Abstract
Transmembrane adenylyl cyclase (AC) generates a cAMP pool within the subplasma membrane compartment that strengthens the endothelial cell barrier. This cAMP signal is steered toward effectors that promote junctional integrity and is inactivated before it accesses microtubules, where the cAMP signal causes phosphorylation of tau, leading to microtubule disassembly and barrier disruption. During infection, Pseudomonas aeruginosa uses a type III secretion system to inject a soluble AC, ExoY, into the cytosol of pulmonary microvascular endothelial cells. ExoY generates a cAMP signal that disrupts the endothelial cell barrier. We tested the hypothesis that this ExoY-dependent cAMP signal causes phosphorylation of tau, without inducing phosphorylation of membrane effectors that strengthen endothelial barrier function. To approach this hypothesis, we first discerned the membrane compartment in which endogenous transmembrane AC6 resides. AC6 was resolved in caveolin-rich lipid raft fractions with calcium channel proteins and the cell adhesion molecules N-cadherin, E-cadherin, and activated leukocyte adhesion molecule. VE-cadherin was excluded from the caveolin-rich fractions and was detected in the bulk plasma membrane fractions. The actin binding protein, filamin A, was detected in all membrane fractions. Isoproterenol activation of ACs promoted filamin phosphorylation, whereas thrombin inhibition of AC6 reduced filamin phosphorylation within the membrane fraction. In contrast, ExoY produced a cAMP signal that did not cause filamin phosphorylation yet induced tau phosphorylation. Hence, our data indicate that cAMP signals are strictly compartmentalized; whereas cAMP emanating from transmembrane ACs activates barrier-enhancing targets, such as filamin, cAMP emanating from soluble ACs activates barrier-disrupting targets, such as tau.
Keywords: compartmentalization, diffusion, endothelium
pulmonary microvascular endothelial cells (PMVECs) interact to form a selectively permeable barrier, thereby limiting the flux of blood components into the underlying tissue. Cytoskeletal elements, such as cortical actin and microtubules, as well as cell-to-cell junctions, regulate the integrity of this endothelial barrier (3, 27, 32, 42). Isoproterenol stimulation of transmembrane adenylyl cyclase (AC) generates subplasma membrane cAMP that stabilizes the cortical actin rim and strengthens cell-to-cell adhesions (6, 22). The evolving paradigm regarding cAMP signaling proposes that this restricted second messenger does not diffuse far from its origin (30). Thus this model suggests that transmembrane ACs generate cAMP to activate proximal barrier protective targets residing within the narrow corridor of the subplasma membrane space. These endothelial barrier protective cAMP targets that act to fortify cortical actin and cell adhesions have not been fully resolved.
Filamin A is a large protein with multiple binding domains, including a filamentous (F) actin-binding domain (14). As a dimer, filamin A binds and cross links F-actin via its actin-binding domain, thus stabilizing cortical actin (23). Filamin has a protein-binding domain capable of interacting with many proteins, such as cell adhesion molecules (12). Therefore, filamin tethers cell adhesion molecules into the peripheral actin rim. Accumulating evidence suggests that filamin A plays a role in endothelial barrier regulation (4, 46). Filamin A deficiency is embryonically lethal, with dramatic increases in vascular permeability attributed to loss of cell-to-cell contacts (11). Furthermore, hydrogen peroxide or bradykinin redistribute filamin from the cell periphery into the cytosol and disrupt the endothelial barrier; however, forskolin activation of transmembrane AC activity increases cAMP, prevents filamin translocation to the cytosol, and reduces permeability (15). In vitro assays identified serine 2152 as a PKA phosphorylation target (17). Serine 2152 resides within the protein-binding domain. However, it is unclear whether isoproterenol activation of transmembrane ACs via G protein signaling is sufficient to induce filamin serine 2152 phosphorylation in pulmonary endothelium. Within the pulmonary endothelium, transmembrane AC activity is inhibited by calcium (37). These calcium-sensitive ACs localize to caveolin-enriched lipid raft compartments within the plasma membrane (44). Lipid rafts and caveolae are cholesterol- and sphingolipid-rich membrane regions, which compartmentalize signaling elements and adhesion molecules (5). Therefore, we were interested to determine whether filamin A is a target of isoproterenol-stimulated transmembrane AC activity that compartmentalizes with AC6 and other barrier-enhancing molecules within lipid rafts.
In contrast to the barrier-protective effects of membrane AC activity, cytosolic AC activity disrupts the endothelial barrier. Pseudomonas aeruginosa ( P. aeruginosa) injects the AC exotoxin, ExoY, into the cytosol of PMVECs, where it generates a cytosolic cAMP pool (34). This ExoY-generated cytosolic cAMP induces endothelial cell gaps and increases permeability in the isolated lung. In a separate study, forskolin stimulation of a mammalian-derived, soluble AC chimera (sACI/II) generates cAMP, not only within the cytosolic compartment, but also at the plasma membrane (33). In these sACI/II-expressing microvascular endothelial cells, simultaneous activation of transmembrane and soluble ACs disrupts the endothelial barrier. Subsequent studies revealed that the endothelial barrier compromise induced by sACI/II generated cytosolic cAMP was mediated through microtubule disruption (26). More specifically, tau, the microtubule-associating protein that stabilizes microtubules, contributes to this cytosolic cAMP microtubule disruption. PKA phosphorylation of tau serine 214 reduces tau association with microtubules and induces microtubule restructuring associated with barrier disruption. Thus, whereas cAMP within the subplasma membrane space acts on the actin cytoskeleton to strengthen the endothelial barrier, cAMP within the cytosolic compartment acts on the microtubule network to disrupt the endothelial barrier. Presently, it is unknown whether ExoY-generated cytosolic cAMP is sufficient to induce phosphorylation of tau at serine 214.
Interestingly, in control cells, forskolin activation of plasma membrane AC activity does not provoke tau phosphorylation at serine 214 (8), suggesting that a peripheral diffusion barricade acts to constrain cAMP within a subplasma membrane space. Phosphodiesterase activity contributes to this diffusion barricade, hydrolyzing cAMP before it escapes the membrane compartment (2). Indeed, forskolin stimulation of PMVECs expressing a dominant-negative phosphodiesterase 4D4 allows cAMP to escape the membrane compartment, phosphorylate tau serine 214, and disrupt the endothelial barrier (8). Thus, in all these examples of elevated cytosolic cAMP, the associated endothelial barrier dysfunction is consistent with microtubule reorganization mediated via tau serine 214 phosphorylation. Presently, it is unclear whether cytosolic cAMP generated via ExoY-AC activity of P. aeruginosa can escape the cytosolic compartment and invade the membrane compartment to activate targets therein, such as filamin A. Our studies reveal that, not only is filamin A a target of transmembrane AC activity in PMVECs, but also it also resides within caveolin-rich, lipid raft membrane microdomains similar to the endogenous AC6. Fluctuations of membrane-localized cAMP signals, using isoproterenol or thrombin, are detected by changes in the phosphorylation status of filamin specifically localized within the membrane compartment. Furthermore, we demonstrate that cAMP generated within the cytosolic compartment cannot penetrate the cAMP diffusion barricades to activate targets of plasma membrane AC activity.
MATERIALS AND METHODS
Isolation and culture of rat PMVECs.
Rat PMVECs were isolated, cultured, and routinely passaged as described in detail by Stevens et al. (36).
Isolation of caveolar and noncaveolar membranes.
PMVECs were grown to confluence in three T75 flasks and caveolar and noncaveolar membranes isolated using a previously described method (25). Cells were rinsed in PBS and DMEM added for 10 min before the addition of either 1 μM isoproterenol (Sigma, St. Louis, MO), 10 U/ml thrombin from rat plasma [reconstituted in 0.1% BSA/PBS (Sigma)], or DMEM alone for 10 min. Cells were rinsed again in PBS, detached from the dish in 1 mM EDTA in PBS, and pelleted (195 g, 5 min). The cell pellet was lysed in 3 ml of ice-cold sodium bicarbonate buffer (500 mM Na2CO3, pH 11) and set on ice for 5 min before sonication on ice (30 s each at power 2, 3, then 4 with 3 min on ice between each sonication) with a final incubation for 15 min on ice. The suspension was Dounce homogenized by 10 strokes with both loose and tight pestle. This lysate was adjusted to 45% sucrose with the addition of 60% sucrose in MES buffered saline (MBS) (25 mM MES, 150 mM NaCl, 250 mM Na2CO3, pH 6.4) and loaded to the bottom of a discontinuous sucrose gradient (5 and 30% sucrose in MBS). The gradients were centrifuged in Beckman optima L-100 (Beckman Coulter, Fullerton, CA) using the SW55Ti rotor for 17 h, at 4°C, and at 64,284 g (23,500 revolution/min). Light-scattering bands were visible at ∼20% and 35% sucrose and represent the caveolar and noncaveolar membranes, respectively. Nine fractions were taken from the top of the gradient, the percent sucrose recorded using a refractometer (Bausch and Lomb, Rochester, NY), and each fraction diluted with ice cold MBS. The fractions were centrifuged for 1 h, at 4°C, and at 194,432 g (40,000 revolution/min). The pelleted membranes were resuspended in 1% SDS, and an aliquot was taken for protein determination (BCA kit, Sigma). Samples were adjusted to equal protein concentration, prepared for Western analysis by the addition of sample buffer (80 mM Tris, pH 6.8, 50% glycerol, 2% β-mercaptoethanol, 2% SDS, trace bromophenol blue), heated at 37°C for 30 min, and stored at −80°C. For fractionation of filamin A, cells were pretreated for 10 min with calpeptin (10 μM; Santa Cruz Biotechnology, Santa Cruz, CA). Calpain I (20 μM) and II (20 μM) inhibitors (Sigma) were included in all buffers.
Immunofluorescence.
Cells were grown to confluence on 25-mm coverslips, washed in HBSS, fixed with ice-cold methanol (20%), and permeabilized with 0.1% Triton X-100 in HBSS each for 10 min. After HBSS wash, nonspecific binding sites were blocked with blocking buffer (5% donkey serum, 5% BSA in HBSS) for 20 min. Cells were incubated with primary antibody (rabbit anti-filamin A; Abcam, Cambridge, MA) at 1:250 in blocking buffer for 2 h and washed in HBSS before incubating with secondary antibody conjugated to fluorescent markers (Alexa Fluor 488 monoclonal antibody; Molecular Probes, Eugene, OR) at 1:500 in 5% BSA-HBSS for 30 min. After a final HBSS rinse, coverslips were mounted with fluorescent mounting media (DAKO) and incubated overnight at 4°C. Fluorescent images were acquired on a Leica TCS SP2 confocal laser-scanning microscope with a ×63 oil-immersion objective at excitation wavelength 488 nm and emission bands set to 500 to 535 nm.
Preparation of whole cell lysates following isoproterenol treatment.
Cells were grown to confluence, rinsed in PBS, and incubated in DMEM for 4 h before the addition of isoproterenol (1 μM) for 10 min. Cells were lysed in radioimmunoprecipitation assay buffer (Boston Bioproducts, Worcester, MA) with protease (Sigma) and phosphatase (Roche, Indianapolis, IN) inhibitor cocktail, and centrifuged (10,000 g, 10 min, 4°C) to remove cellular debris. The supernatant was adjusted to equal protein concentration (Lowry protein assay, Sigma), analyzed by immunoblotting following the addition of sample buffer, and placed at 37°C for 30 min.
Preparation of whole cell lysates following thrombin treatment.
Cells were grown to confluence on 35-mm dishes, rinsed, and then incubated in HBSS for 5 min before the addition of 10, 50, or 100 U/ml thrombin from rat plasma [reconstituted in 0.1% BSA/PBS (Sigma)] for 15 min. Cells were lysed on ice in extraction buffer (5 mM magnesium chloride, 1 mM EDTA, 40 mM Tris, pH 7.4, 130 mM sodium chloride, 50 mM octylglucopyranoside) with protease (Sigma) and phosphatase (Roche) inhibitor cocktails and centrifuged (10,000 g for 10 min at 4°C) to remove cellular debris. Samples were adjusted to equal protein concentration (Lowry protein assay, Sigma) and analyzed by immunoblotting following the addition of sample buffer and incubation at 37°C for 30 min.
Inoculation with P. aeruginosa.
Infection of PMVECs has been described elsewhere (34). Briefly, two strains of P. aeruginosa were used in these studies (45). Both strains express the complete type III secretion system enabling protein transfer into the eukaryotic cell. The ExoY strain encodes the catalytically active protein, whereas the K81M strain contains a point mutation in ExoY (K81M), rendering the protein catalytically inactive. Confluent PMVEC monolayers were rinsed in PBS, and, following a 10-min incubation in DMEM alone, bacteria were added to the cells at a multiplicity of infection of 20:1 for 4 h. Cells where lysed in extraction buffer with protease (Sigma) and phosphatase (Roche) inhibitor cocktails, and centrifuged (10,000 g for 10 min at 4°C) to remove cellular debris. Samples were adjusted to equal protein concentration (Lowry protein assay, Sigma) and analyzed by immunoblotting following the addition of sample buffer and incubation at 37°C for 30 min.
Differential detergent fractionation.
Cells were grown to confluence and treated with isoproterenol (1 μM) or thrombin from rat plasma (100 U/ml) as noted above for preparation of whole cell lysates. After isoproterenol or thrombin treatment, the media was removed and the cells rinsed twice in ice-cold PBS. The fractionation was performed with gentle agitation on ice as described previously (43). First, the cells were extracted in digitonin buffer [0.01% (wt/vol) digitonin in 10 mM PIPES, pH 6.8, 300 mM sucrose, 100 mM NaCl, 3 mM MgCl2, 10 μg/ml phalloidin, protease, and phosphatase inhibitor cocktail (Pierce, Rockford, IL)] for 10 min and the supernatant collected (cytosolic fraction). The remaining cellular components were then extracted in a triton buffer [0.5% (vol/vol) Triton X-100, 10 mM PIPES, pH 7.4, 300 mM sucrose, 100 mM NaCl, 3 mM MgCl2, 3 mM EGTA, 10 μg/ml phalloidin, protease, and phosphatase inhibitor cocktail] for 30 min, the supernatant removed (membrane/organelle fraction), and the nuclear fraction extracted in a Tween/deoxycholate buffer [1% (vol/vol) Tween-40, 0.5% deoxycholate, 10 mM PIPES, pH 7.4, protease, and phosphatase inhibitor cocktail]. Once the supernatant had been removed, the remaining cytoskeletal fraction was extracted in hot (100°C) SDS buffer (2.5% SDS in Tris·HCl, pH 8, 200 mM DTT, protease, and phosphatase inhibitor cocktail) and the supernatant transferred into a microfuge tube. All extractions were performed in equal volumes. Following the addition of sample buffer, fractions were analyzed by Western blot analysis by equal volume loading.
Western blot analysis.
Samples in sample buffer were separated by SDS-PAGE (Invitrogen, Carlsbad, CA) and transferred to nitrocellulose paper (Bio-Rad, Hercules, CA). Nonspecific binding sites where blocked with 5% milk in TBS with 0.1% Tween (TTBS) and subjected to Western blot analysis using appropriate antibodies in 1% milk TTBS [caveolin-1, AC5/6, β-adaptin, β-actin, goat anti-rabbit, and goat anti-mouse secondary antibodies (Santa Cruz Biotechnology), VE-cadherin and β-tubulin (Sigma), E-cadherin (BD Transductions, San Jose, CA), tau phospho-serine 214 (Invitrogen), filamin A and filamin A phospho-serine 2152 (Abcam), N-cadherin (Zymed, Carlsbad, CA), Orai1 (ProSci, Poway, CA), activated leukocyte cell adhesion molecules (ALCAM, gift from S. Ofori Acquah), and transient receptor potential cation channel canonical 4 (TRPC4, gift from M. Zhu)]. Primary antibody binding was detected using horseradish peroxidase-labeled rabbit anti-mouse or goat anti-rabbit IgG and chemiluminescence (GE Healthcare, Piscataway, NJ, and Pierce). Western blots were performed at least three times, and a representative blot is shown.
RESULTS
AC6 resides in caveolin-enriched lipid raft compartments of PMVECs.
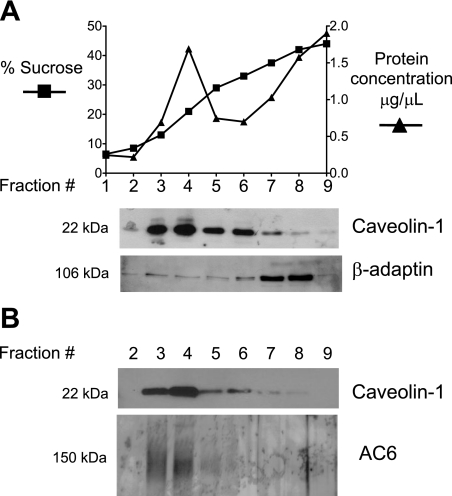
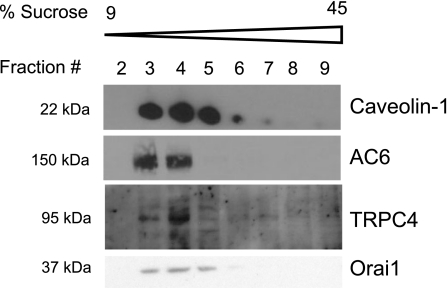
Isolated membranes from PMVECs display calcium-inhibited AC activity, suggestive of AC6 expression (9). The calcium-sensitive ACs have been proposed to reside in caveolin-enriched lipid raft plasma membrane compartments (44). Thus we sought to determine whether caveolin-enriched lipid raft compartments could be isolated in PMVECs. With the use of detergent-free lysis and discontinuous sucrose density gradients, caveolin-1 was enriched in light/buoyant fractions at 15–25% sucrose (corresponding to fractions 3–5, Fig. 1A). Indeed, fractions 3–5 represent lipid raft/caveolae membrane compartments. The nonraft protein, β-adaptin, a component of clathrin-coated pits (10), is excluded from lipid rafts and is isolated in the heavy fractions at 35–45% sucrose (corresponding to fractions 7–8). Thus fractions 7–8 represent the bulk plasma membrane. Using an antibody that recognizes AC6, we were able to detect immunoreactivity in fractions 3 and 4 (Fig. 1B). Thus PMVECs have caveolin-enriched lipid rafts as well as bulk plasma membrane nonraft components, and AC6 localizes to these caveolin-enriched compartments. Furthermore, we detected TRPC4 as well as Orai1 in the caveolin-enriched fraction (Fig. 2), both of which have been implicated in store-operated calcium entry and calcium regulation of AC activity (7, 20). Thus TRPC4 and Orai1 are prominent in lipid raft membrane microdomains with AC6.
Fig. 1.
Adenylyl cyclase 6 (AC6) is detected in the light/buoyant caveolin-1-enriched lipid raft membrane fractions of pulmonary microvascular endothelial cells (PMVECs). A: caveolin-1 is enriched in fractions 3–5 corresponding to 15–25% sucrose. These fractions are enriched in protein. These light/buoyant fractions represent membrane lipid rafts. In contrast, the nonlipid raft protein, β-adaptin, is enriched in the 35–45% sucrose fractions, which are the nonbuoyant/heavy membrane fractions corresponding to fractions 7–8 and referred to as the bulk plasma membrane. B: AC6 is detected in caveolin-1-enriched lipid raft membrane fractions of PMVECs.
Fig. 2.
Transient receptor potential cation channel canonical 4 (TRPC4) and Orai1 reside in caveolin-1-enriched lipid rafts of PMVECs. TRPC4 and Orai1 localize to caveolin-1-enriched lipid raft membrane fractions with AC6.
Select cell adhesion molecules reside in lipid rafts.
If cAMP signaling is compartmentalized, and cell adhesion molecules are also targets of cAMP-induced stabilization of the endothelial barrier, then we would also expect these molecules to reside in close proximity to the cAMP source. Indeed, cell adhesion molecules E-cadherin, N-cadherin, and ALCAM were all detected in caveolin-enriched lipid raft compartments with AC6 (Fig. 3A), whereas VE-cadherin was excluded from rafts residing in the bulk plasma membrane. The lipid raft/bulk plasma membrane distribution of these adhesion molecules did not change with activation of AC activity by isoproterenol (Fig. 3B) or inhibition of AC activity by the inflammatory agonist, thrombin (Fig. 3C) (data not shown). Thus plasma membrane compartmentalization of cell adhesion molecules, either within or outside of lipid rafts, is independent of AC activity.
Fig. 3.
Isoproterenol and thrombin do not affect membrane distribution of cell adhesion molecules. A: cell adhesion molecules E-cadherin, N-cadherin, and activated leukocyte cell adhesion molecules (ALCAM) localize to caveolin-enriched lipid raft membrane fractions with AC6, whereas VE-cadherin is excluded from lipid raft compartments and is detected in the nonlipid raft fractions. B and C: N-cadherin and ALCAM remain in lipid raft membrane fractions, and VE-cadherin remains excluded from lipid rafts following isoproterenol (1 μM) (B) or thrombin (10 U/ml) (C) treatment.
Filamin A resides throughout lipid raft and bulk plasma membrane fractions and is phosphorylated by AC activation.
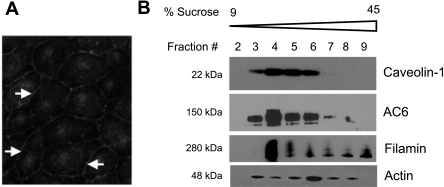
Filamin A is an actin-binding protein, which promotes high-angle F-actin branching and links cell adhesion molecules into the cortical actin rim. Filamin A localizes to cell-to-cell borders of PMVECs (Fig. 4A) and resides both within the same lipid raft fraction as AC6 (Fig. 4B) as well as in the bulk plasma membrane nonraft fractions. Indeed, filamin and actin are distributed through all membrane fractions. Isoproterenol stimulation of transmembrane AC activity leads to an increase in filamin A phosphorylation at serine 2152 (Fig. 5A) without changing total filamin A levels. These data are the first to demonstrate the in vivo phosphorylation of endogenous filamin A following G protein activation of AC activity. In contrast, thrombin, which inhibits AC6 and decreases cAMP, decreases filamin A phosphorylation at serine 2152 (Fig. 5B). With the use of differential detergent fractionation of PMVECs, total filamin as well as filamin phospho-serine 2152 reside primarily in the digitonin-extracted cytosolic fraction with some expression in the Triton X-extracted membrane fraction (Fig. 5C). Following isoproterenol treatment, filamin shifts into the membrane fraction, and phospho-serine 2152 filamin is also elevated within this fraction. Following thrombin treatment, there is no change in filamin distribution between the cytosolic and membrane fractions, but there is a decrease in filamin phospho-serine 2152 within the membrane fraction compared with control. Whereas isoproterenol does not alter the distribution of actin between fractions, thrombin stimulation promotes transfer of cytosolic actin into the cytoskeletal fraction, consistent with previous studies (19). Thus filamin A is detected in caveolin-enriched lipid raft compartments with AC6 and is a phosphorylation target of transmembrane AC activity. Isoproterenol- or thrombin-induced changes in filamin phosphorylation at serine 2152 appear to affect the pool of filamin that is localized to the membrane fraction.
Fig. 4.
Filamin A resides in lipid raft/caveolin-rich membrane fractions with AC6. A: filamin A localizes to cell-to-cell borders of PMVECs (arrows). B: filamin is detected in caveolin-enriched lipid raft membrane fractions of PMVECs with AC6 as well as in nonraft fractions. Actin is distributed through all fractions 3–8.
Fig. 5.
Isoproterenol and thrombin alter filamin serine 2152 phosphorylation in the membrane fraction. A and B: isoproterenol (1 μM for 10 min) (A) activation of transmembrane AC increases filamin A phosphorylation at the PKA phosphorylation site, serine 2152, whereas thrombin (10, 50, 100 U/ml for 15 min) treatment (B) of PMVECs decreases filamin A phosphorylation at serine 2152. C: isoproterenol (1 μM for 10 min) treatment of PMVECs increases filamin A expression and filamin A phospho-serine 2152 in the Triton X-extracted membrane fraction (top). Although thrombin (100 U/ml for 15 min) does not change filamin distribution between fractions, there is a decrease in phospho-serine 2152 in the membrane fraction (bottom).
ExoY-generated cytosolic cAMP phosphorylates tau but does not invade the plasma membrane compartment.
The edema-inducing pulmonary pathogen P. aeruginosa uses a type III secretion system to inject the cytosolic exotoxin, ExoY, into PMVECs (45). ExoY utilizes a eukaryotic factor to stimulate AC activity, generating cAMP in the cytosolic compartment sufficient to induce endothelial cell gaps and increase the filtration coefficient in the isolated lung (34). These barrier-disruptive effects were absent with control bacteria expressing a catalytically inactive ExoY (K81M) unable to generate cAMP. Following a 4-h infection with P. aeruginosa (multiplicity of infection 20:1), we investigated whether ExoY-induced elevated cAMP leads to phosphorylation of the cytosolic cAMP target, tau at serine 214, and whether ExoY-cAMP leads to phosphorylation of the plasma membrane target, filamin at serine 2152. Whereas incubation with ExoY-expressing bacteria induced gaps in PMVEC monolayers within 4 h (data not shown), there was an increase in tau serine 214 phosphorylation (Fig. 6A), yet there was no change in the phosphorylation status of filamin A at serine 2152 at this time point (Fig. 6B). Thus these data reveal that ExoY-generated cytosolic cAMP activates a cytosolic target but does not escape the cytosolic compartment to invade the plasma membrane compartment at least during the evolution of interendothelial cell gap formation (Fig. 7).
Fig. 6.
Cytosolic cAMP does not phosphorylate membrane-localized filamin A. Infection of PMVECs with Pseudomonas aeruginosa ( P. aeruginosa) (multiplicity of infection 20:1) expressing ExoY (ExoY) (A) leads to an increase in tau phospho-serine 214, which is absent with the catalytically inactive ExoY strain (K81M). B: infection with P. aeruginosa (multiplicity of infection 20:1) expressing ExoY (ExoY) or K81M does not increase filamin 2152 phosphorylation. A and B: total filamin expression does not change, and tubulin or actin act as loading controls.
Fig. 7.
Working model illustrating cAMP diffusion barricade. Transmembrane AC6 localizes to caveolin-rich lipid rafts in PMVECs with calcium channel proteins and barrier-enhancing molecules such as filamin A and cell adhesion molecules E-cadherin, N-cadherin, and ALCAM. In contrast, VE-cadherin is in the bulk plasma membrane. A: isoproterenol stimulation of transmembrane AC activity generates cAMP, which is restricted to the subplasma membrane compartment by diffusion barricades and leads to the elevated phosphorylation of filamin A at serine 2152. B: P. aeruginosa uses a type III secretion system to inject ExoY into the cytosolic compartment, where it generates cAMP, leading to phosphorylation of the microtubule associating protein, tau, at serine 214. ExoY-generated cAMP does not traverse the diffusion barricade and is unable to phosphorylate filamin at serine 2152.
DISCUSSION
Actin and microtubule cytoskeletal structures regulate cell shape and are essential for maintenance of the endothelial barrier. The cortical actin rim is a three-dimensional structure composed of highly branched actin filaments, which are networked at branching points by actin-binding proteins. These binding proteins not only stabilize branching points but can also integrate cell adhesion molecules into the cortical actin rim. The ability of actin-binding proteins to bind the actin cytoskeletal network is regulated by phosphorylation events, which in turn control cell shape. Here, we identify the cytoskeletal binding protein, filamin A, as a target of near-membrane AC activity but not of cytosolic AC activity. Furthermore, we show that filamin A compartmentalizes to caveolae with a cAMP source, AC6, as well as select cell adhesion molecules.
The actin-binding protein, filamin, facilitates the formation of actin networks and acts as a platform for transmembrane proteins. Filamin dimers bind actin filaments through the actin-binding domain and induce high-angle branching. This orthogonal cross linking of actin filaments promotes the formation of a gel-like network (39). In addition to actin binding through the actin-binding domain, filamin has been reported to bind over 30 different proteins through the protein-binding domain (38). Some filamin-binding partners are transmembrane proteins, suggesting that filamin A serves as a scaffold coordinating the extracellular environment with cortical actin and vice versa. Previous findings show that filamin colocalizes with caveolin-1 to act as the caveolae link into the cytoskeleton and also to regulate endothelial transcytosis (16, 35, 40). Our studies in PMVECs reveal that filamin resides in caveolin-rich, lipid raft membrane fractions with AC6 and specific cell adhesion molecules. In addition, we demonstrate filamin phosphorylation at serine 2152 in response to isoproterenol stimulation of subplasma membrane cAMP and a decrease in serine 2152 phosphorylation following thrombin treatment. The regulation of filamin phosphorylation at serine 2152 occurs in the Triton X-extracted fraction referred to as the membrane fraction. This serine residue resides within the protein-binding domain, and, although PKA phosphorylation protects against calpain cleavage (17), the physiological significance of serine 2152 phosphorylation has not been resolved. Future studies will be necessary to determine whether serine 2152 phosphorylation favors protein-to-protein interactions that act to strengthen the endothelial barrier.
Our studies reveal that cell adhesion molecules, such as E- and N-cadherin and ALCAM, also reside within lipid rafts, a distribution that is not modulated by agents that affect barrier integrity. Lipid rafts are specialized membrane islands thought to organize cell adhesions. However, a survey of the specific cell adhesion molecules that reside in these rafts within the pulmonary endothelium has not been described. Although previous studies demonstrate plasma membrane targeting of N-cadherin and ALCAM (21, 24), the significance of compartmentalization to lipid rafts is not known. N-cadherin expression in lipid rafts is essential for the adhesive nature of myoblasts, yet, in melanoma cells, N-cadherin within lipid rafts did not participate in cell-to-cell adhesions (5, 31, 41). We also detected E-cadherin in lipid raft compartments. E-cadherin has previously been described in the pulmonary endothelium and implicated in barrier resealing following hyperosmolarity challenge (1, 28, 29). However, the significance of its localization to the lipid rafts is not presently known. In studies presented here, VE-cadherin was excluded from raft membrane fractions. However, in previous studies conducted with PMVECs, the lipid raft adhesion molecule, ALCAM, coimmunoprecipitated with VE-cadherin, suggesting that these two molecules interact across membrane compartments, perhaps through peripheral actin, which spans all membrane compartments (24). VE- and E-cadherin have been well described as cAMP barrier-strengthening targets moderated via the Epac/Rap 1 pathway (13, 18). These data suggest that either lateral dispersion of cAMP occurs between lipid raft and nonlipid raft compartments or that perhaps lateral compartmentation also exists such that nonraft ACs (i.e., calcium insensitive ACs such as AC2, AC4, and AC7) provide the cAMP source for Epac activation of VE-cadherin.
cAMP compartmentalization is established through physical barricades, such as organelles, and biochemical barricades, such as phosphodiesterase activity (30). These restrictions to diffusion steer cAMP to its physiologically relevant effectors underlying the plasma membrane. Here, we have identified filamin A as a target of isoproterenol-stimulated AC activity in PMVECs. Furthermore, filamin A resides in caveolin-rich lipid raft compartments with AC6, suggesting its localization with the cAMP source. Barricades to free diffusion limit the access of cAMP to the cytosolic compartment, where cAMP can activate barrier disruptive targets, such as tau. Indeed, this spatial regulation of cAMP migration is critical for the maintenance of endothelial barrier function. Although it is established that these barricades act to restrict cAMP diffusion from the plasma membrane to cytosol, we provide the first evidence that these barricades also limit cAMP migration from cytosol to the plasma membrane (Fig. 7).
In summary, the endothelial barrier-enhancing properties of subplasma membrane cAMP are transduced via filamin A-mediated stabilization of actin. Within caveolin-rich lipid rafts a potential cAMP axis exists such that AC6 generates cAMP necessary for the phosphorylation of filamin A and strengthening of the endothelial barrier. Furthermore, peripherally localized cAMP barricades are bidirectional barriers acting to restrict cAMP migration between the plasma membrane and the cytosolic compartments and vice versa.
GRANTS
This work was supported by NIH grants HL-60024 and HL-66299 (T. Stevens) and by HL091651-02 (S. L. Sayner).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Linn Ayes and Anna Buford of the Center for Lung Biology Tissue Culture Core for contributions with cell seeding.
REFERENCES
- 1. Adkison JB, Miller GT, Weber DS, Miyahara T, Ballard ST, Frost JR, Parker JC. Differential responses of pulmonary endothelial phenotypes to cyclical stretch. Microvasc Res 71: 175–184, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Baillie GS. Compartmentalized signalling: spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. FEBS J 276: 1790–1799, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Bogatcheva NV, Verin AD. Reprint of “The role of cytoskeleton in the regulation of vascular endothelial barrier function” [Microvascular Research 76 (2008) 202–207]. Microvasc Res 77: 64–69, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bogatcheva NV, Zemskova MA, Kovalenkov Y, Poirier C, Verin AD. Molecular mechanisms mediating protective effect of cAMP on lipopolysaccharide (LPS)-induced human lung microvascular endothelial cells (HLMVEC) hyperpermeability. J Cell Physiol 221: 750–759, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Causeret M, Taulet N, Comunale F, Favard C, Gauthier-Rouviere C. N-cadherin association with lipid rafts regulates its dynamic assembly at cell-cell junctions in C2C12 myoblasts. Mol Biol Cell 16: 2168–2180, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chetham PM, Guldemeester HA, Mons N, Brough GH, Bridges JP, Thompson WJ, Stevens T. Ca2+-inhibitable adenylyl cyclase and pulmonary microvascular permeability. Am J Physiol Lung Cell Mol Physiol 273: L22–L30, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Cioffi DL, Moore TM, Schaack J, Creighton JR, Cooper DM, Stevens T. Dominant regulation of interendothelial cell gap formation by calcium-inhibited type 6 adenylyl cyclase. J Cell Biol 157: 1267–1278, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Creighton J, Zhu B, Alexeyev M, Stevens T. Spectrin-anchored phosphodiesterase 4D4 restricts cAMP from disrupting microtubules and inducing endothelial cell gap formation. J Cell Sci 121: 110–119, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Creighton JR, Masada N, Cooper DM, Stevens T. Coordinate regulation of membrane cAMP by Ca2+-inhibited adenylyl cyclase and phosphodiesterase activities. Am J Physiol Lung Cell Mol Physiol 284: L100–L107, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Crossthwaite AJ, Seebacher T, Masada N, Ciruela A, Dufraux K, Schultz JE, Cooper DM. The cytosolic domains of Ca2+-sensitive adenylyl cyclases dictate their targeting to plasma membrane lipid rafts. J Biol Chem 280: 6380–6391, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Feng Y, Chen MH, Moskowitz IP, Mendonza AM, Vidali L, Nakamura F, Kwiatkowski DJ, Walsh CA. Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis. Proc Natl Acad Sci USA 103: 19836–19841, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feng Y, Walsh CA. The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat Cell Biol 6: 1034–1038, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol 25: 136–146, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gorlin JB, Yamin R, Egan S, Stewart M, Stossel TP, Kwiatkowski DJ, Hartwig JH. Human endothelial actin-binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J Cell Biol 111: 1089–1105, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hastie LE, Patton WF, Hechtman HB, Shepro D. H2O2-induced filamin redistribution in endothelial cells is modulated by the cyclic AMP-dependent protein kinase pathway. J Cell Physiol 172: 373–381, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem 281: 26391–26399, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Jay D, Garcia EJ, Lara JE, Medina MA, de la Luz Ibarra M. Determination of a cAMP-dependent protein kinase phosphorylation site in the C-terminal region of human endothelial actin-binding protein. Arch Biochem Biophys 377: 80–84, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett 579: 4966–4972, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Lim MJ, Chiang ET, Hechtman HB, Shepro D. Inflammation-induced subcellular redistribution of VE-cadherin, actin, and gamma-catenin in cultured human lung microvessel endothelial cells. Microvasc Res 62: 366–382, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Martin AC, Willoughby D, Ciruela A, Ayling LJ, Pagano M, Wachten S, Tengholm A, Cooper DM. Capacitative Ca2+ entry via Orai1 and stromal interacting molecule 1 (STIM1) regulates adenylyl cyclase type 8. Mol Pharmacol 75: 830–842, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Masedunskas A, King JA, Tan F, Cochran R, Stevens T, Sviridov D, Ofori-Acquah SF. Activated leukocyte cell adhesion molecule is a component of the endothelial junction involved in transendothelial monocyte migration. FEBS Lett 580: 2637–2645, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Minnear FL, DeMichele MA, Moon DG, Rieder CL, Fenton JW., 2nd Isoproterenol reduces thrombin-induced pulmonary endothelial permeability in vitro. Am J Physiol Heart Circ Physiol 257: H1613–H1623, 1989 [DOI] [PubMed] [Google Scholar]
- 23. Nakamura F, Osborn TM, Hartemink CA, Hartwig JH, Stossel TP. Structural basis of filamin A functions. J Cell Biol 179: 1011–1025, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ofori-Acquah SF, King J, Voelkel N, Schaphorst KL, Stevens T. Heterogeneity of barrier function in the lung reflects diversity in endothelial cell junctions. Microvasc Res 75: 391–402, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pagano M, Clynes MA, Masada N, Ciruela A, Ayling LJ, Wachten S, Cooper DM. Insights into the residence in lipid rafts of adenylyl cyclase AC8 and its regulation by capacitative calcium entry. Am J Physiol Cell Physiol 296: C607–C619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prasain N, Alexeyev M, Balczon R, Stevens T. Soluble adenylyl cyclase-dependent microtubule disassembly reveals a novel mechanism of endothelial cell retraction. Am J Physiol Lung Cell Mol Physiol 297: L73–L83, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prasain N, Stevens T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc Res 77: 53–63, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quadri SK, Bhattacharjee M, Parthasarathi K, Tanita T, Bhattacharya J. Endothelial barrier strengthening by activation of focal adhesion kinase. J Biol Chem 278: 13342–13349, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Quadri SK, Bhattacharya J. Resealing of endothelial junctions by focal adhesion kinase. Am J Physiol Lung Cell Mol Physiol 292: L334–L342, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Rich TC, Fagan KA, Nakata H, Schaack J, Cooper DM, Karpen JW. Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J Gen Physiol 116: 147–161, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rossier-Pansier L, Baruthio F, Ruegg C, Mariotti A. Compartmentalization in membrane rafts defines a pool of N-cadherin associated with catenins and not engaged in cell-cell junctions in melanoma cells. J Cell Biochem 103: 957–971, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Sayner SL. Emerging themes of cAMP regulation of the pulmonary endothelial barrier. Am J Physiol Lung Cell Mol Physiol 300: L667–L678, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sayner SL, Alexeyev M, Dessauer CW, Stevens T. Soluble adenylyl cyclase reveals the significance of cAMP compartmentation on pulmonary microvascular endothelial cell barrier. Circ Res 98: 675–681, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Sayner SL, Frank DW, King J, Chen H, VandeWaa J, Stevens T. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res 95: 196–203, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Stahlhut M, van Deurs B. Identification of filamin as a novel ligand for caveolin-1: evidence for the organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol Biol Cell 11: 325–337, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stevens T, Creighton J, Thompson WJ. Control of cAMP in lung endothelial cell phenotypes. Implications for control of barrier function. Am J Physiol Lung Cell Mol Physiol 277: L119–L126, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Stevens T, Nakahashi Y, Cornfield DN, McMurtry IF, Cooper DM, Rodman DM. Ca (2+)-inhibitable adenylyl cyclase modulates pulmonary artery endothelial cell cAMP content and barrier function. Proc Natl Acad Sci USA 92: 2696–2700, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, Shapiro SS. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol 2: 138–145, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Stossel TP, Hartwig JH. Interactions of actin, myosin, and a new actin-binding protein of rabbit pulmonary macrophages. II. Role in cytoplasmic movement and phagocytosis. J Cell Biol 68: 602–619, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sverdlov M, Shinin V, Place AT, Castellon M, Minshall RD. Filamin A regulates caveolae internalization and trafficking in endothelial cells. Mol Biol Cell 20: 4531–4540, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taulet N, Comunale F, Favard C, Charrasse S, Bodin S, Gauthier-Rouviere C. N-cadherin/p120 catenin association at cell-cell contacts occurs in cholesterol-rich membrane domains and is required for RhoA activation and myogenesis. J Biol Chem 284: 23137–23145, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann NY Acad Sci 1123: 134–145, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Wang Q, Patton WF, Chiang ET, Hechtman HB, Shepro D. Filamin translocation is an early endothelial cell inflammatory response to bradykinin: regulation by calcium, protein kinases, and protein phosphatases. J Cell Biochem 62: 383–396, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev 87: 965–1010, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci USA 95: 13899–13904, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao J, Singleton PA, Brown ME, Dudek SM, Garcia JG. Phosphotyrosine protein dynamics in cell membrane rafts of sphingosine-1-phosphate-stimulated human endothelium: role in barrier enhancement. Cell Signal 21: 1945–1960, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]