Abstract
Extracellular inorganic pyrophosphate (ePPi) is an important endogenous inhibitor of vascular calcification, but it is not known whether systemic or local vascular PPi metabolism controls calcification. To determine the role of ePPi in vascular smooth muscle, we identified the pathways responsible for ePPi production and hydrolysis in rat and mouse aortas and manipulated them to demonstrate their role in the calcification of isolated aortas in culture. Rat and mouse aortas contained mRNA for ectonucleotide pyrophosphatase/phosphodiesterases (NPP1–3), the putative PPi transporter ANK, and tissue-nonspecific alkaline phosphatase (TNAP). Synthesis of PPi from ATP in aortas was blocked by β,γ-methylene-ATP, an inhibitor of NPPs. Aortas from mice lacking NPP1 (Enpp1−/−) did not synthesize PPi from ATP and exhibited increased calcification in culture. Although ANK-mediated transport of PPi could not be demonstrated in aortas, aortas from mutant (ank/ank) mice calcified more in culture than did aortas from normal (ANK/ANK) mice. Hydrolysis of PPi was reduced 25% by β,γ-methylene-ATP and 50% by inhibition of TNAP. Hydrolysis of PPi was increased in cells overexpressing TNAP or NPP3 but not NPP1 and was not reduced in Enpp1−/− aortas. Overexpression of TNAP increased calcification of cultured aortas. The results show that smooth muscle NPP1 and TNAP control vascular calcification through effects on synthesis and hydrolysis of ePPi, indicating an important inhibitory role of locally produced PPi. Smooth muscle ANK also affects calcification, but this may not be mediated through transport of PPi. NPP3 is identified as an additional pyrophosphatase that could influence vascular calcification.
Keywords: medial calcification, alkaline phosphatase, ectonucleotide pyrophosphorylase, adenosine 5′-triphosphate, ankyrin
extracellular inorganic pyrophosphate (ePPi) is a potent suppressor of vascular calcification both in vitro (8) and in vivo (13, 18) through its ability to inhibit hydroxyapatite formation (10). The importance of endogenous ePPi homeostasis in human cardiovascular function is demonstrated by the severe arterial calcification that occurs in the absence of a synthetic enzyme (17). Cells contain ectoenzymes capable of synthesizing and hydrolyzing ePPi, and our previous studies in cultured vascular smooth muscle cells have implicated them in the control of ePPi levels (15). However, it is not clear whether ePPi levels in vessels in vivo are controlled by local activity of these enzymes rather than by systemic activity via circulating ePPi levels and whether this affects vascular calcification. Although circulating PPi levels are inversely proportional to arterial calcification in patients with advanced chronic kidney disease (14), the correlation is weak and the role of PPi metabolism in the arterial wall has never been investigated.
PPi is synthesized from extracellular ATP by the ectoenzyme nucleotide pyrophosphatase/phosphodiesterase-1 (NPP1), and absence of this enzyme leads to extensive and fatal arterial calcification in children (17) and aortic calcification in mice (6, 11). The ATP analog α,β-methylene-ATP (α,β-meATP) is a competitive inhibitor of this reaction that reduces ePPi production in vascular smooth muscle cells (15), suggesting that NPP1 is a major source of PPi in vascular smooth muscle. However, cultured cells may not be representative of intact tissue and the high inhibitor concentrations raise questions about the specificity, particularly for other members of this enzyme family (NPP2 and NPP3), the role of which in ePPi metabolism is unknown.
An alternative source of ePPi may be transport out of cells through the putative transporter transporter ANK. Mice that are homozygous for a mutation in ANK (ank/ank) develop aortic calcification on a high-phosphate diet (11). Expression in oocytes induces PPi transport (4), and expression in cultured cells decreases intracellular PPi levels and increases PPi levels in the medium (5, 15). However, transport of PPi has never been directly demonstrated in cultured cells or in tissues.
Tissue-nonspecific alkaline phosphatase (TNAP) hydrolyzes PPi to orthophosphate and is a major determinant of hydroxyapatite formation in bone or ectopically by virtue of eliminating inhibitory PPi (11). Calcification of rat aortas in culture is induced by adding alkaline phosphatase (8) and prevented by TNAP inhibitors (12), and TNAP is upregulated in aortas from uremic rats, resulting in increased PPi hydrolysis (9). Whether increased activity of TNAP is sufficient to produce vascular calcification is unknown. The fact that only 50% of ePPi hydrolysis is prevented by TNAP inhibitors is consistent with the fact that PPi hydrolysis is reduced only 50% in aortas from mice lacking TNAP (9) and indicates that additional, but as yet unknown, enzymes are responsible in aorta (9). The recent demonstration that NPP1 can also hydrolyze PPi (1, 20) suggests that its role in ePPi homeostasis is more complex.
Despite the importance of ePPi in arterial calcification, its metabolism in intact vessels has never been studied and the relative importance of the different components in the local control of vascular calcification is unknown. This study examined the expression and activity of the different components of ePPi metabolism in rat and mouse aortas and determined their local role in vascular calcification using an organ culture model.
METHODS
Animals.
Male Sprague-Dawley rats (200–400 g) were obtained form Charles River Laboratories (Wilmington, MA), and ank/ank mice were obtained from Jackson Laboratories (Bar Harbor, ME). The Enpp1−/− mice have been described previously (6, 11). All protocols were approved by the Institutional Animal Care and Use Committee at Emory University.
Vessel studies.
Aortas were perfused with saline and removed, followed by gentle removal of the adventitia. Endothelium was removed by gentle rubbing with a cotton swab except when the vessels were used for culture. Aortic rings were cultured in serum-free DMEM containing 3.8 mM phosphate, and calcification was measured as 45Ca incorporation as previously described (8).
RT-PCR.
Total RNA was isolated from rats or mice aorta using RNAzol RT reagent (Molecular Research Center, Cincinnati, OH). Following DNase treatment, reverse transcription was done by 1 ug of RNA samples using Thermoscript RT-PCR kit (Invitrogen). For each sample, 18S rRNA was used as an internal control. PCR was performed with Platimum PCR SuperMix (Invitrogen) and using the following cycle parameters: 94°C for 2 min and 30 cycles at 94°C for 15 s, 55°C for 30 s, and 72°C for 40 s with final extension at 72°C for 10 min. Products were separated by 1% (wt/vol) agarose gel electrophoresis and visualized by ethidium bromide fluorescence. The primer pairs used for rat have been previously reported (7). Primers for mouse were designed to cross intro-exon boundaries using Clone Manager Suite 7 software (Sci Ed Software, Durham, NC) and are listed in Table 1.
Table 1.
Primer sequences for RT-PCR
| Name | Direction | Sequence (5′-3′) | GenBank | Amplicon |
|---|---|---|---|---|
| NTPD1 mouse | F | CTTTGGCGCTTTGGATCTCG | NM_009848.3 | 355 |
| R | TCTGGTGGCACTGTTCGTAG | |||
| NTPD2 mouse | F | CTGGAGGCAGTGACACAGAC | NM_009849.2 | 396 |
| R | TGGGTGGAGTAGCCCTTTGG | |||
| NTPD3 mouse | F | TGCCTACAGACCCACTAGAC | AY714060.1 | 319 |
| R | TCAGAAGGAGCAACCCTGAC | |||
| ENPP1 mouse | F | CTTTGGCGCTTTGGATCTCG | NM_008813.3 | 410 |
| R | CTTTGGCGCTTTGGATCTCG | |||
| ENPP2 mouse | F | CTGGAGGCAGTGACACAGAC | NM_001136077.1 | 401 |
| R | TGGGTGGAGTAGCCCTTTGG | |||
| ENPP3 mouse | F | CTCCTTCCCTACCATCTAC | NM_134005.2 | 223 |
| R | GACCCTCCATCAACATTCC | |||
| TNAP mouse | F | GCAAGGACATCGCATATCAG | BC065175 | 387 |
| R | CTTCCACCAGCAAGAAGAAG | |||
| ANK mouse | F | CAGTTTCCTGGTGGGATGTG | AF274752.1 | 495 |
| R | TTGATGTGGGCTGAGGTG | |||
| 18S mouse | F | GAAACGGCTACCACATCCAAGG | X00686.1 | 482 |
| R | GTCCCTCTTAATCATGGCCTCAG |
NTPD1-3, nucleotide triphosphate diphosphohydrolase-1–3; ENPP1–3, ectonucleotide pyrophosphatase/phosphodiesterase-1–3; TNAP, tissue-nonspecific alkaline phosphatase; F, forward; R, reverse.
Transfections.
Aortas were infected with an adenovirus (Ad-TNAP) containing full-length human TNAP cDNA with enhanced GFP cDNA. The cDNA coding human TNAP (AB011406) was generated by PCR using primers 1) 5′-Aaa AAGCTT GCC ACC atg ATT TCA CCA TTC-3′; 2) 5′-aaa GGTACC tca GAA CAG GAC GCT CAG GG-3′ from plasmid pDNA3.TNAP, and cloned into the adenovirus vector pAd.track-CMV at the Hind III and Kpn 1 restriction sites. Purified vector was linearized using Pme I and cotransformed with 100 ng pAdEasy-1 supercoiled vector (100 ng/μl) into BJ5183 cells by electroporation. Clones containing the pAd-easy-TNAP were selected using kanamycin and verified by Pac I digestion. The virus was then amplified in HEK293H cells (Invitrogen) and purified by adeno-X virus purification kit (Clontech, Mountain View, CA). Virus titer was determined by serial dilution and calculated as transduction units (TU/ml) as follows: (infected cells/field) × (field/well)/volume virus (ml) × dilution factor. Rat aortas with endothelium removed were cultured as above with 109 TU/ml Ad-TNAP virus.
Transfections of HEK cells for enzyme studies were performed by cloning cDNAs into the pcDNA3 plasmid and transfection with Effectene transfection reagent (Qiagen, Valencia, CA) following the manufacturer's instruction. The eNPP3 plasmid was obtained from Applied Biosystems (Carlsbad, CA).
PPi assay.
PPi was measured enzymatically as previously described using either uridine-diphosphoglucose (UDP-glucose) pyrophosphorylase and UDP[14C]glucose (14) or ATP-sulfurylase and adenosine phosphosulfate to generate ATP, which was measured by a coupled luciferin/luciferase reaction (15).
PPi metabolism.
Aortas or cells were incubated with [γ32P]ATP or 32PPi in a physiologic salt solution (142 mM Na+, 121 Cl−, 5.4 mM K+, 1.8 mM Ca2+, 0.8 mM Mg2+, 5 mM glucose, and 24 mM HEPES pH 7.4). Orthophosphate was separated from ATP or PPi in the medium by the acid-molybdate method as previously described (9). PPi production was measured by chromatography on PEI-cellulose plates developed with 650 mM KH2PO4 pH 3. After autoradiography, spots were scraped and counted by liquid scintillation. Uptake of PPi was measured was measured after the aortas were washed six times in cold salt solution without 32PPi. After assays, the rings were dried and weighed, or the protein content of cultured cells was measured.
Inhibitors.
β,γ-methylene-ATP (β,γ-meATP) and α,β-meATP were obtained from Sigma-Aldrich (St. Louis, MO) and used at 300-μM final concentration. Probenecid was from the same source and used at 2.5 mM. Compound MLS38949 (PubChem CID 2931234) or 2,5-dimethoxy-N-(quinolin-3-yl)benzenesulfonamide is a potent TNAP inhibitor with excellent selectivity over other alkaline phosphatases recently identified through high-throughput screening (3, 19). MLS38949 was used at a final concentration of 30 μM.
Statistics.
Results are expressed as means ± SE. Significance was determined by Student's t-test or by ANOVA. A P value of ≤ 0.05 was considered to be significant.
RESULTS
Expression of mRNA.
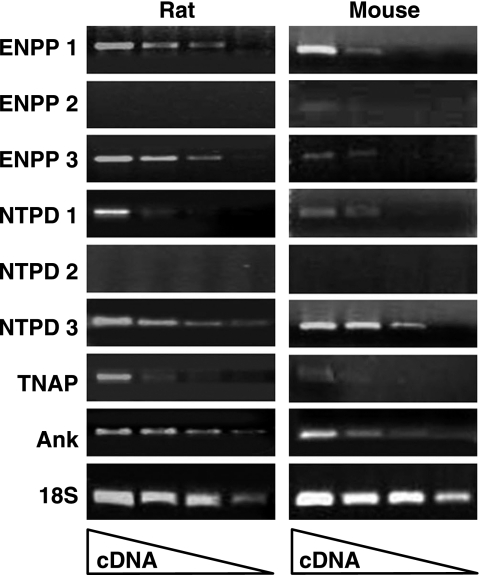
Normal rat aortas were probed for the presence of mRNA for proteins involved in extracellular ATP and PPi metabolism. The relevant ectonucleotidases include the NPPs, which generate PPi from ATP, the nucleotide triphosphate diphosphohydrolases (NTPDs), which cleave ATP to generate Pi, and TNAP, which hydrolyzes ATP and PPi to Pi. In addition, the ANK protein may mediate PPi efflux from cells. As shown in Fig. 1, there was significant expression of NPP1, NPP3, NTPD3, and ANK, and lower levels of NTPD1 and TNAP. There was no significant expression of NPP2 or NTPD2.
Fig. 1.
RT-PCR of RNA from aortas. RNA was prepared from freshly isolated aortas as described in the methods. Reaction volumes contained dilutions of reverse transcription products (1:10 to 1:10,000) synthesized from 5 μg of total RNA. ENPP1–3, ectonucleotide pyrophosphatase/phosphodiesterase-1–3; NTPD, nucleotide triphosphate diphosphohydrolase; TNAP, tissue-nonspecific alkaline phosphatase.
PPi synthesis.
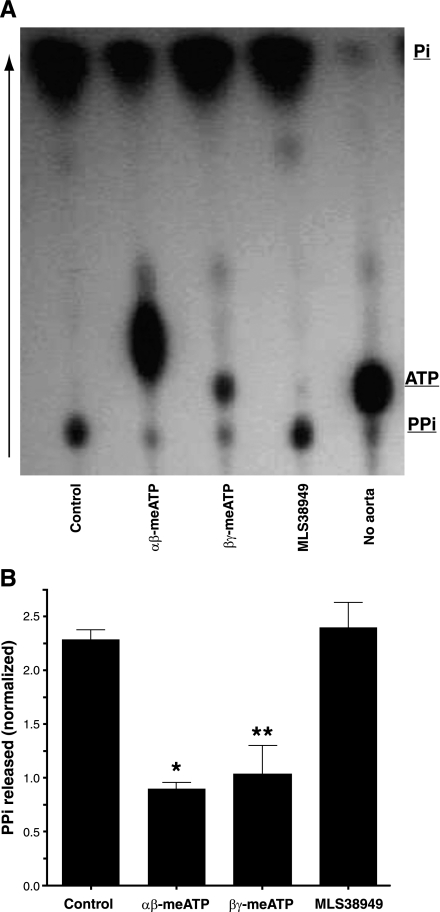
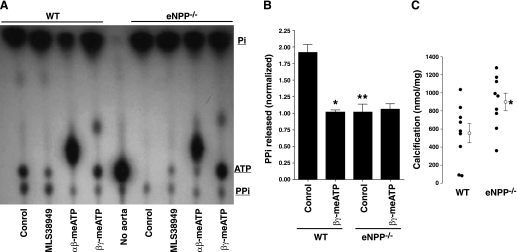
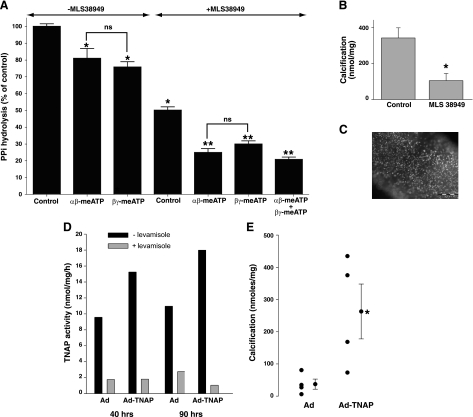
Despite numerous attempts with sensitive assays, we were unable to detect release of PPi from rat aortas into incubation medium under basal conditions. Synthesis of PPi could only be detected when exogenous ATP was added and only as 32PPi synthesized from [γ32P]ATP. ATP hydrolysis was rapid, with 50% hydrolyzed after 20 min and 100% hydrolyzed after 1 h, corresponding to initial rates of 17.4 ± 0.41 and 2.08 ± 0.05 pmol·mg−1·min−1 from 1 and 0.1 μM ATP, respectively. Thin-layer chromatography revealed that only a small proportion (7.3 ± 0.9%) of the ATP was converted to PPi, with the remainder appearing as orthophosphate (Fig. 2). Some of this PPi is a contaminant of the [γ32P]ATP preparation, since a small amount was present in medium incubated without aorta. The rate of PPi production from 1 μM ATP was 1.27 ± 0.03 pmol·mg−1·min−1. Synthesis of PPi was completely prevented by the addition of 300 μM β,γ-meATP, an inhibitor of NPP1 (7), or by α,β-meATP, an inhibitor of NPPs and NTPDs (7), but was not affected by inhibition of TNAP. The additional spot probably represents α,β-me[γ32P]ATP derived from the transfer of 32P from ATP to α,β-meADP via nucleoside diphosphokinases (7). As shown in Fig. 3, A and B, similar findings were obtained in normal mouse aortas, but in aortas from Enpp1−/− mice, PPi levels did not increase above baseline and were not affected by β,γ-meATP. Because the meATPs are substrates for NPPs and NTPDs and are rapidly hydrolyzed, they are not suitable for long-term inhibition of NPP1. Therefore, aortas from Enpp1−/− mice were used to examine the role of NPP1 in vascular calcification. Calcification of these aortas was modestly but significantly greater than of aortas from wild-type littermates (Fig. 3C). The variability in calcification is due to the difficulty in isolating and preparing the mouse aortas without injury, which can induce calcification by itself.
Fig. 2.
Synthesis of pyrophosphate (PPi) from ATP by rat aorta. A: autoradiogram of a representative thin-layer chromatogram of medium after incubation of aortic rings with [γ32P]ATP in 1 μM ATP with or without 300 μM α,β-methylene-ATP (α,β-meATP), 300 μM β,γ-meATP, or 30 μM MLS38949. B: quantification of radioactivity. Results are normalized to inorganic pyrophosphate (PPi) production in the absence of aorta and are the means of 3 experiments. *P < 0.001, **P < 0.01 vs. control.
Fig. 3.
Synthesis of PPi and calcification in aortas from mice lacking NPP1. A: autoradiogram of a representative thin-layer chromatogram of medium after incubation of aortic rings with [γ32P]ATP in 1 uM ATP with or without 30 μM MLS38949, 300 μM α,β-meATP, or 300 μM β,γ-meATP. B: quantification of PPi production. Results are the means of 6 experiments. *P < 0.01 vs. control; **P < 0.01 vs. wild-type (WT). C: calcification of aortas from wild-type mice and mice lacking NPP1 (Enpp1−/−). Symbols represent individual rings and means ± SE. Results are from 1 representative experiment. Similar results were obtained in 2 additional experiments. *P < 0.05.
PPi transport.
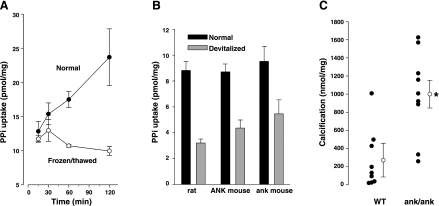
The inability to load sufficient quantities of PPi into smooth muscle coupled with intracellular hydrolysis precluded measurement of PPi efflux. Since influx of PPi has been reported in oocytes expressing ANK (4), measurement of PPi influx was attempted in aortas. To distinguish cellular uptake from binding or trapping of PPi in the extracellular matrix, uptake was also measured in devitalized (frozen and thawed) aortas. As shown in Fig. 4A, there was rapid uptake of 32PPi in the first 15 min that was also present in devitalized aortas and remained constant thereafter, consistent with extracellular binding or trapping. However, uptake continued in a linear fashion in normal aortas and presumably represented cellular uptake. As shown in Fig. 4B, uptake was similar in rat and mouse aortas and was not reduced in aortas from ank/ank mice lacking a functional ANK protein. However, calcification was significantly greater in aortas from ank/ank mice compared with ANK/ANK littermates (Fig. 4C).
Fig. 4.
PP transport and calcification in aortas. A: uptake of PPi in normal and devitalized (frozen and thawed) rat aortas. Results are means ± SE of triplicate measurements; PPi concentration was 5 μM. B: uptake of PPi in rat aortas and in aortas from ank/ank mice and wild-type (ANK/ANK) littermates. Results are means ± SE of triplicate measurements for rat aortas and 8 measurements in mouse aortas; time was 180 min.; PPi concentration was 1 μM. C: calcification of aortas from ank/ank mice and wild-type (ANK/ANK) littermates. Each symbol indicates a separate animal. *P < 0.01.
PPi hydrolysis.
Hydrolysis of ePPi was measured by incubating aortas with 1 or 5 uM PPi containing tracer 32PPi, with resulting rates of 0.24 ± 0.01 and 1.27 ± 0.05 pmol·mg−1·min−1. Approximately 50% was inhibited by MLS38949, a potent and highly specific inhibitor of TNAP that inhibits <10% of NPP1 activity (3). Half of the remaining hydrolysis was inhibited by α,β-meATP or β,γ-meATP (Fig. 5A). The effect of the inhibitors was additive. The role of TNAP in calcification was tested in cultured aortas. Since normal aortas do not calcify in culture, even in the presence of high-phosphate concentrations, it was necessary to induce calcification to test the effect of TNAP inhibition. This was accomplished by injuring the aortas through rubbing with a cotton swab as previously described (8). The subsequent calcification was inhibited by the TNAP inhibitor MLS38949 (Fig. 5B). The effect of increased TNAP activity was tested by viral transduction of TNAP into cultured aortas using a GFP-labeled construct, which resulted in extensive cellular labeling (Fig. 5C). This produced an increase in activity compared with transduction of the empty virus (Fig. 5D) that ranged from 2.08-fold to 3.43-fold in three experiments and a significant increase in calcification (Fig. 5E).
Fig. 5.
Hydrolysis of PPi and calcification in aortas. A: hydrolysis of PPi (1 μM) by rat aorta with or without 30 μM MLS38949, 300 μM α,β-meATP, or 300 μM β,γ-meATP. Results are the means ± SE of 3–5 experiments. *P < 0.001 vs. control; **P < 0.001 vs. MLS38949, by paired analysis. B: calcification of injured aortas cultured without or with 30 μM MLS38949. Results are the means ± SE of 8 aortic rings. *P < 0.005. C: fluorescence microscopy of rat aorta transduced with GFP-containing adenovirus showing expression of GFP in individual cells. Line indicates 500 μm. D: alkaline phosphatase activity in aortas exposed to Ad-TNAP or empty virus. Concentration of levamisole was 1 mM. Results from a representative experiment are shown. E: calcification of aortic rings cultured with Ad-TNAP or empty virus. Symbols represent individual rings and means ± SE. *P < 0.05.
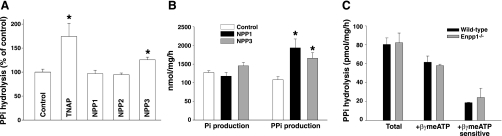
The inhibition of PPi hydrolysis by the meATPs suggested involvement of an NPP, and this was investigated by overexpressing these enzymes in HEK cells (Fig. 6A). Hydrolysis of PPi was significantly increased after transfection of TNAP or NPP3 but not NPP1. To ensure that transfection of NPP1 resulted in increased activity, hydrolysis of [γ32P]ATP was examined, using treatment of the medium with inorganic pyrophosphatase before extraction to identify PPi (Fig. 6B). Production of orthophosphate from ATP did not vary with the transfections, but PPi production was significantly greater in cells transfected with NPP1 or NPP3, confirming successful transfection. PPi hydrolysis did not differ between aortas from Enpp1−/− mice and aortas from wild-type littermates and was still inhibited by β,γ-meATP in the Enpp1−/− aortas (Fig. 6C).
Fig. 6.
Role of NPP1 and NPP3 in PP hydrolysis. A: hydrolysis of PPi (5 μM) in HEK cells overexpressing ectoenzymes. Results are the means ± SE of 5 experiments, each performed in duplicate or triplicate. *P < 0.02 vs. control, by paired, one-tailed testing. B: hydrolysis of ATP (1 μM) in the same cells. Results are the means ± SE of a single experiment performed in triplicate. Similar results were obtained in an additional experiment. *P < 0.05, by one-tailed t-test. C: hydrolysis of PPi (1 μM) by aortas from Enpp1 mice and wild-type littermates without and with 300 μM β,γ-meATP. Results are the means ± SE of 3–4 different mice, each assayed in duplicate.
DISCUSSION
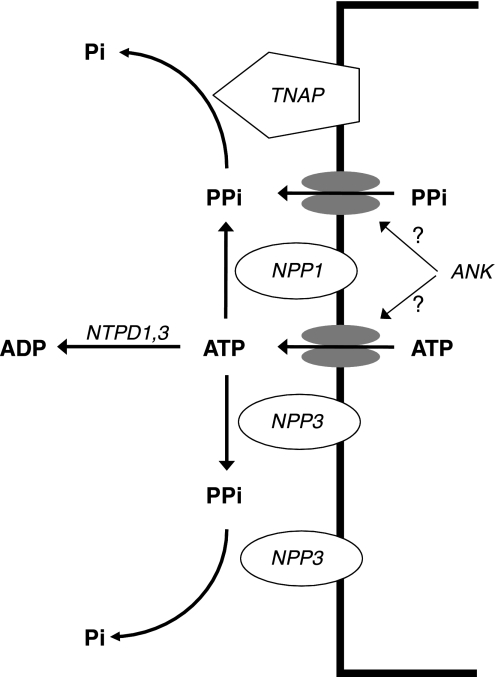
This is the first examination of ePPi and ATP metabolism and its role in smooth muscle calcification in intact vessels. The results indicate that ectoenzymes and transporters capable of producing and clearing ePPi are present in vascular smooth muscle (Fig. 7) and influence vascular calcification in a manner consistent with an important, local inhibitory role of PPi.
Fig. 7.
Diagram of ePPi metabolism in vascular smooth muscle. PPi is synthesized by NPP1 and 3 from ATP released from cells. Another source is transport of PPi across the cell membrane, possibly mediated by ANK, which may also mediate ATP release. PPi is hydrolyzed by TNAP and NPP3. NTPDs are very active in vascular smooth muscle and could limit availability of ATP for synthesis of PPi.
Analysis of mRNA transcripts from aorta indicated expression of NPP1, TNAP, and ANK, all of which have been implicated in ePPi metabolism. There was also expression of NPP3 but not NPP2. The NPP family consists of seven single-span transmembrane proteins except for NPP2, which is secreted (21), and only NPPs 1–3 have been implicated in the hydrolysis of nucleotides. Expression of NTPD1 and NTPD3 was also detected. Members of the NTP (apyrase) family can hydrolyze nucleoside 5′-triphosphates and diphosphates with varying preferences for the type of nucleotide (16). Of these, NTPDs 1–3 are ectoenzymes. Although they do not directly participate in ePPi metabolism, they could influence it by reducing the availability of extracellular ATP. The pattern of expression of these ectoenzymes in rat and mouse aorta parallels that in vascular smooth muscle cells in culture (15).
We (15) have previously demonstrated that extracellular ATP is the major source of ePPi in vascular smooth muscle cells, presumably mediated by NPP1 based on inhibition by β,γ-meATP. This inhibitor also prevented PPi production from ATP in aortas, and the absence of PPi synthesis from ATP in Enpp1−/− aortas definitively demonstrates that NPP1 is the sole source of PPi from extracellular ATP in vascular smooth muscle. The results also indicate that NPP1 was completely inhibited at the concentration of β,γ-meATP that was used. Since neither [γ32P]ATP nor β,γ-meATP is likely to enter cells, intracellular NPP1 would not be detected or inhibited. However, this enzyme is not known to function intracellularly and this would not contribute to extracellular PPi levels. The rate constant of PPi hydrolysis in aortas was fivefold less than the rate constant of ATP hydrolysis, and, since the concentration of PPi produced from ATP was far less than the starting ATP concentration, hydrolysis of PPi should not have interfered with the measurement of PPi synthesis. This is borne out by the fact that PPi synthesis was not altered by inhibition of TNAP.
Aortas exhibited a progressive uptake of PPi that could not be accounted for by binding to the extracellular matrix and presumably represents cellular uptake. This is the first demonstration of PPi transport in a tissue and the first in vascular cells. However, uptake did not differ between aortas from ANK/ANK and ank/ank mice, indicating that it is not mediated by ANK. Although it is possible that ANK mediates only efflux and not influx of PPi, its expression in oocytes resulted in measurable influx (4), consistent with bidirectional transport. However, it is possible that ANK is misoriented in oocytes and does not mediate influx under normal conditions. Despite having similar PPi uptake, aortas from ank/ank mice exhibited significantly greater calcification than wild-type aortas, indicating an important local role for ANK in vascular calcification. In addition to ANK mediating efflux but not influx of PPi, another possible explanation is that ANK transports another compound involved in ePPi metabolism such as ATP. The latter is supported by recent data showing that overexpression of ANK in chondrocytes leads to an increase in extracellular ATP (2).
Based on inhibition by MLS38949, a potent and specific inhibitor of TNAP (3, 19), this enzyme is responsible for half the PPi hydrolysis in vascular smooth muscle. This is consistent with our previous results (9) using the less potent and specific inhibitor levamisole and the measurement of PPi hydrolysis in aortas from mice lacking TNAP. Approximately 25% of PPi hydrolysis was blocked by α,β-meATP and β,γ-meATP, both in absence and presence of TNAP inhibitor, suggesting involvement of one of the NPPs. The fact that NPP3 but not NPP1 increased PPi hydrolysis in HEK cells and the fact that PPi hydrolysis was not altered in aortas from Enpp1−/− mice suggest that NPP3 rather than NPP1 contributes to PPi hydrolysis in vascular smooth muscle. Although purified NPP1 has been shown to hydrolyze PPi at millimolar concentrations in osteoblast-derived matrix vesicles (1) and in proteoliposomes (20), the results in aorta indicate that NPP1 does not hydrolyze PPi in vascular smooth muscle under physiologic conditions. Expression of NPP3 in HEK cells also increased PPi production from ATP, indicating that it may also contribute to ePPi synthesis. These potential roles of NPP3 in ePPi metabolism have not previously been demonstrated.
Genetic studies have implicated ePPi metabolism in the development of vascular calcification but whether these findings are due to alterations in local or systemic ePPi metabolism is unknown. The cultured aorta model provides a means to test this and demonstrated increased calcification in the absence of NPP1 or ANK, or in the presence of excess TNAP, suggesting that these proteins can act locally to affect extracellular PPi levels in vascular smooth muscle. In particular, the results indicate an important role for TNAP, which is consistent with its critical role in bone mineralization (11) and the upregulation of vascular TNAP in uremia, a condition associated with medial vascular calcification (9).
The results also indicate an important role for extracellular ATP as a source of ePPi in vascular smooth muscle. Nucleotides can be released from cells by vesicular exocytosis or selective transport via ATP channels and nucleotide transporters and can be generated extracellularly by adenylate kinase and nucleoside diphosphokinase. The source in vascular smooth muscle is unknown. In addition to NPPs, ATP can be cleaved by NTPDs. This was the major pathway in rat and mouse aortas comprising ∼90% of the ATP clearance rate. This contrasts with cultured smooth muscle cells, wherein ∼50% of added ATP is cleaved to PPi (15). This indicates that cultured cells may not accurately reflect extracellular ATP metabolism in arteries and also suggests that changes in the relative abundance of NPP1 and NTPs could alter ePPi metabolism and vascular calcification.
Unlike cultured cells, there was no measurable release of PPi from cultured aortas in the absence of added ATP. It is unlikely that this is due to the absence of ATP release or PPi release, since calcification was increased in aortas from Enpp1−/− and ank/ank mice. This implies that there is basal production of ePPi but it is too low to measure. However, some estimates can be made from the data obtained in this study. Based on the reported range of 10−9-10−7 M for extracellular ATP (22) and the rate constant determined in this study for PPi production from ATP (and assuming equilibration of medium ATP with extracellular ATP), PPi production via NPP1 should be 1.27–127 pmol·mg−1·min−1. Assuming an extracellular PPi concentration of 0.3–0.5 μM, which is the minimum level for inhibition of hydroxyapatite formation (10), and the rate constant measured in this study, the rate of PPi hydrolysis should be ≥72–120 pmol·g−1·min−1, which exceeds the rate of PPi synthesis except at the highest extracellular ATP concentration. This is consistent with the lack of PPi release from aortas and the role of ePPi hydrolysis as the major determinant of calcification. The fact that vascular smooth muscle may be a net PPi-consuming tissue suggests that circulating ePPi could be an important determinant of extracellular PPi levels and calcification in vascular smooth muscle in vivo.
GRANTS
This work was supported by National Institutes of Health Grants DK-069681, GM-36387, and HL-101899 and a Predoctoral Fellowship from the Government of Aragón, Spain (B086/2007 to R. Villa-Bellosta).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Ciancaglini P, Yadav MC, Simao AM, Narisawa S, Pizauro JM, Farguharson C, Hoylaerts MF, Millan JL. Kinetic analysis of substrate utilization by native and TNAP-, NPP1- or PHOSPHO1-deficient matrix vesicles. J Bone Miner Res 25: 716–723, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Costello JC, Rosenthal AK, Kurup IV, Masuda I, Medhora M, Ryan LM. Parallel regulation of extracellular ATP and inorganic pyrophosphate: roles of growth factors, transduction modulators, and ANK. Connect Tissue Res 52: 139–146, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Dahl R, Sergienko EA, Su Y, Mostofi YS, Yang L, Simao AM, Narisawa S, Brown B, Mangravita-Novo A, Vicchiarelli M, Smith LH, O′Neill WC, Millan JL, Cosford ND. Discovery and validation of a series of aryl sulfonamides as selective inhibitors of tissue-nonspecific alkaline phosphatase (TNAP). J Med Chem 52: 6919–6925, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gurley KA, Reimer RJ, Kingsley DM. Biochemical and genetic analysis of ANK in arthritis and bone disease. Am J Hum Genet 79: 1017–1029, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science 289: 265–270, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Johnson K, Polewski M, van Etten D, Terkeltaub R. Chondrogenesis mediated by PPi depletion promotes spontaneous aortic calcification in NPP1−/− mice. Arterioscler Thromb Vasc Biol 25: 686–691, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Joseph SM, Pifer MA, Przybylski RJ, Dubyak GR. Methylene ATP analogs as modulators of extracellular ATP metabolism and accumulation. Br J Pharmacol 142: 1002–1014, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lomashvili KA, Cobbs S, Hennigar RA, Hardcastle KI, O'Neill WC. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J Am Soc Nephrol 15: 1392–1401, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Lomashvili KA, Garg P, Narisawa S, Millan JL, O'Neill WC. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int 73: 1024–1030, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meyer JL. Can biological calcification occur in the presence of pyrophosphate? Arch Biochem Biophys 231: 1–8, 1984 [DOI] [PubMed] [Google Scholar]
- 11. Murshed M, Harmey D, Millan JL, McKee MD, Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev 19: 1093–1104, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Narisawa S, Harmey D, Yadav MC, O'Neill WC, Hoylaerts MF, Millan JL. Novel inhibitors of alkaline phosphatase suppress vascular smooth muscle cell calcification. J Bone Miner Res 22: 1700–1710, 2007 [DOI] [PubMed] [Google Scholar]
- 13. O'Neill WC, Lomashvili KA, Malluche HH, Faugere MC, Riser BL. Treatment with pyrophosphate inhibits uremic vascular calcification. Kidney Int 79: 512–517, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Neill WC, Sigrist MK, McIntyre CW. Plasma pyrophosphate and vascular calcification in chronic kidney disease. Nephrol Dial Transplant 25: 187–191, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prosdocimo DA, Douglas DC, Romani AM, O'Neill WC, Dubyak GR. Autocrine ATP release coupled to extracellular pyrophosphate accumulation in vascular smooth muscle cells. Am J Physiol Cell Physiol 296: C828–C839, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2: 409–430, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rutsch F, Vaingankar S, Johnson K, Goldfine I, Maddux B, Schauerte P, Kalhoff H, Sano K, Boisvert WA, Superti-Furga A, Terkeltaub RA. PC-1 nucleotide triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification. Am J Pathol 158: 543–554, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schibler D, Russell GG, Fleisch H. Inhibition by pyrophosphate and polyphosphate of aortic calcification induced by vitamin D3 in rats. Clin Sci 35: 363–372, 1968 [PubMed] [Google Scholar]
- 19. Sergienko EA, Su Y, Garcia X, Brown B, Hurder A, Narisawa S, Millan JL. Identification and characterization of novel tissue-nonspecific alkaline phosphatase inhibitors with diverse modes of action. J Biomol Screen 14: 824–837, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simao AMS, Yadav MC, Narisawa S, Bolean M, Pizauro JM, Hoylaerts MF, Ciancaglini P, Millan JL. Proteoliposomes harboring alkaline phosphatase and nucleotide pyrophosphatase as matrix vesicle biomimetics. J Biol Chem 285: 7598–7609, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stefan C, Jansen S, Bollen M. Modulation of purinergic signaling by NPP-type ectophosphodiesterases. Purinergic Signal 2: 361–370, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783: 673–694, 2008 [DOI] [PubMed] [Google Scholar]