Abstract
Thrombospondin-1 (TSP1) is a multidomain protein that contains epidermal growth factor (EGF)-like repeats that indirectly activate the EGF receptor (EGFR) and selected downstream signaling pathways. In these studies, we show that TSP1 opens the paracellular pathway in human lung microvascular endothelial cells (HMVEC-Ls) in a dose-, time-, and protein tyrosine kinase (PTK)-dependent manner. TSP1 increased tyrosine phosphorylation of proteins enriched to intercellular boundaries including the zonula adherens (ZA) proteins, vascular endothelial-cadherin, γ-catenin, and p120 catenin. In HMVEC-Ls, EGFR and ErbB2 are expressed at low levels, and both heterodimerize and tyrosine autophosphorylate in response to TSP1. Prior EGFR-selective PTK inhibition with AG1478 or ErbB2-selective PTK inhibition with AG825 protected against TSP1-induced tyrosine phosphorylation of ZA proteins and barrier disruption. Preincubation of HMVEC-Ls with an EGFR ectodomain-blocking antibody also prevented TSP1-induced opening of the paracellular pathway. Therefore, in HMVEC-Ls, TSP1 increases tyrosine phosphorylation of ZA proteins and opens the paracellular pathway, in part, through EGFR/ErbB2 activation. Surprisingly, recombinant TSP1 EGF-like repeats 1–3 and the high-affinity EGFR ligands, EGF, TGF-α, and amphiregulin, each failed to increase paracellular permeability. However, HMVEC-Ls in which EGFR was overexpressed became responsive to the EGF-like repeats of TSP1 as well as to EGF. These studies indicate that TSP1 disrupts the endothelial barrier through EGFR/ErbB2 activation although additional signals are necessary in cells with low receptor expression.
Keywords: zonula adherens, vascular endothelial-cadherin, catenins, tyrosine phosphorylation, endothelial growth factor-like repeats
thrombospondin-1 (TSP1) is a multidomain glycoprotein (6) that recognizes multiple endothelial cell (EC) receptors that are coupled to diverse signaling pathways and biological responses (56, 61). TSP1 is secreted by numerous host tissues, including ECs, and is present in the underlying extracellular matrix (ECM) (5, 31). In the lung, TSP1 is also expressed by type II alveolar epithelial cells and fetal lung fibroblasts (31). TSP1 is not only expressed in tissues relevant and anatomically proximal to the vasculature and lung but is also present within the intravascular compartment as a circulating plasma protein (31) and in polymorphonuclear neutrophils, monocytes, and the α-granules of platelets (25, 30, 31, 35). These cells continuously traffic through the microvasculature, where they intimately interact with the endothelial surface. Although ECs can both produce (45) and respond (16) to TSP1, whether TSP1 is presented to the pulmonary microvascular endothelium in vivo through an endocrine, paracrine, and/or autocrine pathway is unknown. TSP1 expression is increased in vascular tissues in response to injurious stimuli in vitro (12, 55) and in the bronchoalveolar lavage fluids of patients with the acute respiratory distress syndrome (ARDS) (23). In an in vitro system that reflects changes in intercellular junctions and the paracellular pathway, we have demonstrated that exogenous TSP1 increases paracellular movement of macromolecules across postconfluent bovine pulmonary artery EC monolayers (16). At the same time, TSP1 increased tyrosine phosphorylation of proteins enriched to EC-EC boundaries and several of these substrates for tyrosine phosphorylation were identified as the zonula adherens (ZA) proteins, γ-catenin, and p120ctn.
The ZA is a specialized structure that couples the actin cytoskeleton to the cytoplasmic domain of cadherins, surface receptors that mediate homophilic cell-to-cell adhesion (33). Cadherins are a superfamily of single-chain glycoproteins comprised of an NH2-terminal extracellular domain that dictates homophilic adhesive specificity, a hydrophobic membrane-spanning domain, and a highly conserved COOH-terminal cytoplasmic domain essential to homophilic adhesion and cytoskeletal linkage. Although multiple cadherins can be coexpressed in ECs, vascular endothelial (VE)-cadherin appears to be unique to ECs and is localized to their intercellular junctions (33). At least three cytoplasmic proteins, collectively termed catenins, form multiprotein complexes that participate in anchoring the cytoplasmic domain of cadherins to actin microfilaments (33). These include β-catenin, γ-catenin, also known as plakoglobin, and p120 catenin. β-, γ- and p120 catenins each contain multiple 42-amino-acid (aa) repeats originally identified in the Drosophila segment polarity gene product, armadillo. These three proteins bind directly to cadherins. β- and γ-catenin appear to compete for the same binding site, whereas p120 catenin associates with cadherin at a more juxtamembranous location. β- and γ-catenin each, directly and/or indirectly, couple the cadherin-catenin complex to the actin cytoskeleton. Increased tyrosine phosphorylation of ZA proteins can be coincident with their uncoupling from their binding partners, reduction of homophilic adhesion between opposing VE-cadherin ectodomains, and opening of the paracellular pathway (16, 32).
We previously reported that prior broad-spectrum protein tyrosine kinase (PTK) inhibition protects against TSP1-induced opening of the paracellular pathway and loss of barrier function (16). The operative PTK(s) had not been identified. Each TSP1 monomer contains three epidermal growth factor (EGF)-like repeats (6), each of which contains the six spatially conserved cysteine residues that form the three intramolecular disulfide bonds required to engage the EGF receptor (EGFR) (20). TSP1 increases ZA protein tyrosine phosphorylation (16), reorganizes the actin cytoskeleton (1), and enhances cell motility (59), all activities that can occur downstream of EGFR activation (11, 22, 40, 54, 63). In fact, we recently reported that the EGF-like repeats of TSP1 activate EGFR in human A431 epidermoid carcinoma cells (37).
The four members of the ErbB receptor PTK family each contain an NH2-terminal ligand-binding ectodomain coupled to an intracellular catalytic domain and its tyrosine phosphorylation sites (47, 65). Ligand binding to the ectodomain of EGFR (also referred to as ErbB1 or HER1), ErbB3, or ErbB4, induces receptor homodimerization and heterodimerization with other ErbB family members, intrinsic kinase activity, and autotransphosphorylation of specific tyrosine residues, which, in turn, serve as a docking site within the cytoplasmic domain for signaling molecules (47). ErbB2, an orphan receptor that does not directly recognize any known ligand, responds only through heterodimerization with other ErbB receptors (47, 65). In the hierarchy of ErbB receptor-receptor interactions, ErbB2 is the preferred heterodimerization partner for the other ErbB proteins (19) and in general potentiates ErbB signaling (19, 47, 65). High-affinity EGFR ligands share a 45–55-aa EGF motif with six spatially conserved cysteine residues that form three intramolecular disulfide bonds that dictate their tertiary conformation (20, 47, 65). These ligands are synthesized as transmembrane precursor proteins that are proteolytically cleaved to release mature growth factors for autocrine/paracrine stimulation. In addition to these “authentic” ErbB ligands, EGF-like sequences are present in many other proteins (3, 14, 24, 26), including TSP1 (37). EGFR and the other ErbB family members are known to participate in host-cell embryogenesis and development, proliferation, differentiation, wound healing, and malignant transformation.
In the present studies, we have defined ErbB receptor expression in human lung microvascular endothelial cells (HMVEC-Ls) and established that TSP1 activates one or more of these ErbB receptors to increase tyrosine phosphorylation of ZA proteins and regulates the paracellular pathway. These studies identify a novel mechanism through which TSP1 regulates endothelial barrier integrity.
MATERIALS AND METHODS
Preparation of human TSP1 and recombinant TSP1 EGF-like repeats.
TSP1 was purified from pooled outdated human platelets purchased from the American Red Cross as described (16). Purity was assessed by PAGE in SDS. Baculovirus-expressed recombinant human TSP1 EGF-like repeats 1–3 (E123) (aa 549–691 numbered from the initiating methionine of the full-length subunit) were purified after secretion as described (37).
Cell culture.
HMVEC-Ls (Lonza Walkersville, Walkersville, MD) were cultured in EC growth medium (Lonza) containing 5% fetal bovine serum (FBS) (Hyclone Laboratories, Logan, UT), as described (18). HMVEC-Ls were studied in passages 2 to 7. Human epidermoid carcinoma A431 cells and human lung A549 alveolar type II cells derived from a lung adenocarcinoma (American Type Culture Collection, Manassas, VA) were cultured in DMEM (ATCC) enriched with 10% FBS, as described (37).
Assay for endothelial barrier function.
Transendothelial 14C-BSA flux was used as a measure of endothelial paracellular permeability as described (18). Briefly, gelatin-impregnated polycarbonate filters (Nucleopore, Pleasanton, CA) mounted in chemotactic chambers (ADAPS, Dedham, MA) were inserted into wells of 24-well plates. HMVEC-Ls were cultured to confluence in each upper compartment. The baseline barrier function of each monolayer was established by applying 14C-BSA to each upper compartment for 1 h, after which the lower compartment was counted for 14C activity. Only monolayers retaining ≥97% of the 14C-BSA were studied. The monolayers were then exposed for 6 h to increasing concentrations of TSP1, EGF, transforming growth factor (TGF)-α, and amphiregulin (Sigma Chemical, St. Louis, MO) or recombinant TSP1 EGF-like repeats (E123). On the basis of the established dose-response relationship, other monolayers were exposed to a fixed TSP1 concentration (30 μg/ml or 214 nM) or medium alone for increasing exposure times. Transfer of 14C-BSA across EC monolayers was again assayed and expressed in picomoles/hour. In selected experiments, EC monolayers were pretreated for 2 h with the broad-spectrum PTK inhibitor, erbstatin (5 μM) (Calbiochem, San Diego, CA), the EGFR-selective tyrphostin, AG1478 (5 μM) (Calbiochem) (37, 64), the ErbB2-selective tyrphostin, AG825 (5 μM) (Calbiochem) (64), the murine anti-human EGFR ectodomain blocking antibody, GR13L (Calbiochem) (15), or either of two species- and isotype-matched irrelevant antibody controls, murine anti-human TNF-α antibody (R&D Systems, Minneapolis, MN) and murine anti-human B7–1/CD80 antibody (R&D Systems) or medium alone.
F-actin and phosphotyrosine fluorescence microscopy.
Postconfluent HMVEC-Ls cultured to postconfluence on glass coverslips were exposed for 6 h to TSP1 (30 μg/ml, i.e., 214 nM) or medium alone, washed, fixed (formaldehyde 3.7%, 20 min), and permeabilized (Triton X-100 0.5% in HEPES buffer, 5 min, 4°C). To detect intercellular gaps, monolayers were stained for 20 min with the F-actin probe, rhodamine-phalloidin (1.65 × 10−7 M) (Molecular Probes, Eugene, OR), as described (16). To immunolocalize substrates for TSP1-induced tyrosine phosphorylation to one or more subcellular compartments, monolayers were incubated with FITC-conjugated murine anti-phosphotyrosine 4G10 IgG (Upstate Biotech, Lake Placid, NY) as described (18). The phalloidin-stained and immunostained monolayers were photographed through a Nikon Eclipse TE-2000-E fluorescence microscope.
Phosphotyrosine immunoblotting of EC and ZA proteins.
Postconfluent HMVEC-L monolayers were exposed for 1 h to TSP1 (30 μg/ml, i.e., 214 nM) or medium alone in the presence of sodium orthovanadate (vanadate) (200 μM) and phenylarsine oxide (PAO) (1.0 μM), only during the last 0.25 h of incubation, after which they were solubilized with ice-cold lysis buffer as described (18). The samples were resolved by electrophoresis on an 8–16% gradient SDS-polyacrylamide gel (Invitrogen, Carlsbad, CA) and were transferred onto PVDF membranes (Millipore, Bedford, MA). Each blot was blocked with 1% BSA in PBS-Tween 20, incubated with horseradish peroxidase (HRP)-conjugated, antiphosphotyrosine antibodies (0.25 μg/ml) (PY-plus; Zymed, Carlsbad, CA), and developed with enhanced chemiluminescence (ECL). To confirm equivalent protein loading and transfer, blots were stripped and reprobed with anti-β-tubulin antibody followed by HRP-conjugated anti-mouse IgG and developed with ECL.
In other experiments, an immunoprecipitation strategy was employed to identify ZA substrates for tyrosine phosphorylation as described (16, 18, 57). HMVEC-L monolayers were exposed for 10 min, 1 h, 2 h, and 6 h to TSP1 (30 μg/ml, i.e., 214 nM) or medium alone and lysed. The lysates were precleared by incubation with either anti-murine or anti-goat IgG cross-linked to agarose (Sigma) for 1 h at 4°C and then incubated overnight at 4°C with specific murine monoclonal antibodies raised against β-, γ-, or p120-catenin (Transduction Laboratories, Lexington, KY), or a goat polyclonal antibody raised against VE-cadherin (Santa Cruz Biotechnology, Santa Cruz, CA). The resultant immune complexes were immobilized by incubation with IgG cross-linked to agarose for 2 h at 4°C, centrifuged, washed, boiled for 5 min in sample buffer, and again centrifuged. The supernates were then processed for phosphotyrosine immunoblotting as described above (16, 18, 57). To control for discrepancies in immunoprecipitation efficiency and protein loading and transfer, the blots were stripped and reprobed with the immunoprecipitating antibody. The blots were subsequently incubated with HRP-conjugated anti-mouse IgG (Transduction Laboratories) or HRP-conjugated anti-goat IgG (Santa Cruz) and developed with ECL. Blots were scanned by laser densitometry, and the phosphotyrosine-containing bands were normalized to the immunoprecipitated protein of interest.
ErbB mRNA and protein expression in endothelia.
Total RNA was isolated from postconfluent HMVEC-Ls, using the Absolutely RNA Miniprep Kit (Stratagene, La Jolla, CA) as described (18, 37). Complementary DNA was generated from total RNA by annealing it to poly (dT) primer at 65°C and then reversibly transcribing it with Superscript III reverse transcriptase (Invitrogen) (20 μl, 50°C, 45 min). This cDNA was used as a template for amplification with Ex Tag DNA polymerase (5U) (TakaRa) and sense and antisense primers for human EGFR, ErbB2, ErbB3, and ErbB4 in a PCR reaction that cycled with annealing temperatures at 58°C and extension at 72°C × 35 s. These sense and antisense primers included: for EGFR, TGATGGCTAGTGTGGACAACC and CATGATATTCTTTCTCTTCAGCA; for ErbB2, GGCTGCTGGACATTGACGAG and GGGGCTGGGGCAGCCGCTC; for ErbB3, GGAGTACAAATTGCCAAGGGTA and CAGGTCTGGCAAGTATGGAT; and for ErbB4, CTCTGATCATGGCAAGTATGGAT and CATTGTATTCTTTTTCATCTCCTTC. The anticipated sizes of PCR products were 318 bp for EGFR, 231 bp for ErbB2, 327 bp for ErbB3, 324 bp for ErbB4, and 452 bp for GAPDH, a housekeeping gene control. Postconfluent HMVEC-Ls were lysed, and the lysates were processed for immunoblotting with murine anti-EGFR, anti-ErbB2, anti-ErbB3, or anti-ErbB4 antibodies (BD Biosciences Pharmingen, San Diego, CA) followed by HRP-conjugated anti-mouse IgG (Transduction Laboratories) and developed with ECL. In selected experiments, to enrich for EGFR and ErbB2, HMVEC-L lysates (up to 1 mg) were immunoprecipitated with either murine anti-EGFR or murine anti-ErbB2 antibodies (2.5 μg antibody/500 μg of lysate) (BD Biosciences Pharmingen). The blots of EGFR immunoprecipitates were probed with anti-EGFR antibody, and the blots of ErbB2 immunoprecipitates were probed with anti-ErbB2 antibodies.
EGFR/ErbB2 heterodimerization and activation.
To determine whether TSP1 induces EGFR/ErbB2 heterodimerization in HMVEC-Ls, reciprocal coimmunoprecipitation assays were performed as described (57). HMVEC-Ls were solubilized with a low-stringency lysis buffer containing 20 mmol/l Tris, pH 7.5, 2 mmol/l CaCl2, 1% Triton X-100, 5 mg/ml leupeptin, 5 mg/ml aprotinin, 1 mmol/l benzamidine, 200 μmol/l PAO, 1 mmol/l vanadate, and 0.1 mmol/l molybdate. The EC lysates were immunoprecipitated with either murine anti-EGFR or anti-ErbB2 antibodies and the immunoprecipitates processed for immunoblotting. Here, the blots of EGFR immunoprecipitates were probed with anti-ErbB2 antibody, and the blots of ErbB2 immunoprecipitates were probed with anti-EGFR antibodies. The blots were subsequently incubated with HRP-conjugated anti-mouse IgG (Transduction Laboratories) and developed with ECL. To detect EGFR/ErbB2 activation in HMVEC-Ls, cells were exposed for 1 h to TSP1 30 μg/ml or medium alone and lysed, and the lysates were immunoprecipitated with either anti-EGFR or anti-ErbB2 antibodies or an equivalent concentration of the irrelevant antibody control. The immunoprecipitates were processed for phosphotyrosine immunoblotting as described (18, 57).
EGFR overexpression in HMVEC-Ls.
Adenovirus (Ad) encoding for human EGFR (Ad-EGFR) or green fluorescent protein (GFP) (Ad-GFP) (4) were packaged and amplified in human embryonic kidney 293 cells. HMVEC-Ls cultured to confluence in 60-mm dishes were incubated with packaged Ad-EGFR or Ad-GFP at 105-108 plaque forming units per dish, i.e., at approximate multiplicity of infections (MOIs) of 1, 10, 50, 100, 150, 200, and 500, respectively. At 24 h, the cells were lysed and the lysates processed for immunoblotting for EGFR. The blots were stripped and first reprobed for phospho-EGFR (Y1068), as an indicator of activation, followed by β-tubulin to indicate protein loading and transfer. For barrier assays, postconfluent HMVEC-Ls cultured in barrier assay chambers were infected with increasing MOIs of Ad-EGFR or Ad-GFP, after which baseline barrier function was established. The infected monolayers were exposed for 6 h to EGF (100 ng/ml) or increasing concentrations of recombinant TSP1 EGF-like repeats, or medium alone, after which barrier function was assayed.
Statistical methods.
Analysis of variance was used to compare the mean responses among experimental and control groups for all experiments. The Dunnett and Scheffé F-tests were used to determine between which group's significant differences existed. A P value of <0.05 was considered significant.
RESULTS
TSP1 induces dose-, time- and PTK-dependent increases in transendothelial 14C-BSA flux.
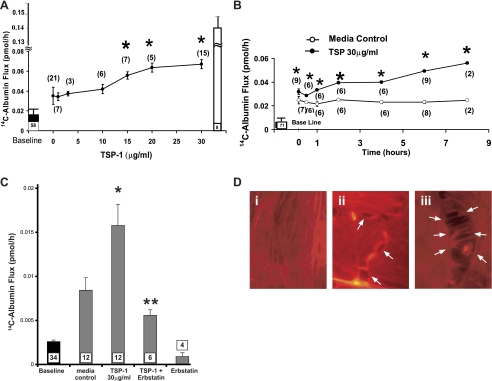
TSP1 increased 14C-BSA flux across HMVEC-L monolayers in a concentration-dependent manner (Fig. 1A). The mean (± SE) pretreatment transendothelial 14C-BSA flux was 0.013 ± 0.006 pmol/h (n = 58), and the mean (± SE) 14C-BSA transfer across naked filters without EC monolayers was 13.54 ± 1.01 pmol/h (n = 8). The lowest TSP1 concentration that increased 14C-BSA flux compared with the medium control was 15 μg/ml or 0.107 μM. The maximum mean (± SE) 14C-BSA flux of 0.067 ± 0.005 pmol/h was seen with TSP1 30 μg/ml (214 nM), at which point the TSP1-induced effect had begun to plateau or saturate (Fig. 1A). Transendothelial 14C-BSA flux was assayed after increasing exposure times to TSP1 (30 μg/ml) or medium alone, from 10 min to 8 h. Here, the mean (± SE) pretreatment barrier function was 0.005 ± 0.001 pmol/h (n = 71). 14C-BSA flux across medium control monolayers did not change throughout the 8-h study period. TSP1 (30 μg/ml) increased 14C-BSA flux compared with the simultaneous medium controls at ≥10 min with further time-dependent increments at 2–8 h (Fig. 1B). Prior broad-spectrum PTK inhibition with erbstatin (5 μM) completely protected against the TSP1-induced increase in 14C-albumin flux (Fig. 1C). Finally, TSP1, at the same concentration and after the same exposure times that increase transendothelial 14C-BSA flux (Fig. 1, A and B), induced intercellular gaps in postconfluent monolayers (Fig. 1D, ii and iii). Therefore, TSP1 disrupted barrier integrity in a dose-, time-, and PTK-dependent manner coincident with opening of the paracellular pathway.
Fig. 1.
Dose-, time-, and protein tyrosine kinase (PTK)-dependent effects of thrombospondin-1 (TSP1) on transendothelial 14C-albumin flux. Postconfluent human lung microvascular endothelial cells (HMVEC-L) monolayers were exposed to increasing concentrations of TSP1 or medium alone for increasing exposure times. A: ●, mean (±SE) 14C-BSA flux in pmol/h immediately after 6-h exposures to increasing concentrations of TSP1. B: circles represent mean (±SE) 14C-BSA flux in pmol/h immediately after increasing exposure times to TSP1 (30 μg/ml, i.e., 214 nM) (●) or medium alone (○). C: vertical bars represent mean (±SE) transendothelial 14C-BSA flux in pmol/h immediately after 6-h exposures to medium alone, TSP1 (30 μg/ml, i.e., 214 nM), TSP1 + erbstatin (5 μM), or erbstatin alone. In A, B, and C, mean (±SE) pretreatment baseline transendothelial 14C-BSA flux is shown by the closed bars and flux across naked filters by the open bar (A); n indicates number of monolayers studied and is indicated next to each symbol (A + B) or within each bar (C). *Significantly increased compared with the simultaneous medium control at P < 0.05. **Significantly decreased compared with TSP1 alone at P < 0.05. D: postconfluent HMVEC-L monolayers were exposed for 6 h to TSP1 (30 μg/ml, i.e., 214 nM) (ii and iii) or medium alone (i), after which they were probed with the F-actin probe, rhodamine-phalloidin, and processed for fluorescence microscopy. Arrows indicate intercellular gaps in TSP1-exposed monolayers. Magnification = ×800.
Identification of substrates for TSP1-induced protein tyrosine phosphorylation.
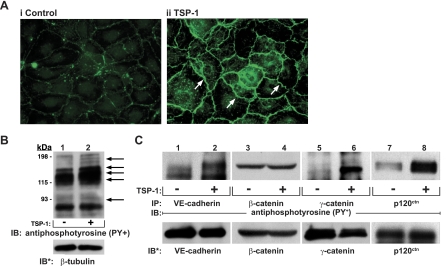
As a first step to determine which EC proteins might be tyrosine phosphorylated in response to TSP1, postconfluent HMVEC-L monolayers were exposed for 1 h to TSP1 (30 μg/ml) or medium alone and probed with FITC-conjugated anti-phosphotyrosine antibody for fluorescence microscopy (Fig. 2A). TSP1-exposed ECs displayed enhanced fluorescence signal almost exclusively restricted to intercellular boundaries compared with the medium control (see arrows in Fig. 2A, ii). These data suggest that TSP1 preferentially stimulates tyrosine phosphorylation of proteins that are either enriched to or upon phosphorylation translocate to cell-to-cell junctions in postconfluent EC monolayers. In other experiments, TSP1-treated and medium control HMVEC-Ls were lysed, and the were lysates processed for phosphotyrosine immunoblotting. TSP1 increased phosphotyrosine signal compared with the medium control (Fig. 2B). Phosphotyrosine-containing bands that migrated with apparent ∼Mr of 185,000, 170,000, 140,000–120,000, and 95,000 were revealed. We have identified EGFR as a substrate for TSP1-induced tyrosine phosphorylation in A431 cells (37). EGFR and possibly ErbB2 likely explain the ∼170-kDa and/or ∼185-kDa phosphotyrosine-containing bands (Fig. 2B).
Fig. 2.
Identification of substrates for TSP1-induced protein tyrosine phosphorylation. A: postconfluent HMVEC-L monolayers were exposed for 1 h to TSP1 (30 μg/ml, i.e., 214 nM) (ii) or medium alone (i), after which they were probed with FITC-conjugated antiphosphotyrosine antibodies and processed for fluorescence microscopy. Arrows indicate increased phosphotyrosine signal at intercellular boundaries. Magnification = ×400. In other experiments, HMVEC-Ls were incubated for 1 h with TSP1 (30 μg/ml, i.e., 214 nM) (+) or medium alone (-) in the presence of vanadate (200 μM) and phenylarsine oxide (PAO) (1.0 μM) only during the last 0.25 h of incubation. B: TSP1-exposed and medium control endothelial cells (ECs) were lysed, and total cell lysates were resolved by SDS-PAGE, transferred to PVDF, and the blots probed with antiphosphotyrosine antibody. To indicate protein loading and transfer, the blots were stripped and reprobed with anti-β-tubulin antibody. C: lysates of TSP1-exposed and medium control ECs were immunoprecipitated with antibodies raised against vascular endothelial (VE)-cadherin, β-catenin, γ-catenin, or p120ctn. The immunoprecipitates were resolved by SDS-PAGE and transferred to PVDF, and the blots were probed with antiphosphotyrosine antibody. For normalization of phosphotyrosine signal to loading of the immunoprecipitated protein, blots were stripped and reprobed with the immunoprecipitating antibodies. IP, immunoprecipitate; IB, immunoblots; IB*, immunoblots after stripping. Each blot is representative of 3 independent experiments.
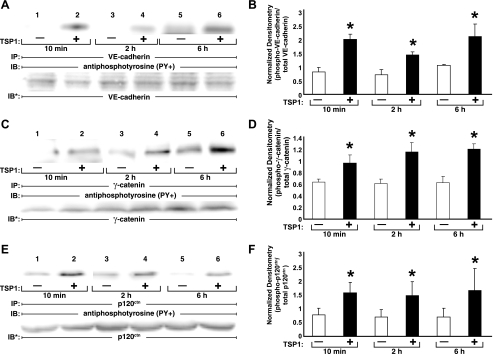
On the basis of subcellular localization and gel mobility, we adopted an immunoprecipitation immunoscreening strategy to determine whether ZA proteins are substrates for TSP1-induced tyrosine phosphorylation in HMVEC-Ls (Fig. 2C). TSP1 increased tyrosine phosphorylation of VE-cadherin (lanes 1 and 2), γ-catenin (lanes 5 and 6), and p120ctn (lanes 7 and 8), but not β-catenin (lanes 3 and 4). Whether one or more p120ctn isoforms were preferentially phosphorylated in response to the TSP1 stimulus was unclear. We then studied tyrosine phosphorylation of these three ZA proteins, whose phosphorylation increased in response to a 1-h TSP1 exposure, overtime (Fig. 3). TSP1 increased tyrosine phosphorylation of VE-cadherin (Fig. 3, A and B), γ-catenin (Fig. 3, C and D), and p120ctn (Fig. 3, E and F) each at 10 min, 2 h, and 6 h compared with their respective simultaneous medium controls. Therefore, tyrosine phosphorylation of each of these three ZA proteins, VE-cadherin, γ-catenin, and p120ctn, is detectible at ≤10 min following the TSP1 stimulus, temporarily proximal to or coincident with TSP1-induced barrier disruption (Fig. 1B).
Fig. 3.
Tyrosine phosphorylation of zonula adherens (ZA) proteins over time. HMVEC-Ls were incubated with TSP1 (30 μg/ml, i.e., 214 nM) or medium alone for 10 min, 2 h, or 6 h in the presence of vanadate (200 μM) and PAO (1.0 μM) only during the last 10 min of incubation. At each time point, ECs were lysed and the lysates immunoprecipitated with antibodies raised against VE-cadherin (A), γ-catenin (C), or p120ctn (E), and each immunoprecipitate was processed for phosphotyrosine immunoblotting. To control for protein immunoprecipitation, loading, and transfer, blots were stripped and reprobed with each immunoprecipitating antibody. Each blot is representative of 3 independent experiments. B, D, and F: densitometry for each ZA phosphotyrosine signal was normalized to the densitometry for the respective total ZA protein signal in the same lane in the same gel. B: normalized densitometry for VE-cadherin (n = 3). D: normalized densitometry for γ-catenin (n = 3). F: normalized densitometry for p120ctn (n = 3). *Significantly increased compared with the simultaneous medium control at P < 0.05.
ErbB expression in endothelia.
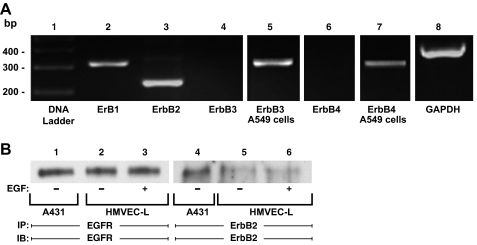
In HMVEC-Ls, we applied an RT-PCR approach to detect mRNAs for the four ErbB family members (Fig. 4A). In HMVEC-Ls, EGFR and ErbB2 mRNAs were detected (lanes 2 and 3), whereas mRNAs for ErbB3 and ErbB4 were not (lanes 4 and 6). A549 cells were used as a positive control for ErbB3 and ErbB4 mRNAs (lanes 5 and 7). We then used antibodies specific for EGFR and ErbB2 to probe for ErbB proteins. In total HMVEC-L lysates, neither EGFR or ErbB2 protein could be detected (data not shown). Only after prior enrichment through immunoprecipitation of large quantities of EC protein, could we detect EGFR (lanes 2 and 3) or ErbB2 (lanes 5 and 6) protein (Fig. 4B). A431 cells were used as positive controls (lanes 1 and 4). Prior treatment with EGF did not alter abundance of either protein (Fig. 4B, lanes 2 vs. 3 and lanes 5 vs. 6).
Fig. 4.
Epidermal growth factor receptor (EGFR) and ErbB2 expression in HMVEC-Ls. A: RNA was isolated from postconfluent HMVEC-Ls. Complementary DNA was generated from RNA using oligo (dT) primers and reverse transcriptase. This cDNA was used as a template for amplification with DNA polymerase and primers corresponding to EGFR or ErbB1 (lane 2), ErbB2 (lane 3), ErbB3 (lanes 4-5), and ErbB4 (lanes 6-7), as well as GAPDH as a housekeeping gene control (lane 8). The control DNA ladder is shown on the left (lane 1). A549 cells served as the positive controls for both ErbB3 (lane 5) and ErbB4 (lane 7). The DNA ladder, ErbB1, ErbB2, ErbB3, ErbB4 and GAPDH (lanes 1-4, 6, and 8) were run on the same gel, whereas the ErbB3 and ErbB4 positive controls (lanes 5 and 7) were run on separate gels. B: total cell lysates from EGF-exposed (lanes 3 and 6) and control (lanes 2 and 5) HMVEC-Ls were immunoprecipitated with either anti-EGFR or anti-ErbB2 antibodies. The immunoprecipitates were resolved by SDS-PAGE and transferred to PVDF, and each blot was probed with the immunoprecipitating antibody. The total cell lysates from A431 epithelial cells served as the positive controls for both EGFR and ErbB2 proteins (lanes 1 and 4).
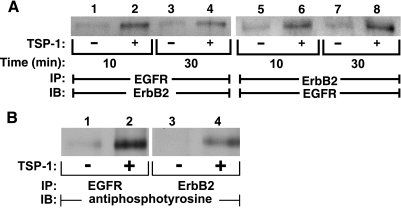
TSP1 activates EGFR and ErbB2.
Prior broad-spectrum PTK inhibition protects against TSP1-induced opening of the endothelial paracellular pathway (Fig. 1C) (16). TSP1 contains EGF-like repeats (37) and evokes multiple activities (1, 16, 59) that could be ascribed to EGFR signaling (11, 22, 40, 54, 63). In A431 cells, TSP1 activates EGFR (37). In HMVEC-Ls, after 10- and 30-min exposures to TSP1 (30 μg/ml), immunoprecipitation of EGFR coimmunoprecipitated ErbB2 (lanes 2 and 4) and immunoprecipitation of ErbB2 coimmunoprecipitated EGFR (lanes 6 and 8) (Fig. 5A). Coincident with EGFR/ErbB2 heterodimerization, TSP1 increased tyrosine phosphorylation of both EGFR (lane 2) and ErbB2 (lane 4) (Fig. 5B). In these studies, extremely low levels of ErbB protein expression precluded stripping and probing for total EGFR/ErbB2. These combined data indicate that TSP1 induces heterodimerization and activation of EGFR and ErbB2 in HMVEC-Ls.
Fig. 5.
TSP1 induces heterodimerization and activation of EGFR and ErbB2 in HMVEC-Ls. Postconfluent HMVEC-Ls were exposed for 10 or 30 min to TSP1 (30 μg/ml, i.e., 214 nM) (lanes 2, 4, 6, and 8) or medium alone (lanes 1, 3, 5, and 7). A: lysates from these ECs were immunoprecipitated with either anti-EGFR (lanes 1-4) or anti-erbB2 (lanes 5-8) antibodies. The immunoprecipitates were resolved by SDS-PAGE and transferred to PVDF, and the EGFR blots were incubated with anti-ErbB2 antibodies (lanes 1-4) and the ErbB2 blots incubated with anti-EGFR antibodies (lanes 5-8). B: lysates from HMVEC-Ls exposed for 1 h to TSP1 (30 μg/ml, i.e., 214 nM) (lanes 2 and 4) or media alone (lanes 1 and 3) were immunoprecipitated with either anti-EGFR (lanes 1-2) or anti-ErbB2 (lanes 3-4) antibodies. The immunoprecipitates were resolved by SDS-PAGE and transferred to PVDF, and the blots were probed with anti-phosphotyrosine antibodies. Each blot is representative of 3 independent experiments.
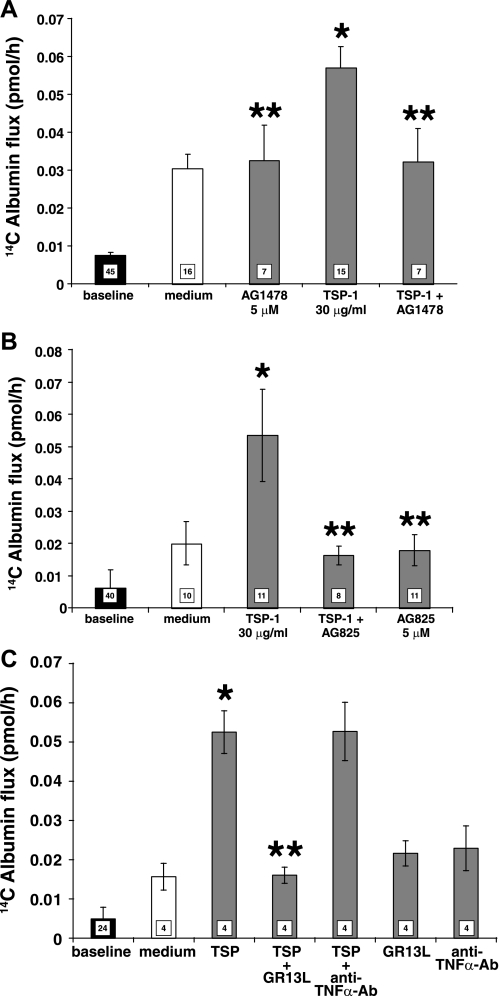
TSP1 opens the endothelial paracellular pathway through EGFR/ErbB2 activation.
TSP1 activates EGFR/ErbB2 (Fig. 5B) (37), and prior broad-spectrum PTK inhibition protects against TSP1-induced barrier disruption (Fig. 1C) (16). We asked whether EGFR and/or ErbB2 catalytic activities might be operative in this EC response to TSP1. Pretreatment of HMVEC-Ls with either the EGFR-selective (AG1478) (Fig. 6A) or ErbB2-selective (AG825) (Fig. 6B) inhibitor protected against opening of the paracellular pathway in response to TSP1. Preincubation of monolayers with the EGFR ectodomain-blocking antibody, GR13L, blocked the TSP1-induced increases in paracellular permeability, whereas the species- and isotype-matched antibody control had no such effect (Fig. 6C). These data indicate that accessibility to the ligand-binding portion of the EGFR ectodomain and EGFR and ErbB2 kinase activities are required for TSP1-induced barrier disruption.
Fig. 6.
TSP1 opens the endothelial paracellular pathway through EGFR/ErbB2 activation. HMVEC-Ls cultured to postconfluence in barrier assay chambers were exposed for 6 h to TSP1 (30 μg/ml, i.e., 214 nM) or medium alone in the presence or absence of the EGFR-selective tyrphostin, AG1478 (5 μM) (A), the ErbB2-selective tyrphostin, AG825 (5 μM) (B), or the GRI3L antibody that blocks the ligand-binding portion of the EGFR ectodomain or a species- and isotype-matched antibody control (C). Vertical bars represent mean (± SE) transendothelial 14C-BSA flux in pmol/h immediately after the 6-h study period. The mean (± SE) pretreatment baselines are shown by the closed bars; n, the number of monolayers studied, is indicated in each bar. *Significantly increased compared with the simultaneous media control at P < 0.05; **significantly decreased compared with TSP1 alone at P < 0.05.
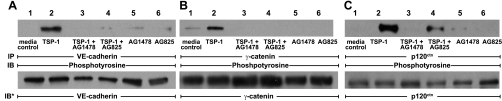
TSP1 increases ZA protein tyrosine phosphorylation through EGFR/ErbB2 activation.
TSP1 activates EGFR/ErbB2 (Fig. 5B) and increases tyrosine phosphorylation of the ZA proteins, VE-cadherin, γ-catenin, and p120ctn (Fig. 2C). We asked whether EGFR/ErbB2 kinase activity participates in ZA protein phosphorylation in response to TSP1. Prior EGFR-selective PTK inhibition with AG1478 or ErbB2-selective PTK inhibition with AG825 each markedly diminished tyrosine phosphorylation of all three of these ZA proteins (Fig. 7, A–C). Prior PTK inhibition with AG825 only blocked p120ctn phosphorylation by ∼70% (Fig. 7C). These results indicate that TSP1 increases tyrosine phosphorylation of these three ZA proteins through EGFR/ErbB2 activation.
Fig. 7.
TSP1 increases ZA protein tyrosine phosphorylation through EGFR/ErbB2 activation. HMVEC-Ls were incubated for 1 h with TSP1 (30 μg/ml, i.e., 214 nM) or medium alone, in the presence or absence of either AG1478 (5 μM) or AG825 (5 μM), as well as in the presence of vanadate (200 μM) and PAO (1.0 μM), only during the last 0.25 h of incubation. ECs were lysed and immunoprecipitated with antibodies raised against VE-cadherin (A), γ-catenin (B), or p120ctn (C). The immunoprecipitates were resolved by SDS-PAGE and transferred to PVDF, and the blots were probed with antiphosphotyrosine antibody. Blots were stripped and reprobed with the immunoprecipitating antibodies for normalization of phosphotyrosine signal to the loaded immunoprecipitated protein. These blots are representative of 2 independent experiments.
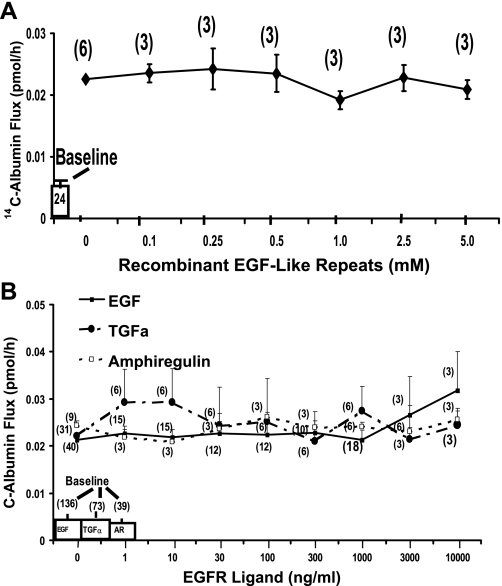
Failure of EGFR ligands and TSP1 EGF-like repeats to open the paracellular pathway.
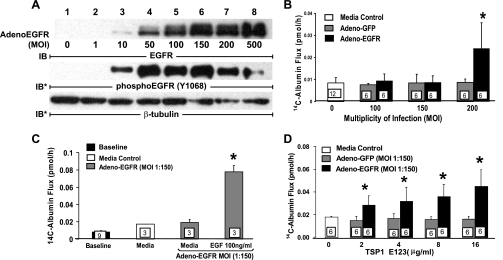
In A431 cells, treatment with baculovirus-derived recombinant TSP1 domains corresponding to overlapping sequences from the full-length protein mapped the ability to activate EGFR to the tandem EGF-like repeats (37). Because TSP1 opens the endothelial paracellular pathway through EGFR/ErbB2 activation (Fig. 6, B, C, and E), we asked whether recombinant TSP1 EGF-like repeats alone could increase paracellular permeability. When postconfluent HMVEC-L monolayers were exposed for 6 h to increasing concentrations of TSP1 EGF-like repeats, EGF-like repeats at concentrations up to ∼50-fold greater than those required for native TSP1 to open the paracellular pathway (Fig. 1A) failed to increase 14C-BSA flux (Fig. 8A). Postconfluent HMVEC-L monolayers were exposed for 6 h to increasing concentrations of the EGFR ligands, EGF, TGF-α, and amphiregulin, after which they were assayed for transendothelial 14C-albumin flux (Fig. 8B). Not one of these three high-affinity EGFR ligands, at concentrations up to 1,000-fold greater than the concentrations required to activate EGFR, increased 14C-BSA flux (Fig. 8B). This failure of recombinant TSP1 EGF-like repeats or authentic high-affinity EGFR ligands to open the endothelial paracellular pathway could be explained by the extremely low levels of EGFR and ErbB2 expression in HMVEC-Ls and/or the requirement of one or more other TSP1 domains in addition to the type 2 repeats. To begin to address this issue, EGFR was overexpressed in HMVEC-Ls before exposure to recombinant TSP1 EGF-like repeats. Infection of HMVEC-Ls with Ad-EGFR at MOIs >10 dramatically increased levels of EGFR expression (Fig. 9A). In these same infected ECs, EGFR Y1068 autophosphorylation also was evident at MOIs ≥10 (Fig. 9A), whereas barrier function was lost only at MOIs ≥200 (Fig. 9B). In HMVEC-Ls infected with Ad-EGFR at MOI = 150, the cells became barrier responsive to EGF (100 ng/ml) (Fig. 9C) as well as recombinant TSP1 EGF-like repeats, whereas those infected with an equivalent MOI of Ad-GFP did not (Fig. 9D). Therefore, in unmanipulated HMVEC-Ls, in which EGFR protein is expressed at low, almost undetectable levels, EGFR activation alone is insufficient to open the paracellular pathway. This finding indicates that, under physiological conditions, one or more TSP1 domains outside of the EGF-like repeats are also required for the barrier-disrupting effect. However, after EGFR expression is sufficiently elevated, activation of the receptor opens the endothelial paracellular pathway.
Fig. 8.
Effect of TSP1 EGF-like repeats and EGFR ligands on transendothelial 14C-albumin flux. Postconfluent HMVEC-L monolayers were exposed for 6 h to increasing concentrations of recombinant TSP1 EGF-like repeats (A) or recombinant human EGF, TGF-α, or amphiregulin (B). Mean (± SE) pretreatment baseline transendothelial 14C-BSA flux is shown by the open bars. Each symbol represents mean (± SE) 14C-BSA flux in pmol/h immediately after 6-h exposures to increasing concentrations of the TSP1 EGF-like repeats (A) or EGFR ligands (B); n indicates number of monolayers studied and is indicated next to each symbol.
Fig. 9.
EGFR overexpression in HMVEC-Ls increases EC responsiveness for TSP1 EGF-like repeats. Adenovirus encoding for human EGFR (Ad-EGFR) or green fluorescent protein (GFP) (Ad-GFP) were packaged and amplified in human embryonic kidney 293 cells. A: HMVEC-Ls cultured to confluence in 60-mm dishes were incubated with media alone or infected with packaged Ad-EGFR at 105-108 plaque forming units per dish, i.e., at approximate multiplicities of infection (MOIs) of 1, 10, 50, 100, 150, 200, and 500, respectively. At 24 h, the cells were lysed and the lysates processed for immunoblotting for EGFR. The blots were stripped and reprobed for phosphoEGFR (Y1068) and again for β-tubulin to indicate protein loading and transfer. B: HMVEC-Ls were cultured to postconfluence in barrier assay chambers, after which they were infected with either Ad-EGFR or Ad-GFP at increasing MOIs (0–200), after which transendothelial 14C-BSA flux was measured. C and D: postconfluent HMVEC-Ls were infected with Ad-EGFR or Ad-GFP at an MOI of 150. After the baseline barrier integrity for each infected monolayer was established, the cells were exposed for 6 h to EGF (100 ng/ml) (C), increasing concentrations of recombinant TSP1 EGF-like repeats (D), or medium alone (C and D), after which barrier function was assayed. *Significantly increased compared with either the simultaneous Ad-GFP (B and D) or medium (C) controls at P < 0.05.
DISCUSSION
In this study, we demonstrate that exogenous soluble TSP1 increases paracellular movement of macromolecules across human lung microvascular EC monolayers through a PTK-dependent mechanism. Because these ECs express EGFR and ErbB2 at the mRNA and protein levels, we investigated the role of these ErbB receptors in mediating TSP1-induced barrier disruption. TSP1 increased tyrosine phosphorylation of EGFR, ErbB2, and the ZA proteins, VE-cadherin, γ-catenin, and p120ctn. Prior EGFR-selective and ErbB2-selective PTK inhibition each protected against increased ZA protein tyrosine phosphorylation and barrier disruption in response to the TSP1 stimulus. Prior immunoblockade of the EGFR ectodomain also completely protected against the loss of barrier function. We previously found that the EGF-like repeats of TSP1 indirectly activate EGFR in high-EGFR-expressing A431 cells (37). However, in HMVEC-Ls, recombinant TSP1 EGF-like repeats 1–3 and the high-affinity EGFR ligands, EGF, TGF-α, and amphiregulin, each failed to open the paracellular pathway. ECs, in which EGFR was overexpressed, became responsive to the TSP1 EGF-like repeats and the EGFR ligand, EGF. These combined data indicate that, in human lung microvascular endothelia, TSP1 regulates the ZA multiprotein complex and the paracellular pathway through EGFR and ErbB2 activation. Under physiological conditions where EGFR expression is low, EGFR activation is necessary but insufficient to open the paracellular pathway in response to TSP1.
In our studies, native TSP1 increased transendothelial albumin flux at concentrations as low as 15 μg/ml (0.107 μM) (Fig. 1A). In normal subjects, the mean (± SD) concentration of TSP1 in whole blood is reportedly 18.00 ± 3.39 μg/ml with a range of 13.5–26.5 μg/ml (n = 21) (10). During platelet aggregation and release, TSP1 levels are further elevated (58). In one report, the TSP1 concentrations in the bronchoalveolar lavage fluids obtained from patients with ARDS were elevated compared with those from normal subjects (23). On the basis of these combined studies, the TSP1 concentrations used in the present studies are physiologically relevant, especially under conditions of platelet activation. At a fixed concentration of 214 nM, TSP1 increased transendothelial 14C-BSA flux as early as 10 min with further gradual time-dependent increases throughout the 8-h study period (Fig. 1B). TSP1 is a multidomain protein (6) that contains multiple binding motifs that are known to recognize up to eight distinct cognate receptors expressed on host cells, including endothelia (56, 61). Each of these receptors are coupled to their own signaling events. It is conceivable that the time requirements for the endothelial barrier response to TSP1 is dictated by the net effect of multiple cross-talking signaling pathways functioning in concert.
The level of expression and function of ErbB proteins in various endothelia is unclear. ErbB protein expression in most normal nonmalignant cells, including endothelia, is extremely low and not consistently detectable (27). In cells cultured in growth factor- and/or serum-containing medium, at least for EGFR, this may be explained, in part, through ligand-induced internalization and lysosomal degradation (41). Nevertheless, EGFR ligands appear to be important or even necessary for the maintenance of ECs in culture (9). Although a few studies have indicated distinct, and in some cases contradictory, patterns of ErbB receptor expression in various endothelia (8, 29), their expression in HMVEC-Ls was unknown. In selected reports, EC responsiveness to EGFR ligands was described (42, 46), whereas, in another study, multiple EC responses were inhibitable by selective EGFR PTK inhibition (43). At least in one report, the endothelium was consistently stained by anti-EGFR antibody in normal human bronchial mucosa (51). ErbB2 has been detected in human aortic (28) and bone marrow-derived (21) endothelia. We found that, in HMVEC-Ls, EGFR and ErbB2 were expressed at mRNA and protein levels, whereas ErbB3 and ErbB4 were not detected (Fig. 4, A and B). Nevertheless, TSP1-induced EC responses were inhibitable by either AG1478 (Fig. 6A) or AG825 (Fig. 6B). It is conceivable that each of these two proteins, EGFR and ErbB2, expressed almost below levels of detection, together heterodimerize and synergize to regulate HMVEC-L responses to TSP1.
In these studies, we identified the ZA proteins, VE-cadherin, γ-catenin, and p120ctn but not β-catenin (Figs. 2C and 3) as substrates for TSP1-induced tyrosine phosphorylation. Tyrosine phosphorylation of VE-cadherin regulates its binding to catenins and reduces the homophilic adhesion between opposing ectodomains, through inside-out signaling (36). Multiple established mediators of increased vascular permeability, like TSP1, have been shown to increase tyrosine phosphorylation of ZA proteins, including VE-cadherin (16, 18). p120ctn has emerged as a key regulator of cadherin expression and function (36). p120ctn not only regulates VE-cadherin turnover at the plasma membrane but also influences lateral clustering of VE-cadherin (36). β- and γ-catenin compete for the same binding site on the intracellular domain of VE-cadherin (32). In postconfluent ECs, the formation of mature and cytoskeleton-connected junctions is accompanied by increases in γ-catenin association with VE-cadherin with competitive displacement of β-catenin (32). Increased γ-catenin association with VE-cadherin may explain preferential γ-catenin vs. β-catenin phosphorylation by one or more ZA-associated PTKs, including EGFR (22, 40, 54). Prior ErbB2-selective PTK inhibition with AG825 also completely blocked VE-cadherin and γ-catenin phosphorylation (Fig. 7, A and B) but only partially protected against p120ctn phosphorylation (Fig. 7C, lane 4). p120ctn was first identified as a substrate for c-src (52), and EGFR activation can be associated with c-src recruitment (60). It is conceivable that one or more src family PTKs contribute to TSP1-induced p120ctn phosphorylation. Either EGFR-selective or ErbB2-selective PTK inhibition each protected against TSP1-induced barrier disruption (Fig. 6, A and B). Because only EGFR and ErbB2 but not ErB3 or 4 are expressed in HMVEC-Ls (Fig. 4) and no ligand has ever been established for ErbB2, ErbB2 is likely transactivated through EGFR activation. That EGFR ectodomain-blocking antibody protects against the TSP1 effect supports the absolute requirement for EGFR engagement.
We then tested the portion of TSP1 that activates EGFR, the EGF-like repeats, in the barrier assay. Exposure to increasing concentrations of recombinant TSP1 EGF-like repeats (up to 5 μM) failed to increase transendothelial 14C-BSA flux (Fig. 8A). Although this baculovirus-derived recombinant domain can clearly activate EGFR (37), we asked whether an altered tertiary structure could explain its inability to open the paracellular pathway. We tested three high-affinity EGFR ligands, EGF, TGF-α, and amphiregulin; each at concentrations up to 10 μg/ml failed to open the endothelial paracellular pathway (Fig. 8B). These combined data indicate that, under conditions where EGFR expression is low, as in HMVEC-Ls, engagement and activation of EGFR alone are insufficient to open the endothelial paracellular pathway and suggest that TSP1 must also interact with another EC surface receptor or receptors to do so. We then asked whether the inability of EGFR activation alone to open the paracellular pathway could be explained by its extremely low expression in these cells. HMVEC-Ls, in which EGFR expression was elevated to levels that in itself did not alter barrier function, became barrier responsive to EGF and the TSP1 EGF-like repeats (Fig. 9C). Our data demonstrate that, if expressed at sufficient levels, EGFR can regulate the paracellular pathway in HMVEC-Ls.
EGFR may participate in TSP1-induced opening of the paracellular pathway through multiple mechanisms. First, EGFR increases tyrosine phosphorylation of ZA proteins (22, 40, 54). EGFR activation also recruits and activates src family kinases (60), which, in turn, tyrosine phosphorylate ZA proteins and open the paracellular pathway (18). EGFR activation is also coupled to Erk1/2 activation, which has been implicated in loss of barrier function (50). EGFR activation provokes profound cell-shape changes and actin reorganization (11, 63), and a structure-function relationship exists between actin reorganization and changes in barrier function (17). Activation of EGFR generates H2O2, which, through oxidation of a crucial cysteine residue within the conserved catalytic domain, inactivates protein tyrosine phosphatase (PTP) activity (34). We had demonstrated that PTP inhibition, at levels that do not in themselves alter barrier function, enhances TSP1-induced loss of barrier function (16). Although EGFR activation alone, through its ability to inactivate PTPs, may be insufficient to open the paracellular pathway, like pharmacological PTP inhibition (16, 66), it may enhance barrier dysfunction in response to TSP1.
EGFR not only responds to direct binding of ligands but can be transactivated by a wide range of diverse stimuli (7). First, several heterologous receptors, including PDGF-βR (53), G protein-coupled receptors (38), members of the cytokine receptor superfamily (13), E-cadherin (49), and integrins (44), can associate with and/or transactivate EGFR. Environmental stressors, including hyperosmotic conditions, heat shock, UV and γ-radiation, oxidant stress with H2O2, heavy metals, and alkylating agents, all can activate EGFR (7). Interestingly, several established mediators of increased vascular permeability and/or lung injury, including thrombin, TNF-α, platelet-activating factor, bradykinin, angiopoietin, and H2O2, each transactivate EGFR (2, 7, 38, 48). Furthermore, EGFR ligands, such as TGF-α, are increased in both the lungs of experimental animals (62) and the pulmonary edema fluids of patients (39) with acute lung injury. In addition to authentic high-affinity EGFR ligands, other proteins that participate in hemostasis, wound healing, and tissue remodeling, like TSP1, also contain EGF-like sequences (3, 14). It is conceivable that EGFR is a pivotal signaling element in a final common pathway for the host response to a subset of injurious stimuli for acute lung injury.
GRANTS
This work was supported in part by grants HL084223 (S. Goldblum), HL079644 (J. Murphy-Ullrich), and HL54462 (D. Mosher) from the National Institutes of Health. This work was performed in University of Alabama at Birmingham facilities supported by the Research Facilities Improvement Program Grant C06RR15490 from the National Center for Research Resources.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Ms. Shirley Taylor for excellent secretarial support.
REFERENCES
- 1. Adams JC, Kureishy N, Taylor AL. A role for syndecan-1 in coupling fascin spike formation by thrombospondin-1. J Cell Biol 152: 1169–1182, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adomeit A, Granesis A, Gross S, Seedorf K, Wetzker R, Liebmann C. Bradykinin B(2) receptor-mediated mitogen-activated protein kinase activation in COS-7 cells requires dual signaling via both protein kinase C pathway and epidermal growth factor receptor transactivation. Mol Cell Biol 19: 5289–5297, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Apella E, Weber IT, Blasi F. Structure and function of epidermal growth factor-like regions in proteins. FEBS Lett 231: 1–4, 1988 [DOI] [PubMed] [Google Scholar]
- 4. Bonner JA, Buchsbaum DJ, Russo SM, Fiveash JB, Trummell HQ, Curiel DT, Raisch KP. Anti-EGFR-mediated radiosensitization as a result of augmented EGFR expression. Int J Radiat Oncol Biol Phys 59: 2–10, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of Thrombospondin 1. J Cell Biol 130: 503–506, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carlson CB, Lawler J, Mosher DF. Structures of thrombospondins. Cell Mol Life Sci 65: 672–686, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carpenter G. Employment of the epidermal growth factor receptor in growth factor-independent signaling pathways. J Cell Biol 146: 697–702, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen K, Vita JA, Berk BC, Keaney JF., Jr c-Jun N-terminal kinase activation by hydrogen peroxide in endothelial cells involves SRC-dependent epidermal growth factor receptor transactivation. J Biol Chem 276: 16045–16050, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Couch JM, Cullen P, Casey TA, Fabre JW. Mitotic activity of corneal endothelial cells in organ culture with recombinant human epidermal growth factor. Opthalmology 94: 1–6, 1987 [DOI] [PubMed] [Google Scholar]
- 10. Dawes J, Clemetson KJ, Gogstad GO, McGregor J, Clezardin P, Prowse CV, Pepper DS. A radioimmunoassay for thrombospondin, used in a comparative study of thrombospondin, beta-thromboglobulin and platelet factor 4 in healthy volunteers. Thromb Res 29: 569–81, 1983 [DOI] [PubMed] [Google Scholar]
- 11. Diakonova M, Payrastre B, van Velzen AG, Hage WJ, van Bergen en Henegouwen PMV, Boonstra J, Cremers FFM, Humble BM. Epidermal growth factor induces rapid and transient association of phospholipase C-γ1 with EGF-receptor and filamentous actin at membrane ruffles of A431 cells. J Cell Sci 108: 2499–2509, 1995 [DOI] [PubMed] [Google Scholar]
- 12. DiPietro LA, Nissen NN, Gamelli RL, Koch AE, Pyle JM, Polverini PJ. Thrombospondin 1 synthesis and function in wound repair. Am J Pathol 148: 1851–1860, 1996 [PMC free article] [PubMed] [Google Scholar]
- 13. Donato NJ, Gallick GE, Steck PA, Rosenblum MG. Tumor necrosis factor modulates epidermal growth factor receptor phosphorylation and kinase activity in human tumor cells. J Biol Chem 264: 20474–20481, 1989 [PubMed] [Google Scholar]
- 14. Engel J. EGF-like domains in extracellular matrix proteins: localized signals for growth and differentiation? FEBS Lett 251: 1–7, 1989 [DOI] [PubMed] [Google Scholar]
- 15. Gill GN, Kawamoto T, Cochet C, Le A, Sato JD, Masui H, McLeod C, Mendelsohn J. Monoclonal anti-epidermal growth factor receptor antibodies which are inhibitors of epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem 259: 7755–7760, 1984 [PubMed] [Google Scholar]
- 16. Goldblum SE, Young BA, Wang P, Murphy-Ullrich JE. Thrombospondin-1 induces tyrosine phosphorylation of adherens junction proteins and regulates an endothelial paracellular pathway. Mol Biol Cell 10: 1537–1551, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldblum SE, Ding X, Campbell-Washington J. Tumor necrosis factor induces endothelial cell F-action depolymerization, new actin synthesis, and barrier dysfunction. Am J Physiol Cell Physiol 264: C894–C905, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Gong P, Angelini DJ, Yang S, Xia G, Cross AS, Mann D, Bannerman DD, Vogel SN, Goldblum SE. Toll-like receptor 4 signaling is coupled to src family kinase activation, tyrosine phosphorylation of zonula adherens proteins, and opening of the paracellular pathway in human lung microvascular endothelia. J Biol Chem 283: 13437–13449, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J 16: 1647–1655, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res 284: 2–13, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Hoffmann I, Eugene E, Nassif X, Couraud PO, Bourdoulous S. Activation of ErbB2 receptor tyrosine kinase supports invasion of endothelial cells by Neisseria meningitidis. J Cell Biol 155: 133–143, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoschuetzky H, Aberle H, Kemler R. β-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol 127: 1375–1380, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Idell S, Maunder R, Fein AM, Switalska HI, Tuszynski GP, McLarty J, Niewiarowski S. Platelet-specific α-granule proteins and thrombospondin in bronchoalveolar lavage in the adult respiratory distress syndrome. Chest 96: 1125–1132, 1989 [DOI] [PubMed] [Google Scholar]
- 24. Iozzo RV, Moscatello DK, McQuillan DJ, Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem 274: 4489–4492, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Jaffe EA, Ruggiero JT, Falcone DJ. Monocytes and macrophages synthesize and secrete thrombospondin. Blood 65: 79–84, 1985 [PubMed] [Google Scholar]
- 26. Jones PL, Crack J, Rabinovitch M. Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the alpha v beta 3 integrin to promote epidermal growth factor receptor phosphorylation and growth. J Cell Biol 139: 279–293, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jorissen RN, Walker F, Pouliot N, Garrett TPJ, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res 284: 31–53, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Kenny TP, Keen CL, Jones P, Kung HJ, Schmitz HH, Gershwin ME. Pentameric procyanidins isolated from Theobroma cacao seeds selectively downregulate ErbB2 in human aortic endothelial cells. Exp Biol Med (Maywood) 229: 255–263, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Kim HS, Shin HS, Kwak HJ, Cho CH, Lee CO, Koh GY. Betacellulin induces angiogenesis through activation of mitogen-activated protein kinase and phosphatidylinositol 3′-kinase in endothelial cell. FASEB J 17: 318–320, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Kreis C, La Fleur M, Menard C, Paquin R, Beaulieu AD. Thrombospondin and fibronectin are synthesized by neutrophils in human inflammatory joint disease and in a rabbit model of in vivo neutrophil activation. J Immunol 143: 1961–1968, 1989 [PubMed] [Google Scholar]
- 31. Lahav J. The functions of thrombospondin and its involvement in physiology and pathophysiology. Biochim Biophys Acta 1182: 1–14, 1993 [DOI] [PubMed] [Google Scholar]
- 32. Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol 129: 203–217, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lampugnani MG, Dejana E. Interendothelial junctions: structure, signalling and functional roles. Curr Opin Cell Biol 9: 674–682, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem 273: 15366–15372, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Leung LLK. Role of thrombospondin in platelet aggregation. J Clin Invest 74: 1764–1772, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lilien J, Balsamo J, Arregui C, Xu G. Turn-off, drop-out: functional state switching of cadherins. Dev Dyn 224: 18–29, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Liu A, Garg P, Yang S, Gong P, Annis DS, Pallero MA, Passaniti A, Mann D, Mosher DF, Murphy-Ullrich J, Goldlum SE. The EGF-like repeats of thrombospondins activate phospholipase Cγ and increase epithelial cell migration through indirect epidermal growth factor receptor activation. J Biol Chem 284: 6389–6402, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol 11: 177–183, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Madtes DK, Rubenfeld G, Klima LD, Milberg JA, Steinberg KP, Martin TR, Raghu G, Hudson LD, Clark JG. Elevated transforming growth factor-alpha levels in bronchoalveolar lavage fluid of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 158: 424–430, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Mariner DJ, Davis MA, Reynolds AB. EGFR signaling to p120-catenin through phosphorylation at Y228. J Cell Sci 117: 1339–1350, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Marmor MD, Yarden Y. Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene 23: 2057–2070, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Matsuda T, Okamura K, Sato Y, Morimoto A, Ono M, Kohno K, Kuwano M. Decreased response to epidermal growth factor during cellular senescence in cultured human microvascular endothelial cells. J Cell Physiol 150: 510–516, 1992 [DOI] [PubMed] [Google Scholar]
- 43. Michaelis UR, Fisslthaler B, Medhora M, Harder D, Fleming I, Busse R. Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (EGFR). FASEB J 17: 770–772, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: Roles of integrin aggregation and occupancy of receptors. J Cell Biol 135: 1633–1642, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mosher DF, Doyle MJ, Jaffe EA. Synthesis and secretion of thrombospondin by culture human endothelial cells. J Cell Biol 93: 343–348, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Okamura K, Morimoto A, Hamanaka R, Ono M, Kohno K, Uchida Y, Kuwano M. A model system for tumor angiogenesis: involvement of transforming growth factor-alpha in tube formation of human microvascular endothelial cells induced by esophageal cancer cells. Biochem Biophys Res Commun 186: 1471–1479, 1992 [DOI] [PubMed] [Google Scholar]
- 47. Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 19: 3159–3167, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pan Z, Kravchenko VV, Ye RD. Platelet-activating factor stimulates transcription of the heparin-binding epidermal growth factor-like growth factor in monocytes. J Biol Chem 270: 7787–7790, 1995 [DOI] [PubMed] [Google Scholar]
- 49. Pece S, Gutkind JS. Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J Biol Chem 275: 41227–41233, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Petrache I, Birukova A, Ramirez SI, Garcia JG, Verin AD. The role of the microtubules in tumor necrosis factor-alpha-induced endothelial cell permeability. Am J Respir Cell Mol Biol 28: 574–581, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Polosa R, Prosperini G, Leir SH, Holgate ST, Lackie PM, Davies DE. Expression of c-erbB receptors and ligands in human bronchial mucosa. Am J Respir Cell Mol Biol 20: 914–923, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol 14: 8333–8342, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Saito Y, Haendeler J, Hajo Y, Yamamoto K, Berk BC. Receptor heterodimerization: Essential mechanism for platelet-derived growth factor-induced epidermal growth factor receptor transactivation. Mol Cell Biol 21: 6387–6394, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Oku N, Miyazawa K, Kitamura N, Takeichi M, Ito F. Tyrosine phosphorylation of β-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhes Commun 1: 295–305, 1994 [DOI] [PubMed] [Google Scholar]
- 55. Siano JP, Grady KK, Millet P, Swerlick RA, Wick TM. Plasmodium falciparum: soluble thrombospondin increases cytoadherence of parasitized erythrocytes to human microvascular endothelium under shear flow conditions. Exp Parasitol 87: 69–72, 1997 [DOI] [PubMed] [Google Scholar]
- 56. Streit M, Velasco P, Riccardi L, Spencer L, Brown LF, Janes L, Lange-Asschenfeldt B, Yano K, Hawighorst T, Iruela-Arispe L, Detmar M. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J 19: 3272–3282, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sui X, Kiser TD, Hyun SW, Angelini DJ, Del Vecchio RL, Young BA, Hasday JD, Romer LH, Passaniti A, Tonks NK, Goldblum SE. Receptor protein tyrosine phosphatase μ regulates the paracellular pathway in human lung microvascular endothelia. Am J Pathol 166: 1247–1258, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Switalska HI, Niewiarowski S, Tuszynski GP, Rucinski B, Schmaier AH, Morinelli TA, Cierniewski CS. Radioimmunoassay of human platelet thrombospondin: different patterns of thrombospondin and beta-thromboglobulin antigen secretion and clearance from the circulation. J Lab Clin Med 106: 690–700, 1985 [PubMed] [Google Scholar]
- 59. Taraboletti G, Roberts DD, Liotta LA. Thrombospondin-induced tumor cell migration: haptotaxis and chemotaxis are mediated by different molecular domains. J Cell Biol 105: 2409–2415, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tice DA, Biscardi JS, Nickles AL, Parson SJ. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci USA 96: 1415–1420, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Uno K, Hayashi H, Kuroki M, Uchida H, Yamauchi Y, Kuroki M, Oshima K. Thrombospondin-1 accelerates wound healing of corneal epithelia. Biochem Biophys Res Commun 315: 928–934, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Vivekananda J, Lin A, Coalson JJ, King RJ. Acute inflammatory injury in the lunch precipitated by oxidant stress induces fibroblasts to synthesize and release transforming growth factor-α. J Biol Chem 269: 25057–25061, 1994 [PubMed] [Google Scholar]
- 63. Xie H, Pallero MA, Gupta K, Chang P, Ware MF, Witke W, Kwiatkowski DJ, Lauffenburger DA, Murphy-Ullrich JE. EGF receptor regulation of cell motility: EGF induces disassembly of focal adhesions independently of the motility-associated PLCγ signaling pathway. J Cell Sci 111: 615–624, 1998 [DOI] [PubMed] [Google Scholar]
- 64. Yaish P, Gazit A, Gilon C, Levitzki A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science 242: 933–935, 1988 [DOI] [PubMed] [Google Scholar]
- 65. Yarden Y, Sliwkowski MX. Untangling the ErbB signaling network. Nat Rev Mol Cell Biol 2: 127–137, 2001 [DOI] [PubMed] [Google Scholar]
- 66. Young BA, Sui X, Kiser TD, Hyun SW, Wang P, Sakarya S, Angelini DJ, Schaphorst KL, Hasday JD, Cross AS, Romer LH, Passaniti A, Goldblum SE. Protein tyrosine phosphatase activity regulates endothelial cell-cell interactions, the paracellular pathway and capillary tube stability. Am J Physiol Lung Cell Mol Physiol 285: L63–L75, 2003 [DOI] [PubMed] [Google Scholar]