Abstract
Alveolar barrier function depends critically on the claudin family tight junction proteins. Of the major claudins expressed by alveolar epithelial cells, claudin (Cldn)-3 and Cldn-4 are the most closely related by amino acid homology, yet they differ dramatically in the pattern of expression. Previously published reports have shown that Cldn-3 is predominantly expressed by type II alveolar epithelial cells; Cldn-4 is expressed throughout the alveolar epithelium and is specifically upregulated in response to acute lung injury. Using primary rat alveolar epithelial cells transduced with yellow fluorescent protein-tagged claudin constructs, we have identified roles for Cldn-3 and Cldn-4 in alveolar epithelial barrier function. Surprisingly, increasing expression of Cldn-3 decreased alveolar epithelial barrier function, as assessed by transepithelial resistance and dye flux measurements. Conversely, increasing Cldn-4 expression improved alveolar epithelial transepithelial resistance compared with control cells. Other alveolar epithelial tight junction proteins were largely unaffected by increased expression of Cldn-3 and Cldn-4. Taken together, these results demonstrate that, in the context of the alveolar epithelium, Cldn-3 and Cldn-4 have different effects on paracellular permeability, despite significant homology in their extracellular loop domains.
Keywords: tight junction, alveolus, lung barrier
in the healthy lung, the alveolar epithelium acts as a barrier to fluid leak and regulates ion transport to promote fluid absorption from the alveolar space (17, 26, 33). Conversely, insults that compromise the alveolar barrier promote the accumulation of fluid within the air space, leading to acute lung injury. Importantly, the severity of injury is directly related to the level of lung epithelial barrier dysfunction (49). Epithelial barrier function is critically dependent on tight junctions, structures localized to sites of cell-cell contact. Tight junctions control fluid diffusion between cells by creating a barrier that specifically regulates paracellular permeability to ions and small molecules.
Claudins, a large family of tetraspan transmembrane proteins, form the structural basis for regulated tight junction permeability (2, 3, 30). Overall paracellular permeability is determined by the pattern of claudin expression in a particular epithelial cell type. Of the 24 claudin family proteins, >6, including claudin (Cldn)-1, Cldn-3, Cldn-4, Cldn-5, Cldn-7, and Cldn-18, have been found to be expressed by alveolar epithelial cells (8, 14, 29, 51). Of these, type II alveolar epithelial cells are enriched for Cldn-3; type I cells have low Cldn-3 protein content (24, 31, 48). In contrast, Cldn-4, which is closely homologous to Cldn-3, is expressed by type I and type II cells. While patterns of claudin expression in the alveolar epithelium are emerging, our understanding of the functional roles for each of the specific claudins remains incomplete.
Recently, Wray et al. (51) determined that, in the lung, Cldn-4 is upregulated in response to ventilator-induced lung injury. The finding of a specific increase in Cldn-4 expression allowed the investigators to use a peptide fragment (CPEBD) derived from Clostridium perfringens enterotoxin (CPE) as a probe for Cldn-4 function (35). CPEBD administered in vivo significantly decreased Cldn-4 content of the lung. Functionally, CPEBD treatment dramatically increased pulmonary edema and bulk alveolar protein permeability (leak) in response to severe ventilator-induced lung injury, underscoring a role for Cldn-4 in protecting the lung from mechanical injury to tight junctions (51). Cultured alveolar epithelial cells treated with CPEBD also exhibited a decrease in Cldn-4 and a concomitant decrease in transepithelial resistance (TER). In addition, a small interfering RNA-mediated reduction in Cldn-4 led to a similar decrease in TER, suggesting that the changes in lung alveolar epithelium after treatment with CPEBD are specific to Cldn-4 (51). Consistent with these observations, alveolar Cldn-4 is also decreased in a cecal ligation-and-puncture model of sepsis, which could also exacerbate lung function due to a decrease in alveolar epithelial barrier function (9).
Although CPEBD has considerable utility as a tool for manipulating claudins in vivo, one drawback is that CPEBD binds strongly to Cldn-3 and Cldn-4, both of which are present in the alveolus (28). Indeed, Wray et al. (51) showed that, in lungs treated with CPEBD, Cldn-3 and Cldn-4 were decreased, and a similar result was obtained in cultured epithelial cells. Since the first extracellular loop domains of Cldn-3 and Cldn-4 are 94% identical at the amino acid level (15), these two claudins would be expected to serve similar roles in regulating paracellular ion permeability. However, this possibility has not been directly examined in the context of the alveolar epithelium.
To identify roles for Cldn-3 and Cldn-4 in regulating alveolar epithelial barrier function, we generated adenovector expression constructs to transduce primary alveolar epithelial cells to increase expression of Cldn-3 or Cldn-4. Increasing Cldn-4 expression augmented alveolar epithelial TER; however, increasing Cldn-3 expression had the opposite effect. Moreover, increasing Cldn-3 increased short-circuit current (Isc) and paracellular permeability to aqueous molecules. These results indicate that Cldn-3 and Cldn-4 have distinct roles in regulating alveolar epithelial barrier function, despite a nearly identical amino acid sequence in the extracellular loop domain responsible for determining the charge specificity of their paracellular permeability.
MATERIALS AND METHODS
Isolation and culture of rat type II alveolar epithelial cells.
Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Emory University. Sprague-Dawley rat type II alveolar epithelial cells were isolated from lungs lavaged and perfused with elastase using the method of Dobbs et al. (16) with modifications (1). Preparations routinely contained >90–95% type II alveolar epithelial cells. Freshly isolated cells were cultured in Earle's MEM (Life Technologies, Rockville, MD) containing 10% FBS, 25 μg/ml gentamicin, and 0.25 μg/ml amphotericin B (Life Technologies) in Transwell supports coated with rat tail type I collagen (Roche Diagnostics, Mannheim, Germany) at 7.5 × 105 cells/ml, as previously described (29).
Adenoviral vector production and transduction.
NH2-terminal, enhanced yellow fluorescent protein (YFP)-tagged Cldn-3 and Cldn-4 cDNAs were produced as previously described (15), removed using KpnI and XbaI, and then ligated into pAdLox using standard molecular biological techniques. YFP-Cldn-3/AdLox, YFP-Cldn-4/AdLox, and YFP control vector were packaged and amplified by the National Heart, Lung, and Blood Institute Viral Vector Core at the University of Pittsburgh.
Alveolar epithelial cells cultured for 4 days on Transwell supports were incubated with adenovector constructs added to the apical and basal sides and then analyzed at day 6. Adenovector titers were adjusted to yield comparable levels of cell transduction and transgene protein expression.
Barrier function measurements.
TER of cells in medium cultured on permeable supports was measured using an Ohmmeter (World Precision Instruments, Sarasota, FL), as previously described, with cells in Ringer buffer (150 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 10 mM HEPES, pH 7.4) (14, 48). Ussing chamber measurements were performed as previously described (29) with a VCC MC6/A&A II data analysis system (Physiologic Instruments, San Diego, CA). For measurement of Isc, cells on Snapwell inserts (Corning Life Sciences, Lowell, MA) were analyzed in Ringer buffer at 37°C. The ratio of Na permeability to Cl permeability (PNa/PCl) was calculated by the method of Hou et al. (21) from dilution potentials measured from monolayers with Ringer buffer on one side and Ringer buffer diluted 1:1 with Ringer solution in which 300 mM mannitol was substituted for NaCl to maintain osmotic neutrality on the other side. Apical-to-basal and basal-to-apical dilution potentials were measured. Paracellular dye permeability was assessed by simultaneous measurement of the diffusion of two different-sized fluorescent dyes across the cell monolayer, as previously described (48). Briefly, the medium in the bottom chamber was replaced with PBS + 0.675 mM CaCl2 + 0.2 mM MgCl2, and the medium in the top chamber was replaced with PBS + Ca + Mg containing 0.1 mg/ml calcein (0.66 kDa) and 1.0 mg/ml Texas Red dextran (10 kDa). The cells were further incubated at 37°C for 2 h. At varying intervals, the cell chamber was removed, and the amount of fluorophore that diffused into the lower chamber was measured using a multiwell plate fluorometer (PE Biosystems, Foster City, CA).
Immunofluorescence staining.
Immunofluorescence staining was performed as previously described (14, 48). Unless otherwise stated, antibodies were obtained from Invitrogen (Carlsbad, CA). After 6 days in culture, the cells were washed with PBS three times, fixed in 1:1 methanol-acetone for 2 min at room temperature, and washed three times with PBS, once with PBS + 0.5% Triton X-100, and once with PBS + 0.5% Triton X-100 + 2% normal goat serum. Cells were incubated with primary anti-rabbit and/or anti-mouse antibodies in PBS + 2% normal goat serum for 1 h, washed, incubated with Cy2-conjugated goat anti-rabbit IgG and Cy3-conjugated goat anti-mouse IgG (Jackson Immunoresearch) in PBS + 2% normal goat serum, washed, and then mounted in Mowiol (Kuraray, Houston, TX) under a glass coverslip. Cells were imaged by phase-contrast and fluorescence microscopy using an Olympus IX70 microscope with a U-MWIBA filter pack (BP460–490, DM505, BA515–550) or U-MNG filter pack (BP530–550, DM570, BA590–800+) or by confocal immunofluorescence microscopy using an Olympus Fluoview FV1000 system. Minimum and maximum intensities were adjusted for images in parallel, so that the intensity scale remained linear to maximize dynamic range.
Biochemical analysis.
After 6 days in culture, cells on permeable supports were harvested and lysed in 2× sample buffer containing 50 mM DTT, resolved by SDS-PAGE, transferred to Immobilon membranes (Millipore, Billerica, MA), and blotted using primary antibodies and horseradish peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG. Specific signals corresponding to a given protein were detected by immunoblot analysis using enhanced chemiluminescence reagent (GE Healthcare, Pittsburgh, PA) and quantified with an electrophoresis documentation and analysis system (Eastman Kodak, Rochester, NY). Normalization for protein content was done using parallel samples analyzed for actin. Tight junction protein content for samples from cells treated with YFP, YFP-Cldn-3, and YFP-Cldn-4 adenovectors was further normalized to values of samples from untreated cells. The amount of Cldn-4 relative to Cldn-3 was calculated using anti-Cldn-3, anti-Cldn-4, and anti-YFP immunoblots by the following formula: (endogenous Cldn-4/endogenous Cldn-3) = (endogenous Cldn-4anti-Cldn-4/YFP-Cldn-4anti-Cldn-4) × (YFP-Cldn-4anti-YFP/YFP-Cldn-3anti-YFP) × (YFP-Cldn-3anti-Cldn-3/endogenous Cldn-3anti-Cldn-3).
For biochemical analysis of Triton X-100 extractability (14), cells were harvested by scraping into 1 ml of PBS at 4°C, which was then brought to 0.5% Triton X-100 by addition of 25 μl of 20% Triton X-100. The samples were incubated for 30 min at 4°C, centrifuged at 100,000 g for 30 min, and separated into Triton-soluble (supernatant) and -insoluble (pellet) fractions, which were equivalently diluted and then analyzed by SDS-PAGE and immunoblotting (see above). Statistical significance was calculated by t-test.
RESULTS
Two closely related claudins, Cldn-3 and Cldn-4, show significant differences in expression by alveolar epithelium. Cldn-3 is preferentially expressed by type II cells, whereas Cldn-4 is expressed throughout the alveolus and is upregulated in response to acute lung injury (8, 14, 29, 51). Given the structural similarities between the two proteins, particularly in the extracellular loop domains, we sought to determine the effect of increased expression of Cldn-3 and Cldn-4 on alveolar epithelial cell barrier function. We developed adenoviral vectors that enabled transduction of primary alveolar epithelial cells with YFP-tagged Cldn-3 or Cldn-4. The YFP tag was added to the NH2 terminus of each claudin, a modification previously shown not to interfere with proper claudin function, since it leaves the COOH terminus able to interact with tight junction scaffold proteins (e.g., ZO-1). Additionally, an NH2-terminal green fluorescent protein tag does not affect the ability of the extracellular loop domains to form functional tight junctions (32).
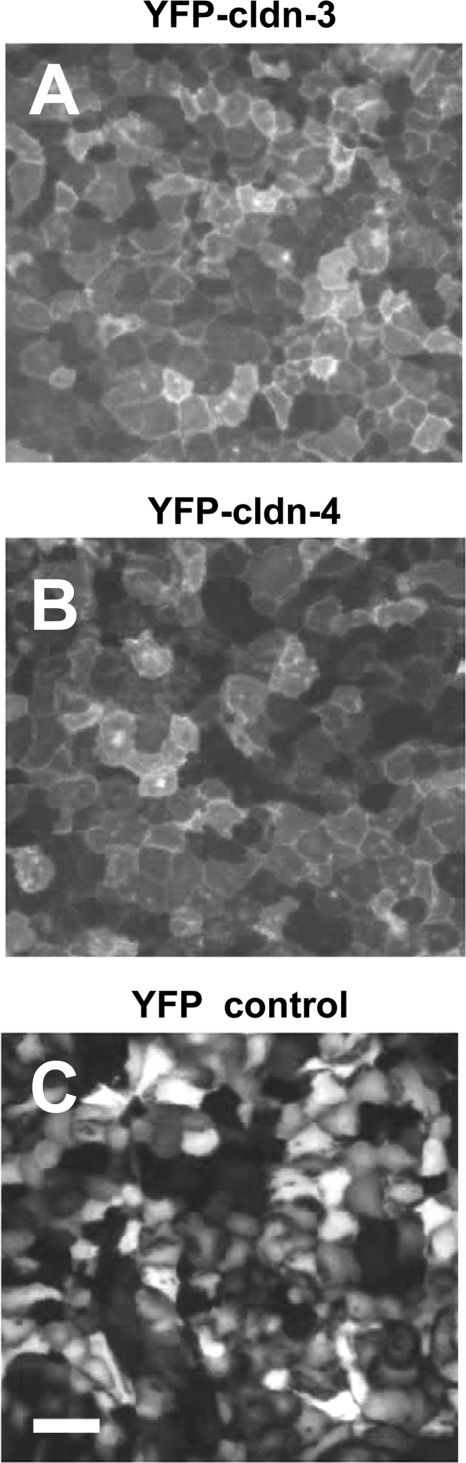
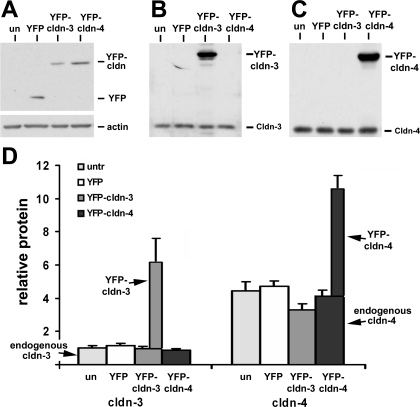
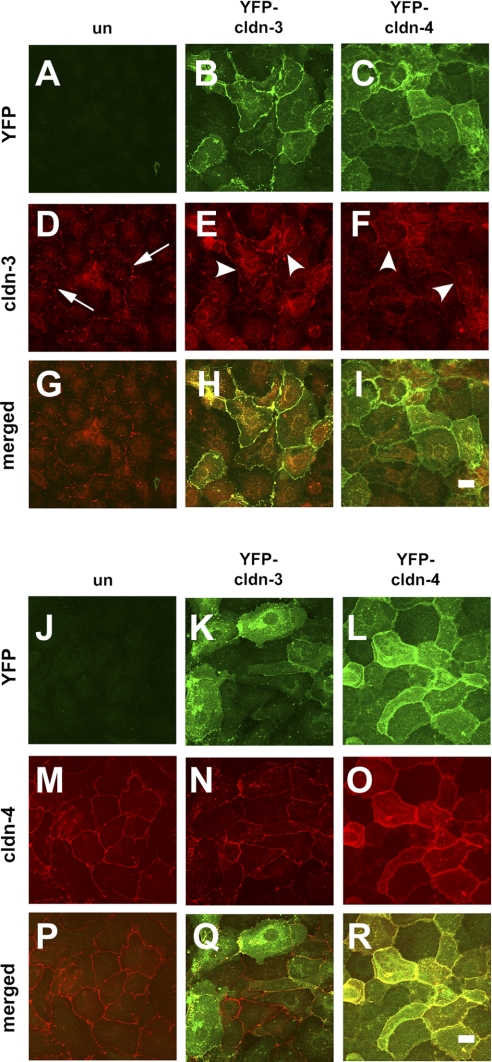
To determine the effect of expression on alveolar epithelial function, primary type II alveolar epithelial cells were plated onto Transwell permeable supports to generate a model alveolar epithelial cell system (20) in which claudins found in type I cells are expressed (29, 48). On day 4 of culture, cells were treated overnight with adenovectors to enable expression of YFP-Cldn-3, YFP-Cldn-4, or YFP control. Cells were further incubated for 2 days to achieve optimal transgene expression (Fig. 1) and analyzed on day 6 of culture, similar to our previous analysis (29). Under these conditions, expression of YFP-Cldn-3 relative to actin was 1.17 ± 0.37 (n = 5), and expression of YFP-Cldn-4 relative to actin was 0.86 ± 0.07 (n = 6). YFP-Cldn-3 and YFP-Cldn-4 were properly processed by alveolar epithelial cells, given that both localized to tight junctions at the plasma membrane, similar to untagged endogenous claudins (Fig. 1, A and B). We found that transducing alveolar epithelial cells with YFP, YFP-Cldn-3, or YFP-Cldn-4 did not have a significant effect on the total protein level of endogenous Cldn-3 or Cldn-4 (Fig. 2).
Fig. 1.
Yellow fluorescent protein (YFP)-tagged claudin (Cldn)-3 and Cldn-4 are expressed and properly transported by alveolar epithelial cells. Primary rat alveolar epithelial cells were transduced on day 4 with YFP-Cldn-3 (A), YFP-Cldn-4 (B), or YFP control vector (C) and then imaged live by fluorescence microscopy on day 6. Note efficient cell transduction and prominent plasma membrane localization of YFP-Cldn-3 and YFP-Cldn-4. Scale bar, 100 μm.
Fig. 2.
Transduced alveolar epithelial cells express YFP-Cldn-3 and YFP-Cldn-4 within a physiological range. A: immunoblot analysis using anti-green fluorescent protein antibodies showed comparable expression of YFP-Cldn-3 and YFP-Cldn-4 in cells collected on day 6. B and C: immunoblot analysis of samples using anti-Cldn-3 (B) or Cldn-4 (C) antibodies demonstrates the presence of the claudin-specific epitope for both constructs. Note relative difference in expression of endogenous Cldn-3 (B) and Cldn-4 (C). un, Untreated. D: use of YFP-tagged constructs enabled Cldn-3 and Cldn-4 protein content to be uniformly normalized relative to each other. Note ∼5-fold more endogenous Cldn-4 than endogenous Cldn-3 in alveolar epithelial cells on day 6. Transduction with any of the adenovector constructs had little effect on endogenous Cldn-3 or Cldn-4 compared with untreated (untr) cells. Values are means ± SE (n = 4–6).
Using anti-YFP to normalize for YFP-Cldn-3 and YFP-Cldn-4, we were able to calculate the ratio of endogenous Cldn-4 to Cldn-3 expression (see materials and methods). On the basis of these results, we found that model type I alveolar epithelial cells expressed about fourfold more Cldn-4 than Cldn-3 protein (Fig. 2D). Model type I cells express low levels of endogenous Cldn-3, consistent with type I cells in vivo (31). In fact, the amount of Cldn-3 expressed by type II cells was found by LaFemina and co-workers (31) to be ∼16-fold higher than the amount of Cldn-3 expressed by type I cells.
Transducing cells with YFP-Cldn-3 increased total Cldn-3 expression by 6.8 ± 3.7-fold (n = 5) over baseline (Fig. 2D). Thus the increase in total Cldn-3 in cells expressing YFP-Cldn-3 was consistent with the physiological difference in Cldn-3 expression between type II and type I cells. In YFP-Cldn-4-transduced cells, total Cldn-4 protein increased to 2.7 ± 0.2-fold (n = 6) higher than in nontransduced cells (Fig. 2D). This level was ∼50% higher than the increase in Cldn-4 protein observed by Wray and co-workers (51) in response to ventilator-induced lung injury, which caused Cldn-4 to increase to ∼1.8-fold higher than baseline.
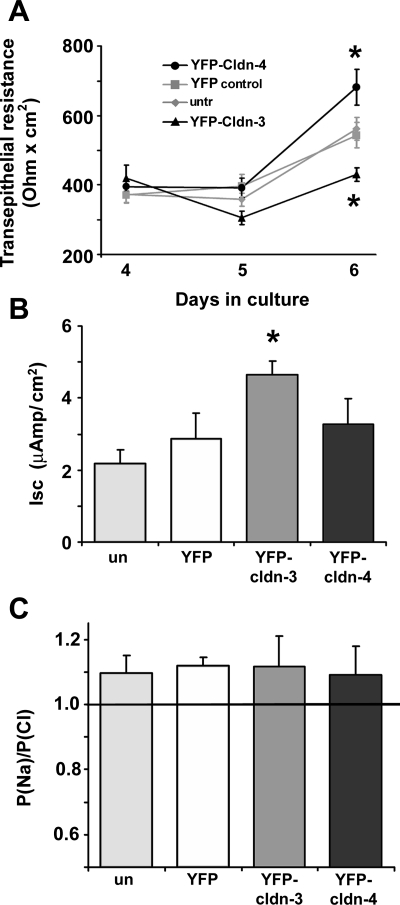
To examine the effect of increased Cldn-3 and Cldn-4 on alveolar epithelial barrier function, we initially measured TER. On day 4, prior to transduction, all alveolar epithelial cells had comparable TER, ∼400 Ω·cm2 (Fig. 2A). However, by day 6, alveolar epithelial cells expressing YFP-Cldn-4 showed a significant increase in TER relative to untreated and control YFP-treated cells. Surprisingly, the TER of cells expressing YFP-Cldn-3 was significantly lower than that of untreated or control YFP-treated cells (Fig. 3A), indicating that Cldn-3 induced a decrease in alveolar barrier function. Importantly, TER of untreated and control YFP-treated cells was comparable throughout the course of the experiment, demonstrating that the adenovector transduction did not alter alveolar barrier function.
Fig. 3.
Cldn-3 and Cldn-4 differentially affect alveolar epithelial barrier function. A: primary rat alveolar epithelial cells were untreated (untr) or transduced on day 4 with YFP-Cldn-3, YFP-Cldn-4, or YFP alone and then analyzed for changes in transepithelial resistance (TER). By day 6, TER was significantly higher in cells expressing YFP-Cldn-4 than in cells expressing YFP alone or untreated controls. TER was significantly lower in cells expressing YFP-Cldn-3 than in cells expressing YFP alone or untreated cells. Values are means ± SE (n = 16–32). *P < 0.05. B: short-circuit current (Isc) was significantly higher on day 6 in cells expressing YFP-Cldn-3 than controls. Values are means ± SE (n = 6). *P < 0.05. Isc was comparable in cells expressing YFP-Cldn-4 and YFP alone on day 6. C: epithelial permeability to Na relative to Cl (PNa/PCl) was comparable in cells expressing YFP-Cldn-3 or YFP-Cldn-4 and YFP alone, reflecting a slight preference for Na over Cl permeability. Values are means ± SE (n = 6).
We also determined that Isc measurements for alveolar epithelial monolayers were comparable to previously published values (25, 27) (Fig. 3B). Isc was significantly higher for cells expressing YFP-Cldn-3 than for controls. By contrast, Isc was comparable in cells expressing YFP-Cldn-4 and control cells. While expression of YFP-Cldn-3 and YFP-Cldn-4 had different effects on Isc and TER, increased expression of these two claudins did not alter the relative charge specificity of alveolar epithelial tight junctions (Fig. 3C) PNa/PCl of cells expressing YFP-Cldn-3 and YFP-Cldn-4 was comparable to that of control cells (PNa/PCl ∼1.1), reflecting a slight preference for Na over Cl permeability (7). PNa/PCl was comparable when dilution potentials were determined in the apical-to-basal direction, or vice versa (data not shown). This result is consistent with the high degree of homology between the first extracellular loop domain of Cldn-3 and Cldn-4 (15), which is responsible for claudin charge specificity (11).
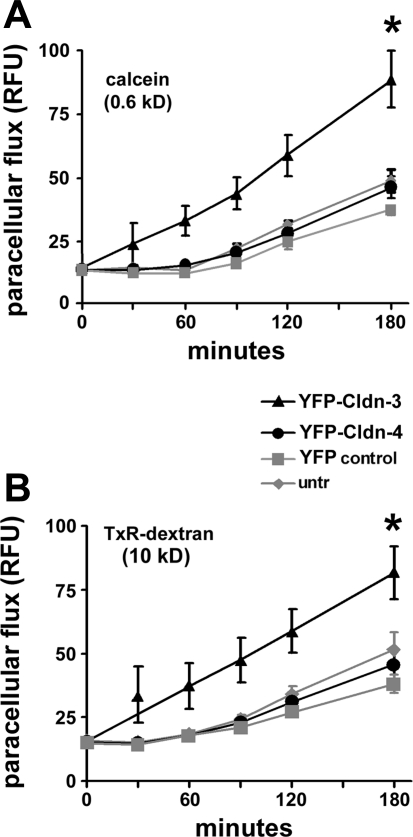
As another measure of alveolar barrier function, we examined paracellular permeability to two fluorescent aqueous tracers: a small molecule (0.62 kDa), calcein, and a macromolecule (10 kDa), Texas Red dextran (Fig. 4). Cells expressing YFP-Cldn-3 showed significant increases in the paracellular permeability of both fluorescent dyes, a result that further supports a decreased barrier function in response to increased Cldn-3. In contrast, paracellular permeability to fluorescent dyes was comparable in cells expressing YFP-Cldn-4 or YFP alone and untreated control cells.
Fig. 4.
Cldn-3 and Cldn-4 differentially affect alveolar epithelial paracellular permeability. Primary rat alveolar epithelial cells were untreated (untr) or transduced on day 4 with YFP-Cldn-3, YFP-Cldn-4, or YFP control vector and then analyzed for changes in paracellular permeability to fluorescent tracer molecules. By day 6, paracellular permeability to calcein (A) and Texas Red (TxR) dextran (B) was significantly greater for cells expressing YFP-Cldn-3, indicating an overall decrease in permeability to small and macromolecular tracers. YFP-Cldn-4 expression had little effect on paracellular dye permeability. Values are means ± SE (n = 8). *P < 0.05.
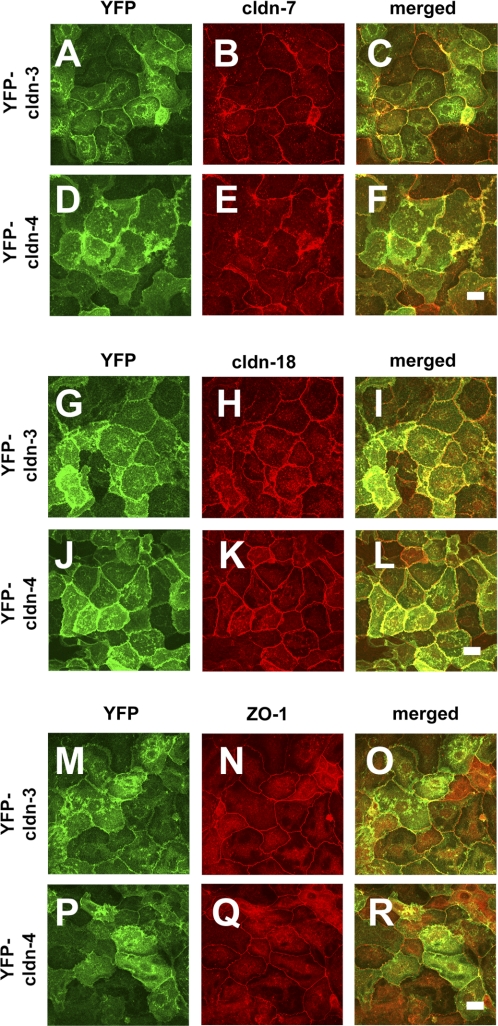
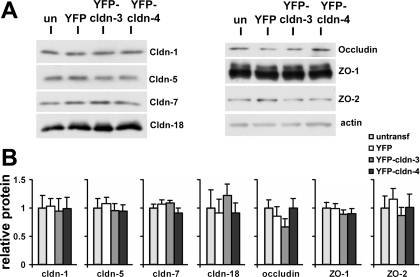
Given these findings, we performed a detailed analysis to determine how increasing Cldn-3 or Cldn-4 had affected other, endogenous tight junction proteins. Immunofluorescence microscopy analysis showed that the tight junction morphology of cells expressing YFP-Cldn-3 and YFP-Cldn-4 was comparable to that of control cells (Figs. 5 and 6), demonstrating that expression of these proteins was not deleterious to the cells. YFP-Cldn-3 and YFP-Cldn-4 colocalized with the other claudins, occludin, and ZO-1, consistent with previous results (8, 14, 48, 51). Although YFP-Cldn-3 had little effect on the localization of endogenous Cldn-4 (Fig. 5N), in some areas, we found a more continuous distribution of endogenous Cldn-3 in cells expressing YFP-Cldn-4 along the plasma membrane (Fig. 5E), rather than the more punctate appearance we observed in control cells (Fig. 5D). The change in Cldn-3 induced by YFP-Cldn-4 suggests that, at a certain threshold, Cldn-4 can alter incorporation of Cldn-3 into tight junctions, most likely by a heteromeric interaction (15). Consistent with images obtained by immunofluorescence microscopy (Fig. 6), immunoblot analysis of YFP-Cldn-3 and YFP-Cldn-4 demonstrated comparable levels of several other tight junction proteins, including Cldn-1, Cldn-5, Cldn-7, Cldn-18, occludin, ZO-1, and ZO-2, in whole cell lysates (Fig. 7).
Fig. 5.
YFP-Cldn-3 and YFP-Cldn-4 colocalize with endogenous Cldn-3 and Cldn-4. Primary rat alveolar epithelial cells were untreated (un; A, D, G, J, M, P) or transduced on day 4 with YFP-Cldn-3 (B, E, H, K, N, Q) or YFP-Cldn-4 (C, F, I, L, O, R), further incubated for 2 days, and then fixed and analyzed by immunofluorescence microscopy for Cldn-3 (D–F) or Cldn-4 (M–O). In control cells, Cldn-3 showed a punctate pattern of tight junction labeling (D; arrows). Cldn-3 was more uniform in cells expressing YFP-Cldn-3 or YFP-Cldn-4 (E, F; arrowheads). Scale bars, 10 μm.
Fig. 6.
YFP-Cldn-3 and YFP-Cldn-4 do not disrupt other endogenous tight junction proteins. Primary rat alveolar epithelial cells were transduced on day 4 with YFP-Cldn-3 (A–C, G–I, M–O) or YFP-Cldn-4 (D–F, J–L, P–R), further incubated for 2 days, and then fixed and analyzed by immunofluorescence microscopy for Cldn-7 (B, E), Cldn-18 (H, K) or ZO-1 (N, Q). As previously described (29), distribution of Cldn-7 was more irregular than Cldn-18 or ZO-1, which exhibited a more uniform labeling of tight junctions. Induced expression of YFP-Cldn-3 or YFP-Cldn-4 had little effect on localization of these endogenous tight junction proteins. Scale bars, 10 μm.
Fig. 7.
Total endogenous tight junction protein content was not affected by increased expression of Cldn-3 or Cldn-4. A: primary rat alveolar epithelial cells were untreated (un) or transduced on day 4 with YFP-Cldn-3, YFP-Cldn-4, or YFP alone, further incubated for 2 days, and then subjected to immunoblot analysis for expression of Cldn-1, Cldn-5, Cldn-7, Cldn-18, occludin, ZO-1, and ZO-2. B: expression of YFP-Cldn-3 or YFP-Cldn-4 did not have a significant effect on expression of these endogenous tight junction proteins as measured by densitometric analysis of tight junction protein expression normalized using parallel samples analyzed for actin. Values are means ± SE (n = 3–4).
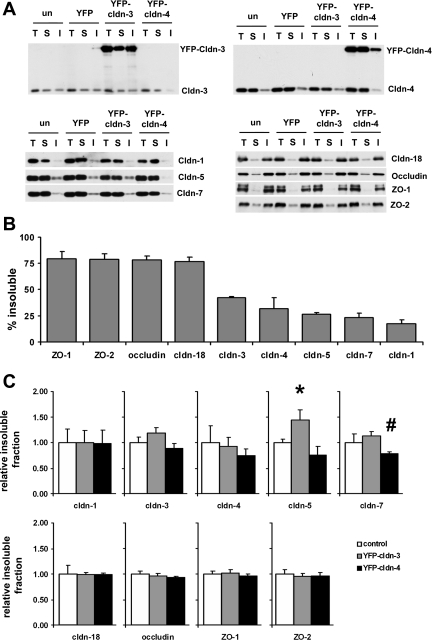
To address the possibility that the junction protein assembly might be affected by expression of YFP-Cldn-3 or YFP-Cldn-4, we used a biochemical assay in which proteins were extracted from cells using 0.5% Triton X-100. At this concentration of Triton X-100, the insoluble fraction primarily reflects proteins incorporated into tight junctions (43, 47). Our rationale in using this approach was that a biochemical assay would allow us to detect changes in tight junction proteins that are undetectable at the level of indirect immunofluorescence microscopy. In untreated alveolar epithelial cells, tight junction proteins ranged from ∼75% insoluble (ZO-1, ZO-2 occludin, and Cldn-18) to <20% insoluble (Cldn-5, Cldn-7, and Cldn-1; Fig. 8, A and B). The cytosolic proteins ZO-1 and ZO-2 were the least soluble of the tight junction proteins, consistent with their role as scaffold proteins that cross-link transmembrane tight junctions to the cytoskeleton (22, 23, 47). Cldn-18 was also significantly more insoluble than the other claudins. Importantly, the array of claudin solubility revealed a much broader range for alveolar epithelial claudins than has been previously demonstrated.
Fig. 8.
Alveolar epithelial tight junction proteins exhibit a range of Triton X-100 insolubility. A: primary rat alveolar epithelial cells were untreated or transduced on day 4 with YFP-Cldn-3, YFP-Cldn-4, or YFP alone, further incubated for 2 days, and then analyzed by Triton X-100 extraction to quantify the insoluble pool of Cldn-1, Cldn-3, Cldn-4, Cldn-5, Cldn-7, Cldn-18, occludin, ZO-1, and ZO-2. T, total protein samples; S, Triton X-100-soluble fractions; I, Triton X-100-insoluble fractions. B: by densitometric analysis, alveolar epithelial tight junction proteins isolated from untreated cells showed a range of insolubility from 75% to 15%. Values are means ± SE (n = 3–4). C: cells expressing YFP-Cldn-3 showed significantly more Cldn-5 associated with the Triton X-100-insoluble pool than controls or cells expressing YFP-Cldn-4 (*P < 0.05). Cells expressing YFP-Cldn-4 exhibited decreased Cldn-7 insolubility compared with cells expressing YFP-Cldn-3 (#P < 0.05).
In keeping with our previous findings, endogenous Cldn-3 had intermediate Triton X-100 solubility, while Cldn-4 was mainly soluble (14). Compared with endogenous Cldn-3 and Cldn-4, YFP-Cldn-3 and YFP-Cldn-4 had comparable solubility characteristics, consistent with the ability of these tagged claudins to properly associate with scaffold proteins [51.1 ± 1.3% for YFP-Cldn-3 (n = 4), 47.4 ± 1.8% for Cldn-3 (n = 3), 29.7 ± 4.0% for YFP-Cldn-4 (n = 3), and 34.3 ± 4.3% for Cldn-4 (n = 3)]. However, we found that that expression of YFP-Cldn-3 and YFP-Cldn-4 had differential effects on the solubility of other alveolar epithelial claudins (Fig. 8C). Cells expressing YFP-Cldn-3 showed a significant increase in the pool of insoluble Cldn-5 compared with untreated control cells or cells expressing YFP-Cldn-4. By contrast, significantly less Cldn-7 was associated with the Triton X-100-insoluble pool in YFP-Cldn-4- than YFP-Cldn-3-expressing cells (Fig. 8C). The Triton X-100 solubility of the other tight junction proteins was not affected by YFP-Cldn-3 or YFP-Cldn-4 expression (Fig. 8C).
Taken together, these data suggest that the predominant effect of YFP-Cldn-3 and YFP-Cldn-4 on alveolar epithelial barrier function was due a specific increase in expression and utilization of Cldn-3 or Cldn-4 in tight junctions as opposed to a major effect of altering these claudins on expression or localization of other alveolar epithelial tight junction proteins.
DISCUSSION
In the uninjured lung, the alveolar epithelium acts as a barrier to fluid leak and regulates ion transport to promote fluid absorption from the alveolar space. The overall paracellular permeability characteristics of an epithelium are determined by the pattern of claudin expression. Several different claudins have been found to be expressed by alveolar epithelial cells; however, our understanding of the functional roles of specific individual claudins in the alveolar epithelium is incomplete.
Roles for two prominent lung claudins, Cldn-3 and Cldn-4, were defined using transduced cultured primary alveolar epithelial cells, enabling the function of these two claudins to be examined in the context of other alveolar epithelial claudins. We found that increasing expression of Cldn-3 or Cldn-4 in alveolar epithelial cells had different effects on paracellular permeability. Increased expression of Cldn-3 was associated with decreasing alveolar epithelial TER from ∼550 to ∼400 Ω·cm2 in our model type I cell system (Fig. 3) and also increased paracellular permeability to aqueous tracers up to 10 kDa (Fig. 4). A role for Cldn-3 in decreasing alveolar barrier function was also proposed by Chen et al. (8), who found that alveolar tightening induced by epidermal growth factor in vitro was associated with decreased Cldn-3 expression.
By contrast, increased Cldn-4 expression increased TER, which is consistent with a role for this claudin in protecting the lung from acute injury (28). Wray et al. (51) used two independent methods to disrupt Cldn-4, the effects of which resulted in decreased epithelial TER. In addition, they found that increasing Cldn-4 expression using phorbol esters increased monolayer TER, although this approach is subject to other effects that occur when protein kinase C is activated (6, 52). Here, we extended these observations by demonstrating that a direct increase in Cldn-4 expression was sufficient to increase alveolar epithelial cell TER. However, increasing Cldn-4 expression did not significantly alter paracellular permeability to aqueous fluorescent tracers by alveolar epithelial cells (Fig. 4). Although changes in TER often parallel changes in paracellular permeability, this is not always the case, as demonstrated using cells expressing mutated claudins or occludin (5, 37). Indeed, it has been proposed that dye flux assays are more a measure of tight junction remodeling/turnover than the instantaneous ion barrier properties that are reflected by TER measurements (39, 40). Thus our results indicate that increasing total Cldn-4 had distinct effects on these two different aspects of the tight junction barrier.
The differential effects of increasing Cldn-3 and Cldn-4 are interesting in light of results demonstrating that in vitro treatment of mice with CPEBD has little effect on the unstressed lung; however, CPEBD dramatically increases pulmonary edema due to ventilator-induced lung injury (51). Given that CPEBD interacts with Cldn-3 and Cldn-4 (35) and our results that show opposing effects of Cldn-3 and Cldn-4 on alveolar barrier function, in vivo application of CPEBD could have offsetting effects on these two claudins in the unstressed lung. However, during lung injury, when Cldn-4 is upregulated and essential for efficient fluid clearance (51), the effect of CPEBD on lung barrier function is much more apparent.
On the basis of amino acid homology, Cldn-3 and Cldn-4 are the two most closely related mammalian claudins. This homology between the two claudins is particularly high in the first extracellular loop domain, which is primarily responsible for regulating the charge specificity of tight junction permeability (4, 11, 44). Although the first extracellular loops of Cldn-3 and Cldn-4 are 94% identical, a single amino acid residue alters the ability of Cldn-3 and Cldn-4 to heterotypically interact with claudins on adjacent cells (15). While Cldn-3 can interact with several other claudins, including Cldn-1, Cldn-2, and Cldn-5, Cldn-4 has only been demonstrated to homotypically interact with itself (12, 15). In contrast, the second extracellular loops of Cldn-3 and Cldn-4 are more divergent (64% identical). Given that the second extracellular loop has also been shown to influence assembly of claudins into tight junctions (15, 37, 38), differences in this domain suggest that Cldn-3 and Cldn-4 would each have a differential influence on tight junction formation.
In addition to the extracellular loop domains, the cytoplasmic COOH terminus is the other major functional claudin domain (45). While Cldn-3 and Cldn-4 have high homology in the first extracellular domain, they show considerably more divergence in the second extracellular loop domain and cytoplasmic COOH-terminal tail, both of which help regulate claudin assembly (10, 15, 38). Although both of these claudins can interact with ZO-1 and ZO-2 (42, 47), Cldn-3 and Cldn-4 are likely to interact with unique scaffolding proteins (13). In fact, claudin stability appears to be controlled by scaffold proteins other than ZO-1 and ZO-2, since removing the PDZ-binding motif has little effect on overall claudin half-life (45). Whether claudins compete for a pool of scaffold proteins or whether specific combinations of claudins are corecruited by the tight junction scaffold remains largely unanswered.
Increased expression of a particular claudin by cells can have a dramatic effect on ion permeability or no effect, depending on the other claudins expressed by the cells (46). This observation is significant, because while we observe a decrease in barrier function when Cldn-3 is increased in alveolar epithelial cells, recent evidence demonstrates that increased Cldn-3 augments barrier function of a low-resistance clone of Madin-Darby canine kidney epithelial cells as measured by TER and paracellular permeability to aqueous tracers (34). However, in the case of Madin-Darby canine kidney cells, increasing Cldn-3 expression enabled an interaction with Cldn-2, which served to increase TER from ∼50 to ∼100 Ω·cm2 (34), whereas we observed a decrease from ∼550 to ∼400 Ω·cm2 (Fig. 2). These results suggest that Cldn-3 may promote the sealing of low-resistance tight junctions while modulating the permeability of high-resistance tight junctions. However, it may be that the role of Cldn-3 is determined by cell-specific factors as opposed to a universal property of Cldn-3 itself.
Triton X-100 insolubility is frequently used as a measure for incorporation of proteins into tight junction strands (18, 36, 41, 50). Interestingly, we found that claudins expressed by alveolar epithelial cells showed a range of Triton X-100 insolubility (Fig. 8), ranging from highly insoluble (Cldn-18) to predominantly soluble (Cldn-1). The finding that alveolar epithelial claudins show differences implies that alveolar tight junctions have a heterogeneous molecular organization that can affect barrier function. Importantly, Triton X-100 solubility of YFP-Cldn-3 and YFP-Cldn-4 was comparable to that of their native counterparts, consistent with the tagged claudins being properly assembled into tight junctions. By and large, transducing cells with YFP-Cldn-3 or YFP-Cldn-4 had a limited effect on Triton X-100 insolubility of other tight junction proteins, although YFP-Cldn-3 induced an increase in Cldn-5 insolubility and YFP-Cldn-4 decreased Cldn-7 insolubility. Whether these changes have a direct impact on tight junction permeability remains to be determined. Given that increased Cldn-5 expression is associated with a decrease in alveolar barrier function (19, 48), we speculate that increased incorporation of Cldn-5 into alveolar epithelial junctions may contribute to the ability of YFP-Cldn-3 to increase tight junction permeability.
Taken together, our results show that differential expression of Cldn-3 and Cldn-4 produced alveolar epithelial monolayers with unique paracellular permeability. By directly transducing primary alveolar epithelial cells, we were able to demonstrate that increased expression of Cldn-4 was sufficient to increase alveolar epithelial cell barrier function, while increased Cldn-3 had the opposite effect. Since type II alveolar epithelial cells in situ express significantly higher levels of Cldn-3 than type I cells (14, 24, 29, 31, 48), we speculate that Cldn-3 plays a specialized role in regulating the permeability of cell junctions formed by type II cells, such as junctions between type II cells and type I cells. One potential implication of this model is that the alveolar barrier may be heterogeneous, particularly when type II-type I cell interfaces are compared with type I-type I cell interfaces. Determining whether type II-type I cell junctions are preferentially more permeable than type I-type I cell junctions is a future challenge that will require novel methods to measure permeability of individual alveolar tight junctions in situ.
GRANTS
This work was supported by Emory Alcohol and Lung Biology Center/National Institutes of Health (NIH) Grant P50-AA-013757 (M. Koval); NIH Grants R01-HL-083120 (M. Koval), R01-HL-57204 (M. Koval and S. S. Margulies), and AA-013528 (C. E. Overgaard and L. A. Mitchell); and the Emory University Research Committee (M. Koval).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Drs. Chris Yun and Asma Nusrat and the Emory Epithelial Barrier Function Core Facility for access to the Ussing chamber system. Adenovectors were packaged and amplified by the National Heart, Lung, and Blood Institute vector core facility at the University of Pittsburgh.
REFERENCES
- 1. Abraham V, Chou ML, DeBolt KM, Koval M. Phenotypic control of gap junctional communication by cultured alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 276: L825–L834, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harbor Perspect Biol 1: a002584, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Renal Physiol 295: F867–F876, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Angelow S, Kim KJ, Yu AS. Claudin-8 modulates paracellular permeability to acidic and basic ions in MDCK II cells. J Physiol 571: 15–26, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol 134: 1031–1049, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Celi A, Cianchetti S, Petruzzelli S, Carnevali S, Baliva F, Giuntini C. ICAM-1-independent adhesion of neutrophils to phorbol ester-stimulated human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 277: L465–L471, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Cheek JM, Kim KJ, Crandall ED. Tight monolayers of rat alveolar epithelial cells: bioelectric properties and active sodium transport. Am J Physiol Cell Physiol 256: C688–C693, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Chen SP, Zhou B, Willis BC, Sandoval AJ, Liebler JM, Kim KJ, Ann DK, Crandall ED, Borok Z. Effects of transdifferentiation and EGF on claudin isoform expression in alveolar epithelial cells. J Appl Physiol 98: 322–328, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Cohen TS, Gray Lawrence G, Margulies SS. Cultured alveolar epithelial cells from septic rats mimic in vivo septic lung. PLoS One 5: e11322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colegio OR, Van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol 284: C1346–C1354, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol 283: C142–C147, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol 285: L1166–L1178, 2003 [DOI] [PubMed] [Google Scholar]
- 13. D'Souza T, Agarwal R, Morin PJ. Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. J Biol Chem 280: 26233–26240, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Daugherty BL, Mateescu M, Patel AS, Wade K, Kimura S, Gonzales LW, Guttentag S, Ballard PL, Koval M. Developmental regulation of claudin localization by fetal alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 287: L1266–L1273, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Daugherty BL, Ward C, Smith T, Ritzenthaler JD, Koval M. Regulation of heterotypic claudin compatibility. J Biol Chem 282: 30005–30013, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis 134: 141–145, 1986 [DOI] [PubMed] [Google Scholar]
- 17. Eaton DC, Helms MN, Koval M, Bao HF, Jain L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annu Rev Physiol 71: 403–423, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Farshori P, Kachar B. Redistribution and phosphorylation of occludin during opening and resealing of tight junctions in cultured epithelial cells. J Membr Biol 170: 147–156, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Fernandez AL, Koval M, Fan X, Guidot DM. Chronic alcohol ingestion alters claudin expression in the alveolar epithelium of rats. Alcohol 41: 371–379, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonzalez R, Yang YH, Griffin C, Allen L, Tigue Z, Dobbs L. Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am J Physiol Lung Cell Mol Physiol 288: L179–L189, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest 118: 619–628, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol 147: 1351–1363, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol 177: 512–524, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaarteenaho-Wiik R, Soini Y. Claudin-1, -2, -3, -4, -5, and -7 in usual interstitial pneumonia and sarcoidosis. J Histochem Cytochem 57: 187–195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim KJ, Borok Z, Ehrhardt C, Willis BC, Lehr CM, Crandall ED. Estimation of paracellular conductance of primary rat alveolar epithelial cell monolayers. J Appl Physiol 98: 138–143, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Kim KJ, Malik AB. Protein transport across the lung epithelial barrier. Am J Physiol Lung Cell Mol Physiol 284: L247–L259, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Kim KJ, Suh DJ. Asymmetric effects of H2O2 on alveolar epithelial barrier properties. Am J Physiol Lung Cell Mol Physiol 264: L308–L315, 1993 [DOI] [PubMed] [Google Scholar]
- 28. Koval M. Tight junctions, but not too tight: fine control of lung permeability by claudins. Am J Physiol Lung Cell Mol Physiol 297: L217–L218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koval M, Ward C, Findley MK, Roser-Page S, Helms MN, Roman J. Extracellular matrix influences alveolar epithelial claudin expression and barrier function. Am J Respir Cell Mol Biol 42: 172–180, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta 1778: 631–645, 2008 [DOI] [PubMed] [Google Scholar]
- 31. LaFemina MJ, Rokkam D, Chandrasena A, Pan J, Bajaj A, Johnson M, Frank JA. Keratinocyte growth factor enhances barrier function without altering claudin expression in primary alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 299: L724–L734, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matsuda M, Kubo A, Furuse M, Tsukita S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J Cell Sci 117: 1247–1257, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Mehta D, Bhattacharya J, Matthay MA, Malik AB. Integrated control of lung fluid balance. Am J Physiol Lung Cell Mol Physiol 287: L1081–L1090, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Milatz S, Krug SM, Rosenthal R, Gunzel D, Muller D, Schulzke JD, Amasheh S, Fromm M. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim Biophys Acta 1798: 2048–2057, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Mitchell LA, Koval M. Specificity of interaction between Clostridium perfringens enterotoxin and claudin-family tight junction proteins. Toxins 2: 1595–1611, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL, 3rd, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol 158: 967–978, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Piehl C, Piontek J, Cording J, Wolburg H, Blasig IE. Participation of the second extracellular loop of claudin-5 in paracellular tightening against ions, small and large molecules. Cell Mol Life Sci 67: 2131–2140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Piontek J, Winkler L, Wolburg H, Muller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J 22: 146–158, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Sasaki H, Matsui C, Furuse K, Mimori-Kiyosue Y, Furuse M, Tsukita S. Dynamic behavior of paired claudin strands within apposing plasma membranes. Proc Natl Acad Sci USA 100: 3971–3976, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 286: C1213–C1228, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Stuart RO, Nigam SK. Regulated assembly of tight junctions by protein kinase C. Proc Natl Acad Sci USA 92: 6072–6076, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 126: 741–754, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFNγ-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell 16: 5040–5052, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest 107: 1319–1327, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van Itallie CM, Colegio OR, Anderson JM. The cytoplasmic tails of claudins can influence tight junction barrier properties through effects on protein stability. J Membr Biol 199: 29–38, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol 285: F1078–F1084, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell 20: 3930–3940, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang F, Daugherty B, Keise LL, Wei Z, Foley JP, Savani RC, Koval M. Heterogeneity of claudin expression by alveolar epithelial cells. Am J Respir Cell Mol Biol 29: 62–70, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1376–1383, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol 136: 399–409, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wray C, Mao Y, Pan J, Chandrasena A, Piasta F, Frank JA. Claudin 4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am J Physiol Lung Cell Mol Physiol 297: L219–L227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yamagata T, Yamagata Y, Nishimoto T, Nakanishi M, Nakanishi H, Minakata Y, Mune M, Yukawa S. The impact of phorbol ester on the regulation of amiloride-sensitive epithelial sodium channel in alveolar type II epithelial cells. Exp Lung Res 28: 543–562, 2002 [DOI] [PubMed] [Google Scholar]