Abstract
Sepsis is a leading cause of acute kidney injury (AKI) and mortality in children. Understanding the development of pediatric sepsis and its effects on the kidney are critical in uncovering new therapies. The goal of this study was to characterize the development of sepsis-induced AKI in the clinically relevant cecal ligation and puncture (CLP) model of peritonitis in rat pups 17–18 days old. CLP produced severe sepsis demonstrated by time-dependent increase in serum cytokines, NO, markers of multiorgan injury, and renal microcirculatory hypoperfusion. Although blood pressure and heart rate remained unchanged after CLP, renal blood flow (RBF) was decreased 61% by 6 h. Renal microcirculatory analysis showed the number of continuously flowing cortical capillaries decreased significantly from 69 to 48% by 6 h with a 66% decrease in red blood cell velocity and a 57% decline in volumetric flow. The progression of renal microcirculatory hypoperfusion was associated with peritubular capillary leakage and reactive nitrogen species generation. Sham adults had higher mean arterial pressure (118 vs. 69 mmHg), RBF (4.2 vs. 1.1 ml·min−1·g−1), and peritubular capillary velocity (78% continuous flowing capillaries vs. 69%) compared with pups. CLP produced a greater decrease in renal microcirculation in pups, supporting the notion that adult models may not be the most appropriate for studying pediatric sepsis-induced AKI. Lower RBF and reduced peritubular capillary perfusion in the pup suggest the pediatric kidney may be more susceptible to AKI than would be predicted using adults models.
Keywords: cecal ligation and puncture, renal blood flow
sepsis is a leading cause of death in children worldwide (16). In the United States, severe sepsis, defined in the pediatric patient as sepsis with cardiovascular distress or multiple organ dysfunction (16), is estimated to be the second leading cause of death in children 1–14 yr of age (39). Sepsis is also the second leading cause of acute kidney injury (AKI) in pediatric patients behind renal ischemic injury (11), and the development of AKI increases mortality in the pediatric septic patient by 20–30% (11, 13). Because current treatments for sepsis-induced AKI in the pediatric patient are mostly supportive (2), understanding the development of sepsis and its effects on the kidney in this specific population is critical for uncovering new treatment modalities.
Most research on sepsis has utilized adult rodent models. Because the cardiovascular system and immune responses are still developing in children (43), the use of adult models may not uncover the most relevant therapeutic targets in the pediatric patient population. This is particularly true for sepsis-induced AKI because the pediatric kidney is still maturing. For example, the developing human, rat, and porcine kidney have decreased renal blood flow (RBF) with higher renal vascular resistance compared with the mature kidney (20, 24, 29). These differences along with a lower systemic mean arterial pressure (MAP) and lower glomerular filtration rate in neonates (20, 24, 29, 35) suggest that adult animals may not be the most appropriate model for studying pediatric sepsis-induced AKI. Moreover, animal studies suggest that the developing kidney may be more susceptible to oxidative stress due to decreased activities of key scavenging enzymes such as superoxide dismutase and catalase (19, 29). This is especially important because decreases in perfusion of the kidney microcirculation accompanied by increases in oxidant generation in the peritubular/capillary microenvironment are key pathogenic features of sepsis-induced AKI in adult mice (34, 40, 42). Increases in NO synthesis in a hypoxic microenvironment favor superoxide production, resulting in reactive nitrogen species (RNS) generation, which can lead to further capillary dysfunction and tubular epithelial cell injury (38, 40, 41).
Few studies have specifically examined renal injury in neonatal/pediatric animal models of sepsis in any species. Furthermore, the initiation of sepsis has primarily been by administration of lipopolysaccharide (LPS) (5, 15, 20, 23), a model of endotoxemia. The goal of our study was to examine the development of sepsis-induced AKI in rat pups induced by cecal ligation and puncture (CLP), a model of bacterial peritonitis that produces generalized sepsis. We monitored changes in systemic hemodynamics, RBF, the renal microcirculation, and oxidative stress during the development of sepsis in rat pups. The CLP model of sepsis displayed characteristics of pediatric severe sepsis, including multiorgan injury, hypothermia, and renal microcirculatory failure leading to AKI. The different hemodynamic responses in the kidney observed after CLP between rat pups and adult rats illustrate the importance of using the appropriate age model to study sepsis-induced AKI.
MATERIALS AND METHODS
Rat model of CLP.
Seven 10-day-old male Sprague-Dawley rat pups and accompanying dam (Harlan, Indianapolis, IN) were acclimated for 7 days with free access to the dam. All studies were performed on pups 17–18 days of age with an average weight of 40.4 g (95% confidence interval = 39.2–41.5 g). To induce sepsis, pups were anesthetized with isoflurane (4% induction, 2% maintenance) and placed on a warming pad. Following laparotomy, the cecum was exteriorized, and the membrane between the cecum and the mesentery was carefully cut to release the cecum. The cecum was ligated 1.5 cm from the tip or just below the ileocecal valve with 4–0 silk. Two punctures were made with an 18-gauge needle, and 1 mm of fecal material was expressed from the punctures. The incision was sutured in two layers with 4–0 silk. In sham pups, the cecum was located but neither ligated nor punctured. Following the procedure, 1 ml of warm saline was administered intraperitoneally, and the animals recovered in individual cages placed on a warming pad with free access to a nutrient gel pack (DietGel Recovery, Clear H2O, Portland, ME). Pups studied at time points >6 h post-CLP received fluid resuscitation (unless otherwise noted) and antibiotics consisting of imipenem/cilastatin administered subcutaneously at 14 mg/kg in 1.5 ml warm saline (38 ml/kg) 6 h after surgery to more closely mimic the clinical setting (10, 27).
Adult male Sprague-Dawley rats (Harlan) weighing 250–300 g where acclimated for 1 wk after arrival. CLP was performed as described above except the cecum was ligated 4 cm from the tip, and four punctures were made with an 18-gauge needle. Following surgery, adult rats received 5 ml of warm saline and recovered in individual cages placed on a warming pad with free access to food and water. In sham rats, the cecum was located but neither ligated nor punctured. In time points >6 h post-CLP, the adults received 14 mg/kg imipenem/cilastatin subcutaneously in 10 ml warm saline at 6 h.
All animals were housed and handled according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals with approval from an internal animal care and use committee.
Intravital videomicroscopy.
Adult or 17- to 18-day-old rats were anesthetized with isoflurane and injected via the tail vein with a solution of fluorescein isothiocyanate (FITC)-labeled dextran (500 kDa; Sigma, St. Louis, MO) to visualize the capillary vascular space and 1,2,3-dihydrorhodamine (DHR; Invitrogen, Carlsbad, CA) to detect RNS generation (17, 40, 41). Adult rats received a dose of 1.4 μmol/kg FITC-dextran and 0.8 mg/kg DHR in 2.1 ml/kg in normal saline. Rat pups received a dose of 2 μmol/kg FITC-dextran and 1.1 mg/kg DHR in 3 ml/kg in normal saline. The left kidney was exposed and positioned on a glass stage atop an inverted Zeiss Axiovert 200M fluorescent microscope equipped with an Axiocam HSm camera (Zeiss). For each rat, videos of 10 s (∼30 frames/s) at ×200 magnification were acquired from five randomly selected nonoverlapping fields of view. Also, a single 500-ms exposure for rhodamine fluorescence (see below) was taken for each field of view. Body temperature was monitored using a rectal thermometer and maintained at 36–37°C with a warming lamp. At the end of the experiment, venous blood was collected, and the right kidney was harvested and fixed in 10% buffered formalin.
Analysis of perfusion status was performed on each of the five 10-s videos/animal. Vessels were categorized as “continuous flow” where red blood cell (RBC) movement was continuous; “intermittent flow” where RBC movement stopped or reversed; and “no flow” where no RBC movement was observed. The data were expressed as the percentage of vessels in each of the three categories.
RBC velocity through the renal microcirculation was calculated using Axiovision 4.7 (Zeiss). RBC velocity through each capillary was determined by measuring the distance traveled by a single RBC over time (μm/s). The average RBC velocity and volumetric blood flow were calculated using only continuous flow vessels with a RBC velocity of <500 μm/s (500 μm/s was the maximum speed measurable with the spatial and temporal resolution of the videos) utilizing the equation V = (Vπr2)/1.6 where the mean RBC velocity (V) and the capillary cross-sectional radius (r) are divided by the Baker-Wayland factor (1.6), as previously described (1). The velocity histograms were generated using RBC velocity by binning of 50 μm/s intervals up to 500 μm/s. All vessels with the velocity higher than 500 μm/s were assigned to the highest bin (>450 μm/s), whereas all vessels with intermittent or no flow were assigned to the lowest bin (<50 μm/s).
The RNS peroxynitrite oxidizes DHR to fluorescent rhodamine that is visualized at 535 nm excitation and 590 nm emission (17). Fluorescence intensity was measured by ImageJ software (National Institutes of Health, Bethesda, MD) after first subtracting background fluorescence intensity. Data are expressed as arbitrary units per square micrometer.
Renal microvascular leakage.
Renal microvascular leakage was assessed using Evans blue dye (EBD; Sigma-Aldrich) as described by Yasuda et al. (45). At 9.5 h post-CLP or sham surgery, rat pups were injected with EBD (1% solution, wt/vol, in saline at 2 ml/kg) via the tail vein. At 10 h, rat pups were anesthetized with isoflurane and perfused with PBS through the left ventricle until all blood was eliminated. The right kidney was rapidly removed, weighed, and stored at −80°C until homogenization in 1 ml formamide and incubation at 55°C for 18 h. The supernatant was collected after centrifugation at 12,000 g for 30 min. The amount of EBD in the supernatant was analyzed by measuring absorbance at 620 nm against a standard curve. Results are expressed as microgram of EBD per milligram of kidney.
Renal blood flow.
Following isoflurane anesthesia, the right kidney was exposed using a flank incision, the renal artery was isolated from the vein, and a Doppler flow probe was positioned around the renal artery. A 0.5PSL renal artery flow probe was used for rat pups, and a 1PRB renal artery flow probe was used for adult rats. Both were purchased from Transonic Systems (Ithaca, NY). Blood flow readings were recorded using PowerLab and LabChart software (AD Instruments). Body temperature was monitored utilizing a rectal probe and maintained between 36 and 37°C with a heating lamp. RBF was calculated as the average flow recorded during the first 10-s interval of each minute over a 10-min period after the flow had stabilized (∼5 min after placement of the probe) and expressed as milliliters per minute per gram kidney weight.
Biotelemetry.
Systemic MAP and heart rate were measured in conscious rats using biotelemetry. Telemetry transmitters (Data Sciences International, Minneapolis, MN) were implanted in the carotid artery in 17-day-old pups or the femoral artery in adult rats. Twenty hours later pups were reanesthetized with isoflurane and received CLP or sham surgery. Sepsis was induced 3 days after transmitter implantation in adult rats. Cardiovascular parameters were recorded for 10 s every 5 min for 24 h following CLP or sham surgery. Data were analyzed by averaging 15-min recordings every 2 h after CLP.
Analysis of serum markers.
Serum nitrate plus nitrite (NOx) concentrations were measured using the Total Nitric Oxide Assay Kit (Enzo Life Sciences, Plymouth Meeting, PA). Data are expressed as concentration of serum NOx in micromolar. Blood urea nitrogen (BUN) concentrations were measured using the QuantiChrom Urea Assay Kit (BioAssay Systems, Hayward, CA). Data are expressed as serum BUN concentration in milligrams per deciliter. Serum creatinine (Cre) concentrations and alanine aminotransferase (ALT) activities were measured using a Roche Cobas Mira Clinical Analyzer (Roche Diagnostic Systems, Branchburg, NJ). Data are expressed as milligrams per deciliter and International Units per liter. Serum interleukin (IL)-1β and tumor necrosis factor (TNF)-α concentrations were measured using a rat cytokine MILLIPLEX MAP kit (Millipore, Billerica, MA). Data are expressed as picograms per milliliter.
Real-time PCR.
RNA was isolated from whole kidney homogenates using the RNeasy kit (Qiagen, Valencia, CA), and 1 μg of total RNA was converted to cDNA using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Each PCR mixture contained 10 ng cDNA, 1× SYBR Green (Bio-Rad), and 200 nmol/l of the primer for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), intercellular adhesion molecule (ICAM)-1, or inducible nitric oxide synthase (iNOS). The primer sequences were as follows: GAPDH forward and reverse, 5′-AGG AAG CTC ACT GGC ATG-3′ and 5′-CTT CTT GAT GTC ATA ATA CTT GGC AG-3′, respectively; ICAM-1 forward and reverse, 5′-CCA AGG AGA TCA CAT TCA CG-3′ and 5′-GGA CCC TAG TCG GAA GAT CG-3′, respectively; and iNOS forward and reverse, 5′-CAG CTG GGC TGT GCA AAC CTT-3′ and 5′-TGA ATG CAA TGT TTG CTT CGA-3′, respectively. The PCR mixture was initially denatured at 95°C for 10 min followed by 40 cycles of 10 s at 95°C to denature and 1 min at 65°C for annealing and extension. The real-time PCR products were analyzed using iCycler software (Bio-Rad).
Immunohistochemistry.
Fixed kidneys embedded in paraffin were cut into 5-μm sections and stained for nitrotyrosine or iNOS. Following rehydration, antigens were unmasked in 10 mM sodium citrate, pH 6.0, heated to 95°C for 30 min and allowed to cool. Slides were then blocked with serum-free protein block (Dako, Carpinteria, CA) followed by addition of primary antibody and then incubated overnight at 4°C in a humid chamber. Polyclonal anti-nitrotyrosine antibody (Millipore) or polyclonal anti-iNOS antibody (Abcam, Cambridge, MA) were diluted 1:500 in 1% BSA, 0.5% milk in 1× TBS, pH 7.6. Slides were then washed in 1× TBS and incubated with secondary antibody from the Dako LSAB +System-HRP kit (Dako). A chromogen reagent supplied in the Dako LSAB +System-HRP kit visualized antigen binding. Nonspecific binding of the antinitrotyrosine antibody was determined by preincubating the antibody with 1 mM 3-nitrotyrosine. Kidney sections were counterstained with Gill's hemotoxylin.
Assessment of kidney morphology.
Paraffin-embedded sections (5 μm) were prepared from the right kidneys fixed in 10% phosphate-buffered formalin. The periodic acid-Schiff stain was used for the analysis of morphology with light microscopy (Nikon E800; Nikon, Melville, NY) by a blinded observer. A semiquantitative score for tubular injury was assigned as described by Wang et al. (37). At least 10 high-power fields were examined. The percentage of tubules that displayed cellular necrosis, loss of brush border, cast formation, vacuolization, and tubule dilation were scored as follows: 0 = none, 1 = <10%, 2 = 11–25%, 3 = 26–45%, 4 = 46–75%, and 5 = >76%.
Statistical analysis.
All data were analyzed with Prism 4 for Mac (GraphPad Software, San Diego, CA) and expressed as means ± SE. Comparisons between two groups of data were analyzed using a nonpaired Student's t-test. Data in groups of three of more were analyzed by one-way ANOVA followed by the Newman-Keuls or Dunnett's multiple-comparison post hoc tests. Two-way ANOVA was used for data comparing two or more groups over time. Survival data were analyzed using the Fisher's exact test. Velocity distributions were analyzed using the Chi square test at both 50- and 100-μm/s binning intervals.
RESULTS
Effects of fluids on survival.
Volume resuscitation improves outcome in murine models of sepsis (9, 46) and remains a key part of goal-directed hemodynamic support in children with sepsis (6, 43). To evaluate the beneficial effects of fluids, pups were administered antibiotics in 0.15 (no fluid resuscitation) or 1.5 (38 ml/kg fluid resuscitation) ml saline subcutaneously at 6 h post-CLP. Table 1 shows survival data for pups through 18 h. All pups survived through the first 6 h following CLP. Administration of antibiotics with fluids at 6 h increased survival at 18 h where survival was increased by ∼40% in pups receiving fluids (P < 0.05 compared with no fluids). Consequently, all further experiments were performed in pups treated with antibiotics plus fluids at 6 h post-CLP.
Table 1.
Survival data for pups through 18 h
| %Survival (live/total) |
|||
|---|---|---|---|
| 6 h | 10 h | 18 h | |
| +Fluids | 100 (42/42) | 83 (29/35) | 66 (22/32) |
| −Fluids | 100 (8/8) | 62.4 (5/8) | 25* (2/8) |
Survival of rat pups when administered antibiotics with (+) or without (−) fluid resuscitation at 6 h post-cecal ligation and puncture.
P < 0.05 compared with +Fluids.
Evidence for severe sepsis and multiorgan failure.
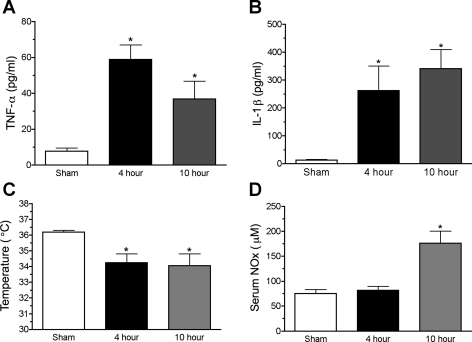
Rat pups subjected to CLP displayed symptoms consistent with severe sepsis (16). Two systemic inflammatory cytokines, TNF-α (Fig. 1A) and IL-1β (Fig. 1B), were both significantly elevated in the serum at 4 and 10 h post-CLP compared with the serum of sham-treated pups. The increases in cytokine levels were also associated with a decrease in core body temperature (Fig. 1C), suggesting the development of cold shock (16). Serum NOx levels, a marker of systemic nitric oxide generation, increased by 10 h post-CLP (Fig. 1D), further supporting the development of a systemic inflammatory response.
Fig. 1.
Cecal ligation and puncture (CLP) produces an inflammatory response in rat pups. Serum levels of the cytokines tumor necrosis factor (TNF)-α (A) and interleukin (IL)-Iβ (B) were elevated at 4 and 10 h after sepsis (n = 6–8). Because preliminary studies indicated there were no differences in cytokine levels in sham animals over the time course, sham values plotted were pooled from the various time points. Appearance of cytokines was associated with a decrease in core temperature (C) (n = 6–11). Serum nitrate + nitrite (NOx) concentration (D) increased by 10 h post-CLP (n = 7–12). Data are expressed as means ± SE. *P < 0.05 compared with sham.
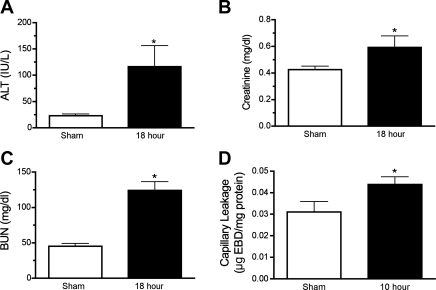
Serum BUN and Cre were measured to assess renal injury, and serum ALT was measured to assess hepatic injury. At 18 h post-CLP, all three markers were elevated significantly compared with sham (Fig. 2). The fivefold rise in serum ALT is consistent with a mild hepatic injury reported for other models of sepsis (25). Also as noted in other models of sepsis, serum BUN increased proportionally greater than Cre (18), suggesting prerenal azotemia. Consistent with this finding was the lack of morphological injury at 18 h post-CLP (data not shown). However, it must be noted that, at 18 h, survival was only 66% (Table 1), suggesting that the sickest pups had already died, so these data may not reflect the full severity of renal injury.
Fig. 2.
CLP produced multiorgan injury in rat pups. Serum alanine aminotransferase (ALT, A), creatinine (B), and blood urea nitrogen (BUN, C) in rat pups were increased at 18 h following CLP. Capillary leakage measured by the presence of Evans blue dye (EBD) in kidney homogenates was elevated by 10 h post-CLP (D). Data are means ± SE (n = 4–13 animals/group). *P < 0.05 compared with sham.
The renal inflammatory response during CLP in the mouse is associated with renal capillary leakage (45). Under normal conditions, EBD binds albumin and is unable to diffuse out of capillaries, but when the capillaries are damaged and “leaky,” EBD will accumulate within the surrounding tissue. In the rat pup, EBD content was elevated significantly at 10 h post-CLP compared with sham-treated pups (P < 0.05, Fig. 2B), indicating capillary damage.
To examine whether CLP-induced sepsis in the rat pup produced an inflammatory response in the kidney, mRNA levels of iNOS and the leukocyte adhesion molecule ICAM-1 were determined from whole kidney RNA. At 6 h post-CLP, iNOS and ICAM-1 mRNA were elevated significantly at 3.7- and 8.2-fold, respectively (P < 0.05, data not shown).
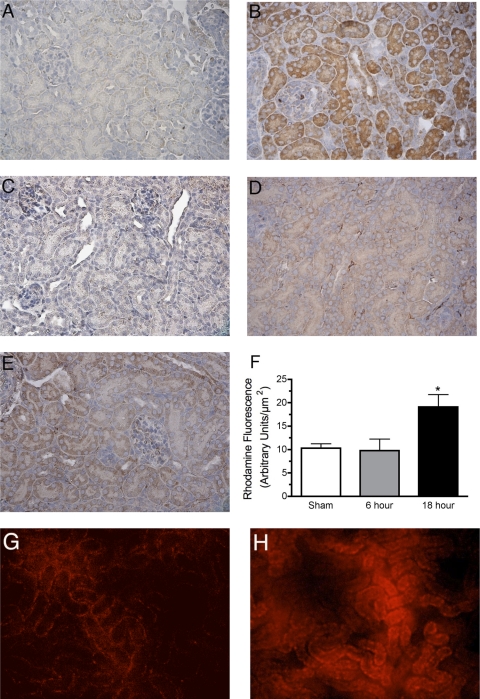
Very low levels of diffuse iNOS immunoreactivity were detected in kidneys from sham pups (Fig. 3A). However, at 6 h post-CLP, levels of immunoreactive iNOS protein were apparent in cortical tubules (Fig. 3B) and medulla (data not shown). Nitrotyrosine immunoreactivity, a marker of peroxynitrite, was not detected in sham pups (Fig. 3C) but was diffuse at 6 h post-CLP (Fig. 3D) and abundant at 18 h in cortical tubules (Fig. 3E) and medulla (data not shown). Rhodamine fluorescence was a second, complementary method used to measure RNS generation in the pup kidney. Representative images of rhodamine fluorescence show low levels of RNS generation in sham (Fig. 3G) but increased RNS at 18 h (Fig. 3H). Analysis of images of rhodamine fluorescence captured during intravital videomicroscopy (IVVM) showed significant increases in fluorescence at 18 h post-CLP but not at 6 h (P < 0.05 compared with sham; Fig. 3F). These data suggest that iNOS induction proceeded RNS generation.
Fig. 3.
Reactive nitrogen species (RNS) generation in the pup kidney. Representative images (from 3 to 4 animals/group) of immunoreactive inducible nitric oxide synthase (iNOS) protein are shown in A (sham) and B (6 h post-CLP). Staining for iNOS was weak and diffuse in sham but more intense in both proximal and distal tubules of the cortex following CLP. Representative images (from 3 to 4 animals/group) of immunoreactive nitrated protein (a marker of peroxynitrite generation) are show in C (sham), D (6 h post-CLP), and E (18 h post-CLP). Specific staining for nitrotyrosine adducts was absent in sham but weak and diffuse at 6 h post-CLP. In contrast, staining for nitrotyrosine adducts was more intense at 18 h post-CLP. The specificity of the antinitrotyrosine antibody was determined by preincubation of the antibody with 10 mM 3-nitrotyrosine (data not shown). Oxidation of 1,2,3-dihydrorhodamine (DHR123) to rhodamine was used as a second indicator of RNS generation. Representative images of rhodamine fluorescence in sham and 18 h CLP are shown in G and H, respectively. Analysis of images of rhodamine fluorescence captured during the intravital videomicroscopy (IVVM) procedure showed an increase in rhodamine fluorescence at 18 h but not at 6 h following CLP (F). Data are means ± SE (n = 5–8 animals/group). *P < 0.05 compared with sham.
Renal microcirculatory failure in pups.
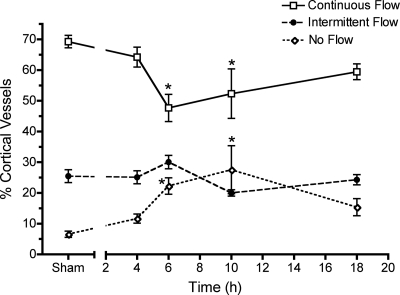
Microcirculatory failure is a hallmark of sepsis (21, 32). In previous studies using aged mice, we found that perfusion of the kidney microcirculation was dramatically decreased following CLP (38, 40). To examine the effects of CLP-induced sepsis on the renal microcirculation in the rat pup, we measured changes in peritubular capillary perfusion status using IVVM. Perfusion analysis showed that the percentage of continuously perfused cortical vessels decreased 31% at 6 h in pups with CLP compared with sham (P < 0.05, Fig. 4). As the percentage of vessels with continuous flow decreased, the percentage of vessels with no flow increased 2.4-fold at 6 h compared with sham (P < 0.05). The overall decline in perfusion status was maintained through 10 h. Perfusion status appeared to recover by 18 h; however, the data are from only pups that survived (66%) and thus may not reflect the full extent of injury.
Fig. 4.
Renal peritubular capillary perfusion status. CLP produced a time-dependent change in the percentage of capillaries with continuous, intermittent, and no flow in pups. Data are means ± SE (n = 6–8 pups/group). *P < 0.05 compared with sham.
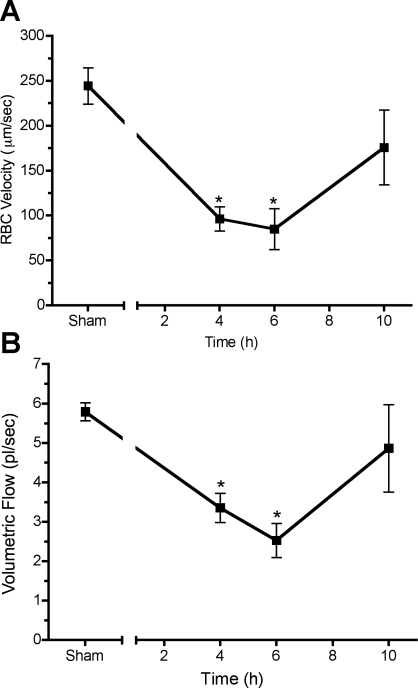
While perfusion status is an overall index of gross perfusion, it does not address indexes of nutritive flow. To identify time-dependent changes in nutritive flow in pups, RBC velocity and volumetric flow were determined over time in continuously flowing capillaries. RBC velocity (Fig. 5A) and volumetric flow (Fig. 5B) in the pup declined significantly at 4 and 6 h after CLP compared with sham (P < 0.05).
Fig. 5.
Time course of peritubular capillary flow in pups. CLP produced a time-dependent change in red blood cell (RBC) velocity (A) and volumetric flow (B) in continuously flowing capillaries. Data are means ± SE (n = 4 pups/group). *P < 0.05 compared with sham.
Comparison of renal hemodynamics between adult rats and pups.
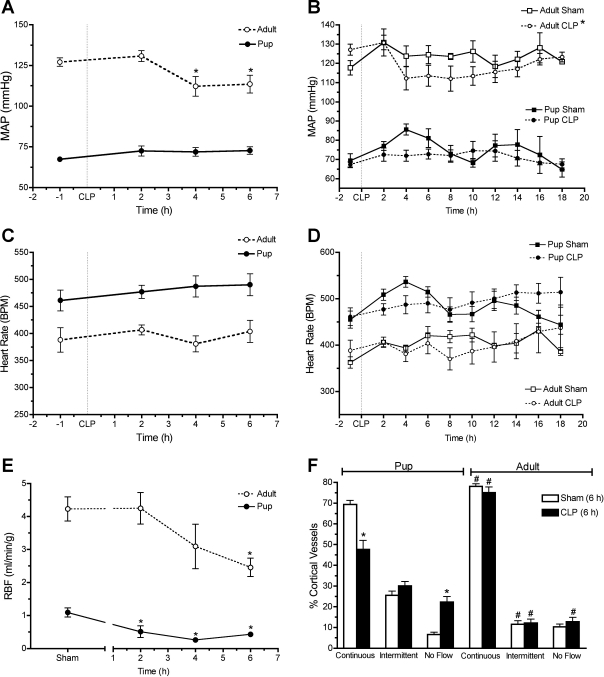
It has been reported that pediatric animals have lower MAP than adults (24, 26, 29). Using biotelemetry, we found MAP in sham pups was much lower than sham adults (69.3 ± 3.6 vs. 117.8 ± 2.7 mmHg; P < 0.05). Systemic blood pressure and RBF are regulators of renal microcirculatory perfusion. Early time-matched changes in MAP, heart rate, and RBF data in adults and pups subjected to CLP are presented in Fig. 6 along with the full time course of MAP and heart rate. Following CLP, MAP decreased in adults over time but not in pups (Fig. 6, A and B) while heart rate remained unchanged in both (Fig. 6, C and D). RBF was also significantly lower in the pup compared with the adult (1.1 ± 0.1 and 4.2 ± 0.4 ml·min−1·g−1, respectively; P < 0.05). CLP caused a rapid time-dependent fall in RBF in both the pup and adult (Fig. 6E); however, the fall in RBF occurred more rapidly in the pup (at 2 h P < 0.05 compared with sham) than in the adult (at 6 h P < 0.05 compared with sham). Moreover, CLP caused a significantly greater percent decrease in RBF in pups than in adults (61 vs. 42%, respectively, at 6 h; P < 0.05).
Fig. 6.
Comparison of systemic and renal hemodynamics between adults and pups. Early changes in mean arterial pressure (MAP), heart rate, and renal blood flow (RBF) are presented for comparisons in A, C, and E, respectively. The full time course for MAP and heart rate is presented in B and D. A comparison of peritubular capillary perfusion at 6 h is presented in F. Data are means ± SE (n = 5–8 pups/group and n = 4–7 adults/group). In A, *P < 0.05 by one-way ANOVA compared with 2 h. In B and E, *P < 0.05 using 2-way ANOVA. In F, *P < 0.05 by 1-way ANOVA compared with sham and #P < 0.05 when adult is compared with pup.
We also determined microcirculatory perfusion status in adult rats subjected to CLP or sham surgery. In Fig. 6F, the 6-h perfusion data for pups from Fig. 4 were replotted with adult perfusion data for comparison. Whereas CLP caused a significant decline in perfusion status at 6 h in pups, CLP did not reduce perfusion status in adults. Interestingly, sham pups had a significantly lower percentage of cortical vessels with continuous flow compared with adults (69.3 ± 2.0 vs. 78.1 ± 1.2%; P < 0.05) and a significantly higher percentage of cortical vessels with intermittent flow compared with adults (25.5 ± 2.1 vs. 11.5 ± 1.8%; P < 0.05).
Systemic blood pressure and RBF are regulators of renal microcirculatory perfusion. Because CLP caused no change in MAP in the rat pup, RBF was measured (Fig. 6E). RBF was significantly higher in the adult rat compared with the rat pup (4.2 ± 0.4 and 1.1 ± 0.1 ml·min−1·g−1, respectively; P < 0.05). CLP caused a rapid time-dependent fall in RBF in the rat pup at 2, 4, and 6 h compared with sham (P < 0.05). CLP also caused a significant fall in RBF at 6 h (P < 0.05) in the adult rat, but the decrease in RBF was delayed in the adult compared with the pup. Moreover, CLP caused a significantly greater decrease in RBF in pups than in adults (61 vs. 42%, respectively, at 6 h; P < 0.05).
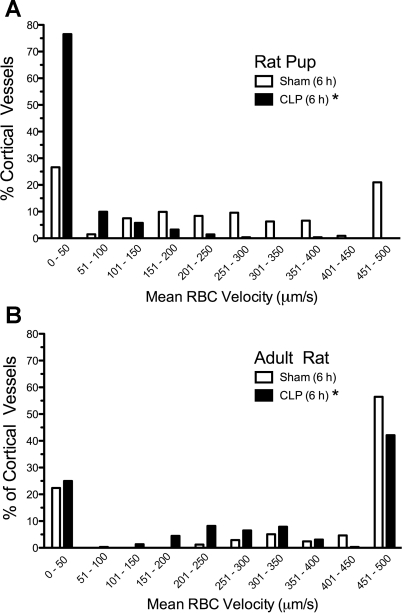
Although perfusion status is an overall index of gross perfusion, it does not address indexes of nutritive flow. To assess overall changes in capillary RBC velocity, the distribution of all capillary velocities was plotted as a histogram for pups (Fig. 7A) and adults (Fig. 7B). CLP produced a shift in the percent distribution of capillaries toward lower velocities in both pups and adults (P < 0.05). Interestingly, in sham animals, the percentage of capillaries with RBC velocities <451 μm/s was greater in the pup than in the adult (79 and 44%, respectively).
Fig. 7.
Frequency distribution of renal cortical capillary RBC velocities at 6 h. CLP shifted the distribution of capillary RBC velocities in pups (A) and adults (B) toward lower velocities (n = 4 animals/group). *P < 0.05, sham vs. CLP.
DISCUSSION
The adult animal may not be the appropriate model for studying sepsis-induced AKI in the pediatric population because the immunological and cardiovascular systems are still developing in the pediatric patient (20, 24, 29, 35). Severe sepsis in the pediatric patient is defined as sepsis with cardiovascular distress or multiple organ dysfunction (16). In contrast to adults with septic shock, hypotension is not always present and is not required for a diagnosis of pediatric septic shock (4). The rat pup CLP model of sepsis displayed many characteristics of pediatric severe sepsis, including cytokine generation, induction of inflammatory markers, multiorgan injury, hypothermia, and renal microcirculatory failure leading to AKI. Children with septic shock typically respond well to aggressive fluid resuscitation (4), and in the rat pup model fluids did significantly increase survival.
Several models of neonatal or pediatric sepsis have been developed using fecal slurry in mice (44), LPS in piglets (5, 12, 23) and rats (20), or zymosan in rats (3). However, there have been no studies that have characterized the development of sepsis in rat pups using the clinically relevant CLP model frequently used in adult models of sepsis. Very early after induction of sepsis in pups by CLP, serum concentrations of the inflammatory cytokines IL-1β and TNF-α were increased along with induction of ICAM-1 and iNOS in the kidney. Thus CLP induced not only a systemic inflammatory response but also activation of the inflammatory response in the kidney. Moreover, induction of sepsis produced a rapidly developing peritubular microcirculatory failure manifested by a decline in perfusion and increased capillary permeability.
The present study is unique because direct comparisons between pups and adults with experimental sepsis-induced AKI have never been reported. Rat pups have a lower resting MAP than adult rats. This difference is also seen in human infants and human adults (8). In the pediatric septic patient, preservation of microcirculatory perfusion is the focus of goal-directed therapy (7), and recent clinical evidence suggests that RBF rather than systemic pressure is the more important factor in maintaining the renal microcirculation (28), at least in adults. Both human and animal studies are suggesting that targeting the renal microcirculation may be the most effective strategy for preventing/treating multiple forms of AKI (22, 33, 45). It is important to note that a lower RBF was also seen in pups compared with adults, suggesting that pups may be more susceptible to renal injury associated with changes in renal perfusion.
CLP in the rat pup caused an increase in hepatic and renal markers consistent with multiorgan injury and severe sepsis. There was a dramatic decline in the average capillary RBC velocity and volumetric flow in continuously flowing capillaries as early as 4 h post-CLP. Moreover, there was a progressive and sustained decline in the percentage of vessels delivering nutritive flow through 10 h. This sustained microcirculatory defect was similar to that observed in aged mice subjected to CLP (40). In contrast, CLP did not alter the overall percentage of vessels with continuous, intermittent, or no flow in adult rats but did cause a shift in the RBC velocity distribution toward lower velocities. These data suggest that the pup kidney may be less able to regulate perfusion of the microcirculation compared with adults.
Peritubular capillary hypoperfusion may result from decreased RBF, which can occur during AKI even in the absence of a decline in MAP (28). Neonates have lower RBF and greater renal vascular resistance compared with adults (35). This is believed to be the result of a complex interaction between the renin-angiotensin system, endothelin, and NO, which are thought to play particularly important roles in regulating renal perfusion in the neonatal kidney (31). Following CLP, MAP in the adult decreased without any apparent compensatory change in heart rate, yet renal peritubular capillary perfusion was sustained. In the pup, peritubular capillary perfusion declined rapidly despite a relatively stable MAP that did not appear to be due to compensatory changes in heart rate. During sepsis, the greater and more rapid fall in RBF in pups compared with adults may be a result of differences in the autoregulatory/compensitory responses triggered by inflammatory mediators and elevated NO production that occur during sepsis despite a relatively stable (although lower than the adult) mean arterial blood pressure in pups. Additional studies are required to establish if there are mechanistic differences in autoregulation between pups and adult and how these might be altered during sepsis.
Importantly, another likely contributor to decreased peritubular capillary perfusion was endothelial activation and the increase in capillary permeability, indicating the development of direct injury to the peritubular capillary network. A decline in peritubular capillary perfusion following CLP may produce a hypoxic microenvironment favoring the generation of oxidants (40–42). This is significant because there is growing recognition of the potential role oxidants may play in the development of cardiovascular failure and organ dysfunction associated with pediatric sepsis (36). The immature kidney has decreased levels of the antioxidant enzymes superoxide dismutase and catalase (19) and thus may be more susceptible to oxidative damage (14, 19). Release of superoxide in a microenvironment where there is increased NO synthesis leads to the generation of the RNS peroxynitrite. Two markers of RNS generation, rhodamine fluorescence and nitrotyrosine immunoreactivity, indicated RNS production at 18 h. However, at 6 h post-CLP, RNS generation was unchanged despite the induction of iNOS mRNA and protein in the kidney. These data are consistent with a sequence of events that progress from hypoperfusion, hypoxia, iNOS induction to RNS generation.
In summary, the rat pup CLP model displays many characteristics of sepsis-induced AKI in the pediatric septic patient, including elevated serum BUN and Cre and decreased RBF (2, 35). Even relatively small changes in serum Cre concentrations like those seen in the rat pup model are associated with increased mortality and morbidity in children (11, 13). The lack of morphological changes in the rat pup kidney following CLP support a prerenal AKI, a form of vasomotor nephropathy to which neonates are particularly vulnerable (35). However, CLP caused a profound injury to the peritubular capillary microcirculation (decrease perfusion and capillary leakage) associated with an increase in oxidant generation by the renal tubules. While control of the renal microcirculation is not fully understood, data from the rat pup model suggest that the renal microcirculation is more easily perturbed than it is in the adult. Differences in the renal hemodynamic responses between rat pups and adults subjected to CLP illustrate the importance of using the appropriate age model to study sepsis-induced AKI, as has been shown in the aged mouse (10). Lower RBF, lower MAP, and reduced peritubular capillary perfusion in the pup coupled with reduced oxidant defenses support the notion that the pediatric kidney may be more susceptible to multiple forms of AKI (30, 35).
GRANTS
This work was supported by National Institutes of Health Grants R01 DK-075991 (to P. R. Mayeux), R01 DK-075991-S1 (to K. A. Seely), and F30 DK-085705 (to J. H. Holthoff) and America Heart Association Grants 10PRE4140065 (to Z. Wang) and SDG 0830060N (to S. W. Rhee).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Russell Melchert and Kerrey Roberto of the University of Arkansas for Medical Sciences Rodent Biotelemetry Core for help with the biotelemetry studies. Cytokine measurements were made using the Flow Cytometry Core, and the Experimental Pathology Core performed the tissue sectioning.
REFERENCES
- 1. Baker M, Wayland H. On-line volume flow rate and velocity profile measurement for blood in microvessels. Microvasc Res 7: 131–143, 1974 [DOI] [PubMed] [Google Scholar]
- 2. Brophy PD. Renal supportive therapy for pediatric acute kidney injury in the setting of multiorgan dysfunction syndrome/sepsis. Sem Nephrol 28: 457–469, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Calkins CM, Bensard DD, Partrick DA, Karrer FM, McIntyre RC. Altered neutrophil function in the neonate protects against sepsis-induced lung injury. J Pediatr Surg 37: 1042–1047, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Carcillo JA, Fields AI. Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med 30: 1365–1378, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Chin A, O'Conner LN, Radhakrishnan J, Fornell L, John E. Endotoxemia and the effects of dopamine on renal functions of neonatal piglets. Biol Neonate 81: 196–202, 2002 [DOI] [PubMed] [Google Scholar]
- 6. de Oliveira CF. Early goal-directed therapy in treatment of pediatric septic shock. Shock 34, Suppl 1: 44–47, 2010 [DOI] [PubMed] [Google Scholar]
- 7. de Oliveira CF, de Oliveira DS, Gottschald AF, Moura JD, Costa GA, Ventura AC, Fernandes JC, Vaz FA, Carcillo JA, Rivers EP, Troster EJ. ACCM/PALS haemodynamic support guidelines for paediatric septic shock: an outcomes comparison with and without monitoring central venous oxygen saturation. Intensive Care Med 34: 1065–1075, 2008 [DOI] [PubMed] [Google Scholar]
- 8. de Swiet M, Fayers P, Shinebourne EA. Systolic blood pressure in a population of infants in the first year of life: the Brompton study. Pediatrics 65: 1028–1035, 1980 [PubMed] [Google Scholar]
- 9. Doi K, Hu X, Yuen PS, Leelahavanichkul A, Yasuda H, Kim SM, Schnermann J, Jonassen TE, Frokiaer J, Nielsen S, Star RA. AP214, an analogue of alpha-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int 73: 1266–1274, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest 119: 2868–2878, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duzova A, Bakkaloglu A, Kalyoncu M, Poyrazoglu H, Delibas A, Ozkaya O, Peru H, Alpay H, Soylemezoglu O, Gur-Guven A, Bak M, Bircan Z, Cengiz N, Akil I, Ozcakar B, Uncu N, Karabay-Bayazit A, Sonmez F. Etiology and outcome of acute kidney injury in children. Pediatr Nephrol 25: 1453–1461, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Fischer D, Nold MF, Nold-Petry CA, Furlan A, Veldman A. Protein C preserves microcirculation in a model of neonatal septic shock. Vasc Health Risk Manag 5: 775–781, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fiser RT, West NK, Bush AJ, Sillos EM, Schmidt JE, Tamburro RF. Outcome of severe sepsis in pediatric oncology patients. Pediatr Crit Care Med 6: 531–536, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Fukumoto K, Pierro A, Spitz L, Eaton S. Cardiac and renal mitochondria respond differently to hydrogen peroxide in suckling rats. J Surg Res 113: 146–150, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Fukumoto K, Pierro A, Spitz L, Eaton S. Neonatal endotoxemia affects heart but not kidney bioenergetics. J Pediatr Surg 38: 690–693, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 6: 2–8, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Gomes A, Fernandes E, Lima JL. Use of fluorescence probes for detection of reactive nitrogen species: a review. J Fluoresc 16: 119–139, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Guo R, Wang Y, Minto AW, Quigg RJ, Cunningham PN. Acute renal failure in endotoxemia is dependent on caspase activation. J Am Soc Nephrol 15: 3093–3102, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Gupta A, Gupta A, Nigam D, Shukla G, Agarwal AK. Profile of reactive oxygen species generation and antioxidative mechanisms in the maturing rat kidney. J Appl Toxicol 19: 55–59, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Hurley RM, Nayyar RP, Goto M, Zeller WP. Renal lesions in young rats induced by Salmonella enteritidis endotoxin. Pediatr Nephrol 3: 156–161, 1989 [DOI] [PubMed] [Google Scholar]
- 21. Ince C. The microcirculation is the motor of sepsis. Crit Care 9, Suppl 4: S13–S19, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Le Dorze M, Legrand M, Payen D, Ince C. The role of the microcirculation in acute kidney injury. Curr Opin Crit Care 15: 503–508, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Lievano G, Nguyen L, Radhakrishnan J, Fornell L, Joshi A, John EG. Significance of fractional excretion of sodium and endothelin levels in the early diagnosis of renal failure in septic neonatal piglets. J Pediatr Surg 33: 1480–1482, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Mathur NB, Agarwal HS, Maria A. Acute renal failure in neonatal sepsis. Indian J Pediatr 73: 499–502, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Miyaji T, Hu X, Yuen PS, Muramatsu Y, Iyer S, Hewitt SM, Star RA. Ethyl pyruvate decreases sepsis-induced acute renal failure and multiple organ damage in aged mice. Kidney Int 64: 1620–1631, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Myers MM, Scalzo FM. Blood pressure and heart rate responses of SHR and WKY rat pups during feeding. Physiol Behav 44: 75–83, 1988 [DOI] [PubMed] [Google Scholar]
- 27. Nguyen HB, Rivers EP, Abrahamian FM, Moran GJ, Abraham E, Trzeciak S, Huang DT, Osborn T, Stevens D, Talan DA. Severe sepsis and septic shock: review of the literature and emergency department management guidelines. Ann Emerg Med 48: 28–54, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Redfors B, Bragadottir G, Sellgren J, Sward K, Ricksten SE. Acute renal failure is NOT an “acute renal success”–a clinical study on the renal oxygen supply/demand relationship in acute kidney injury. Crit Care Med 38: 1695–1701, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Solhaug MJ, Ballevre LD, Guignard JP, Granger JP, Adelman RD. Nitric oxide in the developing kidney. Pediatr Nephrol 10: 529–539, 1996 [DOI] [PubMed] [Google Scholar]
- 30. Solhaug MJ, Bolger PM, Jose PA. The developing kidney and environmental toxins. Pediatrics 113: 1084–1091, 2004 [PubMed] [Google Scholar]
- 31. Solhaug MJ, Wallace MR, Granger JP. Nitric oxide and angiotensin II regulation of renal hemodynamics in the developing piglet. Pediatr Res 39: 527–533, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Spanos A, Jhanji S, Vivian-Smith A, Harris T, Pearse RM. Early microvascular changes in sepsis and severe sepsis. Shock 33: 387–391, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Sutton TA, Kelly KJ, Mang HE, Plotkin Z, Sandoval RM, Dagher PC. Minocycline reduces renal microvascular leakage in a rat model of ischemic renal injury. Am J Physiol Renal Physiol 288: F91–F97, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Tiwari MM, Brock RW, Kaushal GP, Mayeux PR. Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: role of nitric oxide and caspases. Am J Physiol Renal Physiol 289: F1324–F1332, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Toth-Heyn P, Drukker A, Guignard JP. The stressed neonatal kidney: from pathophysiology to clinical management of neonatal vasomotor nephropathy. Pediatr Nephrol 14: 227–239, 2000 [DOI] [PubMed] [Google Scholar]
- 36. von Dessauer B, Bongain J, Molina V, Quilodran J, Castillo R, Rodrigo R. Oxidative stress as a novel target in pediatric sepsis management. J Crit Care 26: 103.e.1–7, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Wang W, Faubel S, Ljubanovic D, Mitra A, Kim J, Tao Y, Soloviev A, Reznikov LL, Dinarello CA, Schrier RW, Edelstein CL. Endotoxemic acute renal failure (ARF) is attenuated in caspase-1 deficient mice. Am J Physiol Renal Physiol 288: F997–F1004, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Wang Z, Herzog C, Kaushal GP, Gokden N, Mayeux PR. Actinonin, a meprin A inhibitor, protects the renal microcirculation during sepsis. Shock 35: 141–147, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Watson RS, Carcillo JA. Scope and epidemiology of pediatric sepsis. Pediatr Crit Care Med 6: S3–S5, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Wu L, Gokden N, Mayeux PR. Evidence for the role of reactive nitrogen species in polymicrobial sepsis-induced renal peritubular capillary dysfunction and tubular injury. J Am Soc Nephrol 18: 1807–1815, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Wu L, Mayeux PR. Effects of the inducible nitric oxide synthase inhibitor L-N6-(1-iminoethyl)-lysine on microcirculation and reactive nitrogen species generation in the kidney following lipopolysaccharide administration in mice. J Pharmacol Exp Ther 320: 1061–1067, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Wu L, Tiwari MM, Messer KJ, Holthoff JH, Gokden N, Brock RW, Mayeux PR. Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am J Physiol Renal Physiol 292: F261–F268, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Wynn J, Cornell TT, Wong HR, Shanley TP, Wheeler DS. The host response to sepsis and developmental impact. Pediatrics 125: 1031–1041, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wynn JL, Scumpia PO, Delano MJ, O'Malley KA, Ungaro R, Abouhamze A, Moldawer KL. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock 28: 675–683, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Yasuda H, Yuen PS, Hu X, Zhou H, Star RA. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int 69: 1535–1542, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zanotti-Cavazzoni SL, Guglielmi M, Parrillo JE, Walker T, Dellinger RP, Hollenberg SM. Fluid resuscitation influences cardiovascular performance and mortality in a murine model of sepsis. Intensive Care Med 35: 748–754, 2009 [DOI] [PubMed] [Google Scholar]