuretic effects via activation of S1P1 in the renal medulla and this natriuretic effect may be through inhibition of epithelial sodium channel, which is nitric oxide independent. It is concluded that S1P is a novel diuretic factor in the renal medulla and may be an important regulator of sodium homeostasis.
Keywords: renal blood flow, sodium transporter, nitric oxide, collecting duct
sphingolipids were originally thought to serve only as structural components of mammalian cell membranes. In recent years, sphingolipid metabolites are emerging as important lipid signaling molecules. Among them, sphingosine-1-phosphate (S1P) is known to play important roles in cellular processes in various organ systems including cardiovascular system and kidney (15, 29). S1P is formed by phosphorylation of sphingosine catalyzed by sphingosine kinase and acts via its G protein-coupled receptors (17, 29). Five members of S1P receptor family have been identified and termed S1P1-S1P5 (17, 29). The S1P1, S1P2, and S1P3 are ubiquitously expressed, while S1P4 is predominantly expressed in the lung and lymphoid system, and S1P5 is mainly in brain tissue (17, 29). The expression and activity of sphingosine kinase have been detected in the kidneys (1, 10) and the mRNAs of S1P receptors are also present in the kidneys (17). It has been shown that S1P protects the kidneys from ischemic injury (2, 27). It has also been demonstrated that S1P regulates vascular functions (14, 23). Therefore, it is possible that S1P may be involved in the regulation of renal function. However, the role of S1P in the regulation of renal function remains unclear. The present study was designed to characterize the expressions of S1P receptors in the kidney and determine whether S1P participates in the control of renal hemodynamics and sodium excretion.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley (Harlan, Madison, WI) rats weighing 250 to 300 g were maintained on a standard pellet diet (Purina Mills) with free access to water in the Animal Resource Center of Virginia Commonwealth University. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University.
RNA extraction and quantitative RT-PCR analysis of the mRNA levels of S1P1, S1P2, and S1P3.
Total RNA from renal cortical and medullary tissues was extracted using TRIzol solution (Life Technologies, Rockville, MD) and then reverse-transcribed (RT; cDNA Synthesis Kit, Bio-Rad, Hercules, CA) (19). The RT products were amplified using real-time quantitative PCR kits. For the analyses of S1P receptor mRNA levels, a SYBR green real-time quantitative PCR kit (Bio-Rad) was used. Primers were designed by using a primer design computer program (Beacon Designer, Bio-Rad) on the basis of sequences of rat S1P1, S1P2, and S1P3 mRNA and 18S ribosomal RNA (rRNA) from GenBank. For S1P1, the sense primer was CCG CTC TAC CAC AAG CAC TA, antisense CCT GCA GTA GAG GAT GAC GA; for S1P2, sense TTG GGA CAG GAT CTC ACG TA, antisense GAG GGT CAG AAG TCC AAA GC; for S1P3, sense TGT CTC CAC AGG TCA AGC TC, antisense CAG ACC TCC AGG GTC AAG AT; for 18S rRNA, sense CGCCGCTAGAGGTGAAATTC, antisense TCTTGGCAAATGCTTTCGC. The real-time quantitative PCR was performed using an iCycler iQ Real-Time PCR Detection System (Bio-Rad) according to the manufacturer's manual. Post-PCR melting curves verified the specificity of single-target amplification, which was also confirmed by separating the PCR product in 1.5% agarose gel electrophoresis and visualized with the use of ethidium bromide staining. Equal amplification efficiencies of 18S rRNA and target genes were tested, and optimization of primers and cDNA concentrations was performed in preliminary experiments. Data were gathered and analyzed by the same Real-Time PCR Detection System. The cycle threshold (Ct) values were exported into a Microsoft Excel worksheet for calculation of gene expression in accordance with the ΔΔCt method. The Ct values were first normalized with respect to 18S to obtain ΔCt values. The ΔCt values in S1P1 from the renal outer medulla were averaged and used as a reference to calculate ΔΔCt values for all other samples. Relative mRNA levels were expressed by the values of 2−ΔΔCt.
Preparation of the homogenates from renal tissues and Western blot analysis of S1P1, S1P2, and S1P3 protein levels.
Tissue homogenate was prepared as we described previously (33). The dissected cortical, outer medullary, and inner medullary tissues were homogenized with a glass homogenator in ice-cold HEPES buffer containing (in mmol/l) 25 Na-HEPES, 1 EDTA, and 0.1 phenylmethylsulfonyl fluoride. After centrifugation of the homogenate at 6,000 g for 5 min at 4°C, the supernatant containing membrane and cytosolic components, termed homogenate, was aliquoted, frozen in liquid nitrogen, and stored at −80°C until used. Western blot analyses were performed as described previously (35). Briefly, protein samples (20 μg) were subjected to 10% SDS-PAGE gel electrophoresis and electrophoretically transferred onto nitrocellulose membranes. The membranes were then probed with antibodies (1:1,000) against S1P1, S1P2, and S1P3 (Santa Cruz Biotechnology; S1PR1: EDG-1, sc-16070, goat IgG; S1PR2: EDG-5, sc-25491, rabbit IgG; S1PR3: EDG-3, sc-22214, goat IgG) overnight in cold room (4°C). After being washed, the membrane was incubated for 1 h with 1:3,000 horseradish peroxidase-labeled secondary antibodies. Then, enhanced chemiluminescence detection solution (ECL, Pierce) was added directly to the blots on the surface carrying proteins, and the membrane was wrapped in Saran wrap and exposed to Kodak Omat film. The intensity of the blots was determined using an imaging analysis program (ImageJ, free download from http://rsbweb.nih.gov/ij/). The membranes were stripped and reprobed with β-actin antibody, which was used as internal control.
Immunohistochemical analysis for S1P1, S1P2, and S1P3 in rat kidney.
The kidneys were removed, cut longitudinally, and fixed in 10% neutral buffered formalin. The kidneys were then embedded in paraffin, and 4-μm sections were cut. Immunostaining were performed as we described before (20) using antibodies against S1P1, S1P2, and S1P3 (1:200 dilution; Santa Cruz Biotechnology), respectively. For negative controls, normal goat or rabbit serum was used instead of the primary antibodies. The negative controls showed no immunoreactivity.
Effect of renal medullary infusion of S1P agonist and/or antagonists on urinary volume, sodium excretion, and renal cortical and medullary blood flows.
Rats were prepared for renal medullary interstitial infusion and measurement of renal tissue blood perfusions as we described previously (21, 35). In brief, after being anesthetized with ketamine (Ketaject; 30 mg/kg body wt im; Phoenix Pharmaceutical, St. Joseph, MO) and thiobutabarbital (Inactin; 50 mg/kg body wt ip; Sigma, St. Louis, MO), the rats were placed on a thermostatically controlled warming table to maintain body temperature at 37°C. After tracheotomy, cannulas were placed in the right femoral vein and artery for intravenous infusions and measurements of arterial pressure.
For renal medullary infusion, the left kidney was immobilized by placement of its dorsal side up in a kidney cup. A catheter (tapered tip, 4–5 mm) was gently implanted into the medulla vertically from the dorsal surface and anchored into place on the kidney surface with Vetbond Tissue Adhesive (3M). The catheter was infused with PBS containing (in mM) 205 NaCl, 40.5 Na2HPO4, and 9.5 NaH2PO4 (pH 7.4, 550 mosM) at a rate of 10 μl/min to maintain the patency of interstitial infusion. A catheter was inserted into the left ureter for urine collection. The urine volume (U·V) was determined gravimetrically and urinary sodium (Na+) and potassium (K+) concentrations were measured using a flame photometer. U·V and urinary Na+ (UNa·V) and K+ (UK·V) excretion were factored per gram kidney weight. For the measurement of cortical and medullary blood flows (CBF and MBF), optical fiber needle probes (Transonic) were implanted to simultaneously measure CBF (1.5-mm depth) and MBF (5-mm depth) using a dual-channel laser-Doppler flowmeter (Transonic).
After the surgery, animals received a continuous intravenous infusion of 0.9% NaCl solution containing 2% albumin at a rate of 1 ml·h−1·100 g body wt−1 throughout the experiment to replace fluid loss and maintain a constant hematocrit (≈42%). At the end of each experiment, animals were euthanized with an excess intravenous dose of pentobarbital sodium (150 mg/kg). The left kidney was excised and the position of the catheter was confirmed.
Experimental protocols.
After a 1.5-h equilibration period and two 20-min control sample collections infused with vehicles, different reagents were infused into renal medulla for 30 min (10-min clearance period and 20-min sample collection). Group 1: infusion of S1P agonist FTY720 (Cayman Chemical) at 36, 72, and 144 μg·kg−1·h−1. Group 2: infusion of S1P1 antagonist W146 (Cayman Chemical) at 4.5, 9, and 18 μg·kg−1·h−1. Group 3: infusion of S1P agonist FTY720 at 36, 72, and 144 μg·kg−1·h−1 plus W146 (18 μg·kg−1·h−1). The FTY720 was first dissolved in DMSO and then in PBS. The final concentration of DMSO was 2%. Previous studies showed that this amount of DMSO does not affect renal function (3, 5, 24). W146 was first dissolved in 20% (2-hydroxypropyl)-β-cyclodextrin (HPβCD) and then in PBS. The final concentration of HPβCD was 0.4%. HPβCD has been widely used as a solubilizing agent. It has been shown that intravenous infusion of 20% HPβCD for 4, 8, and 24 h at a dose of 400 mg/kg does not cause nephrotoxicity and has no influence on urinary Na+ and K+ excretion (28). Vehicle was infused during equilibration period. Additional groups of rats were infused with the antagonists of S1P2 and S1P3 (JTE-013 and CAY10444, 36 μg·kg−1·h−1; Cayman Chemical), respectively, instead of W146. This surgical preparation and protocol procedure have been widely used in the studies of renal physiology and infusion of vehicle alone does not produce time course change in renal function over the period of experiment time.
Effect of renal medullary infusion of FTY720 on different sodium transporters.
Animal preparation was as above. The effect of FTY720 on Na+-K+-Cl− cotransporter was determined by renal medullary infusion of FTY720 (144 μg·kg−1·h−1) and furosemide (Furo; 20 mg·kg−1·h−1) to compare the FTY720-induced increases in UNa·V in the absence and presence of Furo (FTY720 vs. FTY720 + Furo). Similarly, the effects of FTY720 on Na+-Cl− cotransporter, Na+/H+ exchanger, and epithelial sodium channel (ENaC) were determined by comparing the increases in UNa·V induced by FTY720 alone with that by FTY720 plus hydrochlorothiazide (Thia; 1.5 mg·kg−1·h−1), methylisobutyl amiloride (MIA; 50 μg·kg−1·h−1), and amiloride (AMI; 40 μg·kg−1·h−1), respectively. All sodium transporter inhibitors were from Sigma.
Effects of renal medullary infusion of l-NAME on FTY720-induced natriuresis.
Animal preparation was as above. After equilibration, the animals were pretreated with renal medullary infusion of l-arginine methyl ester (l-NAME; 100 μg·kg−1·min−1), an inhibitor of nitric oxide synthase (NOS), and then FTY720 (144 μg·kg−1·h−1). Renal blood flow and urinary sodium excretion were analyzed as above.
Statistics.
Data are presented as means ± SE. The significance of differences in mean values within and between multiple groups was evaluated using an ANOVA followed by a Duncan's multiple range test. Student's t-test was used to evaluate statistical significance of differences between two groups. P < 0.05 was considered statistically significant.
RESULTS
Expression of S1P1, S1P2, and S1P3 in the renal cortex, outer medulla, and inner medulla.
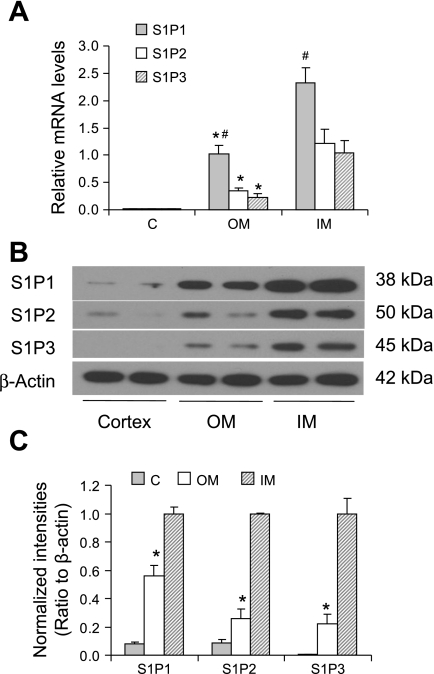
Real-time quantitative RT-PCR analyses for the mRNA expression of S1P1, S1P2, and S1P3 are presented in Fig. 1A, which shows the relative quantitation of S1P1, S1P2, and S1P3 mRNA expressions in different kidney regions. S1P1, S1P2, and S1P3 mRNAs were all detected in three different kidney regions. The mRNA expressions of three S1P receptors were all much higher in medullary area than in cortical area, and S1P1 was shown to be most abundant compared with S1P2 and S1P3.
Fig. 1.
mRNA and protein levels of sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 in the kidneys. A: relative mRNA levels by real-time RT-PCR analysis. The gene expressions were first normalized by the values of 18S rRNA and then calibrated to the levels of S1P1 from the outer medulla (OM). C, cortex; IM, inner medulla. B: representative ECL gel documents of Western blot analyses depicting the protein levels of S1P receptors. C: summarized intensities of Western blots that were normalized by β-actin and then calibrated to the values from IM. *P < 0.05 vs. IM and C. #P < 0.05 vs. S1P2 and S1P3 (n = 5).
The results obtained from Western blot analyses of three S1P receptor proteins are presented in Fig. 1, B and C. Similar to the mRNA expression pattern, the blot intensities of all three S1P receptor proteins were significantly higher in the outer medulla and inner medulla compared with that in the renal cortex.
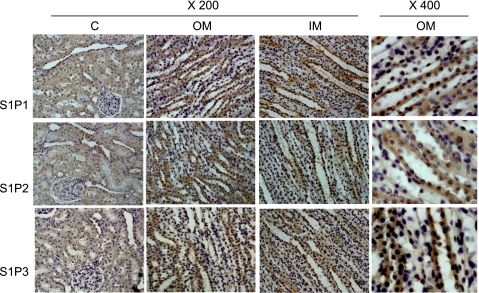
To further define the location of three S1P receptors in the kidney, immunohistochemistry was performed. As shown in Fig. 2, S1P1, S1P2, and S1P3 were detected in all kidney regions, including the cortex, outer medulla and inner medulla, mainly in collecting ducts. Weak staining was observed in proximal tubules and glomeruli. The immunostaining patterns of three S1P receptors were similar in different kidney areas. The positive staining area and intensity showed that three S1P receptors were predominantly expressed in the renal medulla, which was consistent with the results obtained by RT-PCR and Western blot analyses.
Fig. 2.
Immunohistochemical staining of S1P1, S1P2, and S1P3 proteins in the kidneys. Representative staining (brown staining) of S1P receptors in renal cortical (C), outer medullary (OM), and inner medullary (IM) areas in rat kidney (representatives of experiments from 5 rats).
Effects of renal medullary infusion of S1P agonist and/or antagonist on renal hemodynamics and excretory functions.
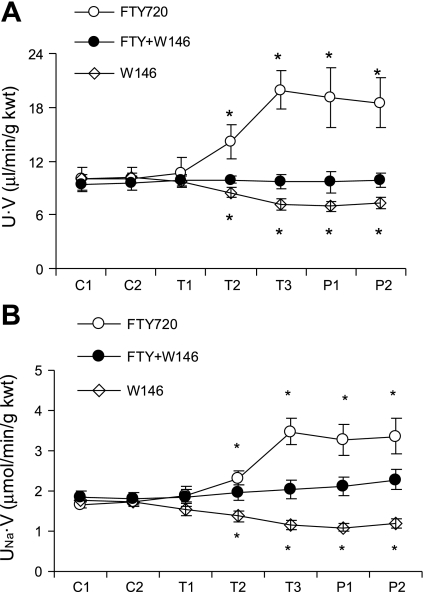
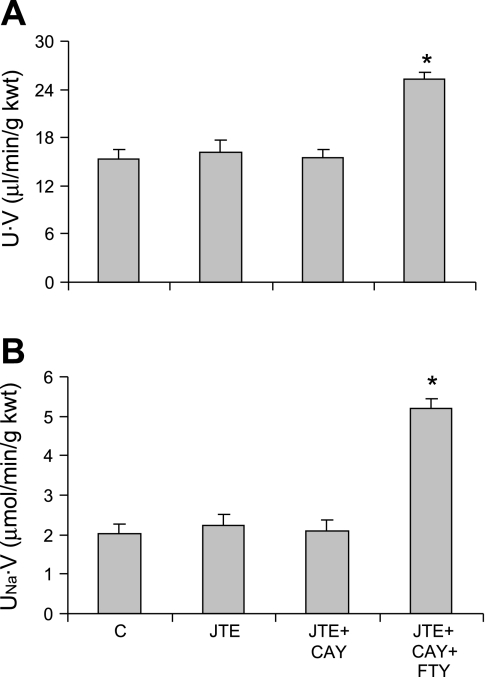
To examine whether S1P has effects on renal hemodynamics and excretory functions, different doses of FTY720, an S1P agonist, were infused into the renal medulla. There was no change in mean arterial pressure after infusion of FTY720. However, infusion of FTY720 significantly increased U·V and UNa·V (Fig. 3). FTY720 had no effect on UK·V (data not shown). FTY720 induced small but significant increases in MBF with no effect on CBF (Fig. 4). The effects of FTY720 on urinary excretion and MBF were abolished when it was infused in combination with W146, a S1P1 antagonist (Figs. 3 and 4). W146 alone induced a significant decrease in U·V and UNa·V (Fig. 3) with no effect on renal blood flow (Fig. 4). Infusion of JTE-013, a S1P2 antagonist, and CAY10444, a S1P3 antagonist, did not show effect on U·V and UNa·V (Fig. 5). In the presence of CAY101444 and JTE-013, FTY720 still significantly increased U·V and UNa·V to the values similar to FTY720 alone (Fig. 5). These data indicated that S1P produced a strong natriuretic effect and a weak vasodilator effect, which was probably through the activation of S1P1 receptor.
Fig. 3.
Effect of S1P agonist and/or S1P1 antagonist on urinary volume (U·V) and sodium excretion (UNa·V). A: U·V. B: UNa·V. FTY720, S1P agonist; W146, S1P1 antagonist; C, control with vehicle infusion; T, treatment with reagent infusions; P, postreagents with vehicle infusions. *P < 0.05 vs. other 2 groups (n = 6).
Fig. 4.
Effect of S1P agonist and/or S1P1 antagonist on renal cortical and medullary blood perfusion. A: cortical blood flow. B: medullary blood flow. *P < 0.05 vs. W146. #P < 0.05 vs. FTY+W146 (n = 6).
Fig. 5.
Effect of S1P agonist and/or S1P2 and S1P3 antagonists on U·V and UNa·V. A: U·V. B: UNa·V. C, control, vehicle infusion; JTE, JTE-013, a S1P2 antagonist; CAY, CAY10444, a S1P3 antagonist. *P < 0.05 vs. others (n = 6).
It should be noted that the doses of W146, JET-013, and CAY10444 used in the present study were prepared using maximally available concentration according to the maximal solubility of the compounds. Based on the IC50 of these compounds, the doses of JET-013 and CAY10444 were comparable with that of W146. Therefore, the absence of effects of JTE-013 and CAY1044 on renal function was not because of the insufficient doses.
Effect of renal medullary infusion of FTY720 on urinary volume and sodium transporters.
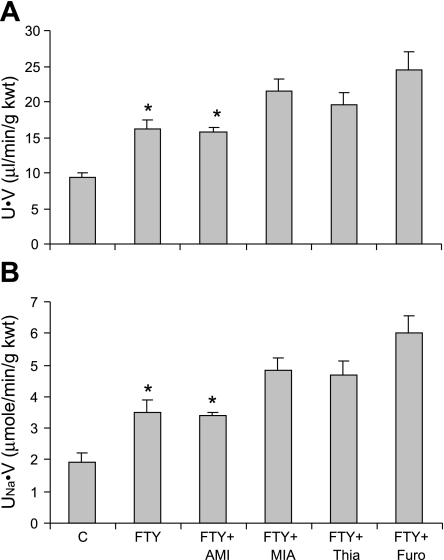
FTY720 in combination with different sodium transporter inhibitors produced additive diuretic and natriuretic effect except with amiloride, an inhibitor of ENaC (Fig. 6). In additional experiments, we confirmed that the dose of amiloride used in the present study achieved the maximal diuretic/natriuretic effect (data not shown). Thus, the lack of additive effect by amiloride in the present study was not due to an insufficient dose. These results demonstrated that inhibitor of ENaC did not further increase urinary volume and sodium excretion in combination with FTY720, which suggested that the diuretic and natriuretic effect of FTY720 may be through inhibition of ENaC but not other sodium transporters including Na+-K+-Cl− cotransporter, Na+-Cl− cotransporter, and Na+/H+ exchanger.
Fig. 6.
Effect of renal medullary infusion of FTY720 on sodium transporters. A: U·V. B: UNa·V. AMI, amiloride, an epithelial Na+ channel blocker; MIA, methylisobutyl amiloride, a Na+/H+ exchanger inhibitor; Thia, hydrochlorothiazide, a Na+-Cl− cotransporter inhibitor; Furo, furosemide, a Na+-K+-Cl− cotransporter inhibitor. *P < 0.05 vs. others (n = 6).
Effects of renal medullary infusion of l-NAME on FTY720-induced natriuresis.
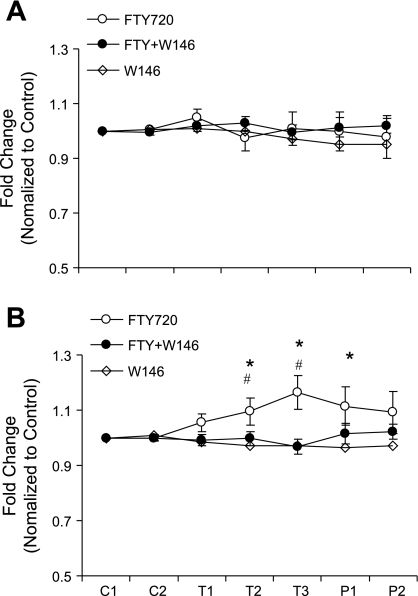
In rats pretreated with renal medullary infusion of l-NAME (100 μg·kg−1·min−1), infusion of FTY720 still significantly increased UNa.V. The values of FTY720-induced increases in UNa.V (ΔUNa.V) were similar between animals pretreated with and without l-NAME. The ΔUNa.V was 1.91 ± 0.25 μmol·min−1·g kidney wt−1 with l-NAME vs. 1.82 ± 0.16 μmol·min−1·g kidney wt−1 without l-NAME (P > 0.05). However, in rats pretreated with renal medullary infusion of l-NAME (100 μg·kg−1·min−1), FTY720-induced increase in MBF was absent. These data indicate the tubular natriuretic effects of FTY720 may be NO independent, while vascular effect of FTY720 may be NO dependent.
DISCUSSION
The present study demonstrated that the expressions of S1P1, S1P2, and S1P3 were much higher in the renal medulla than in the renal cortex, mainly located in collecting duct, and S1P agonist induced a significant natriuresis, which was blocked by S1P1 antagonist but not S1P2 and S1P3 antagonists. In addition, among the inhibitors of major sodium transporters in the renal medulla, inhibitor of ENaC in combination with S1P agonist did not produce additive natriuretic effects and S1P agonist-induced natriuresis was not inhibited by l-NAME. These results indicate that S1P may act as a natriuretic factor via activation of S1P1 receptor and the effect of S1P may be through inhibition of ENaC, which is independent of NO production.
Our results showed that S1P1, S1P2, and S1P3 were predominantly expressed in the renal medulla. We therefore focused our study on the role of S1P in the regulation of renal medullary function by infusing the reagents locally into the renal medulla. It was demonstrated that S1P agonist increased both urinary sodium excretion and MBF. The changes in urinary sodium excretion were more profound than that in MBF, indicating that S1P acts via both vascular and tubular mechanisms and that S1P may regulate tubular and vascular functions in different ways. The effect of S1P agonist on MBF in our results was different from a previous report showing that intravenous injection of S1P reduced renal blood flow (6). It has been shown that S1P causes vessel dilation or constriction in different vascular beds depending on the dominant receptor types expressed (14). S1P1 mediates vessel dilation while S1P2 and/or S1P3 mediates vessel constriction (14). Meanwhile, renal CBF and MBF are regulated differently (9, 22). S1P reduces total renal blood flow and increases MBF, indicating that S1P may participate in the regulations of renal cortical and medullary circulation by different mechanisms. The tubular effect of S1P agonist in our study was consistent with previous reports that showed systemic administration of S1P reduced RBF while increasing urinary sodium excretion (4, 6).
To identify which S1P receptor mediated the natriuretic action of S1P, we utilized S1P antagonists. Our results showed that S1P1 antagonist abolished the effect of FTY720, while S1P2 and S1P3 antagonists did not change the natriuretic effect of FTY720, suggesting that the natriuretic effect of S1P is through the activation of S1P1. Additionally, S1P1 antagonist alone significantly reduced the urinary sodium excretion, suggesting that endogenous S1P plays an important role in the regulation of sodium excretion under basal condition. This result is consistent with the fact that S1P1 is constitutively activated (18, 32, 36). S1P1 antagonist alone reduced urinary sodium excretion but failed to change MBF probably because the vascular effect of S1P was much weak compared with its tubular action in the renal medulla and the effect of S1P1 antagonist on MBF under basal conditions, if any, might be undetectable. S1P2 and S1P3 antagonists did not produce any effect on urinary sodium excretion and renal blood flow when infused into the renal medulla, further suggesting that these two S1P receptors may not mediate the natriuretic effect of S1P. Overall, our data suggest that the natriuretic effect of S1P agonist in the renal medulla is mediated by S1P1 receptor.
To determine which sodium transporter is accountable to natriuretic action of S1P, we performed experiments to dissect the effects of S1P agonist on major sodium transporters in the renal medulla. Our results demonstrated that the tubular effects of S1P agonist might be through inhibition of ENaC activity. These results were in agreement with the expression pattern of S1P1 receptor, i.e., mainly located in collecting ducts. It is speculated that S1P targets ENaC in collecting ducts to inhibit sodium reabsorption.
It should be pointed out that there is a limitation in the in vivo experiment to explore the exact effects of S1P on different sodium transporters and the present study cannot totally rule out the possibility that S1P may affect other sodium transporters in addition to ENaC. It has been known that sodium transporters also participate in the regulation of renal hemodynamics and inhibition of sodium transport may reduce renal blood flow or GFR (7, 8, 26, 30, 31). Therefore, there would be interactions/counteractions between vascular and tubular effects as well as the effects of S1P and sodium transporter inhibitors. These interactions/counteractions may cover some weak effects of S1P on sodium transporters. For example, it has been reported that S1P stimulates Na+/H+ exchanger (NHE) in some nonrenal cells (16, 25). The present study could not conclude whether renal NHE was involved in the effect of S1P because of the complicated interactions/counteractions in the in vivo experiments. Thus, the results from the current study are not conclusive regarding the detailed effects of S1P on specific sodium transporters. A study using isolated and perfused tubular segments may clarify whether S1P exhibits effects on renal NHE and other tubular transporters in future studies. Nevertheless, this limitation would not impact our conclusion that the S1P executes natriuretic effect, which may be mainly through inhibition of ENaC and a weak vasodilator effect in the renal medulla.
Because S1P has been shown to simulate NO production (13), we further detected whether the natriuretic effect of S1P agonist was through the activation of NOS. That S1P agonist still increased the urinary sodium excretion in the presence of l-NAME suggests that the effect of FTY720 is not mediated by NO. This result was consistent with our finding that FTY720 did not affect the activities of other sodium transporters because stimulation of NO production would have inhibited all different sodium transporters (11, 12, 34). The exact mechanisms for S1P to inhibit ENaC need to be clarified in future investigations. In this regard, a possible intracellular signaling pathway is that S1P1 couples to Gi protein and inhibits the production of cAMP (17, 29), a well-known activator of ENaC. S1P1 has been shown to couple only to the heterotrimeric G protein, Gi, while S1P2 and S1P3 can couple to Gi, Gq, and G13 (17, 29). It has also been shown that S1P2 and S1P3 activate adenylyl cylcase activity (17, 29). The different roles of different S1P receptors in the regulation of cAMP production are in agreement with our results, which showed that S1P1 mediated a natiuretic effect.
In summary, the present study demonstrated that S1P agonist increased the urinary sodium excretion through S1P1 receptor by inhibiting the ENaC activity and this natriuretic action was NO independent. It is suggested that S1P is a novel diuretic factor in the renal medulla and may be an important regulator of sodium homeostasis.
GRANTS
This work was supported by National Institutes of Health Grants HL89563 and DK54927.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Alemany R, van Koppen CJ, Danneberg K, Ter Braak M, Meyer Zu Heringdorf D. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch Pharmacol 374: 413–428, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bajwa A, Jo SK, Ye H, Huang L, Dondeti KR, Rosin DL, Haase VH, Macdonald TL, Lynch KR, Okusa MD. Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol 21: 955–965, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bennett WM, Bristol T, Weaver WJ, Muther RS. Lack of nephrotoxicity of dimethyl sulfoxide in man and laboratory animals. Ann NY Acad Sci 411: 43–47, 1983 [DOI] [PubMed] [Google Scholar]
- 4. Bischoff A, Meyer Zu Heringdorf D, Jakobs KH, Michel MC. Lysosphingolipid receptor-mediated diuresis and natriuresis in anaesthetized rats. Br J Pharmacol 132: 1925–1933, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chatterjee PK, Todorovic Z, Sivarajah A, Mota-Filipe H, Brown PA, Stewart KN, Mazzon E, Cuzzocrea S, Thiemermann C. Inhibitors of calpain activation (PD150606 and E-64) and renal ischemia-reperfusion injury. Biochem Pharmacol 69: 1121–1131, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Czyborra C, Bischoff A, Michel MC. Indomethacin differentiates the renal effects of sphingosine-1-phosphate and sphingosylphosphorylcholine. Naunyn Schmiedebergs Arch Pharmacol 373: 37–44, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Dobrowolski L, B dzyńska B, Sadowski J. Differential effect of frusemide on renal medullary and cortical blood flow in the anaesthetised rat. Exp Physiol 85: 783–789, 2000 [PubMed] [Google Scholar]
- 8. Dobrowolski L, Sadowski J. Furosemide-induced renal medullary hypoperfusion in the rat: role of tissue tonicity, prostaglandins and angiotensin II. J Physiol 567: 613–620, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evans RG, Eppel GA, Anderson WP, Denton KM. Mechanisms underlying the differential control of blood flow in the renal medulla and cortex. J Hypertens 22: 1439–1451, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Facchinetti MM, Leocata Nieto F, Marquez MG, Sterin-Speziale N. Stratification of sphingosine kinase-1 expression and activity in rat kidney. Cells Tissues Organs 188: 384–392, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Granger JP, Alexander BT. Abnormal pressure-natriuresis in hypertension: role of nitric oxide. Acta Physiol Scand 168: 161–168, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Herrera M, Ortiz PA, Garvin JL. Regulation of thick ascending limb transport: role of nitric oxide. Am J Physiol Renal Physiol 290: F1279–F1284, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Igarashi J, Michel T. S1P and eNOS regulation. Biochim Biophys Acta 1781: 489–495, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Igarashi J, Michel T. Sphingosine-1-phosphate and modulation of vascular tone. Cardiovasc Res 82: 212–220, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jo SK, Bajwa A, Awad AS, Lynch KR, Okusa MD. Sphingosine-1-phosphate receptors: biology and therapeutic potential in kidney disease. Kidney Int 73: 1220–1230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnstone ED, Speake PF, Sibley CP. Epidermal growth factor and sphingosine-1-phosphate stimulate Na+/H+ exchanger activity in the human placental syncytiotrophoblast. Am J Physiol Regul Integr Comp Physiol 293: R2290–R2294, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Kluk MJ, Hla T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G protein-coupled receptors. Biochim Biophys Acta 1582: 72–80, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Lee MJ, Evans M, Hla T. The inducible G protein-coupled receptor edg-1 signals via the G(i)/mitogen-activated protein kinase pathway. J Biol Chem 271: 11272–11279, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Li N, Chen L, Muh RW, Li PL. Hyperhomocysteinemia associated with decreased renal transsulfuration activity in Dahl S rats. Hypertension 47: 1094–1100, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Li N, Yi F, Sundy CM, Chen L, Hilliker ML, Donley DK, Muldoon DB, Li PL. Expression and actions of HIF prolyl-4-hydroxylase in the rat kidneys. Am J Physiol Renal Physiol 292: F207–F216, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Li N, Zhang G, Yi FX, Zou AP, Li PL. Activation of NAD(P)H oxidase by outward movements of H+ ions in renal medullary thick ascending limb of Henle. Am J Physiol Renal Physiol 289: F1048–F1056, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Mattson DL. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol 284: R13–R27, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Morris AJ, Panchatcharam M, Cheng HY, Federico L, Fulkerson Z, Selim S, Miriyala S, Escalante-Alcalde D, Smyth SS. Regulation of blood and vascular cell function by bioactive lysophospholipids. J Thromb Haemost 7, Suppl 1: 38–43, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nesic Z, Todorovic Z, Stojanovic R, Basta-Jovanovic G, Radojevic-Skodric S, Velickovic R, Chatterjee PK, Thiemermann C, Prostran M. Single-dose intravenous simvastatin treatment attenuates renal injury in an experimental model of ischemia-reperfusion in the rat. J Pharm Sci 102: 413–417, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Nikmo A, Bjorklund S, Vainio M, Ekokoski E, Tornquist K. Sphingosylphosphorylcholine activates Gq, Gi-2, and Gi-3 in thyroid FRTL-5 cells: implications for the activation of calcium fluxes and Na+-H+ exchange. Biochem Biophys Res Commun 258: 812–815, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Okusa MD, Persson AE, Wright FS. Chlorothiazide effect on feedback-mediated control of glomerular filtration rate. Am J Physiol Renal Fluid Electrolyte Physiol 257: F137–F144, 1989 [DOI] [PubMed] [Google Scholar]
- 27. Park SW, Kim M, Chen SW, Brown KM, D'Agati VD, Lee HT. Sphinganine-1-phosphate protects kidney and liver after hepatic ischemia and reperfusion in mice through S1P(1) receptor activation. Lab Invest In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pestel S, Martin HJ, Maier GM, Guth B. Effect of commonly used vehicles on gastrointestinal, renal, and liver function in rats. J Pharmacol Toxicol Methods 54: 200–214, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Peters SL, Alewijnse AE. Sphingosine-1-phosphate signaling in the cardiovascular system. Curr Opin Pharmacol 7: 186–192, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Ren Y, Garvin JL, Liu R, Carretero OA. Crosstalk between the connecting tubule and the afferent arteriole regulates renal microcirculation. Kidney Int 71: 1116–1121, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Wang H, Carretero OA, Garvin JL. Inhibition of apical Na+/H+ exchangers on the macula densa cells augments tubuloglomerular feedback. Hypertension 41: 688–691, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Waters CM, Long J, Gorshkova I, Fujiwara Y, Connell M, Belmonte KE, Tigyi G, Natarajan V, Pyne S, Pyne NJ. Cell migration activated by platelet-derived growth factor receptor is blocked by an inverse agonist of the sphingosine 1-phosphate receptor-1. FASEB J 20: 509–511, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Zou AP, Billington H, Su N, Cowley AW., Jr Expression and actions of heme oxygenase in the renal medulla of rats. Hypertension 35: 342–347, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Zou AP, Cowley AW., Jr Role of nitric oxide in the control of renal function and salt sensitivity. Curr Hypertens Rep 1: 178–186, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Zou AP, Yang ZZ, Li PL, Cowley AJ. Oxygen-dependent expression of hypoxia-inducible factor-1alpha in renal medullary cells of rats. Physiol Genomics 6: 159–168, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Zu Heringdorf DM, Vincent ME, Lipinski M, Danneberg K, Stropp U, Wang DA, Tigyi G, Jakobs KH. Inhibition of Ca2+ signalling by the sphingosine 1-phosphate receptor S1P(1). Cell Signal 15: 677–687, 2003 [DOI] [PubMed] [Google Scholar]