Abstract
While orthostatic tachycardia is the hallmark of postural tachycardia syndrome (POTS), orthostasis also initiates increased minute ventilation (V̇e) and decreased end-tidal CO2 in many patients. We hypothesized that chemoreflex sensitivity would be increased in patients with POTS. We therefore measured chemoreceptor sensitivity in 20 POTS (16 women and 4 men) and 14 healthy controls (10 women and 4 men), 16–35 yr old by exposing them to eucapneic hyperoxia (30% O2), eucapneic hypoxia (10% O2), and hypercapnic hyperoxia (30% O2 + 5% CO2) while supine and during 70° head-upright tilt. Heart rate, mean arterial pressure, O2 saturation, end-tidal CO2, and V̇e were measured. Peripheral chemoreflex sensitivity was calculated as the difference in V̇e during hypoxia compared with room air divided by the change in O2 saturation. Central chemoreflex sensitivity was determined by the difference in V̇e during hypercapnia divided by the change in CO2. POTS subjects had an increased peripheral chemoreflex sensitivity (in l·min−1·%oxygen−1) in response to hypoxia (0.42 ± 0.38 vs. 0.19 ± 0.17) but a decreased central chemoreflex sensitivity (l·min−1·Torr−1) CO2 response (0.49 ± 0.38 vs. 1.04 ± 0.18) compared with controls. CO2 sensitivity was also reduced in POTS subjects when supine. POTS patients are markedly sensitized to hypoxia when upright but desensitized to CO2 while upright or supine. The interactions between orthostatic baroreflex unloading and altered chemoreflex sensitivities may explain the hyperventilation in POTS patients.
Keywords: hypoxia, hypercapnia
orthostatic intolerance is defined by symptoms of light-headedness, nausea, cognitive disability, diaphoresis, headache, fatigue, palpitations, and heat, which are present upright and resolve when recumbent. Postural tachycardia syndrome (POTS) is defined by orthostatic intolerance associated with excessive postural tachycardia, and in adults by an increase in sinus heart rate (HR) of >30 beats/min or to a HR > 120 beats/min during 10 min of orthostasis (16).
Many POTS patients have dyspnea and hyperventilation when upright. Our group has reported the association of hyperpnea with thoracic hypovolemia and splanchnic hypervolemia in POTS (33). Large decrements in end-tidal CO2 (ETCO2), often reaching 25 Torr or less, following change of position from supine to upright are driven by increased ventilation (18). This results in cerebral vasoconstriction (25), which may account, in part, for central symptoms of orthostatic intolerance. Evidence of hyperpnea in POTS patients occurs in the absence of any functional or structural cardiac or respiratory abnormalities. Candidate mechanisms for hyperpnea include a defect in chemoreflex response or in ventilatory control in these patients. There is no evidence supporting an intrinsic defect in chemoreception. Thus far baroreflex-chemoreflex interactions have not been studied in patients with POTS.
Respiratory chemoreceptors are of two types: peripheral and central. The peripheral receptors primarily respond to arterial hypoxia and to a lesser extent to hypercapnia (17). The central receptors are far more sensitive to hypercapnia (increase in hydrogen ions) (4). The peripheral chemoreceptors are contained within the carotid and aortic bodies, whereas the central chemoreceptors are currently believed to be distributed mainly in the medulla (5).
Respiratory chemoreceptors can modulate the autonomic nervous system. The modulation can be indirect, via changes in blood O2 and CO2 concentration, or by inhibiting K+ channels, depolarizing chemosensitive neurons, and thereby increasing their firing rate (27, 31). Modulation can also be direct via sympathetic facilitation (30).
Baroreceptors can also modulate chemoreflex activity and ventilation. For example, ventilatory responses to chemoreceptor stimulation are augmented by arterial baroreceptor unloading and are depressed by baroreceptor loading (12).
Based on these relationships, we tested the hypothesis that chemoreflex sensitivities are increased in POTS patients and are potentiated by orthostatic baroreflex unloading causing hyperventilation.
MATERIALS AND METHODS
The study was approved by the Institutional Review Board of New York Medical College, and informed consent was obtained from all subjects.
Subjects
We studied 20 POTS [16 women (W) and 4 men (M)] and 14 healthy subjects (10 W and 4 M) between the ages of 16 and 35 yr and with height of 169 ± 9 cm, weight of 63.8 ± 10.3 kg, and body mass index of 22.4 ± 0.8 kg/m2 (means ± SE). All subjects were nonsmokers and normotensive. Subjects were not taking medications, and none of them had been at high altitude (>1,500 m) for at least 5 mo. Hemoglobin concentrations were normal and ranged from 10.2 to 17.4 mg/dl. Since hypovolemia and dehydration may increase ventilation, POTS patients were screened for evidence of hypovolemia and abnormal renin-angiotensin-aldosterone levels. None of the enrolled patients was hypovolemic or had any other hormonal or hematologic abnormalities. Because of the effects of the menstrual cycle on cardiovascular regulation (21), all female subjects were studied during their early follicular phase (1–4 days after the onset of menstruation).
Experimental Setup
The ventilatory responses to eucapneic hypoxia and hyperoxic hypercapnia were obtained using the “dynamic end-tidal forcing” technique (34). This technique allows for the manipulation of end-tidal concentration of one gas while maintaining the end-tidal concentration for another gas constant. We used four gas conditions intended to separate the effects of hypoxia and hypercapnia on chemoreflex function: eucapneic normoxia (breathing room air), eucapneic hypoxia (10% O2 balanced with N2 while maintaining ETCO2, unchanged from breathing room air), eucapneic hyperoxia (30% O2 while maintaining ETCO2 unchanged from breathing room air), and hypercapnic hyperoxia (30% O2 + 5% CO2).
Eucapneic hypoxia was used to assess the hypoxic response attributed to peripheral chemoreflex sensitivity (PCS). Thirty percent O2 (hyperoxia) was used to minimize the PCS. Hyperoxic hypercapnia was used to measure the hypercapnic [central chemoreceptor sensitivity (CCS)] chemoreflex function by suppressing the effect of peripheral chemoreceptors (carotid body) with O2 and stimulating central chemoreceptors with CO2 (23). An open circuit was used to control inspired gases during each of these gas conditions. Open circuit breathing uses two-way valves to eliminate exhaled CO2 and uses premixed gases for inhalation.
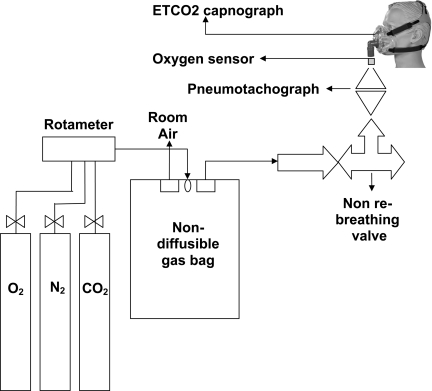
Subjects breathed through a face mask connected to a pneumotachograph (Hans Rudolph, Shawnee, KS), from which we obtained inspired minute volumes. The other end of this pneumotachograph was connected through a low-resistance two-way nonrebreathing valve (Hans Rudolph), which had two outlets. One outlet of this nonrebreathing valve was used for expired gas and connected to a room air exhaust; the other end of the nonrebreathing valve was connected to a 30-liter breathing bag (Rusch, Teleflex Medical). The gas in the bag was constantly replenished with gases (O2, CO2, and N2), mixed to the desired concentration using a rotameter (model FL-6GP, Omega Engineering, Stamford, CT). The inspired content of the bag was changed by varying the concentrations of O2, CO2, and N2. The breathing bag was always fully inflated at the start of inspiration, and excess gas was vented through an exhaust valve. The flow was modulated to ensure that a sufficient amount of gas was available during the increased ventilation induced by hypercapnia.
Two stage gas flow regulators connected to each of the three air cylinders (O2, N2, and CO2) were used to achieve constant flow at the desired concentration of the air mixture, balanced with N2 (5% CO2, 10% O2, 30% O2, balanced by N2). Inhaled O2 concentration was measured continuously and directly by an O2 sensor (S-3A/I Oxygen Analyzer with N-22M Sensor, AEI Technologies, Naperville, IL), attached near the face mask. ETCO2 was measured by nasal prongs connected to a side stream capnograph (Capnocheck sleep capnograph/oximeter, Smiths Medical, St. Paul, MN). The pneumotachograph was calibrated using a 3-liter syringe, and the O2 sensor was calibrated using known concentrations of O2 before each testing session. A schematic diagram of the experimental setup is shown in Fig. 1.
Fig. 1.
Schematic diagram of the experimental setup. ETCO2, end-tidal CO2.
For eucapneic normoxia, the valves were adjusted so that subjects breathed room air. For eucapneic hypoxia, the level of O2 was titrated so that subjects breathed 10% O2 (monitored by O2 sensor) for 8 min. During this time, subjects typically achieved an arterial O2 saturation (SaO2) of ∼80–85% as assessed by pulse oximetry of the earlobe. For hyperoxia, the level of O2 was titrated so that subjects breathed 30% O2 (monitored by O2 sensor) for 8 min. For hypercapnic hyperoxia, the level of O2 and CO2 was titrated so that subjects breathed 30% O2 plus 5% CO2 to increase ETCO2 to 60 Torr or to 10 Torr more than baseline level (monitored by O2 sensor and by capnograph) for 8 min.
The ETCO2 levels were displayed in real time on the capnograph screen, ETCO2 giving an accurate noninvasive measurement of arterial Pco2 (28). Subjects rested and breathed room air in between the hypoxic and hyperoxic conditions. Measurements of all respiratory and cardiovascular parameters were made once a steady state was achieved.
Protocol
Testing began at 10:00 am after 4 h of fast. Subjects refrained from beverages containing xanthine and caffeine for at least 72 h before testing. Subjects were familiarized with the procedures used in the study. Subjects were instrumented for electrocardiography, respiratory plethysmography, SaO2 by pulse oxymetry, pneumotachography via a facemask, impedance plethysmography, continuous blood pressure recording, and capnography. After an initial 20 min of acclimatization, subjects were monitored during a 20-min rest period in which ETCO2 was measured via a nasal cannula. Mean ETCO2 over the last 5 min of rest period was defined as isocapnia for the particular subject for the remainder of the protocol. Subjects then underwent four measurement periods separated by 30-min rest periods. Each measurement period corresponded to one of the following gas conditions presented in random order: eucapneic normoxia (room air), eucapneic hypoxia, eucapneic hyperoxia, and hypercapnic hyperoxia. We randomized the order of conditions between eucapneic normoxia, eucapneic hyperoxia, hyperoxic hypercapnia, and eucapneic hypoxia because the order of gas might effect the responses (13). Subjects breathed gases for 8 min or until the subject requested to stop.
After all supine data collections were complete, the subjects were reintroduced to each gas condition for 1 min while supine and were tilted head-upright to 70° (HUT) for a maximum of 8 min in each gas condition. Continuous HR, mean arterial pressure (MAP), SaO2, ETCO2, and minute ventilation (V̇e) were recorded. Subjects were tilted back to the supine position if fainting ensued or if the subject requested to stop. Cardiac output (CO) was estimated using the model flow method contained within the Finometer.
Data Analysis
Data were digitized at 200 Hz with signal processing software and analyzed off-line. Beat-to-beat data were collected during the last 3 min for HR, MAP (Finometer, TNO, Amsterdam, The Netherlands), SaO2, ETCO2, and expiratory V̇e during supine and HUT in each of the gas conditions.
Cardiovascular responses were further assessed by measuring MAP, systolic blood pressure (SBP), diastolic blood pressure (DBP), HR, CO, total peripheral resistance (TPR), and stroke volume (SV) during ventilatory changes.
MAP was calculated from SBP and DBP with the formula MAP = (SBP + 2·DBP)/3. TPR was calculated as MAP/CO.
Ventilation versus gas content function curves were constructed with V̇e on the ordinate and the gas measure on the abscissa. The operating point was defined by room air (normoxia-eucapnia) for each subject.
Ventilatory PCS (response to hypoxia) was determined by calculating linear regression slopes between V̇e and SaO2 (gas measure) during eucapneic hyperoxia to eucapneic hypoxia (the hypoxic ventilatory response).
Ventilatory central chemoreflex sensitivity (CCS) (response to hypercapnia) was determined by calculating linear regression slopes between V̇e and ETCO2 (gas measure) during eucapneic hyperoxia and hypercapnia.
Statistics
Measurements across the conditions were computed for PCS and CCS for each subject. Individual regression slopes between V̇e, hypoxia, and hypercapnia were averaged across each condition and compared for supine and HUT. Regressions for chemoreflex responses were compared across the two conditions during supine and HUT conditions using a two-way repeated-measures analysis of variance. Likewise, cardiovascular responses (HR, MAP, SBP, DBP, CO, TPR, and SV) were compared across supine and HUT conditions. When appropriate, post hoc comparisons were performed using Tukey's test. Primary outcome variables were correlated to find causal associations. Differences were considered significant when P < 0.05. All values are reported as means ± SE unless otherwise indicated.
RESULTS
Out of 20 POTS (16 W and 4 M) and 14 control (10 W and 4 M) subjects, 19 POTS (15 W and 4 M) and 12 control (8 W and 4 M) subjects completed the eucapneic normoxia (room air HUT) protocol, 13 POTS (9 W and 4 M) and 6 control (5 W and 1 M) subjects completed the hyperoxic HUT protocol, 13 POTS (9 W and 4 M) and 6 control (5 W and 1 M) subjects completed the eucapneic hypoxia HUT protocol, and 9 POTS (5 W and 4 M) and 6 control (5 W and 1 M) subjects completed the hypercapnic hyperoxia HUT protocol. Data of patients completing each gas condition have been averaged and compared across groups. Group-averaged data for POTS and control subjects for every gas condition are shown for the supine position (Table 1) and upright position (Table 2).
Table 1.
Ventilatory and cardiovascular measures for peripheral and central chemoreflex sensitivity in supine position
| Central Chemoreflex |
Peripheral Chemoreflex |
|||||||
|---|---|---|---|---|---|---|---|---|
| Eucapneic normoxia |
Hyperoxic hypercapnia |
Eucapneic hyperoxia |
Eucapneic hypoxia |
|||||
| Supine | Controls | POTS | Controls | POTS | Controls | POTS | Controls | POTS |
| Arterial oxygen saturation, % | 97.5 ± 0.6 | 97.2 ± 0.2 | 99.2 ± 0.6 | 98.9 ± 0.2 | 99.2 ± 0.6 | 98.9 ± 0.2 | 80.8 ± 2.3* | 84.7 ± 1.4*† |
| Minute ventilation, l/min | 4.1 ± 0.4 | 5.5 ± 0.4 | 20.1 ± 2.4* | 14.3 ± 2.1* | 6.1 ± 0.9* | 6.8 ± 0.6* | 6.5 ± 0.6* | 7.5 ± 1.0* |
| Respiratory rate, beats/min | 16.7 ± 1.1 | 16.8 ± 0.7 | 15 ± 1.0 | 14.8 ± 0.8 | 18.6 ± 1.0 | 16.9 ± 0.9 | 14.3 ± 0.9 | 16.9 ± 1.0 |
| ETCO2, Torr | 43.0 ± 0.7 | 42.2 ± 1.0 | 57.3 ± 4.7* | 55.3 ± 6.6* | 44.3 ± 1.6 | 42.2 ± 1.1 | 43.2 ± 0.8 | 42.3 ± 2.0 |
| Heart rate, beats/min | 63.7 ± 3.2 | 75.4 ± 3.2 | 76.5 ± 3.4* | 84.5 ± 3.1* | 60.1 ± 2.7 | 72.8 ± 3.7 | 71.6 ± 3.4 | 83.5 ± 3.2* |
| Systolic arterial pressure, mmHg | 121.2 ± 2.8 | 112.7 ± 1.9† | 129.6 ± 4.2 | 125.25 ± 2.7 | 117.7 ± 3.1 | 120.3 ± 2.3 | 116.6 ± 3.1 | 116.9 ± 1.9 |
| Diastolic arterial pressure, mmHg | 62.9 ± 1.7 | 61.7 ± 1.6 | 70.3 ± 2.1* | 67.9 ± 1.9 | 62.2 ± 1.9 | 66.2 ± 2.0 | 61.4 ± 2.3 | 64.0 ± 2.2 |
| Mean arterial pressure, mmHg | 84.0 ± 1.9 | 79.0 ± 1.5 | 93.6 ± 2.5* | 88.3 ± 1.9 | 83.4 ± 2.4 | 84.1 ± 1.7 | 81.8 ± 2.6 | 84.2 ± 1.9 |
| Cardiac output, l/min | 4.9 ± 0.4 | 4.9 ± 0.2 | 6.2 ± 0.3* | 5.6 ± 0.3 | 5.3 ± 0.4 | 4.8 ± 0.3 | 5.8 ± 0.5 | 5.2 ± 0.3 |
| TPR, mmHg·l−1·min−1 | 17.9 ± 1.3 | 16.6 ± 1.1 | 16.2 ± 1.1 | 18.2 ± 1.1 | 16.3 ± 1.1 | 18.3 ± 1.1 | 14.8 ± 1.1 | 17.5 ± 1.1 |
| Stroke volume, ml | 81.1 ± 8.1 | 66.1 ± 2.1† | 81.1 ± 5.1 | 68.1 ± 3.1 | 93.1 ± 8.1* | 69.1 ± 5.1 | 88.1 ± 11.1 | 66.1 ± 2.1† |
Values are means ± SE.
POTS, postural tachycardia syndrome; ETCO2, end-tidal CO2; TPR, total peripheral resistance.
P < 0.05 compared with eucapneic normoxia;
P < 0.05 compared with controls.
Table 2.
Ventilatory and cardiovascular measures for peripheral and central chemoreflex sensitivity in HUT70
| Central Chemoreflex |
Peripheral Chemoreflex |
|||||||
|---|---|---|---|---|---|---|---|---|
| Eucapneic normoxia |
Hyperoxic hypercapnia |
Eucapneic hyperoxia |
Eucapneic hypoxia |
|||||
| HUT70 | Controls | POTS | Controls | POTS | Controls | POTS | Controls | POTS |
| Arterial oxygen saturation, % | 97.5 ± 0.6 | 97.2 ± 0.2 | 99.2 ± 0.6 | 98.9 ± 0.2 | 98.9 ± 0.6 | 98.7 ± 0.6 | 80.8 ± 2.3* | 84.7 ± 1.4*† |
| Minute ventilation, l/min | 4.6 ± 8.8 | 8.8 ± 1.2† | 26.8 ± 3.3* | 13.6 ± 2.3*† | 6.5 ± 0.4 | 10.1 ± 1.9 | 7.6 ± 1.1* | 11.1 ± 0.9*† |
| Respiratory rate, beats/min | 16.7 ± 1.1 | 18.6 ± 1.4 | 21.4 ± 1.2* | 17.6 ± 1.9 | 14.3 ± 1.4 | 14.9 ± 1.2 | 13.5 ± 0.6 | 15.5 ± 0.9 |
| ETCO2, Torr | 41.2 ± 1.2 | 34.4 ± 1.8† | 60.2 ± 1.7* | 55.1 ± 2.5* | 42.6 ± 1.6 | 42.9 ± 1.6 | 40.1 ± 1.4 | 41.3 ± 2.3 |
| Heart rate, beats/min | 82.8 ± 3.9 | 101.5 ± 5.4†‡ | 84.9 ± 4.2 | 100.9 ± 4.5 | 76.8 ± 4.9 | 94.5 ± 6.5 | 85.9 ± 4.2 | 102.9 ± 4.5†‡ |
| Systolic arterial pressure, mmHg | 118.8 ± 3.9 | 114.1 ± 2.5 | 141.4 ± 10.9* | 133.6 ± 3.9* | 115.3 ± 7.1 | 120.2 ± 4.5 | 119.5 ± 5.2 | 121.8 ± 3.6 |
| Diastolic arterial pressure, mmHg | 67.3 ± 3.8 | 64.3 ± 2.5 | 73.5 ± 4.5* | 73.4 ± 2.7* | 62.7 ± 3.8 | 64.2 ± 2.6 | 66.8 ± 2.6 | 70.5 ± 3.1 |
| Mean arterial pressure, mmHg | 86.2 ± 3.4 | 81.8 ± 2.4 | 99.5 ± 6.4* | 94.7 ± 2.9* | 82.6 ± 5.3 | 88.9 ± 3.3 | 85.3 ± 3.1 | 89.2 ± 3.1 |
| Cardiac output, l/min | 4.3 ± 0.3.1 | 4.9 ± 0.3 | 6.2 ± 0.3* | 5.5 ± 0.3 | 4.9 ± 0.3 | 4.3 ± 0.4 | 5.1 ± 0.5 | 4.9 ± 0.4 |
| TPR, mmHg·l−1·min−1 | 21.1 ± 1.4 | 17.9 ± 1.3‡ | 16.7 ± 1.3 | 17.9 ± 1.7 | 18.3 ± 2.1 | 23.1 ± 2.4‡ | 17.6 ± 1.6 | 20.4 ± 1.9 |
| Stroke volume, ml | 52.1 ± 4.1‡ | 57.1 ± 3.1 | 74.1 ± 3.1* | 57.1 ± 5.1 | 63.1 ± 7.1‡ | 47.1 ± 06‡ | 63.1 ± 7.1* | 47.1 ± 4.1‡ |
Values are means ± SE. HUT70, head-up tilt at 70° position.
P < 0.05 compared with eucapneic normoxia;
P < 0.05 compared with controls;
compared with supine (Table 1).
Eucapneic Normoxia: Room Air
Supine: baseline conditions.
POTS and control subjects had no significant differences in SaO2, ETCO2, and respiratory rate while supine. However, POTS patients had a higher V̇e (P < 0.05), lower SBP (P < 0.05), and higher HR (P < 0.05) than control subjects. CO and TPR were not different, but SV was reduced in POTS compared with control subjects (P < 0.05) (Table 1).
HUT.
Head-up tilt to 70° was used to unload the baroreflexes. Baroreflex unloading during HUT did not affect SaO2 and respiratory rate (Table 2) in either POTS or control subjects. When compared with controls, POTS patients had significantly higher V̇e (P < 0.05), lower ETCO2 (P < 0.01), and higher HR (P < 0.01) than control subjects.
HUT decreased CO (P < 0.01) in controls. SV decreased (P < 0.001), HR increased (P < 0.001), and peripheral resistance increased (P < 0.05) in both POTS and control subjects (Table 2) compared with supine.
PCS: Eucapneic Hypoxia
Hypoxia supine.
Hypoxia in the supine position decreased SaO2 in both POTS and control subjects (P < 0.001). However, controls trended toward larger decreases in SaO2 compared with POTS patients (Table 1). A decrease in SaO2 initiates an increase in ventilation (29) in both POTS and control subjects (P < 0.05). Despite smaller decreases in SaO2, POTS patients had a higher V̇e during hypoxia than control subjects (P < 0.05) (Fig. 2). Thus, in the supine position, POTS patients had a trend toward higher hypoxic ventilatory response/PCS (in l·min−1·%oxygen−1) than controls (POTS vs. controls, 0.2 ± 0.11 vs. 0.15 ± 0.05, P = 0.08). Both POTS and control subjects had an increase in their HR (P < 0.05) (POTS > controls, P < 0.01) and an increase in their COs (Table 1).
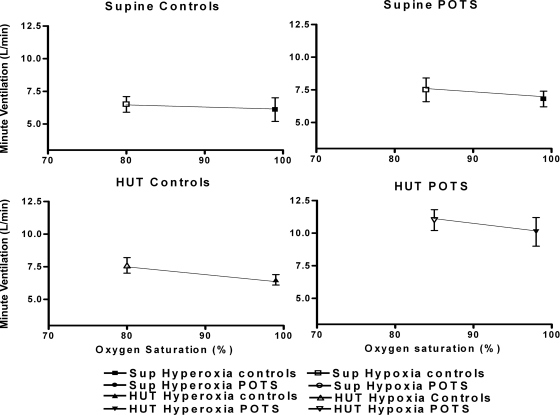
Fig. 2.
Hypoxic ventilatory response/peripheral chemoreceptor sensitivity (in l·min−1·%oxygen−1) averaged over all control subjects (left) and averaged over all postural tachycardia syndrome (POTS) patients (right). Top: results in supine (Sup) the position. Bottom: results during head-up tilt (HUT) position. These data depict the minute ventilation response to decrease in O2 saturation during peripheral chemoreceptor stimulation. Supine ventilation is increased in POTS compared with control subjects (P < 0.05). Baroreflex unloading by HUT further increases minute ventilation in POTS patients and translates the set point upward and toward the right.
Hypoxia HUT.
When compared with room air HUT, hypoxia HUT decreased SaO2 (P < 0.001), increased V̇e, and increased HR in both POTS and control subjects (P < 0.001). However, POTS patients had higher V̇e (P < 0.05) and higher HR (P < 0.05) than controls (Fig. 2). Hypoxia HUT did not change CO, TPR, MAP, CO, or SV (Table 2).
Baroreflex unloading during hypoxia did not change the PCS in controls (supine vs. HUT, 0.15 ± 0.05 vs. 0.19 ± 0.017, P = not significant) but increased PCS in POTS patients (0.2 ± 0.11 to 0.42 ± 0.38, P < 0.05). Thus PCS during HUT was higher in POTS than in control subjects (P < 0.05, Fig. 2). In addition, the hypoxic response was reset, shifted upwards and to the right so that POTS patients had a higher V̇e and higher SaO2 than control subjects (Fig. 2).
CCS: Hyperoxic Hypercapnia
Hyperoxic hypercapnia increased ventilation in both POTS and control subjects. During spontaneous breathing, hypercapnia is some 3–5 times stronger than hypoxia as a ventilatory stimulus under our experimental conditions (Table 1).
Hypercapnia supine.
When supine, POTS and control subjects had no significant differences in ETCO2. Hypercapnia increased ETCO2 in both POTS and control subjects to similar levels with a trend toward higher V̇e in controls compared with POTS patients (P = 0.08) (Fig. 3, and Table 1). The hypercapnic ventilatory response/CCS for POTS patients was lower than that for controls (0.68 ± 0.34 vs. 1.0 ± 0.18, P < 0.05).
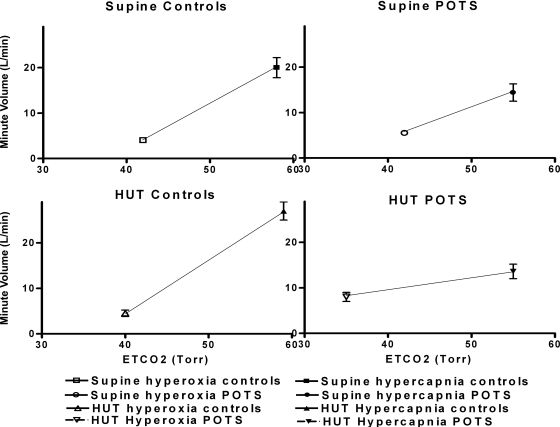
Fig. 3.
Hypercapnic ventilatory response/central chemoreceptor sensitivity (CCS; in l·min−1·Torr−1) averaged over all control subjects (left) and averaged over all POTS patients (right). Top: results during supine position. Bottom: results during HUT position. POTS patients have a decreased supine CCS compared with controls. Baroreflex unloading does not decrease the CCS in controls but decreases the CCS in POTS patients.
At room air supine position, POTS patients had a trend toward higher ventilation than did controls (Table 1). Room air hypercapnia caused a higher increase in ventilation in controls compared with POTS patients. Room air hypercapnia increased MAP and HR in both POTS and control subjects.
Hypercapnia HUT.
Baroreflex unloading during hypercapnia HUT led to higher V̇e in controls compared with POTS patients (P < 0.05, Fig. 3). SaO2 was not different between POTS and control subjects (Table 2).
When compared with room air HUT, hypercapnia HUT did not change CCS in controls (supine vs. HUT, 0.84 ± 0.27 vs. 1.04 ± 0.18, P = not significant) but decreased CCS in POTS patients (0.68 ± 0.34 vs. 0.49 ± 0.38, P < 0.05). Upright CCS for POTS patients was markedly lower than for controls (HUT-CCS, POTS vs. controls, 0.49 ± 0.38 vs. 1.04 ± 0.18, P < 0.05).
DISCUSSION
The results of this study demonstrated an increased respiratory chemoreflex response to hypoxia and a decreased respiratory chemoreflex response to hypercapnia in POTS compared with control subjects, even while supine. These differences were enhanced by orthostasis. We therefore infer that PCS measured by the hypoxic ventilatory response was increased in POTS patients, whereas CCS measured by the hypercapnic ventilatory response was reduced in POTS patients. Baroreflex unloading during HUT potentiated the hypoxic response while further blunting the hypercapnic response. Therefore, in POTS patients, baroreflex unloading during HUT stimulates peripheral O2-dependent chemoreflexes causing hyperventilation that is unopposed by the restraining effects of hypocapnia. Thus it seems that respiratory chemoreflexes are impaired in POTS patients, and their function is further perturbed by baroreflex unloading resulting in hyperventilation.
Increased PCS and Set Point in POTS
Increased ventilation can be induced in healthy humans during severe orthostatic stress that produces extreme reductions in central blood volume and CO (14). The hyperpneic response is therefore physiological in extremis.
In our earlier studies of POTS, we found that modest orthostatic stress initiates hyperpnea and hyperventilation, but not in control subjects. Hyperpnea in POTS patients was related to the enhanced thoracic hypovolemia and reduced CO in these patients (33, 35). Enhanced thoracic hypovolemia results in excessive baroreflex unloading in POTS compared with control subjects, resulting in marked sympathoexcitation (3). In addition to increased peripheral O2 chemoreceptor sensitivity in POTS patients, we observed a resetting of the hypoxic ventilatory response toward higher SaO2 and V̇e. This is directionally similar to orthostatic resetting of the arterial baroreflex known to occur in all subjects but enhanced in POTS patients (10). Thus a resetting of the peripheral chemoreceptor set point is expected when upright.
Baroreflex unloading could increase ventilation through direct sympathoexcitation of the respiratory center (33, 35), through effects on the peripheral chemoreflex (6), or through interactions of baroreflex and chemoreflex afferents in the intermediate area of the nucleus tractus solitarius (22). This could also be due to more localized effects of baroreflex-mediated sympathetic vasoconstriction of arterioles supplying the carotid and aortic bodies. Reduced local blood flow might result in a neural discharge similar to that evoked by asphyxia, anoxemia (2), or nicotine and cyanide (1, 7, 19). Indeed, McCloskey (20) showed that increased impulse traffic of the carotid sinus nerve is easily elicited by hypotension-induced reductions in carotid body blood flow.
Although overt hypotension is uncommon in POTS, intermittently reduced blood flow occurs whenever upright and might engender chemoreflex potentiation similar to potentiation caused by chronic intermittent hypoxia. Indeed, POTS patients have increased V̇e supine, similar to long-lasting increases in baseline respiratory activity and long-term facilitation of respiratory motor output in humans with intermittent hypoxia (26).
Reduced Central Chemoreceptor Sensitivity in POTS
In contrast to the increased response to hypoxia, POTS patients demonstrated depressed V̇e responses to hypercapnia compared with control subjects. Hypercapnia is usually a strong ventilatory stimulus and excites the medullary respiratory center via changes in blood pH (9). Hypercapnia may also influence sympathoexcitation at the rostral ventrolateral medulla (9). We used hyperoxic hypercapnia to examine the ventilatory effects of central chemoreceptor stimulation while minimizing the effects of peripheral chemoreceptors that are primarily O2 sensitive (15, 23). The stimulation of carotid chemoreceptors facilitates central chemoreceptors; conversely, the inhibition of carotid chemoreceptors can blunt central chemoreceptors (24). However, physiological blunting of the central chemoreceptors must have been small because no such effects were observed in control subjects. Thus the peripheral effects on central chemoreceptors cannot account for the reduced central chemoreceptor sensitivity in POTS compared with control subjects. One potential explanation for this reduced chemoreceptor sensitivity may relate to the profound reduction of the cardiovagal baroreflex in POTS patients despite enhanced sympathoexcitation (32). The application of cholinergic agents on the ventrolateral surface of the medulla in areas where the central chemoreceptors may be located stimulates breathing, whereas the application of atropine inhibits ventilation (11). Thus vagal withdrawal could account for central chemoreflex suppression; however, these experiments have only been performed in nonhuman mammals.
Clinical Implications for POTS
POTS patients have increased ventilation, especially when upright, related to sympathetic baroreflex stimulation and almost complete cardiovagal baroreflex withdrawal. Hypocapnia results because POTS patients have decreased sensitivity to CO2, whereas the response of the peripheral chemoreceptors to hypoxia is enhanced. Enhanced hypoxic sensitivity may contribute to the long-term facilitation of sympathoexcitation in these patients. Upright hyperventilation and hypocapnia reduces cerebral blood flow (8) and contributes to light-headedness and to cognitive impairment during daily life.
Limitations
Our study has several limitations. First, the cardiorespiratory effects observed in our study were induced by acute chemoreflex activation but suggest a chronic change in chemoreflex sensitivity whose origins remain unclear. Second, we did not test the effects of hypoxic hypercapnia. However, we were primarily interested in separating the ventilatory effects of O2 and CO2. In retrospect, this could have further delineated the role and contribution of carotid bodies in peripheral and central chemoreceptor interactions.
Summary
The present study demonstrates the enhanced ventilation in response to hypoxia and the blunting of the ventilation response to hypercapnia particularly during baroreflex unloading in POTS patients compared with healthy control subjects.
GRANTS
This study was supported by American Heart Association Grant 0735603T and National Heart, Lung, and Blood Institute Grant 1R21-HL-091948.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Argacha JF, Xhaet O, Gujic M, Adamopoulos D, Beloka S, Dreyfuss C, Degaute JP, van de Borne P. Nicotine increases chemoreflex sensitivity to hypoxia in non-smokers. J Hypertens 26: 284–294, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Bogue J, Stella G. Afferent impulses in the carotid sinus nerve (nerve of hering) during asphyxia and anoxaemia. J Physiol 83: 459–465, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonyhay I, Freeman R. Sympathetic nerve activity in response to hypotensive stress in the postural tachycardia syndrome. Circulation 110: 3193–3198, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bruce EN, Cherniack NS. Central chemoreceptors. J Appl Physiol 62: 389–402, 1987 [DOI] [PubMed] [Google Scholar]
- 5. Coates EL, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol 75: 5–14, 1993 [DOI] [PubMed] [Google Scholar]
- 6. Cunningham DJ. Studies on arterial chemoreceptors in man. J Physiol 384: 1–26, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernandez R, Larrain C, Zapata P. Acute ventilatory and circulatory reactions evoked by nicotine: are they excitatory or depressant? Respir Physiol Neurobiol 133: 173–182, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Fortune JB, Feustel PJ, deLuna C, Graca L, Hasselbarth J, Kupinski AM. Cerebral blood flow and blood volume in response to O2 and CO2 changes in normal humans. J Trauma 39: 463–471, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Guyenet PG, Stornetta RL, Abbott SB, Depuy SD, Fortuna MG, Kanbar R. Central CO2-chemoreception and integrated neural mechanisms of cardiovascular and respiratory control. J Appl Physiol 108: 995–1002, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Halliwill JR, Morgan BJ, Charkoudian N. Peripheral chemoreflex and baroreflex interactions in cardiovascular regulation in humans. J Physiol 552: 295–302, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haxhiu MA, Mitra J, van Lunteren E, Bruce EN, Cherniack NS. Hypoglossal and phrenic responses to cholinergic agents applied to ventral medullary surface. Am J Physiol Regul Integr Comp Physiol 247: R939–R944, 1984 [DOI] [PubMed] [Google Scholar]
- 12. Heistad DD, Abboud FM, Mark AL, Schmid PG. Interaction of baroreceptor and chemoreceptor reflexes. Modulation of the chemoreceptor reflex by changes in baroreceptor activity. J Clin Invest 53: 1226–1236, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Howard LS, Robbins PA. Problems with determining the hypoxic response in humans using stepwise changes in end-tidal Po2. Respir Physiol 98: 241–249, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Landgren S, Neil E. Chemoreceptor impulse activity following haemorrhage. Acta Physiol Scand 23: 158–167, 1951 [DOI] [PubMed] [Google Scholar]
- 15. Llyod BB, Cunningham DJ. A quantitative approach to the regulation of human respiration. In: The Regulation of Human Respiration. Oxford: Blackwell, 1963 [Google Scholar]
- 16. Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA, Rummans TA. Postural tachycardia syndrome (POTS). Neurology 45: S19–S25, 1995 [PubMed] [Google Scholar]
- 17. Marshall JM. Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev 74: 543–594, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Martinon-Torres F, Rodriguez-Nunez A, Fernandez-Cebrian S, Eiris-Punal J, Perez-Munuzuri A, Martinon-Sanchez JM. The relation between hyperventilation and pediatric syncope. J Pediatr 138: 894–897, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Matsuura S. Chemoreceptor properties of glomus tissue found in the carotid region of the cat. J Physiol 235: 57–73, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCloskey DI. Carbon Dioxide and Carotid Body, edited by Torrance RW. Oxford: Blackwell, 1966 [Google Scholar]
- 21. Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Miura M, Reis DJ. The role of the solitary and paramedian reticular nuclei in mediating cardiovascular reflex responses from carotid baro- and chemoreceptors. J Physiol 223: 525–548, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mohan RM, Amara CE, Cunningham DA, Duffin J. Measuring central-chemoreflex sensitivity in man: rebreathing and steady-state methods compared. Respir Physiol 115: 23–33, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Nattie G, Li A. Multiple central chemoreceptor sites: cell types and function in vivo. Adv Exp Med Biol 605: 343–347, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Novak V, Novak P, Opfer-Gehrking TL, Low PA. Postural tachycardia syndrome: time frequency mapping. J Auton Nerv Syst 61: 313–320, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Prabhakar NR, Peng YJ, Kumar GK, Nanduri J, Di GC, Lahiri S. Long-term regulation of carotid body function: acclimatization and adaptation—invited article. Adv Exp Med Biol 648: 307–317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol 287: C1493–C1526, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Rafferty GF, Lou HM, Polkey MI, Greenough A, Moxham J. Effect of hypercapnia on maximal voluntary ventilation and diaphragm fatigue in normal humans. Am J Respir Crit Care Med 160: 1567–1571, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Severinghaus JW. Proposed standard determination of ventilatory responses to hypoxia and hypercapnia in man. Chest 70: 129–131, 1976 [DOI] [PubMed] [Google Scholar]
- 30. Shoemaker JK, Vovk A, Cunningham DA. Peripheral chemoreceptor contributions to sympathetic and cardiovascular responses during hypercapnia. Can J Physiol Pharmacol 80: 1136–1144, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Steinback CD, Salzer D, Medeiros PJ, Kowalchuk J, Shoemaker JK. Hypercapnic vs. hypoxic control of cardiovascular, cardiovagal, and sympathetic function. Am J Physiol Regul Integr Comp Physiol 296: R402–R410, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Stewart JM. Autonomic nervous system dysfunction in adolescents with postural orthostatic tachycardia syndrome and chronic fatigue syndrome is characterized by attenuated vagal baroreflex and potentiated sympathetic vasomotion. Pediatr Res 48: 218–226, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Stewart JM, Medow MS, Cherniack NS, Natelson BH. Postural hypocapnic hyperventilation is associated with enhanced peripheral vasoconstriction in postural tachycardia syndrome with normal supine blood flow. Am J Physiol Heart Circ Physiol 291: H904–H913, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Swanson GD, Bellville JW. Step changes in end-tidal CO2: methods and implications. J Appl Physiol 39: 377–385, 1975 [DOI] [PubMed] [Google Scholar]
- 35. Taneja I, Medow MS, Glover JL, Raghunath NK, Stewart JM. Increased vasoconstriction predisposes to hyperpnea and postural faint. Am J Physiol Heart Circ Physiol 295: H372–H381, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]