Abstract
Mitochondrial matrix cyclophilin D (CyPD) is known to promote development of the mitochondrial permeability transition (MPT). Kidney proximal tubule cells are especially prone to deleterious effects of mitochondrial damage because of their dependence on oxidative mitochondrial metabolism for ATP production. To clarify the role of CyPD and the MPT in proximal tubule injury during ischemia-reperfusion (I/R) and hypoxia-reoxygenation (H/R), we assessed freshly isolated tubules and in vivo injury in wild-type (WT) and Ppif−/− CyPD-null mice. Isolated mouse tubules developed a sustained, nonesterified fatty acid-mediated energetic deficit after H/R in vitro that could be substantially reversed by delipidated albumin and supplemental citric acid cycle substrates but was not modified by the absence of CyPD. Susceptibility of WT and Ppif−/− tubules to the MPT was increased by H/R but was less in normoxic and H/R Ppif−/− than WT tubules. Correction of the energetic deficit that developed during H/R strongly increased resistance to the MPT. Ppif−/− mice were resistant to I/R injury in vivo spanning a wide range of severity. The data clarify involvement of the MPT in oxygen deprivation-induced tubule cell injury by showing that the MPT does not contribute to the initial bioenergetic deficit produced by H/R but the deficit predisposes to subsequent development of the MPT, which contributes pathogenically to kidney I/R injury in vivo.
Keywords: acute kidney injury, membrane potential, mitochondria
the mitochondrial permeability transition (MPT) results from the regulated and reversible opening of an ∼3-nm-diameter pore in the inner mitochondrial membrane with a size exclusion limit of ∼1.5 kDa that is frequently termed the permeability transition pore (PTP) (33, 35, 42, 56). Sustained opening of the PTP dissipates the transmembrane ion gradients necessary for energy conservation and leads to loss of inorganic and small organic matrix solutes and matrix swelling. Pore opening can be prevented and reversed by cyclosporin A (CsA) (7, 13) via binding by CsA of a mitochondrial matrix cyclophilin D (CyPD) (2, 3, 36, 48, 57, 69). Although the specific proteins forming the PTP channel have not been conclusively identified (5, 35, 37), multiple inducers and antagonists are known (33, 35, 56, 72). The endogenous metabolites ADP and Mg2+ and matrix acidification potently antagonize pore opening. Inducers include multiple conditions commonly present during injury states, i.e., decrease of membrane potential (ΔΨm) and increase of Ca2+, phosphate, reactive oxygen species (ROS), and a number of lipid metabolites, including nonesterified fatty acids (NEFA) and their CoA and carnitine esters and ceramides (33, 56, 72). Recent studies have shown that phosphate, in addition to promoting the effect of Ca2+ to induce the MPT, is required for MPT inhibition by CyPD (4) and have implicated the phosphate carrier as a PTP component (43).
Once the glycine-sensitive plasma membrane pores that mediate necrotic cell death (16, 50) form, the MPT is promoted by influx of extracellular Ca2+ and loss of cytosolic metabolites such as ADP and Mg2+, so the process predictably occurs after necrosis is underway (62). The concept that the MPT can also have a prelethal pathogenic role in determining cell fate has been supported by several lines of evidence: the efficacy of CsA to prevent cell killing in a variety of cell models in vitro (32, 35), the demonstration that the MPT occurs in intact cells in which the presence of glycine has prevented plasma membrane pore opening (55), and the findings that mice with targeted deletion of CyPD are resistant to heart and brain ischemia (2, 48, 57), several types of nonischemic neuronal and muscular pathology (17, 27, 45, 52), and pancreatic β-cell death (29) in vivo. Beneficial effects of CsA for myocardial reperfusion injury in humans have been reported (53). Recent work has implicated CyPD and the MPT in the pathogenesis of ischemia-reperfusion (I/R) injury to the kidney (14, 54).
To better define its behavior in proximal tubule cells during acute kidney injury, we previously characterized the MPT and the effect of hypoxia-reoxygenation (H/R) on it in freshly isolated rabbit proximal tubules, which are especially sensitive to mitochondrial damage and dysfunction because of their low capacity for glycolysis (24). We found that NEFA that are endogenously generated by hypoxia prior to the MPT and produced during the MPT and dependence on complex I-requiring substrates would increase the likelihood of development of the MPT prelethally, despite ample levels of the pore antagonists ADP and Mg2+ in intact cells. In these rabbit tubule studies, CsA did not clearly increase resistance to the MPT after H/R. The rabbit system, however, does not allow use of genetic manipulations to further probe the role of the MPT and CyPD in proximal tubules in vitro and in vivo, as can be done with mutant mice. To that end, the present studies were designed 1) to assess regulation of the MPT in mouse proximal tubules and its modification by CyPD deletion and H/R, 2) to characterize the energetic changes that occur in mouse tubules subjected to H/R in vitro under conditions in which they sustain nonlethal reversible injury, and 3) to test the effect of CyPD deletion on the tubule response to H/R in vitro and I/R-induced acute kidney injury in vivo.
MATERIALS AND METHODS
Animal and Surgical Procedures
Mice were cared for before and during the experimental procedures in accordance with the policies of the Institutional Animal Care and Use Committee of the University of Michigan and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols received prior approval from the University of Michigan Institutional Animal Care and Use Committee. Wild-type (WT) C57BL/6J mice were obtained from Jackson Labortory (Bar Harbor, ME). Mice null for Ppif, the gene encoding CyPD, previously backcrossed nine times on the same background (3), were kindly provided by Dr. Michael Forte (University of Oregon, Portland, OR) and maintained for the duration of the study as a colony in our facility. The mice were 10–14 wk of age at the time of the study. Six to 12 mice were used for each isolated tubule preparation, depending on the amount of material needed for particular experiments. Male mice were used for all studies, unless specifically indicated otherwise. For preparation of isolated tubules, mice were anesthetized with intraperitoneal ketamine and xylazine and the kidneys were immediately removed. For studies of I/R in vivo, mice were anesthetized with intraperitoneal ketamine and xylazine and then kept on a warming blanket, with core temperature monitored and maintained at 37 ± 0.2°C. Sterile procedures were used to expose both kidneys via retroperitoneal incisions; both renal pedicles were clamped using atraumatic micro-serrefine clamps (catalog no. 18055-03, Fine Science Tools) for 15, 20, or 25 min and then reperfused, and the wound was closed. Sterile saline (0.5 ml) was injected intraperitoneally, and mice were monitored for recovery, with continued temperature monitoring, on the warming blanket and then returned to individual cages. Buprenorphine was used preoperatively and every 12 h postoperatively for pain control. Tail vein blood was sampled under brief isoflurane anesthesia at 24 and 48 h after ischemia. At 72 h, mice were terminally anesthetized with isoflurane for final blood sampling and removal of kidneys for morphology. One hemisection from each kidney was fixed overnight in 10% alcoholic formalin (BBC Biochemicals, Sherwood, WA) and then transferred to 70% ethanol.
Materials
Type I collagenase was purchased from Worthington Biochemical (Lakewood, NJ), Percoll from Amersham Biosciences (Piscataway, NJ), HPLC-grade acetonitrile from Fisher Scientific (Pittsburgh, PA), high-purity digitonin (catalog no. 300411) from Calbiochem (San Diego, CA), and calcium green-5N from Molecular Probes (Eugene, OR). All other reagents and chemicals, including delipidated bovine serum albumin (catalog no. A6003), were of the highest grade available and were obtained from Sigma-Aldrich (St. Louis, MO). Aqueous stock solutions of experimental reagents were pH-adjusted, so as not to alter the final pH of the experimental medium. Regents that required solubilization in ethanol were delivered from ≥300× stock solutions in volumes of ethanol that did not, by themselves, affect the measured functions.
Isolation of Tubules
Immediately after removal of the kidneys, the parenchyma was injected with 0.3–0.5 ml of a cold 95% O2-5% CO2-gassed solution consisting of 115 mM NaCl, 2.1 mM KCl, 25 mM NaHCO3, 1.2 mM KH2PO4, 2.5 mM CaCl2, 1.2 mM MgCl2, 1.2 mM MgSO4, 25 mM mannitol, 2.5 mg/ml fatty acid-free bovine serum albumin, 5 mM glucose, 4 mM sodium lactate, 1 mM alanine, and 1 mM sodium butyrate (solution A), with the addition of 1 mg/ml collagenase (type I, Worthington Biochemical, Freehold, NJ). The cortices were dissected and minced on an ice-cold tile and then resuspended in additional solution A for 8–10 min of digestion at 37°C followed by enrichment of proximal tubules using centrifugation on self-forming Percoll gradients, as previously described for rabbit tubules (19–22, 66–68).
Experimental Procedures for Isolated Tubules
Incubation conditions were similar to those used previously for rabbit studies (19–22, 66–68). Tubules were suspended at 2.0–3.0 mg tubule protein/ml in a 95% air-5% CO2-gassed medium containing 110 mM NaCl, 2.6 mM KCl, 25 mM NaHCO3, 2.4 mM KH2PO4, 1.25 mM CaCl2, 1.2 mM MgCl2, 1.2 mM MgSO4, 5 mM glucose, 4 mM sodium lactate, 0.3 mM alanine, 5 mM sodium butyrate, 2 mM glycine, and 1.0 mg/ml bovine gelatin (75 bloom) (solution B). For studies limited to normoxic conditions, tubules were preincubated for 15 min at 37°C and then resuspended in fresh solution B containing 2 mM heptanoic acid, instead of sodium butyrate, and 250 μM AMP (66, 68) for an additional 60 min prior to sampling for experiments. For studies comparing normoxia with H/R, at the end of the 15-min preincubation, tubules were resuspended in fresh solution B and regassed with 95% air-5% CO2 (normoxic controls) or 95% N2-5% CO2 (hypoxia). During hypoxia, solution B was kept at pH 6.9 to simulate tissue acidosis during ischemia in vivo, and the usual substrates, glucose, lactate, alanine, and butyrate, were omitted. After 30 min, samples were removed for analysis. The remaining tubules were pelleted and then resuspended in fresh 95% air-5% CO2-gassed, pH 7.4, solution B with experimental agents as needed. Sodium butyrate in solution B was replaced with 2 mM heptanoic acid during reoxygenation, and, to ensure availability of purine precursors for ATP resynthesis, 250 μM AMP was included, unless specifically indicated. After 60 min of reoxygenation, tubules were sampled for studies.
Measurement of Lethal Plasma Membrane Damage by Lactate Dehydrogenase Release
Lactate dehydrogenase (LDH) activity was measured before and after the addition of 0.1% Triton X-100, as described elsewhere (63).
Measurement of ATP Levels
Tubule samples were immediately deproteinized in trichloroacetic acid, neutralized with trioctylamine-CFC 113, and stored at −20°C, as previously described (19–22). Purine nucleotides and their metabolites in 20-μl aliquots of the neutralized extracts were separated and quantified using a reverse-phase ion-pairing, gradient HPLC method, as previously described (19–22).
Assessment of ΔΨm by JC-1 Fluorescence
An aliquot of JC-1 from a frozen 2 mg/ml stock solution in dimethylsulfoxide was mixed 1:4 with calf serum, dispersed as an intermediate stock solution in phosphate-buffered saline, and then used at a final concentration of 9 μg/ml in the tubule suspension (23).
Measurements on suspended tubules.
At the end of the desired experimental period, JC-1 was added to the flask, and the suspension was regassed with air-CO2 and incubated in darkness for an additional 15 min at 37°C. Then tubules were pelleted and washed three times in an ice-cold solution containing 110 mM NaCl, 25 mM Na-HEPES, pH 7.2, 1.25 mM CaCl2, 1.0 mM MgCl2, 1.0 mM KH2PO4, 3.5 mM KCl, 5.0 mM glycine, and 5% polyethylene glycol (average mol wt 8,000) (solution C). Fluorescence was measured immediately on a 300-μl aliquot of the tubules containing 1.2–1.5 mg of tubule cell solution protein brought up to 2.3 ml with additional ice-cold solution C and then scanned using a fluorometer (Alphascan, Photon Technology International, Monmouth Junction, NJ) at 488-nm excitation and 500- to 625-nm emission collected using the right-angle mode of the fluorometer during continuous gentle stirring. After smoothing of the resulting curve, peak green fluorescence of the monomeric form of the dye was measured at 535 nm, and peak red fluorescence of the J aggregates was measured at 595 nm.
Viewing of cellular fluorescence.
An aliquot (25 μl) of the suspension as used for the fluorometer measurements was placed between two coverslips and viewed on a fluorescence microscope (DM IRB, Leica, Bensheim, Germany) using a ×20 HC PL Fluotar lens and an I3 filter set consisting of a 450- to 490-nm band-pass excitation filter, a 510-nm dichroic mirror, and 520-nm long-pass emission filters. Images were captured using an Olympus DP70 camera and DP controller software (Olympus, Center Valley, PA).
Assessment of ΔΨm With Safranin O
At the end of normoxic control incubation or H/R, samples of tubule suspension were immediately diluted into ice-cold solution C supplemented with 2.0 mg/ml bovine gelatin, washed once in the same solution, and then held in the solution at 4°C until use. For the safranin O uptake measurements (19–22), the tubules in the holding solution were pelleted and resuspended at a final concentration of 0.10–0.15 mg/ml in an intracellular buffer-type solution containing 120 mM KCl, 1 mM KH2PO4, 5 μM safranin O, 100–150 μg digitonin/mg protein, and 10 mM K-HEPES, pH 7.2, at 37°C (solution D) supplemented with 4 mM concentrations of potassium salts of the substrates that are described with the data and EGTA as described below. Fluorescence was measured once every second at 485-nm excitation and 586-nm emission using fluorometers (Deltascan and Alphascan, Photon Technology International, Lawrenceville, NJ) equipped with temperature-controlled (37°C), magnetically stirred cuvette holders to follow safranin O uptake by the mitochondria. For studies of baseline energization in the absence of the MPT, the permeabilization medium contained 1 mM EGTA. Safranin O uptake was followed until maximal, then 0.5 mg/ml delipidated albumin was added, and uptake was monitored until the response was complete. For studies of the MPT, the permeabilization medium contained 40 μM EGTA to provide a small excess above the Ca2+ carried over from the holding medium (20–30 μM). When safranin O uptake was maximal, the MPT was induced by addition of CaCl2 using a constant concentration for all experiments each day based on a preliminary titration to establish the minimum amount needed to initiate rapid deenergization and light scattering with a single addition. The required concentration of Ca2+ ranged from 35 to 45 μM. Fluorescence values for safranin uptake were normalized to the value at the start of uptake when safranin O was entirely in the medium, which was set at 1.0. Uptake of safranin O into the matrix of energized mitochondria results in quenching of its fluorescence, so the measured signal decreases. To make it easier to follow the tracings with respect to high and low ΔΨm, they are inverted in the figures.
Measurement of Medium Ca2+ With Calcium Green-5N
Tubules in cold solution C were washed quickly with ice-cold solution D containing 40 μM EGTA just before being placed into the cuvette for the experiment to prevent carryover of Ca2+ from solution C and allow greater consistency of Ca2+ increments produced by additions of Ca2+ for induction of the MPT. Otherwise, experiments were run exactly as described for measurements of ΔΨm with safranin O, except safranin O was replaced with calcium green-5N (0.15 μM, 506-nm excitation and 536-nm emission). To allow calculation of medium Ca2+ from the measured fluorescence values, each experiment was ended by addition of the uncoupler carbonyl cyanide 3-chlorophenylhydrazone (10 μM) followed by 400 μM EGTA to be in excess of total Ca2+ added during the study to determine the minimum fluorescence (Fmin) followed by 2 mM CaCl2 to determine the maximum fluorescence (Fmax). Ca2+ values for each experimental point were then calculated from its fluorescence (Fx) as follows: Ca = Kd × (Fx − Fmin)/(Fmax − Fx). The dissociation constant (Kd) determined in the experimental buffer (solution D) without tubules was 22.5 μM for calcium green-5N. Ca2+ uptake and retention were further quantified by factoring the maximal change in Ca2+ concentration by milligrams of tubule protein measured by the Lowry assay.
Assessment of Changes of Matrix Volume by Light Scattering
Fluorescence was followed at 520-nm excitation and 520-nm emission (24, 34) simultaneously with the safranin O uptake measurements of ΔΨm or calcium green-5N measurements of medium Ca2+ (24).
Blood Urea Nitrogen and Creatinine Assays
Blood urea nitrogen (BUN) was assayed colorimetrically (Bioassay Systems, Hayward, CA). Creatinines were assayed by HPLC using the column described for the nucleotide assays and a method adapted from Hewavitharana and Bruce (38). Serum was deproteinized using acetonitrile, then dried down, resuspended in a mobile phase consisting of 10 mM potassium phosphate, pH 6.8, and injected to run isocratically at room temperature and a flow rate of 0.6 ml/min with detection at 234 nm. Creatinine eluted at 9.2 min. Total run time was 48 min to allow the column to clear before the next injection.
Renal Morphology
Fixed kidney hemisections were dehydrated in graded ethanol, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. The extent of injury was assessed in a blinded fashion by quantification of the increased eosin fluorescence (10, 44) of damaged tubules containing casts and necrotic debris in the outer medulla of both kidneys. Eight to 12 images covering the entire outer stripe of the outer medulla were collected for each kidney using a ×20 objective and a Leica L5 filter set (460- to 400-nm band pass excitation, 505-nm dichroic mirror, 512- to 542-nm emission). The percentage of area of the image with the more brightly fluorescent injured tubules was quantified using the threshold and particle analysis functions of National Institutes of Health ImageJ. If injury was substantially less in one kidney than the other, incomplete ischemia was presumed, and functional and morphological data from that mouse were not considered further. This occurred with only one mouse.
Genotyping WT and Ppif−/− Mice
Total DNA was extracted from tail and proximal tubules using the Wizard SV genomic DNA purification kit (Promega, Madison, WI). Multiplex PCR was done to simultaneously assess the presence of Ppif intron 2 to exon 3 using the primer sequences 3′-gtagatgtcgtgccaaagactgcag-5′(forward) and 3′-agctgcaggcccttgtcaccagtgca-5′ (reverse) and of the neomycin cassette used to replace it in the knockouts using the primer sequences 3′-cacggatccgaccgcttcctcgtgct-5′ (forward) and 3′-gccgctctggattttggtatt-5′ (reverse) based on information from Dr. Michael Forte, who generated the mice (3). The PCR was carried out using GoTaq green Taq polymerase (Promega; 1.5 U), each primer at 250 nM, and 1 μM dNTP mix. Products were separated by 1% agarose gel electrophoresis together with a set of size markers.
Statistics
Paired and unpaired t-tests were used as appropriate. All experiments consisted of multiple groups, so they were analyzed statistically by analysis of variance for repeated measures or independent group designs as needed. Individual group comparisons for the multigroup studies were then made using the Holm-Sidak test for multiple comparisons (SigmaStat 3, SPSS, Chicago, IL). P < 0.05 was considered to be statistically significant. Values are means ± SE of no less than three to five experiments on separate tubule preparations or are traces representative of the behavior in at least that many experiments.
RESULTS
Occurrence of the MPT in Tubules From WT and Ppif−/− CyPD Knockout Mice
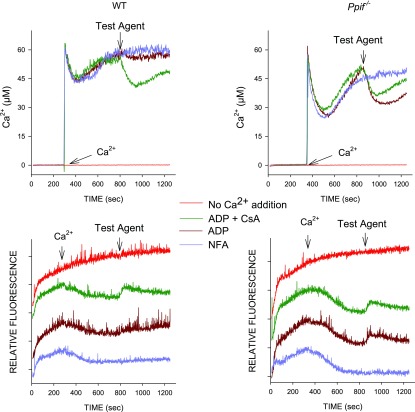
Genotyping of proximal tubule DNA from WT and knockout mice confirmed the absence of the deleted portion of the Ppif gene and the presence of the inserted neomycin cassette in the knockout mice (see Supplemental Fig. S1 in Supplemental Material for this article, available online at the Journal website). This was stable throughout the course of the work, as were the differences of MPT sensitivity from WT mice. Supplemental Fig. S2 illustrates use of changes of ΔΨm and assessment of matrix volume by light scattering to follow induction of the MPT with Ca2+ in permeabilized tubules from WT mice. Results were similar to our previous findings with rabbit tubules, which we described in more detail elsewhere (24). In our previous study (24), we also developed, for the permeabilized tubules, a method for measurement of medium Ca2+ to follow Ca2+ movements into and out of the mitochondria to allow for better quantitation of the effects of determinants of the MPT. The latter approach was used for all subsequent studies comparing the MPT in tubules from WT and Ppif−/− mice. Figure 1 shows representative experiments of this type comparing WT and knockout tubules. Initial uptake of Ca2+ following its addition at 300 s is followed by spontaneous release as the PTP opens, precluding retention of the Ca2+ (24). In tubules from Ppif−/− mice, Ca2+ uptake before release is substantially greater, consistent with resistance to development of the MPT. At 800 s, when most Ca2+ was released in both types of tubules, test agents (100 μM ADP or ADP + 1 μM CsA) were added to assess their efficacy to close the PTP and allow reenergization and reuptake of Ca2+. Tubules from WT mice did not respond to ADP alone (Fig. 1) or CsA alone (not shown) but took up Ca2+ with ADP + CsA. In contrast, in Ppif−/− tubules, Ca2+ reaccumulation was strong with ADP alone, while addition of CsA slightly decreased the response to ADP. The downward deflections of the light-scattering traces indicate increases of matrix volume as the MPT develops (24). The upward deflections of the traces upon addition of the test agents correspond precisely to the ability of the agents to induce Ca2+ reuptake, indicating that the reuptake was accompanied by decreased matrix volume.
Fig. 1.
Mitochondrial permeability transition (MPT) in tubules from wild-type (WT) and knockout (Ppif−/−) mice. Top: medium Ca2+ measured using calcium green-5N; bottom: corresponding 520/520 light-scattering traces, which are offset from each other for clarity. Delipidated albumin (0.5 mg/ml) was present in the medium from the start. Ca2+ was added at 300 s to induce the MPT, except in the trace labeled “no Ca2+ addition,” which shows the spontaneous change of light scattering in the absence of experimental maneuvers. Test additions at 800 s were 100 μM ADP, 100 μM ADP + 1 μM cyclosporin A (CsA), or buffer [no further addition (NFA)]. Data are from studies in which protein from WT and Ppif−/− tubules was closely matched to allow direct comparison of traces. Light-scattering changes after addition of Ca2+ are more gradual than those in Supplemental Fig. S2B, because delipidated albumin, which was present prior to Ca2+ addition in these studies, delays development of the MPT (24). Albumin was used prior to Ca2+ addition to avoid shifts of the baseline in the middle of experiments from Ca2+-independent interactions of albumin and calcium green-5N.
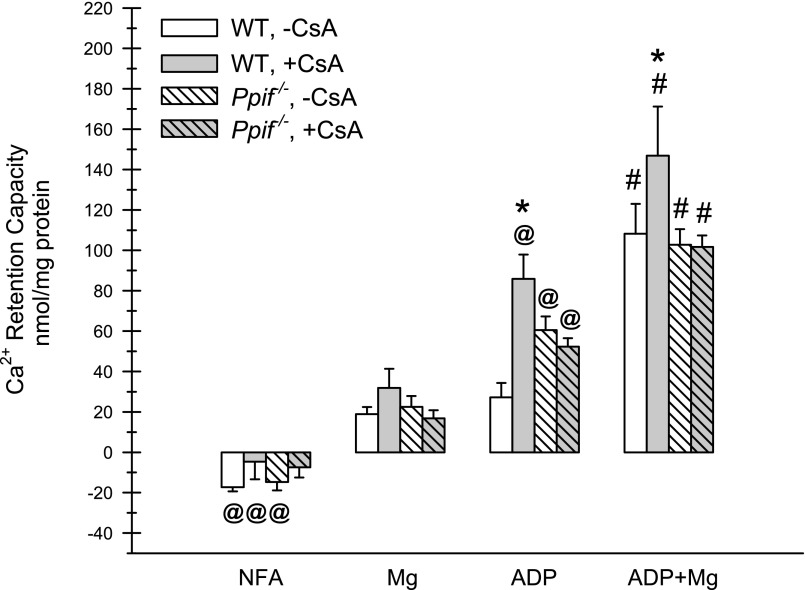
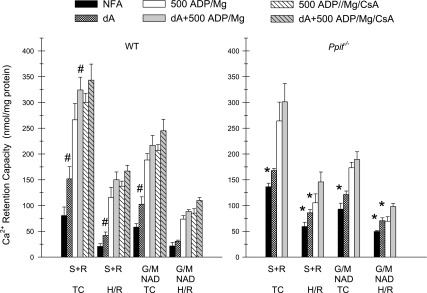
Figure 2 summarizes quantitative results for a series of such studies that included comparisons of additional MPT-modifying test additions. Under all conditions, Ca2+ retention capacity, indicating closure of the PTP, was significantly better with ADP + Mg2+ than with ADP, Mg2+, or no further addition (NFA). ADP alone resulted in significantly better reuptake than Mg2+ alone in all but the WT mice without CsA. Mg2+ alone resulted in better reuptakes than all corresponding NFA groups, except Ppif−/− tubules with CsA. CsA significantly increased uptake when added along with ADP or ADP + Mg2+ in WT mice but had no benefit for tubules from Ppif−/− mice. The concentration dependence of the CsA effects under these experimental conditions is shown in Supplemental Fig. S3. On the basis of data from Fig. 2 that CsA closed the PTP only in the presence of ADP, 100 μM ADP was used in Supplemental Fig. S3 studies to allow detection of CsA-induced effects. In WT tubules, Ca2+ retention capacity was significantly increased with 0.1 μM CsA, with a maximal effect at 1 μM and a trend toward lesser efficacy at higher concentrations. Ca2+ retention capacity was increased with ADP alone in tubules from Ppif−/− mice compared with WT mice but was not further increased by CsA.
Fig. 2.
Reversal of the MPT by modifying agents in tubules from WT and Ppif−/− mice. Protocol is similar to that described in Fig. 1 legend. Ca2+ reuptake was factored for tubule protein (Ca2+ retention capacity), which was measured after addition at 800 s of buffer (NFA), 1 mM Mg2+, 100 μM ADP, or ADP + Mg2+ to tubules from WT or knockout mice with and without 1 μM CsA. NFA groups have negative values because of small amounts of continuing Ca2+ release. Values are means ± SE (n = 3–4). #P < 0.001 vs. corresponding ADP, Mg2+, and NFA groups. @P < 0.016 vs. corresponding Mg2+ groups. *P < 0.001 vs. the same condition without CsA.
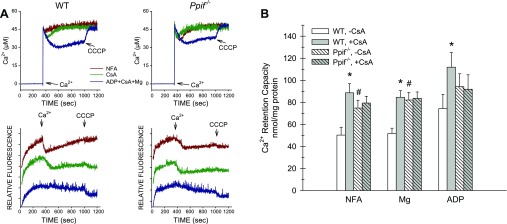
As shown in Fig. 2, CsA alone did not reverse the MPT after it developed in WT tubules. Figure 3 summarizes the results of further studies testing the effects of CsA and other modifying agents present prior to Ca2+ to prevent PTP opening in the mouse tubules. The representative traces in Fig. 3A show that CsA increased initial Ca2+ uptake and delayed matrix swelling in WT, but not Ppif−/−, tubules. Ca2+ uptake was increased in Ppif−/− tubules, as in Fig. 1, prior to PTP opening. The light-scattering traces for these experiments also show the delay in matrix swelling in the Ppif−/− compared with WT tubules, which was not evident in Fig. 1, because delipidated albumin was present prior to Ca2+ addition. Quantitative data from a series of these experiments are shown in Fig. 3B. CsA significantly increased Ca2+ retention capacity under all three conditions tested in the WT tubules but was without effect in the Ppif−/− tubules.
Fig. 3.
Prevention of the MPT by modifying agents in tubules from WT and Ppif−/− mice. A: traces from representative studies. Top: medium Ca2+ measured using calcium green-5N; bottom: corresponding 520/520 light-scattering traces, which are offset from each other for clarity. Ca2+ was added at 300 s to induce the MPT. Test additions present from the start were 1 μM CsA, 100 μM ADP + 1 mM Mg2+ + 1 μM CsA, or buffer (NFA). Delipidated albumin was not used in these experiments. Data are from studies in which protein from WT and Ppif−/− tubules was closely matched to allow direct comparison of traces. CCCP, carbonyl cyanide 3-chlorophenylhydrazone. B: quantitative data for experiments comparing prevention of the MPT. Ca2+ retention capacity was measured after NFA or after addition of 1 mM Mg2+ or 100 μM ADP to tubules from WT or Ppif−/− mice with and without 1 μM CsA. Values are means ± SE (n = 6–11). *P < 0.008 vs. corresponding group without CsA. #P < 0.015 vs. corresponding WT group.
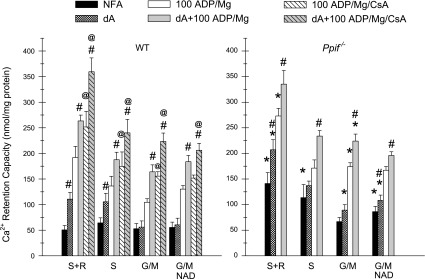
Our previous studies using rabbit tubules showed that NEFA generated during the MPT strongly promote the process and that susceptibility to opening of the PTP is higher with complex I-dependent substrates than with the complex II-dependent substrate succinate (24); therefore, it is necessary to assess both conditions to adequately profile tubule behavior and to extrapolate to likely determinants of tubule responses in vivo. Figure 4 addresses these issues and related questions for the WT and Ppif−/− mouse tubules. Test agents were present prior to induction of the MPT by Ca2+. WT and Ppif−/− tubules respiring on succinate or glutamate + malate with NFA were compared with tubules treated with delipidated albumin to bind NEFA, ADP + Mg2+, and ADP + Mg2+ + delipidated albumin. WT tubules were also studied with ADP + Mg2+ + CsA and ADP + Mg2+ + CsA + delipidated albumin. In contrast to rabbit tubules (24), the mouse tubules did not show greater resistance to the MPT with succinate as the substrate than with the complex I-dependent substrate combination of glutamate + malate. However, rotenone + succinate significantly increased the benefit provided by ADP + Mg2+ in WT and Ppif−/− tubules relative to succinate alone and glutamate + malate. Similar to prior rabbit tubule studies (24), addition of NAD to glutamate + malate-treated tubules to replace NAD lost from the matrix during the MPT (15) did not modify susceptibility. Delipidated albumin significantly increased Ca2+ retention capacity when used in combination with ADP + Mg2+ or ADP + Mg2+ + CsA, irrespective of the substrate present, but when used without the other MPT-modifying agents, its effects were weaker in the presence of glutamate + malate than with succinate as the substrate. As in Fig. 3, in Ppif−/− tubules, resistance to the MPT was greater under most conditions.
Fig. 4.
Modification of susceptibility to the MPT by nonesterified fatty acid (NEFA) binding with delipidated albumin (dA) as a function of substrate conditions in tubules from WT and Ppif−/− mice. Ca2+ retention capacity was measured with NFA to the indicated substrates or with addition of 0.5 mg/dl dA, 100 μM ADP + 1 mM Mg2+, 100 μM ADP + 1 mM Mg2+ + 1 μM CsA, ADP + Mg2+ + dA, or ADP + Mg2+ + CsA + dA in tubules from WT and Ppif−/− mice. Succinate (S) was tested alone and with rotenone (S + R): 1 μM without dA, 5 μM with dA. Glutamate + malate (G/M) was tested with and without 3 mM NAD. All test agents were present from the start of the experiments prior to addition of Ca2+. Values are means ± SE (n = 5–9). *P < 0.035 vs. corresponding WT group. #P < 0.025 vs. corresponding group without dA. @P < 0.04 vs. corresponding group without CsA. Values were significantly greater for all groups with ADP + Mg2+ than corresponding groups without ADP + Mg2+ (P < 0.009). Values were significantly greater for all groups with S + R and ADP + Mg2+ and the Ppif−/− group with S + R and dA than corresponding groups without rotenone and corresponding G/M groups (P < 0.022). Ca2+ retention capacity was significantly better in WT tubules with dA alone and Ppif−/− tubules with NFA or dA alone with S + R than with G/M (P < 0.005).
Response of WT and Ppif−/− Tubules to H/R
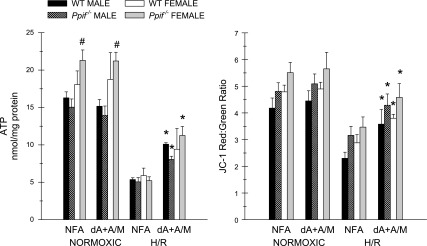
ATP levels and changes of energization with the ΔΨm-sensitive probe JC-1 were assessed using tubules from male and female mice. ATP levels tended to be higher in normoxic tubules from female than male mice (Fig. 5; see Supplemental Table S1). Decreases of ATP and of the JC-1 red-to-green ratio were similar in male and female mice at the end of 30 min of hypoxia followed by 60 min of reoxygenation (Fig. 5). Supplementation of the reoxygenation medium with delipidated albumin + α-ketoglutarate + malate significantly improved recovery of ATP and energization, as described previously for rabbit tubules (19, 21). No differences were noted in any of the parameters studied between Ppif−/− and WT tubules (Fig. 5; see Supplemental Table S1). The appearance of JC-1-loaded tubules is illustrated in Supplemental Fig. S4, which shows excellent preservation of overall tubule morphology in the preparations, loss of the high energization-associated red signal in the unsupplemented H/R tubules, which results in a yellowish green color, and restoration of the signal in H/R tubules by delipidated albumin + α-ketoglutarate + malate.
Fig. 5.
Response of WT and Ppif−/− tubules to hypoxia-reoxygenation (H/R). After 30 min of hypoxia followed by 60 min of reoxygenation, ATP was measured and membrane potential (ΔΨm) was assessed using JC-1. Tubules were incubated during reoxygenation with NFA or with 5 mg/dl dA + 4 mM α-ketoglutarate + 4 mM malate (dA + A/M). Values are means ± SE (n = 3–7). *P < 0.004 vs. corresponding 30-min H/R NFA group. #P < 0.002 vs. corresponding male group.
Table 1 provides additional metabolic information about the mouse tubules under normoxic conditions, at the end of hypoxia, and after H/R. In this study, normoxic and H/R Ppif−/− tubules were again compared with respect to the effect of supplementation with delipidated albumin + α-ketoglutarate + malate vs. NFA to the reoxygenation medium, and to provide more information about cellular adenine nucleotide profiles, they were studied with addition of AMP to the reoxygenation medium as usual and also without AMP. Under the conditions studied, i.e., glycine in all solutions and medium pH decreased during hypoxia, there were no increases of LDH release at the end of hypoxia, and although an upward trend was seen, LDH release by the reoxygenated tubules was not significantly different from LDH release by the normoxic tubules. ATP and JC-1 results for the groups with AMP alone and delipidated albumin + α-ketoglutarate + malate + AMP during reoxygenation showed the same benefit of delipidated albumin + α-ketoglutarate + malate for recovery of cellular ATP and energization measured by JC-1 fluorescence observed in the comparable groups in Fig. 5. Omission of AMP from the medium substantially lowered cellular ATP levels in normoxic tubules and in tubules with supplemental delipidated albumin + α-ketoglutarate + malate during reoxygenation, but not in tubules without delipidated albumin + α-ketoglutarate + malate. Omission of AMP did not affect energization as measured by JC-1 in any group.
Table 1.
Metabolic parameters during hypoxia-reoxygenation
| LDH, %free | JC1, red-to-green ratio | ATP, nmol/mg protein | ADP, nmol/mg protein | AMP, nmol/mg protein | ATP, mM | ADP, mM | AMP, mM | |
|---|---|---|---|---|---|---|---|---|
| Normoxia | ||||||||
| NFA | 12.20 ± 2.54 | 4.56 ± 0.10 | 6.73 ± 0.80 | 1.02 ± 0.05 | 0.56 ± 0.01 | 2.69 ± 0.32 | 0.41 ± 0.02 | 0.23 ± 0.00 |
| AMP | 10.68 ± 2.49 | 5.03 ± 0.14 | 22.86 ± 4.69*‡ | 2.26 ± 0.42*‡ | 1.35 ± 0.32*‡ | 9.14 ± 1.88 | 0.90 ± 0.17 | 0.54 ± 0.13 |
| dA + A/M | 13.07 ± 1.44 | 4.88 ± 0.21 | 5.76 ± 0.26 | 0.87 ± 0.09 | 0.81 ± 0.32 | 2.30 ± 0.11 | 0.35 ± 0.04 | 0.32 ± 0.13 |
| dA + A/M + AMP | 11.67 ± 3.53 | 5.24 ± 0.11 | 17.10 ± 4.78*‡ | 1.80 ± 0.19*‡ | 2.10 ± 0.84*‡ | 6.84 ± 1.91 | 0.72 ± 0.08 | 0.32 ± 0.13 |
| 30 min of hypoxia | 7.97 ± 2.48 | 0.37 ± 0.03§ | 0.49 ± 0.04§ | 1.88 ± 0.16§ | 0.15 ± 0.01 | 0.19 ± 0.01 | 0.75 ± 0.06 | |
| 60 min of reoxygenation | ||||||||
| NFA | 15.28 ± 3.42 | 2.42 ± 0.15§ | 1.89 ± 0.22§ | 0.68 ± 0.02§ | 1.58 ± 0.16§ | 0.75 ± 0.09 | 0.27 ± 0.01 | 0.63 ± 0.07 |
| AMP | 14.38 ± 3.64 | 2.79 ± 0.19§ | 2.09 ± 0.26§ | 0.77 ± 0.03§ | 1.72 ± 0.25 | 0.84 ± 0.10 | 0.31 ± 0.01 | 0.69 ± 0.10 |
| dA + A/M | 15.48 ± 4.26 | 4.29 ± 0.20*†§ | 2.70 ± 0.03*§ | 0.69 ± 0.04§ | 0.33 ± 0.09*†§ | 1.08 ± 0.01 | 0.28 ± 0.02 | 0.13 ± 0.03 |
| dA + A/M + AMP | 15.27 ± 5.66 | 4.78 ± 0.22*†‡§ | 11.01 ± 2.72*†‡§ | 1.39 ± 0.29*†‡§ | 0.97 ± 0.31 | 4.40 ± 1.09 | 0.56 ± 0.12 | 0.39 ± 0.13 |
Values are means ± SE (n = 3). Ppif−/− tubules were subjected to 30 min of hypoxia followed by 60 min of reoxygenation or an equivalent period of normoxic incubation in medium supplemented with no further additions (NFA), 250 μM AMP, delipidated bovine serum albumin + α-ketoglutarate + malate (dA + A/M) or dA + A/M + AMP. Millimolar (mM) data for adenine nucleotides were calculated from nmol/mg protein values based on 2.5 μl cell water/mg protein. LDH, lactate dehydrogenase.
P < 0.03 vs. corresponding NFA group.
P < 0.04 vs. corresponding AMP group.
P < 0.04 vs. corresponding dA + A/M group.
P < 0.04 vs. corresponding normoxic group for all parameters except ATP, where P < 0.001.
End-hypoxia ATP and ADP were different from all other points (P < 0.001 and P < 0.02, respectively). Statistics for nucleotide (mM) values (not shown) are the same as for protein-factored (nmol/mg protein) data.
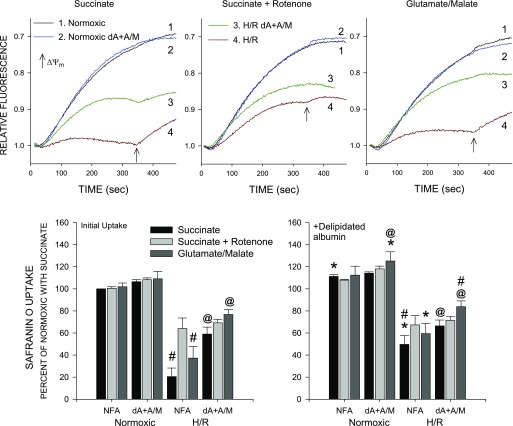
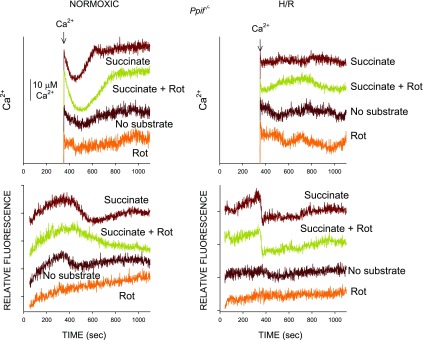
Changes of energization induced by H/R can also be assessed using safranin O uptake by permeabilized tubules (19, 21–25). Figure 6 summarizes such data from studies comparing normoxic and H/R tubules with and without delipidated albumin + α-ketoglutarate + malate during the reoxygenation period. Normoxic tubules showed similarly high levels of energization when studied during safranin O uptake with succinate or succinate + rotenone or glutamate + malate as substrates. Energization of the normoxic tubules was not affected by prior incubation with delipidated albumin + α-ketoglutarate + malate or addition of delipidated albumin during the safranin O uptake period. After H/R, safranin O uptake was severely impaired in tubules respiring on succinate alone but was significantly improved by addition of delipidated albumin at the end of the permeabilization period or inclusion of rotenone but reached only ∼60% of normoxic levels. Energization was stronger in H/R tubules supported by glutamate + malate during safranin O uptake than in tubules supported by succinate alone but was weaker than in tubules supported by succinate + rotenone. Energization was significantly better in H/R tubules that received delipidated albumin + α-ketoglutarate + malate during reoxygenation than in unsupplemented tubules as measured by safranin O uptake under all the substrate conditions and was not further improved by delipidated albumin during safranin O uptake.
Fig. 6.
Effects of H/R on ΔΨm measured using safranin O uptake. Top: representative traces from studies using safranin O to follow ΔΨm. Traces are from a representative experiment comparing tubules at the end of normoxia or H/R under 3 substrate conditions: succinate, succinate + rotenone, and glutamate + malate. Prior to these measurements, tubules had been incubated with NFA or dA + A/M during 60 min of reoxygenation or the equivalent preceding period of normoxic incubation. Arrows indicate addition of dA (0.5 mg/ml) at 350 s during the permeabilization period. Results are from a study using Ppif−/− tubules but are similar to results with WT tubules. Bottom: group data. Values are means ± SE for 3 experiments with Ppif−/− tubules calculated as percent uptake relative to normoxic NFA tubules from the same preparation studied with succinate. Left: summary of values for initial uptake period; right: safranin uptake after addition of dA at 350 s. #P < 0.05 vs. corresponding succinate + rotenone group. *P < 0.05 vs. the same condition prior to dA addition at 350 s. @P < 0.01 vs. corresponding NFA group. All H/R groups were significantly different from corresponding normoxic group (P < 0.001).
Susceptibility to the MPT of WT and Ppif−/− Tubules After H/R
Figures 7 and 8 summarize results of studies assessing the changes induced by H/R in susceptibility of WT and Ppif−/− tubules to the MPT. Based on the Figs. 4 and 6 results, succinate was used in combination with rotenone. NAD was included with glutamate + malate to avoid complicating effects of loss of NAD to the dilute medium of the permeabilized tubules (15). In the Fig. 7 studies, where ADP was increased to 500 μM from 100 μM (Figs. 4 and 6), normoxic WT and Ppif−/− tubules behaved similarly to the comparable experiments in Fig. 4 and 6, except the increase of Ca2+ retention capacity (decreased MPT sensitivity) produced by delipidated albumin and CyPD deletion in the groups with ADP + Mg2+ was no longer present at the higher ADP concentration. The higher ADP concentration also eliminated the increases of Ca2+ retention capacity produced by CsA in the normoxic WT tubules. Without added ADP + Mg2+, delipidated albumin significantly increased Ca2+ retention capacity with both types of substrate in WT, but not Ppif−/− tubules. H/R strongly decreased Ca2+ retention capacity (increased MPT sensitivity) with both substrate conditions and in WT and Ppif−/− tubules (P < 0.005, H/R vs. corresponding normoxic time control). Delipidated albumin significantly increased Ca2+ retention after H/R only in WT tubules with succinate + rotenone. CsA did not affect Ca2+ retention after H/R in the WT tubules. After H/R, Ca2+ retention was higher in Ppif−/− tubules with NFA than in WT tubules but was not different from WT tubules in the presence of 500 μM ADP + Mg2+.
Fig. 7.
Effects of H/R on MPT susceptibility in WT and Ppif−/− tubules. Protocol is similar to that described in Fig. 4 legend, except 500 μM, rather than 100 μM, ADP was used in the ADP + Mg2+ groups and substrate conditions were limited to S + R and G/M + NAD. Values are means ± SE (n = 4 for WT experiments and n = 3 for Ppif−/− experiments). *P < 0.02 vs. corresponding WT group. #P < 0.032 vs. corresponding group without dA. All H/R groups were significantly different from corresponding normoxic time-control (TC) groups (P < 0.012). All S + R groups with ADP + Mg2+, except the Ppif−/− H/R tubules, were significantly different from corresponding G/M + NAD group (P < 0.044). Among the S + R groups with NFA or dA alone, only TC tubules were different from corresponding G/M + NAD group (P < 0.015).
Fig. 8.
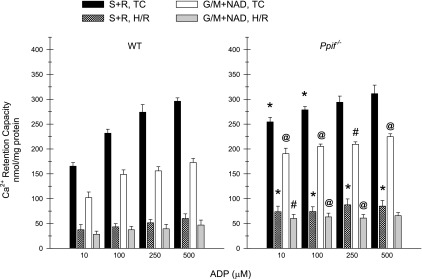
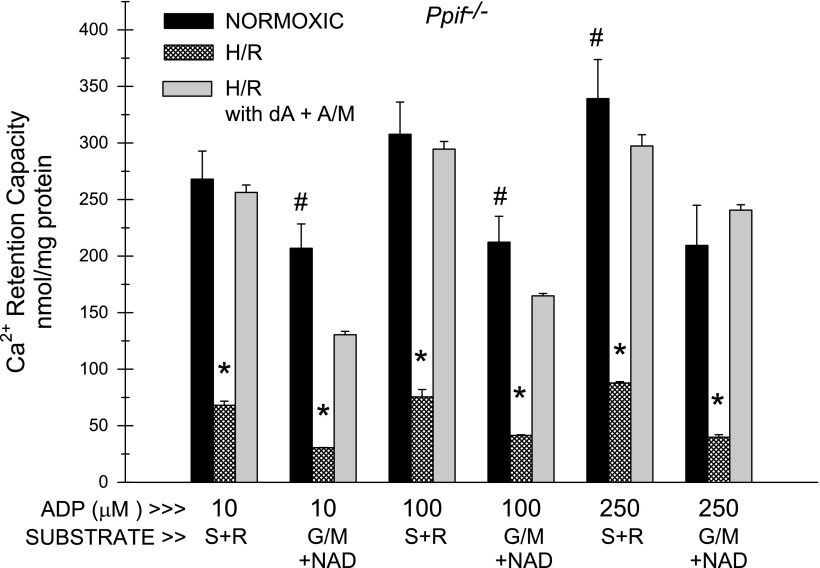
Effects of H/R on occurrence of the MPT: ADP dependence of Ca2+ retention capacity. Ca2+ retention capacity was measured in WT and Ppif−/− tubules after normoxic or H/R incubation, as described in Fig. 7, in the presence of the indicated concentrations of ADP and S + R or G/M + 3 mM NAD as substrates. MgCl2 (2 mM) was present in all experiments. Values are means ± SE (n = 7). *P < 0.001 vs. corresponding WT condition. #P < 0.01 vs. corresponding WT condition. @P < 0.05 vs. corresponding WT condition. All H/R groups were significantly different from corresponding normoxic (TC) groups (P < 0.001). In all normoxic (TC) studies, sensitivity was significantly less with S + R than with G/M + NAD (P < 0.001). In H/R studies, differences between the two substrates were much smaller but were still significant (P < 0.05) for all conditions, except 10 μM ADP.
Figure 8 summarizes the results of additional experiments to further clarify the dependence of MPT sensitivity on ADP concentration in WT and Ppif−/− tubules. The differences in Ca2+ retention between normoxic WT and Ppif−/− tubules were smaller and less consistent at >100 μM ADP but were still significant under all conditions, except 500 μM ADP with succinate + rotenone as substrate. Sensitivity of H/R tubules to the MPT was greater than in Fig. 7 and only minimally improved as ADP was increased from 10 to 500 μM. MPT sensitivity was significantly less in Ppif−/− than WT tubules under all conditions, except 500 mM ADP with glutamate + malate as substrate.
Susceptibility of the permeabilized mouse tubules to the MPT after H/R in Figs. 7 and 8, although improved with ADP + Mg2+, was still high and was not further improved by delipidated albumin. In Figs. 5 and 6, supplementation of tubules during 60 min of reoxygenation with delipidated albumin + α-ketoglutarate + malate strongly reversed the energetic deficit produced by H/R. Figure 9 was designed to assess the effect of this approach on the H/R-induced increase of susceptibility to the MPT. Delipidated albumin + α-ketoglutarate + malate during reoxygenation strongly ameliorated the H/R-induced increase of MPT sensitivity, completely eliminating it for some of the test conditions.
Fig. 9.
Effect of dA + A/M during reoxygenation on susceptibility to the MPT after H/R. Tubules from Ppif−/− mice were treated as described in Fig. 7 and 8 legends, except at the end of hypoxia, flasks were split into pairs, and one of each pair was supplemented during reoxygenation with dA + A/M (H/R with dA + A/M), while the other had no further additions (H/R). Indicated amounts of ADP (μM) + 2 mM Mg2+ were present during the MPT testing period. Values are means ± SE (n = 3). *P < 0.001 vs. normoxic and H/R with dA + A/M. #P < 0.05 vs. H/R with dA + A/M.
Ca2+ is required for the MPT (33, 39, 42, 64, 71), and in the present studies, we use Ca2+ uptake to elicit it and assess susceptibility to it. However, after H/R, tubules are deenergized due to NEFA accumulation (Fig. 7) (19, 21, 25), which could decrease Ca2+ uptake and, paradoxically, protect against the MPT. This raises the possibility that the markedly decreased Ca2+ retention capacity in Figs. 7–9 does not represent increased sensitivity to the MPT but, rather, is simply due to limited Ca2+ uptake that never elicits the MPT. Light-scattering changes such as those illustrated in Figs. 1 and 3 were evident in the H/R studies in Figs. 7 and 8 (not shown), indicating the presence of the MPT. Further experiments to explore this behavior are shown in Fig. 10, which compares development of the MPT as a function of substrate availability in normoxia and H/R in Ppif−/− tubules. After Ca2+ addition, normoxic tubules in the presence of succinate show Ca2+ uptake from the medium followed by release and changes in light scattering, indicating matrix expansion identical to the similar studies with Ppif−/− tubules in Figs. 1 and 3. Addition of rotenone to the succinate increases maximal Ca2+ uptake and decreases the rate of the light-scattering change, consistent with lower susceptibility to the MPT, as in Fig. 4. On the other hand, decreasing Ca2+ uptake of normoxic tubules by incubating them with no added substrate limits the light-scattering changes, and adding rotenone to the no-substrate medium to further inhibit metabolism of endogenous complex I-dependent substrates almost completely blocks Ca2+ uptake and completely prevents the light-scattering change, illustrating the dependence of the MPT on Ca2+ uptake. However, after H/R, even though tubules show marked impairment of Ca2+ uptake in the presence of succinate, they have an enhanced swelling response, consistent with increased sensitivity to the MPT. That substrate-driven Ca2+ uptake is required for the MPT in the H/R tubules, despite the limited amount of uptake, is shown by the absence of swelling responses to Ca2+ by H/R tubules incubated with no substrate and no substrate + rotenone.
Fig. 10.
Occurrence of the MPT as a function of substrate availability in tubules after normoxic and H/R incubation. Results are from a representative study using Ppif−/− mouse tubules subjected to normoxic incubation or H/R followed by MPT measurements with succinate, succinate + rotenone (Rot), no substrate, or no substrate plus rotenone. Medium did not contain any other MPT-modifying agents. Top: medium Ca2+ measured using calcium green-5N; bottom: corresponding 520/520 light-scattering measurements, which indicate matrix volume changes. For both types of data, traces are offset from each other for clarity. For studies to quantify Ca2+ uptake, such as those in Figs. 8 and 9, concentration of tubules was increased for the H/R conditions to allow cleaner measurements of their lower levels of uptake relative to baseline noise. Unequivocal Ca2+ uptake relative to baseline is not clearly evident in the H/R tubules here because in order to permit direct comparison of light-scattering traces between normoxic and H/R tubules, the same densities were used for both.
Effect of CyPD Absence on Acute Kidney Injury In Vivo
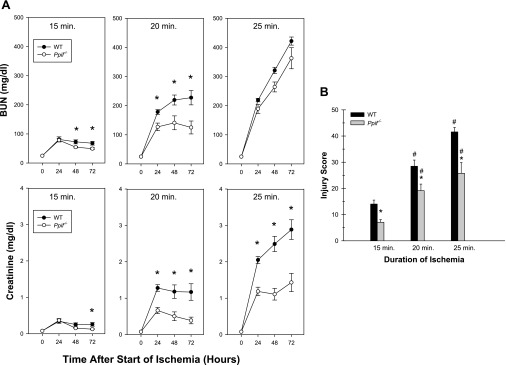
Graded levels of I/R injury were produced by varying the duration of ischemia between 15 and 25 min. Ischemia for 15 and 20 min produced mild and moderate injury, respectively, with survival of all mice for the full 72 h (Fig. 11). With 25 min of ischemia, six of the seven WT mice and three of the eight Ppif−/− mice became moribund between 48 and 72 h and were euthanized ahead of schedule. In the 15- and 20-min groups, the increases of BUN and creatinine were significantly ameliorated in the knockout mice. With the 25-min insult, BUN was not different in the two types of mice, but creatinine was significantly improved in the Ppif−/− mice. Morphological changes typical for I/R were observed, with the most prominent damage in the outer stripe of the outer medulla (not shown). The structural injury tracked very closely with the creatinines and was significantly decreased in the Ppif−/− mice at all durations of ischemia (Fig. 11).
Fig. 11.
Sensitivity of WT and Ppif−/− tubules to ischemia-reperfusion in vivo. A: time courses of changes of blood urea nitrogen (BUN) and creatinine over 72 h after 15, 20, and 25 min of bilateral clamp ischemia in vivo in WT and Ppif−/− mice. Values are means ± SE (n = 5–6 for 15-min studies, 8–9 for 20-min studies, and 7–8 for 25-min studies). *P < 0.05, WT vs. Ppif−/− at corresponding sampling time. All 20- and 25-min ischemia values were significantly greater than corresponding time points for the same type of mice at the shorter durations (P < 0.007), except BUN for 25-min study of WT mice vs. 20-min study of WT mice. B: morphological injury scores at 72 h. Values are means ± SE (n = 5–6 for 15-min studies, 8–9 for 20-min studies, and 7–8 for 25-min studies). *P < 0.012, WT vs. Ppif−/− at corresponding duration of ischemia. #P < 0.049 vs. the same type of mice at shorter durations. In 9 of the 25-min studies, mice were euthanized early, between 48 and 72 h, because they were moribund, so final values for those mice were at the time of death.
DISCUSSION
CyPD is a mitochondrial matrix peptidyl-prolyl cis-trans isomerase that was recognized as the mitochondrial target of CsA (35, 36, 49, 69) after CsA was found to be a potent inhibitor of the development of the MPT (7, 13, 28, 33). Its role in controlling the PTP was conclusively established more recently by studies of strains of mutant mice lacking the gene (2, 3, 48, 57); one of these strains (3) was used for the present work. Although benefit from CsA may derive from other effects of CsA on cyclophilins and calcineurin, such as inhibition of the mitochondrial fission-promoting protein Drp1 (11), and an increasing number of mitochondrial targets of CyPD that could affect processes other than the MPT are emerging (18, 30, 31), the available data from studies using CsA and CyPD-null mice continue to favor an important pathogenic role for the MPT. However, the complexity of the interactions involved dictate care in application of these tools to understanding organ-specific effects of the MPT and caution against simple extrapolation of results from one cell type to others.
CsA antagonized the MPT in tubules from WT mice on the basis of the increased Ca2+ retention capacity in its presence (Figs. 1–3) and its ability to reverse (Figs. 1 and 2) and prevent (Fig. 3) Ca2+-induced changes in matrix volume as assessed by light scattering. In contrast, CsA was without benefit for Ppif−/− tubules lacking CyPD, but those tubules displayed enhanced Ca2+ retention capacity relative to WT tubules in the absence of CsA. Taken together, these data document involvement of CyPD in the MPT in the tubules and specificity of the effects of CsA for actions on CyPD. They cannot be explained in this system by other recently reported, potentially confounding, effects of CsA and CyPD on mitochondria (11, 18, 30, 31). As in isolated mitochondria (3), the effects of CsA and CyPD deletion were partial, in that they increased resistance to the MPT but did not totally prevent it. This finding has been taken to indicate that CyPD is not, itself, an essential integral component of the PTP but, rather, serves as a modulator of whichever protein(s) form(s) the channel (33, 35, 42).
ADP and Mg2+ are potent endogenous antagonists of the MPT that synergize with each other and with CsA (24, 33, 39, 51, 64). This behavior was evident in the mouse tubules. CsA in WT tubules was effective in ameliorating the MPT when it was present prior to MPT induction by Ca2+ and the resulting loss of matrix ADP and Mg2+ (Fig. 3), but addition of ADP (100 μM) to the medium was required for CsA to strongly close the PTP (Figs. 1 and 2). In Ppif−/− tubules, ADP alone produced substantial PTP closing, which was not the case in the WT tubules treated with ADP alone. In WT and Ppif−/− tubules, ADP + 1 mM Mg2+ produced the most effective closing of the PTP and eliminated the benefit of CyPD deletion. If we consider 1–2 mM free Mg2+ in cells and averaged cellular ADP concentrations of 0.2–0.5 mM (Table 1; see below), these observations emphasize the importance of accounting for availability of these metabolites in assessing the likelihood of MPT development under injury conditions relevant to the behavior of intact cells.
Other endogenous metabolites that can affect MPT susceptibility are the type of substrate available to support electron transport. This has often not been systematically assessed. However, in our prior studies using isolated rabbit tubules, we found a much lower susceptibility to the MPT when respiration was supported by succinate than when it was supported by complex I-dependent substrates (24). Similar behavior has been described for muscle mitochondria (26). In the present study, mouse tubules supported by succinate alone were not different from those supported by glutamate + malate (Fig. 5); however, addition of rotenone to the succinate to suppress reverse electron transport (41) and generation of oxaloacetate, which inhibits succinate dehydrogenase (47), markedly increased resistance to the MPT. The differences in MPT resistance between WT and Ppif−/− tubules were seen with all the substrate combinations tested.
When glycine is present to prevent rapid lethal plasma membrane damage, the condition that pertains during ischemia in vivo (65), isolated rabbit and rat tubules subjected to hypoxia develop a sustained energetic deficit, characterized by failure to recover ATP during reoxygenation, that is associated with persistent partial mitochondrial deenergization mediated by accumulation of NEFA (19, 21, 25). The energetic deficit can be prevented and reversed by supplementation during hypoxia and/or reoxygenation with citric acid cycle substrates, which act at least in part by mediating anaerobic ATP generation, which lowers NEFA levels (19, 21, 25). Delipidated albumin strongly reverses deenergization when added to permeabilized tubules tested at the end of reoxygenation and has some efficacy even in nonpermeabilized tubules when present during reoxygenation (21). Mouse tubules, assessed for the first time under these conditions in the present study, exhibited similar behavior, in that ATP recovery was impaired during reoxygenation in the presence of glycine, despite suppression of LDH release during H/R, and mitochondria showed persistent, but partial deenergization, as measured by JC-1 and safranin O uptake (Fig. 5, Table 1). ATP recovery and deenergization were improved by delipidated albumin and supplemental citric acid cycle substrates during reoxygenation, and energization was partially restored by delipidated albumin in permeabilized tubules tested at the end of reoxygenation (Fig. 6). Because the energetic deficit is associated with partial, rather than complete, deenergization, it cannot be explained by a sustained MPT. However, incomplete forms of the MPT have been described (8, 9, 39, 40, 46) and could contribute to the energetic deficit. Moreover, our earliest observations on the energetic deficit suggested benefit of combinations of MPT-modifying pharmacological agents (66). The present studies, by comparing tubules from WT and Ppif−/− mice, provide an informative test of MPT involvement in the energetic deficit. The energetic deficit developed and was subject to modification similarly in the WT and Ppif−/− tubules, which strongly argues against any role for the MPT in it. Rather, as indicated by earlier work, reversible transmembrane cycling of NEFA plays the major role (19, 21, 25). CyPD directly interacts with the lateral stalk of the mitochondrial F1F0-ATPase to inhibit enzyme activity, which could serve to decrease ATP production during normoxia and inhibit ATP depletion during hypoxia (31). ATP levels in Ppif−/− tubules, however, were remarkably similar to those in WT tubules during normoxia and hypoxia (see Supplemental Table S1).
When the isolated mouse tubules are incubated without glycine (or alanine) in the medium, LDH release reaches 80–90% during the first 15 min of hypoxia, even at pH 6.9, and we have not found that to be measurably different in tubules from Ppif−/− mice compared with WT tubules (data not shown). This cell death is occurring during hypoxia when ATP depletion results from failure of oxidative phosphorylation due to the oxygen deprivation, so it does not reflect any effects on the capacity for oxidative phosphorylation, as would result from different susceptibility to the MPT in WT and Ppif−/− tubules. In fact, ATP levels as measured in Fig. 5 at the end of hypoxia were not different in the WT and Ppif−/− tubules (see Supplemental Table S1). As discussed above, during reoxygenation in the presence of glycine, oxidative phosphorylation in the tubules continues to be impaired due to NEFA-mediated mitochondrial uncoupling without immediate development of the MPT. The data show that the NEFA-mediated uncoupling and resulting ATP depletion are also not influenced by CyPD, so this condition does not reflect any differences that would result from altered MPT susceptibility in the null mice. We previously showed using rabbit tubules that the ATP depletion that persists during reoxygenation is severe enough to produce necrosis if glycine is withdrawn before the energetic deficit is corrected (68). In the present studies, we have also tested whether this occurs in the mouse tubules and whether Ppif−/− tubules would be protected relative to WT tubules from the effect of glycine withdrawal during reoxygenation. Ppif−/− and WT tubules were allowed to recover for 1 h of reoxygenation in the presence of glycine; then the glycine was withdrawn and LDH release was measured. Glycine withdrawal after 60 min of reoxygenation (see Supplemental Fig. S5) leads to similarly severe LDH release in WT and Ppif−/− tubules, consistent with a continuing primary role for the ATP depletion that was induced by the initial NEFA-mediated energetic deficit and not requiring development of the MPT. The importance of this ATP depletion is further indicated by the data in Supplemental Fig. S5 showing that delipidated albumin + α-ketoglutarate + malate, which corrects the ATP depletion (Fig. 5), strongly ameliorated this LDH release in WT and Ppif−/− tubules.
Adenine nucleotide levels measured during the H/R studies summarized in Table 1 are relevant to occurrence of the MPT in the tubule cells. In normoxic control tubules without nucleotide supplementation, ADP levels were calculated to be 0.41 mM when averaged for total cell water. The level decreased to 0.19 mM at the end of hypoxia and then only partially recovered to 0.27 mM at the end of reoxygenation. Actual ADP levels in the mitochondrial matrix are higher than these whole cell averages, while cytosolic levels are lower (58). However, once the PTP begins to open in cells, the two compartments will tend to equilibrate, so the average values obtained from the whole cell measurements provide a reasonable approximation of levels in cells while the MPT would be developing. The data in Table 1 also bear on considerations relating to our routine supplementation of the reoxygenation medium with AMP. The presence of AMP did not have a substantial effect on the underlying impairment of mitochondrial energization or its amelioration by delipidated albumin + α-ketoglutarate + malate in the reoxygenation medium, because JC-1 ratios were only minimally lower without than with AMP under both conditions. However, AMP allowed better discrimination of the difference in ability to restore ATP between unprotected and protected tubules. AMP in the medium also moderately increased cellular ADP from 0.35–0.40 to 0.70–0.90 mM in normoxic tubules and from 0.28 to 0.56 in protected H/R tubules. There was only a marginal effect of the AMP on ADP levels (0.31 mM) of unprotected H/R tubules.
Although the MPT did not develop in the isolated proximal tubules during the 60 min of reoxygenation in the presence of glycine, studies of its occurrence in permeabilized tubules sampled at the end of reoxygenation allowed assessment of susceptibility to the MPT at that time and the changes resulting from the absence of CyPD. A substantial decrease in Ca2+ retention capacity of WT and Ppif−/− tubules occurred after H/R and, as importantly shown in Fig. 10, was attributable to increased sensitivity to the MPT, not to impairment of Ca2+ uptake after H/R. In the initial series of studies, which assessed WT and Ppif−/− tubules in the absence of ADP and Mg2+ compared with 0.5 mM ADP + Mg2+, resistance to the MPT was increased in normoxic Ppif−/− tubules and, after H/R, only in the absence of ADP and Mg2+ (Fig. 8). Further studies with additional ADP concentrations showed benefit of CyPD deletion at a range of concentrations, but effects after H/R were maximal at ≤250 μM ADP (Fig. 9).
As in normoxic tubules, resistance of WT and Ppif−/− tubules after H/R was much less with the complex I-dependent substrate combination of glutamate + malate than with succinate + rotenone, even in the presence of ADP + Mg2+. This is similar to our previous studies with rabbit tubules (24) and, as proposed in that work, provides an explanation for sensitivity to the MPT in vivo, despite the presence of ADP + Mg2+, because complex I-dependent substrates predominate there. Mouse tubules in the present work differed from the rabbit tubules we previously studied (24), in that the increased susceptibility to the MPT after H/R was not substantially ameliorated by delipidated albumin during induction of the MPT in permeabilized tubules, even in the presence of maximally effective amounts of ADP + Mg2+ (Figs. 8 and 9), suggesting additional effects of H/R to promote the MPT in them, such as species differences in ROS generation (54). However, susceptibility of H/R tubules to the MPT dramatically benefited if they were supplemented with delipidated albumin + α-ketoglutarate + malate during the reoxygenation period (Fig. 9). This observation importantly emphasizes the ability to restore normal energetic function after H/R by that approach. The greater benefit of delipidated albumin + α-ketoglutarate + malate during reoxygenation compared with delipidated albumin only during permeabilization likely derives from additional effects of the high intracellular concentrations of ATP generated in the cells during the 60 min of reoxygenation. Tubules generate ATP after permeabilization under the conditions studied (21); however, the final medium ATP concentrations reached are relatively low at the tubule densities used during permeabilization, and the time frame for ATP generation during those protocols is short, so effects such as those seen from its restoration in the cells prior to permeabilization would not necessarily be expected.
CyPD deletion has been reported by Devalaraja-Narashimha et al. (14) to be protective against a mild I/R insult (BUN peak of 60 at 24 h) in a mixed-strain knockout. The present studies using mice extensively backcrossed onto C57BL/6J document protective effects of CyPD knockout across a wider range of insult severity and indicate that endogenous antagonists of the MPT do not prevent its pathogenic occurrence during injury in vivo. Inflammatory injury plays a major role in the progression of I/R in vivo, and our studies of Ppif−/− mice and the study of Devalaraja-Narashimha et al. use global knockouts, which affect cells other than proximal tubules. However, there are no clear pathways by which modification of the MPT in inflammatory cells would be expected to modify this process. When overexpressed naturally in tumor cells or experimentally, CyPD associates with Bcl2 and is antiapoptotic (18), so theoretically its absence in the Ppif−/− mice could be proapoptotic and promote injury, but the Ppif−/− mice in the present study and the study of Devalaraja-Narashimha et al. were protected against I/R, as in models of I/R affecting other nonmalignant tissues without increased CyPD (17, 27, 45, 52). Also, occurrence of the MPT in vivo will not necessarily require the full amounts of Ca2+ used to elicit it and measure resistance to it in the permeabilized tubules, since multiple factors during injury other than Ca2+, such as ROS production and signaling via glycogen synthase kinase-3β activation and other pathways (56, 72), sensitize mitochondria to development of the MPT. Glycogen synthase kinase-3β has recently been implicated in renal I/R injury via promotion of apoptotic pathways, but in vivo studies of its inhibition show very early generalized ameliorative effects that are not clearly explained only by apoptosis (61).
The benefit for in vivo I/R in the Ppif−/− mice differs from the lack of effect on H/R in the isolated tubules. The morphological changes we have quantified at 72 h in vivo in Fig. 11B largely represent a decrease in extent of necrotic cell death, consistent with prevention of necrosis by increased resistance to the MPT in the Ppif−/− mice. Most necrotic cell death during in vivo clamp models of I/R, such as those studied here, begins only after several hours (1), allowing for the contribution of additional factors not readily modeled in the isolated tubules. These include prolonged periods of variable continued hypoxia due to impaired reflow that would favor incomplete recovery from the early energetic deficit, variation in the glycine availability due to washout of the high levels present in the control kidney prior to ischemia followed by reflow at low circulating glycine levels, the increased possibility of injury due to oxidant production over time, and, perhaps most importantly, the recruitment of additional pathways for cell death mediated by apoptosis and the newly identified programmed necrosis (necroptosis) pathways that are subject to modification by the MPT (56, 60). Combinations of these processes could allow for conditions in which tubule cells with partial recovery of energetic function are at increased risk for progression to the MPT that is opposed by the absence of CyPD. This mix of events developing over hours to days is not modeled by the short-term studies with the isolated tubules that are limited to the initial events in the process.
The MPT contributes importantly to the immediate reperfusion injury that occurs after cardiac ischemia (2, 35), and amelioration of reperfusion injury by CsA in humans has been reported (53). Use of CsA to prevent MPT-mediated acute kidney injury is complicated by the potential for hypoxia from CsA-induced vasoconstriction, which could oppose any primary benefit of blocking the MPT at the tubule cell level. It has been reported to be protective in clamp I/R models in the kidney (12, 59, 70), but these studies involved pretreatment, so those effects could have been secondary to preconditioning from the pretreatment and were interpreted in that context.
In summary, regulation and susceptibility to the MPT can be readily studied in freshly isolated mouse proximal tubules by following energization, movements of Ca2+ added to induce the MPT, and changes in matrix volume. Normoxic tubules lacking CyPD show resistance to the MPT that is most prominent when the endogenous MPT antagonists ADP and Mg2+ are absent or reduced. The sustained energetic deficit induced by H/R in isolated tubules and mediated by accumulated NEFA is not modified by the absence of CyPD. Resistance to the MPT after H/R is decreased, particularly when energization is supported by complex I-dependent substrates and, in these mouse tubules, is not substantially ameliorated by removal of fatty acids during induction of the MPT but is dramatically improved by maneuvers that reverse the energetic deficit during reoxygenation. The effect of CyPD deletion to increase resistance to the MPT is maintained after H/R. CyPD deletion confers resistance to I/R in vivo over a wide range of insult severity, indicating that conditions develop during in vivo injury that allow it to play a pathogenic role. The data clarify involvement of the MPT in oxygen deprivation-induced tubule cell injury by showing that the MPT does not contribute to the initial bioenergetic deficit produced by H/R, but the deficit predisposes to subsequent development of the MPT, which contributes pathogenically to kidney I/R injury in vivo. The work also provides information about baseline behavior and generally useful paradigms for assessing the impact of other bioenergetic alterations in genetically modified mouse tubules.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-34275 (J. M. Weinberg), the Research Service of the Department of Veterans Affairs (J. M. Weinberg), and German Research Foundation Grant Fe 929 (T. Feldkamp).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to Drs. Michael Forte and Paolo Bernardi, who graciously provided the Ppif−/− mice and reviewed the manuscript, and to Drs. Zheng Dong and Qingqing Wei for guidance in developing the in vivo I/R model. We thank Tiffany Ostrowski, Sheila Madipelli, Srinivas Gullapalli, and Ruth Senter for assistance with several aspects of this work.
Some of the studies were reported in abstract form (J Am Soc Nephrol 19: 174A, 2008).
REFERENCES
- 1. Arnold PE, VanPutten VJ, Lumlertgul D, Burke TJ, Schrier RW. Adenine nucleotide metabolism and mitochondrial Ca2+ transport following renal ischemia. Am J Physiol Renal Fluid Electrolyte Physiol 250:F357–F363, 1986. [DOI] [PubMed] [Google Scholar]
- 2. Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434:658–662, 2005. [DOI] [PubMed] [Google Scholar]
- 3. Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem 280:18558–18561, 2005. [DOI] [PubMed] [Google Scholar]
- 4. Basso E, Petronilli V, Forte MA, Bernardi P. Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation. J Biol Chem 283:26307–26311, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernardi P, Rasola A. Calcium and cell death: the mitochondrial connection. Subcell Biochem 45:481–506, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Broekemeier KM, Dempsey ME, Pfeiffer DR. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J Biol Chem 264:7826–7830, 1989. [PubMed] [Google Scholar]
- 8. Broekemeier KM, Klocek CK, Pfeiffer DR. Proton selective substate of the mitochondrial permeability transition pore: regulation by the redox state of the electron transport chain. Biochemistry 37:13059–13065, 1998. [DOI] [PubMed] [Google Scholar]
- 9. Brustovetsky N, Dubinsky JM. Limitations of cyclosporin A inhibition of the permeability transition in CNS mitochondria. J Neurosci 20:8229–8237, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carle BN. Autofluorescence in the identification of myocardial infarcts. Hum Pathol 12:643–646, 1981. [DOI] [PubMed] [Google Scholar]
- 11. Cereghetti GM, Stangherlin A, Martins de BO, Chang CR, Blackstone C, Bernardi P, Scorrano L. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA 105:15803–15808, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cologna AJ, Lima LVD, Tucci S, Suaid HJ, Reis RB, Tirapelli LF, Antunes A, Martins ACP. Cyclosporine action on kidneys of rats submitted to normothermic ischaemia and reperfusion. Acta Cir Bras 23:36–41, 2008. [DOI] [PubMed] [Google Scholar]
- 13. Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J 255:357–360, 1988. [PMC free article] [PubMed] [Google Scholar]
- 14. Devalaraja-Narashimha K, Diener AM, Padanilam BJ. Cyclophilin D gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol 297:F749–F759, 2009. [DOI] [PubMed] [Google Scholar]
- 15. Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of mycoytes in postischemic reperfusion of the heart. J Biol Chem 276:2571–2574, 2001. [DOI] [PubMed] [Google Scholar]
- 16. Dong Z, Patel Y, Saikumar P, Weinberg JM, Venkatachalam MA. Development of porous defects in plasma membranes of ATP-depleted Madin-Darby canine kidney cells and its inhibition by glycine. Lab Invest 78:657–668, 1998. [PubMed] [Google Scholar]
- 17. Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan YL, Wang CY, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Du Yan S. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med 14:1097–1105, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eliseev RA, Malecki J, Lester T, Zhang Y, Humphrey J, Gunter TE. Cyclophilin D interacts with Bcl2 and exerts an anti-apoptotic effect. J Biol Chem 284:9692–9699, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feldkamp T, Kribben A, Roeser NF, Ostrowski T, Weinberg JM. Alleviation of fatty acid- and hypoxia-reoxygenation-induced proximal tubule deenergization by ADP/ATP carrier inhibition and glutamate. Am J Physiol Renal Physiol 292:F1606–F1616, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Feldkamp T, Kribben A, Roeser NF, Senter RA, Kemner S, Venkatachalam MA, Nissim I, Weinberg JM. Preservation of complex I function during hypoxia-reoxygenation-induced mitochondrial injury in proximal tubules. Am J Physiol Renal Physiol 286:F749–F759, 2004. [DOI] [PubMed] [Google Scholar]
- 21. Feldkamp T, Kribben A, Roeser NF, Senter RA, Weinberg JM. Accumulation of nonesterified fatty acids causes the sustained energetic deficit in kidney proximal tubules after hypoxia-reoxygenation. Am J Physiol Renal Physiol 290:F465–F477, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Feldkamp T, Kribben A, Weinberg JM. F1F0-ATPase activity and ATP dependence of mitochondrial energization in proximal tubules after hypoxia/reoxygenation. J Am Soc Nephrol 16:1742–1751, 2005. [DOI] [PubMed] [Google Scholar]
- 23. Feldkamp T, Kribben A, Weinberg JM. Assessment of mitochondrial membrane potential in proximal tubules after hypoxia/reoxygenation. Am J Physiol Renal Physiol 288:F1092–F1102, 2005. [DOI] [PubMed] [Google Scholar]
- 24. Feldkamp T, Park JS, Pasupulati R, Amora D, Roeser NF, Venkatachalam MA, Weinberg JM. Regulation of the mitochondrial permeability transition in kidney proximal tubules and its alteration during hypoxia-reoxygenation. Am J Physiol Renal Physiol 297:F1632–F1646, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feldkamp T, Weinberg JM, Horbelt M, Von KC, Witzke O, Nurnberger J, Kribben A. Evidence for involvement of nonesterified fatty acid-induced protonophoric uncoupling during mitochondrial dysfunction caused by hypoxia and reoxygenation. Nephrol Dial Transplant 24:43–51, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fontaine E, Eriksson O, Ichas F, Bernardi P. Regulation of the permeability transition pore in skeletal muscle mitochondria. Modulation by electron flow through the respiratory chain complex I. J Biol Chem 273:12662–12668, 1998. [DOI] [PubMed] [Google Scholar]
- 27. Forte M, Gold BG, Marracci G, Chaudhary P, Basso E, Johnsen D, Yu XL, Fowlkes J, Bernardi P, Bourdette D. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc Natl Acad Sci USA 104:7558–7563, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fournier N, Ducet G, Crevat A. Action of cyclosporine on mitochondrial calcium fluxes. J Bioenerg Biomembr 19:297–303, 1987. [DOI] [PubMed] [Google Scholar]
- 29. Fujimoto K, Chen Y, Polonsky KS, Dorn GW. Targeting cyclophilin D and the mitochondrial permeability transition enhances beta-cell survival and prevents diabetes in Pdx1 deficiency. Proc Natl Acad Sci USA 107:10214–10219, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ghosh JC, Siegelin MD, Dohi T, Altieri DC. Heat shock protein 60 regulation of the mitochondrial permeability transition pore in tumor cells. Cancer Res 70:8988–8993, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giorgio V, Bisetto E, Soriano ME, Dabbeni-Sala F, Basso E, Petronilli V, Forte MA, Bernardi P, Lippe G. Cyclophilin D modulates mitochondrial F0F1-ATP synthase by interacting with the lateral stalk of the complex. J Biol Chem 284:33982–33988, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giorgio V, Soriano ME, Basso E, Bisetto E, Lippe G, Forte MA, Bernardi P. Cyclophilin D in mitochondrial pathophysiology. Biochim Biophys Acta 1797:1113–1118, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol Cell Physiol 258:C755–C786, 1990. [DOI] [PubMed] [Google Scholar]
- 34. Hackenbrock CR, Rehn TG, Weinbach EC, Lemasters JJ. Oxidative phosphorylation and ultrastructural transformation in mitochondria in the intact ascites tumor cell. J Cell Biol 51:123–137, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Halestrap AP. A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans 38:841–860, 2010. [DOI] [PubMed] [Google Scholar]
- 36. Halestrap AP, Davidson AM. Inhibition of Ca2+-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J 268:153–160, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett 512:1–7, 2002. [DOI] [PubMed] [Google Scholar]
- 38. Hewavitharana AK, Bruce HL. Simultaneous determination of creatinine and pseudouridine concentrations in bovine plasma by reversed-phase liquid chromatography with photodiode array detection. J Chromatogr B Analyt Technol Biomed Life Sci 784:275–281, 2003. [DOI] [PubMed] [Google Scholar]
- 39. Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch Biochem Biophys 195:468–477, 1979. [DOI] [PubMed] [Google Scholar]
- 40. Koshkin V, Dai FF, Robson-Doucette CA, Chan CB, Wheeler MB. Limited mitochondrial permeabilization is an early manifestation of palmitate-induced lipotoxicity in pancreatic beta-cells. J Biol Chem 283:7936–7948, 2008. [DOI] [PubMed] [Google Scholar]
- 41. Lambert AJ, Brand MD. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J 382:511–517, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta 1366:177–196, 1998. [DOI] [PubMed] [Google Scholar]
- 43. Leung AW, Varanyuwatana P, Halestrap AP. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J Biol Chem 283:26312–26323, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liang HL, Hilton G, Mortensen J, Regner K, Johnson CP, Nilakantan V. MnTMPyP, a cell-permeant SOD mimetic, reduces oxidative stress and apoptosis following renal ischemia-reperfusion. Am J Physiol Renal Physiol 296:F266–F276, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Martin LJ, Gertz B, Pan Y, Price AC, Molkentin JD, Chang Q. The mitochondrial permeability transition pore in motor neurons: involvement in the pathobiology of ALS mice. Exp Neurol 218:333–346, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mokhova EN, Khailova LS. Involvement of mitochondrial inner membrane anion carriers in the uncoupling effect of fatty acids. Biochemistry (Mosc) 70:159–163, 2005. [DOI] [PubMed] [Google Scholar]
- 47. Muller FL, Liu YH, bdul-Ghani MA, Lustgarten MS, Bhattacharya A, Jang YC, Van Remmen H. High rates of superoxide production in skeletal-muscle mitochondria respiring on both complex I- and complex II-linked substrates. Biochem J 409:491–499, 2008. [DOI] [PubMed] [Google Scholar]
- 48. Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434:652–658, 2005. [DOI] [PubMed] [Google Scholar]
- 49. Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, a cyclosporin A-sensitive channel. J Biol Chem 271:2185–2192, 1996. [DOI] [PubMed] [Google Scholar]
- 50. Nishimura Y, Lemasters JJ. Glycine blocks opening of a death channel in cultured hepatic sinusoidal endothelial cells during chemical hypoxia. Cell Death Differ 8:850–858, 2001. [DOI] [PubMed] [Google Scholar]
- 51. Novgorodov SA, Gudz TI, Brierley GP, Pfeiffer DR. Magnesium ion modulates the sensitivity of the mitochondrial permeability transition pore to cyclosporin A and ADP. Arch Biochem Biophys 311:219–228, 1994. [DOI] [PubMed] [Google Scholar]
- 52. Palma E, Tiepolo T, Angelin A, Sabatelli P, Maraldi NM, Basso E, Forte MA, Bernardi P, Bonaldo P. Genetic ablation of cyclophilin D rescues mitochondrial defects and prevents muscle apoptosis in collagen VI myopathic mice. Hum Mol Genet 18:2024–2031, 2009. [DOI] [PubMed] [Google Scholar]
- 53. Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med 359:473–481, 2008. [DOI] [PubMed] [Google Scholar]
- 54. Plotnikov EY, Kazachenko AV, Vyssokikh MY, Vasileva AK, Tcvirkun DV, Isaev NK, Kirpatovsky VI, Zorov DB. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int 72:1493–1502, 2007. [DOI] [PubMed] [Google Scholar]
- 55. Qian T, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial permeability transition in pH-dependent reperfusion injury to hepatocytes. Am J Physiol Cell Physiol 273:C1783–C1792, 1997. [DOI] [PubMed] [Google Scholar]
- 56. Rasola A, Sciacovelli M, Pantic B, Bernardi P. Signal transduction to the permeability transition pore. FEBS Lett 584:1989–1996, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA 102:12005–12010, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schwenke WD, Soboll S, Seitz HJ, Sies H. Mitochondrial and cytosolic ATP/ADP ratios in rat liver in vivo. Biochem J 200:405–408, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Singh D, Chander V, Chopra K. Cyclosporine protects against ischemia/reperfusion injury in rat kidneys. Toxicology 207:339–347, 2005. [DOI] [PubMed] [Google Scholar]
- 60. Vandenabeele P, Galluzzi L, Vanden BT, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11:700–714, 2010. [DOI] [PubMed] [Google Scholar]
- 61. Wang ZY, Havasi A, Gall J, Bonegio R, Li ZJ, Mao HP, Schwartz JH, Borkan SC. GSK3β promotes apoptosis after renal ischemic injury. J Am Soc Nephrol 21:284–294, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Weinberg JM. Calcium as a mediator of renal tubule cell injury. Semin Nephrol 4:179–191, 1984. [Google Scholar]
- 63. Weinberg JM, Davis JA, Abarzua M, Kiani T. Relationship between cell ATP and glutathione content and protection by glycine against hypoxic proximal tubule cell injury. J Lab Clin Med 113:612–623, 1989. [PubMed] [Google Scholar]
- 64. Weinberg JM, Humes HD. Calcium transport and inner mitochondrial membrane damage in renal cortical mitochondria. Am J Physiol Renal Fluid Electrolyte Physiol 248:F876–F889, 1985. [DOI] [PubMed] [Google Scholar]
- 65. Weinberg JM, Nissim I, Roeser NF, Davis JA, Schultz S, Nissim I. Relationships between intracellular amino acid levels and protection against injury to isolated proximal tubules. Am J Physiol Renal Fluid Electrolyte Physiol 260:F410–F419, 1991. [DOI] [PubMed] [Google Scholar]
- 66. Weinberg JM, Roeser NF, Davis JA, Venkatachalam MA. Glycine-protected, hypoxic, proximal tubules develop severely compromised energetic function. Kidney Int 52:140–151, 1997. [DOI] [PubMed] [Google Scholar]
- 67. Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci USA 97:2826–2831, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Weinberg JM, Venkatachalam MA, Roeser NF, Saikumar P, Dong Z, Senter RA, Nissim I. Anaerobic and aerobic pathways for salvage of proximal tubules from hypoxia-induced mitochondrial injury. Am J Physiol Renal Physiol 279:F927–F943, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Woodfield KY, Price NT, Halestrap AP. cDNA cloning of rat mitochondrial cyclophilin. Biochim Biophys Acta 1351:27–30, 1997. [DOI] [PubMed] [Google Scholar]
- 70. Zhu T, Au-Yeung KK, Siow YL, Wang G, O K. Cyclosporine A protects against apoptosis in ischaemic/reperfused rat kidneys. Clin Exp Pharmacol Physiol 29:852–854, 2002. [DOI] [PubMed] [Google Scholar]
- 71. Zoratti M, Szabo D, De Marchi U. Mitochondrial permeability transitions: how many doors to the house? Biochim Biophys Acta 1706:40–52, 2005. [DOI] [PubMed] [Google Scholar]
- 72. Zorov DB, Juhaszova M, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc Res 83:213–225, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.