Abstract
In the heart, nitric oxide (NO) modulates contractile function; however, the mechanisms responsible for this effect are incompletely understood. NO can elicit effects via a variety of mechanisms including S-nitrosylation and stimulation of cGMP synthesis by soluble guanylate cyclase (sGC). sGC is a heterodimer comprised of a β1- and an α1- or α2-subunit. sGCα1β1 is the predominant isoform in the heart. To characterize the role of sGC in the regulation of cardiac contractile function by NO, we compared left ventricular cardiac myocytes (CM) isolated from adult mice deficient in the sGC α1-subunit (sGCα1−/−) and from wild-type (WT) mice. Sarcomere shortening under basal conditions was less in sGCα1−/− CM than in WT CM. To activate endogenous NO synthesis from NO synthase 3, CM were incubated with the β3-adrenergic receptor (β3-AR) agonist BRL 37344. BRL 37344 decreased cardiac contractility in WT CM but not in sGCα1−/− myocytes. Administration of spermine NONOate, an NO donor compound, did not affect sarcomeric shortening in CM of either genotype; however, in the presence of isoproterenol, addition of spermine NONOate reduced sarcomere shortening in WT but not in sGCα1−/− CM. Neither BRL 37344 nor spermine NONOate altered calcium handling in CM of either genotype. These findings suggest that sGCα1 exerts a positive inotropic effect under basal conditions, as well as mediates the negative inotropic effect of β3-AR signaling. Additionally, our work demonstrates that sGCα1β1 is required for NO to depress β1/β2-AR-stimulated cardiac contractility and that this modulation is independent of changes in calcium handling.
Keywords: cyclic-3′,5′-guanosine monophosphate; β3-adrenergic receptor; cardiac contractility; nitric oxide; soluble guanylate cyclase
nitric oxide (no) modulates cardiac contractile function. However, the precise mechanisms by which NO impacts cardiac contractility remain to be determined. In cardiac myocytes (CM), NO is generated by neuronal, inducible, and endothelial NO synthases (NOS1, NOS2, and NOS3, respectively). NO binds to and activates soluble guanylate cyclase (sGC) to synthesize the second messenger molecule cGMP. cGMP signals by activating cGMP-dependent protein kinase (PKG), as well as by modulating the activity of cGMP-regulated phosphodiesterases (PDEs; Refs. 9, 17). Cyclic nucleotides are degraded by PDEs, some of which are cGMP specific, such as PDE5 (9, 17, 25). The effects of NO may be sGC dependent, but NO has also been suggested to regulate cardiac contractile function by cGMP-independent mechanisms, including by nitrosylation of protein thiol groups (13).
Previous studies (19, 22, 33, 41, 50, 51) have demonstrated that NO, endogenously produced by NOS or released from NO-donor compounds, can attenuate the ability of β1/β2-adrenergic receptor (β1/β2-AR) agonists to augment cardiac contractility. In the heart, β3-adrenergic receptor (β3-AR) signaling activates NOS3 and inhibition of NOS3 blocks β3-AR signaling (11). BRL 37344, a β3-AR agonist, exerts a negative inotropic effect on ventricular myocardium through the activation of endogenous NOS3 and has been suggested to signal via sGC (11, 12, 23). In isolated myocytes, high NO levels appear to elicit negative inotropic effects under basal conditions, while low levels of NO have been associated with positive inotropic effects (20, 21, 27).
Whether alterations in calcium levels or myofilament sensitivity to calcium are responsible for the NO/cGMP-mediated impact on contractile function is controversial. The amplitude of the calcium transient has been reported to either decrease or remain unchanged after challenge with NO in the presence of β1/β2-AR stimulation in isolated CM (8, 49, 51). Alternatively, phosphorylation of the myofilament protein troponin I, possibly by PKG, has been suggested to be responsible for the ability of NO and cGMP to decrease calcium sensitivity (23, 37).
The specific intracellular compartment where cGMP is generated and metabolized appears to be critical for its impact on cardiac contractility (28, 42). However, the intracellular location of sGC in CM has not been demonstrated. Of the two heterodimeric sGC isoforms, sGCα1β1 appears to be the predominant form expressed in CM (4, 26). We (5) have previously generated mice deficient in sGCα1 on an SV129 genetic background (sGCα1−/−), and we reported that both basal and NO-stimulated sGC enzyme activity of the left ventricle were greatly diminished in sGCα1−/− mice. Male sGCα1−/− mice are hypertensive and exhibit increased left ventricular (LV) systolic function, as well as delayed LV relaxation (5).
The aim of the current study was to further elucidate the role of sGC in cardiac contractile function. We isolated CM from wild-type (WT) and sGCα1−/− mice and compared the contractile properties of WT and sGCα1−/− CM under baseline conditions, as well as after NO stimulation. Because sGCα1−/− mice exhibited enhanced cardiac inotropy in vivo, we hypothesized that CM from mice with reduced sGC activity would exhibit increased contractile function (5). Surprisingly, sGCα1 deficiency was associated with impaired sarcomere shortening under basal conditions. Our data furthermore suggest that activation of the β3-AR depresses cardiac contractility via the NOS3/sGC pathway. Finally, we report that the ability of NO to depress β1/β2-AR cardiac contractility requires sGCα1 without alterations in calcium handling.
MATERIALS AND METHODS
Reagents.
Spermine NONOate (Enzo Life Sciences, Plymouth Meeting, PA) was dissolved in ice-cold 10 mM NaOH and kept on ice. Before use, spermine NONOate was preincubated at 37°C for 45 min at a final concentration of 100 μM. BRL 37344, nadolol, Bay 41–2272, and dl-isoproterenol (ISO) HCl were purchased from Sigma-Aldrich (St. Louis, MO). 1H-(1,2,4)oxadiazolo(4,3-a)-quinoxalin-1-one (ODQ) was obtained from Enzo Life Sciences, and nitro-l-arginine methyl ester (l-NAME) was purchased from Acros Organics (Morris Plains, NJ). DT-2 was purchased from Axxora (San Diego, CA).
Isolation of adult mouse CM.
The animal experimental protocol was approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital, which conforms to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996). CM were isolated from 8- to 12-wk-old male WT SV129 mice (Taconic Farms, Germantown, NY) and from male and female sGCα1−/− mice generated on an SV129 background (5). The animals were heparinized and anesthetized with 100 mg/kg of pentobarbital sodium. The heart was rapidly excised and placed in ice-cold calcium-free Tyrode buffer consisting of 137 mM NaCl, 5.4 mM KCl, 0.5 mM MgCl2, and 10 mM HEPES (pH 7.4) and supplemented with 10 mM d-glucose, 10 mM 2,3-butanedione monoxime, and 5 mM taurine. LV myocytes were isolated by Langendorff perfusion, as previously described (24). CM were transferred through a series of increasing calcium solutions to a final concentration of 1.2 mM CaCl2.
Immunocytochemistry of adult murine CM.
Freshly isolated CM were fixed and permeabilized with a solution of 2% paraformaldehyde/0.75% Triton X-100. Antibodies directed against sGCβ1 (1:1,000, ab5033, rabbit) or α-actinin (1:1,000, ab18061, mouse) were purchased from AbCam (Cambridge, MA). sGCα1 antibody was a gift from S. Janssens and was used at 1:100 (36). Bound primary antibodies were detected with DyLight 594-conjugated AffiniPure donkey anti-rabbit IgG (1:100) or FITC-conjugated AffiniPure donkey anti-rabbit IgG. (1:50; both from Jackson Immunochemicals). Cells were visualized with a Nikon E800 microscope equipped with a Bio-Rad Radiance 2000 confocal head.
Imaging of sarcomere length and calcium transients in CM.
Freshly isolated CM were loaded with 1 μM fura-2 AM (Molecular Probes) in Tyrode buffer for 20 min at 25°C. CM were perfused with Tyrode buffer supplemented with 1.2 mM CaCl2, 5.5 mM d-glucose, and 0.5 mM probenecid and maintained at 35–37°C. Cells were field stimulated at 1, 2, 4, and 6 Hz by a MyoPacer Field Stimulator (IonOptix, Milton, MA). Sarcomere length data were acquired by SarcLen software (IonOptix), and intracellular calcium ratio data were acquired using a dual-excitation fluorescence photomultiplier system (Hyperswitch Light Source; IonOptix). Sarcomere length and calcium transient data were analyzed with IonWizard software.
RESULTS
sGCα1 localizes to the sarcomeric Z-line in CM.
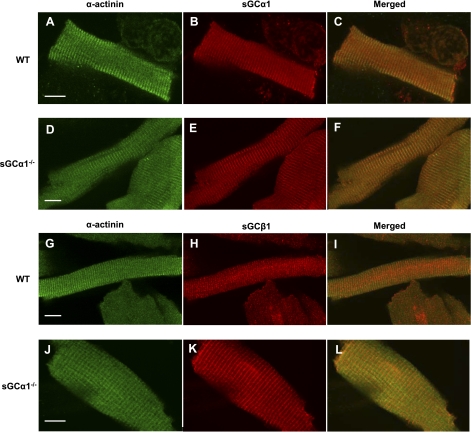
The sarcomeres of CM are organized into repeating myofibrillar structures: the Z-line, and the I-, M-, and A-bands. To determine where sGC resides within CM, we performed immunocytochemistry using antibodies directed against the α1- and β1-subunits of sGC and visualized the cells using confocal microscopy. Coimmunostaining with antibodies directed against sGCα1 and α-actinin revealed that sGCα1 localizes to the sarcomeric Z-line in WT CM (Fig. 1, A–C). The sGCα1−/− mice were generated by deleting the sixth exon of the sGCα1 gene (5). The expressed, nonfunctional mutant enzyme remains localized to the Z-line in CM (Fig. 1, D–F). These results suggest that sequences other than those encoded by the sixth exon are responsible for sGCα1 localization. Similarly, sGCβ1, the obligate binding partner of sGCα1, also colocalized to Z-lines in CM isolated from WT or sGCα1−/− mice (Fig. 1, G-L). Taken together, these results suggest that sGCα1β1 localizes to the compartment previously identified to contain NOS3 and PDE5 in CM (35, 43).
Fig. 1.
Localization of soluble guanylate cyclase-α1 (sGCα1) in cardiac myocytes (CM). CM isolated from wild-type (WT; A–C and G–I) and sGCα1−/− (D–F and J–L) mice were reacted with primary antibodies recognizing sGCα1 (B and E), sGCβ1 (H and K), and α-actinin (A, D, G, and J), and bound antibody was visualized by confocal microscopy using fluorescently labeled secondary antibodies. α-Actinin was detected with FITC-labeled anti-mouse IgG (green), and sGCα1 and sGCβ1 were detected using Dylight 594-labeled anti-rabbit IgG (red). Merged view (C, F, I, and L) demonstrates that sGCα1 colocalizes with α-actinin on Z-lines. Scale bars (white) = 10 μm.
sGCα1 deficiency decreases contractile function in a calcium-independent manner.
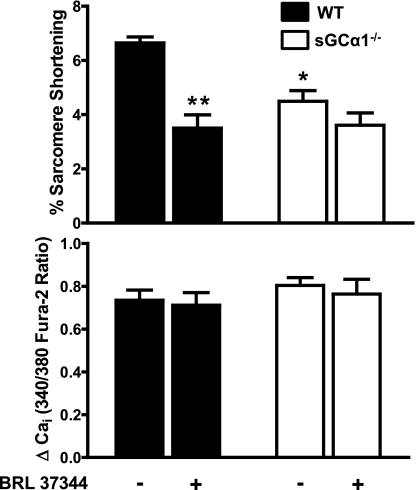
To determine if basal sGCα1β1 activity is important for contractile function, isolated CM from WT and sGCα1−/− mice were electrically stimulated at various pacing frequencies, and sarcomere lengths and calcium transients were recorded and analyzed. CM from sGCα1−/− mice shortened 32% less than CM from WT mice at a pacing frequency of 2 Hz (P < 0.05; Fig. 2, top). Despite the defect in sarcomeric shortening, the amplitude of the calcium transients was similar in CM from both genotypes (Fig. 2, bottom).
Fig. 2.
Effect of BRL 37344 on WT and sGCα1−/− CM contractility and calcium handling. %Sarcomere shortening (top) and calcium transient amplitude (ΔCai; bottom) are shown for WT and sGCα1−/− CM incubated in the absence and presence of BRL 37344 (1 nM). CM were incubated for 10 min and perfused with Tyrode buffer containing 1 nM of the β3-AR agonist BRL 37344. Cells were paced at 2 Hz. *P < 0.001 for WT BRL vs. WT basal. **P < 0.01 for sGCα1−/− basal vs. WT basal.
We considered the possibility that the impaired sarcomere shortening observed in CM from male sGCα1−/− was attributable to the hypertension seen in these mice. To examine this hypothesis, we studied CM from female sGCα1−/− mice that do not have hypertension. CM from normotensive female sGCα1−/− also exhibited a decreased ability to shorten compared with WT CM, indicating that the contractile defect in the sGCα1−/− mice is due to deficiency of sGCα1 and not due to secondary effects of hypertension on the heart (Supplemental Fig. S1; Supplemental Material for this article is available online at the Am J Physiol Heart Circ Physiol website). These results suggest that under basal conditions sGC activity exerts a positive inotropic effect in isolated CM.
Activation of sGC by NOS3-derived NO in CM decreases contractility.
To test whether sGCα1β1 mediates the effect of NO on cardiac contractility, we activated endogenous NOS3 in WT and sGCα1−/− CM by stimulating the β3-AR with BRL 37344 (1 nM). BRL 37344 diminished sarcomere shortening by 47% in WT cells (P < 0.01; Fig. 2, top). In contrast, BRL 37344 did not decrease sarcomere shortening in sGCα1−/− CM. The BRL 37344-induced decrease in sarcomere shortening in WT cells occurred without changes in the magnitude of calcium transients, indicating that BRL 37344 influences cardiac contractility without altering calcium flux (Fig. 2, bottom).
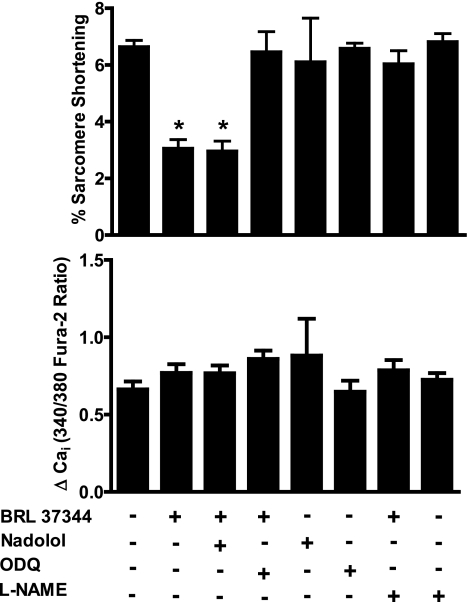
To confirm that the effect of BRL 37344 on sarcomere shortening was independent of β1/β2-AR signaling, we tested the effect of the β1/β2-AR antagonist nadolol on sarcomere shortening, both in the absence and presence of BRL 37344. Nadolol (10 μM) alone had no effect on sarcomere shortening in WT CM. In CM treated with both BRL 37344 (1 nM) and nadolol, the sarcomere shortening was indistinguishable from cells treated with BRL 37344 alone (Fig. 3, top), indicating β1/β2-AR activation did not modulate the observed sarcomere shortening response to BRL 37344.
Fig. 3.
Effect of nadolol, nitro-l-arginine methyl ester (l-NAME), and 1H-(1,2,4)oxadiazolo(4,3-a)-quinoxalin-1-one (ODQ) on contractile function and calcium handling after β3-adrenergic receptor stimulation in WT CM. %Sarcomere shortening (top) and calcium transient amplitude (bottom) from WT CM incubated with BRL 37344 and 10 μM nadolol, 10 mM l-NAME, or 10 μM ODQ and paced at 2 Hz. *P value of < 0.01 for indicated groups vs. WT basal in Bonferroni posttests.
To confirm that BRL 37344 depressed contractility through activation of NOS and sGC, we examined the effects on CM contractility of l-NAME, a nonselective NOS inhibitor, as well as ODQ, an inhibitor of sGC. Incubation of WT CM with l-NAME (10 μM) prevented BRL 37344 from decreasing sarcomere shortening, confirming that BRL 37344 depressed contractility by activating NOS (Fig. 3, top). Similar to sGCα1 deficiency, ODQ prevented the decrease in sarcomere shortening in response to BRL 37344 (Fig. 3, top). Taken together, these results confirm that β3-AR signaling requires NOS3 and sGC to attenuate cardiac contractility. Of note, incubation of CM with BRL 37344, nadolol, l-NAME, or ODQ did not alter Ca2+ transients (Fig. 3, bottom). These results indicate that NO/cGMP signaling impacts cardiac contractility independently of calcium handling.
sGC activity inhibits β1/β2-AR-stimulated contractile function in CM.
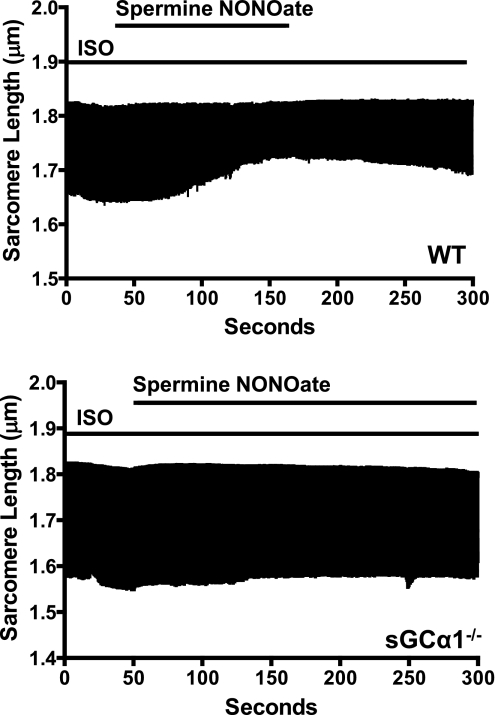
To evaluate the role of sGCα1 in the ability of NO to modulate β1/β2-AR-stimulated contractile function, we studied the impact of NO on WT and sGCα1−/− CM in the absence and presence of ISO. In the absence of ISO, perfusion of WT CM with 100 μM spermine NONOate did not alter sarcomere shortening (data not shown). Sarcomeric shortening and Ca2+ transients were similar in WT and sGCα1−/− CM in the presence of 10 nM ISO. This dose of ISO produced a submaximal increase in sarcomere shortening (data not shown) and was previously used to reveal the impact of NO/cGMP on CM function (43). In WT cells, perfusion with the NO donor compound elicited a dramatic reduction of ISO-stimulated sarcomere shortening. The maximal decrease (39%) in sarcomere shortening occurred at 150 ± 30 s after addition of the NO donor compound. As confirmation that the change in contractile function was due to perfusion of spermine NONOate, sarcomere contraction increased rapidly upon removal of the NO donor compound (Fig. 4, top). However, in sGCα1−/− CM, perfusion with spermine NONOate did not alter sarcomere shortening in cells stimulated with ISO (Fig. 4, bottom). The absence of a response to NO in sGCα1−/− CM suggests that sGCα1 is required for NO to decrease β1/β2-AR-stimulated myocardial contractility.
Fig. 4.
Effect of nitric oxide on β-adrenergic receptor-stimulated sarcomere shortening in WT and sGCα1−/− CM. Representative tracing demonstrating time-dependent change in sarcomere length of a single isoproterenol (ISO)-stimulated, paced WT (top) and sGCα1−/− (bottom) CM during perfusion with 100 μM spermine NONOate.
To confirm that sGC activity is able to decrease β1/β2-AR-stimulated cardiac contractility, CM were treated with the sGC activator Bay 41–2272 (39, 40). In ISO-stimulated WT CM, perfusion with either 5 or 10 μM Bay 41–2272 reduced sarcomere shortening without altering Ca2+ transients. Bay 41–2272 did not decrease sarcomere shortening in ISO-stimulated myocytes from sGCα1−/− mice (Supplemental Fig. S2), confirming that sGCα1β1 is able to attenuate β1/β2-AR-stimulated myocardial contractility.
The muscarinic-cholinergic receptor 2 (M2-Chr), the predominant muscarinic receptor in the heart, exerts negative inotropic effects via pertussis toxin-sensitive Gi/o proteins. Activation of Gi/o inhibits adenylate cyclase and lowers cAMP levels leading to decreased contractility (10, 15). To demonstrate that ISO-stimulated sarcomere shortening could be decreased in both genotypes by a cGMP-independent mechanism, we administered the muscarinic-cholinergic agonist carbachol (10 μM) to WT and sGCα1−/− CM incubated with ISO. In contrast to our experiments using an NO donor compound, carbachol decreased sarcomere shortening in both WT and sGCα1−/− myocytes (Supplemental Fig. S3). These results suggest that sGCα1−/− myocytes retain the ability to decrease contractility in response to negative inotropic signals from a cGMP-independent pathway.
PKG is required for the NO-induced decrease in cardiac contractility.
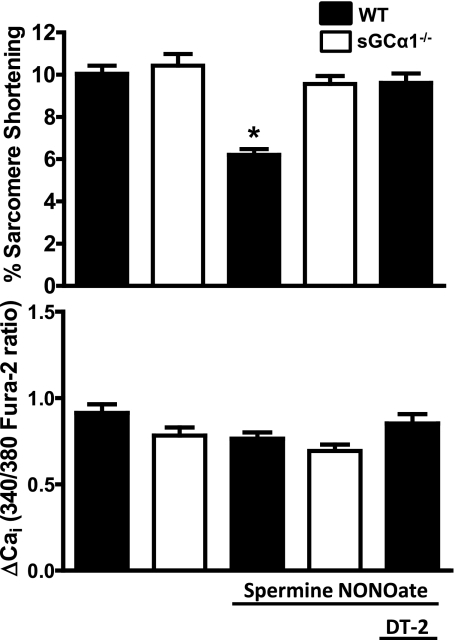
NO donor compounds were previously shown to decrease contractile force in myocardial tissues from WT mice but not in those from mice deficient in PKG I, a major downstream target of cGMP, indicating that PKG is critical to NO-mediated control of contractile force (48). We further investigated whether PKG is responsible for attenuating CM contractility in response to NO. Pretreatment of WT CM with 125 nM DT-2, a cell-permeable inhibitor of PKG, completely prevented the decrease in ISO-induced sarcomere shortening induced by perfusion with NO (7). These results confirm that PKG is a critical mediator of the negative inotropic effects of NO (Fig. 5, top). DT-2 alone had no effect on sarcomere shortening (data not shown).
Fig. 5.
Summary data of %sarcomere shortening and corresponding Ca2+ transient peak height amplitudes of WT and sGCα1−/− CM. All CM were preincubated with ISO (10 nM) for 10 min before imaging. Graphs represent percentage of sarcomere shortening (top) and Ca2+ transient amplitude (bottom) measured at 150 s after start of perfusion buffer with or without spermine NONOate (100 μM). Additional CM were preincubated and continuously perfused with 125 nM DT-2 where indicated before administration of spermine NONOate. *P < 0.05 WT vs. WT with spermine NONOate.
The addition of NO had no effect on Ca2+ transients in ISO-stimulated WT or sGCα1−/− CM (Fig. 5, bottom). Similarly, inhibition of PKG by DT-2 did not alter the height of the Ca2+ transient in WT CM incubated with 10 nM ISO alone (Fig. 5, bottom). Therefore, the negative inotropic effects of NO/cGMP signaling appear to be calcium independent.
DISCUSSION
Data from both animal and human studies have supported the idea that elevating cardiac cGMP levels may elicit beneficial effects in a variety of cardiovascular diseases, such as heart failure and cardiac hypertrophy (14, 44). Elucidating the precise functions of cGMP in the heart is important because of the development of cGMP-elevating drugs, including those that activate sGC or inhibit PDE5 (32, 34, 40). In vivo experiments comparing the cardiac function of WT and sGCα1−/− mice yield important physiologic insights, but interpretation of studies using intact animals is complicated by the impact of neurohumoral responses and non-CM cells in the heart. Studies using isolated CM allow the dissection of cGMP signaling in cardiac contractile function in the absence of compensatory responses and the influence of other cell types. The contractile performance of CM from sGCα1−/− mice yields insight into the role of NO and cGMP in cardiac function. We report the unexpected finding that contractile function is impaired in CM from sGCα1−/− mice at baseline. Additionally, sGCα1β1 is required for NO to regulate the ability of β1/β2-AR to stimulate contractile function in the heart. With the use of the β3-AR agonist BRL 37344, we confirm that the β3-AR utilizes the NOS/cGMP pathway to limit contractile amplitude. Additionally, we provide evidence that the sGC/cGMP pathway regulates cardiac contractile function independently of calcium modulation.
CM exhibit precise localization of proteins that modulate cyclic nucleotide levels and their signaling partners. Both NOS3 and PDE5 are expressed at the Z-line of CM, while NOS1 appears to be targeted to the sarcoplasmic reticulum (1, 29, 35, 43). The β3-AR also appears to colocalize with NOS3 and is compartmentalized to the Z-line in CM (2, 18, 28). We demonstrate that sGCα1β1, similar to NOS3, PDE5, and the β3-AR, is spatially restricted to the Z-line of CM. Mutation of the α1-subunit does not disrupt Z-line localization of sGCβ1, indicating that the deleted portion of sGCα1 in our mutant mouse is not responsible for cellular localization of the enzyme.
While high levels of NO and cGMP depress β1/β2-AR-stimulated cardiac contractility (6, 43), considerable evidence suggests that a low level increase in cGMP can have a positive inotropic effect on the heart (20, 21, 27). In vivo both endogenous and exogenous NO have been shown to increase cardiac function (16, 30). In isolated heart studies, NO and cGMP also induced positive inotropy (21, 31). In isolated CM, the addition of low concentrations of cGMP increased contractility (20, 27). Similarly, our work demonstrates that deletion of sGCα1 decreases basal contractility. Taken together, these results support the hypothesis that low levels of cGMP generated by sGCα1β1 augment cardiac contractile function.
We (5) previously reported that LV end systolic pressure and systemic arterial pressure were greater in male sGCα1−/− mice on an SV129 background than in female sGCα1−/− mice or WT mice of either gender. Since elevated blood pressure can induce LV hypertrophy and may decrease contractile function, we studied myocytes derived from nonhypertensive sGCα1−/− females to exclude the possibility that systemic hypertension was responsible for the impaired contractile function observed in CM isolated from male sGCα1−/− mice. Similar to their male littermates, CM from sGCα1−/− female mice contracted less than WT CM. These results confirm that the decreased contractile function observed in the sGCα1−/− CM is not a result of secondary effects of hypertension in these mice.
A possible explanation for the reduced basal contractile phenotype of the sGCα1−/− myocytes is reduced modulation of PDEs by cGMP (45) The cAMP-hydrolyzing enzyme PDE3 is competitively inhibited by cGMP (28). In a recent study (38), NO-induced increases in cGMP concentrations resulted in elevated cAMP levels in a specific compartment of myocytes. Therefore, a reduction in cGMP synthesis due to sGCα1 deficiency may increase cAMP degradation leading to decreased basal contractile function.
The β3-AR agonist BRL 37344 decreased sarcomere shortening in WT CM but not in sGCα1−/− cells. Furthermore, inhibiting NOS activity with l-NAME or sGC activity with ODQ prevented the BRL 37344-induced reduction in sacromere shortening in WT cells, confirming that the β3-adrenergic receptor functions to depress cardiac contractility through NOS and sGC. These results further support the idea that the effects of NO on cardiac contractility are mediated by cGMP and not by S-nitrosylation or other cGMP-independent mechanisms.
In the absence of ISO, exposure to spermine NONOate did not alter basal contractility in either WT or sGCα1−/− CM. This finding is in agreement with a previous report (33) that NO donor compounds do not impact contractility of isolated CM. This insensitivity to exogenous NO is not surprising considering that CM are known to express high levels of the NO-scavenger myoglobin. In fact, CM from myoglobin-deficient mice have a greater sensitivity to NO donors (47). In contrast, BRL 37344 was able to exert negative inotropic effects in the absence of β1/β2-AR stimulation. We hypothesize that colocalization of β3-ARs, NOS3, and sGCα1β1 in CM allows direct activation of sGC in response to β3-AR stimulation. In contrast, when NO is not generated by NOS3 in direct proximity to β3-AR and sGCα1β1, NO modulates cardiac contractility only in the context of β1/β2-AR stimulation.
Exposure to NO after β1/β2-AR stimulation with ISO decreased sarcomere shortening in WT but not sGCα1−/− CM. The finding that the ability of NO to blunt β-adrenergic signaling is impaired in sGCα1−/− CM may explain why load-independent indexes of cardiac contractile function in vivo are greater in sGCα1−/− mice than in WT mice (5), while contractility was decreased in CM isolated from sGCα1−/− mice than from WT mice. We speculate that in vivo adrenergic stimulation produces a phenotype of increased cardiac inotropy in sGCα1−/− mice, which have decreased cGMP signaling and, thus, are less equipped to oppose β1/β2-AR stimulation.
Inhibiting PKG with DT-2 prevented the ability of NO to reduce sarcomere shortening in WT CM. This result, as well as our data showing that either NO or Bay 41–2272 can depress β1/β2-AR stimulated CM contractile function in WT CM but not sGCα1−/− CM, suggests that NO and Bay 41–2272-mediated generation of cGMP by sGCα1β1 and activation of PKG are responsible for the NO-mediated inhibition of β1/β2-AR stimulated cardiac contractile function.
We observed no alterations in calcium handling after perfusion with an NO donor compound. Similar to our findings, PDE5 inhibition was shown to increase basal contractility in CM in the absence of concomitant changes in the Ca2+ transient (43, 46). It is conceivable that cGMP, via PKG, exerts a direct effect on the myofilament machinery to modulate contractility. Since the phosphorylation site serine 23/24 of the myofilament protein troponin I was suggested to regulate calcium sensitivity, we examined the phosphorylation status of this protein (23, 37). However, we did not detect differences in troponin I phosphorylation between WT and sGCα1−/− CM under the conditions that produced an NO-dependent decrease in β1/β2-AR-stimulated sarcomeric shortening in WT CM but not in sGCα1−/− CM (i.e., ∼150 s after perfusion with spermine NONOate in ISO-stimulated CM).
In summary, we utilized mice deficient in sGCα1 to focus on long-standing questions regarding the roles of NO and cGMP in cardiac physiology. sGCα1β1-derived cGMP has a positive inotropic role in basal CM contractility. Furthermore, we report that sGCα1β1 mediates the negative inotropic effect of β3-AR stimulation on cardiac contractile function. Additionally, our work demonstrates that sGCα1β1 is required for NO to depress β1/β2-AR-stimulated cardiac contractility and that this modulation is independent of changes in calcium handling.
GRANTS
S. M. Cawley was supported by a Ruth L. Kirschtein National Research Service Award Grant HL-07208-29, E. S. Buys was supported by a Scientist Development Grant 10SDG2610313 from the American Heart Association, F. Ichinose was supported by National Institute of General Medical Sciences Grant RO1-GM-079360, and K. D. Bloch was supported by National Heart, Lung, and Blood Institute R01 HL74352.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Robert Searles for sharing expertise in CM isolations, and we thank Dr. Peter Pokreisz for advice on immunocytochemistry. We also thank Robert Tyzkowski for technical support at the Program in Membrane Biology Microscopy Core Facility.
REFERENCES
- 1. Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O'Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 416: 337–339, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Boivin B, Lavoie C, Vaniotis G, Baragli A, Villeneuve LR, Ethier N, Trieu P, Allen BG, Hebert TE. Functional beta-adrenergic receptor signaling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes. Cardiovasc Res 71: 69–78, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Borlaug BA, Melenovsky V, Marhin T, Fitzgerald P, Kass DA. Sildenafil inhibits beta-adrenergic-stimulated cardiac contractility in humans. Circulation 112: 2642–2649, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Budworth J, Meillerais S, Charles I, Powell K. Tissue distribution of the human soluble guanylate cyclases. Biochem Biophys Res Commun 263: 696–701, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Buys ES, Sips P, Vermeersch P, Raher MJ, Rogge E, Ichinose F, Dewerchin M, Bloch KD, Janssens S, Brouckaert P. Gender-specific hypertension and responsiveness to nitric oxide in sGCalpha1 knockout mice. Cardiovasc Res 79: 179–186, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Champion HC, Georgakopoulos D, Takimoto E, Isoda T, Wang Y, Kass DA. Modulation of in vivo cardiac function by myocyte-specific nitric oxide synthase-3. Circ Res 94: 657–663, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Dostmann WR, Taylor MS, Nickl CK, Brayden JE, Frank R, Tegge WJ. Highly specific, membrane-permeant peptide blockers of cGMP-dependent protein kinase Ialpha inhibit NO-induced cerebral dilation. Proc Natl Acad Sci USA 97: 14772–14777, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ebihara Y, Karmazyn M. Inhibition of beta- but not alpha 1-mediated adrenergic responses in isolated hearts and cardiomyocytes by nitric oxide and 8-bromo cyclic GMP. Cardiovasc Res 32: 622–629, 1996 [PubMed] [Google Scholar]
- 9. Feil R, Lohmann SM, de Jonge H, Walter U, Hofmann F. Cyclic GMP-dependent protein kinases and the cardiovascular system: insights from genetically modified mice. Circ Res 93: 907–916, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Fischmeister R, Hartzell HC. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J Physiol 376: 183–202, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gauthier C, Leblais V, Kobzik L, Trochu JN, Khandoudi N, Bril A, Balligand JL, Le Marec H. The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J Clin Invest 102: 1377–1384, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gauthier C, Tavernier G, Charpentier F, Langin D, Le Marec H. Functional beta3-adrenoceptor in the human heart. J Clin Invest 98: 556–562, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gonzalez DR, Beigi F, Treuer AV, Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci USA 104: 20612–20617, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5-inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry and clinical status in patients with stable systolic heart failure: results of a 1-year prospective, randomized, placebo-controlled study. Circ Heart Fail 4: 8–17, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Hescheler J, Kameyama M, Trautwein W. On the mechanism of muscarinic inhibition of the cardiac Ca current. Pflügers Arch 407: 182–189, 1986 [DOI] [PubMed] [Google Scholar]
- 16. Heusch G, Post H, Michel MC, Kelm M, Schulz R. Endogenous nitric oxide and myocardial adaptation to ischemia. Circ Res 87: 146–152, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Hofmann FBM, Feil R, Kleppisch T. Mouse models of NO/natriuretic peptide/cGMP kinase signaling. Handbook of Experimental Pharmacology, edited by Offermanns S. Amsterdam, The Netherlands: Elsevier, vol. 159, 2004, p. 95–130 [Google Scholar]
- 18. Janssens S, Pokreisz P, Schoonjans L, Pellens M, Vermeersch P, Tjwa M, Jans P, Scherrer-Crosbie M, Picard MH, Szelid Z, Gillijns H, Van de Werf F, Collen D, Bloch KD. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circ Res 94: 1256–1262, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Joe EK, Schussheim AE, Longrois D, Maki T, Kelly RA, Smith TW, Balligand JL. Regulation of cardiac myocyte contractile function by inducible nitric oxide synthase (iNOS): mechanisms of contractile depression by nitric oxide. J Mol Cell Cardiol 30: 303–315, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Kojda G, Kottenberg K, Nix P, Schluter KD, Piper HM, Noack E. Low increase in cGMP induced by organic nitrates and nitrovasodilators improves contractile response of rat ventricular myocytes. Circ Res 78: 91–101, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Kojda G, Kottenberg K, Noack E. Inhibition of nitric oxide synthase and soluble guanylate cyclase induces cardiodepressive effects in normal rat hearts. Eur J Pharmacol 334: 181–190, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Layland J, Li JM, Shah AM. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J Physiol 540: 457–467, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee DI, Vahebi S, Tocchetti CG, Barouch LA, Solaro RJ, Takimoto E, Kass DA. PDE5A suppression of acute beta-adrenergic activation requires modulation of myocyte beta-3 signaling coupled to PKG-mediated troponin I phosphorylation. Basic Res Cardiol 105: 337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lim CC, Apstein CS, Colucci WS, Liao R. Impaired cell shortening and relengthening with increased pacing frequency are intrinsic to the senescent mouse cardiomyocyte. J Mol Cell Cardiol 32: 2075–2082, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol 91: 1421–1430, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Mergia E, Russwurm M, Zoidl G, Koesling D. Major occurrence of the new alpha2beta1 isoform of NO-sensitive guanylyl cyclase in brain. Cell Signal 15: 189–195, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Mohan P, Brutsaert DL, Paulus WJ, Sys SU. Myocardial contractile response to nitric oxide and cGMP. Circulation 93: 1223–1229, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Mongillo M, McSorley T, Evellin S, Sood A, Lissandron V, Terrin A, Huston E, Hannawacker A, Lohse MJ, Pozzan T, Houslay MD, Zaccolo M. Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circ Res 95: 67–75, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Pokreisz P, Vandenwijngaert S, Bito V, Van den Bergh A, Lenaerts I, Busch C, Marsboom G, Gheysens O, Vermeersch P, Biesmans L, Liu X, Gillijns H, Pellens M, Van Lommel A, Buys E, Schoonjans L, Vanhaecke J, Verbeken E, Sipido K, Herijgers P, Bloch KD, Janssens SP. Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation 119: 408–416, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Preckel B, Kojda G, Schlack W, Ebel D, Kottenberg K, Noack E, Thamer V. Inotropic effects of glyceryl trinitrate and spontaneous NO donors in the dog heart. Circulation 96: 2675–2682, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Prendergast BD, MacCarthy P, Wilson JF, Shah AM. Nitric oxide enhances the inotropic response to beta-adrenergic stimulation in the isolated guinea-pig heart. Basic Res Cardiol 93: 276–284, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Reffelmann T, Kloner RA. Therapeutic potential of phosphodiesterase 5 inhibition for cardiovascular disease. Circulation 108: 239–244, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Sandirasegarane L, Diamond J. The nitric oxide donors, SNAP and DEA/NO, exert a negative inotropic effect in rat cardiomyocytes which is independent of cyclic GMP elevation. J Mol Cell Cardiol 31: 799–808, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Schmidt P, Schramm M, Schroder H, Stasch JP. Mechanisms of nitric oxide independent activation of soluble guanylyl cyclase. Eur J Pharmacol 468: 167–174, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Senzaki H, Smith CJ, Juang GJ, Isoda T, Mayer SP, Ohler A, Paolocci N, Tomaselli GF, Hare JM, Kass DA. Cardiac phosphodiesterase 5 (cGMP-specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J 15: 1718–1726, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Sinnaeve P, Chiche JD, Nong Z, Varenne O, Van Pelt N, Gillijns H, Collen D, Bloch KD, Janssens S. Soluble guanylate cyclase alpha(1) and beta(1) gene transfer increases NO responsiveness and reduces neointima formation after balloon injury in rats via antiproliferative and antimigratory effects. Circ Res 88: 103–109, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Solaro RJ, Moir AJ, Perry SV. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature 262: 615–617, 1976 [DOI] [PubMed] [Google Scholar]
- 38. Stangherlin A, Gesellchen F, Zoccarato A, Terrin A, Fields LA, Berrera M, Surdo NC, Craig MA, Smith G, Hamilton G, Zaccolo M. cGMP signals modulate cAMP levels in a compartment-specific manner to regulate catecholamine-dependent signaling in cardiac myocytes. Circ Res 108: 929–939, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stasch JP, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, Gerzer R, Minuth T, Perzborn E, Pleiss U, Schroder H, Schroeder W, Stahl E, Steinke W, Straub A, Schramm M. NO-independent regulatory site on soluble guanylate cyclase. Nature 410: 212–215, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Stasch JP, Schmidt P, Alonso-Alija C, Apeler H, Dembowsky K, Haerter M, Heil M, Minuth T, Perzborn E, Pleiss U, Schramm M, Schroeder W, Schroder H, Stahl E, Steinke W, Wunder F. NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br J Pharmacol 136: 773–783, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Su J, Scholz PM, Weiss HR. Differential effects of cGMP produced by soluble and particulate guanylyl cyclase on mouse ventricular myocytes. Exp Biol Med (Maywood) 230: 242–250, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Takimoto E, Belardi D, Tocchetti CG, Vahebi S, Cormaci G, Ketner EA, Moens AL, Champion HC, Kass DA. Compartmentalization of cardiac beta-adrenergic inotropy modulation by phosphodiesterase type 5. Circulation 115: 2159–2167, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Takimoto E, Champion HC, Belardi D, Moslehi J, Mongillo M, Mergia E, Montrose DC, Isoda T, Aufiero K, Zaccolo M, Dostmann WR, Smith CJ, Kass DA. cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res 96: 100–109, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med 11: 214–222, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Vandecasteele G, Verde I, Rucker-Martin C, Donzeau-Gouge P, Fischmeister R. Cyclic GMP regulation of the l-type Ca(2+) channel current in human atrial myocytes. J Physiol 533: 329–340, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang H, Kohr MJ, Traynham CJ, Ziolo MT. Phosphodiesterase 5 restricts NOS3/soluble guanylate cyclase signaling to L-type Ca(2+) current in cardiac myocytes. J Mol Cell Cardiol 47: 304–314 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wegener JW, Godecke A, Schrader J, Nawrath H. Effects of nitric oxide donors on cardiac contractility in wild-type and myoglobin-deficient mice. Br J Pharmacol 136: 415–420, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wegener JW, Nawrath H, Wolfsgruber W, Kuhbandner S, Werner C, Hofmann F, Feil R. cGMP-dependent protein kinase I mediates the negative inotropic effect of cGMP in the murine myocardium. Circ Res 90: 18–20, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Ziolo MT, Harshbarger CH, Roycroft KE, Smith JM, Romano FD, Sondgeroth KL, Wahler GM. Myocytes isolated from rejecting transplanted rat hearts exhibit a nitric oxide-mediated reduction in the calcium current. J Mol Cell Cardiol 33: 1691–1699, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Ziolo MT, Katoh H, Bers DM. Expression of inducible nitric oxide synthase depresses beta-adrenergic-stimulated calcium release from the sarcoplasmic reticulum in intact ventricular myocytes. Circulation 104: 2961–2966, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Ziolo MT, Lewandowski SJ, Smith JM, Romano FD, Wahler GM. Inhibition of cyclic GMP hydrolysis with zaprinast reduces basal and cyclic AMP-elevated L-type calcium current in guinea-pig ventricular myocytes. Br J Pharmacol 138: 986–994, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.