Abstract
Carbon monoxide (CO) is produced by heme oxygenase (HO)-catalyzed heme degradation to CO, iron, and biliverdin. HO has two active isoforms, HO-1 (inducible) and HO-2 (constitutive). HO-2, but not HO-1, is highly expressed in endothelial and smooth muscle cells and in adjacent astrocytes in the brain. HO-1 is expressed basally only in the spleen and liver but can be induced to a varying extent in most tissues. Elevating heme, protein phosphorylation, Ca2+ influx, and Ca2+/calmodulin-dependent processes increase HO-2 activity. CO dilates cerebral arterioles and may constrict or dilate skeletal muscle and renal arterioles. Selected vasodilatory stimuli, including seizures, glutamatergic stimulation, hypoxia, hypotension, and ADP, increase CO, and the inhibition of HO attenuates the dilation to these stimuli. Astrocytic HO-2-derived CO causes glutamatergic dilation of pial arterioles. CO dilates by activating smooth muscle cell large-conductance Ca2+-activated K+ (BKCa) channels. CO binds to BKCa channel-bound heme, leading to an increase in Ca2+ sparks-to-BKCa channel coupling. Also, CO may bind directly to the BKCa channel at several locations. Endothelial nitric oxide and prostacyclin interact with HO/CO in circulatory regulation. In cerebral arterioles in vivo, in contrast to dilation to acute CO, a prolonged exposure of cerebral arterioles to elevated CO produces progressive constriction by inhibiting nitric oxide synthase. The HO/CO system is highly protective to the vasculature. CO suppresses apoptosis and inhibits components of endogenous oxidant-generating pathways. Bilirubin is a potent reactive oxygen species scavenger. Still many questions remain about the physiology and biochemistry of HO/CO in the circulatory system and about the function and dysfunction of this gaseous mediator system.
Keywords: endothelium, gasotransmitter, heme oxygenase, potassium channels, vascular smooth muscle
this review article is part of a collection on Hypertension and Novel Modulators of Vascular Tone. Other articles appearing in this collection, as well as a full archive of all Review collections, can be found online at http://ajpheart.physiology.org/.
Endogenously produced gaseous messengers, or gasotransmitters (119), include nitric oxide (NO), carbon monoxide (CO), hydrogen sulfide (H2S), and several reactive oxygen species (ROS). These molecules, critical to the physiology of all organisms examined, function by both intra- and intercellular communication. CO is not constrained by cell membranes because it is a stable, lipid soluble gas. Therefore, CO cannot be stored in vesicles for later release. While CO can be bound to heme containing proteins that may release it (23, 40, 115), the predominant determination of the signal strength is de novo synthesis. Being a gas, CO diffuses down partial pressure gradients so that the uptake or metabolizing processes to terminate the signals are unnecessary. Falling concentrations of CO when production is slowed result from binding to a ferrous heme and diffusion away. The present short review will favor the cerebrovascular system where the constitutive enzyme for CO production is expressed at very high levels, particularly in endothelium and perivascular astrocytes. The roles of heme oxygenase (HO) and CO in regulation of the circulation to other organs and tissues, cardiovascular disease, and hypertension was recently reviewed by Abraham and Kappas (1).
Control of CO Production
Endogenous CO is produced in the body as the product of heme degradation to CO, iron, and biliverdin, catalyzed by HO with the oxidation of NADPH. The highest HO expression is in the brain and cerebral circulation. Overall, HO activity of the brain tissue exceeds that of systemic organs, including even the liver and spleen (79). CO production by the brain in vivo can be estimated by measuring extracellular CO in cortical periarachnoid cerebrospinal fluid (pCSF), collected from the brain surface using the closed cranial window technique. In newborn pigs, the baseline CO in pCSF detected by gas chromatography/mass spectrometry is about 5–8 × 10−8 M (19, 147) and may rise to 1 μM with strong stimulation (19) (see Functional Significance of CO in Control of Cerebrovascular Circulation).
In the brain, there are multiple sources of CO that may contribute to the regulation of cerebral blood flow. These cellular components include cerebral vascular cells, neurons, and glia. Freshly isolated cerebral vessels and cortical astrocytes, as well as cultured endothelial cells and astrocytes, produce significant amounts of CO that are acutely increased in response to glutamate, agonists of ionotropic glutamate receptors (iGluRs) (64, 93, 100), and the proinflammatory cytokine tumor necrosis factor-α (TNF-α) (12).
Catalytically active HO is represented as two major isoforms, HO-1 (inducible) and HO-2 (constitutive). HO-1 and HO-2, the products of two distinctly regulated genes mapped to chromosomes 22g12 and 16p13.3, respectively, share only 40% similarity in amino acid sequence (77). However, a common heme catalytic pocket domain (24 amino acids) is evolutionally conserved in both HO-1 and HO-2 proteins (79, 110). Although catalytic mechanisms of heme degradation by the isoforms are similar, the enzymatic activities of HO-1 and HO-2 are regulated via distinct mechanisms. In cerebral vessels, HO-2 is expressed in endothelial and smooth muscle cells and in the adjacent astrocytes of the neurovascular unit (Fig. 1) (68, 69, 96, 111). Both HO-1 and HO-2 are membrane-bound proteins, anchored to the endoplasmic reticulum membrane via a COOH-terminal hydrophobic tail (107, 140). In cerebral vascular endothelial cells, as in most other cell types, HO-1 and HO-2 have similar intracellular localization with a strong preference for the endoplasmic reticulum and nuclear envelope (Fig. 1) (96). In the carotid body, HO-2 colocalizes with large-conductance Ca2+-activated K+ (BKCa) channels (59) that may be functionally significant because CO activates BKCa channels to cause dilation (see Mechanism of CO-Induced Vasodilation). In pulmonary artery endothelial cells, HO-1 was found in plasma membrane caveolae, and caveolin binding to HO-1 can regulate its activity (53). HO-2 participates in the immediate regulation of CO production in the brain and cerebral vessels, whereas HO-1 may be responsible for long-term changes in endogenous CO.
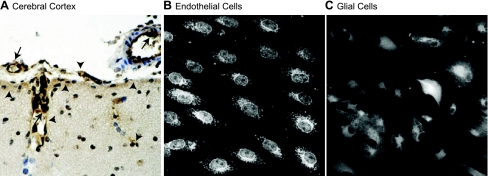
Fig. 1.
Heme oxygenase-2 (HO-2) immunostaining in the cerebral cortex (A), cultured cerebral microvascular endothelial cells (B), and glial cells (C) from newborn pigs. A: in the cerebral cortex, HO-2 immunoperoxidase staining (brown) is localized in endothelial cells in pial arterioles and penetrating arterioles (arrows) and in glia limitans and in glial cells adjacent to penetrating vessels (arrowheads). Nuclear counterstaining, hematoxylin (blue). In cultured cerebral vascular endothelial cells (B) and in cortical glial cells (C), HO-2 immunofluorescence staining is localized in the nuclear envelope, perinuclear area of the cytoplasm, and endoplasmic reticulum.
The structure and functional significance of the third more recently discovered HO-encoding gene, HO-3, still remains elusive (34, 79). HO-3 genes may be processed pseudogenes derived from HO-2 transcripts (34).
HO-1 Regulation
Under basal conditions, HO-1 is highly expressed only in the spleen and liver where it participates in the degradation of red blood cells and in the elimination of toxic heme from the body (77, 79). However, under oxidative stress conditions, HO-1 can be induced in various tissues, including the vasculature and brain. HO-1 is an early response gene (heat shock protein 32) that is regulated via stress- and antioxidant-response elements in the promoter region in conjunction with the redox-sensitive transcription factor nuclear factor-E2-related factor 2 (33, 89), nuclear factor-κB (62), and cAMP-responsive element (60). The list of cell-specific inducers of HO-1 includes heme, other metalloporphyrins, transition metals (iron and cobalt), NO, ROS, and oxidized lipids (2, 26, 32, 60, 77, 79).
HO-1 is not expressed in the brain under basal conditions, except in selected neuronal populations (21, 27, 79, 91, 92, 111). HO-1 upregulation in the brain has been observed in patients with chronic neurodegenerative disorders, such as Alzheimer's and Parkinson's diseases (105, 106, 117). HO-1 induction in vivo occurs in response to stress conditions that include hemorrhage, hyperthermia, and ischemia (28, 105, 106, 111, 118). However, oxidative stress caused by epileptic seizures in newborn pigs in vivo failed to upregulate HO-1 in cerebral vessels or in brain parenchyma (91).
In cultured cerebral vascular endothelial cells, HO-1 is induced by serum and a limited array of strong pro-oxidant stimuli, including cobalt protoporphyrin, hemin, and peroxynitrite (11, 91, 92, 113). Pharmacological intervention with cobalt protoporphyrin has been also successfully used to upregulate HO-1 expression in cerebral vessels and brain in vivo (91, 92, 94). In contrast to other cell types, purported potent HO-1 inducers (reviewed in Ref. 101) including hydrogen peroxide, arachidonic acid, NO donors (3-morpholinosydnonimine and S-nitroso-N-acetyl-dl-penicillamine), transition metals (CoCl2, FeCl2, FeCl3), TNF-α, and glutamate failed to induce HO-1 in brain endothelial cells (11, 12, 92, 113). Overall, with a few exceptions, HO-1 is not expressed in cerebral vessels or the brain under physiological or pathological conditions. Furthermore, HO-1 does not contribute to acute vascular responses that involve rapid CO-mediated increases in blood flow.
Regulation of HO-2 Activity
HO-2 is abundantly expressed in the brain of various mammalian species, including mice, rats, pigs, and humans. In newborn and mature animals, HO-2 is detected in neurons (20, 21, 24, 25, 27, 28, 111, 142), glial cells (69, 104), and cerebral vessels (30, 68, 96).
In response to various physiological/pathophysiological stimulations, including glutamate, seizures, hypoxia, and hypotension, HO-2 activity in vivo is rapidly increased without altering HO-2 expression (19, 64, 65, 93, 96, 100) (see Functional Significance of CO in Control of Cerebrovascular Circulation). The HO-2 gene is not responsive to transcriptional modulation. The only exception to this rule is the ability of adrenal glucocorticoid hormones to induce HO-2 via a glucocorticoid response element in the gene promoter region (74, 96, 99). Prolonged treatment with corticosteroids in vivo (2–4 days) increased HO-2 in neurons in newborn and adult rats (74, 99) and in cerebral microvessels in newborn pigs (96). Taking into account that corticosteroids are slow-acting weak inducers of HO-2, it is unlikely that HO-2 induction contributes to HO-mediated responses in the cerebral vasculature. Recently, an increased HO-2 expression in the brain has been reported in the rat model of prolonged cerebral ischemia-reperfusion (111).
Functional mechanisms that acutely increase endogenous CO include the activation of preexisting HO-2 enzyme by posttranslational modifications. Posttranslational activation of HO-2 is Ca2+/calmodulin dependent (14) and involves protein phosphorylation (14, 63, 64). In hippocampal and olfactory neurons, HO-2 is activated during neuronal and odorant stimulation by phosphorylation of serine-79 by casein kinase 2 via participation of protein kinase C (PKC) and calmodulin (13, 14). In endothelial cells, HO-2 activation by agonists of iGluRs is mediated by protein tyrosine kinases but is independent of PKC- or casein kinase 2-mediated phosphorylation (63, 64). Ca2+/calmodulin-dependent mechanisms are also involved in HO-2 activation in cerebral vessels and endothelial cells by glutamate and iGluR agonists (63, 64). Ca2+ and calmodulin are important regulators of HO-2 activity in freshly isolated cortical astrocytes and in cultured cortical glial cells (132). Negative feedback regulation of HO-2 activity by bilirubin may also occur (78).
NO can affect HO-2 activity. NO can inhibit HO-2 activity by binding to a heme regulatory motif on HO-2 (23). On the other hand, NO increases HO-2 catalytic activity in cerebral microvessels by a cGMP-dependent mechanism (63). Similarly, in isolated heart and aortic endothelial cells, NO stimulated CO production (81, 86). Thus NO may directly inhibit HO-2 catalytic activity but indirectly stimulate the activity via the elevation of cGMP.
Factors that increase the heme substrate availability also increase CO production by cerebral vessels, indicating that HO-2 activity is substrate dependent (64, 65). Therefore, the regulation of cellular heme production can be involved in the regulation of CO production. Cellular heme production is a multistep process that includes both mitochondrial and cytoplasmic reactions. The rate-limiting step is the mitochondrial synthesis of δ-aminolevulinic acid from succinyl-CoA and glycine catalyzed by δ-aminolevulinic acid synthase (52, 82). δ-Aminolevulinic acid synthase activity is controlled by a negative feedback inhibition by heme and oxidized heme (hemin). In intact cerebral microvessels, CO production is increased by elevated intracellular Ca2+ concentration ([Ca2+]i) and PKC activity-induced heme availability (64, 65).
Recent findings suggest that HO-2 activity is regulated via a redox-dependent mechanism. In isolated cerebral vessels and in cerebral vascular endothelial cells, TNF-α rapidly activates HO-2 via a ROS-mediated mechanism, and NADPH oxidase 4 (Nox4) is a major source of ROS that activate HO-2 via a posttranslational modification (12). The mechanism by which ROS modulate HO-2 activity may involve a thiol/disulfide redox molecular switch that controls the heme substrate binding to the HO-2 protein (137).
Overall, acute increases in HO-2 activity, which is the major enzyme of the neurovascular unit, endothelial cells, astrocytes, and neurons, involve posttranslational mechanisms initiated by physiologically relevant factors that affect heme substrate availability, protein phosphorylation, Ca2+ influx, and Ca2+/calmodulin-dependent molecular events.
Mechanism of CO-Induced Vasodilation
CO dilates arteries and arterioles by activating vascular smooth muscle cell BKCa channels. CO and CO donors activate recombinant and vascular smooth muscle cell BKCa channels in excised plasma membrane patches under conditions where cytosolic signaling proteins would be absent and kinases inactive (39, 43, 48, 123, 131, 133). In excised arterial smooth muscle cell membrane patches, CO elevates BKCa channel-apparent Ca2+ sensitivity, particularly within the micromolar concentration range (123, 133). Arterial smooth muscle cell BKCa channels are composed of a pore-forming α-subunit and an auxiliary β1-subunit that elevates channel Ca2+ sensitivity (16). CO activates recombinant BKCa channel α-subunits since the coexpression of auxiliary β-subunits was not required for channel stimulation (39, 43, 127, 131, 133). Data also indicate that NO and CO activate vascular smooth muscle cell BKCa channels via distinct mechanisms that involve effects on different channel subunits (131). The mechanism by which CO activates BKCa channel α-subunits is not fully resolved, with several mechanisms having been proposed. These include the following: 1) direct CO binding to an external BKCa channel histidine residue (122), 2) CO binding to reduced (ferrous, Fe2+) heme that is bound to the BKCa channel heme-binding domain (43, 138), 3) CO binding to His/Asp residues in the high-affinity divalent cation sensor in the regulator of conductance for K+ type-1 domain (39), and 4) an undefined role for cysteine 911 in the BKCa channel COOH-terminus (114). Following their introduction, each of these mechanisms has received subsequent critical evaluation. Under physiological conditions, CO should not bind directly to amino acids, and no direct experimental evidence or chemical mechanism to support such binding has been obtained. Only one mechanism has received independent support, namely, binding of CO to BKCa channel-associated heme (43, 138). Therefore, additional discussion of this mechanism will be provided here.
Cellular heme is produced in the reduced state, and being hydrophobic incorporates readily into lipid bilayers (7). Binding of heme to the heme-binding domain (Cys-Lys-Ala-Cys-His) of the α-subunit located between amino acids 612 and 616 inhibits BKCa channels (36, 43, 112). The affinity of heme for the arterial smooth muscle cell BKCa channel heme-binding domain is high with a half-maximal inhibitory concentration of ∼5 nM at physiological voltage and [Ca2+]i (43). Therefore, the BKCa channel is functionally a heme protein. CO, by binding to channel-bound reduced heme, changes the association of heme with the channel, leading to channel activation (43). Thus BKCa channel-bound heme is a receptor for CO, and CO binding increases BKCa channel Ca2+ sensitivity (43, 133). HO and BKCa channels are membrane colocalized, and HO-2 may also interact directly with the heme-binding domain (127, 138). Conceivably, HO-1 and HO-2 may not only generate CO, a BKCa channel activator, but may also reduce membrane-associated inhibitory heme to generate CO. Both of these HO-mediated effects would elevate BKCa channel activity. Although local HO-2-derived CO generation has been proposed to mediate O2 sensitiviy of BKCa channels in carotid body glomus cells, O2 regulates BKCa channel activity in the absence of other reactants required for HO-2-induced heme metabolism, and O2 sensing was not altered in carotid body and adrenal medulla cells of HO-2 knockout mice (90, 127). Thus whether HO-2 is an O2 sensor that regulates BKCa channel activity through CO generation is unresolved.
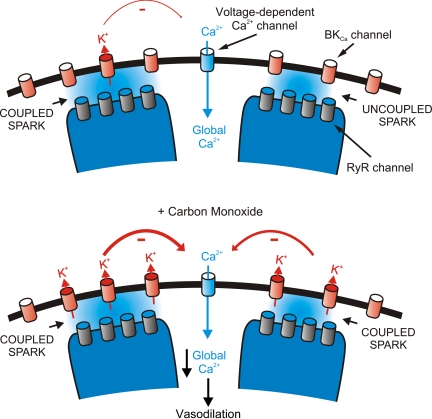
In arterial smooth muscle cells, BKCa channels are sensitive to [Ca2+]i within the micromolar range (97, 133, 145). Local Ca2+ transients termed “Ca2+ sparks” activate BKCa channels by generating the necessary micromolar [Ca2+]i at the channel intracellular surface (45). A single Ca2+ spark activates several BKCa channels, leading to an outward BKCa channel transient. In the arterial wall, transient BKCa currents induce membrane hyperpolarization that reduces voltage-dependent Ca2+ channel activity, leading to a decrease in global [Ca2+]i and vasodilation. In cerebral artery smooth muscle cells, CO elevates BKCa channel Ca2+ sensitivity and elevates BKCa channel activity primarily by elevating Ca2+ spark to BKCa channel coupling (Fig. 2) (42, 133). However, CO also elevates Ca2+ spark frequency, which also contributes to the increase in BKCa channel activity by elevating transient BKCa current frequency (42, 133). This combined effect leads to an increase in both fractional and effective coupling of BKCa channels to Ca2+ sparks, thereby elevating transient BKCa current frequency and amplitude (42, 72) (Fig. 2). Essentially, 100% of Ca2+ sparks evoke a transient KCa current in cerebral artery smooth muscle cells of adult rats and mice, indicating that CO will act in these cells primarily by elevating Ca2+ spark to BKCa channel amplitude. In contrast, in human and piglet cerebral artery smooth muscle cells, a significant proportion of Ca2+ sparks (∼30%) does not evoke a transient KCa current and the slope of the amplitude relationship between a spark and evoked KCa transients is low (42, 71, 125). In smooth muscle cells of these vascular preparations, CO would be a significantly more effective vasodilator by elevating both Ca2+ spark frequency and fractional and effective coupling of BKCa channels to Ca2+ sparks. In cremaster muscle artery and arteriole smooth muscle cells, Ca2+ sparks occur infrequently or are absent at rest (126, 136). The effectiveness of CO to dilate skeletal muscle beds remains to be determined. CO can constrict gracilis muscle arterioles (46, 47). Conversely, also in gracilis muscle arterioles, endogenously produced CO reduces pressure-induced vasoconstriction via a K+ channel mechanism (143). Conceivably, an absence of the Ca2+ spark to BKCa channel pathway may contribute to the inability of CO to dilate skeletal muscle arterioles and may explain the vasoconstriction that is proposed to occur because of NO synthase (NOS) inhibition (see Functional Significance of CO in Control of Cerebrovascular Circulation).
Fig. 2.
Carbon monoxide (CO) elevates fractional and effective coupling of Ca2+ sparks to large-conductance Ca2+-activated K+ (BKCa) channels in arterial smooth muscle cells, leading to vasodilation. Ca2+ sparks occur because of the activation of sarcoplasmic reticulum ryanodine-sensitive Ca2+ release channels (RyR channels). Fractional and effective coupling of Ca2+ sparks to BKCa channels varies depending on several factors, including species and anatomical origin of the vasculature. CO elevates BKCa channel Ca2+ sensitivity, leading to more effective BKCa channel activation by coupled sparks and BKCa channel activation by previously uncoupled Ca2+ sparks. Not shown is that CO also elevates Ca2+ spark frequency. The combination of these CO-induced signaling mechanisms stimulates BKCa channels, leading to membrane hyperpolarization, a reduction in voltage-dependent Ca2+ channel activity, a decrease in intracellular Ca2+ concentration ([Ca2+]i), and vasodilation.
Glutamate-induced piglet pial arteriolar dilation results from astrocyte CO that increases Ca2+ sparks and BKCa activity. Glutamate-induced, astrocyte-derived CO elevates Ca2+ spark frequency and reduces global [Ca2+]i in smooth muscle cells of brain slice arterioles (134). The decline in global [Ca2+]i is blocked by guanylyl cyclase inhibition (see Functional Significance of CO in Control of Cerebrovascular Circulation and Fig. 3). In addition, astrocyte-derived CO activates BKCa channels directly and, coupling with increased Ca2+ sparks, elevates transient BKCa currents in juxtaposed cerebral arteriole smooth muscle cells, leading to vasodilation (72). In vivo, inhibitors of Ca2+ sparks and BKCa channels block CO-induced cerebrovascular dilation (42, 68). Thus CO is an astrocyte-derived intercellular vasodilator that causes dilation to glutamatergic stimulation by elevating Ca2+spark-induced BKCa channel coupling in cerebral arteriole smooth muscle cells.
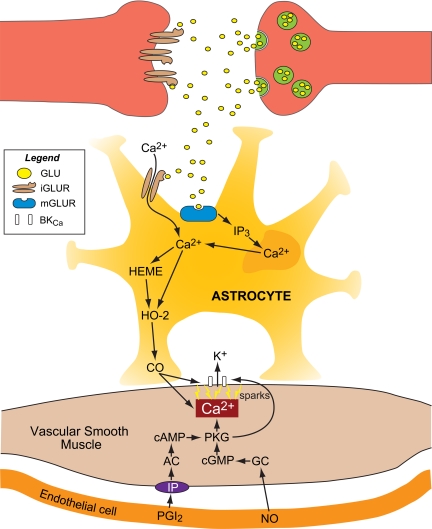
Fig. 3.
CO in neurovascular unit function. From the abluminal side, glutamate binds to astrocyte ionotropic and metabotropic glutamate (GLU) receptors (iGLUR and mGLUR), elevating [Ca2+]i that increases HO-2 activity. The resulting CO binds to BKCa channel-bound heme-elevating channel Ca2+ sensitivity, Ca2+ spark-to-BKCa channel coupling, thereby elevating BKCa channel activity. In addition, CO increases Ca2+ spark frequency, further increasing BKCa channel activity. The resulting smooth muscle cell hyperpolarization decreases [Ca2+]i (see Fig. 2), leading to vasodilation. From the luminal side, permissive enabling contributions of endothelial nitric oxide (NO) and PGI2 involve a necessary level of protein kinase G (PKG) activity that can be provided via NO activation of guanylyl cyclase (GC), producing cGMP and/or activation of PGI2 receptors (IP), adenylyl cyclase (AC), and elevating cAMP. PKG may phosphorylate sarcoplasmic reticulum RyRs, increasing Ca2+ spark frequency and/or the BKCa channel. In the intact vasculature, NO and PGI2 can activate smooth muscle cell GC and AC directly, if a stimulus is sufficient at the endothelial cell. IP3, inositol 1,4,5-trisphosphate.
CO-induced cell signaling has been proposed to occur via guanylyl cyclase activation (38, 80), although CO is far less effective at activating guanylyl cyclase than is NO (54, 55, 57). Treatment of platelets (18) or aorta (33) with CO or a CO donor (32) and elevated HO-1 expression in pulmonary arteries or aorta (3, 85, 103) increase cGMP. The involvement of an endogenous substance that increases guanylyl cyclase sensitivity to CO has been suggested (116). Nevertheless, cGMP as a direct mediator of CO-induced vasodilation under physiological conditions appears unlikely (58). CO activates BKCa channels in smooth muscle cells from a variety of different vascular beds, including cerebral and tail arteries (42, 123). In isolated vascular smooth muscle cells, CO-induced BKCa channel activation is not blocked by inhibitors of soluble guanylyl cyclase and not reproduced by other products of HO-mediated heme metabolism (42, 48, 122, 123, 133). Furthermore, BKCa channel inhibition blocks CO-induced vasodilation (42, 48, 68, 72, 87). Thus vascular smooth muscle cell HO-derived CO or exogenous CO activates arterial smooth muscle cell BKCa channels either directly or via interaction with channel-associated regulatory elements (see Functional Significance of CO in Control of Cerebrovascular Circulation for discussion of role of cGMP in CO-induced dilation).
Functional Significance of CO in Control of Cerebrovascular Circulation
In this review, we primarily focus on the functional significance of CO in cerebrovascular regulation because HO-2 expression, and CO production is highest in the brain and cerebral vasculature. In cerebral vessels, HO-2 is expressed in endothelial cells, adjacent astrocytes, and, to less an extent, the vascular smooth muscle (68, 69, 96, 111). HO-2 is essential for numerous brain functions that include mediation of neuronal activity, regulation of cerebral blood flow, cytoprotection against oxidative stress-related cerebrovascular dysfunction (95), traumatic brain injury (20, 139), and intracranial hemorrhage (121, 124).
In vivo, topical CO (>100 nM) dilates piglet pial arterioles (56). However, at levels at or below 100 nM, the dilation is only transient (56). Brain production of CO results in an accumulation in CSF placed under cranial windows (cortical periarachnoid CSF: pCSF). The basal pCSF CO concentration is 5–8 × 10−8 M (50–80 nM) (19, 49, 56, 70). Brain CO production is acutely increased in response to selected cerebral vasodilator stimulations, including epileptic seizures (19), the excitatory neurotransmitter glutamate (49, 96), acute hypoxia (49), and hypotension (51). In these situations, the level of CO production by brain and vessels is sufficient to provide dilator effects on the cerebral circulation. For example, during seizures the pCSF CO concentration is increased 10- to 20-fold, averaging about 1 μM (19). The elevation of gaseous CO in pCSF during seizures correlates with cerebral vasodilation, consistent with a cause-response relationship (19). Pharmacological inhibition of endogenous CO production reduces pial arteriolar vasodilation in response to seizures, glutamatergic stimulation, hypotension, and hypoxia in newborn pigs (19, 49, 51, 100). In contrast, the elevation of cerebral blood flow in response to hypercapnia occurs in a CO-independent manner and does not involve the elevation of CO in pCSF (66, 68). Although brain CO production was measured only in newborn pigs, the functional role of endogenous CO in the regulation of cerebral blood flow during seizures has also been demonstrated in adult rats (83).
CO can attenuate vascular responses to constrictor stimuli (130, 143). For example, the inhibition of HO accentuates the constriction of pial arterioles produced by hypertension but has minimal effects when piglets are normotensive. In addition, vasoconstriction to a topical application of platelet-activating factor is accentuated following the inhibition of HO (130).
In the rat hypothalamus, CO has been shown to contribute to the regulation of vascular tone that is particularly evident in the absence of NO (37). This finding in adult rats is different from results of newborn pigs where NO and prostacyclin play obligatory permissive roles in CO-induced dilation (67). By “permissive enabling” we mean endothelial NO and/or PGI2 must be present but need not change for CO to cause dilation (9, 66, 67, 70). Thus, in piglet arterioles in vivo or in vitro endothelial removal, COX inhibition, or NOS inhibition blocks dilation to CO (9, 66, 67, 70). Returning NO or PGI2 in the form of a constant, minimally dilator level of sodium nitroprusside (SNP) or iloprost, respectively, totally restores dose-dependent dilation to CO (9, 66, 67, 70). However, in adult rats and 3- to 4-mo-old pigs, NOS inhibition does not inhibit the dilation of pial arterioles to CO (35). These data suggest the permissive action of NO- for CO-induced dilation is age dependent. In the newborn, the permissive enabling functions of both SNP and iloprost involve PKG, but only that of NO involves cGMP (58). It appears likely that the permissive action of PKG involves the elevation of Ca2+ sparks or altering BKCa channel sensitivity (Fig. 3).
While reported actions of acutely (in min) elevated CO on cerebrovascular tone are uniformly dilator, in rat cerebral arterioles prolonged CO (in h) has been shown to be capable of producing vasoconstriction, apparently by inhibiting NOS, but acute CO treatment was not examined (41). However, Holt et al. (35) found acute topical CO dilated adult rat pial arterioles. In isolated rat gracilis muscle arterioles and rat renal arteries, acutely elevated CO produces constriction that appears to involve NOS inhibition (46, 47) and/or CO-induced ROS (61) (see Mechanism of CO-Induced Vasodilation). These data caused us to address the hypothesis that, in contrast to dilation to acute CO, more prolonged exposure of cerebral arterioles to elevated CO produces constriction by reducing NO (56). While treatment with 10−7 M CO caused transient dilation and no effect over 2 h exposure, topical treatment with 2 × 10−7 or 10−6 M CO caused initial dilation, but over 2 h CO exposure pial arteriolar diameter declined to below the basal diameter. Furthermore, the removal of CO caused dilation after 2 h CO exposure. Also, dilation to l-arginine was progressively lost over 90 min CO exposure. If the level of NO was maintained constant by blocking NOS and adding SNP to the CSF, CO caused dilation that was sustained for 2 h and CO removal decreased arteriolar diameter. From this we conclude that a short episodic production of CO allows it to function as a dilator gasotransmitter, whereas a prolonged elevation CO can reduce NO to elevate cerebrovascular tone. Since NO can stimulate CO production (see Regulation of HO-2 Activity), the interaction between HO/CO and NOS/NO could form a negative feedback system in the control of vascular tone.
Pial arterioles of newborn pigs are coated by astrocytic processes (69). Selective astrocyte injury from l-2-α-aminoadipic acid (l-AAA) (135) abolished not only dilation to the astrocyte selective dilator ADP but also dilation in response to glutamate (69). In addition, the increase in pCSF CO concentration that is normally caused by topical glutamate was also absent following l-AAA (69). Of note, the astrocyte-dependent dilator ADP also increased pCSF CO concentration and both the ADP-induced dilation and CO elevation were absent following astrocyte injury (50). Dilations to isoproterenol and CO were not affected by l-AAA. These data suggest both glutamate and ADP stimulate CO production by perivascular astrocytes and this CO causes pial arteriolar dilation in piglets. They also suggest that the predominant cell type that causes CO production and cerebral arteriole dilation to topical glutamate is the astrocyte rather than the endothelial cell. This cell source for glutamate-induced CO signaling may explain the greater dilation of piglet arterioles to glutamate in vivo than in vitro. However, a functional endothelium is still required for the appropriate response to the CO because endothelial-derived NO and/or PGI2 function as permissive enablers for CO dilation (Fig. 3). In other conditions, endothelial CO may signal for dilation directly. For example, mice experiments using HO inhibitors and HO-2 null mice indicate that endothelial-derived CO contributes to cerebrovascular dilation to acetylcholine (98). However, in piglets acetylcholine constricts rather than dilates pial arterioles (5).
To further examine astrocyte-smooth muscle coupling in glutamate-induced smooth muscle responses, we used freshly isolated piglet cerebrovascular smooth muscle cells and brain slices and primary culture astrocytes from piglets and HO-2 null mice (72). Glutamate increased CO production by piglet and wild-type mouse astrocytes but not HO-2-deficient mouse astrocytes. Glutamate-activated BKCa channel currents in piglet cerebral vascular smooth muscle cells in contact with astrocytes but not in the myocytes alone. In addition, if the astrocytes were treated with the HO inhibitor chromium mesophorphrin before being placed with the smooth muscle, glutamate did not activate smooth muscle BKCa channel currents. Similarly, astrocytes from HO-2-deficient mice did not allow the piglet cerebrovascular smooth muscle cells to respond to glutamate (72). With the use of piglet brain slices, it was determined that glutamate reduced arteriole smooth muscle Ca2+ and dilated arterioles in untreated slices but not in slices treated with chromium mesophorphrin; the BKCa channel blocker, paxilline; or the astrocyte toxin, l-AAA. These data confirm that an astrocytic signal, notably HO-2-derived CO, is used by glutamate to stimulate arteriole myocyte BKCa channels and dilate pial arterioles (72).
In addition to effects on vascular tone, the HO/CO system is highly protective to the vasculature during conditions injurious to the brain (92). Epileptic seizures result in prolonged postictal cerebral vascular dysfunction characterized by reduced vasoreactivity to physiologically relevant dilators, including hypercapnia and bradykinin (19, 92). When HO-2 activity is pharmacologically inhibited during seizures, cerebral vascular dysfunction is observed immediately after the ictal episode and is extended for at least 2 days of the postictal period (19, 92). However, seizures do not affect vascular reactivity in the immediate postictal period in control piglets with active HO-2, but cerebral vascular reactivity is greatly reduced 2 days later (19, 92). Thus HO-2 appears necessary for a short-term protection but not sufficient for a long-term protection of the cerebral vasculature from detrimental effects of seizures. In addition, the upregulation of HO-1 expression completely prevents alteration of vascular activity both acutely and 2 days after seizures (92).
CO and bilirubin, the end products of HO-catalyzed heme degradation, have distinct cytoprotective functions (95). HO-2-derived CO is very important in cerebral protection following stroke in mice and can interact positively with NO in providing protection (85). CO suppresses apoptosis (10, 11, 17, 75, 95, 108, 144) although proapoptotic effects of CO also have been reported (109). CO, by binding to a heme prosthetic group, regulates key components of cell signaling, including BKCa channels, guanylyl cyclase, NADPH oxidase, and the mitochondria respiratory chain. CO, by inhibiting the major components of endogenous oxidant-generating machinery, NADPH oxidase, and cytochrome-c oxidase of the mitochondrial respiratory chain, blocks the formation of ROS. Bilirubin, via redox cycling with biliverdin, is a potent ROS scavenger that removes ROS once formed (8, 25, 79, 80). Overall, HO-2 has dual housekeeping cerebroprotective functions by maintaining appropriate blood flow for neuronal activity and by providing an effective defense mechanism that blocks oxidant formation, removes ROS that are formed, and inhibits cell death caused by oxidative stress.
In particular, CO, bilirubin, and biliverdin separately and complimentarily protect against endothelial cell apoptosis caused by ROS. In cerebrovascular endothelial cells from piglets and mice, TNF-α-induced apoptosis, loss of cell-to-cell contacts, and cell detachment were markedly reduced by a CO-releasing molecule (CORM-A1), bilirubin, or HO-1 upregulation (11). Conversely, cerebrovascular endothelial cells from HO-2 null mice had elevated apoptosis caused by TNF-α or serum deprivation. In cerebrovascular endothelial cells challenged with TNF-α, cytoprotective effects of CO and bilirubin are largely due to their antioxidant roles. CO prevents superoxide anion production by inhibiting NOX4, the catalytic subunit of NADPH oxidase, complimenting ROS scavenging by bilirubin (10). Completing the circle, ROS activate HO-2 production of CO, which then inhibits NOX4 via an Akt-dependent pathway promoting endothelial cell survival during inflammation (12). In vivo, the systemic administration of CORM-A1 increased pCSF CO concentration and markedly attenuated the loss of cerebrovascular endothelial function caused by seizures in newborn piglets (147).
CO may affect circulation via regulation of vascular cell proliferation. In rat aortic smooth muscle cells, increasing CO inhibited and scavenging CO increased cell proliferation, actions apparently mediated by cGMP that increases the transcriptional factor E2F-1 (84). CO also reduced hypoxia-induced endothelial cell proliferation by inhibiting adjacent vascular smooth muscle VEGF production (76). This action as well appears to be mediated via cGMP, by decreasing binding of a hypoxic enhancer to hypoxia-inducible factor-1. Following vascular injury as well as hypoxia, CO also suppresses vascular smooth muscle proliferation by increasing cellular cGMP that activates p38 mitogen-activated protein kinase, upregulating caveolin-1 that prevents proliferation (54). Conversely, CO has been shown to promote proliferation of microvascular endothelial cells (73). Thus, depending on the conditions, background stimuli, and specific cell type, CO can either increase or decrease cell proliferation.
Final Remarks
In the preceding pages we have reviewed the HO/CO system with respect to regulation of the blood flow to the brain in particular. CO is a gaseous signaling molecule as are NO, H2S, and various activated oxygen species. These gases diffuse within and among cells down their partial pressure gradients from sites of production to channels and enzymes with which they can interact and alter activity. Of these gases, CO is by far the least chemically reactive. However, CO readily binds to the ferrous iron of heme to potentially alter the activity of all heme proteins. The gaseous signaling molecules interact with each other in regulation of vascular tone. NO increases vascular CO production, but CO inhibits NOS, providing negative feedback between these gasotransmitters. Less is known about the interactions of NO and CO with cystathionine γ-lyase (CSE) and H2S. NO has been shown to be able to simulate CSE (146), CO can inhibit CSE, and H2S can inhibit HO-2 in aortic endothelial cells (45). The interactions among these molecules in vascular signaling are key areas for future research.
Much of the data for present review focusing on cerebrovascular CO was obtained from studies of newborn pigs. In comparing these findings to those of others, principally using adult rodents, a consideration of species and age differences should be made. Also, few data on humans of any age are available. In pigs, the regulatory mechanisms involved in the control of cerebral circulation change with postnatal age (6, 128, 129, 148). Data on CO indicate that age-dependent changes make juvenile pigs more similar to adult rats than newborn pigs (35). These differences are related to permissive-enabling functions of NO and prostanoids and dilator sensitivity, both of which decline with age, rather than qualitative changes in CO-induced dilation of cerebral arterioles. Therefore, model-specific differences do exist and may be very important in understanding physiological functions and worthy of future investigation.
Overall, it is clear that CO is an important signaling molecule in the cerebral circulation and other vascular beds as well. Many of the major mechanisms that control cerebrovascular circulation rely on CO signaling that can come from astrocytes and/or endothelium. In addition, CO and bilirubin, two products of HO metabolism of heme, protect the vasculature and the brain from ROS-induced injury. Future research into interactions with other cerebral vascular regulatory signaling pathways under physiological and pathological conditions is necessary for producing knowledge of the roles of the HO/CO system in health and disease. CORMs, as well as bilirubin and pharmacological formulations, are ripe for clinical investigation in newborns and adults.
GRANTS
The authors were supported by National Institutes of Health Grants HL-042851, HL-34059, HL-99655, NS-63936, HL-67061, and HL-94378.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1. Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 60: 79–127, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Abraham NG, Botros FT, Rezzani R, Rodella L, Bianchi R, Goodman AI. Differential effect of cobalt protoporphyrin on distributions of heme oxygenase in renal structure and on blood pressure in SHR. Cell Mol Biol (Noisy-le-grand) 48: 895–902, 2002 [PubMed] [Google Scholar]
- 3. Abraham NG, Quan S, Mieyal PA, Yang L, Burke-Wolin T, Mingone CJ, Goodman AI, Nasjletti A, Wolin MS. Modulation of cGMP by human HO-1 retrovirus gene transfer in pulmonary microvessel endothelial cells. Am J Physiol Lung Cell Mol Physiol 283: L1117–L1124, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Alam J, Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol 36: 166–174, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Armstead WM, Mirro R, Busija DW, Leffler CW. Permissive role of prostanoids in acetylcholine-induced cerebral constriction. J Pharmacol Exp Ther 251: 1012–1019, 1989 [PubMed] [Google Scholar]
- 6. Armstead WM, Zuckerman SL, Shibata M, Parfenova H, Leffler CW. Different pial arteriolar responses to acetylcholine in the newborn and juvenile pig. J Cereb Blood Flow Metab 14: 1088–1095, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Balla G, Vercellotti GM, Muller-Eberhard U, Eaton J, Jacob HS. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab Invest 64: 648–655, 1991 [PubMed] [Google Scholar]
- 8. Baranano DE, Snyder SH. Neural roles for heme oxygenase: contrasts to nitric oxide synthase. Proc Natl Acad Sci USA 98: 10996–11002, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barkoudah E, Jaggar JH, Leffler CW. The permissive role of endothelial NO in CO-induced cerebrovascular dilation. Am J Physiol Heart Circ Physiol 287: H1459–H1465, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Basuroy S, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-α in cerebral vascular endothelial cells. Am J Physiol Cell Physiol 296: C422–C432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW, Parfenova H. Heme oxygenase-2 provides endogenous protection against oxidative stress and apoptosis caused by TNF-α in cerebral vascular endothelial cells. Am J Physiol Cell Physiol 291: C897–C908, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Basuroy S, Tcheranova D, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase-derived reactive oxygen species, via endogenous carbon monoxide, promote survival of brain endothelial cells during TNF-α-induced apoptosis. Am J Physiol Cell Physiol 300: C256–C265, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boehning D, Moon C, Sharma S, Hurt KJ, Hester LD, Ronnett GV, Shugar D, Snyder SS. Carbon monoxide neurotransmission activated by CK2 phosphorylation of heme oxygenase-2. Neuron 40: 129–137, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Boehning D, Sedaghat L, Sedlak TW, Snyder SS. Heme oxygenase-2 is activated by calcium-calmodulin. J Biol Chem 279: 30927–30930, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Boehning D, Snyder SH. Novel neural modulators. Annu Rev Neurosci 26: 105–131, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature 407: 870–876, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med 192: 1015–1026, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brune B, Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol 32: 497–504, 1987 [PubMed] [Google Scholar]
- 19. Carratu P, Pourcyrous M, Fedinec A, Leffler CW, Parfenova H. Endogenous heme oxygenase prevents impairment of cerebral vascular functions caused by seizures. Am J Physiol Heart Circ Physiol 285: H1148–H1157, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Chang EF, Wong RJ, Vreman HJ, Igarashi T, Galo E, Sharp FR, Stevenson DK, Noble-Haeusslein LJ. Heme oxygenase-2 protects against lipid peroxidation-mediated cell loss and impaired motor recovery after traumatic brain injury. J Neurosci 23: 3689–3696, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen J, Tu Y, Moon C, Nagata E, Ronnett GV. Heme oxygenase-1 and heme oxygenase-2 have distinct roles in the proliferation and survival of olfactory receptor neurons mediated by cGMP and bilirubin, respectively. J Neurochem 85: 1247–1261, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Ding Y, McCoubrey WK, Jr, Maines MD. Interaction of heme oxygenase-2 with nitric oxide donors. Is the oxygenase an intracellular ‘sink’ for NO? Eur J Biochem 264: 854–861, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Doré S, Sampei K, Goto S, Alkayed NJ, Guastella D, Blackshaw S, Gallagher M, Traystman RJ, Hurn PD, Koehler RC, Snyder SH. Heme oxygenase-2 is neuroprotective in cerebral ischemia. Mol Med 5: 656–663, 1999 [PMC free article] [PubMed] [Google Scholar]
- 25. Dore S, Takahashi M, Ferris CD, Zakhary R, Hester LD, Guastella D, Snyder SH. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci USA 96: 2445–2450, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dwyer BE, Nishimura RN, Lu SY. Differential expression of heme oxygenase-1 in cultured cortical neurons and astrocytes determined by the aid of a new heme oxygenase antibody. Response to oxidative stress. Brain Res Mol Brain Res 30: 37–47, 1995 [DOI] [PubMed] [Google Scholar]
- 27. Ewing JF, Maines MD. In situ hybridization and immunohistochemical localization of heme oxygenase-2 mRNA and protein in normal rat brain: differential distribution of isozyme 1 and 2. Mol Cell Neurosci 3: 559–570, 1992 [DOI] [PubMed] [Google Scholar]
- 28. Ewing JF, Maines MD. Rapid induction of heme oxygenase 1 mRNA and protein by hyperthermia in rat brain: heme oxygenase 2 is not a heat shock protein. Proc Natl Acad Sci USA 88: 5364–5368, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ewing JF, Weber CM, Maines MD. Biliverdin reductase is heat resistant and coexpressed with constitutive and heat shock forms of heme oxygenase in brain. J Neurochem 61: 1015–1023, 1993 [DOI] [PubMed] [Google Scholar]
- 30. Fiumana E, Parfenova H, Jaggar JH, Leffler CW. Carbon monoxide mediates vasodilator effects of glutamate in isolated pressurized cerebral arterioles of newborn pigs. Am J Physiol Heart Circ Physiol 284: H1073–H1079, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Foresti R, Hammad J, Clark JE, Johnson TR, Mann BE, Friebe A, Green CJ, Motterlini R. Vasoactive properties of CORM-3, a novel water-soluble carbon monoxide-releasing molecule. Br J Pharmacol 142: 453–460, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Foresti R, Sarathchandra P, Clark JE, Green CJ, Motterlini R. Peroxinitrite induces haem oxygenase-1 in vascular endothelial cells: a link to apoptosis. Biochem J 339: 729–736, 1999 [PMC free article] [PubMed] [Google Scholar]
- 33. Furchgott RF, Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels 28: 52–61, 1991 [DOI] [PubMed] [Google Scholar]
- 34. Hayashi S, Omata Y, Sakamoto H, Higashimoto Y, Hara T, Sagara Y, Noguchi M. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene 336: 241–250, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Holt DC, Fedinec AL, Vaughn AN, Leffler CW. Age and species dependence of pial arteriolar responses to topical carbon monoxide in vivo. Exp Biol Med (Maywood) 232: 1465–1469, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Horrigan FT, Heinemann SH, Hoshi T. Heme regulates allosteric activation of the Slo1 BK channel. J Gen Physiol 126: 7–21, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Horvath B, Hrabak A, Kaldi K, Sandor P, Benyo Z. Contribution of the heme oxygenase pathway to the maintenance of the hypothalamic blood flow during diminished nitric oxide synthesis. J Cereb Blood Flow Metab 23: 653–657, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Hosein S, Marks GS, Brien JF, McLaughlin BE, Nakatsu K. An extracellular source of heme can induce a significant heme oxygenase mediated relaxation in the rat aorta. Can J Physiol Pharmacol 80: 761–765, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Hou S, Xu R, Heinemann SH, Hoshi T. The RCK1 high-affinity Ca2+ sensor confers carbon monoxide sensitivity to Slo1 BK channels. Proc Natl Acad Sci USA 105: 4039–4043, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang TJ, McCoubrey WK, Jr, Maines MD. Heme oxygenase-2 interaction with metalloporphyrins: function of heme regulatory motifs. Antioxid Redox Signal 3: 685–696, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Ishikawa M, Kajimura M, Adachi T, Maruyama K, Makino N, Goda N, Yamaguchi T, Sekizuka E, Suematsu M. Carbon monoxide from heme oxygenase-2 is a tonic regulator against NO-dependent vasodilation in the adult rat cerebral microcirculation. Circ Res 97: e104–e114, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Jaggar JH, Leffler CW, Cheranov SY, Tcheranova D, E S, Cheng X. Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channels. Circ Res 91: 610–617, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Jaggar JH, Li A, Parfenova H, Liu J, Umstot ES, Dopico AM, Leffler CW. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res 97: 805–812, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol 278: C235–C256, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Jin H, Du J, Li X, Wang Y, Liang Y, Tang C. Interaction between hydrogen sulfide/cystathionine γ-lyase and carbon monoxide/heme oxygenase pathways in aortic smooth muscle cells. Acta Pharmacol Sin 27: 1561–1566, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Johnson FK, Johnson RA. Carbon monoxide promotes endothelium-dependent constriction of isolated gracilis muscle arterioles. Am J Physiol Regul Integr Comp Physiol 285: R536–R541, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Johnson RA, Johnson FK. Heme oxygenase-derived endogenous carbon monoxide impairs flow-induced dilation in resistance vessels. Shock 29: 526–530, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Kaide JI, Zhang F, Wei Y, Jiang H, Yu C, Wang WH, Balazy M, Abraham NG, Nasjletti A. Carbon monoxide of vascular origin attenuates the sensitivity of renal arterial vessels to vasoconstrictors. J Clin Invest 107: 1163–1171, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kanu A, Leffler CW. Carbon monoxide and Ca2+-activated K+ channels in cerebral arteriolar responses to glutamate and hypoxia in newborn pigs. Am J Physiol Heart Circ Physiol 293: H3193–H3200, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kanu A, Leffler CW. Roles of glia limitans astrocytes and carbon monoxide in adenosine diphosphate-induced pial arteriolar dilation in newborn pigs. Stroke 40: 930–935, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kanu A, Whitfield J, Leffler CW. Carbon monoxide contributes to cerebrovascular vasodilation in piglets. Am J Physiol Heart Circ Physiol 291: H2409–H2414, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kikuchi G, Hayashi N. Regulation by heme of synthesis and intracellular translocation of delta-aminolevinulinate synthase in the liver. Mol Cell Biochem 37: 27–41, 1981 [DOI] [PubMed] [Google Scholar]
- 53. Kim HP, Wang X, Galbiati F, Ryter SW, Choi AM. Caveolae compartmentalization of heme oxygenase-1 in endothelial cells. FASEB J 18: 1080–1089, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Kim HP, Wang X, Nakao A, Kim SI, Murase N, Choi ME, Ryter SW, Choi AM. Caveolin-1 expression by means of p38 beta mitogen-activated protein kinase mediates the antiproliferative effect of carbon monoxide. Proc Natl Acad Sci USA 102: 11319–11324, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kimura H. Hydrogen sulfide as a neuromodulator. Mol Neurobiol 26: 13–19, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Knecht KR, Milam S, Wilkinson DA, Fedinec AL, Leffler CW. Time-dependent action of carbon monoxide on the newborn cerebrovascular circulation. Am J Physiol Heart Circ Physiol 299: H70–H75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Koehler RC, Traystman RJ. Cerebrovascular effects of carbon monoxide. Antioxid Redox Signal 4: 279–290, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Koneru P, Leffler CW. Role of cyclic GMP in carbon monoxide induced vasodilation in piglets. Am J Physiol Heart Circ Physiol 286: H304–H309, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Kooli A, Kermorvant-Duchemin E, Sennlaub F, Bossolasco M, Hou X, Honoré JC, Dennery PA, Sapieha P, Varma D, Lachapelle P, Zhu T, Tremblay S, Hardy P, Jain K, Balazy M, Chemtob S. Arachidonic acids induce a heme oxygenase-dependent vasorelaxation of cerebral microvasculature. Free Radic Biol Med 44: 815–825, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Kronke G, Bochkov VN, Huber J, Gruber F, Bluml S, Furnkranz A, Kadl A, Binder BR, Leitinger N. Oxidized phospholipids induce expression of human heme oxygenase-1 involving activation of cAMP-responsive element-binding protein. J Biol Chem 278: 51006–51014, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Lamon BD, Zhang FF, Puri N, Brodsky SV, Goligorsky MS, Nasjletti A. Dual pathways of carbon monoxide-mediated vasoregulation: modulation by redox mechanisms. Circ Res 105: 775–783, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lavrovsky Y, Schwartzman ML, Abraham NG. Novel regulatory sites of the human heme oxygenase-1 promoter region. Biochem Biophys Res Commun 196: 336–341, 1993 [DOI] [PubMed] [Google Scholar]
- 63. Leffler CW, Balabanova L, Fedinec AL, Parfenova H. Nitric oxide increases carbon monoxide production by piglet cerebral microvessels. Am J Physiol Heart Circ Physiol 289: H1442–H1447, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Leffler CW, Balabanova L, Fedinec AL, Waters CM, Parfenova H. Mechanism of glutamate stimulation of CO production in cerebral microvessels. Am J Physiol Heart Circ Physiol 285: H74–H80, 2003 [DOI] [PubMed] [Google Scholar]
- 65. Leffler CW, Balabanova L, Sullivan CD, Wang X, Fedinec AL, Parfenova H. Regulation of CO production in cerebral microvessels of newborn pigs. Am J Physiol Heart Circ Physiol 285: H292–H297, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Leffler CW, Fedinec AL, Parfenova H, Jaggar JH. Permissive contributions of NO and prostacyclin in CO-induced cerebrovascular dilation in piglets. Am J Physiol Heart Circ Physiol 289: H432–H438, 2005 [DOI] [PubMed] [Google Scholar]
- 67. Leffler CW, Nasjletti A, Johnson RA, Fedinec AL. Contributions of prostacyclin and nitric oxide to carbon monoxide induced cerebrovascular dilation in newborn pigs. Am J Physiol Heart Circ Physiol 280: H1490–H1495, 2001 [DOI] [PubMed] [Google Scholar]
- 68. Leffler CW, Nasjletti A, Yu C, Johnson RA, Fedinec AL, Walker N. Carbon monoxide and cerebral microvascular tone in newborn pigs. Am J Physiol Heart Circ Physiol 276: H1641–H1646, 1999 [DOI] [PubMed] [Google Scholar]
- 69. Leffler CW, Parfenova H, Fedinec AL, Basuroy S, Tcheranova D. Contributions of astrocytes and CO to pial arteriolar dilation to glutamate in newborn pigs. Am J Physiol Heart Circ Physiol 291: H2897–H2904, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Leffler CW, Parfenova H, Jaggar JH, Wang R. Carbon monoxide and hydrogen sulfide: gaseous messengers in cerebrovascular circulation. J Appl Physiol 100: 1065–1076, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li A, Adebiyi A, Leffler CW, Jaggar JH. KCa channel insensitivity to Ca2+ sparks underlies fractional uncoupling in newborn cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol 291: H1118–H1125, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li A, Xi Q, Umstot ES, Bellner L, Schwartzman ML, Jaggar JH, Leffler CW. Astrocyte-derived CO is a diffusible messenger that mediates glutamate-induced cerebral arteriolar dilation by activating smooth muscle Cell KCa channels. Circ Res 102: 234–241, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li Volti G, Sacerdoti D, Sangras B, Vanella A, Mezentsev A, Scapagnini G, Falck JR, Abraham NG. Carbon monoxide signaling in promoting angiogenesis in human microvessel endothelial cells. Antioxid Redox Signal 7: 704–710, 2005 [DOI] [PubMed] [Google Scholar]
- 74. Liu N, Wang X, McCoubrey WK, Maines MD. Developmentally regulated expression of two transcripts for heme oxygenase-2 with a first exon specific to rat testis; control by corticosterone of the oxygenase protein expression. Gene 241: 175–183, 2000 [DOI] [PubMed] [Google Scholar]
- 75. Liu XM, Chapman GB, Peyton KJ, Schafer AI, Durante W. Carbon monoxide inhibits apoptosis in vascular smooth muscle cells. Cardiovasc Res 55: 396–405, 2002 [DOI] [PubMed] [Google Scholar]
- 76. Liu Y, Christou H, Morita T, Laughner E, Semenza GL, Kourembanas S. Carbon monoxide and nitric oxide suppress the hypoxic induction of vascular endothelial growth factor gene via the 5′ enhancer. J Biol Chem 273: 15257–15262, 1998 [DOI] [PubMed] [Google Scholar]
- 77. Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J 2: 2557–2268, 1988 [PubMed] [Google Scholar]
- 78. Maines MD. Carbon monoxide: an emerging regulator of cGMP in the brain. Mol Cell Neurosci 4: 389–397, 1993 [DOI] [PubMed] [Google Scholar]
- 79. Maines MD. The heme oxygenase system and its functions in the brain. Cell Mol Biol 46: 573–585, 2000 [PubMed] [Google Scholar]
- 80. Marks GS, Brien JF, Nakatsu K, McLaughlin BE. Does carbon monoxide have a physiological function? Trends Pharmacol Sci 12: 185–188, 1991 [DOI] [PubMed] [Google Scholar]
- 81. Maulik N, Engelman DT, Watanabe M, Engelman RM, Das DK. Nitric oxide-a retrograde messenger for carbon monoxide signaling in ischemic heart. Mol Cell Biochem 157: 75–86, 1996 [DOI] [PubMed] [Google Scholar]
- 82. May BK, Bhasker CR, Bawden MJ, Cox TC. Molecular regulation of 5-aminolevulinate synthase. Diseases related to heme biosynthesis. Mol Biol Med 7: 405–421, 1990 [PubMed] [Google Scholar]
- 83. Montecot C, Seylaz J, Pinard E. Carbon monoxide regulates cerebral blood flow in epileptic seizures but not in hypercapnia. Neuroreport 9: 2341–2346, 1998 [DOI] [PubMed] [Google Scholar]
- 84. Morita T, Mitsialis SA, Koike H, Liu Y, Kourembanas S. Carbon monoxide controls the proliferation of hypoxic vascular smooth muscle cells. J Biol Chem 272: 32804–32809, 1997 [DOI] [PubMed] [Google Scholar]
- 85. Morita T, Perrella MA, Lee ME, Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci USA 92: 1475–1479, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Morley P, Small DL, Murray CL, Mealing GA, Poulter MO, Durkin JP, Stanimirovic DB. Evidence that functional glutamate receptors are not expressed on rat or human cerebromicrovascular endothelial cells. J Cereb Blood Flow Metab 18: 396–406, 1998 [DOI] [PubMed] [Google Scholar]
- 87. Naik JS, Walker BR. Heme oxygenase-mediated vasodilation involves vascular smooth muscle cell hyperpolarization. Am J Physiol Heart Circ Physiol 285: H220–H228, 2003 [DOI] [PubMed] [Google Scholar]
- 88. Namiranian K, Koehler RC, Sapirstein A, Doré S. Stroke outcomes in mice lacking the genes for neuronal heme oxygenase-2 and nitric oxide synthase. Curr Neurovasc Res 2: 23–27, 2005 [DOI] [PubMed] [Google Scholar]
- 89. Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 43: 233–260, 2003 [DOI] [PubMed] [Google Scholar]
- 90. Ortega-Saenz P, Pascual A, Gomez-Diaz R, Lopez-Barneo J. Acute oxygen sensing in heme oxygenase-2 null mice. J Gen Physiol 128: 405–411, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Parfenova H, Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW. Glutamate induces oxidative stress and apoptosis in cerebral vascular endothelial cells: contributions of HO-1 and HO-2 to cytoprotection. Am J Physiol Cell Physiol 290: C1399–C1410, 2006 [DOI] [PubMed] [Google Scholar]
- 92. Parfenova H, Carratu P, Tcheranova D, Fedinec A, Pourcyrous M, Leffler CW. Epileptic seizures cause extended postictal cerebral vascular dysfunction that is prevented by HO-1 overexpression. Am J Physiol Heart Circ Physiol 288: H2843–H2850, 2005 [DOI] [PubMed] [Google Scholar]
- 93. Parfenova H, Fedinec A, Leffler CW. Ionotropic glutamate receptors in cerebral microvascular endothelium are functionally linked to heme oxygenase. J Cereb Blood Flow Metab 23: 190–197, 2003 [DOI] [PubMed] [Google Scholar]
- 94. Parfenova H, Leffler CW, Tcheranova D, Basuroy S, Zimmermann A. Epileptic seizures increase circulating endothelial cells in peripheral blood as early indicators of cerebral vascular damage. Am J Physiol Heart Circ Physiol 298: H1687–H1698, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Parfenova H, Leffler CW. Cerebroprotective functions of HO-2. Curr Pharm Des 14: 443–453, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Parfenova H, Neff RA, 3rd, Alonso JS, Shlopov BV, Jamal CN, Sarkasova SA, Leffler CW. Cerebrovascular endothelial heme oxygenase: expression, intracellular compartmentalization, and activation by glutamate. Am J Physiol Cell Physiol 281: C1954–C1963, 2001 [DOI] [PubMed] [Google Scholar]
- 97. Perez GJ, Bonev AD, Nelson MT. Micromolar Ca2+ from sparks activates Ca2+-sensitive K+ channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol 281: C1769–C1775, 2001 [DOI] [PubMed] [Google Scholar]
- 98. Qin X, Kwansa H, Bucci E, Doré S, Boehning D, Shugar D, Koehler RC. Role of heme oxygenase-2 in pial arteriolar response to acetylcholine in mice with and without transfusion of cell-free hemoglobin polymers. Am J Physiol Regul Integr Comp Physiol 295: R498–R504, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Raju VS, McCoubrey WK, Jr, Maines MD. Regulation of heme oxygenase-2 by glucocorticoids in neonatal rat brain: characterization of a functional glucocorticoid response element. Biochim Biophys Acta 1351: 89–104, 1997 [DOI] [PubMed] [Google Scholar]
- 100. Robinson JS, Fedinec AL, Leffler CW. Role of CO in glutamate receptor-induced dilation of newborn pig pial arterioles. Am J Physiol Heart Circ Physiol 282: H2371–H2376, 2002 [DOI] [PubMed] [Google Scholar]
- 101. Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 86: 583–650, 2006 [DOI] [PubMed] [Google Scholar]
- 102. Ryter SW, Choi AM. Heme oxygenase-1: molecular mechanisms of gene expression in oxygen-related stress. Antioxid Redox Signal 4: 625–632, 2002 [DOI] [PubMed] [Google Scholar]
- 103. Sammut IA, Foresti R, Clark JE, Exon DJ, Vesely MJ, Sarathchandra P, Green CJ, Motterlini R. Carbon monoxide is a major contributor to the regulation of vascular tone in aortas expressing high levels of haeme oxygenase-1. Br J Pharmacol 125: 1437–1444, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Scapagnini G, D'Agata V, Calabrese V, Pascale A, Colombrita C, Alkon D, Cavallaro S. Gene expression profiles of heme oxygenase isoforms in the rat brain. Brain Res 954: 51–59, 2002 [DOI] [PubMed] [Google Scholar]
- 105. Schipper HM. Heme oxygenase expression in human central nervous system disorders. Free Radic Biol Med 37: 1995–2011, 2004 [DOI] [PubMed] [Google Scholar]
- 106. Schipper HM. Heme oxygenase-1: transducer of pathological brain iron sequestration under oxidative stress. Ann NY Acad Sci 1012: 84–93, 2004 [DOI] [PubMed] [Google Scholar]
- 107. Schuller DJ, Wilks A, Ortiz de Montellano PR, Poulos TL. Crystal structure of human heme oxygenase-1. Nat Struct Biol 6: 860–867, 1999 [DOI] [PubMed] [Google Scholar]
- 108. Soares MP, Usheva A, Brouard S, Berberat PO, Gunther L, Tobiash E, Bach FH. Modulation of endothelial cell apoptosis by heme oxygenase-1-derived carbon monoxide. Antioxid Redox Signal 4: 321–328, 2002 [DOI] [PubMed] [Google Scholar]
- 109. Song R, Zhou Z, Kim PK, Shapiro RA, Liu F, Ferran C, Choi AM, Otterbein LE. Carbon monoxide promotes Fas/CD95-induced apoptosis in Jurkat cells. J Biol Chem 279: 44327–44334, 2004 [DOI] [PubMed] [Google Scholar]
- 110. Sugishima M, Hagiwara Y, Zhang X, Yoshida T, Migita CT, Fukuyama K. Crystal structure of dimeric heme oxygenase-2 from Synechocystis sp. PCC 6803 in complex with heme. Biochemistry 44: 4257–4266, 2005 [DOI] [PubMed] [Google Scholar]
- 111. Sutherland BA, Rahman RM, Clarkson AN, Shaw OM, Nair SM, Appleton I. Cerebral heme oxygenase 1 and 2 spatial distribution is modulated following injury from hypoxia-ischemia and middle cerebral artery occlusion in rats. Neurosci Res 65: 326–334, 2009 [DOI] [PubMed] [Google Scholar]
- 112. Tang XD, Xu R, Reynolds MF, Garcia ML, Heinemann SH, Hoshi T. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature 425: 531–535, 2003 [DOI] [PubMed] [Google Scholar]
- 113. Tcheranova D, Leffler CW, Parfenova H. Distinct protein kinase pathways are involved in HO-1 induction by severe oxidative stress in cerebral microvascular endothelium of newborn pigs (Abstract). FASEB J 19: A1252, 2005 [Google Scholar]
- 114. Telezhkin V, Brazier SP, Mears R, Muller CT, Riccardi D, Kemp PJ. Cysteine residue 911 in C-terminal tail of human BKCa a channel subunit is crucial for its activation by carbon monoxide. Pflügers Arch. 2011. February 8 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 115. Thorup C, Jones CL, Gross SS, Moore LC, Goligorsky MS. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am J Physiol Renal Physiol 277: F882–F889, 1999 [DOI] [PubMed] [Google Scholar]
- 116. Tulis DA. Salutary properties of YC-1 in the cardiovascular and hematological systems. Curr Med Chem Cardiovasc Hematol Agents 2: 343–359, 2004 [DOI] [PubMed] [Google Scholar]
- 117. van Horssen J, Schreibelt G, Drexhage J, Hazes T, Dijkstra CD, van der Valk P, de Vries HE. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic Biol Med 45: 1729–1737, 2008 [DOI] [PubMed] [Google Scholar]
- 118. Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab 23: 629–652, 2003 [DOI] [PubMed] [Google Scholar]
- 119. Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16: 1792–1798, 2002 [DOI] [PubMed] [Google Scholar]
- 120. Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal 5: 493–501, 2003 [DOI] [PubMed] [Google Scholar]
- 121. Wang J, Doré S. Heme oxygenase 2 deficiency increases brain swelling and inflammation after intracerebral hemorrhage. Neuroscience 155: 1133–1141, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wang R, Wu L. The chemical modification of KCa channels by carbon monoxide in vascular smooth muscle cells. J Biol Chem 272: 8222–8226, 1997 [DOI] [PubMed] [Google Scholar]
- 123. Wang R, Wu L, Wang Z. The direct effect of carbon monoxide on KCa channels in vascular smooth muscle cells. Pflügers Arch 434: 285–291, 1997 [DOI] [PubMed] [Google Scholar]
- 124. Wang J, Zhuang H, Dore S. Heme oxygenase 2 is neuroprotective against intracerebral hemorrhage. Neurobiol Dis 22: 473–476, 2006 [DOI] [PubMed] [Google Scholar]
- 125. Wellman GC, Nathan DJ, Saundry CM, Perez G, Bonev AD, Penar PL, Tranmer BI, Nelson MT. Ca2+ sparks and their function in human cerebral arteries. Stroke 33: 802–808, 2002 [DOI] [PubMed] [Google Scholar]
- 126. Westcott EB, Jackson WF. Heterogeneous function of ryanodine receptors, but not IP3 receptors, in hamster cremaster muscle feed arteries and arterioles. Am J Physiol Heart Circ Physiol 300: H1616–H1630, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C, Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science 306: 2093–2097, 2004 [DOI] [PubMed] [Google Scholar]
- 128. Willis AP, Leffler CW. NO and prostanoids: age dependence of hypercapniaand histamine-induced dilations of pig pial arterioles. Am J Physiol Heart Circ Physiol 277: H299–H307, 1999 [DOI] [PubMed] [Google Scholar]
- 129. Willis AP, Leffler CW. Endothelial NO and prostanoid involvement in newborn and juvenile pig pial arteriolar vasomotor responses. Am J Physiol Heart Circ Physiol 281: H2366–H2377, 2001 [DOI] [PubMed] [Google Scholar]
- 130. Winestone JS, Bonner C, Leffler CW. Carbon monoxide as an attenuator of vasoconstriction in piglet cerebral circulation. Exp Biol Med (Maywood) 228: 46–50, 2003 [DOI] [PubMed] [Google Scholar]
- 131. Wu L, Cao K, Lu Y, Wang R. Different mechanisms underlying the stimulation of KCa channels by nitric oxide and carbon monoxide. J Clin Invest 110: 691–700, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Xi Q, Tcheranova D, Basuroy S, Parfenova H, Jaggar JH, Leffler CW. Glutamate-stimulated, astrocyte-derived carbon monoxide production is intracellular calcium concentration-dependent in piglets (Abstract). FASEB J 24: lb556, 2011 [Google Scholar]
- 133. Xi Q, Tcheranova D, Parfenova H, Horowitz B, Leffler CW, Jaggar JH. Carbon monoxide activates KCa channels in newborn cerebral arteriole smooth muscle cells by increasing the apparent Ca2+-sensitivity of α-subunits. Am J Physiol Heart Circ Physiol 286: H610–H618, 2004 [DOI] [PubMed] [Google Scholar]
- 134. Xi Q, Umstot E, Zhao G, Narayanan D, Leffler CW, Jaggar JH. Glutamate regulates Ca2+ signals in smooth muscle cells of newborn piglet brain slice arterioles through astrocyte- and heme oxygenase-dependent mechanisms. Am J Physiol Heart Circ Physiol 298: H562–H569, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Xu HL, Mao L, Ye S, Paisansathan C, Vetri F, Pelligrino DA. Astrocytes are a key conduit for upstream signaling of vasodilation during cerebral cortical neuronal activation in vivo. Am J Physiol Heart Circ Physiol 294: H622–H632, 2008 [DOI] [PubMed] [Google Scholar]
- 136. Yang Y, Murphy TV, Ella SR, Grayson TH, Haddock R, Hwang YT, Braun AP, Peichun G, Korthuis RJ, Davis MJ, Hill MA. Heterogeneity in function of small artery smooth muscle BKCa: involvement of the beta1-subunit. J Physiol 587: 3025–3044, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Yi L, Jenkins PM, Leichert LI, Jakob U, Martens JR, Ragsdale SW. The heme regulatory motifs in heme oxygenase-2 form a thiol/disulfide redox switch that responds to the cellular redox state. J Biol Chem 284: 20556–20561, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yi L, Morgan JT, Ragsdale SW. Identification of a thiol/disulfide redox switch in the human BK channel that controls its affinity for heme and CO. J Biol Chem 285: 20117–20127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yoneyama-Sarnecky T, Olivas AD, Azari S, Ferriero DM, Manvelyan HM. Heme oxygenase-2 modulates early pathogenesis after traumatic injury to the immature brain. Dev Neurosci 32: 81–90, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Yoshida T, Sato M. Posttranslational and direct integration of heme oxygenase into microsomes. Biochem Biophys Res Commun 163: 1086–1092, 1989 [DOI] [PubMed] [Google Scholar]
- 141. Zakhary R, Gaine SP, Dinerman JL, Ruat M, Flavahan NA, Snyder SH. Heme oxygenase 2: endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc Natl Acad Sci USA 93: 795–798, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zakhary R, Poss KD, Jaffrey SR, Ferris CD, Tonegawa S, Snyder SH. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc Natl Acad Sci USA 94: 14848–14853, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zhang F, Kaide J, Wei Y, Jiang H, Yu C, Balazy M, Abraham NG, Wang W, Nasjletti A. Carbon monoxide produced by isolated arterioles attenuates pressure- induced vasoconstriction. Am J Physiol Heart Circ Physiol 281: H350–H358, 2001 [DOI] [PubMed] [Google Scholar]
- 144. Zhang X, Shan P, Otterbein LE, Alam J, Flavell RA, Davis RJ, Choi AM, Lee PJ. Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 mitogen-activated protein kinase pathway and involves caspase 3. J Biol Chem 278: 1248–1258, 2003 [DOI] [PubMed] [Google Scholar]
- 145. Zhao G, Neeb ZP, Leo MD, Pachuau J, Adebiyi A, Ouyang K, Chen J, Jaggar JH. Type 1 IP3 receptors activate BKCa channels via local molecular coupling in arterial smooth muscle cells. J Gen Physiol 136: 283–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous KATP channel opener. EMBO J 20: 6008–6016, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zimmermann A, Leffler CW, Tcheranova D, Fedinec AL, Parfenova H. Cerebroprotective effects of the CO-releasing molecule CORM-A1 against seizure-induced neonatal vascular injury. Am J Physiol Heart Circ Physiol 293: H2501–H2507, 2007 [DOI] [PubMed] [Google Scholar]
- 148. Zuckerman SL, Armstead WM, Hsu P, Shibata M, Leffler CW. Age dependence of cerebrovascular response mechanisms in domestic pigs. Am J Physiol Heart Circ Physiol 271: H535–H540, 1996 [DOI] [PubMed] [Google Scholar]