Abstract
Endothelial cells respond to fluid flow stimulation through transient and sustained signal pathway activation. Smad2 is a signaling molecule and transcription factor in the Smad signaling pathway, traditionally associated with TGF-β. Although phosphorylation of Smad2 in the receptor-dependent COOH-terminal region is the most appreciated way Smad2 is activated to affect gene expression, phosphorylation may also occur in the MH1-MH2 linker region (L-psmad2). Here, we show that in human aortic endothelial cells (HAEC), Smad2 was both preferentially phosphorylated in the linker region and localized to the nucleus in a flow-dependent manner. The Smad corepressor transforming growth interacting factor (TGIF) was also found to have flow-dependent nuclear localization. Tissue studies confirmed this L-psmad2 generation trend in rat aorta, indicating likely importance in arterial tissue. HAEC-based inhibitor studies demonstrated that L-psmad2 levels were not related to MAPK phosphorylation, but instead followed the pattern of pAkt473, both with and without the phosphatidylinositol 3-kinase inhibitor PI-103. Akt and Smad species were also shown to directly interact under flow relative to static controls. To further evaluate impacts of PI-103 treatment, expression profiles for two TGF-β and shear stress-dependent genes were determined and showed that mRNAs were lower from untreated 10 dyn/cm2 than 2 dyn/cm2 average shear stress cultures. However, upon exposure to PI-103, this trend was reversed, with a stronger response observed at 10 dyn/cm2. Taken together, the results of this work suggest that fluid flow exposure may influence endothelial gene expression by a novel mechanism involving Akt, L-psmad2, and TGIF.
Keywords: shear stress, mitogen activated protein kinases, plasminogen activator inhibitor 1, platelet-derived growth factor subunit B, mechanotransduction, transforming growth interacting factor
fluid flow is known to impact the physiology of endothelial cells, both in vivo and in vitro, as evidenced by the display of flow-dependent phenotypes. In particular, patterns of gene expression show a shear stress dependency, with altered profiles seen for cells from static culture, low shear stress (≤4 dyn/cm2), and moderate shear stress (≥10 dyn/cm2) (1, 18, 20). Cells exposed to prolonged moderate shear stress (24 h) exhibit an antiapoptotic and antiproliferative phenotype compared with those from static culture, and these characteristics are similar to those exhibited by cells in vivo (39). Furthermore, it has been found that endothelial cells exposed to prolonged fluid flow (12–24 h) align with flow and have suppressed inflammatory responses (2, 4, 30, 37).
We previously reported that human aortic endothelial cells (HAEC) exhibited prolonged phosphorylation of multiple MAPK species in a flow-dependent manner (31). This finding suggests that physiological responses related to shear stress-derived MAPK phosphorylation may be an important part of tissue adaptation to the flow environment. Therefore, in this study, we undertook an investigation of possible consequences of prolonged MAPK signaling and focused upon linker region phosphorylation of Smad2.
Smads are signaling molecules and transcription factors with well-defined roles in transforming growth factor-β (TGF-β) signaling. But they have also been implicated in non-TGF-β-dependent mechanisms, including responses to shear stress. When TGF-β binds to surface receptors, phosphorylation of COOH-terminal Smad2 serine sites is initiated and enables association with Smad4 (27, 29, 44). These complexes translocate to the nucleus where they can participate in diverse gene expression responses (9, 22, 23, 32, 35). However, it has also been shown that Smad2 can be phosphorylated in its MH1-MH2 linker region, with several kinase pathways implicated in this activity, including the MAPKs ERK (17) and JNK (3, 25). Depending upon the context, linker phosphorylated Smad2 has been shown to both be arrested in the cytoplasm and to form Smad4 complexes and translocate to the nucleus. As such, linker region phosphorylation of Smad2 may result in either activation or inhibition of gene transcription (3, 15, 17).
The role of Smads in shear stress-induced gene expression has been investigated only to a limited extent. Although shear stress has not been found to affect the level of Smad2 mRNA (36), it has been shown to alter Smad2-dependent gene transcription. Brown et al. (3) found that 18 h of steady laminar 10 dyn/cm2 shear stress activated Gal4-Smad2 reporter constructs in transformed bovine aortic endothelial cells. Cotransfecting cells with dominant negative MAP kinase kinase kinase 1 (MEKK-1) inhibited this activity, implying a dependence on constituents of the JNK signaling pathway. However, to our knowledge, no studies of linker phosphorylated Smad2 and fluid flow have been conducted in human endothelium or in arterial tissue sections.
Several genes have been shown to exhibit sensitivity to both shear stress and TGF-β signaling, including serpine-1 and platelet-derived growth factor subunit B (PDGF-B). Serpine-1 [formerly known as plasminogen activator inhibitor 1 (PAI-1)] has shown reductions in both mRNA and soluble protein levels following a day of shear stress exposure (24, 45), and PDGF-B has been observed to have a similar type of shear stress mRNA regulation (21). Both gene promoters have also been shown to interact directly with Smad proteins (9, 34). As such, these genes make good candidates to evaluate the potential effects of shear flow and any subsequently phosphorylated Smad2.
As reviewed by Ross and Hill (27), a multitude of species can either phosphorylate or interact with Smads, resulting in diverse responses that appear to be dependent upon physiological context, additional signaling pathways, and cell type. When Smads result in repression of gene transcription, there are corepressors that participate in the formation of regulatory complexes on or near gene promoters. One of the earliest characterized of these corepressors is transforming growth interacting factor (TGIF). TGIF has been shown to function by associating with Smads in the nucleus and recruiting histone deacetylases (43). Additionally, TGIF may also function as a target for other proteins that participate in gene repression (42), indicating that TGIF is an important regulator of Smad-based gene repression.
The work presented here was undertaken to evaluate the consequences of the prolonged MAPK phosphorylation we previously reported and is largely focused upon the flow-dependent generation of linker phosphorylated Smad2 in HAEC. Additionally, we examined potential roles for linker phosphorylated Smad2 in flow-exposed endothelial cultures and analyzed tissue from two regions in the rat aorta to determine whether linker phosphorylated Smad2 was differentially regulated with flow in vivo.
MATERIALS AND METHODS
Cell culture and flow experiments.
For protein and RNA extraction and analysis experiments, HAEC (Lonza, Walkersville, MD) were cultured on glass plates precoated with collagen I, and the parallel plate flow system was set up and operated as described previously (31). Briefly, the volumetric flow rates (Q) of flow medium that corresponded to average wall shear stresses of 2 dyn/cm2 and 10 dyn/cm2 shear stress (τ) were determined using the following equation, τ = 6Qμ/wh2, where μ is the fluid viscosity (3 cP), w is the channel width (6.2 cm), and h is the channel height (0.036 cm). Flow medium viscosity elevation to 3 cP was achieved by adding 3% 500,000 MW dextran (Spectrum Chemical Manufacturing, New Brunswick, NJ). Flow was provided by a Masterflex peristaltic pump and tubing (Cole Parmer, Montreal, QC, Canada) and yielded pulsatility indexes of 0.80 and 0.55 for the 2 dyn/cm2 and 10 dyn/cm2 cases, respectively, with corresponding frequencies of 0.33 Hz and 0.80 Hz. Hydrostatic pressure was maintained at 80 ± 5 mmHg by continuous sparging of 5% CO2 in air (Praxair, Calgary, AB, Canada). For inhibitor experiments, the compounds were added to media bottles before flow initiation and remained there for the entire experimental time of 20 h. The MEK-1 inhibitor PD-98059 (10 μM) and the phosphatidylinositol 3-kinase (PI3K) inhibitors LY294002 (40 μmol/l) and PI-103 (1 μmol/l) were used at the indicated concentrations, and all were obtained from EMD Biosciences (Gibbstown, NJ). HAEC for Duolink Assays were grown on standard glass microscope slides coated with collagen using the procedure referenced above. Flow chambers with appropriately sized top plates were then assembled with 0.01-in. (0.025 cm)-thick silicone rubber gaskets (described in Ref. 38) and operated identically to the larger chambers. For static experiments, the growth medium was replaced with the same flow media used for flow studies for 20 h before cell harvest.
Protein extraction.
Cytoplasmic/membrane protein fractions were obtained as described previously (31), with all solutions prechilled on ice and samples stored at −80°C until analysis. Following these steps, nuclear proteins were harvested by first rinsing the plates/nuclei twice with cold PBS to remove any remaining cytosolic protein. Cold-inhibited MES buffered saline [MBS; 25 mmol/l MES; 0.15 mol/l NaCl (pH 6.5); 4% complete protease inhibitor (Roche Applied Science, Laval, QC, Canada), and 1% halt phosphatase inhibitor cocktail (Thermo Scientific-Pierce, Rockford, IL)] was added to the attached nuclei. Nuclei were then removed from the glass by scraping with a cell scraper, collected, and stored on ice. A second volume of MBS was added, and the scraping and collection repeated. A final wash of the glass plates was performed with an additional MBS volume, and this was also added to the nuclei sample. A volume of 10% SDS was added to achieve a final SDS concentration of 0.1% in the samples, and the nuclei were extracted 5 min at room temperature with vortex. Following extraction, nuclear protein samples were concentrated to a final volume of <100 μl using Amicon Ultra Centrifugal concentration tubes (Millipore, Billerica, MA) and stored at −80°C until analysis.
Western blotting.
Protein concentrations were determined by BCA kit (Thermo Scientific-Pierce), and samples denatured, run on SDS-PAGE gels, and electroblotted as previously described (31). Blot membranes were blocked for 1 h in ECL Advance Blocking Agent (GE Healthcare, Baie D'urfe, QC, Canada), and primary antibodies were applied at an appropriate dilution (1:1,000–1:2,000) in blocking agent and incubated overnight at 4C on a shaker. The following primary antibodies, obtained from Cell Signaling Technology (Danvers, MA), were used: phospho-ERK, ERK, phospho-JNK, JNK, phospho-p38, p38, L-psmad2 (phospho-Smad2 Ser245/250/255), R-psmad2 (phospho-Smad2 Ser465/467), Smad2, Smad4, phospho-Akt Ser473, phospho-Akt Thr308, Akt, and α-tubulin. Appropriate secondary horseradish peroxidase-labeled antibodies (Jackson ImmunoResearch, West Grove, PA) were applied at 40 ng/ml in blocking agent for 1 h at room temperature. Blots were developed with ECL Advance Detection Reagents (GE Healthcare) and imaged with a Chemi-Smart gel-doc system (Vilber Lourmat, Marne-la-Vallée, France). Band quantification was performed with Labworks Software (UVP, Upland, CA).
Duolink assay.
Slides supporting static control and flow-exposed HAEC were removed from either static culture or the flow chambers, respectively, rinsed twice with 12 ml cold PBS, and fixed with 4% p-formaldehyde (PFA; Sigma-Aldrich, Oakville, ON, Canada) in PBS for 20 min at room temperature. Following fixation, slides were rinsed four times with 12 ml PBS and washed once for 15 min in another PBS volume. Cells were then permeablized with 4 ml 0.1% Triton X-100 (Sigma-Aldrich) in PBS for 15 min at room temperature. Following permeablization, cells were washed two times with 4 ml PBS, and assay wells were created along the flow path with an Elite Pap Pen (Diagnostic Biosystems, Pleasanton, CA). Cells within each well were blocked and assayed with the Duolink II Kit (Olink Bioscience, Uppsala, Sweden) with volumes of 15 μl per well. Primary antibodies to the targets of interest for each assay were used at the recommended immunofluorescence dilution (or 1:400 if no dilution given) and were from Cell Signaling Technology (pAKT473, Smad2 raised in rabbit, Akt) or Santa Cruz Biotechnology (Smad2 raised in mouse; Santa Cruz, CA). Detection was accomplished with the Duolink Orange Fluorphore, with excitation and emission wavelengths analogous to Cy3 dye. Samples were imaged on an Olympus FluoView FV1000 confocal laser scanning microscope (Markham, ON, Canada) and accompanying FluoView software (version 2.1b), using an Olympus 40× objective. Image sorting and conversion was performed with Image Pro Software, version 6 (Media Cybernetics, Bethesda, MD).
RNA extraction.
Cells on glass plates exposed to 20 h of flow, with or without PI-103 (1 μM), were removed from the flow system, washed twice with cold PBS, trypsinized, scraped from the plate, collected, and stored on ice. Cells were pelleted by spinning at 250 g, the supernatant was removed, and RNA was extracted via the Bio-Rad Aqua-Pure Kit (Mississauga, ON, Canada). Following extraction, hydrated RNA samples were stored at −80°C until analysis.
Real-time PCR.
RNA was assayed with the Ribogreen Kit (Invitrogen, Burlington, ON, Canada). RNA (1 μg) was converted to cDNA via the qScript Kit (Quanta Biosciences, Gaithersburg, MD) and diluted to a final volume of 200 μl. A further 10 μl 1:10 cDNA template dilution (5 ng) was loaded per analysis well, and quantitative PCR was carried out with Perfecta SYBR Green SuperMix (Quanta Biosciences) on a Bio-Rad iCycler iQ real-time PCR instrument. Primers for human serpine-1 and PDGF-B were obtained from Qiagen-SA Biosciences (Frederick, MD) and were used according to the manufacturer's instructions. Human PPIH (cyclophilin H) was used as the housekeeping gene, and relative expression was determined by the 2−ΔΔCT method. Primers for PPIH were: 5′-CGGACCGGGCTTTAGGTTCG-3′ (forward) and 5′-TTCCTGACCGCCAATACTGACAT-3′ (reverse).
Tissue immunofluorescence.
As a kind gift from Dr. Quentin Pittman, University of Calgary, secondary tissue was obtained from Wistar rats (Charles River Laboratories International, Wilmington, MA) subjected to primary experiments approved by the University of Calgary Health Sciences Animal Care Committee. Briefly, the rats were deeply anesthetized with pentobarbital sodium (65 mg/kg) and perfused transcardially with 120 ml cold PBS followed by 120 ml 4% PFA (Acros Organics-Thermo Fisher Scientific, Ottawa, ON, Canada) in PBS. Following fixation, whole aortas were dissected and postfixed in cold 4% PFA-PBS for 24 h. Aortas were then washed twice in 1.5 ml PBS, cleaned, and cut into sections (arch and thoracic) for staining. Before staining, each section was transferred to a well of a 48-well plate and permeablized 10 min at room temperature with 500 μl 0.1% Triton X-100 in PBS. Following permeablization, sections were washed four times with 500 μl PBS. The tissue was then blocked overnight at 4C with 5% rabbit serum (Sigma-Aldrich) in PBS and gentle rocking. After this step, the tissue was rinsed twice with 3% bovine serum albumin fraction V (BSA; Thermo Fisher Scientific) in PBS and blocked in this solution for 2 h at 4C. A working solution of L-psmad2 antibody (1:500) was prepared in BSA-PBS, and a 250-μl aliquot was applied to each section after removal of the blocking volume. Primary antibody incubation was performed overnight at 4C with gentle rocking. Following incubation, the antibody solution was removed, and the tissue was rinsed four times with 500 μl 3% BSA-PBS. A fifth volume was added to each well, and the tissue was washed for 15 min at room temperature. A working solution of goat anti-rabbit Alexa 555 secondary antibody (Invitrogen, Burlington, ON, Canada) was prepared (1:400) and aliquoted to each sample (250 μl/well) following removal of wash buffer. Secondary antibody was incubated in the dark for 1 h at room temperature with gentle rocking. Following secondary labeling, the wash steps were repeated, but only with PBS. A working solution of Hoechst 33258 Dye (Invitrogen) was prepared at 1 μg/ml, and nuclear staining was conducted with 250 μl/well in the dark for 15 min at room temperature. After removal of the Hoechst solution, PBS wash steps were repeated and the samples were stored in a fresh 500-μl volume of PBS until mounting. Fully labeled tissue samples were trimmed as required under a stereomicroscope to enable them to lay flat and were mounted en face on a microscope slide with Prolong Gold Antifade Reagent (Invitrogen). Slides were allowed to dry in the dark overnight, and the samples were imaged as described for the Duolink Assay.
Statistical analysis.
Data are reported as means ± SE. For Western blotting results, the ratios of active to total species were calculated for each lane, and/or α-tubulin was used as a loading control, unless otherwise indicated. Blot to blot comparison was accomplished via standard samples, and statistical significance of results was determined by ANOVA.
RESULTS
Levels and nuclear translocation of L-pSmad2 varied with flow condition.
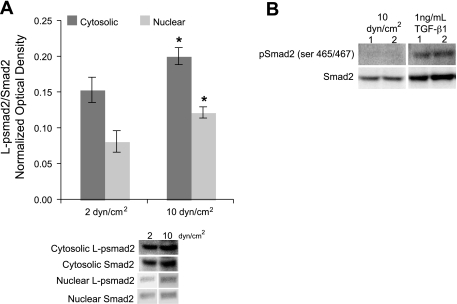
Smad2 linker phosphorylation (ser245/250/255) was measured by Western blot to determine whether the imposed flow conditions influenced its generation. As shown in Fig. 1A, linker phosphorylated Smad2 (L-psmad2) in the cytosol was 31% higher from 10 dyn/cm2 cultures than those exposed to 2 dyn/cm2. This species was also found in the nucleus, with nuclear levels 50% higher in cells from 10 versus 2 dyn/cm2 average shear stress, indicating that the protein was able to translocate to the nucleus upon phosphorylation. Differences in L-psmad2 levels were not due to changes in expression of Smad2 protein, as it was found to be constant under both average shear stress conditions (data not shown), but were related to phosphorylation of linker region sites. Similarly, both cytosolic and nuclear levels of Smad4 were also found to be invariant in response to the different flow conditions (data not shown).
Fig. 1.
Smad protein levels for human aortic endothelial cells (HAEC) exposed to 20 h of 2 and 10 dyn/cm2 average shear stresses at 80 ± 5 mmHg and exemplary Western blot lanes. Each plot point represents the average value for the indicated number of samples. Western blot quantification was performed on raw image data and normalized to total kinase levels for each lane. A: cytosolic (n = 10) and nuclear (n = 3) L-psmad2 profiles. B: Western blot lanes showing COOH-terminal phosphorylated Smad2 levels under flow for 20 h or treated with 1 ng/ml transforming growth factor (TGF)-β1 for 1 h. *P < 0.05.
Flow exposure preferentially phosphorylated Smad2 in the linker, not COOH-terminal, region.
Because phosphorylation of Smad2 in the linker region is not the primary mechanism by which this protein is known to be functionally activated and induced to translocate to the nucleus, experiments were conducted to verify that L-psmad2, and not COOH-terminal TGF-β receptor 1 (TGFR1) phosphorylated Smad2, was the primary species identified under flow conditions. COOH-terminal phosphorylated Smad2 (ser465/467) was not detected in flow-exposed cultures, but it was identified upon TGF-β1 treatment (Fig. 1B). These findings indicate that L-psmad2 was indeed the primary species in both cytosolic and nuclear protein fractions from flow experiments and that the antibody systems employed in these studies were capable of allowing discrimination between the differentially phosphorylated forms of Smad2.
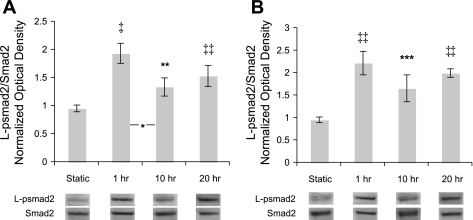
L-psmad2 was generated following flow initiation, and levels remained relatively constant.
To investigate the appearance and maintenance of L-psmad2 expression over the 20-h duration of the flow experiments, the intermediate time points of 1 and 10 h were studied for both flow conditions. As shown in Fig. 2, L-psmad2 was significantly elevated versus static control in both cases following 1 h of flow exposure, and the level of expression was relatively constant for each flow condition through the terminal time point. A significant decrease was observed for 2 dyn/cm2 between 1 and 10 h (Fig. 2A). However, by 20 h, levels were again statistically similar to that observed at 1 h, and as discussed for Fig. 1A, more abundant in 10 versus 2 dyn/cm2 cultures. These results demonstrate that once L-psmad2 is generated in response to flow stimulation, it is maintained for at least a day.
Fig. 2.
L-psmad2 time course over 20 h of flow exposure at 80 ± 5 mmHg and exemplary Western blot lanes. Plot points and blot quantification was performed as in Fig. 1. All flow points had significantly more L-psmad2 than static control. A: 2 dyn/cm2 average shear stress. A significant drop in L-psmad2 levels was measured between 1 and 10 h samples. B: 10 dyn/cm2 average shear stress (n = 4 all conditions). *P < 0.05; **P < 0.01; ***P < 0.005; ‡P < 0.001; ‡‡P < 0.0001.
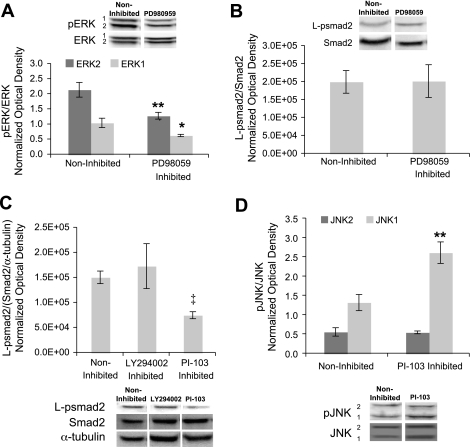
ERK inhibition did not alter L-psmad2 levels in flow-exposed cells.
Because ERK is known to phosphorylate the Smad2 linker region, and we have previously found prolonged ERK phosphorylation in shear-exposed cells (31) that follows a pattern similar to that of L-psmad2 described above, the role of ERK in generating the L-psmad2 profiles observed at 20 h was investigated. The small molecule inhibitor of MEK1 (upstream of ERK) PD98059 was introduced to the 10 dyn/cm2 experimental medium before the onset of flow (10 μmol/l) and retained for the duration of the experiments. As shown in Fig. 3A, the levels of phosphorylated ERK1 and ERK2 after 20 h of flow were each reduced by 40% in the presence of the inhibitor; however, the amount of L-psmad2 (Fig. 3B) was not affected.
Fig. 3.
MAPK and Smad2 activity with chemical inhibitors of the ERK and phosphatidylinositol 3-kinase (PI3K) pathways at 20 h of 10 dyn/cm2, average shear stress at 80 ± 5 mmHg, and exemplary Western blot lanes. Plot points and blot quantification was performed as in Fig. 1. A: ERK phosphorylation in the presence of 10 μmol/l PD98059. For 10 dyn/cm2 and 10 dyn/cm2 + PD98059: n = 3. B: L-psmad2 levels in the presence of 10 μmol/l PD98059. For 10 dyn/cm2, n = 4, and for 10 dyn/cm2 + PD98059: n = 3. C: L-psmad2 levels in the presence of 40 μmol/l LY294002 and 1 μmol/l PI-103. For 10 dyn/cm2, n = 3, for 10 dyn/cm2 + LY294002, n = 2, and for 10 dyn/cm2 + PI-103, n = 3. D: JNK phosphorylation in the presence of 1 μmol/l PI-103. For 10 dyn/cm2, n = 3, and for 10 dyn/cm2 + PI-103, n = 3. *P < 0.05; **P < 0.01; ‡P < 0.0001.
PI-103, but not LY294002, decreased L-psmad2 levels in flow-exposed cells; JNK and p38 phosphorylation did not correlate to L-psmad2 abundance.
Because ERK inhibition did not alter L-psmad2 levels in the experimental system, the other MAPKs we previously identified to have prolonged phosphorylation under shear flow, JNK and p38, were considered for their involvement in Smad2 phosphorylation. Most chemical inhibitors of the JNK and p38 pathways function downstream of these proteins, but evidence suggests the species that interact with the Smad2 linker region are upstream or inclusive of JNK and p38 (3). Therefore, the PI3K inhibitors LY294002 and PI-103 were selected to take advantage of overlap and cross talk between PI3K and MAPK upstream of JNK and p38. LY294002 (40 μmol/l) and PI-103 (1 μmol/l) were added directly to the experimental medium, as was done for PD98059. Following 20 h of flow exposure, L-psmad2 levels were reduced 50% in the presence of PI-103, but not affected by LY294002 (Fig. 3C). JNK phosphorylation was similarly not influenced by LY294002 after 20 h of flow/inhibitor exposure (data not shown). However, PI-103 treatment did affect phosphorylation of JNK1 (but not JNK2), yielding an increase of twofold over noninhibited levels (Fig. 3D). This increase in JNK1 phosphorylation was in opposition to the effect of PI-103 on L-psmad2. This finding, along with the lack of an effect on JNK2, indicates that JNK was not directly involved in the generation of the observed L-psmad2. Phosphorylation of p38 did not respond significantly to either inhibitor, indicating that it too was not directly involved with Smad2 phosphorylation under the conditions investigated (data not shown). The general lack of response to LY294002 treatment may be due to differences in inhibitor effectiveness, molecule half-life (assays were conducted 20 h after addition of inhibitor), or functionality between PI-103 and LY294002.
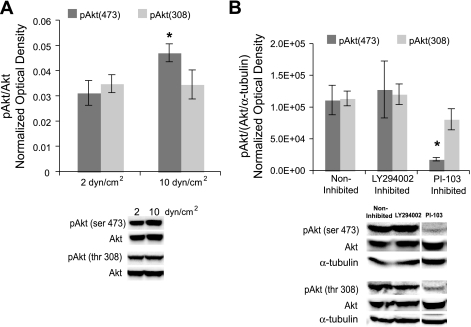
Levels of phosphorylated Akt were flow dependent, and its modulation was similar to that of L-psmad2.
Akt has been shown to exhibit increased activity with elevated shear stress (10). This, along with the reduction in L-psmad2 observed upon PI-103 treatment, prompted an examination of the behavior of Akt in both flow conditions and PI-103-treated cells. PI-103 is a specific, potent, and unique inhibitor of PI3K, and it strongly affects Akt phosphorylation (13). The levels of Akt phosphorylated at thr308 [pAkt308] were not affected by changes in flow; however, Akt phosphorylated at ser473 [pAkt473] was increased 51% in cultures exposed to 10 versus 2 dyn/cm2 average shear stress magnitude (Fig. 4A). This result matched the observed increase in L-psmad2 under the same flow condition (Fig. 1A). Similarly, PI-103 inhibition of 10 dyn/cm2 cultures decreased pAkt473 levels by 83% (Fig. 4B). This result again matched the behavior of L-psmad2 (Fig. 3C) for the same condition. Treatment with LY294002 did not alter either Akt phosphorylation level at 20 h, and PI-103 did not significantly affect pAkt308 (Fig. 4B). These findings, when considered with the other data presented in this study, suggest that pAkt473, or its upstream activators/downstream targets, could be involved with producing the L-psmad2 observed with flow exposure.
Fig. 4.
Flow-derived phosphorylated (p)Akt activity, and exemplary Western blot lanes. Plot points and blot quantification was performed as in Fig. 1. A: pAkt473 and pAkt308 levels in cultures from 20 h of 2 and 10 dyn/cm2 average shear stress at 80 ± 5 mmHg. For pAkt473 2 dyn/cm2, n = 5; for pAkt473 10 dyn/cm2, n = 6; and for pAkt308 2 and 10 dyn/cm2, n = 5. B: pAkt from 20 h of 10 dyn/cm2 average shear stress at 80 ± 5 mmHg with 40 μmol/l LY294002 and 1 μmol/l PI-103. In all cases, n = 3. *P < 0.05.
Akt and Smad2, as well as pAKT473 and Smad 2, directly associated under flow stimulation.
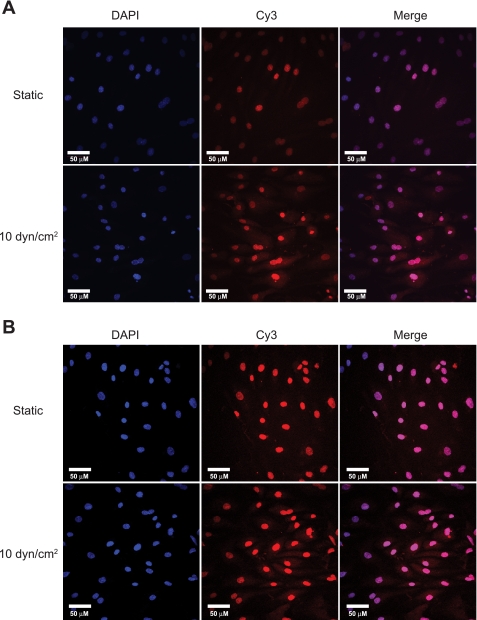
To further test the potential relationship between Akt and Smad2, Duolink Assays were performed on cells from 10 dyn/cm2 flow exposure and static controls. This in situ technique provides signal enhancement only when two target proteins are colocated, allowing improved sensitivity and confidence versus traditional techniques (e.g., Co-IP) for establishing protein-protein interactions. As shown by the cytosolic staining in Fig. 5A, total Akt and total Smad2 were found to associate in response to 20 h of flow exposure relative to static control. An additional experiment was conducted with antibodies to pAkt473 and Smad2, and, as shown in Fig. 5B, indicates that those species also preferentially associated following flow exposure. These results demonstrate that there can be direct interaction between Akt and Smad2 species in response to flow stimulation in cultured HAEC and are consistent with results of the PI-103 experiments in suggesting that flow-dependent pAkt473 may be responsible for the generation of at least a portion of the observed L-psmad2.
Fig. 5.
Duolink Assay results for static and flow exposed HAEC. Protein associations determined by Cy3 channel confocal microscopy signal. Nuclei are counterstained with 4',6-diamidino-2-phenylindole (DAPI). A: total Akt and Smad2 association, n = 2. B: Smad2 and pAkt473 association. Enhanced cytoplasmic staining is evident with both sets of antibodies, indicating that colocalization of the proteins was occurring preferentially under flow stimulation.
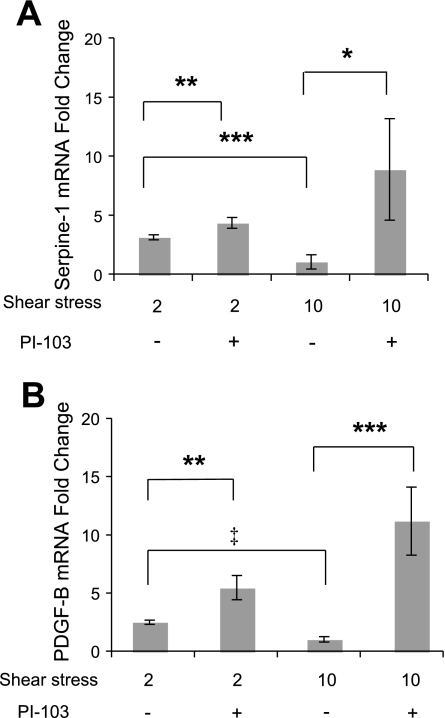
PI-103 affected mRNA expression for shear stress and TGF-β sensitive genes.
Given the effect PI-103 had on L-psmad2, we performed an initial examination of the potential functional consequence of treatment with this inhibitor on two genes that are both shear stress and TGF-β sensitive, serpine-1 (serpine) and PDGF-B (PDGF). As shown in Fig. 6, A and B, mRNA expression for both genes was found to trend as expected with shear stress, being significantly lower (serpine, 2.9-fold; PDGF, 2.4-fold) from 10 versus 2 dyn/cm2 cultures following 20 h of flow exposure. However, upon treatment with PI-103 during flow experiments, this trend was reversed. Additionally, the response observed at 10 dyn/cm2 was much stronger (serpine, 8.9-fold; PDGF, 10.5-fold) than that from 2 dyn/cm2 (serpine, 2.4-fold; PDGF, 2.2-fold). Taken together, these findings indicate that culture treatment with PI-103 during flow exposure reduced flow-induced repression of serpine and PDGF mRNA and that effect of PI-103 on a factor present under the higher average shear stress resulted in a much stronger effect.
Fig. 6.
Real-time PCR gene expression with 1 μmol/l PI-103 treatment at both 2 and 10 dyn/cm2 average shear stress at 80 ± 5 mmHg. Analysis was performed on cDNA pooled from 3 × 106 cells exposed to each condition. Relative expression analysis was performed by the 2−ΔΔCt method with PPIH as the housekeeping gene. A: serpine-1 mRNA expression. B: platelet-derived growth factor subunit B (PDGF-B) mRNA expression. *P < 0.05; **P < 0.01; ***P < 0.005; ‡P < 0.001.
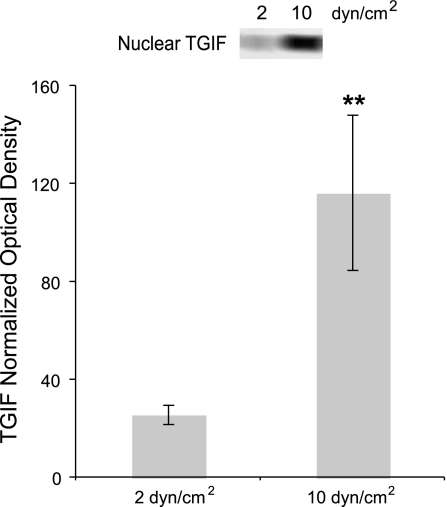
Nuclear TGIF levels were increased in a flow-dependent manner.
Based upon PI-103 gene expression analyses and their correlation to the PI-103-induced reduction in L-psmad2 levels, it was decided to examine cofactors that could participate in Smad2-dependent inhibition of serpine and PDGF mRNA expression. The Smad corepressor TGIF was selected for specific analysis due to its documented role in this capacity. Examination of TGIF expression in the cytoplasm of flow-exposed cells showed no correlation to the flow condition (data not shown). However, as presented in Fig. 7, the amount of TGIF measured in the nucleus was 4.5-fold higher from 10 versus 2 dyn/cm2 average shear stress cultures. This result correlates well with the other findings of this work (that for uninhibited cultures serpine and PDGF gene expression was lower at 10 vs. 2 dyn/cm2, whereas L-psmad2 levels were higher) and supports the idea that a reduction in L-psmad2 by PI-103 may have contributed to the alteration of mRNA expression profiles through a decreased association with nuclear TGIF.
Fig. 7.
Nuclear transforming growth interacting factor (TGIF) levels after 20 h of 2 and 10 dyn/cm2 average shear stress at 80 ± 5 mmHg, and exemplary Western blot lanes. Plotted data represent average relative amounts as determined by combining Western blot normalization versus same lane p53, α-tubulin, and backstained gel optical densities. For each case, n = 3. **P < 0.01.
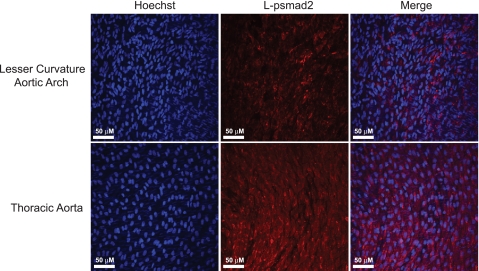
Increased L-psmad2 staining was evident in rat aortic tissue exposed to high/uniform versus low/oscillatory average shear stress profiles.
To determine whether the flow-dependent generation L-psmad2 observed in vitro had relevance to an in vivo setting, secondary use rat aortic tissue was obtained and stained for L-psmad2 in both the lesser curvature of the aortic arch and thoracic aorta sections. Tissue from these locations provides relative models of low/oscillatory and high/uniform shear stress and flow profiles that have been well characterized in anatomically similar mice (14, 33) and has been used to analyze biomarkers related to these flow conditions (5, 33). Figure 8 presents representative results of this work and clearly shows that L-psmad2 was more abundant in endothelial cells from the high/uniform shear stress thoracic region compared with the lower/oscillating shear stress arch.
Fig. 8.
Representative en face confocal microscopy images of rat aortic sections stained with antibody to L-psmad2. Nuclei are counterstained with Hoechst 33258 dye. Enhanced signal from thoracic sections indicates that more L-psmad2 was generated in this high shear stress region compared with the low shear stress arch; n = 5 (intact aorta dissections divided into arch and thoracic samples).
DISCUSSION
This study represents the first report of Smad2 linker region phosphorylation dependence on fluid flow in human endothelial cells and shows that the flow-dependent generation of this species translates to the rat in vivo aortic environment. Additionally, for cultured cells, results indicate that L-psmad2 was able to enter the nucleus, establish flow-dependent TGIF nuclear levels, and suggest that flow-dependent gene expression may be mediated by nuclear L-psmad2 and TGIF. These conclusions are supported by several findings. First, differential flow stimulation did not alter the total expressed levels of Smad2 protein, indicating that the changes in L-psmad2 were due to upstream kinase activity derived from flow conditions, likely involving pAkt473. Second, L-psmad2 was the active form of Smad2 detected in cell extracts; COOH-terminal phosphorylation was not observed in response to flow stimulation. Finally, serpine and PDGF mRNA expression were altered in response to PI-103 in a manner that modeled changes in L-psmad2 and pAkt473 levels.
An important finding of this study is that L-psmad2 was expressed in rat aortic tissue with levels that varied according to flow characteristics/shear stress distribution. This both confirms and extends the results of our culture work by demonstrating that differences in L-psmad2 abundance are generated and maintained over the long-term in a flow-dependent manner in rodent aorta. Whereas other factors differ between the lesser curvature of the aortic arch and the thoracic aorta, e.g., the possible presence of arch oscillating flow (33), this consistency of results suggests a role for average wall shear stress in determining L-psmad2 expression. When taken together with the other data presented in this study, it is possible to conclude that the generation and actions of L-psmad2 may have direct consequences on endothelial physiology, including the development of atherosclerosis.
Results from cultured HAEC show that MAPK activity was not primarily responsible for L-psmad2 generation, as might have been expected based upon previously published data describing linker phosphorylation (8, 17, 26), its relationship to MAPKs (12, 15), and our own work showing prolonged flow-dependent phosphorylation of ERK, JNK, and p38 species (31). Inhibition of MEK/ERK with PD98059 produced a 40% reduction in the activity of both ERK1 and ERK2 without generating a similar response in L-psmad2 levels. Additionally, treatment with both LY294002 and PI-103 did not affect the JNK or p38 pathways in a manner that correlated to L-psmad2 behavior. But the reduction in pAkt473 upon PI-103 treatment matched that observed for L-psmad2, indicating that this kinase species, or its associated upstream activators/downstream targets, are likely participating in L-psmad2 generation. This hypothesis was given additional support by the demonstration of flow-dependent interactions between total Akt and Smad2, as well as pAkt473 and Smad2, as determined by Duolink Assays on static and flow-exposed cells.
The possibility of a correlation between linker region Smad2 phosphorylation and Akt has not been previously demonstrated. Phosphorylation of Akt as a consequence of shear stress-dependent PI3K activity has been reported in human umbilical vein endothelial cells (11), and this is in agreement with our pAkt473 data. PI3K/Akt activation has also been found to stimulate interaction among components of those pathways and Smads 2 and 3 in cells with active TGF-β receptor signaling (28). Additional work has shown a dependence of Smad3 linker region phosphorylation on Akt (7) and also demonstrated Akt can participate in the nuclear behavior of Smad3 by influencing interactions with CREB binding protein as well as its acetylation. These findings are particularly interesting in light of our L-psmad2 and Duolink results and support our conclusion that that there is most likely a direct relationship between flow-derived pAkt473 and L-psmad2. Further studies are needed to expand upon the Duolink results presented here to evaluate how Akt and/or components of its phosphorylation cascade and L-psmad2 may be related in flow-exposed endothelium.
PI-103 treatment produced additional effects on flow-exposed HAECs as evidenced through evaluation of serpine and PDGF mRNA expression, indicating that there were consequences to its actions on the cells beyond the reduction in pAkt473 and L-psmad2 species. The influence on mRNA expression was especially pronounced at 10 dyn/cm2, yielding order of magnitude fold changes (PDGF), or nearly so (serpine), versus untreated cultures. This increase was between four and five times that seen for the same genes with PI-103 at 2 dyn/cm2, indicating that a factor involved with flow-mediated gene repression was affected by PI-103. This finding correlates well with the reduction in L-psmad2 seen under the same conditions and, because of the shear stress/TGF-β sensitivity of these genes, implies a relationship between increased levels of mRNA and lower amounts of L-psmad2. We have made additional observations of endothelial cells cotreated with PI-103 and TGF-β under flow stimulation that demonstrate widespread cell detachment and/or death, whereas introduction of either of these compounds alone does not affect cell viability or retention (RD Shepherd, SM Kos, unpublished observations). Although the specific interactions remain to be determined, all of these results indicate that PI-103 alters endothelial cell responses to flow stimulation in an important way that appears to involve Smad signaling.
The discovery that nuclear levels of TGIF were also flow sensitive fits well with a model of flow-derived L-psmad2, impacting HAEC gene expression. The presence of L-psmad2 in nuclear protein fractions, and the coincident presence of Smad4, suggests that L-psmad2 should have been capable of functionally interacting with Smad-specific DNA binding sites (22, 23, 35) and/or additional transcription factors (19, 26) and coactivators/repressors (23). Nuclear TGIF levels were found to be nearly five times higher from 10 versus 2 dyn/cm2 average shear stress, correlating well with the expression of serpine and PDGF mRNA expression before PI-103 treatment. Upon reduction of L-psmad2 generation with PI-103 treatment, it follows that TGIF/Smad2-dependent gene repression could be alleviated. Given the data presented here, a model can be proposed that describes a flow-dependent generation of L-psmad2 and its nuclear translocation, while similarly influencing nuclear localization of TGIF. These events would allow L-psmad2/TGIF to affect gene expression through association with promoter sequences and various partners, depending on the specific physiological environment.
Although not conclusive, the results of our preliminary work are intriguing and lay the foundation for additional investigations into the flow-derived physiological properties of endothelial cells by establishing the presence of several novel phenomena. Due to the nature of our experimental system, we cannot explicitly correlate our in vitro findings to differences in average shear stress exposure, since variations in frequency and pulsatility index were also present between the conditions. It is possible that these characteristics contributed to the observed behavior of L-psmad2 and TGIF (6, 16, 40, 41). However, our findings present the first evidence that prolonged differential flow exposure can influence both tissue-based and cultured endothelial L-psmad2 levels, as well as subsequent L-psmad2 and TGIF nuclear translocation in cultured cells. Given the apparent in vivo regulation of L-psmad2 observed in rat aortic tissue, specific investigations into the influence of individual flow parameters (e.g., waveform and frequency) upon the generation and actions of L-psmad2 in endothelial cell systems are indicated and may provide new targets for vascular disease intervention. Additionally, the possibility that Akt is involved in the flow-dependent generation of L-psmad2, and that nuclear L-psmad2 and the Smad corepressor TGIF are agents of flow-dependent gene expression, is quite interesting. The larger potential for Smads and their partner proteins to be adaptable effectors of mechanical forces in endothelial cells opens new avenues of research that may have implications in multiple areas of human development, health, and disease.
GRANTS
This work was supported by funding from National Heart, Lung, and Blood Institute Grant R01 HL-68916, a University of Calgary Starter Grant, Canadian Foundation for Innovation Grant 10261, The Alberta Science and Research Investment Program, a Natural Sciences and Engineering Research Council of Canada Discovery Grant, and an Alberta Heritage Foundation for Medical Research Undergraduate Student Award for Jennifer Irwin.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the laboratory of Dr. Michael Walsh, and specifically Dr. Jingti Deng for her assistance with the Duolink Assays. We also thank Dr. Linda Andersen for her guidance and help with the tissue immunofluorescence studies. Finally, we thank Jennifer Irwin for her help with the initial Smad2 studies and Nicole Marshal for assistance in the gene expression studies.
REFERENCES
- 1. Andersson M, Karlsson L, Svensson PA, Ulfhammer E, Ekman M, Jernas M, Carlsson LMS, Jern S. Differential global gene expression response patterns of human endothelium exposed to shear stress and intraluminal pressure. J Vasc Res 42: 441–452, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Azuma N, Akasaka N, Kito H, Ikeda M, Gahtan V, Sasajima T, Sumpio BE. Role of p38 MAP kinase in endothelial cell alignment induced by fluid shear stress. Am J Physiol Heart Circ Physiol 280: H189–H197, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Brown JD, DiChiara MR, Anderson KR, Gimbrone MA, Jr, Topper JN. MEKK-1, a component of the stress (stress-activated protein kinase/c-jun n-terminal kinase) pathway, can selectively activate smad2-mediated transcriptional activation in endothelial cells. J Biol Chem 274: 8797–8805, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Chiu JJ, Chen LJ, Lee CI, Lee PL, Lee DY, Tsai MC, Lin CW, Usami S, Chien S. Mechanisms of induction of endothelial cell E-selectin expression by smooth muscle cells and its inhibition by shear stress. Blood 110: 519–528, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Conway DE, Sakurai Y, Weiss D, Vega JD, Taylor WR, Jo H, Eskin SG, Marcus CB, McIntire LV. Expression of CYP1A1 and CYP1B1 in human endothelial cells: regulation by fluid shear stress. Cardiovasc Res 81: 669–677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vauhn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci USA 101: 14871–14876, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Das F, Ghosh-Choudhury N, Venkatesan B, Li X, Mahimainathan L, Choudhury GG. Akt kinase targets association of CBP with smad3 to regulate TGF-beta induced expression of plasminogen activator inhibitior-1. J Cell Physiol 214: 513–527, 2008 [DOI] [PubMed] [Google Scholar]
- 8. de Caestecker MP, Parks WT, Frank CJ, Castagnino P, Bottaro DP, Roberts AB, Lechleider RJ. Smad2 transduces common signals from receptor serine-threonine and tyrosine kinases. Genes Dev 12: 1587–1592, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGFb-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J 17: 3091–3100, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dimmeler S, Assmus B, Hermann C, Haendeler J, Zeiher AM. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ Res 83: 334–341, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Engel ME, McDonnell MA, Law BK, Moses HL. Interdependent smad and JNK signaling in transforming growth factor B-mediated transcription. J Biol Chem 274: 37413–37420, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, Shokat KM, Weiss WA. A dual PI3 kinase/mTOR inhibitor revels emergent efficacy in glioma. Cancer Cell 9: 341–349, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feintuch A, Ruengsakulrach P, Lin A, Zhang J, Zhou YQ, Bishop J, Davidson L, Courtman D, Foster FS, Steinman DA, Henkelman RM, Ethier CR. Hemodynamics in the mouse aortic arch as assessed by MRI, ultrasound, and numerical modeling. Am J Physiol Heart Circ Physiol 292: H884–H892, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Funaba F, Zimmerman CM, Mathews LS. Modulation of smad2-mediated signaling by extracellular signal-regulated kinase. J Biol Chem 277: 41361–41368, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Himburg HA, Dowd SE, Friedman MH. Frequency-dependent response of the vascular endothelium to pulsatile shear stress. Am J Physiol Heart Circ Physiol 293: H645–H653, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Kretzschmar M, Doody J, Timokhina I, Massague J. A mechanism of repression of TGF-B/smad signaling by oncogenic Ras. Genes Dev 13: 804–816, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. LaMack JA, Friedman MH. Individual and combined effects of shear stress magnitude and spatial gradient on endothelial cell gene expression. Am J Physiol Heart Circ Physiol 293: H2853–H2859, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Lopez-Rovira T, Chalaux E, Rosa JL, Bartrons R, Ventura F. Interaction and functional cooperation of NF-kB with smads: transcriptional regulation of the junB promoter. J Biol Chem 275: 28937–28946, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Malek AM, Izumo S. Control of endothelial cell gene expression by flow. J Biomech 28: 1515–1528, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Malek AM, Gibbons GH, Dzau VJ, Izumo S. Fluid shear stress differentially modulates expression of genes encoding basic fibroblast growth factor and platelet-derived growth factor B chain in vascular endothelium. J Clin Invest 92: 2013–2021, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Massague J, Wotton D. Transcriptional control by the TGF-B/smad signaling system. EMBO J 19: 1745–1754, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Massague J, Seaoane J, Wotton D. Smad transcription factors. Genes Dev 19: 2783–2810, 2005 [DOI] [PubMed] [Google Scholar]
- 24. McCormick SM, Eskin SG, McIntire LV, Teng CL, Lu CM, Russell CG, Chittur KK. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci USA 98: 8955–8960, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mori S, Matsuzaki K, Yoshida K, Furukawa F, Tahashi Y, Yamagata H, Sekimoto G, Seki T, Matsui H, Nishizawa M, Fujisawa J, Okazaki K. TGF-β and HGF transmit the signals through JNK-dependent smad2/3 phosphorylation at the linker regions. Oncogene 23: 7416–7429, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Rodriguez-Pascual F, Redondo-Horcajo M, Lamas S. Functional cooperation between smad proteins and activator protein-1 regulates transforming growth factor-β-mediated induction of endothelin-1 expression. Circ Res 92: 1288–1295, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Ross S, Hill CS. How the smads regulate transcription. Int J Biochem Cell Biol 40: 383–408, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Runyan CE, Schnaper HW, Poncelet AC. The phosphatidylinositol 3-kinase/Akt pathway enhances smad3-stimulated mesangial cell collagen I expression in response to transforming growth factor-beta1. J Biol Chem 279: 2632–2639, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Schmierer B, Hill CS. TGFβ-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol 8: 970–982, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Sheikh T, Rahman M, Gale Z, Luu NT, Stone PCW, Matharu NM, Rainger GEL, Nash GB. Differing mechanisms of leukocyte recruitment and sensitivity to conditioning by shear stress for endothelial cells treated with tumour necrosis factor-α or interleukin-1β. Br J Pharmacol 145: 1052–1061, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shepherd RD, Kos SM, Rinker KD. Long term shear stress leads to increased phosphorylation of multiple MAPK species in cultured human aortic endothelial cells. Biorheology 46: 529–538, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Simonsson M, Kanduri M, Grönroos E, Heldin CH, Ericsson J. The DNA binding activities of Smad2 and Smad3 are regulated by coactivator-mediated acetylation. J Biol Chem 281: 39870–39880, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Suo J, Ferrara DE, Sorescu D, Guldberg RE, Taylor WR, Giddens DP. Hemodynamic shear stresses in mouse aortas: implications for atherogenesis. Arterioscler Thromb Vasc Biol 27: 346–351, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Taylor LM, Khachigian LM. Induction of platelet-derived growth factor B-chain expression by transforming growth factor-β involve transactivation by smads. J Biol Chem 275: 16709–16716, 2000 [DOI] [PubMed] [Google Scholar]
- 35. ten Dijke P, Hill CS. New insights into TGF-β-smad signaling. Trends Biochem Sci 29: 265–273, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Topper JN, Cai J, Qiu Y, Anderson KR, Xu YY, Deeds JD, Feeley R, Gimeno CJ, Woolf EA, Tayber O, Mays GG, Sampson BA, Schoen FJ, Gimbrone MA, Jr, Falb D. Vascular MADs: two novel MAD-related genes selectively inducible by flow in human vascular endothelium. Proc Natl Acad Sci USA 94: 9314–9319, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Urbich C, Mallat Z, Tedgui A, Clauss M, Zeiher AM, Dimmeler S. Upregulation of TRAF-3 by shear stress blocks CD40-mediated endothelial activation. J Clin Invest 108: 1451–1458, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Viegas KD, Dol SS, Salek MM, Shepherd RD, Martinuzzi RM, Rinker KD. Methicillin resistant Staphylococcus aureus adhesion to human umbilical vein endothelial cells demonstrates wall shear stress dependent behavior. Biomed Eng Online 10: 20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wasserman SM, Topper JN. Adaptation of the endothelium to fluid flow: in vitro analyses of gene expression and in vivo implications. Vasc Med 9: 35–45, 2004 [DOI] [PubMed] [Google Scholar]
- 40. White CR, Haidekker M, Bao X, Frangos JA. Temporal gradients in shear, but not spatial gradients, stimulate endothelial cell proliferation. Circulation 103: 2508–2513, 2001 [DOI] [PubMed] [Google Scholar]
- 41. White CR, Stevens HY, Haidekker M, Frangos JA. Temporal gradients in shear, but not spatial gradients, stimulate ERK1/2 activation in human endothelial cells. Am J Physiol Heart Circ Physiol 289: H2350–H2355, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Wotton D, Knoepfler PS, Laherty CD, Eisenman RN, Massague J. The smad transcriptional corepressor TGIF recruits mSin3. Cell Growth Differ 12: 457–463, 2001 [PubMed] [Google Scholar]
- 43. Wotton D, Lo RS, Lee S, Massague J. A smad transcriptional corepressor. Cell 97: 29–39, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Xu L. Regulation of smad activities. Biochim Biophys Acta 1759: 503–513, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang Z, Wang J, Wang L, Chen L, Tu C, Luo C, Tang A, Wang SM, Tao J. In vitro shear stress modulates antithrombogenic potentials of human endothelial progenitor cells. J Thromb Thrombolysis 23: 121–127, 2007 [DOI] [PubMed] [Google Scholar]