Abstract
We tested the hypothesis that 1) prostaglandins (PGs) contribute to compensatory vasodilation in contracting human forearm subjected to acute hypoperfusion, and 2) the combined inhibition of PGs and nitric oxide would attenuate the compensatory vasodilation more than PG inhibition alone. In separate protocols, subjects performed forearm exercise (20% of maximum) during hypoperfusion evoked by intra-arterial balloon inflation. Each trial included baseline, exercise before inflation, exercise with inflation, and exercise after deflation. Forearm blood flow (FBF; ultrasound) and local (brachial artery) and systemic arterial pressure [mean arterial pressure (MAP); Finometer] were measured. In protocol 1 (n = 8), exercise was repeated during cyclooxygenase (COX) inhibition (Ketorolac) alone and during Ketorolac-NOS inhibition [NG-monomethyl-l-arginine (l-NMMA)]. In protocol 2 (n = 8), exercise was repeated during l-NMMA alone and during l-NMMA-Ketorolac. Forearm vascular conductance (FVC; ml·min−1·100 mmHg−1) was calculated from FBF (ml/min) and local MAP (mmHg). The percent recovery in FVC during inflation was calculated as (steady-state inflation + exercise value − nadir)/[steady-state exercise (control) value − nadir] × 100. In protocol 1, COX inhibition alone did not reduce the %FVC recovery compared with the control (no drug) trial (92 ± 11 vs. 100 ± 10%, P = 0.83). However, combined COX-nitric oxide synthase (NOS) inhibition caused a substantial reduction in %FVC recovery (54 ± 8%, P < 0.05 vs. Ketorolac alone). In protocol 2, the percent recovery in FVC was attenuated with NOS inhibition alone (69 ± 9 vs. 107 ± 10%, P < 0.01) but not attenuated further during combined NOS-COX inhibition (62 ± 10%, P = 0.74 vs. l-NMMA alone). Our data indicate that PGs are not obligatory to the compensatory dilation observed during forearm exercise with hypoperfusion.
Keywords: prostaglandins, blood flow, nitric oxide, vasodilation, exercise, hypoperfusion
during acute experimental hypoperfusion, skeletal muscle blood flow is partially restored in the exercising forearm through local dilator mechanisms and/or a myogenic response (4, 5). Along these lines, nitric oxide synthase (NOS) inhibition blunts the magnitude of restoration of forearm blood flow (FBF) and vascular conductance (FVC) during exercise with acute hypoperfusion by 10–20% (4). The fact that a substantial flow restoration still occurs despite NOS inhibition suggests that additional vasodilator signals are likely involved in this response.
In general, prostaglandins (PGs) do not appear to contribute significantly to the exercise hyperemic response to dynamic contractions (9, 16, 20, 29, 31). These findings are based on little to no change in muscle blood flow following PG synthesis inhibition. However, PGs have been reported to be involved in the regulation of skeletal muscle blood flow following periods of ischemia (2, 10, 14, 20) and during systemic hypoxia (25). Therefore, it is possible that the PGs may become a more important vasodilator signal during exercise under conditions of reduced oxygen availability. Thus, the aim of the current study was to investigate whether PGs contribute to the compensatory vasodilation observed during acute hypoperfusion in exercising human skeletal muscle. Because there is an interaction between the PG and nitric oxide (NO) systems (17, 19, 25, 27), a secondary aim of the study was to examine this potential interaction during exercise with hypoperfusion. We hypothesized that PGs would contribute to the compensatory vasodilator response; however, the contribution would be enhanced in the absence of NO.
METHODS
Subjects
A total of 16 young healthy male subjects volunteered to participate in two separate protocols (eight subjects in each protocol). Subjects gave written informed consent and were nonobese, nonsmokers, and were not taking any medications. Studies were performed after an overnight fast and after the subjects refrained from exercise and caffeine for at least 24 h. All study protocols were approved by the Mayo Institutional Review Board and in accordance with the Declaration of Helsinki.
Heart Rate and Systemic Blood Pressure
Heart rate (HR) was measured by three-lead electrocardiography. Systemic blood pressure was assessed (beat-to-beat) with a finger plethysmograph (Finometer) on the nonexercising hand and verified with an automated cuff on the same arm. The systemic pressure was used as an index of pressure proximal (upstream) from the balloon. Cardiac output (CO) was estimated using the Modelflow technique, which has been validated against other techniques and used in exercise studies (24, 33).
Arterial Catheterization and Balloon Placement
Brachial catheter placement and balloon insertion have been described in detail previously (5). Briefly, a 20-gauge, 5-cm catheter was placed in the brachial artery in the experimental arm using ultrasound guidance under aseptic conditions after local anesthesia (2% lidocaine). A guide wire was then placed in the artery, which was then cannulated with a 4-Fr introducer (Cook, Bloomington, IN) that permitted insertion of a 2-Fr Fogarty balloon catheter in the brachial artery. A port and stopcock system allowed the measurement of arterial pressure, administration of study drugs, and drawing of arterial blood samples. The system was continuously flushed (3 ml/h) with heparinized saline. The configuration of the balloon upstream from the lumen of the introducer allowed measurement of the arterial pressure distal to the balloon that was perfusing the contracting forearm muscles.
FBF
Brachial artery mean blood velocity (MBV) and brachial artery diameter were determined with a 12-MHz linear-array Doppler probe (model M12L, Vivid 7; General Electric, Milwaukee, WI). Brachial artery blood velocity was measured throughout each condition with a probe insonation angle previously calibrated to 60°. Brachial artery and balloon diameter measurements were obtained at end diastole and between contractions during steady-state conditions. Diameter measurement typically results in the loss of the pulse wave signal for 15–20 s. Velocity and diameter measurements were made 2–3 cm proximal to the balloon. FBF was calculated as the product of MBV (cm/s) and brachial artery cross-sectional area (cm2) and multiplied by 60 to present as milliliters per minute (ml/min).
Forearm Exercise
Rhythmic forearm exercise was performed with a hand grip device by the nondominant arm at 20% of each subject's maximal voluntary contraction (MVC, mean 52 ± 2 kg, range 36–71 kg). Forearm exercise consisted of squeezing and releasing two handles together 4–5 cm to raise and lower a weight over a pulley at a duty cycle of 1 s contraction and 2 s relaxation (20 contractions/min) using a metronome to ensure correct timing. The average weights used for forearm exercise in protocols 1 and 2 were 10.3 ± 0.4 and 10.2 ± 0.7 kg, respectively.
Brachial Artery Balloon Inflation
To reduce FBF, the brachial artery was partially occluded via inflation of the Fogarty balloon catheter with saline using a calibrated microsyringe for tight control of balloon volume. Balloon inflations were targeted to initially reduce blood velocity by ∼50%. A meter visible to the investigators provided real-time MBV readings from the Doppler signal and instant feedback at the onset of balloon inflation, which allowed for accurate manipulations of flow.
Pharmacological Infusions
NG-monomethyl-l-arginine (l-NMMA; NOS inhibitor) was infused at a loading dose of 5 mg/min for 5 min and then at a maintenance dose of 1 mg/min for the remainder of the study. This dose of l-NMMA has been shown to effectively attenuate the forearm vasodilator response to exogenous acetylcholine (ACh) administration (4, 6). Ketorolac [cyclooxygenase (COX) inhibitor] was infused at a dose of 600 μg/min for 5 min. To test the efficacy of the NOS inhibition, exogenous ACh (a nonspecific muscarinic agonist) was infused intra-arterially at 2.0, 4.0, and 8.0 μg·dl forearm volume−1·min−1 for 2 min before and after l-NMMA (protocol 2) and combined Ketorolac-l-NMMA (both protocols). ACh dose responses were also performed before and after Ketorolac (protocol 1) to quantify the amount of ACh-induced vasodilation due to PG-mediated mechanisms.
Experimental Protocol
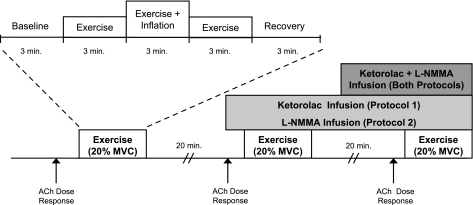
A schematic of the general experimental design is shown in Fig. 1. Each subject completed three forearm exercise trials at 20% MVC. Each exercise trial consisted of 3 min of rest, exercise, exercise with balloon inflation, exercise following balloon deflation, and recovery (15 min total; 9 min of total exercise). In protocol 1, forearm exercise was performed during no drug, followed by Ketorolac alone, and then combined Ketorolac-l-NMMA. In protocol 2, exercise was performed during no drug, followed by l-NMMA alone, and then combined l-NMMA-Ketorolac. Each exercise trial was separated by 20 min of rest to allow FBF to return to baseline.
Fig. 1.
Schematic diagram of experimental protocol. Subjects completed three exercise trials. Each exercise trial consisted of baseline, exercise (control), exercise during inflation, exercise following deflation, and recovery measurements (3 min each). Exercise trials were performed during control (no drug), cyclooxygenase (COX) inhibition (Ketorolac, protocol 1), NG-monomethyl-l-arginine (l-NMMA; protocol 2), and combined Ketorolac-l-NMMA infusions (both protocols). Each trial was separated by at least 20 min of rest to allow forearm blood flow (FBF) to return to baseline values. Acetylcholine (ACh) dose-response infusions were performed at the start of the study under control (no drug) conditions and repeated during Ketorolac (protocol 1), l-NMMA (protocol 2), and combined l-NMMA-Ketorolac (both protocols). MVC, maximal voluntary contraction.
Data Analysis and Statistics
Data were collected at 200 Hz, stored on a computer, and analyzed off-line with signal-processing software (WinDaq; DATAQ Instruments, Akron, OH). Local mean arterial pressure [brachial artery pressure (BAP)] was determined from the brachial artery pressure waveform measured distal to the balloon, systemic mean arterial pressure (MAP) (e.g., pressure proximal to the balloon) was derived from the Finometer pressure waveform, and HR was determined from the electrocardiogram. FBF, BAP, MAP, CO, and HR were determined by averaging values during the last 30 s of rest, exercise, exercise with inflation, exercise following deflation, and recovery. In addition, all values were analyzed and averaged during the first 10 s of target balloon inflation (nadir) and the first 10 s immediately following balloon deflation. FVC was calculated as (FBF/BAP) × 100 and expressed as milliliters per minute per 100 mmHg.
All values are expressed as means ± S.E. Within a given protocol, the FBF, FVC, BAP, systemic MAP, HR, and CO during rest, exercise, the nadir after balloon inflation, exercise at the end of the balloon inflation, exercise following deflation, and recovery were analyzed by repeated-measures ANOVA. When significance was detected, Tukey's post hoc test was used to identify individual differences and adjust P values to account for multiple comparisons, to preserve an overall type I error rate of 0.05.
Percent recovery in FBF and FVC was calculated as [steady-state inflation + exercise value − nadir/steady-state exercise (control) value − nadir] × 100. To investigate the role of PGs and NO on percent recovery of blood flow and conductance, one-way repeated-measures ANOVA were performed between drug conditions. To further explore the contribution of local vasodilatation to any restoration of flow, we analyzed balloon resistance and forearm vascular resistance and considered them individually and in series (4, 5, 23). Using systemic arterial pressure (SAP; Finometer), brachial artery pressure distal to the balloon (BAP; catheter), and brachial artery blood flow, we calculated the resistance of the balloon (SAP-BAP/flow) and vascular resistance (BAP/flow). The total resistance was calculated as the sum of these two resistors. Changes in vascular and balloon resistance were analyzed from the onset of balloon inflation (nadir) until the end of the inflation period and expressed as a percent change. One-way repeated-measures ANOVA were used to compare the percent change in resistance between drug conditions. Statistical significance was set a priori at P < 0.05.
RESULTS
Seven of the eight subjects completed all of the exercise trials in protocol 1. One subject did not complete the final exercise trial (combined Ketorolac-l-NMMA) because of a severely attenuated blood flow response during exercise before balloon inflation that, in turn, prevented the subject from continuing forearm exercise. Therefore, all subjects (n = 8) were included in the group analysis for trials 1 and 2 (no drug and Ketorolac alone, respectively) and only seven for trial 3 (combined Ketorolac-l-NMMA).
The subjects were 27 ± 2 yr of age, 184 ± 3 cm in height, and weighed 87 ± 4 kg [body mass index (BMI): 26 ± 1 kg/m2]. All eight subjects completed protocol 2. The subjects were 25 ± 2 yr of age, 179 ± 3 cm in height, and weighed 81 ± 3 kg (BMI: 25 ± 1 kg/m).
FBF and Vasodilatation During Exercise With Balloon Inflation
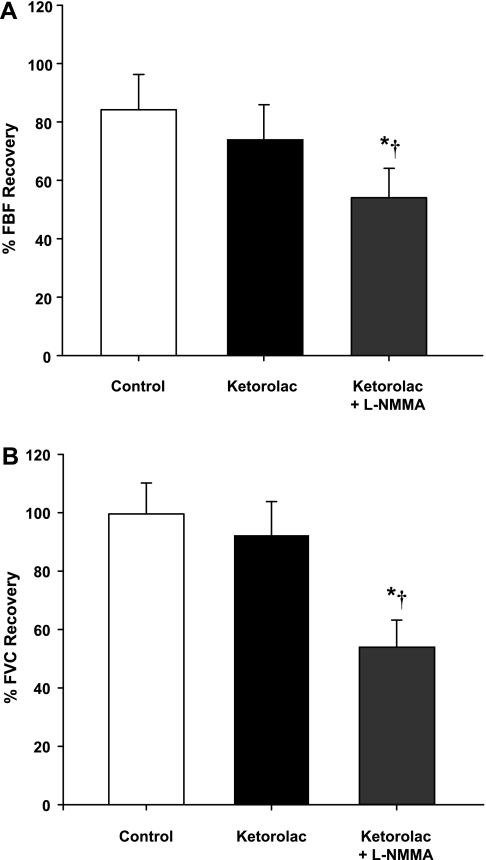
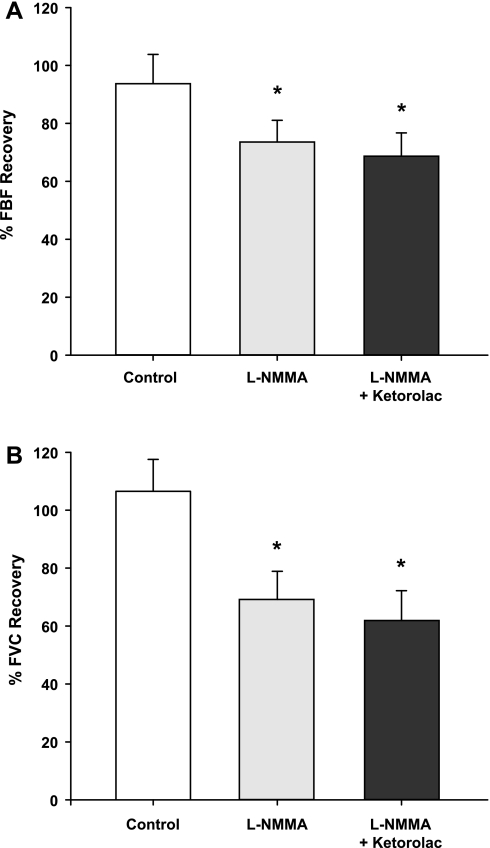
Group mean data for FBF and FVC responses are presented in Table 1. As expected, during both protocols, exercise increased FBF and FVC in all three exercise trials (P < 0.001). In protocol 1, balloon inflation (nadir) during the exercise trial with no drug acutely reduced FBF by 56% and FVC by 35% (P < 0.01). In protocol 2, FBF and FVC were acutely reduced by 54 and 38%, respectively (P < 0.01). In both protocols, FBF and FVC at the end of inflation were restored to the exercise (control) levels, which were substantially higher than their respective nadir values (P < 0.01). The percent recovery of FBF and FVC during the exercise trials are presented in Fig. 2, A and B (protocol 1) and Fig. 3, A and B (protocol 2).
Table 1.
Forearm blood flow and vasodilation during exercise with balloon inflation
| Inflation |
Deflation |
|||||
|---|---|---|---|---|---|---|
| Baseline | Exercise (Control) | Nadir | Steady state | Acute | Steady state | |
| Protocol 1 | ||||||
| 20% MVC (no drug) | ||||||
| FBF, ml/min | 95 ± 16 | 461 ± 67* | 195 ± 29† | 399 ± 51§ | 573 ± 89§b | 554 ± 77§ |
| FVC, ml·min−1·100 mmHg−1 | 107 ± 17 | 519 ± 73* | 330 ± 46† | 512 ± 66§ | 608 ± 83§ | 597 ± 74§ |
| 20% MVC (Ketorolac) | ||||||
| FBF, ml/min | 83 ± 15 | 423 ± 56* | 202 ± 37‡ | 347 ± 46a,d | 596 ± 113§b | 498 ± 76§ |
| FVC, ml·min−1·100 mmHg−1 | 93 ± 17 | 473 ± 61* | 305 ± 51‡ | 448 ± 58a,d | 638 ± 106‡§b | 542 ± 75§ |
| 20% MVC (Ketorolac + l-NMMA) | ||||||
| FBF, ml/min | 73 ± 13d | 400 ± 66* | 193 ± 35‡ | 296 ± 45d | 567 ± 166§b | 424 ± 59a,d |
| FVC, ml·min−1·100 mmHg−1 | 81 ± 14d | 433 ± 61* | 304 ± 49‡ | 379 ± 58c | 591 ± 150§b | 452 ± 51d |
| Protocol 2 | ||||||
| 20% MVC (no drug) | ||||||
| FBF, ml/min | 77 ± 9 | 447 ± 50* | 202 ± 26† | 409 ± 45a | 624 ± 120§b | 496 ± 51§ |
| FVC, ml·min−1·100 mmHg−1 | 89 ± 10 | 480 ± 45* | 290 ± 25‡ | 465 ± 32a | 649 ± 123§b | 517 ± 47§ |
| 20% MVC (l-NMMA) | ||||||
| FBF, ml/min | 60 ± 8d | 421 ± 44* | 188 ± 23† | 348 ± 40a,d | 522 ± 90§b | 491 ± 56§b |
| FVC, ml·min−1·100 mmHg−1 | 67 ± 10d | 451 ± 45* | 278 ± 25† | 389 ± 41d | 541 ± 90§b | 503 ± 52§ |
| 20% MVC (l-NMMA + Ketorolac) | ||||||
| FBF, ml/min | 50 ± 6d | 414 ± 48* | 177 ± 29† | 332 ± 52d | 529 ± 103§b | 482 ± 62§ |
| FVC, ml·min−1·100 mmHg−1 | 54 ± 9d | 441 ± 54* | 255 ± 34‡ | 369 ± 57d | 531 ± 97§b | 493 ± 66§ |
Values are means ± SE.
FBF, forearm blood flow; FVC, forearm vascular conductance; l-NMMA, NG-monomethyl-l-arginine; MVC, maximal voluntary contraction.
P < 0.001 vs. baseline.
P < 0.01 vs. exercise (control).
P < 0.05 vs. exercise.
P < 0.01 vs. nadir.
P < 0.05 vs. nadir.
P < 0.05 versus inflation (steady state).
P < 0.05 vs. other trials.
P < 0.05 vs. no drug trial.
Fig. 2.
Percent recovery in FBF (A) and forearm vascular conductance (FVC; B) during balloon inflation (protocol 1). COX inhibition did not reduce the %recovery of FBF and FVC compared with the respective %recovery during the control (no drug) trial (P = 0.21 and P = 0.83, respectively). The %recovery of FBF and FVC was reduced with combined Ketorolac-l-NMMA infusions compared with the control and Ketorolac alone trials. *P < 0.01 vs. control (no drug). †P < 0.05 vs. Ketorolac alone.
Fig. 3.
Percent recovery in FBF (A) and FVC (B) during balloon inflation (protocol 2). Nitric oxide synthase (NOS) inhibition (l-NMMA) reduced the %recovery of FBF and FVC compared with the respective %recovery during the control (no drug) trial. Combined infusion of l-NMMA and Ketorolac did not reduce the %recovery of FBF and FVC compared with l-NMMA alone (P = 0.61 and P = 0.74, respectively). *P < 0.01 vs. control (no drug).
Contribution of PGs and NO to Blood Flow Recovery During Hypoperfusion
Protocol 1.
Balloon inflation (nadir) during the exercise trial with COX inhibition acutely reduced FBF by 52% and FVC by 36% (P < 0.05). Similar to the control trials, the FBF and FVC at the end of inflation were partially restored to exercise (control) levels, which were substantially higher than their respective nadir values (P < 0.05). The FBF and FVC at the end of inflation under COX inhibition were less than the values observed during the no drug trial (P < 0.05; Table 1). However, the percent recovery of FBF and FVC during the trial following Ketorolac was similar to the percent recovery values observed during the control (no drug) trial (P = 0.21 for FBF and P = 0.83 for FVC; Fig. 2, A and B). Combined infusion of Ketorolac-l-NMMA decreased baseline (resting) FBF and FVC compared with values observed during the control trial (P < 0.05). Balloon inflation (nadir) during the combined Ketorolac-l-NMMA trial acutely reduced FBF by 53% and FVC by 31% (P < 0.05). Unlike the control and COX inhibition trials, FBF and FVC at the end of the inflation period were not significantly different compared with the respective nadir levels (P = 0.80 for FBF and P = 0.90 for FVC; Table 1). Consequently, the percent recovery of FBF and FVC with combined Ketorolac-l-NMMA was substantially lower than the compensatory responses observed during the control (no drug) trial (P < 0.01) and Ketorolac alone trial (P <0.05; Fig. 2, A and B).
Vascular resistance during balloon inflation (from nadir to the end of inflation) decreased during the control (no drug) trial and with COX inhibition alone (P < 0.01 for both) but not with combined Ketorolac-l-NMMA (P = 0.08; Table 2). Consequently, the percent reduction in vascular resistance was less with combined Ketorolac-l-NMMA compared with the Ketorolac alone (P <0.05) and no drug (P <0.01) trials. Balloon resistance decreased (from nadir to the end of inflation) in the no drug trial, with COX inhibition, and with combined Ketorolac-l-NMMA (P < 0.01 for all, Table 2). However, the absolute (P = 0.83) and relative (P = 0.96) changes in balloon resistance were not different between drug conditions.
Table 2.
Changes in vascular and balloon resistance during inflation for all trials
| Resistance, mmHg·ml−1·min−1 |
|||
|---|---|---|---|
| Nadir | End Inflation | Change, % | |
| Protocol 1 | |||
| 20% MVC (no drug) | |||
| Vascular | 0.36 ± 0.06 | 0.22 ± 0.03* | −35 ± 5 |
| Balloon | 0.16 ± 0.02 | 0.09 ± 0.03* | −54 ± 10 |
| 20% MVC (Ketorolac) | |||
| Vascular | 0.39 ± 0.05 | 0.25 ± 0.03* | −32 ± 5 |
| Balloon | 0.17 ± 0.06 | 0.10 ± 0.06* | −52 ± 9 |
| 20% MVC (Ketorolac + l-NMMA) | |||
| Vascular | 0.39 ± 0.08 | 0.30 ± 0.04* | −20 ± 5†§ |
| Balloon | 0.15 ± 0.03 | 0.08 ± 0.03* | −53 ± 8 |
| Protocol 2 | |||
| 20% MVC (no drug) | |||
| Vascular | 0.36 ± 0.03 | 0.23 ± 0.01* | −37 ± 2 |
| Balloon | 0.11 ± 0.02 | 0.06 ± 0.02* | −61 ± 9 |
| 20% MVC (l-NMMA) | |||
| Vascular | 0.38 ± 0.03 | 0.27 ± 0.03* | −28 ± 4‡ |
| Balloon | 0.12 ± 0.02 | 0.06 ± 0.02* | −57 ± 6 |
| 20% MVC (l-NMMA + Ketorolac) | |||
| Vascular | 0.43 ± 0.05 | 0.31 ± 0.04* | −28 ± 5 |
| Balloon | 0.13 ± 0.03 | 0.06 ± 0.02* | −60 ± 6 |
Values are means ± SE.
P < 0.01 vs. nadir.
P < 0.01 vs. no drug trial.
P < 0.05 vs. no drug trial.
P < 0.05 vs. Ketorolac alone trial.
Protocol 2.
Infusion of l-NMMA decreased resting FBF and FVC compared with the control (no drug) trial (P < 0.05) but did not impact steady-state exercise (control) values (Table 1). Balloon inflation (nadir) during the exercise trial with l-NMMA acutely reduced FBF by 54% and FVC by 37% (P < 0.01). The FBF and FVC at the end of inflation under NOS inhibition were less than the values observed during the no drug trial (P < 0.05; Table 1). Consequently, the percent recovery of FBF and FVC during the trial following l-NMMA was substantially lower than the percent recovery values observed during the no drug trial (Fig. 3, A and B). Balloon inflation (nadir) during the combined l-NMMA-Ketorolac trial acutely reduced FBF by 57% and FVC by 40% (P < 0.01). Combined infusion of l-NMMA-Ketorolac did not reduce the percent recovery of FBF and FVC compared with l-NMMA alone (P = 0.61 and P = 0.74, respectively; Fig. 3, A and B).
Vascular resistance during balloon inflation (from nadir to the end of inflation) decreased during the control (no drug) trial, with l-NMMA alone, and with combined l-NMMA-Ketorolac (P < 0.01 for all, Table 2). Consequently, the percent reduction in vascular resistance was less with l-NMMA compared with the no drug trial (P < 0.05). Combined infusion of l-NMMA-Ketorolac did not result in a further percent reduction in vascular resistance (P = 0.97 vs. l-NMMA alone, Table 2). Balloon resistance decreased (from nadir to the end of inflation) in the no drug trial, with l-NMMA, and with combined l-NMMA-Ketorolac (P < 0.01 for all, Table 2). However, the absolute (P = 0.65) and relative (P = 0.83) changes in balloon resistance were not different between drug conditions.
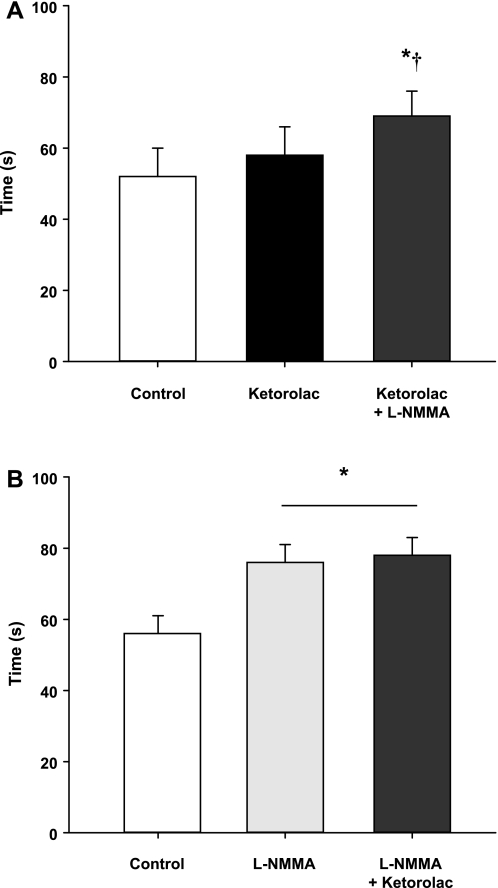
Timing of Compensatory Vasodilatation
In protocol 1, COX inhibition failed to increase the time to reach steady-state FBF during balloon inflation compared with the no drug trial (P = 0.35, Fig. 4A). However, combined COX and NOS inhibition (Ketorolac-l-NMMA) increased the timing of compensation compared with control (no drug) and Ketorolac alone trials. In protocol 2, NOS inhibition alone increased the time to reach steady-state FBF during balloon inflation compared with the no drug trial (Fig. 4B). There was no difference in the timing of compensation between the l-NMMA alone and combined l-NMMA-Ketorolac trials (P = 0.75).
Fig. 4.
Timing of flow restoration. A: protocol 1. COX inhibition (Ketorolac) does not increase the time to reach steady-state FBF during balloon inflation compared with the control (no drug) trial. Combined COX-NOS inhibition (l-NMMA) delayed the time to reach steady-state blood flow compared with the no drug and Ketorolac alone trials. B: protocol 2. The time to reach steady-state blood flow during balloon inflation under NOS inhibition is delayed. The combined NOS-COX inhibition (l-NMMA-Ketorolac) does not delay the restoration of flow beyond l-NMMA alone. *P < 0.01 vs. control (no drug). †P < 0.05 vs. Ketorolac alone.
Effect of COX and NOS Inhibition on Vasodilator Responses to Exogenous Acetylcholine
Protocol 1.
Repeated-measures ANOVA revealed a significant time, drug, and time × drug effect during the ACh dose-response trials (P < 0.01 for all). COX inhibition did not attenuate the vasodilator response (change in FVC from baseline) to exogenous ACh infusion at the low (183 ± 47 vs. 206 ± 58 ml·min−1·100 mmHg−1; P = 0.83) and medium (204 ± 55 vs. 267 ± 70 ml·min−1·100 mmHg−1; P = 0.23) dose compared with no drug (saline). However, the vasodilator response to the high dose of ACh was attenuated with COX inhibition (293 ± 81 vs. 405 ± 93 ml·min−1·100 mmHg−1; P < 0.01). Furthermore, combined Ketorolac-l-NMMA attenuated the responsiveness to ACh (108 ± 44, 125 ± 37, 183 ± 62 ml·min−1·100 mmHg−1; low to high dose) compared with no drug (saline; P < 0.01) and Ketorolac alone (P < 0.05).
Protocol 2.
The vasodilator response to exogenous ACh was substantially reduced in the presence of l-NMMA (76 ± 15, 79 ± 12, and 110 ± 10 ml·min−1·100 mmHg−1; P < 0.05) and combined l-NMMA-Ketorolac (69 ± 15, 65 ± 5, and 102 ± 27 ml·min−1·100 mmHg−1; P < 0.01) compared with no drug (133 ± 16, 153 ± 14, and 173 ± 21 ml·min−1·100 mmHg−1). The combined infusion of l-NMMA-Ketorolac did not reduce the responsiveness to ACh compared with l-NMMA alone (P = 0.18).
Hemodynamic Changes
Systemic hemodynamic responses during exercise are presented in Table 3. Exercise resulted in an increase in MAP in all trials (P < 0.05). MAP remained elevated above baseline values throughout each trial (P < 0.05). Estimated CO and HR did not change with exercise (control) in any of the trials. MAP, HR, and CO did not change with balloon inflation compared with exercise (control) values in any of the trials.
Table 3.
Systemic hemodynamic responses
| Inflation |
Deflation |
|||||
|---|---|---|---|---|---|---|
| Baseline | Exercise (Control) | Nadir | Steady state | Acute | Steady state | |
| Protocol 1 | ||||||
| 20% MVC (no drug) | ||||||
| Mean arterial pressure, mmHg | 85 ± 5 | 91 ± 5* | 92 ± 4* | 93 ± 4* | 95 ± 4* | 94 ± 4* |
| Brachial artery pressure, mmHg | 84 ± 3† | 87 ± 2† | 56 ± 3*‡ | 75 ± 4†‡ | 89 ± 3† | 88 ± 2† |
| Heart rate, beats/min | 61 ± 2 | 61 ± 1 | 61 ± 1 | 62 ± 1 | 61 ± 1 | 60 ± 1 |
| Cardiac output, l/min | 5.5 ± 0.4 | 5.8 ± 0.5 | 6.0 ± 0.4* | 6.0 ± 0.5* | 5.9 ± 0.6 | 6.0 ± 0.6* |
| 20% MVC (Ketorolac) | ||||||
| Mean arterial pressure, mmHg | 85 ± 3 | 90 ± 3* | 91 ± 3* | 94 ± 3* | 94 ± 4* | 92 ± 3* |
| Brachial artery pressure, mmHg | 84 ± 2† | 86 ± 2† | 60 ± 4*‡ | 73 ± 5†‡ | 87 ± 3† | 87 ± 1† |
| Heart rate, beats/min | 60 ± 2 | 61 ± 2 | 61 ± 2 | 61 ± 2 | 61 ± 2 | 60 ± 1 |
| Cardiac output, l/min | 5.6 ± 0.5 | 5.8 ± 0.4 | 5.9 ± 0.4 | 6.0 ± 0.4* | 6.1 ± 0.5* | 6.0 ± 0.5* |
| 20% MVC (Ketorolac + l-NMMA) | ||||||
| Mean arterial pressure, mmHg | 87 ± 4 | 92 ± 4* | 95 ± 4* | 97 ± 4* | 97 ± 4* | 96 ± 3* |
| Brachial artery pressure, mmHg | 85 ± 3† | 87 ± 3† | 58 ± 4*†‡ | 74 ± 3*†‡ | 89 ± 3† | 88 ± 3† |
| Heart rate, beats/min | 58 ± 1 | 59 ± 1 | 58 ± 1 | 58 ± 1 | 58 ± 1 | 58 ± 1 |
| Cardiac output, l/min | 5.4 ± 0.4 | 5.6 ± 0.3 | 5.6 ± 0.3 | 5.6 ± 0.4 | 5.8 ± 0.3* | 5.9 ± 0.5* |
| Protocol 2 | ||||||
| 20% MVC (no drug) | ||||||
| Mean arterial pressure, mmHg | 87 ± 2 | 96 ± 4* | 97 ± 5* | 101 ± 5* | 101 ± 5*† | 99 ± 5* |
| Brachial artery pressure, mmHg | 84 ± 3† | 90 ± 4† | 65 ± 4*†‡ | 84 ± 5† | 92 ± 3*† | 93 ± 5*† |
| Heart rate, beats/min | 61 ± 2 | 62 ± 2 | 63 ± 2 | 62 ± 2 | 62 ± 2 | 62 ± 2 |
| Cardiac output, l/min | 5.5 ± 0.4 | 5.8 ± 0.3 | 6.0 ± 0.5* | 6.2 ± 0.4* | 6.2 ± 0.4* | 6.3 ± 0.4*‡ |
| 20% MVC (l-NMMA) | ||||||
| Mean arterial pressure, mmHg | 89 ± 3 | 97 ± 2* | 97 ± 2* | 101 ± 3* | 100 ± 3* | 101 ± 4* |
| Brachial artery pressure, mmHg | 87 ± 2† | 91 ± 3† | 65 ± 4*†‡ | 85 ± 3† | 93 ± 3*† | 94 ± 3*† |
| Heart rate, beats/min | 61 ± 2 | 61 ± 2 | 61 ± 2 | 62 ± 2 | 62 ± 2 | 62 ± 2 |
| Cardiac output, l/min | 5.4 ± 0.4 | 5.7 ± 0.4 | 5.8 ± 0.4* | 5.9 ± 0.3* | 6.0 ± 0.3* | 5.9 ± 0.3* |
| 20% MVC (l-NMMA + Ketorolac) | ||||||
| Mean arterial pressure, mmHg | 88 ± 4 | 95 ± 4* | 95 ± 4* | 99 ± 4* | 100 ± 4*†‡ | 100 ± 5*†‡ |
| Brachial artery pressure, mmHg | 87 ± 2† | 91 ± 2† | 64 ± 4*‡ | 85 ± 3† | 93 ± 2† | 94 ± 3† |
| Heart rate, beats/min | 62 ± 2 | 64 ± 2 | 64 ± 2 | 64 ± 2 | 64 ± 2 | 64 ± 2 |
| Cardiac output, l/min | 5.4 ± 0.3 | 5.7 ± 0.3 | 5.7 ± 0.4 | 6.0 ± 0.3* | 6.0 ± 0.3* | 5.9 ± 0.3* |
Values are means ± SE.
P < 0.05 vs. baseline.
P < 0.05 vs. nadir.
P < 0.05 vs. exercise (control).
DISCUSSION
The primary novel finding from this study is that COX inhibition with Ketorolac failed to reduce the compensatory vasodilation in the contracting human forearm subjected to acute hypoperfusion. This was observed both in the presence (protocol 2) and in the absence (protocol 1) of NO. Thus PGs are not obligatory to the compensatory dilation observed during forearm exercise with hypoperfusion.
The role of vasodilating PGs during exercise in humans remains uncertain (1). Inhibition of PG synthesis has been shown to reduce FBF following isometric (14) and dynamic (34) contractions. Conversely, Naylor and colleagues (18) found that inhibition of PG synthesis increases the hyperemic response following ischemic exercise. These aforementioned studies have been limited to measurements of postexercise blood flow using venous occlusion plethysmography. The use of Doppler ultrasound and thermodilution techniques to measure muscle blood flow during dynamic contractions has revealed that inhibition of PG formation has little or no effect on the exercise hyperemic response (16, 20, 29, 31). Taken together, it seems reasonable to conclude that PGs do not play an essential role in skeletal muscle vasodilation during dynamic exercise under normoxic conditions. However, available evidence in humans suggests that PGs contribute to the rise in skeletal muscle blood flow following periods of limb ischemia (2, 10, 14, 20). Moreover, COX inhibition has been shown to attenuate muscle vasodilation evoked by systemic hypoxia in rats (25). Therefore, it is plausible that the role of PGs in the regulation of muscle blood flow during exercise under conditions of reduced oxygen availability may be enhanced. Interestingly, the acute reactive hyperemia response (absolute change from inflation steady state to acute deflation) was larger during COX inhibition compared with the control (no drug) trial (protocol 1, Table 1). This might be explained by a significantly lower absolute flow before balloon deflation during the Ketorolac trial. However, there is some evidence that suggests PG inhibition augments reactive hyperemia after ischemic exercise in the human forearm (18).
To our knowledge, this is the first study to examine the role of PGs in the regulation of skeletal muscle blood flow during exercise under conditions of acute hypoperfusion. Our findings are in agreement with available data in the coronary circulation of pigs. Ruocco et al. (26) demonstrated COX inhibition with indomethacin did not alter coronary blood flow in response to an experimentally induced flow-limiting coronary artery stenosis in pigs. However, COX inhibition in patients with coronary artery disease causes coronary vasoconstriction (8, 11), thus suggesting that vasodilator PGs may play a greater role in the regulation of coronary vasomotor control in chronic vs. acute ischemia and in blood vessels subjected to atherosclerotic disease processes. By contrast, we recently demonstrated that adenosine receptor inhibition with aminophylline blunts the compensatory vasodilator response in the contracting human forearm subjected to acute hypoperfusion and is independent of NO (3). Given the recent evidence from Mortensen et al. (17) and Nyberg et al. (21) that showed adenosine contributes to the regulation of skeletal muscle blood flow by stimulating PG synthesis from endothelial cells, we were somewhat surprised that COX inhibition did not attenuate the compensatory vasodilation in the present study. It is possible that a role of PGs in the restoration of flow may have been masked by a compensatory increase in adenosine production in the present study. However, our recent evidence suggests that NOS inhibition does not enhance the contribution of adenosine to the compensatory vasodilator response during exercise with hypoperfusion (3). The integration of our previous (3) and current findings suggests adenosine contributes to the compensatory vasodilation in hypoperfused skeletal muscle in a manner that is at least partially independent of NO and PG pathways.
Restoration of Flow via Local Vasodilator Mechanisms
Similar to our previous studies (3–5), skeletal muscle blood flow was restored following acute hypoperfusion during control (no drug) conditions despite the absence of a pressor response. This suggests that humans rely on local vasodilator mechanisms, and not a reflex increase in pressure, to restore blood flow to hypoperfused contracting muscle. In contrast, there is overwhelming evidence that suggests a pressor response is essential in the restoration of blood flow to underperfused hindlimbs of exercising dogs (12, 22, 23, 30, 35). The discrepant findings between our series of studies in humans and those performed in dogs may simply be related to species differences. However, a larger mass of active muscle (hindlimbs vs. forearm), greater vascular occlusion, and higher exercise intensity used in the dog model likely explain the differences in the mechanisms responsible for restoring blood flow to hypoperfused contracting muscle. Along these lines, the exercise intensity (20% MVC), reduction in FBF (∼50%) induced by balloon inflation, or a combination of the two used in our model is not sufficient enough to augment the pressor response beyond that of exercise alone.
Role of PGs in ACh-Induced Vasodilation
Previous studies have suggested that PGs contribute to ACh-induced vasodilation (13, 15) while others have shown no role for PGs (28, 32). In then present study, COX inhibition decreased the vasodilator responses to exogenous ACh by 11–28%. However, the decreased vasodilator response was only significant at the highest dose of infused ACh (8.0 μg·dl forearm volume−1·min−1). The lack of significant change at the lower doses of ACh used in the current study (2.0 and 4.0 μg·dl forearm volume−1·min−1) are in agreement with previous findings from our laboratory that used lower doses of ACh (1–4 μg·dl forearm volume−1·min−1) (28). Taken together, our findings suggest that the ACh-induced vasodilation becomes more dependent on PG-mediated pathways during infusions of higher doses of exogenous ACh.
Experimental Considerations
We have previously demonstrated that NO and adenosine contribute to the compensatory vasodilation in hypoperfused contracting muscles (3, 4). These findings are based on a blunted %FBF and FVC recovery following NOS inhibition with l-NMMA and adenosine receptor blockade with aminophylline. In the current study, COX inhibition did not reduce the percent recovery of FBF and FVC compared with the control (no drug) condition (protocol 1, Fig. 2). Conversely, the absolute FBF and FVC during steady-state inflation were significantly lower during COX inhibition compared with the control (no drug) trial (protocol 1, Table 1). By simply looking at the absolute FBF and FVC values at steady-state inflation under COX inhibition, it appears that PGs contribute to the restoration of flow to hypoperfused exercising skeletal muscle. With the use of this approach, an alternative explanation might be that any PG contribution to this response is NO mediated. Along these lines, combined COX-NOS inhibition did not further attenuate the absolute recovery compared with NOS inhibition alone (protocol 2, Table 2). However, this approach does not take into account any differences in flow before inflation or the degree of flow reduction at the onset of balloon inflation. In contrast, analyzing the percent recovery accounts for any drug-related changes in flow throughout the exercise trial and is more appropriate for addressing the aims of the current study.
The ability to deliver sufficient pharmacological inhibitors to examine pathways of interest is always a concern in these types of vascular studies. In the present study, the efficacy of NOS inhibition with l-NMMA was confirmed via intra-arterial ACh. Similar to some of our previous findings (4, 6), l-NMMA reduced the vasodilator response to exogenous ACh by ∼40–50%. Unfortunately, the effectiveness of COX inhibition was not assessed directly. However, the local dose of Ketorolac used in the present study was equal to or higher than either systemic or local doses of related compounds that have been used in previous studies that clearly either limited production of PGs (7, 14, 34) or had an obvious effect on blood flow responses (9, 14, 29, 34).
Last, in the current study, we used Ketorolac, a nonselective COX inhibitor, to examine the contribution of PGs in the compensatory vasodilator response to acute hypoperfusion in exercising human skeletal muscle. Therefore, it is possible that Ketorolac not only blocked vasodilator PG production but also inhibited thromboxane A2-mediated vasoconstriction and thus masked the vasodilator role of PGs in the compensatory response. Unfortunately, measurements of PG and thromboxane metabolites were not performed in the current study to determine the magnitude of change in each of these pathways following COX inhibition.
In conclusion, we found that PGs do not contribute to the compensatory vasodilation (%recovery) observed during forearm exercise with hypoperfusion. This occurred in the presence and absence of available NO. Taken together with our previous studies (3, 4), our data suggest that adenosine and NO contribute to the regulation of skeletal muscle blood flow during exercise with hypoperfusion, whereas PGs are not obligatory.
GRANTS
This study was supported by National Institutes of Health Research Grants HL-46493 (to M. J. Joyner), AR-55819 (to D. P. Casey), and by CTSA RR-024150. The Caywood Professorship via the Mayo Foundation also supported this research.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Branton Walker, Shelly Roberts, Jean Knutson, Karen Krucker, Chistopher Johnson, and Pam Engrav for technical assistance. We also thank the volunteers for their time.
REFERENCES
- 1. Boushel R, Langberg H, Risum N, Kjaer M. Regulation of blood flow by prostaglandins. Curr Vasc Pharmacol 2: 191–197, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Carlsson I, Wennmalm A. Effect of different prostaglandin synthesis inhibitors on post-occlusive blood flow in human forearm. Prostaglandins 26: 241–252, 1983 [DOI] [PubMed] [Google Scholar]
- 3. Casey DP, Joyner MJ. Contribution of adenosine to the compensatory dilation in hypoperfused contracting human muscles is independent of nitric oxide. J Appl Physiol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casey DP, Joyner MJ. NOS inhibition blunts and delays the compensatory dilation in hypoperfused contracting human muscles. J Appl Physiol 107: 1685–1692, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casey DP, Joyner MJ. Skeletal muscle blood flow responses to hypoperfusion at rest and during rhythmic exercise in humans. J Appl Physiol 107: 429–437, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casey DP, Madery BD, Curry TB, Eisenach JH, Wilkins BW, Joyner MJ. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J Physiol 588: 373–385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments alpha-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol 287: H2576–H2584, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Duffy SJ, Castle SF, Harper RW, Meredith IT. Contribution of vasodilator prostanoids and nitric oxide to resting flow, metabolic vasodilation, and flow-mediated dilation in human coronary circulation. Circulation 100: 1951–1957, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Duffy SJ, New G, Tran BT, Harper RW, Meredith IT. Relative contribution of vasodilator prostanoids and NO to metabolic vasodilation in the human forearm. Am J Physiol Heart Circ Physiol 276: H663–H670, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol 81: 1807–1814, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Friedman PL, Brown EJ, Jr, Gunther S, Alexander RW, Barry WH, Mudge GH, Jr, Grossman W. Coronary vasoconstrictor effect of indomethacin in patients with coronary-artery disease. N Engl J Med 305: 1171–1175, 1981 [DOI] [PubMed] [Google Scholar]
- 12. Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O'Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Kamper AM, Paul LC, Blauw GJ. Prostaglandins are involved in acetylcholine- and 5-hydroxytryptamine-induced, nitric oxide-mediated vasodilatation in human forearm. J Cardiovasc Pharmacol 40: 922–929, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Kilbom A, Wennmalm A. Endogenous prostaglandins as local regulators of blood flow in man: effect of indomethacin on reactive and functional hyperaemia. J Physiol 257: 109–121, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meeking DR, Browne DL, Allard S, Munday J, Chowienczyck PJ, Shaw KM, Cummings MH. Effects of cyclo-oxygenase inhibition on vasodilatory response to acetylcholine in patients with type 1 diabetes and nondiabetic subjects. Diabetes Care 23: 1840–1843, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Mortensen SP, Gonzalez-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol 581: 853–861, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mortensen SP, Nyberg M, Thaning P, Saltin B, Hellsten Y. Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension 53: 993–999, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Naylor HL, Shoemaker JK, Brock RW, Hughson RL. Prostaglandin inhibition causes an increase in reactive hyperaemia after ischaemic exercise in human forearm. Clin Physiol 19: 211–220, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Nicholson WT, Vaa B, Hesse C, Eisenach JH, Joyner MJ. Aging is associated with reduced prostacyclin-mediated dilation in the human forearm. Hypertension 53: 973–978, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nowak J, Wennmalm A. A study on the role of endogenous prostaglandins in the development of exercise-induced and post-occlusive hyperemia in human limbs. Acta Physiol Scand 106: 365–369, 1979 [DOI] [PubMed] [Google Scholar]
- 21. Nyberg M, Mortensen SP, Thaning P, Saltin B, Hellsten Y. Interstitial and plasma adenosine stimulate nitric oxide and prostacyclin formation in human skeletal muscle. Hypertension 56: 1102–1108, 2010 [DOI] [PubMed] [Google Scholar]
- 22. O'Leary DS, Augustyniak RA. Muscle metaboreflex increases ventricular performance in conscious dogs. Am J Physiol Heart Circ Physiol 275: H220–H224, 1998 [DOI] [PubMed] [Google Scholar]
- 23. O'Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle in dogs? Am J Physiol Heart Circ Physiol 268: H980–H986, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol 550: 317–324, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ray CJ, Abbas MR, Coney AM, Marshall JM. Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies. J Physiol 544: 195–209, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruocco NA, Most AS, Sasken H, Steiner M, Gewirtz H. Role of endogenous prostacyclin in myocardial blood flow regulation distal to a severe coronary stenosis. Cardiovasc Res 22: 511–519, 1988 [DOI] [PubMed] [Google Scholar]
- 27. Saunders NR, Dinenno FA, Pyke KE, Rogers AM, Tschakovsky ME. Impact of combined NO and PG blockade on rapid vasodilation in a forearm mild-to-moderate exercise transition in humans. Am J Physiol Heart Circ Physiol 288: H214–H220, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Schrage WG, Dietz NM, Eisenach JH, Joyner MJ. Agonist-dependent variablity of contributions of nitric oxide and prostaglandins in human skeletal muscle. J Appl Physiol 98: 1251–1257, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol 557: 599–611, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sheriff DD, Wyss CR, Rowell LB, Scher AM. Does inadequate oxygen delivery trigger pressor response to muscle hypoperfusion during exercise? Am J Physiol Heart Circ Physiol 253: H1199–H1207, 1987 [DOI] [PubMed] [Google Scholar]
- 31. Shoemaker JK, Naylor HL, Pozeg ZI, Hughson RL. Failure of prostaglandins to modulate the time course of blood flow during dynamic forearm exercise in humans. J Appl Physiol 81: 1516–1521, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Tomai F, Crea F, Gaspardone A, Versaci F, Ghini AS, Parma A, Chiariello L, Gioffre PA. Acetylcholine-induced vasodilatation in the human peripheral circulation is independent of ATP-sensitive K+ channels and prostacyclin. G Ital Cardiol 27: 1237–1244, 1997 [PubMed] [Google Scholar]
- 33. Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol 74: 2566–2573, 1993 [DOI] [PubMed] [Google Scholar]
- 34. Wilson JR, Kapoor SC. Contribution of prostaglandins to exercise-induced vasodilation in humans. Am J Physiol Heart Circ Physiol 265: H171–H175, 1993 [DOI] [PubMed] [Google Scholar]
- 35. Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol 245: H481–H486, 1983 [DOI] [PubMed] [Google Scholar]