Abstract
Hypoxia enhances transforming growth factor-β (TGF-β) signaling, inhibiting alveolar development and causing abnormal pulmonary arterial remodeling in the newborn lung. We hypothesized that, during chronic hypoxia, reduced peroxisome proliferator-activated receptor-γ (PPAR-γ) signaling may contribute to, or be caused by, excessive TGF-β signaling. To determine whether PPAR-γ was reduced during hypoxia, C57BL/6 mice were exposed to hypoxia from birth to 2 wk and evaluated for PPAR-γ mRNA and protein. To determine whether rosiglitazone (RGZ, a PPAR-γ agonist) supplementation attenuated the effects of hypoxia, mice were exposed to air or hypoxia from birth to 2 wk in combination with either RGZ or vehicle, and measurements of lung histology, function, parameters related to TGF-β signaling, and collagen content were made. To determine whether excessive TGF-β signaling reduced PPAR-γ, mice were exposed to air or hypoxia from birth to 2 wk in combination with either TGF-β-neutralizing antibody or vehicle, and PPAR-γ signaling was evaluated. We observed that hypoxia reduced PPAR-γ mRNA and protein, in association with impaired alveolarization, increased TGF-β signaling, reduced lung compliance, and increased collagen. RGZ increased PPAR-γ signaling, with improved lung development and compliance in association with reduced collagen and TGF-β signaling. However, no reduction was noted in hypoxia-induced pulmonary vascular remodeling. Inhibition of hypoxia-enhanced TGF-β signaling increased PPAR-γ signaling. These results suggest that hypoxia-induced inhibition of lung development is associated with a mutually antagonistic relationship between reduced PPAR-γ and increased TGF-β signaling. PPAR-γ agonists may be of potential therapeutic significance in attenuating TGF-β signaling and improving alveolar development.
Keywords: transforming growth factor-β, lung development, infant
lung development is inhibited by exposure to chronic hypoxia (8, 21). We have recently shown that excessive transforming growth factor (TGF)-β signaling plays a critical role in hypoxia-induced inhibition of lung development and abnormal vascular remodeling (4). Peroxisome proliferator-activated receptor-γ (PPAR-γ) is a member of the retinoid X-receptor heterodimer family of the retinoid/steroid/thyroid hormone superfamily of ligand-activated nuclear receptors that is involved in lipid storage and metabolism in the lung and plays a role in normal lung development (28, 32). Interactions of TGF-β and PPAR-γ in the newborn lung during hypoxia exposure have not been determined, but data from other models of increased TGF-β signaling in the lung [e.g., hyperoxia exposure (27), bleomycin administration (23)] suggest that PPAR-γ ligands may reduce TGF-β signaling. We hypothesized that a reduction in a PPAR-γ may contribute to excessive TGF-β signaling during chronic hypoxia exposure and that a PPAR-γ ligand such as rosiglitazone (RGZ) may attenuate hypoxia-inhibited alveolarization and abnormal vascular remodeling. Because there is evidence that TGF-β signaling may inhibit PPAR-γ signaling in other models (14, 18), we also wished to test the hypotheses that hypoxia-enhanced TGF-β signaling may reduce PPAR-γ in the newborn mouse lung and that inhibition of TGF-β signaling would prevent the reduction of PPAR-γ.
MATERIALS AND METHODS
All protocols were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham and were consistent with the PHS policy on Humane Care and Use of Laboratory Animals (Office of Laboratory Animal Welfare, 2002) and the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, 1996). All experiments, unless otherwise specified, were done with a minimum of six mice from at least two litters for each experimental condition.
Animal model.
C57BL/6 mice were exposed to either normobaric 12% O2 (hypoxia) or air (normoxia) from soon after birth for 14 days (the period of maximal alveolar development) as described previously (2, 4, 5, 25) in combination with either vehicle (PBS) or RGZ (Cayman Chemical, Ann Arbor, MI). RGZ or an equivalent volume of vehicle was administered at a dose of either 1 or 3 μg/g body wt (1 or 3 mg/kg) using a P10 pipette orally once daily from birth for 14 days. These doses were selected on the basis of previous studies in newborn rat pups (13). To prepare the doses, 10 mg of RGZ powder were completely dissolved in 100% ethanol to make a stock solution with a concentration of 1 mg/ml. Before being administered orally to the pups, RGZ was further diluted to a working solution of 0.1 or 0.3 mg/ml in PBS (10 or 30% ethanol in PBS). A 10-μl volume of RGZ working solution was administered orally to 1-day-old newborn pups weighing ∼1 g (1 or 3 μg/g body wt). RGZ volume was gradually increased as pups gained weight.
Additional litters of pups exposed to either air or hypoxia were administered TGF-β-neutralizing antibody (Clone 1D11, MAB1835; R&D Systems, Minneapolis, MN), which neutralizes all three isoforms of TGF-β (-β1, -β2, and -β3), at a dose of 20 μg by intraperitoneal injection on postnatal days 1, 5, and 10 (∼20 μg/g body wt on postnatal day 1; 6 μg/g on day 5, and 4 μg/g on day 10). As described previously by us (25) and others (24), this dosing results in a marked reduction (50–75%) of TGF-β signaling.
Analysis of lung function.
After completion of hypoxia or air exposure, the mice were sedated with ketamine/xylazine, and pulmonary function was evaluated on a flexiVent as previously described (25). Briefly, a 24G Angiocath was inserted into the trachea and fixed with a ligature of 3–0 silk. The flexiVent apparatus (SCIREQ, Montreal, QC, Canada) equipped with a Module 1 was used to perform measurement maneuvers including perturbations, (predefined pressure of volume waveforms) such as forced oscillations, using room air in the closed-chest animal. The tidal volume was set at 6 ml/kg, similar to that clinically used, with a respiratory rate of 150/min. Calibration of the flexiVent was done using the tracheal cannula to be used, before each experiment. Lung volumes were measured by volume displacement after completion of the flexiVent measurements.
Mice were then euthanized, and the lungs were inflation-fixed for histology, or lung homogenates were prepared for RNA and protein analysis (4, 5, 25). Additional newborn mice were exposed for shorter durations (1, 3, or 7 days) to air or hypoxia for evaluation of RNA and protein in lung homogenates. Estimation of TGF-β signaling was done by quantitation of phosphorylated Smad2/β-tubulin by Western blotting and of PPAR-γ signaling by measurement of adipose differentiation-related protein (ADRP) and leptin by quantitative real-time PCR.
Analysis of mRNA.
Quantitative real-time PCR (qPCR) was performed using the Bio-Rad iCycler System (Hercules, CA) as described previously (4, 5, 25) for genes involved in TGF-β and PPAR-γ signaling pathways (Table 1 showing primer sequences). RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA) from homogenized lung from mice at 14 days of age, treated with proteinase K and DNase I, and then quantified and reverse transcribed using the SYBR Green RT-PCR kit (Applied Biosystems, Foster City, CA). cDNA was amplified by PCR in the Bio-Rad iCycler for 30–40 cycles.
Table 1.
Mouse primer sequences (3′-5′) for real-time quantitative RT-PCR
| Primer Name | Sequence |
|---|---|
| 18S Forward | GTC TGC CCT ATC AAC TTT CG |
| 18S Reverse | ATG TGG TAG CCG TTT CTC A |
| ADRP Forward | CTG GAC CGT GCC GAC TTG |
| ADRP Reverse | GCT CTG TTG GGG ATC CAC TAC |
| PPAR-γ Forward | GAG TGT GAC GAC AAG ATT TG |
| PPAR-γ Reverse | GGT GGG CCA GAA TGG CAT CT |
| Col la1 Forward | CCA AGG GTA ACA GCG GTG AA |
| Col la1 Reverse | CCT CGT TTT CCT TCT TCT CCG |
| Col 3a1 Forward | TCA AGT CTG GAG TGG GAG G |
| Col 3a1 Reverse | TCC AGG ATG TCC AGA AGA ACC A |
| Leptin Forward | GAT GGA CCA GAC TCT GGC AG |
| Leptin Reverse | AGA GTG AGG CTT CCA GGA CG |
| TGF-β1 Forward | GCC CTG GAT ACC AAC TAT TGC TT |
| TGF-β1 Reverse | AGT TGG CAT GGT AGC CCT TG |
| TGF-β2 Forward | CTT CAC CAC AAA GAC AGG AAC CT |
| TGF-β2 Reverse | TGC CAT CAA TAC CTG CAA ATC T |
| TGF-β3 Forward | GGA AAT CAA ATT CAA AGG AGT GG |
| TGF-β3 Reverse | AGT TGG CAT AGT AAC CCT TAG G |
ADRP, adipose differentiation-related protein; PPAR, peroxisome proliferator-activated receptor; Col, collagen; TGF, transforming growth factor.
Immunohistochemical staining of lung sections.
The primary antibodies for phosphorylated Smad2 (AB3849; Chemicon International/Millipore, Billerica, MA) and PPAR-γ (sc-7196; Santa Cruz Biotechnology, Santa Cruz, CA) were used at 1:100 dilution for 30 min, and the secondary antibody and diaminobenzidine staining kit were used as described in the product manual (DAKO Envision+HRP-DAB; DakoCytomation, Carpinteria, CA). Antigen retrieval was performed by heating in pH 6.0 citrate buffer (LabVision, Fremont, CA) for 20 min before addition of primary antibody. Nonspecific IgG and omission of primary antibody were used as controls for staining specificity. Whole lung sections were imaged at ×400. Six random high-power fields from each of six different animals per group were evaluated for each experiment. The identity of the sample was masked to observers estimating immunohistochemical staining to avoid bias.
Western blot analysis.
Newborn mouse lungs were homogenized in 1 ml of a tissue protein extraction reagent (Pierce Biotechnology, Rockford, IL) containing complete proteinase inhibitor cocktail (Roche Diagnostics, Indianapolis, IN), centrifuged at 7,000 g for 5 min, and the supernatant was frozen at −80°C until analysis. Protein concentrations were measured using the Bio-Rad Bradford Protein Assay (Bio-Rad). Ten micrograms of protein per lane were fractionated by 10% Tris-Glycine SDS-PAGE electrophoresis, followed by transfer to a PVDF membrane (Millipore). Western Blot analysis was done using specific primary antibodies (developed in rabbit or goat, reactive against mouse) for PPAR-γ (1:1,000; 07–466, Millipore), TGF-β1, -2, and -3 (1:500; ab9758, ab80059, and ab15537 respectively; Abcam, Cambridge, MA), Smad2/3 (1:1,000; no. 3102, Cell Signaling Technology, Danvers, MA), and pSmad2 (Ser465/467) (1:500; no. 3108, Cell Signaling Technology), ADRP (1:500; sc-32450, Santa Cruz Biotechnology), leptin (1:1,000; AB1673, Chemicon/Millipore), and β-tubulin (1:1,000; sc-9104, Santa Cruz Biotechnology) overnight at 4°C.
The secondary antibody was a goat anti-rabbit secondary antibody (1:10,000; no. 7074, Cell Signaling) or donkey anti-goat (1:8,000; no. 6420; SouthernBiotech, Birmingham, AL) for 1 h at room temperature. Immunoreactive bands were visualized by treatment with Immun-Star Western blotting detection reagents (Bio-Rad) according to the manufacturer's instructions. Densitometry was done, normalizing for β-tubulin, a protein that did not change significantly with hypoxia in this model.
ELISA.
All lung homogenates were analyzed as a single batch for total TGF-β1 by ELISA as described in the manufacturer's protocol (MB100B, R&D Systems). The range of measurement of this ELISA was 5–2,000 pg/ml. Total (latent + active) TGF-β1 (not TGF-β2 or TGF-β3) was measured by the addition of hydrochloric acid to activate latent TGF-β to active TGF-β, followed by neutralization with sodium hydroxide, as described in the product manual. TGF-β1 concentrations were normalized by protein concentration.
Collagen measurement.
Collagen was measured using the Sircol soluble collagen assay (Biocolor, Newtonabbey, Northern Ireland) as described previously (3, 25). The dye reagent (Sirius Red in picric acid) binds specifically to the [Gly-X-Y]n helical structure found in all collagens.
Lung and heart morphometry.
Lung alveolar and vascular morphometry was performed as previously described (4, 5, 25) using the MetaMorph software (v.6.2r4; Universal Imaging, West Chester, PA) interfaced with a Nikon TE2000U microscope equipped with a QiCam Fast Cooled high-resolution CCD camera.
Alveolar development was evaluated by mean linear intercepts (MLI, an estimate of alveolar size as increased development and septation is associated with smaller alveoli) (22) and radial alveolar counts (RAC, an estimate of the number of alveolar septae from the terminal bronchiole to the nearest connective tissue septum) (11). Images from six random ×100 lung fields were taken from each animal, with one image from the apex, middle, and base of each lung for MLI measurement, and six RAC measurements were performed on each animal. Alveolar septal thickness was determined near the center of septae (avoiding areas with nuclei or red blood cell) using the measurement calipers in MetaMorph in six random ×400 lung fields from each animal, with six measurements per field.
Vascular morphometry was done on pulmonary arteries, defined as vascular structures that accompanied airways and were between 20 and 150 μm in external diameter. At least 20 pulmonary arteries from each section were evaluated. Vessels cut transversely were measured along both axes, and the average wall thickness was obtained. Vessels cut obliquely or longitudinally were measured along the short axis. The wall thickness of each artery was expressed as a percentage of the vessel diameter.
Hearts were sectioned transversely just below the level of the mitral leaflet, and the thickness of the free wall of the right ventricle (RV) compared with that of the left ventricle (LV) (RV/LV free wall thickness ratio) was determined as an index of RV hypertrophy secondary to pulmonary hypertension, as described previously (4, 5, 25). Of note, this measure was used instead of the conventional RV/(LV + S) weight ratio, which is not accurate in newborn mice.
Statistical analysis.
Data were expressed as means ± SE. Data were analyzed by two-way ANOVA to test for separate and combined effects of therapy (RGZ or 1D11) and hypoxia on measurements. Multiple-comparisons testing (Student-Newman-Keuls) was performed if statistical significance (P < 0.05) was noted by two-way ANOVA. For mRNA analysis, normalization was done by considering the average of the measurements (mRNA for gene of interest/18S RNA) in the air-vehicle group at 14 days as 1.0; measurements in other groups were expressed relative to the expression in this group. Other measurements (e.g., protein as measured by ELISA, Western blots, Sircol) were shown as measured in absolute amounts (mg/mg protein or as a ratio to internal standard).
RESULTS
RGZ at 3 mg/kg led to increased mortality (50%) in the mouse pups in the second week (P7-P12), associated with ascites and poor growth even in air-exposed animals, whereas the same volume of vehicle was well tolerated. RGZ at 1 mg/kg was well tolerated and not associated with a change in mortality or change in weight. Hypoxia was not associated with increased mortality (>85% survival in each litter) but led to impaired growth in the mouse pups (weight lower by ∼20%, mean weight in hypoxia group 6 g vs. 7.5 g in air group), which was unchanged by RGZ administration at 1 mg/kg. Only results from RGZ at 1 mg/kg were used for this study.
Chronic hypoxia exposure reduces PPAR-γ and PPAR-γ signaling in newborn mouse lung.
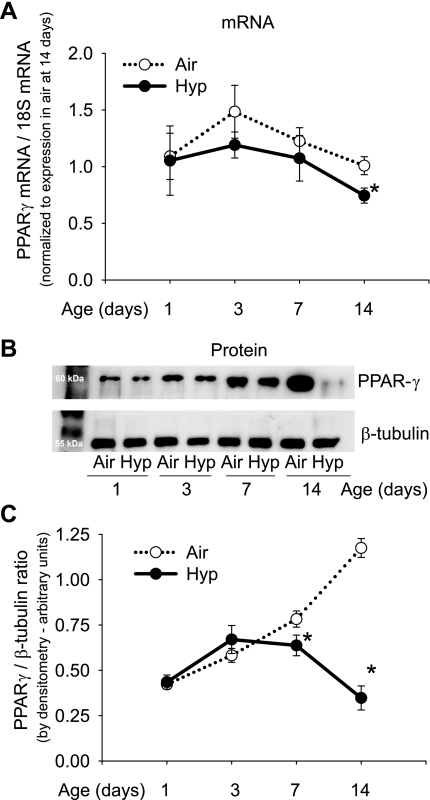
Hypoxia reduced PPAR-γ mRNA expression and PPAR-γ protein in the newborn mouse lung (Fig. 1). PPAR-γ mRNA was significantly reduced by hypoxia only at postnatal day 14. PPAR-γ protein increased over time from postnatal day 1 through day 14 in air-exposed mice; hypoxia was associated with reduced PPAR-γ protein at 7 and 14 days (Fig. 1). A hypoxia-induced reduction in PPAR-γ protein was also noted by immunohistochemistry (Fig. 2). PPAR-γ was diffusely present in the lung parenchyma (alveolar and airway epithelial cells, endothelium, and interstitium), with most staining in interstitial locations at alveolar junctions.
Fig. 1.
Effect of chronic hypoxia exposure on peroxisome proliferator-activated receptor (PPAR)-γ expression in newborn mouse lung. A: mRNA for PPAR-γ by qPCR in homogenized lungs from mice exposed to air or hypoxia (Hyp) from birth for up to 14 days (n = 6 mice/group; means ± SE, *P < 0.05 vs. air at same time point). B: Western blots for PPAR-γ and β-tubulin on lung homogenates of C57BL/6 mice during 14 days of air or hypoxia exposure. Each lane represents a pooled sample from 3 mouse pups of the same postnatal age. C: PPAR-γ/β-tubulin ratio in Western blots quantitated by densitometry (n = 6 mice/group at each time point; means ± SE, *P < 0.05 vs. air at same time point).
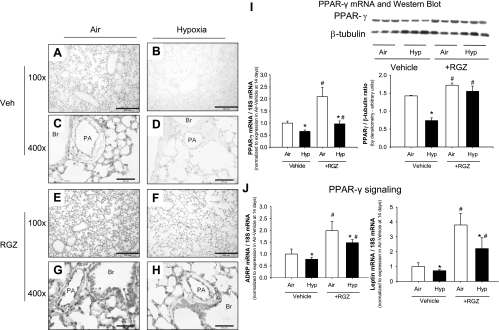
Fig. 2.
Rosiglitazone (RGZ) administration restores PPAR-γ during hypoxia exposure. A–H: representative photomicrographs at ×100 and ×400 magnification demonstrating PPAR-γ staining in lung sections from 14-day-old mouse pups exposed to air (A, C, E, and G) or hypoxia (B, D, F, and H) from birth while being administered RGZ (E–H) or vehicle (A–D) [pulmonary artery (PA); bronchus (Br)]. Effects of RGZ (1 mg/kg per day) on PPAR-γ mRNA expression (I) and protein amounts by Western blot (J and K) in lung homogenates of C57BL/6 mice exposed to air or hypoxia while administered RGZ or vehicle, with PPAR-γ/β-tubulin ratio in Western blots quantitated by densitometry (K). Each lane in Western blot represents a sample from a different mouse pup (means ± SE, n = 6 mice/group; *P < 0.05 corresponding air group, #P < 0.05 vs. corresponding vehicle group). ADRP, adipose differentiation-related protein.
In addition to a reduction in PPAR-γ protein during hypoxia exposure, the downstream indicators of PPAR-γ signaling ADRP and leptin were also decreased (Fig. 2).
RGZ administration restores PPAR-γ during hypoxia exposure.
Administration of RGZ increased PPAR-γ mRNA and protein levels as well as indicators of PPAR-γ signaling (ADRP and leptin) in both air-exposed and hypoxia-exposed mice (Fig. 2). The RGZ-induced increases in PPAR-γ, ADRP, and leptin were blunted in hypoxia-exposed mice compared with air-exposed mice but were higher than hypoxia-vehicle group (Fig. 2). Immunohistochemistry indicated that RGZ increased PPAR-γ in the interstitium as well as in the airway epithelium and pulmonary arterial endothelium.
RGZ attenuates hypoxia-induced inhibition of alveolar development.
Hypoxia inhibited alveolar development, as evidenced by a reduction in RAC and an increase in the MLI in hypoxia-vehicle mice compared with air-vehicle mice, associated with larger alveoli and fewer secondary septations (Fig. 3). Air-RGZ mice had alveolar development similar to air-vehicle mice by MLI and RAC, indicating no effects of RGZ on alveolar development during normoxia. Hypoxia-RGZ mice had RAC higher than hypoxia-vehicle mice but lower than air-vehicle mice, indicating attenuation but not complete prevention of hypoxia-induced inhibition of alveolar septation (Fig. 3). MLI in hypoxia-RGZ mice was lower than hypoxia-vehicle mice and similar to air-vehicle and air-RGZ mice, indicating smaller alveoli and improved alveolarization during hypoxia. Alveolar septal thickness was not significantly altered in this study by hypoxia, exposure to RGZ, or by their combination (means ± SE; in μm; air-vehicle mice: 2.9 ± 0.11; hypoxia-vehicle mice: 3.3 ± 0.22; air-RGZ mice: 3.0 ± 0.07; hypoxia-RGZ mice: 3.1 ± 0.12; P = 0.25).
Fig. 3.
RGZ attenuates hypoxia-induced inhibition of alveolar development. A–D: representative photomicrographs (×100, hematoxylin and eosin stain; calibration bars = 250 μm) of lung sections from 14-day-old mice exposed to air (A and C), or hypoxia (B and D) in combination with either RGZ (C and D) or vehicle (A and B). Radial alveolar count (E) and mean linear intercept (F) of pups administered RGZ or vehicle at 14 days while exposed to air or hypoxia are shown, demonstrating a reduction in radial alveolar count and an increase in mean linear intercept with hypoxia, which are attenuated by RGZ (means ± SE; n = 6 mice/group; *P < 0.05 vs. corresponding air; #P < 0.05 vs. corresponding vehicle).
RGZ does not significantly attenuate hypoxia-induced pulmonary vascular remodeling or RV hypertrophy.
Hypoxia increased wall thickness in resistance pulmonary arteries of hypoxia-vehicle pups compared with air-vehicle pups, and RGZ did not change wall thickness in either air or hypoxia compared with the vehicle group (Fig. 4). A nonsignificant trend toward reduction (0.05 < P < 0.2) was noted in RV/LV thickness ratio in hypoxia-RGZ pups (Fig. 4).
Fig. 4.
RGZ does not significantly attenuate hypoxia-induced pulmonary arterial remodeling or right ventricular (RV) hypertrophy. Pulmonary arterial remodeling and RV/left ventricle (LV) ratios in C57BL/6 mouse pups at 14 days of age. A–D: representative photomicrographs of a resistance pulmonary artery and a bronchus from mouse pups given either vehicle (A and B) or RGZ (C and D) during 14 days of air (A and C) or hypoxia (B and D) exposure (×400; calibration bars = 50 μm). In mouse pups given vehicle, pulmonary arterial wall thickness (arrow) is increased by hypoxia (B) compared with air (A). Administration of RGZ does not significantly attenuate the hypoxia-induced increase in wall thickness (D) or change wall thickness of air-exposed animals (C). Wall thickness (%) (E) of pulmonary arteries and RV/LV thickness ratio (F) are shown at 14 days of age in mice given either vehicle or RGZ, while being exposed to air or hypoxia from birth (means ± SE; n = 6 mice/ group; *P < 0.05 vs. corresponding air; #P < 0.05 vs. corresponding vehicle).
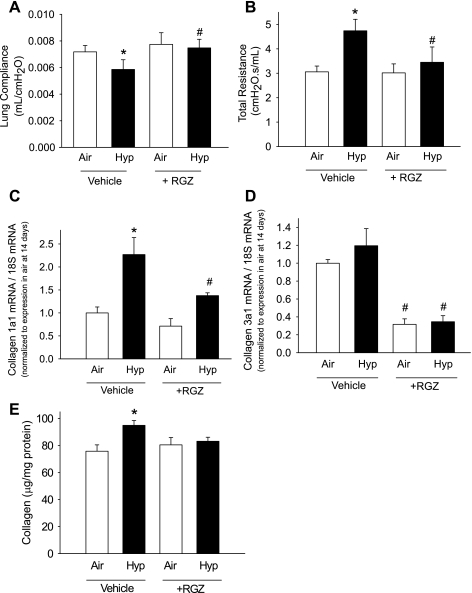
RGZ prevents hypoxia-induced alterations in lung function and reduces hypoxia-induced increases in collagen accumulation.
Hypoxia reduced lung compliance and increased total lung resistance in vehicle-administered mice (Fig. 5). RGZ administration prevented the reduction in lung compliance and increase in resistance during hypoxia and did not change compliance or resistance during air exposure (Fig. 5). Hypoxia-vehicle mice had increased collagen mRNA and collagen content compared with air-vehicle mice (Fig. 5). RGZ administration reduced collagen 1a1 mRNA during hypoxia compared with hypoxia-vehicle mice and reduced collagen 3a1 mRNA during both air and hypoxia exposure compared with vehicle controls. RGZ reduced collagen content (by Sircol assay) during hypoxia compared with hypoxia-vehicle mice and did not change collagen content during air exposure (Fig. 5).
Fig. 5.
RGZ prevents hypoxia-induced alterations in lung function by reducing collagen accumulation. Lung compliance (A) and total lung resistance (B) in 14-day-old mouse pups exposed from birth to air or hypoxia, in combination with either vehicle or RGZ are shown. Effects of chronic hypoxia exposure on collagen 1a1 (C) and collagen 3a1 (D) mRNA expression in mice exposed from birth to air or hypoxia, in combination with either vehicle or RGZ are shown. Total collagen protein was measured by Sircol collagen assay (E) (means ± SE, n = 6 mice/group; *P < 0.05 corresponding air, #P < 0.05 vs. corresponding vehicle).
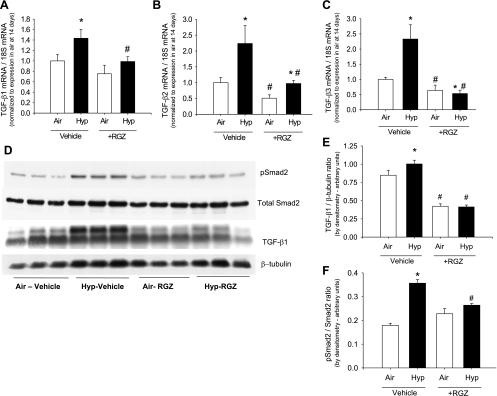
Hypoxia increases TGF-β signaling, which is attenuated by RGZ.
Hypoxia increased TGF-β1, -β2, and -β3 mRNA expression although the magnitude of increase in TGF-β1 mRNA was relatively modest (Fig. 6). RGZ attenuated the hypoxia-induced increases in TGF-β1, -β2, and -β3 mRNA and, in addition, also reduced TGF-β2 and -β3 mRNA expression in air (Fig. 6). By Western blot analysis, hypoxia increased TGF-β1/β-tubulin, pSmad2/β-tubulin, and pSmad2/Smad2 in lung homogenates, consistent with an increase in TGF-β signaling. RGZ attenuated the hypoxia-induced increases in both TGF-β1 and pSmad2 (Fig. 6). A similar hypoxia-induced increase in TGF-β1 was noted by ELISA, which was attenuated by RGZ (data not shown).
Fig. 6.
Hypoxia increases transforming growth factor (TGF)-β signaling, which is attenuated by RGZ. TGF-β1, -β2, and -β3 mRNA are increased in hypoxia and ameliorated by oral RGZ administration (A–C). Western blot for pSMAD2, total SMAD2, TGF-β1, and β-tubulin in lung homogenates from 14-day-old pups exposed to air or hypoxia, in combination with either vehicle or RGZ (D) with TGF-β1/β-tubulin ratio (E) and pSmad2/Smad2 ratio (F) in Western blots quantitated by densitometry are shown. Each lane represents a sample from a different mouse pup (means ± SE, n = 6 mice/group; *P < 0.05 corresponding air, #P < 0.05 vs. corresponding vehicle).
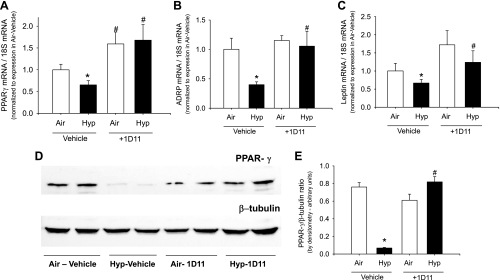
Hypoxia decreases PPAR-γ signaling, which is prevented by inhibition of TGF-β signaling.
Hypoxia-induced reductions in PPAR-γ and its signaling molecules ADRP and leptin were prevented by treatment with the TGF-β-neutralizing antibody 1D11 (Fig. 7). An increase in PPAR-γ was also noted by Western blot in lung homogenates of hypoxia-exposed mice treated with 1D11, such that air-vehicle, air-1D11, and hypoxia-1D11 groups had similar PPAR-γ/β-tubulin, whereas hypoxia-vehicle mice had marked reduction in PPAR-γ (Fig. 7).
Fig. 7.
Hypoxia decreases PPAR-γ signaling, which is prevented by 1D11, a panspecific TGF-β-neutralizing antibody. mRNA of PPAR-γ and its signaling indicators ADRP and leptin are decreased in hypoxia and ameliorated by 1D11 administration (A–C). Western blot for PPAR-γ and β-tubulin in lung homogenates from 14-day-old pups exposed to air or hypoxia, in combination with either vehicle or 1D11 (D) with PPAR-γ/β-tubulin ratio in Western blots quantitated by densitometry (E) are shown. Each lane represents a sample from a different mouse pup (means ± SE, n = 6 mice/group; *P < 0.05 corresponding air, #P < 0.05 vs. corresponding vehicle).
DISCUSSION
The present study is the first to determine the role of chronic postnatal hypoxia in regulating PPAR-γ expression in the newborn mouse lung and the interactions of PPAR-γ with TGF-β. We have previously shown that excessive TGF-β signaling contributes to hypoxia-induced inhibition of alveolar development and abnormal pulmonary arterial remodeling (4), probably attributable to a reduction in Thy-1 by hypoxia, which leads to increased TGF-β activation (25). In the present study, we have shown that chronic hypoxia reduces PPAR-γ and its signaling and that restoration of PPAR-γ signaling using rosiglitazone, a PPAR-γ agonist, was associated with reduced TGF-β signaling as well as attenuation of hypoxia-induced inhibition of alveolar development. This improvement in lung development was associated with less hypoxia-induced accumulation of lung collagen and an improvement in lung compliance and reduction in resistance. Additionally, we have also shown that inhibition of hypoxia-enhanced TGF-β signaling is associated with greater PPAR-γ and its signaling molecules ADRP and leptin. This mutually antagonistic relationship of PPAR-γ to TGF-β signaling that is modulated by hypoxia is a novel finding in the setting of the newborn lung. A schematic of such a possible relationship is shown in Fig. 8, which requires additional study for determination of the underlying mechanisms in this model.
Fig. 8.
Possible schematic of interactions between PPAR-γ with TGF-β signaling in the newborn lung during normoxia (A) and hyperoxia (B). During normal postnatal lung development in air (normoxia) (A), PPAR-γ signaling is high and TGF-β signaling is not excessive, because of inhibition by PPAR-γ. However, during hypoxia (B), an increase in TGF-β signaling inhibits PPAR-γ, reversing the balance between PPAR-γ and TGF-β signaling.
PPAR-γ is necessary for differentiation of the placental cytotrophoblast as well as cardiac and adipose tissue development in utero, and PPAR-γ absence results in embryonic death in midgestation (7). Simon et al. (28) ablated PPAR-γ specifically in airway epithelial cells to study the role of PPAR-γ in lung development and observed enlarged airspaces at 8 wk of age resulting from insufficient postnatal lung development as well as a reduction in structural extracellular matrix gene expression. In addition to the lung epithelial effects and effects on other cell types, PPAR-γ is a modulator of the lung alveolar interstitial fibroblast phenotype (27). In our study, we evaluated mice from birth to 2 wk of age because mouse-lung development in the first two postnatal weeks parallels lung development in humans from week 24 of gestation through the first 2 yr of age (6, 10) because alveolar development is postnatal in mice, whereas it begins in utero and continues postnatally in humans (31, 34).
The increase in PPAR-γ with increasing postnatal age, associated with advancing lung maturation, and the inhibition of this increase with hypoxia and concomitant impairment in lung development, which is attenuated by the PPAR-γ agonist rosiglitazone, suggest that a hypoxia-mediated reduction in the normal postnatal increase in PPAR-γ may contribute to the inhibition of lung development noted with hypoxia. Our findings that greater TGF-β signaling during hypoxia is associated with less PPAR-γ and that inhibition of TGF-β improves PPAR-γ signaling suggest that hypoxia-mediated reductions in PPAR-γ may be secondary, at least in part, to excessive TGF-β signaling. Our results also demonstrate that reductions in PPAR-γ mRNA and protein changes during hypoxia are not of the same magnitude (Figs. 1, 2, and 7); the reduction in PPAR-γ protein with hypoxia exposure was greater than the reduction in PPAR-γ mRNA. These results suggest that the amount of PPAR-γ protein is regulated at a posttranscriptional level, perhaps at the protein stabilization level (16). We observed that the PPAR-γ agonist RGZ, in addition to increasing ADRP and leptin as expected, also increased its receptor PPAR-γ, a phenomenon that has been observed earlier in neonatal rats (27, 32) and has been described in the literature (30).
We demonstrated that hypoxia-induced pulmonary vascular remodeling and RV hypertrophy in the newborn mouse were not significantly affected by RGZ even in the presence of reduced TGF-β signaling. This contrasts with our previous observation that hypoxia-induced pulmonary vascular remodeling is reduced when TGF-β signaling is inhibited (4). Furthermore, studies in adult animal models have shown that RGZ attenuates hypoxia-induced pulmonary vascular remodeling (12, 26). Crossno et al. (12) showed that RGZ treatment of adult rats at 5 mg/kg per day prevented and reversed chronic hypoxia-induced pulmonary vascular remodeling and RV hypertrophy. Nisbet et al. (26) showed that RGZ treatment of adult mice at 10 mg/kg for 3 or 5 wk attenuated chronic hypoxia-induced pulmonary hypertension (26). It is likely that age/developmental effects may be responsible for the differences between our study and that of Crossno et al. (12) and Nisbet et al. (26), as hypoxia-induced pulmonary vascular remodeling in adult animals is an active process of thickening of resistance pulmonary arteries, whereas in newborn animals hypoxia inhibits the normal postnatal reduction in arterial wall thickness. Another possibility is that the dose of RGZ in our study, while sufficient for improving alveolar development and partial inhibition of TGF-β signaling, was inadequate to prevent pulmonary vascular remodeling. We noted that a RGZ dose of 1 mg/kg was well tolerated, whereas a dose of 3 mg/kg was associated with high mortality in newborn mice, compared with the dose of 10 mg/kg, which was well tolerated in adult mice in the study by Nisbet et al. (26). The mechanism of the increased toxicity of RGZ in newborn mice requires further investigation but may be related to age-related immaturity of metabolic pathways in other organ systems, as PPAR-γ expression is normally low in fetal life and at birth (28, 29). This finding is important because toxicity of RGZ and other thiazolidinediones is receiving increasing scrutiny (33), and these drugs have not been inadequately evaluated in the pediatric population.
PPAR-γ ligands have been shown to reduce TGF-β1-mediated increases in α-smooth muscle actin expression and collagen synthesis by lung fibroblasts (9, 23) and reduce lung fibrosis in the bleomycin model in adult mice in vivo (23). This inhibition of TGF-β signaling by PPAR-γ occurs through interference with Smad2 and Smad3 signaling pathways, leading to inhibition of the transcription factor activator protein-1 (17), and by reduction in ERK1/2 phosphorylation (35). We have previously shown that chronic hypoxia exposure increases TGF-β signaling in newborn mouse lung and that attenuation of this increased TGF-β signaling, using a transgenic mouse model with inducible dominant-negative TGF-β receptors, improves lung development and reduces pulmonary vascular remodeling (4). This study extends these observations with the novel findings that the PPAR-γ ligand RGZ inhibits Smad signaling and collagen synthesis with resulting improvements in lung development and function in newborn mice exposed to chronic hypoxia. We also noted that RGZ reduced the hypoxia-induced increases in gene expression of TGF-β1, -β2, and -β3. Similar reductions in pathologically increased TGF-β1 synthesis by PPAR-γ ligands have been reported in the adult mouse models of bleomycin-induced fibrosis (23) and in models of allergic asthma (20). Simon et al. (28) have demonstrated that ablation of PPAR-γ in airway epithelial cells led to impaired lung development similar to that observed with chronic hypoxia exposure, associated with increased TGF-β1 and extracellular matrix proteins such as collagen and elastin. Taken together, these data suggest that chronic hypoxia-induced reduction in PPAR-γ permits increased TGF-β signaling and that RGZ administration dampens TGF-β signaling to normal levels.
Our study has multiple strengths. We evaluated PPAR-γ mRNA and protein expression at multiple time points during a critical phase of postnatal lung development, during the process of alveolar septation. Both lung structure and function were evaluated following exposure to normoxia as well as hypoxia, in combination with either vehicle or RGZ. We confirmed the increase in TGF-β signaling with hypoxia exposure and its attenuation by RGZ in this newborn mouse model and have thus identified a possible mechanism underlying the improvement in alveolarization with RGZ. In addition to evaluating the effect of a PPAR-γ agonist on TGF-β signaling, we also determined the effect of inhibiting TGF-β on PPAR-γ signaling. However, our study has a number of limitations. Mouse models may not closely resemble human disease because of interspecies differences. Chronic hypoxia exposure, although a reproducible and useful animal model, does not closely mimic bronchopulmonary dysplasia but may more accurately simulate the impairment of lung development seen with chronic intrauterine hypoxemia and growth restriction. Experiments using whole lung homogenates do not permit the identification of changes in gene expression or protein synthesis in selected cell populations, such as airway epithelia, endothelial cells, or interstitial cells. RGZ, one of the thiazolidinedione derivatives, is generally considered one of the most potent and selective synthetic ligands of PPAR-γ but may also exhibit some non-PPAR-γ-mediated effects (15, 19). Our studies demonstrate a reduction in TGF-β signaling with a PPAR-γ agonist and an increase in PPAR-γ and its signaling with inhibition of TGF-β but do not identify a mechanism by which these processes occur. The ability of PPAR-γ to inhibit TGF-β signaling may be tissue and cell specific, and the mechanism of inhibition may therefore depend on the specific context.
In conclusion, chronic hypoxia is associated with impairment of lung development and lung function, associated with reduction in PPAR-γ and increase in TGF-β signaling. RGZ, a PPAR-γ agonist, attenuates effects of hypoxia on TGF-β signaling and improves alveolar development but not pulmonary arterial remodeling. PPAR-γ agonists may therefore be a potential therapeutic option in situations of increased TGF-β signaling and impairment of lung development, such as in bronchopulmonary dysplasia (1). However, the risk of toxicity and the risk-to-benefit ratio need to be carefully evaluated in newborn infants and young children because this population may be at higher risk. Inhibition of TGF-β, which we have previously shown to attenuate hypoxia-induced inhibition of alveolarization and vascular remodeling, and now demonstrated to improve PPAR-γ signaling, also has potential as a therapeutic approach.
GRANTS
This work was supported by the Translational Research in Normal and Disordered Development (TReNDD) Program, in part by R01 HL092906, HL044195, HL082818, HL-50147, HL007457, HL56046, a CCRI grant, American Thoracic Society/Pulmonary Hypertension Association grant ATS PH-06-006, a Research Facilities Improvement Program grant C06 RR 15490, and an American Heart Association-0455197B grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Alejandre-Alcazar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Perez J, Wygrecka M, Eul B, Kobrich S, Hesse M, Schermuly RT, Seeger W, Eickelberg O, Morty RE. Hyperoxia modulates TGF-beta/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L537–L549, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Ambalavanan N, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Endothelin-A receptor blockade prevents and partially reverses neonatal hypoxic pulmonary vascular remodeling. Pediatr Res 57: 631–636, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ambalavanan N, Li P, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Endothelin-1 mediates hypoxia-induced increases in vascular collagen in the newborn mouse lung. Pediatr Res 61: 559–564, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ambalavanan N, Nicola T, Hagood J, Bulger A, Serra R, Murphy-Ullrich J, Oparil S, Chen YF. Transforming growth factor-β signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung. Am J Physiol Lung Cell Mol Physiol 295: L86–L95, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ambalavanan N, Nicola T, Li P, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Role of matrix metalloproteinase-2 in newborn mouse lungs under hypoxic conditions. Pediatr Res 63: 26–32, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amy RW, Bowes D, Burri PH, Haines J, Thurlbeck WM. Postnatal growth of the mouse lung. J Anat 124: 131–151, 1977 [PMC free article] [PubMed] [Google Scholar]
- 7. Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 4: 585–595, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Blanco LN, Massaro D, Massaro GD. Alveolar size, number, and surface area: developmentally dependent response to 13% O2. Am J Physiol Lung Cell Mol Physiol 261: L370–L377, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Burgess HA, Daugherty LE, Thatcher TH, Lakatos HF, Ray DM, Redonnet M, Phipps RP, Sime PJ. PPARγ agonists inhibit TGF-β induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol 288: L1146–L1153, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Burri PH. Structural aspects of postnatal lung development— alveolar formation and growth. Biol Neonate 89: 313–322, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1-postnatal lung growth. Thorax 37: 572–579, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crossno JT, Jr, Garat CV, Reusch JE, Morris KG, Dempsey EC, McMurtry IF, Stenmark KR, Klemm DJ. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am J Physiol Lung Cell Mol Physiol 292: L885–L897, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Dasgupta C, Sakurai R, Wang Y, Guo P, Ambalavanan N, Torday JS, Rehan VK. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-β and Wnt signaling and is protected by rosiglitazone. Am J Physiol Lung Cell Mol Physiol 296: L1031–L1041, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fu M, Zhang J, Lin Y, Zhu X, Zhao L, Ahmad M, Ehrengruber MU, Chen YE. Early stimulation and late inhibition of peroxisome proliferator-activated receptor gamma (PPAR gamma) gene expression by transforming growth factor beta in human aortic smooth muscle cells: role of early growth-response factor-1 (Egr-1), activator protein 1 (AP1) and Smads. Biochem J 370: 1019–1025, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galli A, Ceni E, Crabb DW, Mello T, Salzano R, Grappone C, Milani S, Surrenti E, Surrenti C, Casini A. Antidiabetic thiazolidinediones inhibit invasiveness of pancreatic cancer cells via PPARgamma independent mechanisms. Gut 53: 1688–1697, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gani OA, Sylte I. Ligand-induced stabilization and activation of peroxisome proliferator-activated receptor gamma. Chem Biol Drug Des 72: 50–57, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Guo B, Koya D, Isono M, Sugimoto T, Kashiwagi A, Haneda M. Peroxisome proliferator-activated receptor-gamma ligands inhibit TGF-beta 1-induced fibronectin expression in glomerular mesangial cells. Diabetes 53: 200–208, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Han J, Hajjar DP, Tauras JM, Feng J, Gotto AM, Jr, Nicholson AC. Transforming growth factor-beta1 (TGF-beta1) and TGF-beta2 decrease expression of CD36, the type B scavenger receptor, through mitogen-activated protein kinase phosphorylation of peroxisome proliferator-activated receptor-gamma. J Biol Chem 275: 1241–1246, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Han S, Zheng Y, Roman J. Rosiglitazone, an agonist of PPARgamma, inhibits non-small cell carcinoma cell proliferation in part through activation of tumor sclerosis complex-2 (Abstract). PPAR Res 2007: 29632, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Honda K, Marquillies P, Capron M, Dombrowicz D. Peroxisome proliferator-activated receptor gamma is expressed in airways and inhibits features of airway remodeling in a mouse asthma model. J Allergy Clin Immunol 113: 882–888, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Massaro D, Massaro GD. Invited Review: pulmonary alveoli: formation, the “call for oxygen,” and other regulators. Am J Physiol Lung Cell Mol Physiol 282: L345–L358, 2002 [DOI] [PubMed] [Google Scholar]
- 22. McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambon P, Snyder JM. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol 23: 162–167, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Milam JE, Keshamouni VG, Phan SH, Hu B, Gangireddy SR, Hogaboam CM, Standiford TJ, Thannickal VJ, Reddy RC. PPAR-gamma agonists inhibit profibrotic phenotypes in human lung fibroblasts and bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L891–L901, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakanishi H, Sugiura T, Streisand JB, Lonning SM, Roberts JD., Jr TGF-β-neutralizing antibodies improve pulmonary alveologenesis and vasculogenesis in the injured newborn lung. Am J Physiol Lung Cell Mol Physiol 293: L151–L161, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Nicola T, Hagood JS, James ML, Macewen MW, Williams TA, Hewitt MM, Schwiebert L, Bulger A, Oparil S, Chen YF, Ambalavanan N. Loss of Thy-1 inhibits alveolar development in the newborn mouse lung. Am J Physiol Lung Cell Mol Physiol 296: L738–L750, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol 42: 482–490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rehan VK, Wang Y, Patel S, Santos J, Torday JS. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, prevents hyperoxia-induced neonatal rat lung injury in vivo. Pediatr Pulmonol 41: 558–569, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Simon DM, Arikan MC, Srisuma S, Bhattacharya S, Tsai LW, Ingenito EP, Gonzalez F, Shapiro SD, Mariani TJ. Epithelial cell PPARγ contributes to normal lung maturation. FASEB J 20: 1507–1509, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Simon DM, Mariani TJ. Role of PPARs and retinoid x receptors in the regulation of lung maturation and development (Abstract). PPAR Res 2007: 91240, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Su JL, Winegar DA, Wisely GB, Sigel CS, Hull-Ryde EA. Use of a PPAR gamma-specific monoclonal antibody to demonstrate thiazolidinediones induce PPAR gamma receptor expression in vitro. Hybridoma 18: 273–280, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Thurlbeck WM. Postnatal growth and development of the lung. Am Rev Respir Dis 111: 803–844, 1975 [DOI] [PubMed] [Google Scholar]
- 32. Wang Y, Santos J, Sakurai R, Shin E, Cerny L, Torday JS, Rehan VK. Peroxisome proliferator-activated receptor gamma agonists enhance lung maturation in a neonatal rat model. Pediatr Res 65: 150–155, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Woodcock J, Sharfstein JM, Hamburg M. Regulatory action on rosiglitazone by the U.S. Food and Drug Administration. N Engl J Med 363: 1489–1491, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Zeltner TB, Burri PH. The postnatal development and growth of the human lung. II. Morphol Respir Physiol 67: 269–282, 1987 [DOI] [PubMed] [Google Scholar]
- 35. Zhao C, Chen W, Yang L, Chen L, Stimpson SA, Diehl AM. PPARgamma agonists prevent TGFbeta1/Smad3-signaling in human hepatic stellate cells. Biochem Biophys Res Commun 350: 385–391, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]