Abstract
The contribution of medial calcification to vascular dysfunction in renal failure is unknown. Vascular function was measured ex vivo in control, noncalcified uremic, and calcified uremic aortas from rats with adenine-induced renal failure. Plasma urea was 16 ± 4, 93 ± 14, and 110 ± 25 mg/dl, and aortic calcium content was 27 ± 4, 29 ± 2, and 4,946 ± 1,616 nmol/mg dry wt, respectively, in the three groups. Maximal contraction by phenylephrine (PE) or KCl was reduced 53 and 63% in uremic aortas, and sensitivity to KCl but not PE was increased. Maximal relaxation to acetylcholine was impaired in uremic aortas (30 vs. 65%), and sensitivity to nitroprusside was also reduced, indicating some impairment of endothelium-independent relaxation as well. None of these parameters differed between calcified and noncalcified uremic aortas. However, aortic compliance was reduced in calcified aortas, ranging from 17 to 61% depending on the severity of calcification. We conclude that uremic vascular calcification, even when not severe, significantly reduces arterial compliance. Vascular smooth muscle and endothelial function are altered in renal failure but are not affected by medial calcification, even when severe.
Keywords: vascular contractility, renal failure, vascular relaxation
the incidence of cardiovascular disease is markedly increased in patients with advanced kidney disease and is associated with changes in vascular function. Arterial compliance is decreased (16) and correlates strongly with death (3). Endothelial function is also abnormal, manifest primarily as reduced endothelium-dependent vasodilatation (9, 10, 12, 20, 21) and may contribute to hypertension and ischemia. Renal failure may affect the function of vascular smooth muscle as well, but the data are disparate with some studies showing enhanced responsiveness and other showing reduced responsiveness to α-adrenergic stimulation (2, 7, 19, 27, 32).
The etiology of vascular dysfunction in renal failure is not known. Histological studies have shown changes in the medial layer of arteries consisting of both hypertrophy and hyperplasia of smooth muscle cells as well as increased matrix (1), which could explain the decrease in compliance. There is also a high incidence of atherosclerosis (8), which might also lower compliance and is associated with endothelial dysfunction. Increased oxidant stress in renal failure (6) is another potential cause of endothelial dysfunction.
Smooth muscle calcification is another common arterial lesion in advanced kidney disease that could also contribute to vascular dysfunction. Although it has been considered to be benign, recent data have shown that vascular calcification correlates with cardiovascular events and death (15). This may be related to a decrease in arterial compliance since increased pulse-wave velocity correlates with vascular calcification and cardiovascular disease in advanced kidney disease (4, 26). However, pulse-wave velocity can be influenced by smooth muscle tone in addition to elasticity (18), and interpretation of these studies is also complicated by the fact that they cannot distinguish between the medial calcification (Monckeberg's sclerosis) commonly associated with renal failure and the intimal (atherosclerotic) calcification that frequently coexists. Thus medial calcification in uremia has not been directly correlated with arterial compliance, and its effect on endothelial or smooth muscle function is also unclear.
To address the effects of medial vascular calcification in uremia on vascular function, we performed ex vivo measurements of compliance, contraction, and relaxation in calcified and noncalcified aortas from uremic rats and in control aortas from nonuremic rats. Uremia and vascular calcification were induced by feeding a high-phosphate diet containing adenine (24, 30), and comparisons were made between control, pair-fed rats and uremic rats with and without calcification.
METHODS
Animals.
Renal failure was induced by feeding adenine as previously described (14), with modifications. Male Sprague-Dawley rats weighing ∼300 g were fed a standard rodent diet supplemented with 0.66% phosphorus (from a 2:1 mixture of dibasic and monbasic sodium phosphate). The final content of the diet was 23% protein, 0.95% calcium, and 1.06% phosphorus. To produce renal failure, adenine was added at a concentration of 0.75%. NaCl (0.22%) was added to the drinking water to counteract the salt wasting that occurs with this model. Since rats lose weight on this diet, food was restricted in the control rats so that aggregate weights were similar. For the studies of vessel compliance, the model was altered to produce a wider range of calcification. Dietary protein was reduced to 2.5% (25) or calcitriol (40 ng/kg 3 times/wk) was added to enhance calcification, and dietary phosphorus was lowered to 0.73% to reduce calcification. Rats were killed after 28–30 days. Plasma was obtained by aortic puncture at the bifurcation, and aortas were removed, gently cleaned, and stored in Hanks' solution. All studies were completed in compliance with protocols reviewed and approved by the Institutional Animal Care and Use Committee.
Vessel studies.
One or two rings (4–5 mm in length) were prepared from the straight section of the descending thoracic aortas, without regard to the degree of visible calcification. Contractile and relaxation studies in response to agonists were completed as we have described previously (13). For each aorta, resting tension was adjusted to 50 mN over a 1-h period. All vessels demonstrated maximal active force generation at this tension (data not shown). Data were recorded using PowerLab digital acquisition and analyzed using Chart Software (AD Instruments, Mountain View, CA). Vascular contractility was assessed by generating concentration-response curves to KCl (0–80 mM) and phenylephrine (PE; 0.1 nM–10 μM). Agonist sensitivity was determined by plotting the individual concentration-response curves as the percentage of maximum and comparing the average of the EC50 values obtained. For comparisons of maximal contraction, isometric force is normalized to cross-sectional area (CSA) which is estimated based on vessel geometry and wet weight [CSA = 2 × wet weight/(circumference)] as described (31).Vascular relaxation was also determined. Following precontraction with 2–5 μM PE, a concentration which yields 80–90% maximum contraction, relaxation responses were examined in response to the endothelium-dependent vasorelaxant acetylcholine (0.1 nM–100 μM) and the endothelium-independent NO-donor sodium nitroprusside (SNP; 0.1 nM–1 μM).
In a separate study, passive mechanical properties of the aorta were assessed using methodologies adapted from Blough et al. (5) as an indication of vessel compliance. Aortic rings were maximally dilated with 30 μM SNP, and tension was applied until a deflection in force measurement was observed. This tension was set as L0, and the length was increased in 25-μm increments while the force was monitored. The length that resulted in maximal force development or resulted in tearing of the vessel was used as an index of the passive mechanical properties of the vessel, or compliance.
After the measurements were completed, the rings were dried and the calcium content was measured as below.
Chemical assays.
Plasma urea was assayed enzymatically using urease and glutamate dehydrogenase (ThermoDMA, Arlington, TX). Plasma phosphate was measured colorimetrically by the molybdate method and plasma calcium was determined colorimetrically using 100 μM o-cresolphthalein complexone in 270 mM aminomethylpropanol buffer, pH 10.0, with 5.2 mM 8-hydroxyquinolone added to complex magnesium. Absorbance was measured at 575 nm. To measure aortic calcium content, dried aortas were incubated in 1 M HCl overnight and calcium was measured as described above. Aortas were dried after extraction and weighed, and results are expressed as nanomoles per milligram dry weight. For ex vivo vessel studies, calcium content was measured in the individual rings.
Statistical analysis.
Results are presented as means ± SE. EC50 values and maximum force were determined for each sample, and differences between groups were assessed by ANOVA using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA). Concentration response curves were analyzed using two-way ANOVA followed by Bonferroni tests to compare individual points. Significance was defined as P < 0.05.
RESULTS
Plasma chemistries and aortic calcium content for the animals used in the contraction and relaxation studies are shown in Table 1. These rats were fed standard rat chow (23% protein) supplemented with sodium phosphate to a final content of 1.06% phosphorus with or without adenine to induce renal failure. Plasma urea and phosphate were significantly elevated in adenine-treated rats, with no significant difference between rats with and without aortic calcification. Plasma calcium did not differ between control and uremic rats or between uremic rats with and without aortic calcification.
Table 1.
Biochemical values in control and uremic rats fed a 23% protein and 1.06% phosphorus diet
| Adenine-Fed Rats |
|||
|---|---|---|---|
| Control Rats (n = 6) | Noncalcified (n = 5) | Calcified (n = 6) | |
| Aortic calcium, nmol/mg | 20.8 ± 2.2 | 24.9 ± 2.7 | 2453 ± 746* |
| Plasma BUN, mg/dl | 14.4 ± 3.3 | 58.7 ± 10.6† | 65.4 ± 8.6† |
| Plasma phosphorus, mM | 0.82 ± 0.21 | 2.64 ± 0.75† | 2.57 ± 0.63† |
| Plasma calcium, mM | 1.87 ± 0.56 | 1.80 ± 0.25 | 1.78 ± 0.17 |
Values are means ± SE.
BUN, blood urea nitrogen.
P < 0.002 vs. noncalcified or control.
P < 0.0001 vs. control.
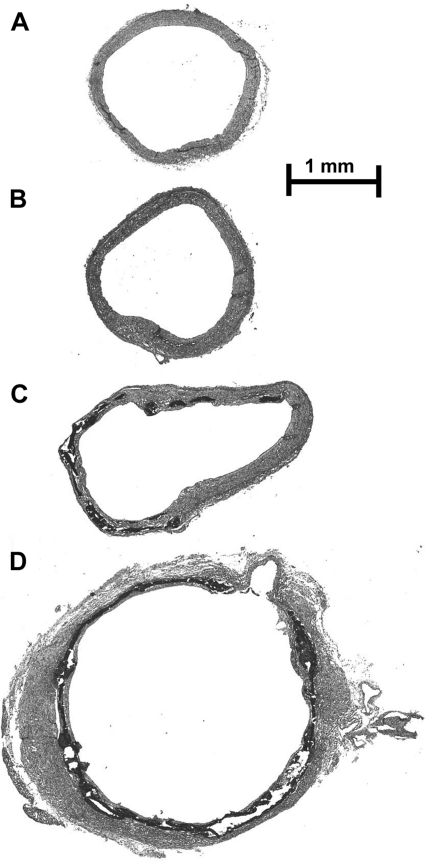
Aortic calcium content was variable in uremic rats fed standard rat chow with 1.06% phosphorus, with only about one-half the aortas exhibiting any calcification. Aortas were considered to be calcified when the calcium content was >37 nmol/mg, which is three SD above the mean content in whole aortas from nonuremic rats. There was a clear separation between calcified and noncalcified aortas, with the range in calcified aortas being 577–4,794 nmol/mg. Calcification was visually apparent when the calcium content exceeded 300 nmol/mg and was diffuse and severe when the content exceeded 1,500 nmol/mg. Staining of the aortas for calcification (von Kossa stain) is shown in Fig. 1. There was no staining in control or noncalcified uremic aortas, and they appeared otherwise similar. Moderately calcified aortas showed patches of medial calcification, with the remainder of the aorta appearing normal. Heavily calcified aortas showed calcification within the vascular media that was histologically similar but fully circumferential. The fracturing that is present is an artifact of the tissue preparation.
Fig. 1.
Aortic histology in control and uremic rats fed a 23% protein and 1.06% phosphorus diet. Aortas were stained by the von Kossa method, in which calcium phosphate appears black. No calcification was observed in aortas from control, nonuremic rats (A), and in uremic aortas with a normal Ca content (B). Aortas with increased calcium content exhibited both patchy (C) and diffuse, circumferential (D) calcification restricted to the medial layer. The same magnification was used for all images.
The heterogeneous distribution of the calcification makes it is impossible to assign a calcium content to each image, but these sections are representative of the rings used for measurement of contraction and relaxation. Although the vascular wall appears thickened in portions of Fig. 1D, heavy calcification was usually associated with thinning of the wall and dilatation, which is readily apparent in the calcified portion of Fig. 1C. Medial thickness was 130 ± 14 and 134 ± 5 μm in control and noncalcified uremic aortas, respectively, indicating that smooth muscle mass was not altered in uremic vessels. Calcification precluded accurate measurement of medial thickness, but the amount of normal smooth muscle appeared to be reduced in the heavily calcified aortas.
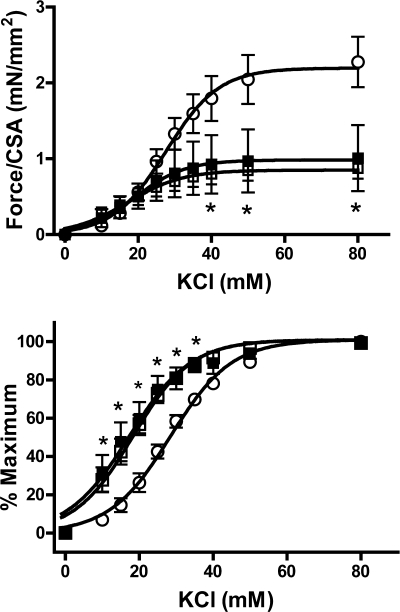
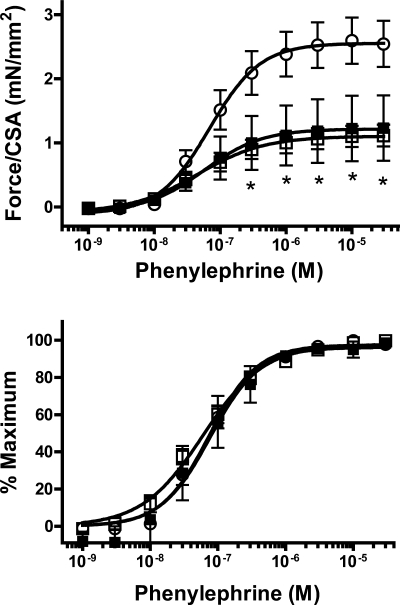
Smooth muscle contractile function was investigated by measuring force generation in response to depolarization (using KCl) and to the α-adrenoceptor agonist PE. Sensitivity to KCl was increased in uremic aortas (Fig. 2), with no difference between noncalcified and calcified aortas (EC50 28.1 ± 1.0, 18.0 ± 1.7, 17.5 ± 2.8 nM in control, uremic noncalcified, and uremic calcified, respectively) (P < 0.01 vs. control for either noncalcified or calcified uremic aortas). In addition, the maximum force per cross-sectional area obtained with KCl was markedly decreased in uremic aortas, again with no difference between noncalcified and calcified aortas (2.38 ± 0.33, 0.87 ± 0.14, and 1.01 ± 0.43 mN/mm2 in control, uremic noncalcified, and uremic calcified respectively; P < 0.05 vs. control; n = 6–8). In the case of PE, there were no changes in sensitivity (Fig. 3) but the same profound reduction in maximum force was observed (2.60 ± 0.36, 1.12 ± 0.17, and 1.23 ± 0.51 mN/mm2 in control, uremic noncalcified, and uremic calcified, respectively) (P < 0.05 vs. control for either noncalcified or calcified uremic aortas; n = 6–8). Again, there were no differences between calcified and noncalcified aortas in response to PE.
Fig. 2.
Contractile response of uremic aortas to KCl. Top: absolute force as a function of KCl concentration. Bottom: relative force as a function of KCl concentration. ○, Control (n = 6); □, uremic, noncalcified (n = 8); ■, uremic, calcified (n = 6). Rats were fed a 23% protein and 1.06% phosphorus diet. CSA, cross-sectional area. *P < 0.05 compared with control by 2-way, repeated-measures ANOVA followed by Bonferroni correction.
Fig. 3.
Contractile response of uremic aortas to phenylephrine (PE). Top: absolute force as a function of PE concentration. Bottom: relative force as a function of PE concentration. ○, Control (n = 6); □, uremic, noncalcified (n = 8); ■, uremic, calcified (n = 6). Rats were fed a 23% protein and 1.06% phosphorus diet. *P < 0.05 compared with control by 2-way, repeated-measures ANOVA followed by Bonferroni correction.
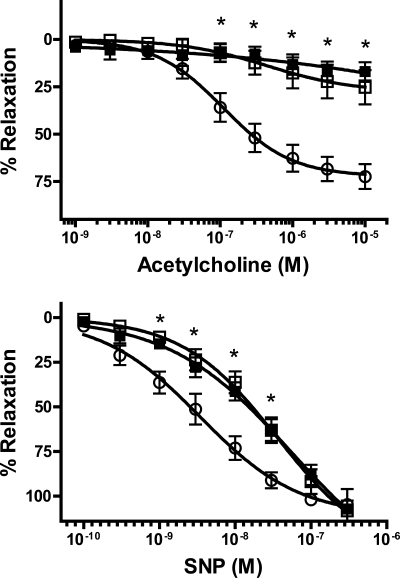
Vasorelaxation studies are shown in Fig. 4. Relaxation responses to acetylcholine, an endothelium-dependent vasodilator, were profoundly depressed in uremic aortas. Maximal relaxation with SNP, a direct smooth muscle dilator, was not altered in uremic aortas, but sensitivity was significantly reduced. There were no significant differences between calcified and noncalcified aortas.
Fig. 4.
Relaxation of uremic aortas. Aortas were preconstricted with 3 μM PE, and relaxation was measured in response to increasing concentrations of acetylcholine (top) or sodium nitroprusside (SNP; bottom). Results are expressed as a percentage of relaxation referenced to the PE contraction. ○, Control (n = 6); □, uremic, noncalcified (n = 8); ■, uremic, calcified (n = 6). Rats were fed a 23% protein and 1.06% phosphorus diet. *P < 0.05 compared with control by 2-way, repeated-measures ANOVA followed by Bonferroni correction.
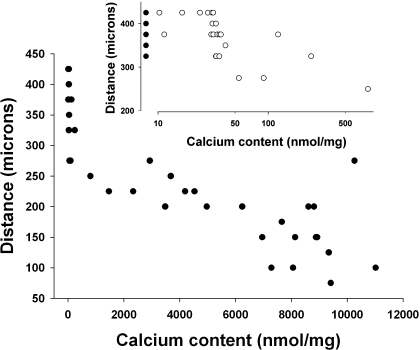
Aortic compliance was measured directly ex vivo as tension vs. distance in aortic rings maximally relaxed with SNP. Compliance was reduced >50%, but the aortas were either severely calcified or not calcified at all. To determine whether lesser degrees of calcification also reduce compliance, changes were made in the model to obtain intermediate degrees of calcification. First, dietary protein was reduced to 2.5%, which increases aortic calcification in adenine-treated rats by an unknown mechanism (25). This increased the prevalence of calcification and yielded some aortas with intermediate calcium contents. Greater success was achieved by administering graded doses of calcitriol (up to 40 ng/kg 3 times/wk) to rats fed standard chow with 0.73% phosphorus. There was no apparent difference in compliance between aortic rings with similar calcium contents generated with the different models, so the data were combined to show compliance as a function of calcium content. As shown in Fig. 5, compliance was reduced in almost all calcified vessels and was inversely related to calcium content. Compared with noncalcified aortic rings (n = 17), minimally calcified rings (Ca content 41–803 nmol/mg, n = 6) showed a 20% decrease in compliance (308 ± 22 vs. 387 ± 9 μm) (P < 0.01), while in the most heavily calcified rings (Ca content 8,061–11,022 nmol/mg, n = 10) the decrease was 61% (153 ± 20 μm) (P < 0.001). Compliance did not differ between control aortas and noncalcified uremic aortas.
Fig. 5.
Effect of calcification on aortic passive mechanical properties. Aortic rings (n = 45) were maximally dilated with SNP, and tension was measured while transverse length was increased. Aortas were dried, and calcium content was determined. Data are plotted as the number of micrometers at which maximum tension was achieved or the vessel ruptured. The regression line for the data is 358 − 0.024 × aortic calcium content (r = 0.86), and the slope is significantly less than zero by linear regression (P < 0.0001). Inset: values for aortas with calcium contents <900 nmol/mg. ●, Nonuremic aortas. Data are from rats fed a 23 or 2.5% protein diet with 1.06% phosphorus diet and from rats fed a 23% protein, 0.73% phosphorus diet, and treated with calcitriol up to 40 ng/kg 3 times/wk.
DISCUSSION
This is the first study to directly measure the effect of uremic medial calcification on vascular function. Of all the parameters tested, only compliance, an index of the passive mechanical properties, differed between calcified and noncalcified aortas. Compliance was reduced at all levels of calcification and was inversely related to the degree of calcification. Although it is not possible to quantitatively compare vascular calcification in patients and animals, it is likely that vascular calcification in patients falls within the range achieved in this study. Vascular calcification in humans is often visible to the naked eye, as was the case in aortas from uremic rats. Furthermore, circumferential calcification as seen in many aortas in this study is frequently observed in humans by computed tomography. Thus the reduction in compliance observed in this experimental model of renal failure is likely to occur in patients with medial calcification.
It is widely believed that medial calcification decreases arterial compliance in patients with advanced kidney disease and end-stage renal disease, but this remains unproved. Existing data are correlative and are based primarily on pulse-wave velocity, which can be affected by factors other than aortic structure (18). Associations with calcification, although significant, are weak (26) and have not been found in all studies (16). Previous studies in humans have not distinguished between medial calcification and the intimal (atherosclerotic) calcification that frequently coexists, so the contribution of medial calcification to aortic stiffness has been uncertain. This study indicates that at least some of the reduced arterial compliance in chronic kidney disease and end-stage renal disease can be explained by medial calcification. This is consistent with the correlation between pulse-wave velocity and medial calcification in nonuremic rats treated with nicotine and toxic doses of vitamin D (22) and with the increased pulse wave velocity in uremic rats treated with doses of doxercalciferol that produce aortic calcification (23).
A recent study in uremic ApoE−/− and wild-type mice, in which most of the aortic calcification was intimal, found no correlation between pulse-wave velocity and aortic calcification (17). This finding and the decreased compliance observed in the current study suggest that only medial calcification reduces arterial compliance, which may be related to the loss of elastin in medial calcification (22). Pulse-wave velocity was increased in uremic mice in the absence of calcification or other structural changes in the aorta, demonstrating that pulse-wave velocity is not necessarily indicative of changes in arterial structure. In the current study, compliance was not altered in uremic aortas that were not calcified, possibly due to different hemodynamics, different species, or the shorter duration of uremia.
Although marked changes in smooth muscle and endothelial function were observed in calcified vessels, the changes were qualitatively and quantitatively the same in noncalcified uremic vessels, indicating that the effects on vascular function were the result of uremia rather than calcification. It is possible that intimal calcification, which did not occur in this study, can alter vascular function. A severe reduction in maximal force generation in calcified aortas was also noted in a previous study of rats made uremic by nephrectomy and given a very high calcium diet and high doses of calcitriol (29). The calcification was exclusively medial and histologically identical to that obtained in adenine-fed rats in this study. Both calcification and force generation were improved in rats treated with etidronate, suggesting that medial calcification affects smooth muscle function. However, noncalcified uremic aortas were not studied and calcium content was not measured or correlated with force generation. In another study, treatment of uremic rats with paricalcitol increased aortic calcification and reduced force generation in superior mesenteric arteries (11), but it is not clear whether these vessels were calcified. Vasodilatation of these arteries was not altered by paricalcitol, consistent with the present results showing no effect of medial calcification on relaxation.
The lack of effect of medial calcification on vascular function despite extensive and grossly visible calcification with reduced compliance may be explained by the fact that contraction and relaxation were measured under isometric conditions in which very little wall motion is necessary to measure force. It is also possible that the profound effects of uremia alone on contraction and relaxation masked any additional effect of calcification. Changes in vessel function might therefore be more apparent when medial calcification occurs in the absence of renal failure, such as in diabetes and aging. Hemodynamics are also determined by chronic changes in vessel caliber related to remodeling of smooth muscle, and this could be altered by medial calcification in the absence of changes in acute vasomotor responses.
This study demonstrated marked alterations in both smooth muscle and endothelial function in uremic rats. Maximal force was substantially reduced in response to either KCl or PE, and sensitivity to KCl was increased. Previous studies of arterial function in animals and in humans with renal failure have yielded contradictory results, with both increased (2, 32) and decreased (7, 11, 19, 27) α-adrenergic sensitivity noted. The mechanisms underlying the changes in smooth muscle function in uremia have not been investigated. The severe reduction in maximum force in the absence of reduced smooth muscle mass indicates a significant impairment in contractile capacity. The underlying mechanism is unknown but could involve changes in vasoactive substances or the intracellular signaling cascades that regulate calcium sensitivity in vascular smooth muscle (28).
Relaxation in response to acetylcholine, an endothelium-dependent dilator, was severely impaired in the uremic rats in this study. Although relaxation to sodium nitroprusside, a direct vasodilator, was also impaired, the reduction was not severe and could not entirely explain the impaired response to acetylcholine. This impairment in endothelium-dependent relaxation has been observed in prior studies, both in animals (10, 11) and in humans (21) ex vivo, and in humans in vivo (9, 12, 20), but the impaired direct vasodilation has not previously been described.
Heavily calcified aortas exhibited significant dilatation, likely due to the destruction of elastin that has been demonstrated in the vitamin d-nicotine model of medial calcification (22). However, this is unlikely to have artifactually lowered compliance. Measurements of compliance were based on an initial length that was set for each ring based on the development of tension and the fact that distance was not normalized to initial distance would tend to increase apparent compliance in dilated aortas. Measurements of force generation were also based on an initial tension and normalized to cross-sectional surface area, which would tend to lower force generation in dilated aortas. Measurements of vasorelaxation are independent of vessel geometry.
Caution must be exercised in extrapolating these results to patients with kidney disease. Severe uremia and vascular calcification developed within a month in this model, clearly faster than the development of vascular calcification in humans and without time for remodeling of the vessel wall that could affect vascular function in chronic kidney disease. However, this model does demonstrate that medial vascular calcification can significantly reduce arterial compliance without affecting the function of smooth muscle or endothelium.
GRANTS
This work was supported by National Institutes of Health Grants HL070892 (R. L. Sutliff) and DK069681 (W. C. O'Neill) and an Amgen Junior Faculty Award (K. A. Lomashvili).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Amann K, Wolf B, Nichols C, Törnig J, Schwarz U, Zeier M, Mall G, Ritz E. Aortic changes in experimental renal failure. Hyperplasia or hypertrophy of smooth muscle cells? Hypertension 29: 770–775, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Beretta-Piccoli C, Weidmann P, Schiffl H, Cottier C, Reubi FC. Enhanced cardiovascular pressor reactivity to norepinephrine in mild renal parenchymal disease. Kidney Int 22: 297–303, 1982 [DOI] [PubMed] [Google Scholar]
- 3. Blacher J, Guerin AP, Pannier B, Marchias SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation 99: 2434–2439, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38: 938–942, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Blough ER, Rice KM, Desai DH, Wehner P, Wright GL. Aging alters mechanical and contractile properties of the Fisher 344/Nnia × Norway/Binia rat aorta. Biogerontology 8: 303–313, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Boaz M, Mataz Z, Biro A, Katzir Z, Green M, Fainaru M, Smetana S. Serum malondialdehyde and prevalent cardiovascular disease in hemodialysis. Kidney Int 56: 1078–1083, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Fox AW, May RE, Mitch WE. Comparison of peptide and nonpeptide receptor-mediated responses in rat tail artery. J Cardiovas Pharmacol 20: 282–289, 1992 [DOI] [PubMed] [Google Scholar]
- 8. Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. New Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Hand MF, Haynes WG, Webb DJ. Hemodialysis and l-arginine, but not d-arginine, correct renal failure-associated endothelial dysfunction. Kidney Int 53: 1068–1077, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Kalliovalkama J, Jolma P, Tolvanen JP, Kahonen M, Hutri-Kahonen N, Saha H, Tuorila S, Moilanen E, Porsti I. Potassium channel-mediated vasorelaxation is impaired in experimental renal failure. Am J Physiol Heart Circ Physiol 277: H1622–H1629, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Karavalakis E, Eraranta A, Vehmas TI, Koskela JK, Koobi P, Mustonen J, Niemela O, Rysa J, Ruskoaho H, Porsti I. Paricalcitol treatment and arterial tone. Nephron Exp Nephrol 109: e84–e93, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Kari JA, Donald AE, Vallance DT, Bruckdorfer KR, Leone A, Mullen MJ, Bunce T, Dorado B, Deanfield JE, Rees L. Physiology and biochemistry of renal function in children with chronic renal failure. Kidney Int 52: 468–472, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Kline ER, Kleinhenz DJ, Liang B, Dikalov S, Guidot DM, Hart CM, Jones DP, Sutliff RL. Vascular oxidative stress and nitric oxide depletion in HIV-1 transgenic rats are reversed by glutathione restoration. Am J Physiol Heart Circ Physiol 294: H2792–H2804, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lomashvili KA, Monier-Faugere MC, Wang X, Malluche HH, O'Neill WC. Effect of bisphosphonates on vascular calcification and bone metabolism in experimental renal failure. Kidney Int 75: 617–625, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 16. London GM, Marchais SJ, Safar ME, Genest AF, Guerin AP, Metivier F, Chedid K, London AM. Aortic and large artery compliance in end-stage renal failure. Kidney Int 37: 137–142, 1990 [DOI] [PubMed] [Google Scholar]
- 17. Maizel J, Six I, Slama M, Tribouilloy C, Sevestre H, Poirot S, Giummelly P, Atkinson J, Choukroun G, Andrejak M, Kamel S, Maziere JC, Massy ZA. Mechanisms of aortic and cardiac dysfunction in uremic mice with aortic calcification. Circulation 119: 306–313, 2009 [DOI] [PubMed] [Google Scholar]
- 18. McDonald D. Regional pulse-wave velocity in the arterial tree. J Appl Physiol 24: 73–78, 1968 [DOI] [PubMed] [Google Scholar]
- 19. Meggs LG, Ben-Ari J, Gammon D, Choudhury M, Goodman AI. Effect of chronic uremia on the cardiovascular alpha1 receptor. Life Sci 39: 169–179, 1986 [DOI] [PubMed] [Google Scholar]
- 20. Morris STW, McMurry JJV, Rodger RSC, Jardine AG. Impaired endothelium-dependent vasodilatation in uraemia. Nephrol Dial Transplant 15: 1194–1200, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Morris STW, McMurry JJV, Spiers A, Jardine AG. Impaired endothelial function in isolated human uremic resistance arteries. Kidney Int 60: 1077–1082, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Niederhoffer N, Lartaud-Idjouadiene I, Giummelly P, Duvivier C, Peslin R, Atkinson J. Calcification of medial elastic fibers and aortic elasticity. Hypertension 29: 999–1006, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Noonan W, Koch K, Nakane M, Ma J, Dixon D, Bolin A, Reinhart G. Differential effects of vitamin D receptor activators on aortic calcification and pulse wave velocity in uraemic rats. Nephrol Dial Transplant 23: 3824–3830, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Okada H, Kaneko Y, Yawata T, Uyama H, Ozono S, Motomiya Y, Hirao Y. Reversibility of adenine-induced renal failure in rats. Clin Exper Nephrol 3: 82–88, 1999 [Google Scholar]
- 25. Price PA, Roublick AM, Williamson MK. Artery calcification in uremic rats is increased by a low protein diet and prevented by treatment with ibandronate. Kidney Int 70: 1577–1583, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Raggi P, Bellasi A, Ferramosca E, Islam T, Muntner P, Block GA. Association of pulse wave velocity with vascular and valvular calcification in hemodialysis patients. Kidney Int 71: 802–807, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Rascher W, Schomig A, Kreye VA, Ritz E. Diminished vascular response to noradrenaline in experimental chronic uremia. Kidney Int 21: 20–27, 1982 [DOI] [PubMed] [Google Scholar]
- 28. Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Tamura K, Suzuki Y, Matsushita M, Fujii H, Miyaura C, Aizawa S, Kogo H. Prevention of aortic calcification by etidronate in the renal failure rat model. Eur J Pharmacol 558: 159–166, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Yokozawa T, Zheng PD, Oura H, Koizumi F. Animal model of adenine-induced chronic renal failure in rats. Nephron 44: 230–234, 1986 [DOI] [PubMed] [Google Scholar]
- 31. Zhao G, Sutliff RL, Weber CS, Wang J, Lorenz J, Paul RJ, Fagin JA. Smooth muscle-targeted overexpression of insulin-like growth factor I results in enhanced vascular contractility. Endocrinology 142: 623–632, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Zimlichman RR, Chaimovitz C, Chaichenco Y, Goligorsky MS, Rapoport J, Kaplanski J. Vascular hypersensitivity to noradrenaline: a possible mechanism of hypertension in rats with chronic uraemia. Clin Sci 67: 161–166, 1984 [DOI] [PubMed] [Google Scholar]