Abstract
Recent epidemiological reports showed that smoking has a negative impact on renal function and elevates the renal risk not only in the renal patient but perhaps also in the healthy population. Studies suggested that nicotine, a major tobacco alkaloid, links smoking to renal dysfunction. While several studies showed that smoking/chronic nicotine exposure exacerbates the progression of chronic renal diseases, its impact on acute kidney injury is virtually unknown. Here, we studied the effects of chronic nicotine exposure on acute renal ischemic injury. We found that chronic nicotine exposure increased the extent of renal injury induced by warm ischemia-reperfusion as evidenced by morphological changes, increase in plasma creatinine level, and kidney injury molecule-1 expression. We also found that chronic nicotine exposure elevated markers of oxidative stress such as nitrotyrosine as well as malondialdehyde. Interestingly, chronic nicotine exposure alone increased oxidative stress and injury in the kidney without morphological alterations. Chronic nicotine treatment not only increased reactive oxygen species (ROS) production and injury but also exacerbated oxidative stress-induced ROS generation through NADPH oxidase and mitochondria in cultured renal proximal tubule cells. The resultant oxidative stress provoked injury through JNK-mediated activation of the activator protein (AP)-1 transcription factor in vitro. This mechanism might exist in vivo as phosphorylation of JNK and its downstream target c-jun, a component of the AP-1 transcription factor, is elevated in the ischemic kidneys exposed to chronic nicotine. Our results imply that smoking may sensitize the kidney to ischemic insults and perhaps facilitates progression of acute kidney injury to chronic kidney injury.
Keywords: smoking, oxidative stress, JNK/AP-1 activation
smoking is a major and preventable contributor to excess morbidity and mortality in the United States. Although the pathological role of smoking in the development of cardiovascular diseases, cancer, or chronic obstructive pulmonary diseases is widely studied, its impact on kidney function has only recently been recognized (38). Epidemiological studies have concluded that smoking accelerates the rate of progression of renal failure to end-stage renal disease in the renal patient (36). It also elicits a negative impact on renal function and may elevate the risk of chronic renal injury even in the healthy population (5, 37). However, while it has been suggested that smoking may have a negative impact on experimental and human radiocontrast-induced nephropathy (17, 28), whether smoking has a detrimental effects on acute kidney injury (AKI) is unknown. This is important not only because of the increased mortality that is associated with AKI but also because AKI itself is an important risk factor for the development and progression of chronic kidney disease (34). Thus smoking may exacerbate AKI-induced tubulointerstitial injury and progression to chronic renal disease.
The mechanisms of smoking-related renal damage are poorly understood but are likely due to both vascular and tubular effects. For instance, smoking-induced oxidative stress leads to endothelial dysfunction (35) and vascular injury (36). Indeed, studies have found increased renal vascular resistance (11, 45), decreased glomerular filtration rate (GFR), and biochemical evidence of smoking or chronic nicotine-induced renal toxicity, even in the absence of histological changes (26). Increased oxidative stress and morphological abnormalities have also been observed in the proximal tubular epithelium after exposure to chronic cigarette smoke or nicotine (NIC) (8–10, 12), and low-grade damage of proximal tubules has also been observed among chronic smokers in the general population (16, 20). These alterations may sensitize the kidney to acute ischemic AKI.
While the harmful effects of smoking may be due to many different components of tobacco smoke, one of the more likely culprits is the alkaloid NIC (19). NIC is excreted by glomerular filtration and tubular secretion (19) and has been found in high concentration in the serum and kidneys of smokers (reviewed in Ref. 19). Chronic exposure to NIC increases oxidative stress in the kidney (22, 43), cultured proximal tubule (25), or mesangial cells (23), thus linking smoking and NIC to renal injury (23). Consequently, smoking or chronic NIC exposure might exacerbate acute renal injury through increasing oxidative stress as observed in experimental radiocontrast-induced AKI (17).
Therefore, our first aim was to determine whether chronic NIC exposure exacerbates ischemia-reperfusion-induced oxidative stress and kidney injury in mice and in cultured mouse proximal tubule cells. We then performed in vitro studies to determine the mechanism by which chronic NIC exposure enhanced oxidative stress and consequent tubular injury.
MATERIALS AND METHODS
Animals, chronic NIC exposure, and renal eschemia-reperfusion.
Nine- to 10-wk-old male C57Bl/6J mice (Jackson Laboratories) were divided into two groups (n = 12/group). The first group of mice received nicotine bitartrate (Sigma-Aldrich, St. Louis, MO) in a 2% saccharine solution at 200 μg/ml concentration as their drinking source for 4 wk as suggested by others (6). The second group received a 2% saccharine solution for 4 wk. Our pilot studies showed that this length of NIC exposure was needed to reproducibly mimic plasma cotinine levels in C57BL/6J mice that are comparable to those found in chronic smokers (19). Eight animals from each group were subjected to 18 min of warm ischemia followed by 24-h reperfusion. Briefly, renal ischemia was imposed under pentobarbital sodium anesthesia as described elsewhere (40). Kidneys were exposed through an abdominal incision and subjected to bilateral ischemia by clamping both renal pedicles with nontraumatic vascular microclamps (Micro Aneurysm clip, straight, 10 mm, 125-g pressure, RS-5426, Roboz Surgical Instruments, Rockville, MD). Mice were kept on a heating pad, and their rectal temperature was monitored to maintain the body temperature at 37°C. After 18 min the clamps were removed, reperfusion of the kidneys was visually confirmed, and the incisions were closed. After the surgery, the animals were monitored for recovery then returned to their cages and allowed free access to food and water with NIC or saccharine, respectively. Four animals from each group (saccharine or NIC) underwent a sham operation; i.e., kidneys were exposed similar to the ischemia-reperfusion group, but renal pedicle clamping was not employed. Twenty-four hours after the ischemia, all animals were euthanized, blood was drawn, and the kidneys were removed for further purposes such as histological and molecular studies. All these procedures were done in accordance with guidelines of the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center.
Cotinine content of serum and the kidney.
Serum and kidney cotinine content was determined by a Cotinine ELISA kit (Calbiotech, Spring Valley, CA) according to the manufacturer's recommendations.
Renal histology.
Formalin-fixed and paraffin-embedded kidneys were assessed for tubulointerstitial injury in 5-μm paraffin sections stained with periodic acid-Schiff (PAS) according to a standard protocol as described elsewhere (29). Accordingly, 60 randomly selected fields of view of the renal cortex/medulla (at magnification of ×200) in 4–4 sections were subjected to a semiquantitative scoring with a light microscope with a scale of 0–4 (grade 0, normal; grade 1, affected area <10%; grade 2, affected area 10–25%; grade 3, affected area 25–75%; and grade 4, affected area >75%).
Plasma creatinine assay.
A Quantichrom Creatinine Assay kit was used (BioAssay Systems, Hayward, CA) as recommended by the manufacturer.
Malondialdehyde assay.
A malondialdehyde (MDA) assay kit was provided by Northwest Life Science Specialties (Vancouver, WA). Equal amounts of kidney lysates (200 μg) were processed according to the manufacturer's recommendation, and the MDA content was determined spectrophotometrically and expressed as nanomoles per milligram protein.
Cell line and establishment of oxidative injury in vitro.
The immortalized mouse proximal tubule line (TKPTS) (15) was used. Oxidative injury was induced by treatment of cells with 200 μM H2O2 (3). Some cells were pretreated with 200 μM NIC (Sigma-Aldrich) for 24 h before treatment with H2O2. The dose of NIC was chosen based on our preliminary studies that showed that this dose exacerbated oxidative stress-related injury in vitro.
Adenoviral infection.
TKPTS cells grown in 24-well plates were infected with 50 multiplicities of infection/ml dominant-negative JNK (dnJNK) (24) or control adenovirus (Ad-Null, Vector BioLabs, Philadelphia, PA) for 24 h as described elsewhere (3). The dnJNK adenovirus was a kind gift of Dr. H. Kaneto (Osaka University).
Assessment of cell viability/injury in vitro.
Cell injury was assessed by a fluorescent CytoTox-One Homogenous Membrane Integrity Assay Kit (Promega, Madison, WI). Briefly, after the appropriate treatment an aliquot of the growth medium was removed and saved. The monolayer was lysed according to the manufacturer's recommendations and lactate dehydrogenase (LDH) content was determined by a fluorescent substrate in both the medium and cell lysate. LDH release was calculated as a percentage of LDH content in the medium compared with the total LDH content (medium+lysate). We also determined the extent of cell viability by a LIVE/DEAD Viability/Cytotoxicity Assay Kit (Invitrogen). The kit contains two fluorescent dyes: calcein AM, which is retained by live cells and emits green fluorescence, and ethidium-1 (EthD-1), which is taken up by damaged cells but excluded by live cells (it emits red fluorescence). Briefly, cells grown in six-well plates were treated as desired, and monolayers were washed with PBS and stained with calcein AM and EthD-1 for 20 min. After repeated washing with PBS, red and green fluorescence was observed under a fluorescence microscope. Calcein exhibits green fluorescence, and it is a marker of intact cells. EthD-1 exhibits red fluorescence that indicates compromised membrane integrity.
Fluorescence microscopy.
For imaging of live cells, a Nikon Eclipse TS100F inverted fluorescence microscope equipped with CY3, FITC, and DAPI filters was used at ×10–40 magnification. Images were captured by a DS Cooled Camera head (DS-Ql1) using NIS Elements for Basic Research 3.0 software (Nikon).
Protein isolation, Western blotting.
Kidneys were removed and homogenized in a RIPA buffer as described earlier (2). Similarly, monolayers of cells were lysed in a RIPA buffer. SDS-PAGE and Western blotting were performed using conventional techniques as described elsewhere (2).
Measurement of reactive oxygen species production in vitro.
Intracellular generation of reactive oxygen species (ROS) was determined by a microplate assay using oxidant-sensitive 2′,7′-dichlorofluorescein-diacetate (DCFDA; Invitrogen, Grand Island, NY). Cells grown in T25 flasks were pretreated as needed and isolated with trypsinization and then loaded with 100 μM DCFDA in HBSS for 30 min at 37°C. After incubation, the dye was washed away with fresh HBSS and placed in wells of a 96-well plate (0.5 × 106 cells/well). H2O2 was added to the wells, and the increase in fluorescence was monitored in a fluorescence plate reader (Fluorocount, Packard) at 485 nmexc/530 nmem. ROS production was calculated as changes in fluorescence/30 min/0.5 × 106 cells and expressed as the percentage of untreated values. To assess the source of generated ROS, chronic NIC-exposed TKPTS cells were pretreated with the xanthine oxidase inhibitor allopurinol (100 μM), the NADPH inhibitor diphenilenediodium (5 μM), or the mitochondrial inhibitor antimycin A (10 μM) for 30 min before treatment with 200 μM H2O2 similar to that described elsewhere (1).
Reporter luciferase assay.
TKPTS cells were transfected with a pAP-1-Luc plasmid (Agilent Technology Wilmington, DE) together with a Renilla luciferase plasmid (Promega) using Xfect reagent (Clontech, Mountain View, CA) according to the manufacturer's recommendations. Twenty-four hours after transfection, cells were treated as needed and 24 h later the firefly and Renilla luciferase activities were determined by a Dual Luciferase Assay Kit (Promega).
Statistical analysis.
Continuous variables were expressed as means and SD. Statistical differences between the treated and control groups were determined by Student's t- or Mann-Whitney rank sum tests. Differences between means were considered significant if P < 0.05. All analyses were performed using the SigmaStat 3.5 software package (Systat, San Jose, CA).
RESULTS
Chronic NIC exposure exacerbates renal ischemia-reperfusion injury in mice and oxidative stress-induced injury in cultured renal proximal tubule cells.
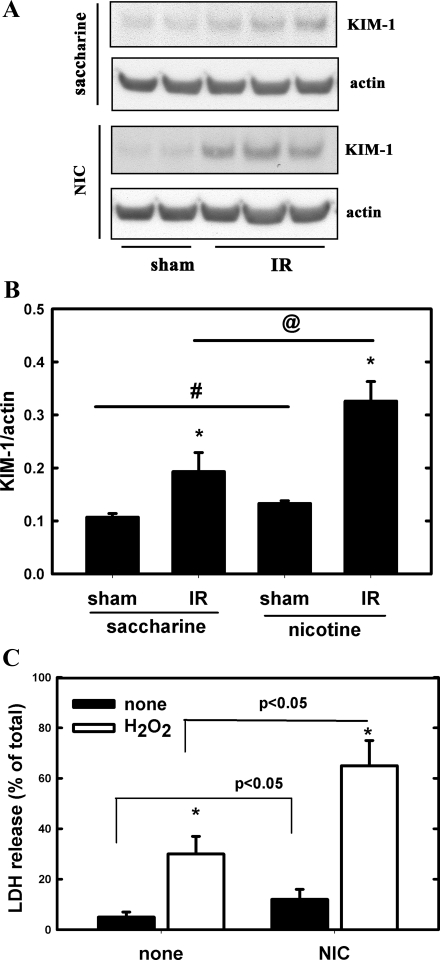
We adopted a model of chronic NIC exposure using C57Bl/6J mice as described by others (6). Plasma cotinine levels in the NIC-exposed mice (Table 1) were comparable to that found in the serum of chronic smokers (150–300 ng/ml) (19). In addition, the kidneys also contained significant amounts of cotinine (Table 1). NIC did not alter basal plasma creatinine levels but caused a moderate increase in expression of kidney injury molecule-1 (KIM-1), a known marker of renal proximal tubular injury (27), in kidney lysates (Fig. 1), suggesting that mild kidney injury may be present.
Table 1.
Effects of chronic nicotine exposure on cotinine levels of the plasma and kidney, plasma creatinine levels, as well as tubulointerstitial injury in ischemic mice
| Saccharine |
Chronic NIC |
|||
|---|---|---|---|---|
| Sham | IR | Sham | IR | |
| Plasma cotinine, ng/ml | ND | ND | 127.4 ± 9.2 | 134.2 ± 14.3 |
| Kidney cotinine, ng/g protein | ND | ND | 16.4 ± 7.3 | 14.8 ± 9.2 |
| Plasma creatinine, mg/dl | 0.35 ± 0.003 | 1.36 ± 0.32* | 0.38 ± 0.01 | 2.03 ± 0.19*† |
| Injury index | 0.025 ± 0.04 | 1.85 ± 0.14* | 0 | 2.59 ± 0.3*‡ |
Values are means ± SD.
NIC, nicotine; IR, ischemia-reperfusion; ND, not done.
P < 0.001 compared with sham.
P < 0.001 compared with saccharine+IR.
P = 0.029 compared with saccharine+IR.
Fig. 1.
Chronic nicotine (NIC) exposure exacerbates injury of the kidney after acute ischemia-reperfusion (IR) and cultured proximal tubule cells after treatment with H2O2. A: expression of kidney injury molecule (KIM)-1 in kidney lysates from C57BL/6J mice that underwent renal IR injury was determined by Western blotting. One group received saccharine, while the other NIC as described in materials and methods. B: densitometry of results from A. Values are means ± SD of ratios of KIM-1/actin; n = 4 (sham) and n = 8 (IR). *P < 0.05 compared with sham. #P < 0.05 compared with saccharine group. @P < 0.05 compared with the saccharine group. C: TKPTS cells were pretreated or not with 200 μM NIC for 24 h before treatment with 200 μM H2O2. Lactate dehydrogenase (LDH) release was determined 24 h after treatment with H2O2. Values represent percentage of released LDH and expressed as means ± SD; n = 3. *P < 0.05 compared with untreated (none).
Subjecting the animals to 18 min of warm ischemia caused significant AKI, as demonstrated by an increase in plasma creatinine (Table 1) and in renal KIM-1 expression (Fig. 1). Chronic NIC exacerbated ischemia-reperfusion-induced AKI; it caused plasma creatinine and renal KIM-1 expression to increase to a greater extent than in the saccharin-treated mice.
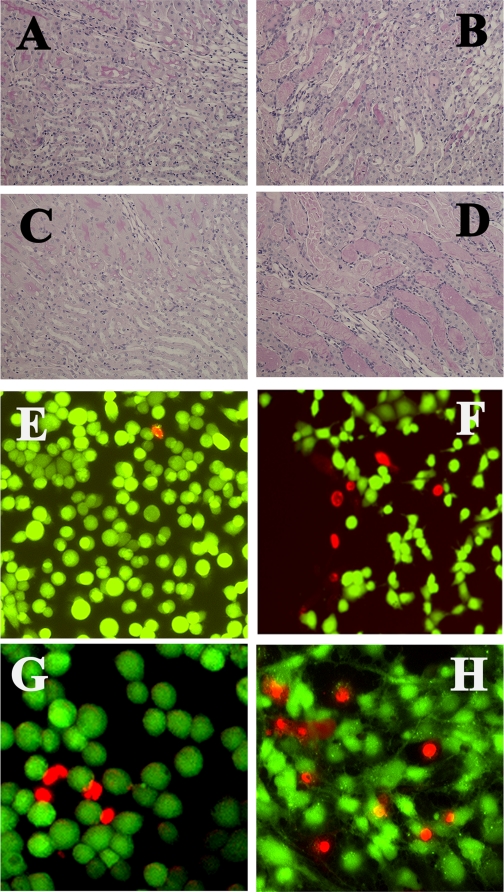
Chronic NIC also exacerbated ischemia-reperfusion-induced renal morphological changes. The extent of tubulointerstitial injury was assessed and scored in 5-μm paraffin sections stained with PAS according to a standard protocol (29). While no injury was observed in the saccharine+sham and NIC+sham animals (Fig. 2, A and B), prominent injury to tubular epithelial cells was observed in the saccharine+ischemia-reperfusion (Fig. 2C and Table 1: injury score 1.85 ± 0.14) and NIC+ischemia-reperfusion (Fig. 2D and Table 1: injury score 2.59 ± 0.3) groups, with the degree of injury being greater in the nicotine+ischemia-reperfusion group (Fig. 2D and Table 1). The injury was most apparent in the outer medullary and juxtamedullary region of the renal cortex.
Fig. 2.
Effects of chronic NIC exposure on kidney morphology and integrity of cultured proximal tubule. Sections of kidneys form saccharine+sham (A), saccharine+IR (B), NIC+sham (C), and NIC+IR (D) were stained with PAS and evaluated for injury as described in materials and methods. TKPTS cells were treated or not with 200 μM NIC for 24 h followed by 200 μM H2O2 for 3 h and stained with calcein/ethidium as described in materials and methods. Pictures are representatives of 3 independent experiments. E: control, untreated. F: H2O2-treated; G: NIC alone; H: NIC+H2O2.
Next, we studied the effects of chronic NIC exposure (24 h) on an established in vitro model of oxidative injury in mouse renal proximal tubule cells (TKPTS) (2, 3). Accordingly, TKPTS cells were pretreated with 200 μM NIC for 24 h before treatment with 200 μM H2O2. Twenty-four hours after treatment with H2O2, the extent of LDH release and calcein/EthD-1 uptake was determined. As seen in Fig. 1C, oxidative stress (H2O2 treatment) significantly increased LDH release, which was further increased by chronic pretreatment with NIC. We also assessed the extent of injury by calcein/EthD-1 staining. While calcein uptake (green fluorescence) is a sign of live/intact cells, EthD-1 uptake (red fluorescence) is a sign of impaired cell membrane integrity. Values were expressed as percentage of cells with EthD-1 uptake compared with the total number of cells. Figure 2, E and F, shows that EthD-1 uptake was significantly increased upon exposure to H2O2 (from <1% to 7.5 ± 1.2%), which was further aggravated by pretreatment with NIC (25.3 ± 3.5% Fig. 2H). Interestingly, chronic NIC exposure alone moderately but significantly increased both LDH release (Fig. 1C) and EthD-1 uptake (Fig. 2G: 9.2 ± 1.5%). These data suggest that chronic NIC exposure alone is mildly toxic to proximal tubule cells in vitro. These in vitro findings corroborate with KIM-1 results in vivo (Fig. 1, A and B), suggesting a mild injury in renal cells upon chronic NIC exposure both in vivo and in vitro.
Chronic NIC exposure increases ischemic injury by exacerbating oxidative stress in the kidney as well as in cultured renal proximal tubule cells.
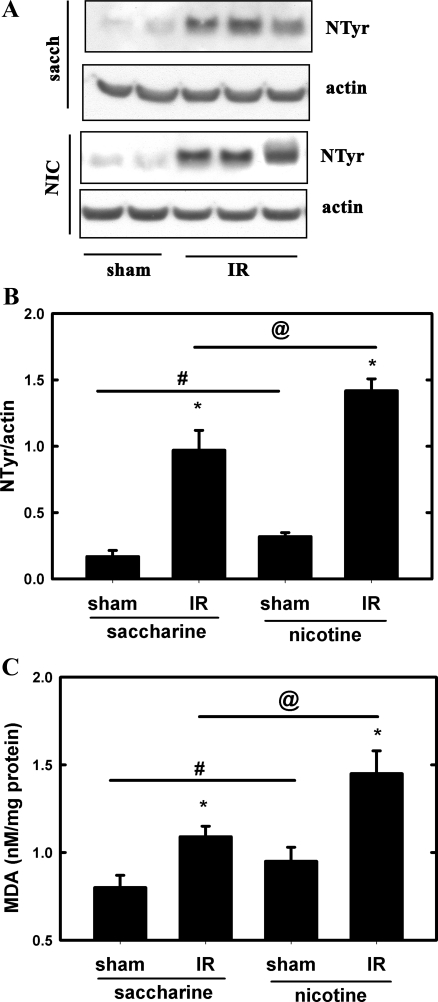
Renal nitrotyrosine expression, a marker of oxidative stress (33), was determined by Western blotting. Nitrotyrosine expression was elevated in the ischemic kidneys from saccharine-treated mice, which further increased in the ischemic kidneys from NIC-exposed mice (Fig. 3, A and B). Chronic NIC exposure alone also elevated expression of nitrotyrosine. In addition, MDA content (7) was also determined. Figure 3C shows that renal MDA content was significantly higher in ischemic kidneys from NIC-exposed mice compared with their saccharine-exposed counterparts. Interestingly, MDA content of kidneys from NIC-exposed mice was also higher than in their saccharine-treated counterparts. These results suggest the presence of a mild oxidative stress in the kidneys of NIC-treated mice and significant exacerbation of oxidative stress in the ischemic kidneys.
Fig. 3.
Chronic NIC exposure increases oxidative stress in the kidney. A: expression of nitrotyrosine (NTyr) in kidney lysates from C57BL/6J mice that underwent renal IR injury was determined by Western blotting. One group received saccharine while the other NIC as described in materials and methods. B: densitometry of results from A. Values are means ± SD of NTyr/actin ratios; n = 4 (sham) and n = 8 (IR). *P < 0.05 compared with sham. #P < 0.05 compared with saccharine group. @P < 0.05 compared with the saccharine group. C: renal malondialdehyde (MDA) content of kidney lysates from C57BL/6J mice that underwent renal IR injury was determined by spectrophotometry as described in materials and methods. One group received saccharine while the other NIC as described in materials and methods. Values represent MDA content in nmol/mg protein and expressed as means ± SD; n = 4 (sham) and n = 8 (IR). *P < 0.05 compared with sham. #P < 0.05 compared with saccharine group. @P < 0.05 compared with saccharine group.
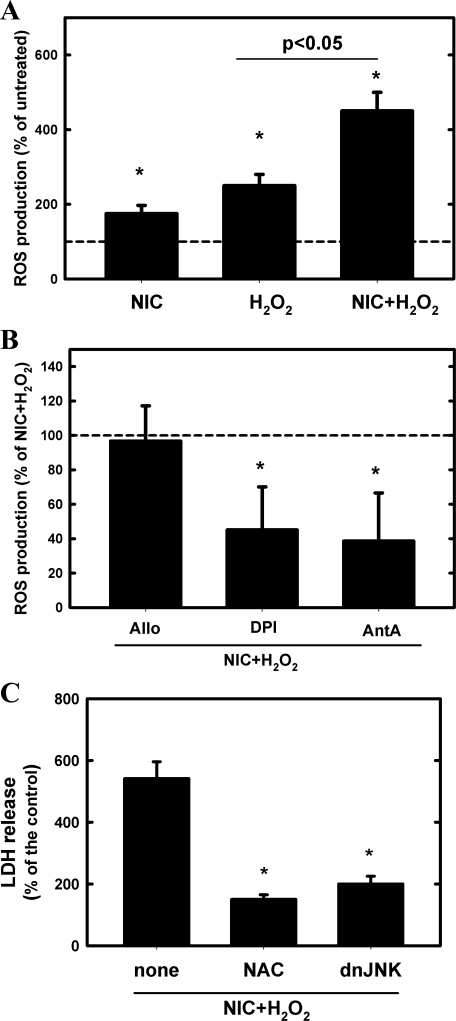
Next, TKPTS cells were pretreated or not with 200 μM NIC for 24 h and 200 μM H2O2-induced ROS production was determined. As Fig. 4A shows, chronic NIC pretreatment significantly increased H2O2-induced ROS production in an additive manner. In addition, chronic NIC exposure itself also increased ROS production (Fig. 4A), which underscores the in vivo findings (Fig. 3).
Fig. 4.
Chronic NIC exposure exacerbates H2O2-induced reactive oxygen species (ROS) production and resultant injury of cultured proximal tubule cells. A: TKPTS cells were pretreated or not with 200 μM NIC for 24 h, and then 200 μM H2O2-induced ROS production was determined. Values represent ROS production as percentage of the corresponding untreated values and are expressed as means ± SD; n = 3. *P < 0.05 compared with untreated. B: NIC-exposed TKPTS cells were pretreated with the xanthine oxidase inhibitor allopurinol (Allo; 100 μM), the NADPH inhibitor diphenilenediodium (DPI; 5 μM), or the mitochondrial inhibitor antimycin A (AntA; 10 μM) for 30 min before treatment with 200 μM H2O2. ROS production was expressed as percentage of NIC+H2O2-treated values; n = 3; means ± SD. *P < 0.05 compared with NIC+H2O2-treated value. C: TKPTS cells were pretreated with 100 μM N-acetylcysteine (NAC) 1 h before treatment with 200 μM NIC for 24 h followed by 200 μM H2O2 for 24 h; then, LDH release was determined. Similarly, some cells were infected with a dominant-negative JNK (dnJNK) adenovirus for 24 h before treatment with 200 μM NIC for 24 h followed by 200 μM H2O2. In these cells, LDH release was also determined. Values represent the pcrcentage of the control values and are expressed as means ± SD; n = 3. *P < 0.05 compared with untreated.
As the main source of ROS in renal cells is xanthine oxidase, NADPH oxidase, or the mitochondria, we employed various inhibitors, similar to our study published earlier (1), to assess the contribution of those pathways to NIC+H2O2-induced ROS generation in vitro. Figure 4B shows that while the xanthine oxidase inhibitor allopurinol does not affect NIC+H2O2-induced ROS production, then both the NADPH oxidase inhibitor diphenyliodium and mitochondrial inhibitor antimycin A significantly inhibited it. Thus we concluded that both NADPH oxidase and the mitochondria are important players in ROS generation.
To determine whether NIC-induced oxidative stress mediates injury, cells were pretreated with 100 μM N-acetylcysteine (NAC) before treatment with NIC+H2O2 and LDH release was determined. In these experiments, the antioxidant NAC abrogated NIC+H2O2-induced LDH release (Fig. 4C).
Chronic NIC exposure aggravates acute renal ischemia- or oxidative stress-induced stress kinase signaling in the kidney or in cultured renal proximal tubule cells.
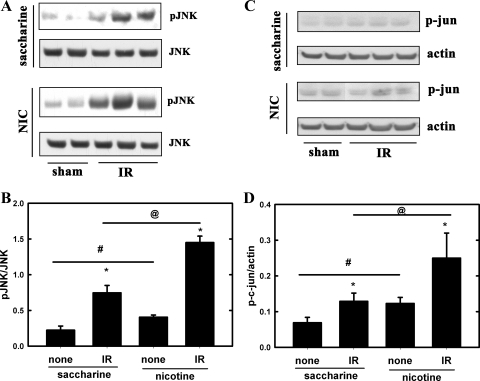
Oxidative stress activates (phosphorylates) various MAPKs, including JNK both in the kidney (13, 21) and in cultured renal proximal tubule cells (3, 21), which, through the transcription factor AP-1 (14, 30), is involved in injury (3, 13, 21). Accordingly, we analyzed kidney lysates from ischemic or sham-operated mice for phosphorylation of JNK by Western blotting. Representative blots in Fig. 5A show increased phosphorylation of JNK in the ischemic kidneys, which was exacerbated upon chronic exposure to NIC. Interestingly, chronic NIC exposure alone also elevated JNK phosphorylation in the kidney (Fig. 5, A and B), suggesting a higher level of stress in the kidneys of NIC-treated animals. These results suggest that chronic NIC exposure provokes stronger stress, and consequent prodeath, responses to ischemia in the kidneys. In addition, phosphorylation of c-jun, a component of the AP-1 transcription factor (44), is also aggravated in the ischemic kidneys from NIC-treated animals (Fig. 5, C and D).
Fig. 5.
Chronic NIC exposure exacerbates acute renal ischemia-induced phosphorylation of JNK and c-jun in the mouse kidney. A: levels of phospho-JNK and JNK in kidney lysates from C57BL/6J mice that underwent renal IR injury were determined by Western blotting. One group received saccharine while the other NIC as described in materials and methods. B: densitometry of results from A. Values are expressed as means ± SD of ratios of pJNK/JNK; n = 4 (sham) and n = 8 (IR). *P < 0.05 compared with sham. #P < 0.05 compared with saccharine group. @P < 0.05 compared with the saccharine group. C: levels of phospho-c-jun and actin were also determined as described in A. D: densitometry of results from C. Values are expressed as means ± SD of p-c-jun/actin ratios; n = 4 (sham) and n = 8 (IR). *P < 0.05 compared with sham. #P < 0.05 compared with saccharine group. @P < 0.05 compared with the saccharine group.
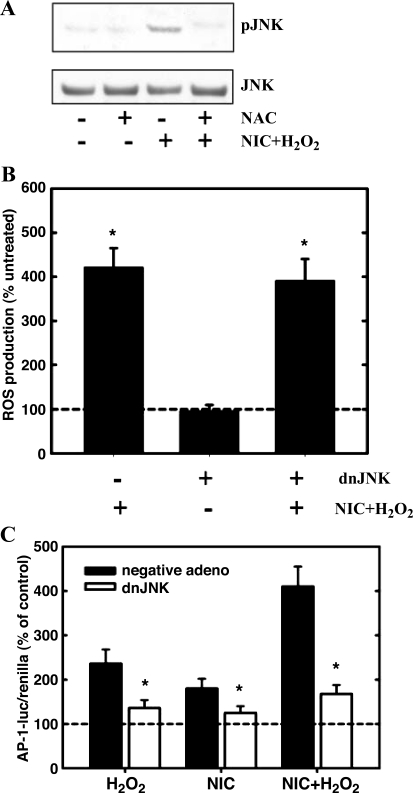
We also found that the ROS scavenger NAC attenuated NIC+H2O2-induced phopshorylation of JNK (Fig. 6A) but dnJNK inhibition of JNK function did not significantly inhibit NIC+H2O2-induced ROS production (Fig. 6B). These results suggest that phosphorylation of JNK is the consequence but not the cause of NIC+H2O2-induced ROS production.
Fig. 6.
Oxidative stress mediates phosphorylation of JNK leading to activation of activator protein-1 (AP-1) in cultured proximal tubule cells. A: TKPTS cells were pretreated with 100 μM NAC before treatment with 200 μM NIC for 24 h followed by treatment with 200 μM H2O2 for 30 min. Phosphorylation of JNK was determined by Western blotting. The blot shown is representative of 3 independent experiments. B: TKPTS cells were infected with a dnJNK adenovirus for 24 h before exposure to 200 μM NIC for 24 h. In these cells, 200 μM H2O2-induced ROS production was determined. Values represent changes in DCFDA fluorescence/30 min/0.5 × 106 cells as described in materials and methods and expressed as means ± SD (n = 3). *P < 0.05 compared with untreated. C: TKPTS cells were transiently transfected with an AP-1 luciferase plasmid together with a Renilla luciferase. After 24 h, some cells were infected with the dnJNK adenovirus and some with a negative (empty) adenovirus. These cells were treated with 200 mM H2O2 or 200 μM NIC for 24 h. In another set of experiments, cells were pretreated with 200 μM NIC for 24 h followed by treatment with 200 μM H2O2 for 24 h, and firefly (AP-1) as well as Renilla luciferase activities were determined. Values represent relative luciferase activities (firefly/Renilla) expressed as percentage of their own controls in means ± SD; n = 3. *P < 0.05 compared with the negative virus-infected counterparts.
Activated JNK, possibly through AP-1, mediates injury of renal proximal tubule cells.
Further experiments were designed to demonstrate the role of JNK and its downstream target, AP-1, in NIC+oxidative stress-induced injury in vitro. Accordingly, TKPTS cells were infected with a dnJNK adenovirus as described earlier (3) and treated with 200 μM NIC for 24 h followed by treatment with 200 μM H2O2. LDH release was determined 24 h later. As is shown in Fig. 4C, dnJNK significantly inhibited NIC+H2O2-induced injury. dnJNK also inhibited H2O2- as well as NIC-induced LDH release (data not shown).
In addition, NIC or H2O2 alone significantly increased activity of the AP-1-luciferase reporter, which was further increased by their combination (NIC+H2O2). Importantly, all those AP-1 activities were significantly attenuated by dnJNK (Fig. 6C). These results imply that the deleterious effects of activated JNK are mediated through the AP-1 transcription factor in chronic NIC+H2O2 treated cells.
DISCUSSION
NIC is a major component of cigarette smoke (19): it has been found in high concentration in the blood (19) and kidneys of chronic smokers (19, 48). It is excreted by glomerular filtration and tubular secretion (19). Thus the renal tubules are exposed to high levels of NIC and/or its major metabolite, cotinine, which may cause direct tubular toxicity. In fact, the presence of NIC or cotinine highly correlates with smoking-induced toxicity (26), suggesting that NIC is likely an important component of smoking-induced renal injury (23). Consequently, the main goal of the present study was to assess the effect of nicotine exposure, as that seen in smokers, on AKI.
In the present study, we used a model of chronic NIC exposure. We selected this model because it has the advantage that 1) the animals get a “bolus” of NIC every time they drink water (like smokers do when they smoke), and 2) their cotinine concentrations stabilize at levels that are similar to those found in chronic smokers. We and others (18) have found that an exposure period of at least 2–3 wk is necessary to consistently reach these levels. Our model went on for 4 wk, a time frame in which blood and tissue levels of cotinine levels are elevated. As before, we found that the cotinine levels in blood and kidney were significantly elevated (Table 1.); the plasma levels were comparable to that found in chronic smokers (19). These increases in cotinine levels were associated with a moderate but significant increase in renal KIM-1 expression in the kidneys exposed to NIC (Fig. 1, A and B), whereas plasma creatinine levels and kidney morphology were unchanged (Table 1). Thus chronic NIC administration appeared to cause early tubular injury without altering GFR or the morphological appearance of the kidneys. This is consistent with studies in humans that have reported that smokers exhibit mild injury of the proximal tubules (16, 20). Further studies are needed to demonstrate whether the integrity of the actin cytoskeleton or activity of endoplasmic reticulum chaperones are involved in this stage of injury (4) as those events may predispose the kidney to further ischemic injury.
The more salient finding of our study was that chronic exposure to NIC exacerbated the severity of acute renal ischemia-reperfusion injury; plasma creatinine levels and renal expression of KIM-1 increased more than in untreated controls (Table 1). Moreover, renal ischemia-reperfusion-induced morphological alterations were worse in the mice that received NIC (Figs. 1A and 2, Table 1). It is interesting to note that previous studies have reported the opposite; NIC ameliorated ischemia-reperfusion-induced AKI (41, 50, 51). However, those studies are not directly comparable to ours because they only exposed their mice to NIC acutely (immediately before subjecting them to AKI). The shorter exposure to NIC (to NIC naive mice/rats) results in significantly lower cotinine levels in the plasma, which may result in different effects and signals. In our study, we not only found that chronic NIC exacerbated AKI in vivo, but we found the same in vitro. Accordingly, chronic NIC treatment of mouse renal proximal tubule cells increased H2O2-induced LDH release (Fig. 1C) as well as EthD staining (Fig. 2, E–H).
Increased production of ROS and the consequent increase in the incidence and severity of several diseases is evident (46). Chronic NIC exposure increases ROS production in mesangial (23) and proximal tubule (25) cells in vitro. In animal models, chronic exposure to cigarette smoke or NIC significantly increased oxidative stress in the kidney and pretreatment with antioxidants prevented renal injury (9, 22, 32, 43). These results clearly connect smoking/chronic NIC to oxidative injury. In our animal model, chronic exposure to NIC increased both basal and acute ischemia-induced oxidative stress in the kidney, as evidenced by increased renal expression of nitrotyrosine (Fig. 3, A and B) and renal MDA content (Fig. 3C). Our in vitro studies also confirmed these observations: H2O2-induced ROS production is further increased upon chronic pretreatment with NIC and NIC itself increased ROS production (Fig. 4A). The source of this NIC-mediated ROS could be the NAPDPH oxidase system (18) or the mitochondria, which we confirmed by utilizing pathway-specific inhibitors (Fig. 4B). Our in vitro studies established a clear connection between NIC-induced enhancement of ROS production and oxidative injury: pretreatment with the ROS scavenger NAC attenuated NIC+H2O2-induced LDH release (Fig. 4B).
Ischemia-reperfusion injury activates the stress kinase JNK through ROS production (14, 30), which is involved in injury of renal proximal tubules in the ischemic kidney (13, 14, 39). The activated JNK increases activity of the transcription factor AP-1 during oxidative stress or renal ischemia-reperfusion injury both in vitro and in vivo (31, 42) and as such supports injury (14, 30). Several studies demonstrated that chronic NIC treatment activates JNK signaling (47, 49) in various cell types, but its effect on the kidney and on renal ischemia is virtually unknown.
Our study shows that chronic NIC exposure exacerbates acute renal ischemia-induced phosphorylation of JNK but also elevates its baseline phosphorylation (Fig. 5, A and B). The elevated stress kinase activation in the chronic NIC-exposed kidneys is probably due to the increased oxidative stress (Fig. 3), and it may also explain the persistent mild injury (Fig. 1A). Our in vitro studies also evidenced that the activated JNK plays a crucial role in injury (Fig. 1C) through activation of AP-1 (Fig. 6C). We also determined that chronic NIC exposure-associated ROS is essential for phosphorylation of JNK and not vice versa (Fig. 6, A and B). A similar scenario might exist in vivo: chronic NIC exposure, in addition to JNK (Fig. 5, A and B), also aggravates phosphorylation of c-jun (Fig. 5, C and D), a component of the AP-1 transcription factor (44).
Our in vitro data on tubular cells demonstrated that NIC has a toxic effect on renal epithelia. However, smoking and NIC also have vascular effects. Thus it is possible that chronic NIC exposure was affecting AKI via affecting blood pressure and/or renal hemodynamics. While we did not study this possibility in the current study, we have separate experiments in which we look at the chronic effects of NIC on blood pressure and intrarenal hemodynamics in Sprague-Dawley rats. We have found that chronic NIC exposure does not alter either blood pressure or renal hemodynamics (unpublished observations). Similar observations were reported by Hua et al. (18), who found no changes in systolic blood pressure after chronic NIC administration in control or diabetic mice. Thus we think that it is unlikely that deleterious effects that are mediated by chronic NIC are secondary to changes in basal blood pressure or intrarenal hemodynamics. Thus these results, taken together with our in vitro data, suggest that NIC may exacerbate renal injury, at least in part, via direct tubular effects.
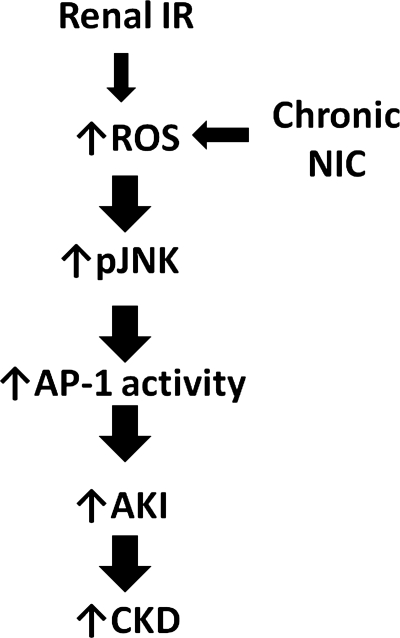
Our results are summarized in Fig. 7: chronic NIC exposure exacerbates acute renal ischemia-reperfusion-induced production of ROS, which in turn aggravates phosphorylation of JNK. The resultant increase in JNK activation leads to increased AP-1 activity and the consequent increase in AKI. Ultimately, this increase in AKI could lead to increased progression to chronic kidney disease in smokers.
Fig. 7.
Summary of results. Chronic NIC exposure exacerbates acute renal IR-induced production of ROS, which in turn aggravates phosphorylation of JNK. The resultant increase in JNK activation leads to increased AP-1 activity and consequent increase in AKI. Ultimately, this increase in AKI could lead to increased progression to chronic kidney disease (CKD) in smokers.
GRANTS
These studies were supported by an American Heart Association Midwest Affiliate Grant-in-Aid (10GRNT3790019, I. Arany), an Intramural Research Support Program Award from the University of Mississippi Medical Center (I. Arany), as well as National Institute of Diabetes and Digestive and Kidney Diseases Grant DK073401 (L. A. Juncos).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Arany I, Faisal A, Clark JS, Vera T, Baliga R, Nagamine Y. p66SHC-mediated mitochondrial dysfunction in renal proximal tubule cells during oxidative injury. Am J Physiol Renal Physiol 298: F1214–F1221, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Arany I, Faisal A, Nagamine Y, Safirstein RL. p66shc inhibits pro-survival epidermal growth factor receptor/ERK signaling during severe oxidative stress in mouse renal proximal tubule cells. J Biol Chem 283: 6110–6117, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Arany I, Megyesi JK, Kaneto H, Tanaka S, Safirstein RL. Activation of ERK or inhibition of JNK ameliorates H2O2 cytotoxicity in mouse renal proximal tubule cells. Kidney Int 65: 1231–1239, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Briganti EM, Branley P, Chadban SJ, Shaw JE, McNeil JJ, Welborn TA, Atkins RC. Smoking is associated with renal impairment and proteinuria in the normal population: the AusDiab kidney study. Australian Diabetes, Obesity and Lifestyle Study. Am J Kidney Dis 40: 704–712, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Brunzell DH, Russell DS, Picciotto MR. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J Neurochem 84: 1431–1441, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Chatterjee PK, Cuzzocrea S, Brown PA, Zacharowski K, Stewart KN, Mota-Filipe H, Thiemermann C. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int 58: 658–673, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Cigremis Y, Turkoz Y, Akgoz M, Sozmen M. The effects of chronic exposure to ethanol and cigarette smoke on the level of reduced glutathione and malondialdehyde in rat kidney. Urol Res 32: 213–218, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Cigremis Y, Turkoz Y, Tuzcu M, Ozen H, Kart A, Gaffaroglu M, Erdogan K, Akgoz M, Ozugurlu F. The effects of chronic exposure to ethanol and cigarette smoke on the formation of peroxynitrite, level of nitric oxide, xanthine oxidase and myeloperoxidase activities in rat kidney. Mol Cell Biochem 291: 127–138, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Cobanoglu B, Ozercan IH, Ozercan MR, Yalcin O. The effect of inhaling thinner and/or cigarette smoke on rat kidneys. Inhal Toxicol 19: 303–308, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Cooper RG. Renal function in male Sprague-Dawley rats concurrently exposed to long-term nicotine (3-{1-methyl-2-pyrrolidinyl}pyridine) and methylated spirits (methyl alcohol). Ren Fail 30: 107–114, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Czekaj P, Palasz A, Lebda-Wyborny T, Nowaczyk-Dura G, Karczewska W, Florek E, Kaminski M. Morphological changes in lungs, placenta, liver and kidneys of pregnant rats exposed to cigarette smoke. Int Arch Occup Environ Health 75 Suppl: S27–S35, 2002 [DOI] [PubMed] [Google Scholar]
- 13. di Mari JF, Davis R, Safirstein RL. MAPK activation determines renal epithelial cell survival during oxidative injury. Am J Physiol Renal Physiol 277: F195–F203, 1999 [DOI] [PubMed] [Google Scholar]
- 14. DiMari J, Megyesi J, Udvarhelyi N, Price P, Davis R, Safirstein R. N-acetyl cysteine ameliorates ischemic renal failure. Am J Physiol Renal Physiol 272: F292–F298, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Ernest S, Bello-Reuss E. Expression and function of P-glycoprotein in a mouse kidney cell line. Am J Physiol Cell Physiol 269: C323–C333, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Gambaro G, Verlato F, Budakovic A, Casara D, Saladini G, Del Prete D, Bertaglia G, Masiero M, Checchetto S, Baggio B. Renal impairment in chronic cigarette smokers. J Am Soc Nephrol 9: 562–567, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Heyman SN, Goldfarb M, Rosenberger C, Shina A, Rosen S. Effect of nicotine on the renal microcirculation in anesthetized rats: a potential for medullary hypoxic injury? Am J Nephrol 25: 226–232, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Hua P, Feng W, Ji S, Raij L, Jaimes EA. Nicotine worsens the severity of nephropathy in diabetic mice: implications for the progression of kidney disease in smokers. Am J Physiol Renal Physiol 299: F732–F739, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev 57: 79–115, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Hultberg B, Isaksson A, Brattstrom L, Israelsson B. Elevated urinary excretion of beta-hexosaminidase in smokers. Eur J Clin Chem Clin Biochem 30: 131–133, 1992 [PubMed] [Google Scholar]
- 21. Hung CC, Ichimura T, Stevens JL, Bonventre JV. Protection of renal epithelial cells against oxidative injury by endoplasmic reticulum stress preconditioning is mediated by ERK1/2 activation. J Biol Chem 278: 29317–29326, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Husain K, Scott BR, Reddy SK, Somani SM. Chronic ethanol and nicotine interaction on rat tissue antioxidant defense system. Alcohol 25: 89–97, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Jaimes EA, Tian RX, Raij L. Nicotine: the link between cigarette smoking and the progression of renal injury? Am J Physiol Heart Circ Physiol 292: H76–H82, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Kaneto H, Xu G, Fujii N, Kim S, Bonner-Weir S, Weir GC. Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J Biol Chem 277: 30010–30018, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Khanna AK, Xu J, Baquet C, Mehra MR. Adverse effects of nicotine and immunosuppression on proximal tubular epithelial cell viability, tissue repair and oxidative stress gene expression. J Heart Lung Transplant 28: 612–620, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Kim KY, Lee YJ, Chung BC, Hong J, Jung BH. Relations between toxicity and altered tissue distribution and urinary excretion of nicotine, cotinine, and hydroxycotinine after chronic oral administration of nicotine in rats. Drug Chem Toxicol 33: 166–172, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Kuehn EW, Park KM, Somlo S, Bonventre JV. Kidney injury molecule-1 expression in murine polycystic kidney disease. Am J Physiol Renal Physiol 283: F1326–F1336, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Lindholt JS. Radiocontrast induced nephropathy. Eur J Vasc Endovasc Surg 25: 296–304, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Mankhey RW, Wells CC, Bhatti F, Maric C. 17β-Estradiol supplementation reduces tubulointerstitial fibrosis by increasing MMP activity in the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 292: R769–R777, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Mehta A, Sekhon CP, Giri S, Orak JK, Singh AK. Attenuation of ischemia/reperfusion induced MAP kinases by N-acetyl cysteine, sodium nitroprusside and phosphoramidon. Mol Cell Biochem 240: 19–29, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Morooka H, Bonventre JV, Pombo CM, Kyriakis JM, Force T. Ischemia and reperfusion enhance ATF-2 and c-Jun binding to cAMP response elements and to an AP-1 binding site from the c-Jun promoter. J Biol Chem 270: 30084–30092, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Muthukumaran S, Sudheer AR, Menon VP, Nalini N. Protective effect of quercetin on nicotine-induced prooxidant and antioxidant imbalance and DNA damage in Wistar rats. Toxicology 243: 207–215, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, Fujita T, Brodsky S, Goligorsky MS. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol 281: F948–F957, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Okusa MD, Chertow GM, Portilla D. The nexus of acute kidney injury, chronic kidney disease, and World Kidney Day 2009. Clin J Am Soc Nephrol 4: 520–522, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Orth SR. Effects of smoking on systemic and intrarenal hemodynamics: influence on renal function. J Am Soc Nephrol 15, Suppl 1: S58–S63, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Orth SR. Smoking—a renal risk factor. Nephron 86: 12–26, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Orth SR, Hallan SI. Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients–absence of evidence or evidence of absence? Clin J Am Soc Nephrol 3: 226–236, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Orth SR, Ritz E, Schrier RW. The renal risks of smoking. Kidney Int 51: 1669–1677, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Park KM, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury and JNK, p38, and MAPK kinase activation by remote ischemic pretreatment. J Biol Chem 276: 11870–11876, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Pittock ST, Norby SM, Grande JP, Croatt AJ, Bren GD, Badley AD, Caplice NM, Griffin MD, Nath KA. MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: pathophysiologic correlates. Kidney Int 68: 611–622, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Sadis C, Teske G, Stokman G, Kubjak C, Claessen N, Moore F, Loi P, Diallo B, Barvais L, Goldman M, Florquin S, Le Moine A. Nicotine protects kidney from renal ischemia/reperfusion injury through the cholinergic anti-inflammatory pathway. PLoS ONE 2: e469, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sakai M, Tsukada T, Harris RC. Oxidant stress activates AP-1 and heparin-binding epidermal growth factor-like growth factor transcription in renal epithelial cells. Exp Nephrol 9: 28–39, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Sener G, Sehirli O, Ipci Y, Cetinel S, Cikler E, Gedik N, Alican I. Protective effects of taurine against nicotine-induced oxidative damage of rat urinary bladder and kidney. Pharmacology 74: 37–44, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol 4: E131–E136, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Tamaoki L, Oshiro-Monreal FM, Helou CM. Effects of nicotine exposure on renal function of normal and hypercholesterolemic rats. Am J Nephrol 30: 377–382, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Traber MG, van der Vliet A, Reznick AZ, Cross CE. Tobacco-related diseases. Is there a role for antioxidant micronutrient supplementation? Clin Chest Med 21: 173–187, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Tsai JR, Chong IW, Chen CC, Lin SR, Sheu CC, Hwang JJ. Mitogen-activated protein kinase pathway was significantly activated in human bronchial epithelial cells by nicotine. DNA Cell Biol 25: 312–322, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Urakawa N, Nagata T, Kudo K, Kimura K, Imamura T. Simultaneous determination of nicotine and cotinine in various human tissues using capillary gas chromatography/mass spectrometry. Int J Legal Med 106: 232–236, 1994 [DOI] [PubMed] [Google Scholar]
- 49. Xu Y, Zhang Y, Cardell LO. Nicotine enhances murine airway contractile responses to kinin receptor agonists via activation of JNK- and PDE4-related intracellular pathways. Respir Res 11: 13, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yeboah MM, Xue X, Duan B, Ochani M, Tracey KJ, Susin M, Metz CN. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int 74: 62–69, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yeboah MM, Xue X, Javdan M, Susin M, Metz CN. Nicotinic acetylcholine receptor expression and regulation in the rat kidney after ischemia-reperfusion injury. Am J Physiol Renal Physiol 295: F654–F661, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]