Abstract
The Cre-loxP system is a useful tool to study the physiological effects of gene knockout in the heart. One limitation with using this system in the heart is the toxic effect of chronic expression of the Cre recombinase. To circumvent this limitation, a widely used inducible cardiac-specific model, Myh6-MerCreMer (Cre), using tamoxifen (TAM) to activate Cre has been developed. The current study examined cardiac function in Cre-positive C57B/J6 mice exposed to one, three, or five daily doses of a 40 mg/kg TAM to induce Cre activity specifically in the heart. Echocardiography demonstrated no statistically significant differences in systolic function (SF) at baseline as assessed by fractional shortening. In mice exposed to five injections, a significant fall in all determinants of SF was observed 6 days after TAM was initiated. However, SF returned to baseline levels 10 days after TAM initiation although the hearts exhibited significant hypertrophy. Heart weight-to-tibia length ratios were 73 ± 3, 78.5 ± 6, and 87.6 ± 9 mg/cm for one, three, and five TAM injections, respectively. TAM had no effect on cardiac function or hypertrophy in Cre-negative mice. Cre-positive mice receiving five TAM injections had significant reductions in cardiac mitochondrial ATP and significant reductions in the expression of proteins important for the regulation of cardiac oxidative phosphorylation including peroxisome proliferator-activated receptor-γ coactivator-1α and pyruvate dehydrogenase kinase-4. Thus inducible cardiac-specific activation of Cre recombinase caused a transient decline in SF that was dependent on the number of TAM doses and associated with significant hypertrophy and alterations in mitochondrial ATP and important proteins involved in the regulation of cardiac oxidative phosphorylation.
Keywords: Cre recombinase, tamoxifen, cardiac hypertrophy
tissue-specific gene knockout technology has revolutionized cardiovascular research. The ability to knock genes out in a spatial and temporal fashion has resulted in a greater understanding of organ development and function at an unprecedented pace. The approach that has been most widely used to achieve tissue-specific knockout of genes is the Cre/loxP system adapted from bacteriophage P1 (25, 26). To inactivate a specific gene, an important area such as the transcription start site or an important coding exon is flanked (floxed) by loxP sites that are in the same orientation as determined by the palindromic core sequence of the loxP site. Upon expression of the Cre recombinase, the region between the two loxP sites is deleted and the gene is inactivated. Since the appropriately targeted “floxed” gene is in all cells, the specificity of the system is derived by expression of the Cre recombinase in a cell/tissue-specific and temporal manner with an appropriate promoter.
The Cre/loxP system has been widely used to achieve cardiac-specific knockout of genes using several promoters driving expression of Cre protein specifically to the heart (1, 12, 21, 27). However, high level Cre expression in the heart throughout development using the α-myosin heavy chain promoter has been reported to result in the development of heart failure due to some unknown toxicity of the Cre recombinase in the heart (4). This result is similar to previous reports of high levels of Cre recombinase resulting in alterations in cell proliferation and apoptosis (17, 22). To avoid the potential adverse effects of a high level of Cre expression throughout development, a new generation of fusion proteins was developed in which Cre was fused to mutated estrogen receptor ligand binding domains that were insensitive to endogenous estrogens but sensitive to tamoxifen (TAM; Refs. 8, 9, 20, 29). One major advantage of these fusion proteins is that the activity of the Cre recombinase can be controlled in a temporal fashion by administration of TAM or other estrogen receptor agonists. In the absence of the agonist, the Cre protein is inactive even if expressed at high levels (29). These fusion proteins were used to create cardiac-specific transgenic mice as a means to temporally control gene deletions in the heart and to avoid complications resulting from constitutive expression of Cre throughout development as previously described (4). One widely used model is the Myh6-MerCreMer model in which the Cre fusion protein is expressed in the heart under the control of the human α-myosin heavy chain gene promoter (27). This model has been used to create temporal gene knockout in the heart to study the role of several proteins in the development of cardiac hypertrophy and to study the conduction system of the heart (2, 5, 12, 23). In these studies, control groups only consisted of non-Cre transgenic mice treated with the ligand (TAM) and did not include groups from Cre positive transgenic mice with one or zero copies of the loxP-targeted allele. Furthermore, cardiac function was not monitored daily during the TAM treatment protocol but only at time points from 1 wk to 1 mo following treatment (5, 23). In our use of the Myh6-MerCreMer (Cre+) mice, we noticed that upon activation of the MerCreMer protein with TAM, mice developed transient systolic dysfunction even in the absence of any loxP targeted genes. The main purpose of the present study was to determine the effect of Cre activation on cardiac systolic function using the previously established TAM-dosing method (2, 5, 12, 23).
METHODS
Mice.
Experiments were performed on male and female cardiac-specific inducible Myh6-MerCreMer (Cre) as well as in Cre− mice between 12 and 40 wk of age. Cre mice were obtained from Dr. Jeffrey Molkentin and bred onto a C57BL/6J background for at least six generations before use. Mice were fed a standard diet containing 0.29% NaCl and were provided water ad libitum. All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center. TAM (40 mg/kg) was prepared in ethanol and diluted with corn oil and administered by intraperitoneal injection (100 μl) on 1 (Cre+/1-day; n = 4), 3 (Cre+/3-day; n = 4), or 5 (Cre+/5-day; n = 5) consecutive days, while non-Cre mice were treated with TAM for 5-days (Cre−/5-day; n = 5). Mice were killed 12–14 days after the end of TAM administration.
Echocardiography.
Echocardiographic assessment of cardiac function was conducted using a Vevo 770 high resolution in vivo imaging system (Visualsonics, Toronto, ON, Canada). Measurements were made with a 710B RMV scanhead with a center frequency of 25 MHz and a frequency band ranging from 12.5 to 37.5 MHz. The probe used in these studies is a single-element, mechanical vector probe. Two baseline echocardiography sessions were conducted on each mouse before TAM injections were started. After TAM injection, echocardiography was performed on each mouse every other day until the end of the protocol. All echocardiographic data were obtained within a 20-min session and were made according to the American Society of Echocardiography Guidelines using the leading edge technique (24). Mice were anesthetized with 1.5% isoflourane gas and placed on a prewarmed pad and body temperature was monitored with a rectal probe. Heart rate was monitored via an EKG transducer pad throughout each echocardiography session. Two-dimensional B-mode parasternal long axis views were obtained first to visualize the aortic and mitral valves. The transducer was then rotated clockwise 90° to obtain the parasternal short axis view. At least 3 M-Mode images were obtained at the midpapillary muscle level from this view. Fractional shortening (FS; %) was analyzed and calculated using the Visualsonics advanced cardiovascular measurements package using the mean of 12–15 cardiac cycles derived from three separate M-mode images during each session. Left ventricular (LV) wall dimensions were determined from the M-mode images and included end diastolic dimension (LVEDD), end systolic dimension (LVESD), diastolic LV wall dimensions, and systolic LV wall dimensions.
ATP assay.
Mitochondrial ATP was determined in the hearts of mice following the 5-day TAM protocol. Following euthanization, hearts were minced with a razor blade and homogenized using a glass homogenizer in 1 ml of ice-cold buffer (220 mM mannitol, 70 mM sucrose, 0.5 mM EGTA, 2 mM HEPES, and 0.1% fatty acid-free BSA pH 7.4). The homegenate was centrifuged at 600 g with the resultant supernatant decanted and additionally centrifuged at 10,000 g with both spins at 4°C. Following the second centrifugation, the supernatant was collected with the addition of 5 μl of 10× phosphatase inhibitor (PhosSTOP; Roche) for anlysis of ATP levels and protein expression. The remaining mitochondrial pellet was gently resuspended in 100 μl of the ice-cold buffer. Each individual assay was carried out using 90 μl of reaction mixture from a commercially available ATP firefly-luciferase kit (ATP determination kit; Molecular Probes). A standard ATP curve was determined with each assay using dilutions recommended by the kit. Background luminescence was first determined, and each reaction was initiated with 10 μl of sample added to 90 μl of reaction mixture (100 μl total reaction volume). Determination of ATP mitochondrial content was made using duplicate measures of each sample. The protein concentration was determined by the Bradford assay to standardize the ATP levels to protein added.
Western blot analysis.
Western blots were performed on 35 μg of cytosolic fraction, and mitochondrial protein fractions were prepared as described for the above ATP assay. Cytosolic fractions were used to determine levels of total AMP-activated protein kinase-α (tAMPK-α; no. 2532; 1:500 dilution; Cell-Signaling Technology, Danvers, MA), phosphorylated-AMPK-α (pAMPK; no. 2531, 1:500 dilution; Cell-Signaling Technology), peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α; no. 4259; 1:500 dilution; Cell Signaling Technology) β-tubulin (T5293; 1:5000; Sigma-Aldrich, St. Louis, MO), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; ab8245; 1:10,000 dilution; Cell Signaling Technology), and these samples were resolved on a 10% Criterion Tris·HCl precast gel (Bio-Rad, Hercules, CA). Mitochondrial protein levels of pyruvate dehydrogenase kinase-4 (PDK-4; ab63157; 1:100 dilution; AbCam, Cambridge, MA) and Porin (ab40747, 1:5000, AbCam) were determined in the mitochondrial fraction resolved on a 12.5% Criterion Tris·HCl precast gel (Bio-Rad). The membranes were then incubated with Alexa 680 donkey anti-rabbit IgG (Invitrogen, Carlsbad, CA) or IRDye 800 donkey anti-mouse IgG (Rockland, Gilbertsville, PA) secondary antibodies for 1 h at room temperature and visualized using an Odyssey infrared imager (Li-COR, Omaha, NE), which allows for the simultaneous detection of two proteins. Densitometry analysis was performed using Odyssey software (LI-COR).
Histology.
Following euthanization, hearts were taken from mice, weighed, and fixed with 10% (vol/vol) Formalin. Heart sections were made and stained with hemotoxylin and eosin, periodic acid-Schiff (PAS), and picrosirius red (PSR).
Statistics.
The means ± SE were estimated using generalized linear mixed model fit with categorical group, time, and group-by-time interaction effects; days −2 and 0 were pooled into a “baseline” estimate in final model formulations after finding no significant differences between measurements on these 2 days (6). Primary models utilized linear-link functions and Gaussian response distributions, similar in nature to repeated-measures analysis of covariance. Sensitivity analyses, including log-link γ-distribution models for skewed outcomes and robust Huber-White variance estimates, were conducted to examine potential violations to these assumptions and results were similar in all cases. Reported P values use Bonferroni adjustments for a family-wide 5% error rate. When appropriate, Dunnett's test was used for multiple comparisons of group means with the mean of a control group to maintain an experiment-wise α-error rate of <5%. Power analyses were performed on FS and Western blot data based on previous data, and the number of mice used was calculated to achieve a power (1-β) of 0.8–0.85 and a probability of type I error (α) of <0.05. For FS, we use sufficient mice to detect a 25% difference in the means with an expected SD of 9%. For Western blot data, we used sufficient mice to detect a 40% difference in means with an expected SD of 20%.
RESULTS
LV systolic function.
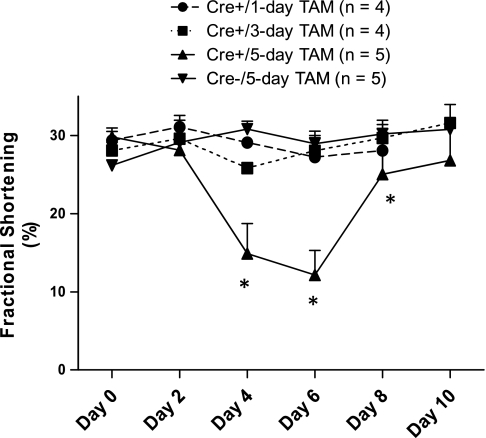
Systolic function data are summarized in Fig. 1. Days on the x-axis represent days following the beginning of TAM administration. Echocardiography demonstrated no statistically significant differences in LV systolic function at baseline in the four groups of mice as assessed by FS (Fig. 1). Overall, there was a significant difference between groups with significant time-groups interaction, due to decreased FS in Cre+/5-day mice as early as day 4 (P < 0.001 vs. Cre−) and persisting through day 8 (P < 0.05 Cre−). Mean FS levels returned to baseline levels 10 days after TAM initiation in Cre+/5-day mice. However, two of the five treated mice did not exhibit complete recovery of FS levels at day 8. No significant within-group decreases in any of the indexes of systolic function were observed in the Cre− mice or in Cre+/1-day and Cre+/3-day mice.
Fig. 1.
Systolic function as represented by fractional shortening following in 1-, 3-, or 5-day tamoxifen (TAM) treatment in animals expressing Myh6-MerCreMer (Cre+) or non-Cre (Cre−) mice. Day 0 is the average of the 2 baseline measurements and days 2, 4, 6, 8, and 10 represent days following the start of TAM administration. *P < 0.05 vs. Cre−/5-day mice.
LV wall dimensions.
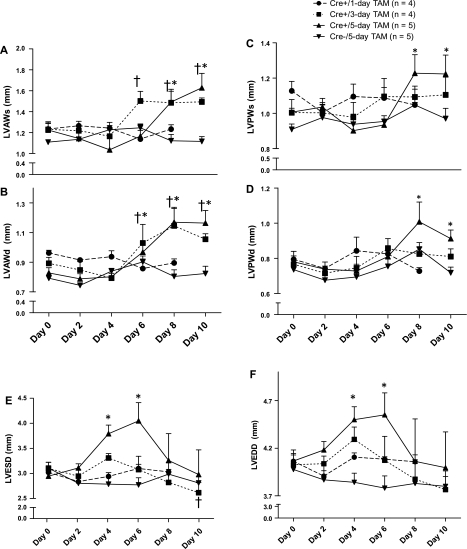
LV wall dimensions exhibited an initial decrease as mice developed LV systolic dysfunction but then exhibited a significant increase compared with baseline levels as LV systolic function improved (Fig. 2). M-mode measurements showed significant thickening of the LV of both the anterior (LVAWs and LVAWd) and posterior walls (LVPWs and LVPWd) of Cre+/5-day mice by day 8 and day 10 (P < 0.05 vs. Cre−). Cre+/3-day mice showed significant hypertrophy of the LVAWs/d at days 6 through 10 (P < 0.05 vs. Cre−). There were significant increases in LVESD and LVEDD (Fig. 2, E and F, respectively) for Cre+/5-day mice beginning at day 4 (P < 0.01 vs. Cre−) and continuing through day 6 (P < 0.05 vs. Cre−/5-day). Both LVESD and LVEDD returned to baseline 10 days after TAM was initiated for all groups. The heart weight-to-tibia length (HW/TL) ratio was utilized as an index of cardiac hypertrophy in mice euthanized at day 14 after the start of TAM treatment. Cre+/5-day mice exhibited a significant increase in HW/TL ratio compared with Cre−/5-day mice, averaging 87.6 ± 9.2 vs. 62.4 ± 3.4 mg/cm in Cre+/5-day vs. Cre−/5-day, respectively (n = 8 vs. n = 7). Cre+/3-day- and Cre+/1-day-treated mice did not exhibit a significant increase in HW/TL ratio compared with Cre−/5-day, averaging 78.5 ± 6.1 and 73 ± 3 mg/cm, respectively (n = 4).
Fig. 2.
Systolic and diastolic left ventricular dimensions for wall thickness and diameter obtained from M-mode measurements of echocardiography following in 1-, 3-, or 5-day tamoxifen treatment in animals expressing myosin heavy chain-MerCreMer or non-Cre controls. A: left ventricular anterior wall systole (LVAWs). B: left ventricular posterior wall systole (LVPWs). C: left ventricular anterior wall diastole (LVAWd). D: left ventricular posterior wall diastole (LVPWd). E: left ventricular end systolic diameter (LVESD). F: left ventricular end diastolic diameter (LVEDD). *P < 0.05 Cre+/5-day vs. Cre−/5-day mice. †P < 0.05 Cre+/3-day vs. Cre−/5-day mice.
Cardiac bioenergetics status.
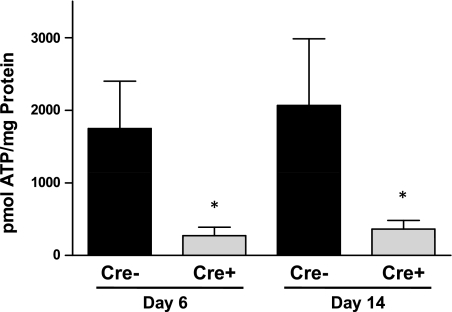
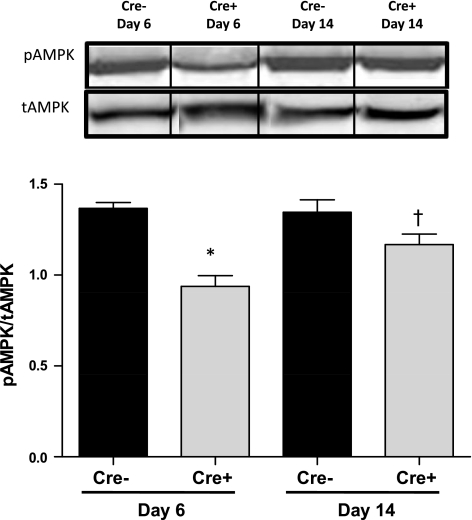
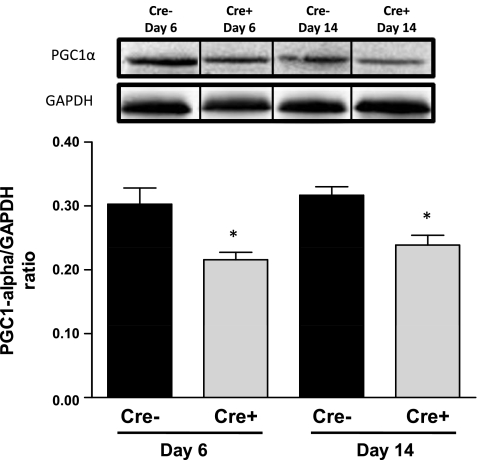
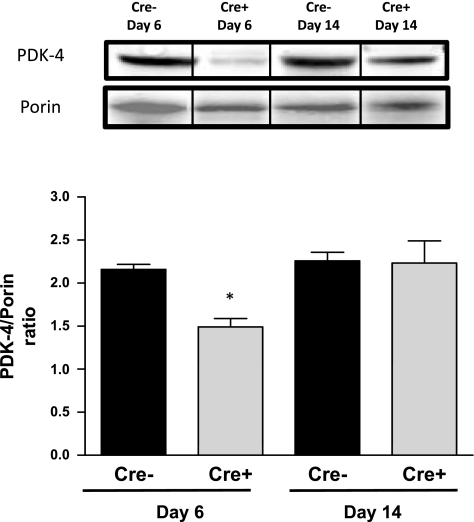
Cardiac mitochondrial ATP levels were measured in Cre+/5-day and Cre−/5-day mice at the nadir of systolic dysfunction at day 6 and following recovery of systolic function at day 14. Cardiac mitochondrial ATP was significantly depleted (P < 0.05 vs. Cre−) in Cre+ mice at both time points studied (Fig. 3). We also measured the levels of proteins important for the regulation of cardiac oxidative phosphorylation. A significant decrease in the levels of phosphorylated AMPK was observed in the hearts of Cre+/5-day mice at day 6 compared with both Cre−/5-day and Cre+/5-day after recovery at day 14 (Fig. 4). The loss of mitochondrial ATP was further confirmed by examination of PGC-1α protein expression in the hearts of Cre+/5-day mice at both days 6 and 14. PGC-1α was significantly reduced (P < 0.05 vs. Cre−) at both days 6 and 14 (Fig. 5). PDK-4 expression was significantly decreased at day 6 in Cre+/5-day mice (P < 0.05 vs. Cre−) but returned to levels similar to Cre− at day 14 (Fig. 6).
Fig. 3.
Cardiac mitochondrial ATP levels determined in Cre−/5-day (n = 6) and Cre+/5-day (n = 8) mice at systolic dysfunction nadir (day 6) and at systolic function recovery (day 14). *P < 0.05, compared with Cre−/5-day mice.
Fig. 4.
Cardiac phosphorylated (p)-AMPK/total (t)AMPK protein levels in Cre−/5-day (n = 4) and Cre+/5-day (n = 4) mice measured by Western blot analysis. *P < 0.05, compared with Cre−/5-day, day 6. †P < 0.05, compared with Cre+/5-day, day 6. Bars represent breaks in original image separating individual groups that were run in quadruplicate.
Fig. 5.
Cardiac peroxisome proliferator-activated receptor-γ coactivator-1α (PGC1-α) protein levels in Cre−/5-day (n = 4) and Cre+/5-day (n = 4) mice measured by Western blot analysis. *P < 0.05, compared with Cre−/5-day mice. Bars represent breaks in original image separating individual groups that were run in quadruplicate.
Fig. 6.
Cardiac mitochondrial pyruvate dehydrogenase kinase-4 (PDK-4) protein levels in Cre−/5-day (n = 4) and Cre+/5-day (n = 4) mice measured by Western blot analysis. *P < 0.05, compared with Cre−/5-day, day 6. Bars represent breaks in original image separating individual groups that were run in quadruplicate.
Cardiac cytokeletal and histological assessment.
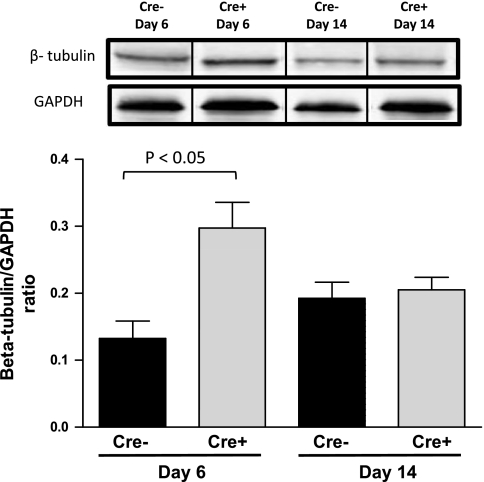
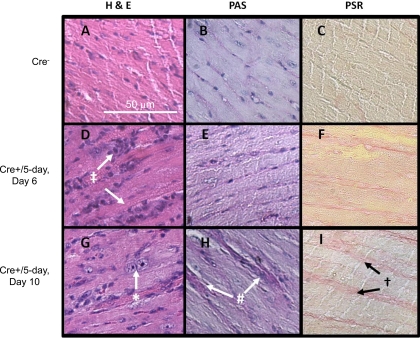
The levels of β-tubulin were examined by Western blot analysis in the hearts of Cre+ and Cre−/5-day mice at day 6 and day 14 following initiation of TAM treatment. Cre+/5-day mice demonstrated a significant increase (P < 0.05 vs. Cre−) in β-tubulin protein expression at the nadir of systolic function, which returned to levels similar to Cre−/5-day mice at the recovery of systolic function at day 14 (Fig. 7). Histological analysis of hearts from Cre+/5-day and Cre−/5-day is summarized in Fig. 8. Hemotoxylin and eosin staining reveals inflammatory cells in Cre+/5-day coinciding with systolic dysfunction, and nuclear chromatin is condensing following recovery of systolic function. After recovery of systolic function in Cre+/5-day, intracellular staining on PAS indicates some loss of cell membrane integrity accompanied by increased PSR staining. Cre+/5-day mice displayed signs of nuclear chromatin condensing, apoptosis, and necrosis that while relatively mild in nature are not seen in Cre− hearts.
Fig. 7.
Cardiac β-tubulin protein levels in Cre−/5-day (n = 4) and Cre+/5-day (n = 4) mice measured by Western blot analysis. P < 0.05, compared with Cre−/5-day, day 6. Bars represent breaks in original image separating individual groups that were run in quadruplicate.
Fig. 8.
Cardiac histology from Cre+/5-day and Cre−/5-day mice at day 6 and day 10 post-TAM treatment. A, D, and G: hemotoxylin and eosin (H&E) staining at ×200 in untreated Cre− (A); Cre+/5-day, day 6 (D); and Cre+/5-day, day 10 (G). B, E, and H: periodic acid-Schiff (PAS) staining at ×200 in untreated Cre− (B); Cre+/5-day, day 6 (E); and Cre+/5-day, day 10 (H). C, F, and I: picrosirius red (PSR) staining at ×200 in untreated Cre− (C); Cre+/5-day, day 6 (F); and Cre+/5-day, day 10 (I). H&E staining reveals inflammatory cells seen on day 6 (‡) and condensed nuclear chromatin noted at Systolic function recovery (*). At day 10, intracellular staining on PAS (#) and appearance of increase collagen on PSR (†).
DISCUSSION
An important finding of this study is that there is a transient, severe reduction in systolic function in mice expressing a ligand-inducible Cre recombinase (Myh6-MerCreMer-Cre+) without any specific loxP target sequence using a previously described and widely utilized TAM treatment protocol consisting of 5 consecutive days of 40 mg/kg TAM injections (2, 5, 12, 23, 27). Despite this significant transient impairment of systolic function, mice were able to recover normal function by day 10 post-TAM treatment. However, this recovery in systolic function was associated with thickening of the anterior and posterior LV walls and an increase in HW/TL ratio. We did not observe any significant impact on systolic function in Cre+ mice treated with TAM for 1 or 3 days or in Cre− mice treated with TAM for 5 days. The LV hypertrophy evident in the Cre+/5-day-treated mice is representative of adaptive or compensatory hypertrophy that occurs in response to high end diastolic pressures and increased ventricular wall stress that accompany acute systolic heart failure. This compensatory wall remodeling helps to maintain normal cardiac output despite impairment of cardiac contractile function.
Cardiac-specific Cre activation utilizing the 5-day TAM treatment protocol results in the development of a dilated cardiomyopathy that occurs in a more severe and rapid fashion than other models of acute systolic dysfunction such as doxorubicin cardiotoxicity (13). The Cre-mediated “cardiotoxicity” is a transient phenomenon with full recovery of systolic function in part due to the compensatory LV hypertrophy. The transient nature of the cardiac dysfunction that occurs with inducible Cre activation in this model could easily be missed unless investigators have a rigorous schedule of echocardiography in which measurements are made at least every other day as was done in the present study. Several other studies have reported similar cardiac hypertrophy after gene deletion using a similar protocol to activate Cre in the heart (5, 23). Whether the subsequent cardiac hypertrophy was due to loss of the specific loxP-targeted gene or the effect of Cre activation was not known, since experiments in Cre+ control mice were not performed in these previous studies. The acute effect of Cre activation on systolic function in these models was also not examined; however, it was reported that loss of dicer was associated with a significant decline in systolic function 1 wk after TAM administration in adult mice (5). These previous reports highlight the importance of studying Cre+ control mice as well as performing careful, daily analysis of cardiac function during the Cre activation period.
Our observations also provide insight into the etiology of the Cre-induced cardiomyopathy. One of the major phenotypes of this model is the alteration in cardiac bioenergetics with a metabolic phenotype similar to that observed in ischemic or idiopathic heart failure. Under normal resting conditions, >90% of ATP produced by the heart is generated in the mitochondria, and fatty acid oxidation accounts for ∼60 to 90% of the ATP synthesized. It is now widely accepted that in cardiac pathologies such as heart failure, there is an initial shift in substrate preference away from fatty acids to glucose metabolism in an effort by the heart to maintain energy levels as myocardial ATP is depleted (14, 18, 19). Our data demonstrate that there is significant depletion of cardiac mitochondrial ATP in Cre+/5-day animals at the nadir in systolic function, but this is not accompanied by increases in AMPK signaling as indicated by decreases pAMPK-to-AMPK ratio. After recovery of systolic function at day 14, cardiac mitochondrial ATP levels were still low despite a significant increase in pAMPK-to-AMPK ratio. The persistent decrease in cardiac mitochondrial ATP levels observed in Cre+/5-day-treated mice may reflect a shift in metabolic and substrate preference away from oxidative phosphorylation toward anaerobic metabolism. This hypothesis is supported by measurement of PGC-1α in the hearts of Cre+/5-day-treated mice, which revealed that PGC-1α protein levels were significantly decreased at the systolic dysfunction nadir and after recovery of systolic function. PGC-1α acts with its coactivator, peroxisome proliferator-activated receptor-γ, to increase expression of genes necessary for mitochondrial biogenesis and oxidative phosphorylation and decrease gene expression involved in anaerobic, glycolytic pathways (28). Our data are consistent with this observation, and mRNA levels for PGC-1α are reduced in heart failure (1, 15). However, PGC-1α did not return to control levels even after systolic function recovery, a finding that could be due to the short recovery time interval of our protocol.
Further support for the loss of oxidative phosphorylation and enhancement of the glycolytic pathway in the hearts of Cre+/5-day-treated mice is the decrease in PDK-4 protein levels at the nadir of systolic function. Decreases in PDK-4 levels are associated with enhancement of glycolysis and glucose oxidation, as observed in myocardial ischemia and other models of heart failure (7). The reductions in both PGC1-α and PDK-4 as well as the decreased levels of mitochondrial ATP levels suggest that recovery of systolic function in Cre+/5-day mice may occur in part due to enhanced energy production through the glycolytic pathway; however, such conclusions require further direct measurements of cardiac substrate preference in this model.
Histological examination of the hearts from Cre+/5-day mice revealed infiltration of mononuclear inflammatory cells, which was similar to the pattern of inflammatory cell infiltration previously observed in this model (15). Further evidence for increased inflammation in the hearts of the Cre+/5-day mice is noted in the PAS staining, which shows some loss of cell membrane integrity as reflected by intracellular staining of myocytes. After recovery of systolic function, we observed increased PSR staining, which indicates increased collagen deposition characteristic of LV hypertrophy and remodeling.
We also found increased protein levels of β-tubulin at the nadir of systolic dysfunction. Previous studies (10, 11) have shown that beta tubulin increases in animal models of heart failure, and it is thought that the accumulation of cytoskeletal proteins like tubulin can reduce contractile function of the heart through interfering with contractile proteins or depleting energy reserves by utilizing ATP for synthesis. Increases in cytoskeletal proteins such as tubulin, however, may be a compensatory mechanism of the cardiomyocyte to adapt to the cellular disruption that occurs in response to increases in diastolic filling pressure and wall stress associated with Cre activation and transient systolic dysfunction in this model (16). Further studies are needed to more fully assess the role of cytoskeletal proteins in the development of systolic dysfunction in this model.
We determined the effect of TAM dosing on the development of transient systolic dysfunction in Cre+ mice. We did not find any significant consequence of either 1- or 3-day TAM treatment on systolic function in Cre+ mice. This suggests that a specific level of nuclear Cre activation is required before effects on systolic function are observed in mice lacking any known loxP sites. We observed that treatment of Cre− mice with TAM for 5 days failed to have any effect on systolic function, which indicates that TAM itself does not cause systolic dysfunction. However, we cannot rule out the possibility that TAM coupled with nuclear localization of the Cre recombinase contributes to the development of cardiotoxicity observed in the present study.
A recent report (15) demonstrated that activation of Cre with raloxifene, an alternative estrogen receptor activator, did not result in acute or chronic alterations in systolic function despite similar Cre activation. However, raloxifene had to be administered three times as long as TAM to achieve similar levels of nuclear localization of Cre as observed with TAM. There are several potential strategies that could be utilized to avoid Cre-mediated cardiotoxicity, including alterations in the dose of TAM, the frequency of administration, as well as the route of administration. To this end, effective cardiac-specific deletion of the SERCA2 gene was reported following two intraperitoneal injections of TAM at a dose of 80 mg/kg as well as oral administration of TAM via cakes or as nonpelleted feed (3). While both of these methods were effective in deleting SERCA2 levels in the hearts, assessment of cardiac function was not performed in the study, so it is difficult to determine if the mice exhibited the transient loss of systolic function observed in the current study. An alternative approach that has recently been reported is to regulate Cre expression with a reverse tetracycline transactivator (rTtA) specifically expressed in the heart (30). With this approach, Cre expression is inhibited by doxycycline that is continuously fed to the mouse either in the food or drink. Once doxycycline is removed, Cre expression is activated. Cardiac-specific elimination of the ClC-3 gene with this approach was reported to cause cardiac hypertrophy and heart failure (30). However, whether these effects were a result of specific loss of ClC-3 or Cre-mediated cardiotoxicity is not known, since experiments were not performed in mice in which only Cre was activated. The effectiveness of this model can only be validated once similar experiments are performed to control for the effects of Cre activation in the heart.
There are some limitations of the present study, including a lack of direct measurement of ventricular pressures in the Cre+/5-day-treated mice. However, direct measurements of ventricular pressures are typically performed in acute experiments in anesthetized mice, which would have precluded performing serial measurements of cardiac function, an essential feature of the experimental design that permitted us to detect the transient effects of Cre induction by 5-day TAM treatment on systolic function. We measured LV dimensions and FS from measurements derived from two-dimensional guided M-mode echocardiography instead of using two-dimensional area measurements or other methods such as Simpson's biplane method. We chose to use M mode for measurements of LV chamber dimensions, which were subsequently used to calculate FS; this is a commonly reported echocardiography modality in rodents due to M-mode's high temporal resolution and M-mode measurements of LV chamber dimensions allow for lower inter- and intraobserver variability of diameter measurements (31).
In summary, our results demonstrate that cardiac-specific induction of Cre recombinase in Myh6-MerCreMer mice, employing a widely used protocol (5 days of injection of TAM at a dose of 40 mg/kg), results in severe, transient systolic dysfunction associated with depletion of cardiac mitochondrial ATP content. Mice eventually recover systolic function after undergoing compensatory LV hypertrophy. Our results suggest that methods of Cre activation with TAM should be used in concert with careful monitoring of systolic function and cardiac hypertrophy to avoid any potential nonspecific effects of Cre activation in the heart that may not be apparent with measurements made 10 days or more after Cre activation. Experiments utilizing inducible cardiac-specific knockout with Myh6-MerCreMer mice should always contain ligand-treated Cre-positive loxP-deficient mice as a control for the nonspecific effects of Cre activation in the heart.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant PO1-HL-51971, as well as a seed grant from the American Medical Association (to M. E. Hall).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Michael Griswold, Biostatistics Center, University of Mississippi Medical Center, for statistical assistance, and Dr. Jeffrey D. Molkentin, Cincinnati Children's Hospital, for the Myh6-MerCreMer transgenic mice.
REFERENCES
- 1. Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest 100: 169–179, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andersson KB, Birkeland JA, Finsen AV, Louch WE, Sjaastad I, Wang Y, Chen J, Molkentin JD, Chien KR, Sejersted OM, Christensen G. Moderate heart dysfunction in mice with inducible cardiomyocyte-specific excision of the Serca2 gene. J Mol Cell Cardiol 47: 180–187, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Andersson KB, Winer LH, Mork HK, Molkentin JD, Jaisser F. Tamoxifen administration routes and dosage for inducible Cre-mediated gene disruption in mouse hearts. Transgenic Res 19: 715–725, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Buerger A, Rozhitskaya O, Sherwood MC, Dorfman AL, Bisping E, Abel ED, Pu WT, Izumo S, Jay PY. Dilated cardiomyopathy resulting from high-level myocardial expression of Cre-recombinase. J Card Fail 12: 392–398, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Costa Martins PA, Bourajjaj M, Gladka M, Kortland M, van Oort RJ, Pinto YM, Molkentin JD, De Windt LJ. Conditional dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation 118: 1567–1576, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Diggle PJ, Heagerty P, Liang KY, Zeger SL. The Analysis of Longitudinal Data. Oxford, England: University Press, 2002 [Google Scholar]
- 7. Doenst T, Pytel G, Schrepper A, Amorim P, Farber G, Shingu Y, Mohr FW, Schwarzer M. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovasc Res 86: 461–470, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P. Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci USA 93: 10887–10890, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun 237: 752–757, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Gupta A, Gupta S, Young D, Das B, McMahon J, Sen S. Impairment of ultrastructure and cytoskeleton during progression of cardiac hypertrophy to heart failure. Lab Invest 90: 520–530, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Hein S, Kostin S, Heling A, Maeno Y, Schaper J. The role of the cytoskeleton in heart failure. Cardiovasc Res 45: 273–278, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Hoesl E, Stieber J, Herrmann S, Feil S, Tybl E, Hofmann F, Feil R, Ludwig A. Tamoxifen-inducible gene deletion in the cardiac conduction system. J Mol Cell Cardiol 45: 62–69, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Kang YJ, Chen Y, Epstein PN. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. J Biol Chem 271: 12610–12616, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, Iwanaga Y, Narazaki M, Matsuda T, Soga T, Kita T, Kimura T, Shioi T. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Heart Fail 3: 420–430, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, Takimoto E, Kass DA. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circ Res 105: 12–15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kostin S, Hein S, Arnon E, Scholz D, Schaper J. The cytoskeleton and related proteins in the human failing heart. Heart Fail Rev 5: 271–280, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Loonstra A, Vooijs M, Beverloo HB, Allak BA, van Drunen E, Kanaar R, Berns A, Jonkers J. Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc Natl Acad Sci USA 98: 9209–9214, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev 90: 207–258, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Luptak I, Yan J, Cui L, Jain M, Liao R, Tian R. Long-term effects of increased glucose entry on mouse hearts during normal aging and ischemic stress. Circulation 116: 901–909, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Metzger D, Clifford J, Chiba H, Chambon P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc Natl Acad Sci USA 92: 6991–6995, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Minamino T, Gaussin V, DeMayo FJ, Schneider MD. Inducible gene targeting in postnatal myocardium by cardiac-specific expression of a hormone-activated Cre fusion protein. Circ Res 88: 587–592, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Naiche LA, Papaioannou VE. Cre activity causes widespread apoptosis and lethal anemia during embryonic development. Genesis 45: 768–775, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Parlakian A, Charvet C, Escoubet B, Mericskay M, Molkentin JD, Gary-Bobo G, De Windt LJ, Ludosky MA, Paulin D, Daegelen D, Tuil D, Li Z. Temporally controlled onset of dilated cardiomyopathy through disruption of the SRF gene in adult heart. Circulation 112: 2930–2939, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Lang RM, Biering M, Devereux RB, Flakschampf FA, Foster E, Pellikka PA, Picard MH, Roman MA, Seward J, Shanewise JA, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Society of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Sauer B, Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci USA 85: 5166–5170, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sauer B, Henderson N. Targeted insertion of exogenous DNA into the eukaryotic genome by the Cre recombinase. New Biol 2: 441–449, 1990 [PubMed] [Google Scholar]
- 27. Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res 89: 20–25, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85: 1093–1129, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Verrou C, Zhang Y, Zurn C, Schamel WW, Reth M. Comparison of the tamoxifen regulated chimeric Cre recombinases MerCreMer and CreMer. Biol Chem 380: 1435–1438, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Xiong D, Heyman NS, Airey J, Zhang M, Singer CA, Rawat S, Ye L, Evans R, Burkin DJ, Tian H, McCloskey DT, Valencik M, Britton FC, Duan D, Hume JR. Cardiac-specific, inducible ClC-3 gene deletion eliminates native volume-sensitive chloride channels and produces myocardial hypertrophy in adult mice. J Mol Cell Cardiol 48: 211–219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scherrer-Crosbie M, Kurtz B. Ventricular remodeling and function: insights using murine echocardiography. J Mol Cell Cardiol 48: 512–517, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]