the prevalence of chronic kidney disease is rising sharply worldwide and affects 13.1% of the population in the US (3). Patients with chronic kidney disease represent a population not only at risk of progression to end-organ failure but also at higher risk for cardiovascular diseases (22). Although inflammation is beneficial for host wound healing and defense toward infection, excessive or altered inflammation often leads to a wide range of tissue injury and human disease, including cardiovascular and kidney disease (13, 20, 29). Inflammation causes oxidative stress by promoting the release of reactive oxygen and other reactive species by inflammatory cells, and this process contributes to tissue injury. Infiltration of inflammatory cells and increased expression of proinflammatory factors are crucial in the development of renal injury (20, 29). Renal tubule cells produce both cytokines and chemokines that are secreted across the apical and basolateral membranes and contribute to the development and progression of interstitial inflammation and glomerular and tubular injury (4, 23, 35).
Cell-to-cell communication, which occurs via gap junctions, is important to cell viability. However, gap junctions have been shown to be disrupted by inflammation (21) and oxidative stress (27). Paired connexins (connexons) play an important role in gap-junctional intercellular communication while unpaired connexins allow communication between the cytosol and extracellular environment. The most common connexins in the renal vasculature are connexin 37 (Cx37), Cx40, Cx43, and Cx45. Cx26, Cx30, Cx30.3, Cx32, and Cx43 are also expressed in renal tubules (12). Several connexins have been implicated in the development of cardiovascular disease, including hypertension and kidney and heart disease (1, 2, 7, 8, 10–12, 14, 15, 17, 18, 24, 25, 28, 30–34).
Cx40 is expressed to a greater extent than Cx45 in renin-producing juxtaglomerular cells while the converse is true in vascular smooth muscle cells. Deletion of Cx40 in renin-producing but not in endothelial cells resulted in hyperreninemia and hypertension (17, 30). Kurt et al. (17) also reported that there is a “reciprocal expression of Cx40 and Cx45 during phenotypical changes in renin-secreting cells”. Toubas et al. (27a) now report in an issue of the American Journal of Physiology-Renal Physiology that in the renal cortex of control mice there is negligible expression of Cx43 while Cx37 is abundantly expressed. However, in three different experimental models of chronic renal disease in mice, i.e., RenTg, anti-glomerular basement membrane glomerulonephritis, and unilateral renal obstruction, there is an early increase in Cx43 expression and a decrease in Cx37 expression in the renal cortex of these mice. The increase in the Cx43-to-Cx37 ratio is associated with an increase in cell adhesion molecules, vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1), followed by inflammatory cell infiltration, with an increased expression of monocyte chemoattractant protein-1 (MCP-1). The increase in Cx43 expression is important in the inflammatory response because deletion of one allele of Cx43 blunted the increase in VCAM-1 expression. Cx37 may counter the effect of Cx43 that could be gleaned from the acceleration of atherosclerosis in Cx37 null mice (8).
The imbalance of renal cortical (Ref. 27a, this report) and vascular Cx43 and Cx37 expression may contribute to the initiation of inflammation (18). However, the balance between Cx43 and Cx37 may play different roles in different tissues and diseases. Thus Cx43 expression is decreased but interstitial collagen fiber is increased in the corpus cavernosum of spontaneously hypertensive rats and rats with chronic renal failure (7). The epithelial-mesenchymal transition (EMT) in embryonic stem cells or human lens is associated with decreased expression of Cx43 (28, 31). However, in agreement with the current study, renal Cx43 mRNA expression is increased 5 days after unilateral ureteral obstruction (25) and myocardial EMT (1). In hypertensive two-kidney, one-clip (2K1C) rats, Cx43 is increased in the unclipped but not the clipped kidney (10). Cx43 mRNA in the heart is not altered 4 wk after 2K1C, DOCA-salt hypertension, or Nω-nitro-l-arginine methyl ester (l-NAME; nitric oxide inhibitor) hypertension. However, Cx43 protein expression is increased in the aorta of rats with 2K1C or DOCA-salt hypertension but decreased in rats with l-NAME hypertension (11). Cx43 has also been reported to be highly expressed in inflammatory, damaged renal tubule, interstitial cells in human kidneys (14), and podocytes of rats with puromycin aminonucleoside nephrosis (32). What makes the kidney and heart different from the other organs, in terms of Cx43 expression? What is responsible for the differential regulation of Cx43 in different organs? Is it related to differential cell expression (2, 13, 33)? More intriguingly, does the differential expression of Cx43 in the same tissue occur with different diseases (11)?
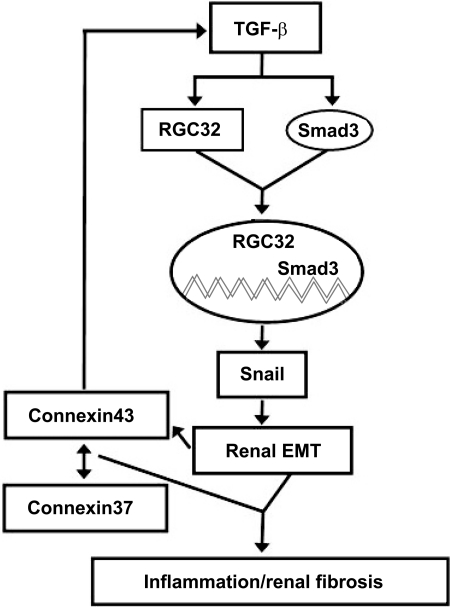
Gap junctions can be regulated by transforming growth factor-β (TGF-β) and vice versa (15). Cx43, the major gap junction protein in the myocardium, may positively regulate TGF-β because deficiency of Cx43 is associated with a decrease in TGF-β signaling (1, 34) (Fig. 1). We have reported that the response gene to complement 32 (RGC-32) is a downstream target of TGF-β signaling, which mediates the EMT in renal proximal tubule cells (9, 16, 19). The SMAD cascade is not implicated in the TGF-β effect on Cx43 expression in the mammary gland (26). However, in cardiomyocytes, Cx43 positively regulates TGF-β by releasing Smads from microtubules (5). SMAD3 also interacts with RGC-32, Slug, and Snail to cause EMT (9). Snail1-mediated EMT results in Cx43 repression (6). The interaction of these different proteins, especially the connexins, in the regulation of EMT and renal fibrosis may lead to a better understanding of how to interfere with the fibrotic process that occurs with renal inflammation and injury.
Fig. 1.
Gap junctions and transforming growth factor (TGF)-β counterregulate each other. Connexin 43 (Cx43), the major gap junction protein in the myocardium, which is also expressed in renal tubules, may positively regulate TGF-β. The response gene to complement 32 (RGC-32) and SMAD 3, downstream targets of TGF-β, regulate Snail, which is important in the epithelial-mesenchymal transition (EMT) of renal proximal tubule cells. Snail-mediated EMT decreases Cx43 expression, resulting in a negative feedback loop. In some tissues, e.g., kidney, an increase in the Cx43-to-Cx37 ratio could be a regulator of renal fibrosis (27a). Whether this interaction also regulates the expression of TGF-β remains to be determined.
GRANTS
This work was supported in part by grants from the National Institutes of Health (HL068686 HL023081, HL074940, HL092196, and DK039308).
REFERENCES
- 1. Asazuma-Nakamura Y, Dai P, Harada Y, Jiang Y, Hamaoka K, Takamatsu T. Cx43 contributes to TGF-beta signaling to regulate differentiation of cardiac fibroblasts into myofibroblasts. Exp Cell Res 315:1190–1199, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Brisset AC, Isakson BE, Kwak BR. Connexins in vascular physiology and pathology. Antioxid Redox Signal 11:267–282, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA 29:2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Daha MR, van Kooten C. Is the proximal tubular cell a proinflammatory cell? Nephrol Dial Transplant 15:41–43, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Dai P, Nakagami T, Tanaka H, Hitomi T, Takamatsu T. Cx43 mediates TGF-β signaling through competitive Smads binding to microtubules. Mol Biol Cell 18:2264–2273, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Boer TP, van Veen TA, Bierhuizen MF, Kok B, Rook MB, Boonen KJ, Vos MA, Doevendans PA, de Bakker JM, van der Heyden MA. Connexin43 repression following epithelium-to-mesenchyme transition in embryonal carcinoma cells requires Snail1 transcription factor. Differentiation 75:208–218, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Fu Q, Lv JJ, Zhang H. Effects of chronic renal failure on the expression of connexin 43 in the rat's corpus cavernosum. Asian J Androl 10:286–289, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Goodenough DA, Kwak BR. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med 12:950–954, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Guo X, Jose PA, Chen SY. Response gene to complement 32 interacts with Smad3 to promote epithelial-mesenchymal transition of human renal tubular cells. Am J Physiol Cell Physiol (First published February 9, 2011) doi: 10.1152/ajpcell.00204.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haefliger JA, Demotz S, Braissant O, Suter E, Waeber B, Nicod P, Meda P. Connexins 40 and 43 are differentially regulated within the kidneys of rats with renovascular hypertension. Kidney Int 60:190–201, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Haefliger JA, Meda P. Chronic hypertension alters the expression of Cx43 in cardiovascular muscle cells. Braz J Med Biol Res 33:431–438, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Hanner F, Sorensen CM, Holstein-Rathlou NH, Peti-Peterdi J. Connexins and the kidney. Am J Physiol Regul Integr Comp Physiol 298:R1143–R1155, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet S, Vinh A, Weyand C. Inflammation, immunity, and hypertension. Hypertension 57:132–140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hillis GS, Duthie LA, Brown PA, Simpson JG, MacLeod AM, Haites NE. Upregulation and co-localization of connexin43 and cellular adhesion molecules in inflammatory renal disease. J Pathol 182:373–379, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Hills CE, Bland R, Bennett J, Ronco PM, Squires PE. TGF-β mediates glucose-evoked up-regulation of connexin-43 cell-to-cell communication in HCD-cells. Cell Physiol Biochem 24:177–186, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Huang WY, Li ZG, Rus H, Wang X, Jose PA, Chen SY. RGC-32 mediates transforming growth factor-beta-induced epithelial-mesenchymal transition in human renal proximal tubular cells. J Biol Chem 284:9426–9432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kurt B, Kurtz L, Sequeira-Lopez ML, Gomez RA, Willecke K, Wagner C, Kurtz A. Reciprocal expression of connexin 40 and 45 during phenotypical changes in renin-secreting cells. Am J Physiol Renal Physiol 300:F743–F748, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kwak BR, Mulhaupt F, Veillard N, Gros DB, Mach F. Altered pattern of vascular connexin expression in atherosclerotic plaques. Arterioscler Thromb Vasc Biol 22:225–230, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Li F, Luo Z, Huang W, Lu Q, Wilcox CS, Jose PA, Chen S. Response gene to complement 32, a novel regulator for transforming growth factor-β-induced smooth muscle differentiation of neural crest cells. J Biol Chem 282:10133–10137, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Massy ZA, Stenvinkel P, Drueke TB. The role of oxidative stress in chronic kidney disease. Semin Dial 22:405–408, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Scheckenbach KE, Crespin S, Kwak BR, Chanson M. Connexin channel-dependent signaling pathways in inflammation. J Vasc Res 48:91–103, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation 116:85–97, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Segerer S, Schlöndorff D. Role of chemokines for the localization of leukocyte subsets in the kidney. Semin Nephrol 27:260–274, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Smyth JW, Hong TT, Gao D, Vogan JM, Jensen BC, Fong TS, Simpson PC, Stainier DY, Chi NC, Shaw RM. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J Clin Invest 120:266–279, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sommer M, Eismann U, Deuther-Conrad W, Wendt T, Mohorn T, Fünfstück R, Stein G. Time course of cytokine mRNA expression in kidneys of rats with unilateral ureteral obstruction. Nephron 84:49–57, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Tacheau C, Fontaine J, Loy J, Mauviel A, Verrecchia F. TGF-β induces connexin43 gene expression in normal murine mammary gland epithelial cells via activation of p38 and PI3K/AKT signaling pathways. J Cell Physiol 217:759–768, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Tomaselli GF. Oxidant stress derails the cardiac connexon connection. J Clin Invest 120:87–89, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a. Toubas J, Beck S, Pageaud AL, Huby AC, Mael-Ainin M, Dussaule JC, Chatziantoniou C, Chadjichristos CE. Alteration of connexin expression is an early signal for chronic kidney disease. Am J Physiol Renal Physiol (First published March 23, 2011) doi:10.1152/ajprenal.00255.2010 [DOI] [PubMed] [Google Scholar]
- 28. Ullmann U, In't Veld P, Gilles C, Sermon K, De Rycke M, Van de Velde H, Van Steirteghem A, Liebaers I. Epithelial-mesenchymal transition process in human embryonic stem cells cultured in feeder-free conditions. Mol Hum Reprod 13:21–32 2007 [DOI] [PubMed] [Google Scholar]
- 29. Vlassara H, Torreggiani M, Post JB, Zheng F, Uribarri J, Striker GE. Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging. Kidney Int Suppl S3–S11, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Wagner C, Jobs A, Schweda F, Kurtz L, Kurt B, Lopez ML, Gomez RA, van Veen TA, de Wit C, Kurtz A. Selective deletion of Connexin 40 in renin-producing cells impairs renal baroreceptor function and is associated with arterial hypertension. Kidney Int 78:762–768, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yao K, Ye PP, Tan J, Tang XJ, Shen Tu XC. Involvement of PI3K/Akt pathway in TGF-beta2-mediated epithelial mesenchymal transition in human lens epithelial cells. Ophthalmic Res 40:69–76, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Yaoita E, Yao J, Yoshida Y, Morioka T, Nameta M, Takata T, Kamiie J, Fujinaka H, Oite T, Yamamoto T. Up-regulation of connexin43 in glomerular podocytes in response to injury. Am J Pathol 161:1597–1606, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang J, Hill CE. Differential connexin expression in preglomerular and postglomerular vasculature: accentuation during diabetes. Kidney Int 68:1171–1185, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y, Wang H, Kovacs A, Kanter EM, Yamada KA. Reduced expression of Cx43 attenuates ventricular remodeling after myocardial infarction via impaired TGF-β signaling. Am J Physiol Heart Circ Physiol 298:H477–H487, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zoja C, Garcia PB, Remuzzi G. The role of chemokines in progressive renal disease. Front Biosci 14:1815–1822, 2009 [DOI] [PubMed] [Google Scholar]