Abstract
Obese Zucker rats (OZR) have elevated sympathetic nerve activity (SNA) and mean arterial pressure (MAP) compared with lean Zucker rats (LZR). We examined whether altered tonic glutamatergic, angiotensinergic, or GABAergic inputs to the rostral ventrolateral medulla (RVLM) contribute to elevated SNA and MAP in OZR. Male rats (14–18 wk) were anesthetized with urethane (1.5 g/kg iv), ventilated, and paralyzed to record splanchnic SNA, heart rate (HR), and MAP. Inhibition of the RVLM by microinjections of muscimol eliminated SNA and evoked greater decreases in MAP in OZR vs. LZR (P < 0.05). Antagonism of angiotensin AT1 receptors in RVLM with losartan yielded modest decreases in SNA and MAP in OZR but not LZR (P < 0.05). However, antagonism of ionotropic glutamate receptors in RVLM with kynurenate produced comparable decreases in SNA, HR, and MAP in OZR and LZR. Antagonism of GABAA receptors in RVLM with gabazine evoked smaller rises in SNA, HR, and MAP in OZR vs. LZR (P < 0.05), whereas responses to microinjections of GABA into RVLM were comparable. Inhibition of the caudal ventrolateral medulla, a major source of GABA to the RVLM, evoked attenuated rises in SNA and HR in OZR (P <0.05). Likewise, inhibition of nucleus tractus solitarius, the major excitatory input to caudal ventrolateral medulla, produced smaller rises in SNA and HR in OZR. These results suggest the elevated SNA and MAP in OZR is derived from the RVLM and that enhanced angiotensinergic activation and reduced GABAergic inhibition of the RVLM may contribute to the elevated SNA and MAP in the OZR.
Keywords: hypertension, sympathetic nerve activity, muscimol, kynurenate, gabazine, losartan
accumulation of excess body fat is an independent risk factor for elevated mean arterial pressure (MAP; Refs. 8, 13, 26). Over activation of sympathetic nerves that regulate MAP is a hallmark of obesity-induced hypertension (8, 11). Obese hypertensive human subjects have increased muscle sympathetic nerve activity (SNA) characterized by a recruitment of previously silent sympathetic fibers (24). Some obese hypertensives present with elevated plasma levels of catecholamines (26), whereas others report increases in norepinephrine spillover only to selective targets such as the kidney (30). Nevertheless, acute elimination of SNA evokes larger decreases in MAP in obese subjects compared with gender and age-matched lean subjects (36), suggesting an enhanced sympathetic contribution to the maintenance of MAP with obesity-related hypertension.
Rat models of obesity mimic the enhanced sympathetic vasomotor tone observed in obese humans. In adult obese Zucker rats (OZR), renal and splanchnic SNA and MAP are elevated (16, 27) compared with age-matched lean Zucker rats (LZR). Furthermore, autonomic ganglionic blockade evokes a larger decrease in basal MAP in OZR than in LZR (31). Similarly, elimination of sympathetic vasomotor tone by inhibition of rostral ventrolateral medulla (RVLM), the primary source of drive for SNA, evokes a greater decrease in MAP in Sprague-Dawley rats made obese by a high-fat diet (40). These observations suggest that obese rats have augmented sympathetic vasomotor tone that is derived from the RVLM.
Elevated SNA is a common attribute for many forms of hypertension, and the RVLM is a critical player in rat models of hypertension. In spontaneously hypertensive rats, Dahl-salt-sensitive rats, and renal injury hypertensive rats, the elevated SNA is associated with an increase in the tonic activation of the RVLM by glutamate and angiotensin II (2, 9, 17, 18, 19, 20). In addition, reduced tonic GABAergic inhibition of the RVLM also appears to contribute to the elevated MAP observed in spontaneously hypertensive rats (38). The mechanisms underlying elevated SNA in OZR have not been elucidated. Although the RVLM is a likely source, the balance of these key tonic excitatory and inhibitory inputs to the RVLM in OZR vs. LZR is not known. The present study examined the contribution of the RVLM to the elevated SNA and MAP in OZR and sought to determine whether tonic contributions of angiotensin II, glutamate, and GABA in the RVLM-mediated drive for SNA were altered in the OZR. These experiments provide the first analysis of the brain stem mechanisms for the regulation of sympathetic vasomotor tone in Zucker rats.
MATERIALS AND METHODS
Animals.
Age-matched adult (14–18 wk old) male OZR and LZR (Harlan, Indianapolis, IN) were fed standard rat chow and tap water ad libitum. Rats were housed (2–4/cage) in the animal care facilities at the Medical College of Georgia or the University of North Texas Health Science Center, which are approved by the American Association for the Accreditation of Laboratory Animal Care. The experiments conformed to guidelines set forth in the Guide for the Care and Use of Laboratory Animals. Institutional Animal Care and Use Committees at the Medical College of Georgia and the University of North Texas Health Science Center reviewed and approved all protocols used in these experiments.
Animal preparation and physiological measures.
Rats were anesthetized with isoflurane via a nose cone for surgical procedures (initially with 5% and then maintained at 1.9–2.5% in 100% oxygen). Adequacy of anesthesia was verified by the absence of a blink to lightly touching the eye and a change in MAP <10 mmHg and lack of withdrawal to a firm toe pinch. A catheter was implanted in a femoral vein for the administration of drugs and in a femoral artery for the measurement of arterial pressure (AP). The LZR were artificially ventilated (55–65 strokes/min of 1 ml/100 g LZR body wt; model 683; Harvard Apparatus). The OZR were initially ventilated at the tidal volume of the age-matched LZR, and then the volume was adjusted slightly upward to maintain an end-tidal CO2 comparable to the LZR (3.5- 4.0%; CapStar-100; CWE), as previously described (33). The rat was placed in a stereotaxic instrument (David Kopf Instruments) with the bite bar set at −11 mm to flex the head downward and facilitate exposure of the dorsal brain stem. The left greater splanchnic nerve was exposed by a retroperitoneal approach, placed on two Teflon-coated silver wires (A-M Systems) bared at the tips, and surrounded by silicone elastomers (kwik-sil; World Precision Instruments) as previously described (16). The nerve was isolated immediately distal to the branch toward the adrenal gland and was left intact for the recordings. The dorsal surface of the brain stem was exposed by partially removing the occipital bone and retracting the underlying dura mater. After surgical procedures were completed, isoflurane anesthesia was replaced by urethane (1.5 g/kg LZR body wt administered intravenously using 1.5 g/5 ml solution at 50 μl/min), as previously described (16, 33). Once anesthetized with urethane, rats were allowed to recover for 30–45 min. Rectal temperature was maintained at 37°C. Shortly before the beginning of the experiment, anesthesia was confirmed as described above and the rat was paralyzed with pancuronium (1 mg/kg iv; Abbott Labs). Hourly supplements of one-third the initial dose of pancuronium were given after confirmation of adequate anesthesia.
Microinjections into brain stem.
Drugs were microinjected into the brain stem using a single-barrel glass micropipette pulled and cut to a tip of 40–50 μm, mounted on a stereotaxic arm, and connected to a pressure microinjection apparatus (Pressure System IIe; Toohey). All drugs were dissolved in artificial cerebrospinal fluid and injected in 50 or 100 nl over a period of 4 to 6 s. The last microinjected drug contained 5% green latex microspheres (Lumiphore) for histological confirmation of the microinjections sites as previously described (25).
Each brain stem site was located using previously established stereotaxic coordinates (25, 32) and by observing changes in MAP with microinjections of glutamate (1 nmol in 50 or 100 nl). The coordinates for RVLM were 1.7–2.1 mm lateral from the midline, 1.4–1.8 mm rostral to the caudal tip of area postrema, and 2.7–3.1 mm below the dorsal surface of the brain stem with the pipette tip angled 20° rostrally. The site producing the largest pressor response to glutamate (>20 mmHg) was selected for further study. The coordinates for caudal ventrolateral medulla (CVLM) were 1.9 mm lateral from the midline, 1.3 mm rostral to caudal tip of the area postrema, and 2.4–2.8 mm below the dorsal surface of the brain stem. The depth producing the largest depressor response to glutamate was selected for further study (>20 mmHg). The coordinates for nucleus tractus solitarius (NTS) were 0.5 mm rostral to the caudal tip of area postrema (calamus scriptorius), 0.5 mm lateral from the midline, and 0.5 mm ventral to the dorsal surface of the brain stem. Glutamate at these coordinates consistently evoked depressor responses (>20 mmHg).
Once the brain stem sites were located, the pipette was withdrawn, rinsed, and filled with the experimental drug. Muscimol (100 pmol), a GABAA agonist, was microinjected to inhibit neuronal cell bodies within the region of the injection. Kynurenate, a broad spectrum ionotropic glutamate receptor antagonist, was microinjected to block glutamatergic inputs to the region to produce functional inhibition (2.7 nmol in RVLM and 5.4 nmol in CVLM). Losartan (1 nmol), an angiotensin II receptor 1 (AT1) antagonist, was microinjected into the RVLM to block tonic influence of angiotensin II. Gabazine (100 pmol), a GABAA receptor antagonist, was microinjected to eliminate GABAergic inputs. These four drugs were injected bilaterally one side at a time with ∼1 min between injections in separate groups of rats. In the experiments where more than one drug was microinjected as part of the design, the efficacy of the antagonist was confirmed and then the second drug was microinjected ∼10–30 min later after the physiological variables had stabilized.
Doses of antagonists for glutamate and GABA were based on pilot experiments showing effective blockade of reflex responses mediated by the brain stem region under study. Maximum responses were measured within 10 min after microinjection of the second side. Effective antagonism was functionally verified in all rats by observing the elimination of established reflexes. For blockade of glutamate receptors, a higher dose was required in the CVLM than in the RVLM to effectively block reflexes. To confirm the blockade of ionotropic glutamate receptors in RVLM, stimulation of the right sciatic nerve (10 s of 1-ms pulses at 20 Hz and 300 μA) was performed. These stimulation parameters produce reliable rises in SNA and MAP that are eliminated or reversed after antagonism of ionotropic glutamate receptors in the RVLM (22). All rats included in the study showed the expected responses before brain stem microinjections and an absence of responses after microinjections of kynurenate into the RVLM (see Fig. 3A). Blockade of GABAergic inputs to the RVLM was confirmed by the elimination of a vagal reflex. Stimulation of a vagal afferent nerve (10 s of 2-ms pulses at 5 Hz and 4 V) with the efferent connection severed produces reflexively mediated decreases in SNA and MAP that are prevented if GABAA receptors are blocked in the RVLM (41). This vagal stimulation was performed to confirm the blockade of GABAA receptors in the RVLM in all rats examined, and only rats with elimination or reversal of the changes in MAP were included in the study (see Fig. 4A). For these experiments, the right cervical vagus nerve was exposed and cut distal to its junction with the superior laryngeal nerve to preserve aortic depressor nerve fibers. The proximal end of the afferent nerve was separated from the superior laryngeal nerve and intervening fibers and was placed on two Teflon-coated silver wires that were bared at the tips. The preparation was isolated and stabilized by kwik-sil. In subsequent experiments, effective blockade of NTS and CVLM were verified by intravenous injection of phenyl biguanide (2 μg in 50 μl saline) to elicit the Bezold-Jarisch reflex (34, 44) to allow the vagus nerve to remain intact. Before brain stem injections, phenyl biguanide reliably produced large reductions in SNA, HR, and MAP, and after inhibition of the NTS or CVLM, these physiological responses were abolished or reversed.
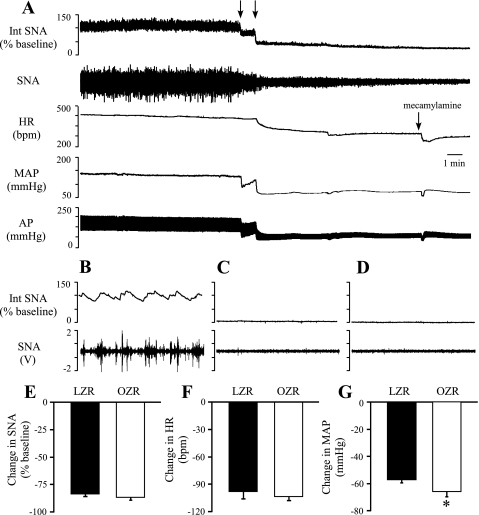
Fig. 3.
Physiological effects of bilateral microinjections of kynurenate into the RVLM. A: representative tracings of Int SNA, raw SNA, HR, MAP, and AP from a lean Zucker rat. Arrows above tracings indicate microinjections of kynurenate into the RVLM. Asterisks below tracings indicate sciatic nerve stimulations used to confirm effective blockade of glutamatergic inputs to the RVLM. Stimulation of the sciatic nerve increased SNA, MAP, and HR before microinjections into the brainstem, and these responses were abolished or reversed after kynurenate into the RVLM. B–D: bilateral microinjections of kynurenate produced no changes in SNA, HR, or MAP in Sprague-Dawley (SD) rats (n = 5) but evoked comparable decreases in all 3 variables OZR (n = 6) and LZR (n = 5). *P < 0.05, different from SD rats.
Fig. 4.
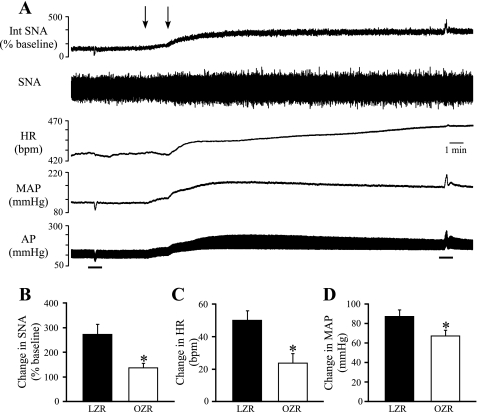
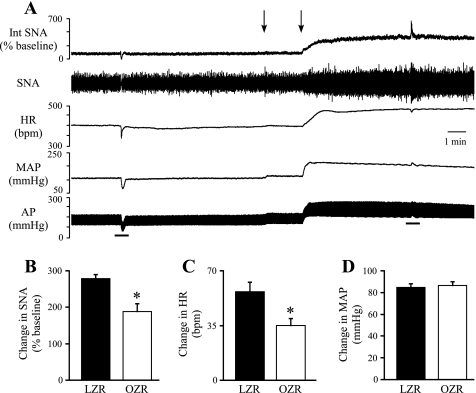
Physiological effects of bilateral microinjections of gabazine into the RVLM. A: representative tracings of Int SNA, raw SNA, HR, MAP, and pulsatile AP from a lean Zucker rat. Arrows above tracings indicate microinjections into the RVLM. Bars below the tracings indicate the stimulation of a vagal afferent nerve to confirm effective blockade of GABAergic inputs to the RVLM. Before brain stem injections the vagal stimulation evoked decreases in SNA and MAP that were reversed after antagonism of GABA receptors in the RVLM. B–D: blockade of GABAergic inputs to the RVLM by microinjections of gabazine produced smaller increases in SNA, HR, and MAP in OZR (n = 12) compared with LZR (n = 11). *P < 0.05, different from LZR.
The dose of losartan was based on a previous report (1) showing that 1 nmol is maximally effective for attenuating pressor responses to local exogenous microinjections of angiotensin II. Higher doses produce large pressor responses unrelated to the AT1 receptor that differ in normotensive and hypertensive rats (1). The responses to losartan were monitored for 30 min based on a previous report (9) showing maximal responses required this time period. Although the hypothalamic paraventricular nucleus appears to be a source of angiotensin II to the RVLM, blockade of AT1 receptors in the RVLM does not abolish the response to manipulation of the paraventricular nucleus (19, 42), rendering it an inconclusive tool for establishing effective blockade of AT1 receptors in the RVLM. In addition, losartan does not completely abolish exogenously microinjected angiotensin II (1). Therefore, the proper placement of losartan into the RVLM was confirmed by subsequent microinjections of muscimol into the same sites to verify abolition of sympathetic vasomotor tone.
Histological processing.
At the completion of the physiological experiments, rats were perfused with 200 ml of PBS (pH 7.4) followed by 500 ml of 4% formaldehyde. Brains were removed and stored in the same fixative for 48 h. The brain stems were sectioned coronally at 50 μm using Vibratome. Sections (1 in 6 series) were mounted onto glass slides, and coverslips were affixed with Krystalon (VWR). Injections sites were visualized under epifluorescence using an Olympus microscope (BX60) to verify correct placement of microinjections as previously shown (25). All rats with microinjections that produced effective antagonism of reflexes also had deposits of latex microspheres in the expected brain stem regions.
Data analysis and statistics.
Amplifiers and filters from the Neurolog system (www.digitimer.com) were used to quantify AP, MAP, and HR. The HR was triggered from the rising phase of the AP pulse (spike trigger, Neurolog). The SNA was amplified and filtered at 10 to 3 kHz with a 60-Hz notch filter (Differential AC amplifier 1700; A-M Systems). The raw SNA was full-wave rectified and averaged into 1-s bins. The baseline integrated SNA (100%) was defined as the activity immediately preceding each stimulus. At the end of the experiment, SNA was eliminated by administration of a ganglionic antagonist (mecamylamine, 10 mg/kg iv) and the noise voltage was defined as 0% SNA. Changes in SNA were estimated as a percent change from baseline. All analog physiological variables were converted to digital signals (Micro 1401; Cambridge Electronic Design) and viewed online (Spike2 software; Cambridge).
All data are expressed as means ± SE. Significant statistical difference was set at P < 0.05. Pairwise comparisons of baseline parameters and changes with a single drug between LZR and OZR were performed using unpaired t-tests. Comparisons of changes among three groups (Sprague-Dawley, LZR, and OZR) were performed using a one-way ANOVA followed by Tukey-Kramer post hoc tests when a significant F value was observed. Comparisons of responses to three doses of GABA into the RVLM in LZR and OZR were performed using a two-way ANOVA. All statistical analyses were performed with SigmaStat software.
RESULTS
The adult OZR weighed significantly more than the LZR at 14–18 wk of age (Table 1). Baseline MAP of the OZR was significantly higher than the LZR under urethane anesthesia (Table 1), as previously reported for conscious adult Zucker rats (5, 29). Rectified SNA voltage was also significantly higher in the OZR compared with the LZR (Table 1), in agreement with previous observations of elevated renal and splanchnic SNA in the OZR (16, 27). In contrast, HR was not significantly different between the LZR and OZR (Table 1).
Table 1.
Baseline values for splanchnic SNA, MAP, and HR for LZR and OZR
| Group | n | Weight, g | SNA, μV | MAP, mmHg | HR, beats/min |
|---|---|---|---|---|---|
| LZR | 59 | 376 ± 4 | 1.69 ± 0.15 | 116 ± 2 | 423 ± 4 |
| OZR | 65 | 584 ± 8* | 2.20 ± 0.16* | 124 ± 2* | 416 ± 3 |
Values are means ± SE. SNA, sympathetic nerve activity; MAP, mean arterial pressure; HR, heart rate; LZR, lean Zucker rats; OZR, obese Zucker rats.
P < 0.05, significantly different from LZR.
Effects of inhibition of the RVLM on SNA, HR, and MAP.
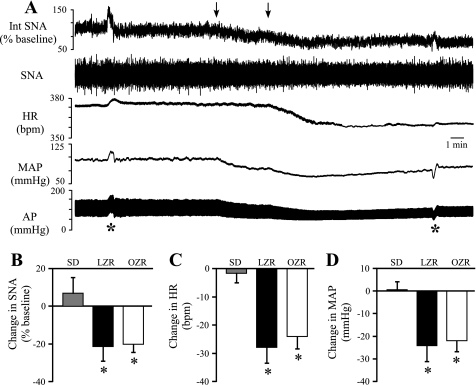
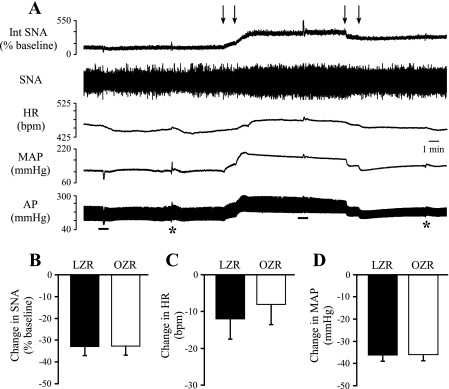
To determine whether the RVLM contributes to the elevated SNA and MAP observed in the OZR, neuronal cell bodies in the RVLM were inhibited by bilateral microinjections of muscimol. The SNA was virtually eliminated, and MAP and HR were significantly reduced (Fig. 1, A–D). Inhibition of the RVLM produced comparable reductions in SNA and HR in LZR and OZR (Fig. 1, E and F) but evoked a greater decrease in MAP in OZR compared with LZR (Fig. 1G). Subsequent injection of the ganglionic blocker mecamylamine produced a transient injection artifact followed by stabilization of all measured parameters within minutes. The SNA and MAP were not further reduced by injection of the ganglionic antagonist mecamylamine (Fig. 1, A, C, and D).
Fig. 1.
Physiological effects of bilateral microinjections of muscimol into the rostral ventrolateral medulla (RVLM). A: representative tracings of integrated splanchnic sympathetic nerve activity (Int SNA), raw splanchnic sympathetic nerve activity (SNA), heart rate (HR), mean arterial pressure (MAP), and pulsatile arterial pressure (AP) from a lean Zucker rat. Arrows above tracings indicate microinjections of muscimol into the RVLM. B–D: segments of tracings shown in A with expanded time scales (5 s) for baseline (B), 10 min after microinjections of muscimol (C), and 5 min after injection of mecamylamine (D). Inhibition of RVLM by bilateral microinjections of muscimol virtually eliminated SNA (E) and produced a larger decrease in MAP (G) in obese Zucker rats (OZR; n = 13) compared with lean Zucker rats (LZR) (n = 13). Decreases in HR were not different between OZR and LZR (F). bpm, Beats/min. *P < 0.05, different from LZR.
Effects of antagonism of AT1 angiotensinergic receptors in the RVLM.
To determine whether angiotensin II provides enhanced tonic excitatory influence in the RVLM of OZR, the AT1 receptor antagonist losartan was microinjected bilaterally into the RVLM. In OZR, losartan produced modest decreases in SNA, HR, and MAP that were maximal within 10 min of the bilateral injections (Fig. 2, B–D). In LZR, the changes in HR were comparable to OZR (Fig. 2C), but the effects of losartan on SNA and MAP were negligible (Fig. 2, B–D). After 30 min, the GABAA agonist muscimol was microinjected bilaterally into the same sites to verify proper placement within the RVLM. Muscimol produced effective inhibition of SNA and significant decreases in MAP (Fig. 2A) that were not further reduced by ganglionic blockade with mecamylamine.
Fig. 2.
Physiological effects of bilateral microinjections of losartan into the RVLM. A: representative tracings of Int SNA, raw SNA, HR, and MAP from a lean Zucker rat. First set of arrows above the tracings indicates microinjections of losartan into the RVLM. Second set of arrows above the tracings indicates microinjections of muscimol into the RVLM. Right arrow above the HR tracing indicates intravenous injection of mecamylamine. B and D: losartan produced no change in SNA or MAP in the LZR but produced small decreases in SNA and MAP in the OZR that were different from the LZR (*P < 0.05). C: losartan produced comparable decreases in HR in LZR and OZR. Subsequent microinjections of muscimol into RVLM virtually eliminated SNA and reduced MAP and HR to levels comparable to those observed after ganglionic blockade with mecamylamine.
Effects of antagonism of ionotropic glutamate receptors in the RVLM on SNA, HR, and MAP.
To determine whether enhanced tonic glutamatergic activation of the RVLM contributes to the elevated SNA and MAP observed in the OZR, glutamatergic inputs to the RVLM were blocked by bilateral microinjections of kynurenate (Fig. 3). Stimulation of the sciatic nerve before the microinjections reliably produced increases in SNA, HR, and MAP, and these responses were eliminated or reversed after microinjections of kynurenate into the RVLM (Fig. 3A). Inhibition of glutamatergic inputs to the RVLM evoked comparable decreases in SNA, HR, and MAP in OZR and LZR (Fig. 3, B–D).
Because several studies (21, 23) have reported that blockade of ionotropic glutamatergic receptors in the RVLM does not alter baseline SNA or MAP in anesthetized Sprague-Dawley rats, an additional set of experiments was performed in Sprague-Dawley rats using our conditions and coordinates. As expected, the bilateral microinjection of kynurenate into the RVLM of Sprague-Dawley rats evoked no changes in SNA, HR, or MAP (SD in Fig. 3, B–D).
Effects of antagonism of GABAA receptors in the RVLM.
To determine whether reduced tonic GABAergic inhibition of the RVLM may contribute to the elevated SNA and MAP observed in OZR, the GABAA receptor antagonist gabazine was microinjected bilaterally into the RVLM (Fig. 4). Stimulation of vagal afferent fibers before the microinjections evoked reflexively mediated reductions in SNA and MAP, and these responses were reversed after antagonism of GABAA receptors in the RVLM (Fig. 4A). Inhibition of GABAA receptors in the RVLM evoked significantly smaller increases in SNA (∼50%), HR (∼52%), and MAP (∼23%) in OZR compared with LZR (Fig. 4, B–D).
Effects of antagonism of ionotropic glutamate receptors in the RVLM after blockade of GABA receptors.
To determine whether the attenuated tonic GABAergic regulation of the RVLM could prevent observation of potential differences in tonic glutamatergic activation of the RVLM, kynurenate was microinjected into the RVLM after the blockade of RVLM GABAA receptors (Fig. 5A). Even after GABAergic receptors were blocked in the RVLM by microinjections of gabazine, kynurenate microinjected in the RVLM evoked comparable decreases in SNA, HR, and MAP (Fig. 5, B–D).
Fig. 5.
Physiological effects of bilateral microinjection of kynurenate into the RVLM after bilateral microinjection of gabazine into RVLM. A: representative tracings of Int SNA, raw SNA, HR, MAP, and pulsatile AP from a lean Zucker rat. First set of arrows above the tracings indicates microinjections of gabazine into the RVLM, and the second set of arrows indicates microinjections of kynurenate into the RVLM. Bars below the tracings indicate vagal afferent nerve stimulation to confirm effective blockade of GABAA receptors in the RVLM. Asterisks below the tracings indicate stimulation of the sciatic nerve to confirm effective antagonism of glutamate receptors in the RVLM. B-D: after elimination of GABAergic inputs to RVLM, blockade of glutamatergic inputs to the RVLM by bilateral microinjection of kynurenate produced comparable decreases in SNA, HR, and MAP between OZR (n = 5) and LZR (n = 5).
Effects of antagonism of ionotropic glutamate receptors in the CVLM on SNA, HR, and MAP.
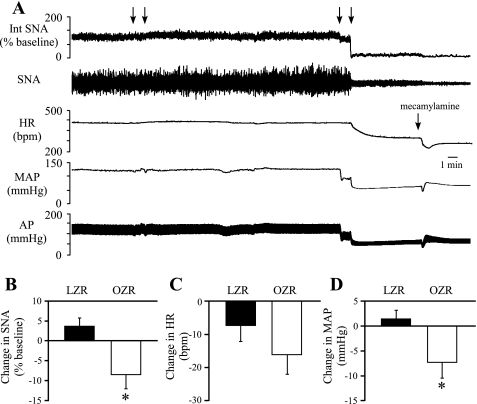
The CVLM was inhibited via disfacilitation by blocking excitatory glutamatergic inputs to determine whether this major source of tonic GABAergic inputs to the RVLM was reduced in OZR. Because glutamate is the primary tonic activator for the CVLM neurons that restrain SNA and MAP, the CVLM neuronal activity was inhibited by microinjections of kynurenate. We (32) have previously shown that increases in MAP after microinjection of kynurenate are comparable to those seen after inhibition of neuronal cell bodies in the region by microinjections of muscimol. The functional blockade of CVLM was confirmed by a reversal of sympathoinhibitory and depressor responses to phenyl biguanide (Fig. 6A) in all rats. As observed after antagonism of GABAA receptors in the RVLM, antagonism of ionotropic glutamate receptors in the CVLM evoked attenuated increases in SNA (∼42%) and HR (∼51%) in OZR compared with LZR (Fig. 6, B and C). The rises in MAP were not different between the groups (Fig. 6D) in this experiment.
Fig. 6.
Physiological effects of bilateral microinjections of kynurenate into the CVLM. A: representative tracings of Int SNA, HR, MAP, and pulsatile AP from a lean Zucker rat. Arrows above tracings indicate microinjections of kynurenate into the CVLM. Bars below the tracings indicate injections of phenyl biguanide (2 μg/50 μl) to confirm effective blockade of glutamatergic inputs to the CVLM. B and C: antagonism of glutamatergic inputs to the CVLM evoked smaller rises in SNA and HR in OZR (n = 14) compared with LZR (n = 13). D: pressor responses were comparable in OZR and LZR. *P <0.05, compared with LZR.
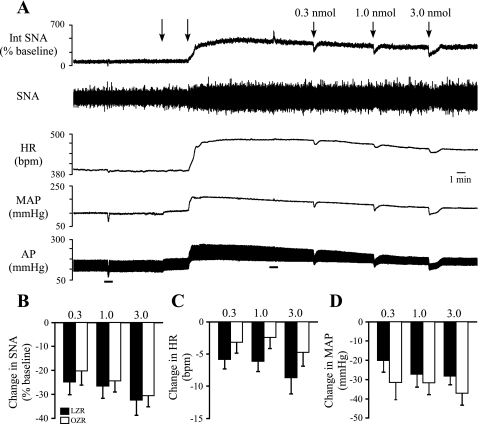
Effects of microinjections of GABA into the RVLM on SNA, HR, and MAP.
To determine whether the attenuated responses to blockade of GABAA receptors in the RVLM with gabazine were due to a reduced ability of GABA to inhibit the RVLM, three doses of GABA (0.3, 1.0, and 3.0 nmol/100 nl) were unilaterally microinjected into the RVLM. Because basal tonic GABAergic inhibition of the RVLM appeared to differ between OZR and LZR, the major endogenous source of GABA to the RVLM was eliminated by functional inhibition of the CVLM. After microinjections of kynurenate into the CVLM (Fig. 7A), microinjections of GABA into the RVLM produced decreases in SNA, HR, and MAP that were not different between OZR and LZR (Fig. 7, B–D).
Fig. 7.
Physiological effects of unilateral microinjection of GABA into the RVLM after microinjections of kynurenate into the CVLM. A: representative tracings of Int SNA, raw SNA, HR, MAP, and pulsatile AP from a lean Zucker rat. Arrows above tracings indicate microinjections of kynurenate into the CVLM to eliminate the major source of endogenous GABA to the RVLM. Bars below tracings indicate injections of phenyl biguanide (2 μg/50 μl) to confirm effective antagonism of glutamatergic inputs to the CVLM. B–D: unilateral microinjection 3 doses of GABA into RVLM produced comparable decreases in SNA, HR, and MAP in OZR (n = 7) and LZR (n = 7).
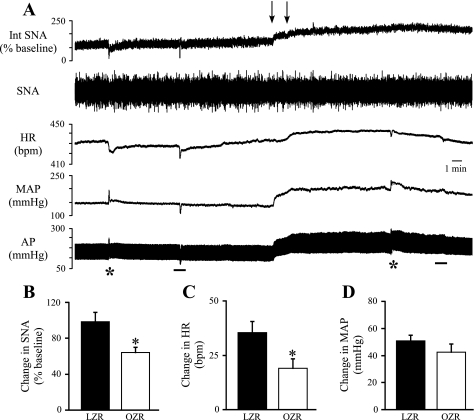
Effects of inhibition of the NTS on SNA, HR, and MAP.
The intermediate NTS is a major source of tonic excitatory input to the CVLM, so we examined whether the contributions of the NTS to resting SNA, HR, and MAP were reduced in OZR compared with LZR. The NTS was inhibited by bilateral microinjections of muscimol. As observed with blockade of glutamatergic inputs to the CVLM, inhibition of the NTS evoked increases in SNA, HR, and MAP and abolished phenylephrine-induced reductions in SNA and HR and phenyl biguanide-induced reductions in SNA, HR, and MAP (Fig. 8A). In addition, as observed with inhibition of glutamatergic inputs to the CVLM, inhibition of the NTS evoked smaller rises in SNA (∼35%) and HR (∼47%) in OZR compared with LZR (Fig. 8, B and C). The rises in MAP were not different between the groups (Fig. 8D).
Fig. 8.
Physiological effects of bilateral microinjections of muscimol into the nucleus tractus solitarius (NTS). A: representative tracings of Int SNA, raw SNA, HR, MAP, and pulsatile AP from a lean Zucker rat. Arrows above tracings indicate microinjections into the NTS. Asterisks below tracings indicate injections of phenylephrine (1 μg/50 μl) to evoke pressor-induced inhibitions of SNA and HR. Although these responses were absent after inhibition of the NTS, tests with phenylephrine were not routinely used, because the pressor response to inhibition of the NTS limited the ability of phenylephrine to effectively raise MAP. Bars below the tracings indicate injections of phenyl biguanide (2 μg/50 μl) to confirm effective inhibition of the NTS. Before brain stem injections, the phenyl biguanide evoked decreases in SNA, HR, and MAP that were eliminated after microinjections of muscimol into the NTS. B and C: inhibition of the NTS by microinjections of muscimol produced smaller increases in SNA and HR in OZR (n = 12) compared with LZR (n = 10). D: pressor responses were comparable in OZR and LZR. *P < 0.05, different from LZR.
DISCUSSION
Adult OZR develop elevated SNA and MAP by unknown mechanisms (16, 33). The present study examined the tonic contributions from brain stem sites that contribute to basal sympathetic vasomotor tone in Zucker rats. The RVLM provides the primary drive for the SNA that maintains MAP (14), and the activity of presympathetic RVLM neurons is powerfully inhibited by GABAergic inputs from the CVLM to restrain SNA, HR, and MAP (32). In spontaneously hypertensive rats the tonic CVLM-mediated GABAergic inhibition of the RVLM appears to be attenuated (37). Furthermore, in many hypertensive rat models, tonic activation of RVLM by glutamate and angiotensin II are augmented (9, 17, 18, 19, 20). The principle observations of the present study are that 1) inhibition of the RVLM evoked a greater decrease in MAP in OZR compared with LZR; 2) antagonism of AT1 receptors in the RVLM evoked modest decreases in SNA and MAP in OZR but not LZR; 3) antagonism of ionotropic glutamate receptors in the RVLM evoked comparable changes in SNA, HR, and MAP in OZR and LZR; 4) antagonism of GABAA receptors in the RVLM evoked smaller rises in SNA, HR, and MAP in OZR compared with LZR; and 5) inhibition of either the CVLM or the intermediate NTS, a primary activator of the CVLM, evoked smaller rises in SNA and HR in OZR compared with LZR. Together these results suggest that elevated SNA to cardiovascular targets in the OZR may be the result of an enhanced tonic excitatory influence of angiotensin II and a reduced tonic inhibitory influence of GABA on the RVLM and that the CVLM and NTS may contribute to the reduced tonic GABAergic influence on the RVLM in OZR.
Many rat models of hypertension exhibit elevated SNA that is associated with an enhanced drive from the RVLM, although precise mechanisms differ depending on the model. In spontaneously hypertensive rats, Dahl-salt sensitive rats, and renal hypertensive rat models, increased tonic activation of the RVLM by angiotensin II appears to contribute to the elevated SNA and MAP (9, 17, 18). In the present study, we observed small but significant decreases in SNA and MAP after microinjections of losartan into the RVLM in OZR but not in LZR. These data suggest that angiotensin II may contribute to the enhanced sympathetic vasomotor tone derived from the RVLM in OZR. Although the mechanisms underlying the difference in responses to antagonism of AT1 receptors were not further explored in the present study, other rodent models of hypertension have increased expression of AT1 receptors and binding of angiotensin II coincident with enhanced efficacy of local angiotensin II (4, 9, 17, 18, 28). Interestingly, the enhanced central angiotensinergic regulation of SNA in OZR is not reflected in their plasma. Unlike some other models of hypertension, OZR do not have elevated plasma renin activity compared with LZR (39). These data indicate that the peripheral and central renin-angiotensin systems are independently regulated and further suggest that antagonism of angiotensin II in OZR may be beneficial for lowering SNA and MAP despite a lack of activation of the peripheral renin-angiotensin system.
Hypertensive rat models with enhanced angiotensinergic activation of the RVLM also appear to have augmented glutamatergic tone (3, 19, 20). However, in the present study we found no evidence to support the notion that enhanced tonic glutamatergic inputs to the RVLM raise SNA in the OZR. Effective antagonism of ionotropic glutamate receptors in the RVLM evoked comparable decreases in SNA, HR, and MAP in OZR and LZR, and this was observed even after the elimination of potentially altered endogenous GABAergic inputs to the RVLM. Unexpectedly, even LZR displayed significant reductions in SNA, HR, and MAP with antagonism of glutamate receptors in the RVLM, in contrast to previous reports of a lack of physiological effects in other normotensive strains (19, 20, 22). The responses to inhibition of glutamatergic inputs to the RVLM observed in LZR were not likely due to specific conditions of our experiments, because under the same conditions blockade of glutamate receptors in Sprague-Dawley rats did not alter SNA, HR, or MAP. Thus, although the tonic glutamatergic regulation of RVLM is enhanced in OZR compared with Sprague-Dawley rats, this mechanism does not appear to explain the differences between OZR and LZR.
In contrast to the comparable tonic influences of glutamate in the RVLM in OZR and LZR, the OZR appeared to have a pronounced reduction in tonic GABAergic inhibition of the RVLM. Specifically, inhibition of GABAA receptors in the RVLM evoked smaller rises in SNA, HR, and MAP in OZR compared with LZR. Reduced tonic GABAergic inhibition of the RVLM has also been observed in spontaneously hypertensive rats compared with normotensive Wistar-Kyoto rats (38), suggesting that other conditions with elevated SNA and MAP may employ this central mechanism. The attenuated responses to blockade of GABAergic inputs to the RVLM in both cases do not appear to reflect a reduction in the ability of GABA in the RVLM to decrease SNA and MAP. In fact, microinjections of GABA into the RVLM evoke larger decreases in MAP in spontaneously hypertensive rats vs. Wistar-Kyoto rats (38). In the present study, endogenous GABAergic inputs from the CVLM were minimized before examination of the effects of GABA in the RVLM to eliminate apparently altered GABAergic tone between the OZR and LZR and prevent baroreceptor-mediated feedback that could influence the magnitude of the responses to GABA in the RVLM. Under these conditions, the microinjections of GABA into the RVLM evoked comparable decreases in SNA, HR, and MAP in OZR and LZR. These data suggest that attenuated responses to blockade of GABAergic inputs to the RVLM in OZR are not due to a reduced ability of GABA to inhibit the RVLM. Instead the blunted response to antagonism of GABAA receptors in the RVLM of OZR may reflect diminished tonic release of GABA in the RVLM.
In agreement with the notion of a reduced tonic GABAergic inhibition of the RVLM, minimizing inputs from the CVLM, the major source of endogenous tonic GABA to the RVLM (32), evoked smaller rises in SNA and HR in the OZR compared with the LZR. Analogous observations have been reported in spontaneously hypertensive rats, where attenuated rises in MAP with blockade of GABAergic inputs to the RVLM were mimicked by inhibition of the CVLM with tetrodotoxin (37, 38). Consistent with the effects of functionally inhibiting the CVLM, inhibition of the intermediate NTS also produced attenuated rises in SNA and HR in OZR compared with LZR. Together, these data suggest that in the OZR the NTS- and CVLM-mediated influences on SNA and HR are reduced in OZR. These effects are likely to be relayed through the RVLM and contribute to the diminished physiological effects of direct blockade of GABAergic receptors in the RVLM.
Unexpectedly, in the present study the significantly attenuated rises in SNA and HR after inhibition of the CVLM or the NTS in the OZR were accompanied by normal pressor responses. Although we have no simple explanation for the dissociation between the responses in this case, especially given the excellent correlation of attenuated SNA, HR, and MAP microinjections of gabazine into the RVLM, it must be acknowledged that the pressor response is a summation of changes in splanchnic SNA with other sympathetic responses. Whether the discrepancies in the effects of altering SNA and MAP by microinjections in the CVLM and RVLM reflect slight differences in the sympathetic targets most affected by each acute manipulation remains to be determined. Another possible explanation for the differences in changes in SNA vs. MAP for the RVLM, CVLM, and NTS could be related to a threshold effect due to changes in adrenergic vascular reactivity. The acute rises in MAP are primarily due to constriction of the mesenteric circulation, and the mesenteric arteries are less responsive to α-adrenergic stimulation in OZR (31). With antagonism of GABAA receptors in the RVLM, a 50% difference in the sympathetic response coincided with a 23% difference in the pressor response in OZR vs. LZR. In the subsequent experiments with the CVLM and NTS, the sympathetic responses were reduced by 42 and 35%, respectively, and were accompanied by no significant difference in the pressor response in the OZR vs. LZR. In all cases, the percent difference in the responses between OZR and LZR exceeds the 25% difference in baseline voltage of SNA. Therefore, the reduced changes in SNA are likely to reflect real differences in the output of the central nervous system that may not be detected by measures of acute changes in MAP.
The varied magnitudes of differences in rises in SNA and MAP between the OZR and LZR with manipulations to the NTS, CVLM, and RVLM may also suggest additional clues for the brain stem mechanisms underlying the regulation of sympathetic vasomotor tone. Elimination of glutamatergic inputs to CVLM to disfacilitate neuronal activity does not fully produce the magnitude of difference in changes in SNA or in MAP observed by blockade of GABAergic inputs to the RVLM. Although the data suggest reduced tonic glutamatergic activation of CVLM contributes the attenuated response to blockade of GABAergic inputs to the RVLM in the OZR, the data also indicate other potential additional mechanisms for the GABAergic regulation of RVLM in OZR vs. LZR. Such changes could include CVLM-independent GABAergic inputs to the RVLM or glutamate-independent inputs to the CVLM. The CVLM is primarily driven by glutamate, but tonic inputs by other neurotransmitters such as angiotensin II or GABA could be altered in the OZR. Alternatively, the microinjections into CVLM may not encompass all CVLM-derived inhibitory inputs to the RVLM. We showed that kynurenate into CVLM blocked acute reflexes but that the CVLM could also contain GABAergic neurons that inhibit the RVLM independent of these reflex-driven neurons (7). Similarly, although the data the NTS contribute to the reduced restraint on SNA in the OZR, the magnitude of responses observed by inhibition of the NTS is smaller than that observed by inhibition of the CVLM. This observation has been reported previously and suggests that NTS-independent tonic glutamatergic inputs to the CVLM also contribute to their basal activity (25). Future studies will be needed to determine the sources of inputs to the CVLM and their potential contributions to the altered tonic regulation of SNA in OZR. Whether the attenuated responses observed by inhibition of the NTS in OZR can totally account for the attenuated response to elimination of glutamatergic inputs to the CVLM in OZR cannot be determined by the experimental designs of the present study (i.e., after inhibition of the NTS, does antagonism of glutamate receptors in the CVLM still evoke smaller rises in SNA in OZR?). Thus these data highlight contributions from the NTS and CVLM for reduced tonic GABAergic inhibition of the RVLM, but indicate that these simplistic relays do not fully convey the complexity of the interactions among the NTS, CVLM, and RVLM.
The underlying causes for obesity-induced brain stem changes that promote chronically elevated sympathetic vasomotor tone are unknown. The adult OZR have augmented inputs from arterial baroreceptor afferent nerves to the NTS that could alter how the brain regulates basal SNA and MAP. Baseline MAP is higher in the adult OZR compared with LZR, but baroreceptor afferent nerve activity does not reset to this modest rise in MAP (16). Specifically, aortic depressor nerve activity is elevated under baseline conditions in OZR compared with LZR, and direct electrical stimulation of these afferent nerve fibers evokes attenuated inhibitions of SNA and MAP in OZR (16). In a renal wrap hypertensive rat model, increased basal MAP is accompanied by altered processing of baroreceptor inputs at the NTS (47). Augmented inputs from excitatory baroreceptor afferent nerves are met with enhanced presynaptic GABAergic inhibition of glutamate release from baroreceptor afferent terminals combined with a postsynaptic GABAB receptor-mediated reduction in the excitability of NTS neurons receiving baroreceptor inputs (46, 48). Whether chronically elevated baroreceptor afferent activity in the OZR is associated with changes in the processing of incoming sensory information at the NTS warrants further study.
The elevated SNA and MAP in OZR could also arise from changes in circulating factors associated with the metabolic syndrome coincident with the obese state. Obesity alters the levels of numerous circulating hormones that have been shown to affect autonomic regulation. Although circulating leptin is elevated with obesity, and exogenous leptin has been shown to acutely raise SNA and MAP in rats (15, 35), the OZR still develop increased SNA and MAP with blunted baroreflexes in the absence of leptin's actions. Moreover, the mutation of the leptin receptor itself cannot provide an explanation for the autonomic deficits observed in the OZR. Juvenile OZR rats lack functional leptin receptors and have normal MAP and baroreflexes (33). In the OZR, autonomic deficits emerge in adulthood long after the onset of obesity as the metabolic syndrome progresses. In addition, SNA is elevated in obese rats by an enhanced drive from the RVLM in the presence or in the absence of functional leptin receptors (16, 40), suggesting that other factors are sufficient to alter brain stem-mediated regulation of sympathetic vasomotor tone with obesity. Although obesity-induced elevation in insulin levels has been proposed as a potential signal, this hormone appears more related to control of lumbar SNA to muscle to promote glucose homeostasis (2). Blood glucose levels are also elevated in the OZR due to insulin resistance. However, barosensitive neurons in the RVLM that are likely to be important for regulation of SNA are not responsive to changes in blood glucose (45). The OZR develop hypothyroidism as they reach adulthood (6), and this condition promotes increases in autonomic contributions to MAP and attenuated baroreflexes (12), but the role of the thyroid for obesity-related autonomic dysfunction has not been explored. Further study is essential to determine whether neural inputs to the brain, circulating factors, or both contribute to obesity-related increases in basal SNA.
Perspectives
The present study begins to unravel changes in contributions from key brain stem nuclei that regulate sympathetic vasomotor tone in OZR. The major findings of this study are that tonic GABAergic and angiotensinergic but not glutamatergic inputs to the RVLM contribute to differences in basal SNA and MAP observed between OZR and LZR. Furthermore, the reduced GABAergic inhibition of the RVLM may stem from attenuated drive by the NTS via the CVLM. These findings are in contrast to other hypertensive models, such as the Dahl-salt-sensitive rat and spontaneously hypertensive rats, where enhanced tonic glutamatergic activation of the RVLM contributes to the elevated resting MAP. The functional significance of these distinct neurochemical mechanisms for increasing RVLM-mediated drive to SNA is not known. Although integrated SNA is increased in all of these models of hypertension, the precise effects on sympathetic preganglionic neurons and the physiological ramifications may differ. In hypertensive humans, the rise in integrated SNA is produced by distinct mechanisms with uniquely affected targets and clinical outcomes. Whereas nonobese hypertensive subjects show increased SNA due to elevated activity in individual sympathetic fibers (24), obese hypertensive subjects have elevated SNA characterized by the recruitment of previously silent fibers instead of increased firing rates (24). Furthermore, whereas cardiac and renal SNA are increased in hypertensives with normal weight (10), obese subjects have elevated renal SNA but reduced cardiac SNA (43). Future studies will be essential to determine whether the neurochemistry underlying the elevated drive for RVLM neurons with hypertensive states affects the mechanisms underlying increased integrated SNA.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-086759 to (A. M. Schreihofer).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Averill DB, Tsuchihashi T, Khosla MC, Ferrario CM. Losartan, nonpeptide angiotensin II-type 1 (AT1) receptor antagonist, attenuates pressor and sympathoexcitatory responses evoked by angiotensin II and l-glutamate in rostral ventrolateral medulla. Brain Res 665: 245–222, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Bardgett ME, McCarthy JJ, Stocker SD. Glutamatergic receptor activation in the rostral ventrolateral medulla mediates the sympathoexcitatory response to hyperinsulinemia. Hypertension 55: 284–290, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergamaschi C, Campos RR, Schor N, Lopes OU. Role of the rostral ventrolateral medulla in maintenance of blood pressure in rats with Goldblatt hypertension. Hypertension 26: 1117–1120, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Bourassa EA, Fang X, Li X, Sved AF, Speth RC. AT1 angiotensin II receptor and novel non-AT1, non-AT2 angiotensin II/III binding site in brainstem cardiovascular regulatory centers of the spontaneously hypertensive rat. Brain Res 1359: 98–106, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carlson SH, Shelton J, White CR, Wyss JM. Elevated sympathetic activity contributes to hypertension and salt sensitivity in diabetic obese Zucker rats. Hypertension 35: 403–408, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Chomard P, Beltramo JL, Ben Cheikh R, Autissier N. Changes in thyroid hormone and thyrotrophin in the serum and thyroid glands of developing genetically obese male and female Zucker rats. J Endocrinol 142: 317–324, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Cravo SL, Morrison SF, Reis DJ. Differentiation of two cardiovascular regions within caudal ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 261: R985–R994, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Davy KP, Hall JE. Obesity and hypertension: two epidemics or one? Am J Physiol Regul Integr Comp Physiol 286: R803–R813, 2004 [DOI] [PubMed] [Google Scholar]
- 9. de Oliveira-Sales EB, Nishi EE, Boim MA, Dolnikoff MS, Bergamaschi CT, Campos RR. Upregulation of AT1R and iNOS in the rostral ventrolateral medulla (RVLM) is essential for the sympathetic hyperactivity and hypertension in the 2K-1C Wistar rat model. Am J Hypertens 23: 708–715, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Esler M, Lambert G, Jennings G. Regional norepinephrine turnover in human hypertension. Clin Exp Hypertens A 11, Suppl 1: 75–89, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Esler M, Rumantir M, Kaye D, Jennings G, Hastings J, Socratous F, Lambert G. Sympathetic nerve biology in essential hypertension. Clin Exp Pharmacol Physiol 28: 986–989, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Foley CM, McAllister RM, Hasser EM. Thyroid status influences baroreflex function and autonomic contributions to arterial pressure and heart rate. Am J Physiol Heart Circ Physiol 280: H2061–H2068, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Garrison RJ, Kannel WB, Stokes J, III, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med 16: 235–251, 1987 [DOI] [PubMed] [Google Scholar]
- 14. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest 100: 270–278, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huber DA, Schreihofer AM. Attenuated baroreflex control of sympathetic nerve activity in obese Zucker rats by central mechanisms. J Physiol 588: 1515–1525, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ito S, Hiratsuka M, Komatsu K, Tsukamoto K, Kanmatsuse K, Sved AF. Ventrolateral medulla AT1 receptors support arterial pressure in Dahl salt-sensitive rats. Hypertension 41: 744–750, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Ito S, Komatsu K, Tsukamoto K, Kanmatsuse K, Sved AF. Ventrolateral medulla AT1 receptors support blood pressure in hypertensive rats. Hypertension 40: 552–529, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Ito S, Komatsu K, Tsukamoto K, Sved AF. Excitatory amino acids in the rostral ventrolateral medulla support blood pressure in spontaneously hypertensive rats. Hypertension 35: 413–417, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Ito S, Komatsu K, Tsukamoto K, Sved AF. Tonic excitatory input to the rostral ventrolateral medulla in Dahl salt-sensitive rats. Hypertension 37: 687–691, 2001 [PubMed] [Google Scholar]
- 21. Ito S, Sved AF. Tonic glutamate-mediated control of rostral ventrolateral medulla and sympathetic vasomotor tone. Am J Physiol Regul Integr Comp Physiol 273: R487–R494, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Kiely JM, Gordon FJ. Role of rostral ventrolateral medulla in centrally mediated pressor responses. Am J Physiol Heart Circ Physiol 267: H1549–H1556, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Koshiya N, Huangfu D, Guyenet PG. Ventrolateral medulla and sympathetic chemoreflex in the rat. Brain Res 609: 174–184, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Lambert E, Straznicky N, Schlaich M, Esler M, Dawood T, Hotchkin E, Lambert G. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension 50: 862–868, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Mandel DA, Schreihofer AM. Glutamatergic inputs to the CVLM independent of the NTS promote tonic inhibition of sympathetic vasomotor tone in rats. Am J Physiol Heart Circ Physiol 295: H1772–H1779, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Masuo K, Mikami H, Itoh M, Ogihara T, Tuck ML. Sympathetic activity and body mass index contribute to blood pressure levels. Hypertens Res 23: 303–310, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Morgan DA, Anderson EA, Mark AL. Renal sympathetic nerve activity is increased in obese Zucker rats. Hypertension 25: 834–838, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Muratani H, Averill DB, Ferrario CM. Effect of angiotensin II in ventrolateral medulla of spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 260: R977–R984, 1991 [DOI] [PubMed] [Google Scholar]
- 29. Osmond JM, Mintz JD, Dalton B, Stepp DW. Obesity increases blood pressure, cerebral vascular remodeling, and severity of stroke in the Zucker rat. Hypertension 53: 381–386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rumantir MS, Vaz M, Jennings GL, Collier G, Kaye DM, Seals DR, Wiesner GH, Brunner-La Rocca HP, Esler MD. Neural mechanisms in human obesity-related hypertension. J Hypertens 17: 1125–1133, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Schreihofer AM, Hair CD, Stepp DW. Reduced plasma volume and mesenteric vascular reactivity in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 288: R253–R261, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Schreihofer AM, Ito S, Sved AF. Brain stem control of arterial pressure in chronic arterial baroreceptor-denervated rats. Am J Physiol Regul Integr Comp Physiol 289: R1746–R1755, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Schreihofer AM, Mandel DA, Mobley SC, Stepp DW. Impairment of sympathetic baroreceptor reflexes in obese Zucker rats. Am J Physiol Heart Circ Physiol 293: H2543–H2549, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Schreihofer AM, Sved AF. Nucleus tractus solitarius and control of blood pressure in chronic sinoaortic denervated rats. Am J Physiol Regul Integr Comp Physiol 263: R258–R266, 1992 [DOI] [PubMed] [Google Scholar]
- 35. Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension 31: 409–414, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, Farley G, Paranjape SY, Davis SN, Biaggioni I. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension 49: 27–33, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Smith JK, Barron KW. Cardiovascular effects of l-glutamate and tetrodotoxin microinjected into the rostral and caudal ventrolateral medulla in normotensive and spontaneously hypertensive rats. Brain Res 506: 1–8, 1990 [PubMed] [Google Scholar]
- 38. Smith JK, Barron KW. GABAergic responses in ventrolateral medulla in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 258: R450–R456, 1990 [DOI] [PubMed] [Google Scholar]
- 39. Stepp DW, Boesen EI, Sullivan JC, Mintz JD, Hair CD, Pollock DM. Obesity augments vasoconstrictor reactivity to angiotensin II in the renal circulation of the Zucker rat. Am J Physiol Heart Circ Physiol 293: H2537–H2542, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Stocker SD, Meador R, Adams JM. Neurons of the rostral ventrolateral medulla contribute to obesity-induced hypertension in rats. Hypertension 49: 640–646, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Sun MK, Guyenet PG. Arterial baroreceptor and vagal inputs to sympathoexcitatory neurons in rat medulla. Am J Physiol Regul Integr Comp Physiol 252: R699–R709, 1987 [DOI] [PubMed] [Google Scholar]
- 42. Tagawa T, Dampney RA. AT(1) receptors mediate excitatory inputs to rostral ventrolateral medulla pressor neurons from hypothalamus. Hypertension 34: 1301–1307, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation 96: 3423–3429, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Verberne AJ, Guyenet PG. Medullary pathway of the Bezold-Jarisch reflex in the rat. Am J Physiol Regul Integr Comp Physiol 263: R1195–R1202, 1992 [DOI] [PubMed] [Google Scholar]
- 45. Verberne AJ, Sartor DM. Rostroventrolateral medullary neurons modulate glucose homeostasis in the rat. Am J Physiol Endocrinol Metab 299: E802–E807, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Zhang W, Herrera-Rosales M, Mifflin S. Chronic hypertension enhances the postsynaptic effect of baclofen in the nucleus tractus solitarius. Hypertension 49: 659–663, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Zhang J, Mifflin SW. Integration of aortic nerve inputs in hypertensive rats. Hypertension 35: 430–436, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Zhang W, Mifflin S. Chronic hypertension enhances presynaptic inhibition by baclofen in the nucleus of the solitary tract. Hypertension 55: 481–486, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]