Abstract
Microvascular rarefaction following an episode of acute kidney injury (AKI) is associated with renal hypoxia and progression toward chronic kidney disease. The mechanisms contributing to microvascular rarefaction are not well-understood, although disruption in local angioregulatory substances is thought to contribute. Matrix metalloproteinase (MMP)-9 is an endopeptidase important in modifying the extracellular matrix (ECM) and remodeling the vasculature. We examined the role of MMP-9 gene deletion on microvascular rarefaction in a rodent model of ischemic AKI. MMP-9-null mice and background control (FVB/NJ) mice were subjected to bilateral renal artery clamping for 20 min followed by reperfusion for 14, 28, or 56 days. Serum creatinine level in MMP-9-null mice 24 h after injury [1.4 (SD 0.8) mg/dl] was not significantly different from FVB/NJ mice [1.5 (SD 0.6) mg/dl]. Four weeks after ischemic injury, FVB/NJ mice demonstrated a 30–40% loss of microvascular density compared with sham-operated (SO) mice. In contrast, microvascular density was not significantly different in the MMP-9-null mice at this time following injury compared with SO mice. FVB/NJ mice had a 50% decrease in tissue vascular endothelial growth factor (VEGF) 2 wk after ischemic insult compared with SO mice. A significant difference in VEGF was not observed in MMP-9-null mice compared with SO mice. There was no significant difference in the liberation of angioinhibitory fragments from the ECM between MMP-9-null mice and FVB/NJ mice following ischemic injury. In conclusion, MMP-9 deletion stabilizes microvascular density following ischemic AKI in part by preserving tissue VEGF levels.
Keywords: kidney failure, chronic kidney disease, ischemia, blood vessels
acute kidney injury (AKI) is a commonly encountered clinical syndrome that is associated with considerable morbidity and mortality (25, 49). In addition to the acute consequences of AKI, there is mounting evidence that an episode of AKI increases the risk for development of chronic kidney disease (CKD) and progression to end-stage renal disease (2, 27, 34, 50). Observations in animal models of AKI reveal that chronic alterations in the renal microvasculature occur following the initial insult that may predispose to the development of CKD. In rodent models of AKI, peritubular capillary loss has been demonstrated to occur weeks after the inciting injury (7, 24, 55). This diminished microvascular reserve has been associated with persistent renal hypoxia (9) that can usher in the development and progression of CKD (18, 19, 30, 37). Indeed, the loss in microvascular density has been demonstrated to precede functional indicators of CKD including diminished urinary concentrating ability and the development of progressive proteinuria in a rat model of ischemic AKI (8).
The underlying mechanisms contributing to loss of microvascular density following AKI are not well-understood, although direct injury to endothelial cells as well as disruption in the balance of local angioregulatory substances are thought to be key contributors (5). Matrix metalloproteinases (MMP) are a group of endopeptidases that are important in degrading and modifying the extracellular matrix (ECM). In addition, MMPs have also been demonstrated to play a role in vascular remodeling (23). MMP-2 and -9, together known as gelatinases, have been found to be upregulated in angiogenic lesions (12) and have garnered much interest in the field of tumor biology. While evidence from tumor biology generally supports a role for increased gelatinase activity, particularly MMP-9, in promoting microvascular growth (1, 12, 20, 54), data from myocardial ischemia-reperfusion injury (IRI) indicate that increased gelatinase activity following injury promotes loss of microvascular density (33). Possible insight into the underlying mechanism for this seemingly paradoxical effect is provided by studies demonstrating the activation of gelatinases can release cryptic fragments from the ECM that serve to inhibit blood vessel stability under physiological conditions (36).
Studies in rodent models of ischemic AKI demonstrate an increase in gelatinase activity occurs following injury (6, 47). Furthermore, increased production of angiostatic factors, potentially generated from gelatinase activation, has also been observed (6, 24). Consequently, in this manuscript we examine the effect of MMP-9 gene deletion on microvascular density in a mouse model of ischemic AKI. We demonstrate that while MMP-9 gene deletion is not acutely protective of renal function, it does confer protection of remote microvascular loss following injury. Furthermore, this protection from microvascular loss is associated with preserved tissue-associated vascular endothelial growth factor (VEGF) levels providing a potential mechanism for microvascular stability following injury in MMP-9-null mice. Our results suggest that MMP-9 activation following AKI plays a role in microvascular integrity and that modulation of MMP-9 activity can provide a novel option in the therapeutic approach to AKI.
MATERIALS AND METHODS
Animals and experimental models.
MMP-9−/− (null) mice and FVB/NJ background control mice were obtained from Jackson Laboratory (Bar Harbor, ME). All experiments were conducted in accordance with The Guide for the Care and Use of Laboratory Animals (Washington, DC: National Academy Press, 1996) and approved by the Institutional Animal Care and Use Committee. Male mice, weighing 20–25 g (10–12 wk), were anesthetized with 5% isoflurane for induction followed by buprenorphine HCl (0.05 mg/kg) subcutaneously and 1.5% isoflurane for maintenance and then placed on a homeothermic table to maintain core body temperature at 37°C. A midline incision was made, the renal pedicles were isolated, and bilateral renal ischemia was induced by clamping the renal pedicles for 20 min with microserrefines. After removal of the microserrifines, reperfusion was monitored visually before closure of the abdominal surgical wound. Five hundred microliters of prewarmed (37°C) sterile saline were instilled into the peritoneum at the time of closing. Animals were allowed to recover on a homeothermic pad to maintain body temperature until the righting reflex was restored. Sham surgery consisted of an identical procedure with the exception of immediate release of the clamps. Reperfusion time was for 14, 28, or 56 days at which time the animals were killed and the kidneys were harvested and subsequently processed for biochemical or microscopy studies as below. In a parallel series of experiments, mice subject to renal IRI as above were fed either regular (0.4% NaCl) or high-sodium (4% NaCl) chow (Dyets, Bethlehem, PA) and monitored over 56 days at which time the animals were killed.
In an additional series of experiments, we examined the relative susceptibility of male MMP-9-null mice on an FVB/NJ background to folate nephrotoxicity. Male mice weighing 20–25 g (10–12 wk) received an intraperitoneal injection of folic acid (500 mg/kg in 0.3 M NaHCO3) or an equivalent volume of vehicle control. Serum samples for serum creatinine determination were obtained at 72 h and 28 days and animals were killed at 56 days.
Measurement of serum and urine creatinine concentrations was performed at the UT Southwestern O'Brien Kidney Research Core Center. Urine protein concentrations were measured using a Coomassie blue assay (Coomassie Plus; Pierce Chemical, Rockford, IL). Urine lipocalin-2/neutrophil gelatinase-associated lipocalin (NGAL) was measured using a mouse lipocalin-2/NGAL Quantikine immunoassay as per the manufacturer's instructions (MLCN20, R&D Systems, Minneapolis, MN).
Tissue staining and microscopy.
At the time of death, kidneys were rapidly fixed ex vivo in either 100% methanol, 4% paraformaldehyde, or by zinc fixation (IHC zinc fixative; BD Biosciences, San Jose, CA) according to the instructions of the manufacturer. Tissues were subsequently processed for immunofluorescence staining, immunohistochemistry, or standard histochemistry. Fifty-micrometer vibratome sections of methanol-fixed kidney tissue were obtained for immunofluorescent staining. Primary rabbit polyclonal antibodies to cablin (15) were utilized for immunostaining of microvasculature. Appropriate secondary antibodies conjugated with Texas Red were purchased from Jackson Immunoresearch Laboratories (West Grove, PA). For immunohistochemistry, kidneys were zinc fixed, paraffin embedded, sectioned at 4 μm, deparaffinized, and stained using a Dako Envision+ System, horseradish peroxidase (HRP; Dako North America, Carpinteria, CA), and a primary antibody to PECAM-1 (BD Biosciences). Negative controls were obtained by incubating kidney tissue sections from sham animals and animals undergoing renal ischemia with secondary antibodies in the absence of primary antibodies. Trichrome staining of kidney tissues was performed by the Indiana University Histopathology Lab utilizing standard histochemistry procedures.
Confocal immunofluorescent images of kidney tissue sections were collected at ×40 magnification using a LSM-510 Zeiss confocal microscope (Heidelberg, Germany) equipped with argon and helium/neon lasers. Immunohistochemistry and trichrome-stained images of kidney tissue sections were obtained at ×40 magnification with a Nikon Diaphot compound microscope (Melville, NY). Five to seven images were collected of a kidney from each animal. Percent of total area (excluding glomeruli) staining positive for cablin (IF) or PECAM-1 (IHC) was determined with Metamorph software (Universal Imaging, West Chester, PA) to determine vessel density. Renal microvascular density in confocal images was also examined by placing a reference grid over acquired images, and a blinded observer counted the number of microvascular (i.e., cablin-positive) structures intersecting the grid normalized to the number of tubules in the field.
Western blotting.
Kidneys were removed without fixation, washed with ice-cold saline, minced, and rapidly transferred into 400 μl of ice-cold PHEM buffer (60 mM PIPES, 25 mM HEPES, 10 mM EGTA, and 2 mM MgSO4; pH 6.9) containing 0.5% Triton X-100, 10 μg/ml chymostatin, 10 μg/ml pepstatin, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 0.5 mM phenylmethylsulfonyl fluoride, and 0.1 mM 1,4-dithiothreitol. Samples were sonicated and then allowed to extract on ice for 10 min. Subsequently, the samples were centrifuged at 4°C and the supernatants were carefully removed for protein determinations and Western blotting. Proteins were measured by a Coomassie blue assay (Coomassie Plus; Pierce Chemical) and resolved on a 12% Tris·HCl gel by electrophoresis. Forty micrograms of protein (80 μg for VEGF blots) were loaded in each lane for a given experiment. After electrophoresis, proteins were transferred to a nitrocellulose filter membrane (BioRad Trans-Blot 0.45-μm nitrocellulose membrane) and equal protein loading was visually confirmed by Ponceau staining and actin immunoblotting. Membranes were probed with either rabbit polyclonal anti-VEGF (1:2,000, sc-507, Santa Cruz Biotechnology, Santa Cruz, CA), rat monoclonal anti-NC1 domain of α-1 collagen (1:500), rat monoclonal anti-NC1 domain of α-2 collagen (1:500) (44), or mouse monoclonal anti-actin (1:5,000, MAB1501R, Millipore, Billerica, MA) followed by HRP-conjugated polyclonal goat anti-rabbit IgG (1:5,000, 4040–05, SouthernBiotech, Birmingham, AL), HRP-conjugated goat polyclonal anti-mouse (1:25,000, 170–6516, Bio-Rad, Hercules, CA), or HRP-conjugated polyclonal donkey anti-rat IgG (1:30,000, Jackson Immunoresearch). Immunoreactive bands were detected by chemiluminescence (SuperSignal West Dura Extended, Pierce Chemical). Blots were scanned on a Bio-Rad Fluor-S Multimager to determine band densities.
Statistical analysis.
Results are expressed as means ± SD. Band density data were analyzed by a one-way ANOVA. Due to the branching nature of the imaging data, immunofluorescence/immunohistochemical data were analyzed for significance by a nested ANOVA and then planned comparisons by a one-way ANOVA. A P = 0.05 was utilized to determine significance. Mortality outcome data at 1 wk were analyzed by Fisher's exact test.
RESULTS
Effect of MMP-9 gene deletion on renal function in a model of ischemic AKI.
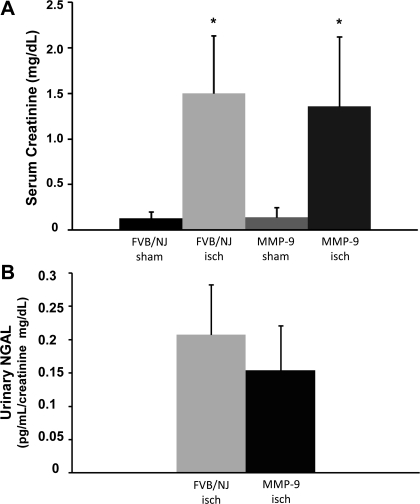
Previous studies in brain and hepatic IRI demonstrated that MMP-9 gene deletion is protective of organ function (4, 21). Consequently, we examined the effect of MMP-9 gene deletion on kidney function in a model of ischemic AKI. The serum creatinine level increased to 1.5 (SD 0.6) mg/dl in FVB/NJ background mice 24 h after a 20-min bilateral renal artery clamp (Fig. 1A), whereas the serum creatinine level increased to 1.4 (SD 0.8) mg/dl in MMP-9-null mice 24 h after a 20-min clamp. While the mean serum creatinine level was lower in MMP-9-null mice following injury, it was not significantly different than in background mice following injury. To further evaluate the susceptibility to IRI between MMP-9-null and background control (FVB/NJ) mice, we examined urinary NGAL excretion 24 h after ischemic injury. Similar to the findings with serum creatinine levels, MMP mice had lower urinary NGAL excretion at 24 h after injury but the values were not significantly different from the values observed in FVB/NJ mice after injury (Fig. 1B). Additionally, although female MMP-9-null mice on a C57/BL6 background are more susceptible to folate-induced AKI (11), we did not find increased susceptibility of male MMP-9-null mice on an FVB/NJ background in this model of acute folate-induced AKI [SCr = 2.3 (SD 1.4) for male FVB/NJ vs. 2.1(SD 1.5) for male MMP-9-null mice, P = 0.8, n = 5].
Fig. 1.
Renal function in a model of acute kidney injury (AKI). Serum creatinine (A) and urinary neutrophil gelatinase-associated lipocalin (NGAL; B) were measured at 24 h after 20 min of bilateral renal ischemia (isch) or sham surgery in control (FVB/NJ) mice and MMP-9-null (MMP-9) mice. Values are expressed as means ± SD. *P < 0.05 vs. control, n = 15 for serum creatinine and n = 9 for urinary NGAL.
MMP-9 gene deletion preserves microvascular density in a model of ischemic AKI.
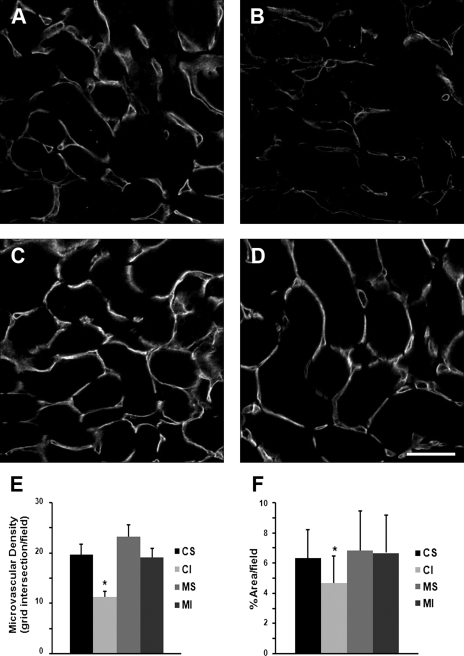
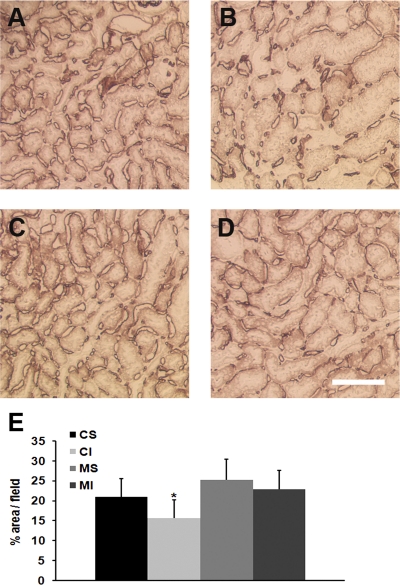
Although MMP-9 gene deletion did not provide acute protection of kidney function in an ischemic model of AKI, we next examined the effect of MMP-9 gene deletion on one of the chronic consequences of AKI, loss of microvascular density. Microvascular structures identified by cablin immunostaining (15) were quantified by two separate methods as described above. Both methods demonstrated a significant 30–40% loss of microvascular density 4 wk after ischemic injury in FVB/NJ background mice that occurred primarily in the outer medullary region of the kidney (Fig. 2). However, microvascular density was not significantly different in the MMP-9-null mice 4 wk after ischemic injury compared with sham-operated MMP-9-null mice. To further validate this finding, microvascular density was examined using PECAM-1 antibodies to identify microvascular structures. The protective effect of MMP-9 gene deletion on microvascular density following ischemic AKI was also observed in PECAM-1 immunostained tissues (Fig. 3).
Fig. 2.
Microvascular density in FVB/NJ background mice and MMP-9-null mice: cablin immunostaining. Kidneys from mice that underwent renal artery clamping for 20 min or sham surgery were harvested 30 days following the procedure. Methanol-fixed tissue sections were immunostained for cablin to identify the peritubular capillaries. Representative images of renal microvasculature, as identified by immunostaining for cablin, 30 days following sham surgery in FVB/NJ mice (A) or MMP-9-null mice (C) and bilateral renal artery clamp in FVB/NJ mice (B) or MMP-9-null mice (D). Bar = 50 μm. E and F: quantitation of microvascular density by 2 methods: first by placing a reference grid over acquired images and having a blinded observer count the number of microvascular (i.e., cablin-positive) structures intersecting the grid normalized to the number of tubules in the field (E) and second by determining the percent of total area (excluding glomeruli) staining positive for cablin (F). CS, FVB/NJ mice following sham surgery; CI, FVB/NJ nice following ischemia-reperfusion injury (IRI); MS, MMP-9-null mice following sham surgery; MI, MMP-9-null mice following IRI. *P < 0.05, n = 4 and 5–7 images per animal.
Fig. 3.
Microvascular density in FVB/NJ background mice and MMP-9-null mice: PECAM-1 immunostaining. Kidneys from mice that underwent renal artery clamping for 20 min or sham operation were harvested 30 days following the procedure. Zinc-fixed tissues were immunostained for PECAM-1 to identify the peritubular capillaries. Representative images of renal microvasculature, as identified by immunostaining for PECAM-1, 30 days following sham operation in FVB/NJ mice (A) or MMP-9-null mice (C) and bilateral renal artery clamp in FVB/NJ mice (B) or MMP-9-null mice (D). Bar = 100 μm. E: quantitation of microvascular density by percent of total area (excluding glomeruli) staining positive for PECAM-1. *P < 0.05, n = 4 and 5–7 images per animal.
MMP-9 gene deletion is associated with a preservation of kidney VEGF levels in a model of AKI.
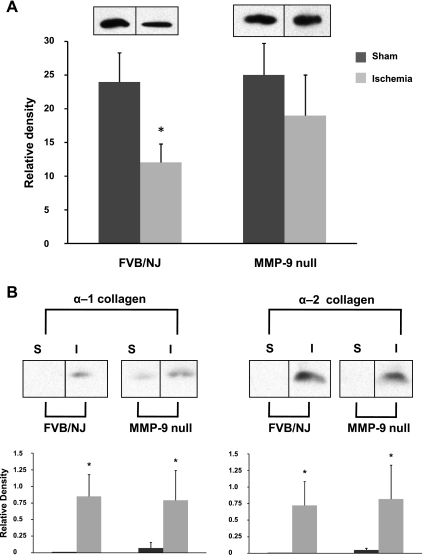
To gain potential insight into a mechanism for the protective effect of MMP-9 gene deletion on microvascular density following ischemic kidney injury, we next quantified the expression levels of key angioregulatory proteins following ischemic injury in MMP-9-null mice. We examined the expression pattern of angioregulatory proteins 2 wk after ischemic injury and thus before the decrease in microvascular density observed in this model of ischemic AKI (24) but after the increase in MMP-9 activity is known to occur (6, 47). In FVB/NJ background mice, we observed a significant decrease of ∼50% in VEGF 2 wk after ischemic insult compared with sham-operated control mice (Fig. 4A). On the contrary, a significant difference in VEGF was not observed in MMP-9-null mice 2 wk after ischemic injury compared with sham-operated MMP-9-null mice.
Fig. 4.
Alterations of angioregulatory proteins in FVB/NJ background mice and MMP-9-null mice following injury. Kidneys from mice that underwent renal artery clamping for 20 min or sham operation were harvested 14 days following the procedure and processed for Western blotting. A: representative lanes from the same immunoblot of tissue-bound vascular endothelial growth factor (VEGF; VEGF188, ∼24-kDa monomer) above the densitometric analysis of the pooled immunoblots for each group. B: representative lanes from the same immunoblot of cryptic extracellular matrix (ECM) peptides corresponding to arresten (∼26 kDa from the NC1 domain of the α-1 chain of collagen IV) and canstatin (∼25 kDa from the NC1 domain of the α-2 chain of collagen IV) above the densitometric analysis of the pooled immunoblots for each group. S, sham-operated; I, ischemia. Values are expressed as mean densities normalized to actin (SD). *P < 0.05 relative to S mice, n = 3.
To examine the release of cryptic fragments from the ECM by MMP-9 that could have an angioinhibitory role following acute ischemic injury, we quantified breakdown products from the NC1 domains of the alpha chains of collagen IV following ischemic injury. Two weeks after ischemia, we observed a significant increase in fragments from the NC1 domain of the α-1 chain of collagen IV (∼26 kDa) and the NC1 domain of the α-2 chain of collagen IV (∼25 kDa) that correspond to the angioinhibitory proteins arresten and canstatin (Fig. 4B). This increase occurred in both FVB/NJ background mice and MMP-9-null mice compared with sham-operated control mice.
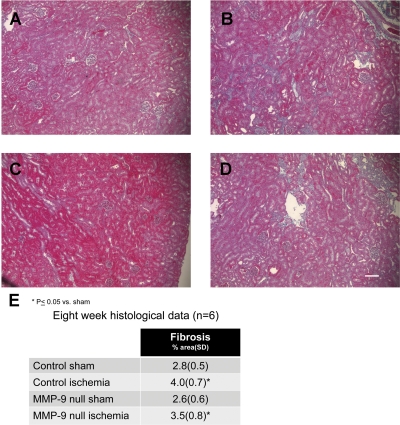
Effect of MMP-9 gene deletion on proteinuria and tissue fibrosis in a model of ischemic AKI.
To determine the impact of microvascular loss on kidney function and morphology, we examined serum creatinine, proteinuria, and tissue fibrosis at 4 and 8 wk following injury. Despite the difference in microvascular density observed, we found no significant difference in serum creatinine or level of proteinuria at 8 wk following injury (Table 1). We observed a significant increase in tissue fibrosis following injury in both FVB/NJ background mice and MMP-9-null mice compared with the respective sham control mice (Fig. 5). The extent of fibrosis observed in the MMP-9-null mice following injury was on average less than the FVB/NJ mice following injury; however, there was no significant difference between the two groups (Fig. 5). Findings at 4 wk for creatinine, proteinuria, and fibrosis (data not shown) were similar to the findings at 8 wk.
Table 1.
Eight-week functional data (n = 6)
| Creatinine, mg/dl (SD) | Proteinuria, Upr/Ucr(SD); mg/mg | |
|---|---|---|
| FVB/NJ sham | 0.10 (0.02) | 0.32 (0.1) |
| FVB/NJ ischemia | 0.10 (0.02) | 0.46 (0.3) |
| MMP-9-null sham | 0.09 (0.02) | 0.35 (0.2) |
| MMP-9-null ischemia | 0.13 (0.03) | 0.41 (0.1) |
MMP-9, matrix metalloproteinase-9; FVB/NJ, background control.
Fig. 5.
Renal fibrosis following injury. Kidneys from mice that underwent renal artery clamping for 20 min or sham operation were harvested 8 wk following the procedure. Representative trichrome-stained images of kidney tissue following sham operation in FVB/NJ mice (A) or MMP-9-null mice (C) and bilateral renal artery clamp in FVB/NJ mice (B) or MMP-9-null mice (D). Bar = 100 μm. E: mean percent area of fibrosis excluding glomeruli (SD). *P < 0.05, n = 6 and 4 images per animal.
Previous studies by Spurgeon-Pechman and co-workers (46) nicely demonstrated the effect of increased dietary sodium intake on kidney function and structure in rodents recovering from ischemic AKI. Given the preservation of microvascular density in the MMP-9-null mice compared with FVB/NJ mice but lack of differences in fibrosis, proteinuria, or serum creatinine at 4 and 8 wk, we next sought to examine the effect of elevated sodium intake during recovery in the MMP-9-null and FVB/NJ mice. Interestingly, we observed a 75% 1-wk mortality in the background control mice on a high-sodium diet compared with a 33% 1-wk mortality in the MMP-9-null mice (P = 0.10); however, the increased mortality in the FVB/NJ mice ultimately limited our analysis of kidney function and structure at 4 and 8 wk.
DISCUSSION
Activation of MMP-9 has been implicated in the pathophysiology of IRI for a variety of organ systems (17, 21, 28, 42, 43, 53). The majority of studies in the kidney showed that MMP-9 increases during IRI (10, 13, 14, 47), although this finding is not universal (57). Activation of MMP-9 in AKI has the potential to trigger a variety of pathophysiologic events through modification of the ECM and alteration of non-ECM proteins. These processes can influence the acute and chronic sequelae of AKI.
We previously demonstrated that the pharmacologic inhibition of MMP-9 concurrent with MMP-2 is protective of acute microvascular injury in a model of ischemic AKI (47). In our current study, the genetic deletion of MMP-9 alone did not confer an overall protective effect on renal function following renal IRI. In contrast, a recent study by Bengatta et al. (11) demonstrated that the genetic deletion of MMP-9 worsened renal function in a model of folate-induced AKI. They provided evidence that MMP-9-mediated release of soluble stem cell factor (SCF) protected proximal tubular cells and intercalated cells from apoptosis in this nephrotoxic model of AKI. They extended their investigation to a model of ischemic AKI and demonstrated similar effects of MMP-9 deletion on tubular cell apoptosis; however, functional data in the ischemic AKI model were not included in their study because they utilized a unilateral renal artery clamp. Whether the overall effect of MMP-9 activation during the acute phase of ischemic AKI is dependent on sex (male vs. female), background strain (FVB/NJ vs. C57/BL6), the ischemic model used (45-min unilateral renal artery clamp vs. 20-min bilateral artery clamp), or that the potential acute beneficial effects of MMP-9 activation on renal function in ischemic AKI are masked by activation of other deleterious pathways requires further investigation. The interaction of MMP-9 with NGAL could provide one potential mechanism for the initiation of competing pathways. NGAL is known to form a complex with MMP-9 that protects MMP-9 from degradation and preserves enzymatic activity (52). MMP-9 activity augmented by complexing with NGAL following renal IRI could enhance the production of SCF thus protecting the tubule from injury. However, NGAL can serve as a prosurvival factor in renal IRI (35, 45, 48) and thus formation of NGAL/MMP-9 complex could hypothetically limit the availability of NGAL to function as a prosurvival factor. In our study with the MMP-9-null mouse, these factors would be reversed (i.e., loss of SCF production and availability of NGAL) following IRI but still opposing. Although we did not observe a difference in urinary NGAL levels between MMP-9-null mice and FVB/NJ mice following ischemia, the assay we utilized does not discern between NGAL complexed with MMP-9 and “free” NGAL per the manufacturer, thus the relative fraction of each could have been different. Another potential situation for opposing pathways in the acute phase of renal IRI involves the relative upregulation of other MMPs in the MMP-9-null mouse. It is known that both MMP-3 and MMP-2 are upregulated in the MMP-9-null mouse (56). We previously demonstrated that MMP-2 activation contributes to injury in the acute phase of ischemic AKI (47) and thus upregulation of MMP-2 in the acute phase may oppose any potential beneficial effect of MMP-9 deletion.
Although MMP-9 gene deletion did not confer an overall protective effect on renal function during the acute phase of ischemic AKI in our study, we did find salutory effects on vascular stability in the chronic phase of ischemic AKI. In this study, we examined nonglomerular microvascular density by immunostaining for both PECAM-1 and cablin [gene sequence (15) homologous to the human gene encoding plasmalemma vesicle-associated protein]. Consistent with prior studies demonstrating no difference in the kidney architecture of MMP-9-null mice compared with background control mice (3) we found no significant difference in the microvascular density between background control mice and MMP-9-null mice, although the mean microvascular density in the MMP-9-null mice was 5–10% greater than that in the background control mice. We observed a 25–30% reduction in microvascular density 1 mo after ischemic injury in the background control mice, which is similar to that previously observed by us and others in rodent models of ischemic AKI (6, 24). In the MMP-9-null mice, there was no statistically significant decrease in microvascular density 1 mo after ischemic injury compared with the sham-operated controls.
As previously mentioned, tumor biology studies generally supported the concept that MMP-9 activation is important for angiogenesis (12, 20, 54) and/or vasculogenesis (1) of tumors. However, some animal studies demonstrated a paradoxical effect of MMP-9 activation on angiogenesis, which has been attributed to the increased production of angiostatic factors by MMP-9 activation in these models (39, 40). Consequently, it has become apparent that the substrate availability in the microenvironment of MMP-9 activation is an important determinant of the overall effect that MMP-9 activation has on blood vessel growth and homeostasis (23). Surprisingly, in this model of ischemic AKI, we did not see a difference in generation of NC-1 fragments consistent with the angiostatic peptides arresten or canstatin in MMP-9-null mice compared with background control mice. Thus, in addition to substrate availability, the pathophysiological stimulus for MMP-9 activation may also play a role. A study by Lindsey et al. (33) underscores this concept. In this study, they demonstrated that MMP-9 gene deletion in a rodent model of myocardial IRI promoted angiogenesis in the injured area of the heart. This angiogenesis of the myocardium in the MMP-9-null mouse following IRI was associated with an alteration of local regulatory factors important in determining the overall angiogenic potential and vascular stability in the injured area of the myocardium. Germane to our study was their examination of tissue VEGF levels. They observed that tissue VEGF levels in background control mice were significantly decreased in injured areas of the myocardium compared with uninjured areas. Interestingly, tissue VEGF levels in the MMP-9-null mice were not significantly decreased in injured areas of the myocardium compared with uninjured areas even though the tissue VEGF levels were overall lower in the MMP-9-null mice. We also observed preservation of tissue VEGF levels in MMP-9-null mice following kidney IRI compared with background control mice following kidney IRI. A potentially important difference is that Lindsey et al. (33) observed increased angiogenesis following ischemic injury, whereas we observed stability of existing vascular structures but not angiogenesis following ischemic injury. The significance of VEGF in maintaining the renal microvasculature is underscored in a study by Kamba et al. (29) that demonstrated up to a 30% reduction in peritbular capillary density with prolonged (21 day) inhibition of VEGF. Additionally, supplementation of VEGF following ischemic injury in a rodent model of AKI preserves renal microvascular structure (32).
VEGF exists as a variety of isoforms varying in length from 121 amino acids to 206 amino acids in humans and that are produced via alternative splicing events (22, 41). VEGF121, VEGF165, and VEGF189 are the three most common isoforms (mouse homologs VEGF120, VEGF164, and VEGF188) and vary in their affinity for the ECM. Upon secretion from VEGF-producing cells, VEGF189 is almost entirely bound to the cell surface or ECM, VEGF121 is freely released, and VEGF165 is 50–70% bound to the cell surface or ECM (26). Evidence suggests that matrix-bound VEGF serves as a biological reservoir of VEGF. Previous studies underscored this notion by demonstrating that endothelial cells grown on a matrix derived from cells transfected with either VEGF189 or VEGF165 stimulated endothelial cell growth (38). In contrast, matrix derived from cells transfected with VEGF121 was no different than matrix derived from cells transfected with vector alone in stimulating proliferation of endothelial cells. Furthermore, there is evidence that the angiogenic signals transmitted by soluble, circulating VEGF and matrix-bound VEGF are different (16). The processing and localized liberation of matrix-bound VEGF by proteases activation, including plasminogen and matrix metalloproteinases, can serve as an important regulator of angiogenesis and vascular patterning (26, 31). Although the role of matrix-bound VEGF in the maintenance and stability of existing vascular structures is to a large extent unknown, our study supports the concept that matrix-bound VEGF is important in this process as it relates to the kidney following injury. In addition, our results are consistent with the possibility that MMP-9 plays an important role in the proteolytic processing of matrix-bound VEGF in ischemic AKI.
Despite the protective effect of MMP-9 deletion on renal microvascular density following IRI, we did not see any benefit in regards to chronic fibrosis. This may be a reflection on the fact that minimal fibrosis occurred with this model. Wang and co-workers (51) demonstrated diminished renal interstitial fibrosis in MMP-9-null mice compared with background controls using a unilateral ureteral obstruction (UUO) model that is a more fibrogenic model than IRI in mice. Breakdown of the tubular basement membrane did not occur in the MMP-9-null mice subjected to UUO which, together with additional data, led the authors to hypothesize that MMP-9 deficiency blocked epithelial cell migration associated with epithelial-to-mesenchymal transformation thus limiting the fibrogenic response. Although we found a statistically significant 30–40% increase in fibrosis at 8 wk following ischemic injury, the total fibrotic area of the kidney was only 3.5–4% as determined by trichrome staining. This degree of fibrosis did not translate into functional renal alteration manifested by proteinuria or elevated serum creatinine as these parameters were not significantly different in the injured animals compared with sham-operated controls. Our attempt to amplify fibrosis and/or unveil chronic functional alterations by intensifying the duration of the initial injury or dietary sodium loading during recovery from ischemia resulted in excessive early mortality. However, the mortality difference in the FVB/NJ mice on a high-sodium diet during recovery compared with the MMP-9-null mice could be considered a de facto indicator of diminished salt sensitivity in the MMP-9-null mice. Nonetheless, the full functional consequences of the difference in microvascular rarefaction in this model or whether MMP-9 gene deletion evokes competing pathways that compensate for one another in IRI remain to be determined and is an area for future research.
In conclusion, we report MMP-9 gene deletion stabilizes microvascular density in a rodent model of ischemic AKI. This protection may occur in part by preservation of tissue VEGF levels. Consequently, therapeutic targeting of MMP-9 during and after ischemic injury may play a role in a multipronged approach to limit the long-term sequelae of AKI.
GRANTS
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants 77124 and 79312, Norman S. Coplon Extramural Research Grant of Satellite Healthcare, and KUFA-ASN Research Grant of the Kidney & Urology Foundation of America and American Society of Nephrology were given to T. A. Sutton. NIDDK Grant 50141 was given to R. L. Bacallao. NIDDK Grant 79328 was provided to UT Southwestern O'Brien Kidney Research Core Center.
Present address of S. Y. Lee: Eulji Hospital, 14 Hangeulbiseok-gil, Nowon-gu, Seoul, Republic of Korea.
Present address of M. Hörbelt: University Hospital, University Duisburg-Essen, Hufelandstr. 55, Essen, Germany.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank David Basile for helpful and insightful discussions.
REFERENCES
- 1. Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell 13: 193–205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in U. S. veterans: focus on acute tubular necrosis. Kidney Int 76: 1089–1097, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Andrews KL, Betsuyaku T, Rogers S, Shipley JM, Senior RM, Miner JH. Gelatinase B (MMP-9) is not essential in the normal kidney and does not influence progression of renal disease in a mouse model of Alport syndrome. Am J Pathol 157: 303–311, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci 21: 7724–7732, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int 72: 151–156, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 13: 1–7, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Basile DP. Transforming growth factor-beta as a target for treatment in diabetic nephropathy. Am J Kidney Dis 38: 887–892, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Basile DP, Donohoe DL, Roethe K, Mattson DL. Chronic renal hypoxia after acute ischemic injury: effects of l-arginine on hypoxia and secondary damage. Am J Physiol Renal Physiol 284: F338–F348, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Basile DP, Fredrich K, Weihrauch D, Hattan N, Chilian WM. Angiostatin and matrix metalloprotease expression following ischemic acute renal failure. Am J Physiol Renal Physiol 286: F893–F902, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Bengatta S, Arnould C, Letavernier E, Monge M, de Preneuf HM, Werb Z, Ronco P, Lelongt B. MMP9 and SCF protect from apoptosis in acute kidney injury. J Am Soc Nephrol 20: 787–797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2: 737–744, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caron A, Desrosiers RR, Beliveau R. Ischemia injury alters endothelial cell properties of kidney cortex: stimulation of MMP-9. Exp Cell Res 310: 105–116, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Caron A, Desrosiers RR, Langlois S, Beliveau R. Ischemia-reperfusion injury stimulates gelatinase expression and activity in kidney glomeruli. Can J Physiol Pharmacol 83: 287–300, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Charron AJ, Xu W, Bacallao RL, Wandinger-Ness A. Cablin: a novel protein of the capillary basal lamina. Am J Physiol Heart Circ Physiol 277: H1985–H1996, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Chen TT, Luque A, Lee S, Anderson SM, Segura T, Iruela-Arispe ML. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J Cell Biol 188: 595–609, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chow AK, Cena J, Schulz R. Acute actions and novel targets of matrix metalloproteinases in the heart and vasculature. Br J Pharmacol 152: 189–205, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eckardt KU, Rosenberger C, Jurgensen JS, Wiesener MS. Role of hypoxia in the pathogenesis of renal disease. Blood Purif 21: 253–257, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Fine LG, Bandyopadhay D, Norman JT. Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney Int Suppl 75: S22–S26, 2000 [PubMed] [Google Scholar]
- 20. Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest 114: 623–633, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamada T, Fondevila C, Busuttil RW, Coito AJ. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology 47: 186–198, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer 8: 880–887, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heissig B, Hattori K, Friedrich M, Rafii S, Werb Z. Angiogenesis: vascular remodeling of the extracellular matrix involves metalloproteinases. Curr Opin Hematol 10: 136–141, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Horbelt M, Lee SY, Mang HE, Knipe NL, Sado Y, Kribben A, Sutton TA. Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am J Physiol Renal Physiol 293: F688–F695, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Hoste EA, Schurgers M. Epidemiology of acute kidney injury: how big is the problem? Crit Care Med 36: S146–S151, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem 267: 26031–26037, 1992 [PubMed] [Google Scholar]
- 27. Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iwata T, Chiyo M, Yoshida S, Smith GN, Jr, Mickler EA, Presson R, Jr, Fisher AJ, Brand DD, Cummings OW, Wilkes DS. Lung transplant ischemia reperfusion injury: metalloprotease inhibition downregulates exposure of type V collagen, growth-related oncogene-induced neutrophil chemotaxis, and tumor necrosis factor-α expression. Transplantation 85: 417–426, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O'Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol 290: H560–H576, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol 13: 806–816, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol 169: 681–691, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leonard EC, Friedrich JL, Basile DP. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Renal Physiol 295: F1648–F1657, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, McClister DM, Jr, Su H, Gannon J, MacGillivray C, Lee RT, Sinusas AJ, Spinale FG. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol 290: H232–H239, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Hsu CY. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 76: 893–899, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D'Agati V, Devarajan P, Barasch J. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 115: 610–621, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol 16: 558–564, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell 4: 1317–1326, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pozzi A, LeVine WF, Gardner HA. Low plasma levels of matrix metalloproteinase 9 permit increased tumor angiogenesis. Oncogene 21: 272–281, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Pozzi A, Moberg PE, Miles LA, Wagner S, Soloway P, Gardner HA. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci USA 97: 2202–2207, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci 114: 853–865, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Rosario HS, Waldo SW, Becker SA, Schmid-Schonbein GW. Pancreatic trypsin increases matrix metalloproteinase-9 accumulation and activation during acute intestinal ischemia-reperfusion in the rat. Am J Pathol 164: 1707–1716, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosell A, Lo EH. Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol 8: 82–89, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Sado Y, Kagawa M, Kishiro Y, Sugihara K, Naito I, Seyer JM, Sugimoto M, Oohashi T, Ninomiya Y. Establishment by the rat lymph node method of epitope-defined monoclonal antibodies recognizing the six different alpha chains of human type IV collagen. Histochem Cell Biol 104: 267–275, 1995 [DOI] [PubMed] [Google Scholar]
- 45. Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 18: 407–413, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Spurgeon-Pechman KR, Donohoe DL, Mattson DL, Lund H, James L, Basile DP. Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol 293: F269–F278, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Sutton TA, Kelly KJ, Mang HE, Plotkin Z, Sandoval RM, Dagher PC. Minocycline reduces renal microvascular leakage in a rat model of ischemic renal injury. Am J Physiol Renal Physiol 288: F91–F97, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Tong Z, Wu X, Ovcharenko D, Zhu J, Chen CS, Kehrer JP. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem J 391: 441–448, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 3: 844–861, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302: 1179–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Wang X, Zhou Y, Tan R, Xiong M, He W, Fang L, Wen P, Jiang L, Yang J. Mice lacking the matrix metalloproteinase-9 gene reduce renal interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 299: F973–F982, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem 276: 37258–37265, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Yeh DY, Lin HI, Feng NH, Chen CF, Wang D, Wang NT. Matrix metalloprotease expressions in both reperfusion lung injury and oleic acid lung injury models and the protective effects of ilomastat. Transplant Proc 41: 1508–1511, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 14: 163–176, 2000 [PMC free article] [PubMed] [Google Scholar]
- 55. Yuan HT, Li XZ, Pitera JE, Long DA, Woolf AS. Peritubular capillary loss after mouse acute nephrotoxicity correlates with downregulation of vascular endothelial growth factor-A and hypoxia-inducible factor-1 alpha. Am J Pathol 163: 2289–2301, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zeisberg M, Khurana M, Rao VH, Cosgrove D, Rougier JP, Werner MC, Shield CF, 3rd, Werb Z, Kalluri R. Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease. PLoS Med 3: e100, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ziswiler R, Daniel C, Franz E, Marti HP. Renal matrix metalloproteinase activity is unaffected by experimental ischemia-reperfusion injury and matrix metalloproteinase inhibition does not alter outcome of renal function. Exp Nephrol 9: 118–124, 2001 [DOI] [PubMed] [Google Scholar]